Abstract
Ghrelin, a natural ligand for the growth hormone (GH)-secretagogue receptor, is primarily produced in the stomach. Administration of ghrelin stimulates food intake and GH secretion in both animals and humans. Ghrelin is the only circulating hormone known to stimulate appetite in humans. As GH is an anabolic hormone, protein stores are spared at the expense of fat during conditions of caloric restriction. Ghrelin also inhibits the production of anorectic proinflammatory cytokines. Thus, ghrelin exhibits anti-cachectic actions via both GH-dependent and -independent mechanisms. Several studies are evaluating the efficacy of ghrelin in the treatment of cachexia caused by a variety of diseases, including congestive heart failure, chronic obstructive pulmonary disease, cancer, and end-stage renal disease. These studies will hopefully lead to the development of novel clinical applications for ghrelin in the future. These studies have also facilitated a better understanding of the molecular basis of the anti-catabolic effects of ghrelin. This review summarizes the recent advances in this area of research.
Keywords: Anorexia, Sarcopenia, Catabolism, Anabolism, Growth hormone, GHS
Introduction
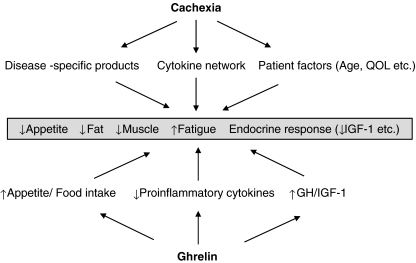
Ghrelin is a natural ligand for the growth hormone (GH)-secretagogue receptor (GHS-R), which possesses a unique fatty acid modification, an n-octanoylation, at Ser 3 [1]. Of the two circulating forms of ghrelin, acylated and unacylated (des-acyl), the acylated form is essential for the biological activity of ghrelin acting via the GHS-R. Ghrelin plays a critical role in a variety of physiological processes, including the stimulation of GH secretion and regulation of energy homeostasis by stimulating food intake and promoting adiposity via a GH-independent mechanism [2–4]. GH, which regulates insulin-like growth factor (IGF)-I levels, is an anabolic hormone that spares protein stores at the expense of fat utilization during conditions of caloric restriction. GH and IGF-1 are the major mediators of metabolism involved in the regulation of energy balance. Ghrelin inhibits the production of anorectic proinflammatory cytokines, including interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α [5, 6]. The combination of these actions suggests this peptide has benefits for the treatment of cachexia (Fig. 1).
Fig. 1.
Mechanisms of cachexia and the therapeutic benefits of ghrelin. QOL quality of life
Cachexia is defined as a complex metabolic syndrome associated with underlying illness and characterized by loss of muscle with or without loss of fat mass [7]. The prominent clinical feature of cachexia is weight loss in adults (corrected for fluid retention) or growth failure in children (excluding endocrine disorders). Anorexia, inflammation, insulin resistance, and increased muscle protein breakdown are frequently associated with cachexia. Cachexia is distinct from starvation, age-related loss of muscle mass, primary depression, malabsorption, and hyperthyroidism and is associated with increased morbidity. Recently, several trials attempting to treat cachexia of different etiologies with ghrelin have been expanded. This review summarizes the recent advances in this area of research.
Physiologic and pharmacologic actions of ghrelin
Orexigenic action
Ghrelin has a well-established role in stimulating appetite and increasing food intake [8, 9]; peripheral administration of ghrelin stimulates GH secretion and food intake in both animals and humans [10, 11]. Ghrelin is the only hormone known to stimulate appetite after peripheral administration. Ghrelin, which increases c-fos expression in the arcuate nucleus, also activates hypothalamic neuropeptide Y/Y1 receptors and agouti-related peptide pathways [12–14]. In addition, ghrelin induces food intake via the orexin pathway [15]. These functions are mediated at least in part by vagal nerve pathways [16]. Repeated administration of ghrelin resulted in significant weight gain in rats [17] and patients with chronic obstructive pulmonary disease (COPD) [18]. Increases in adiposity were associated with body weight gain in animal experiments [17, 19, 20], while adiposity decreased in humans [21–23]. This discrepancy may result from the differences in the doses and frequencies of ghrelin administration. Long-term, twice-weekly injection with low-dose ghrelin (40 μg/kg) significantly decreased fat mass in aged mice [24]. While weekly food intake did not increase under these conditions, ghrelin-induced GH secretion may have contributed to low adiposity. In contrast, lean body mass increased in rodents [24, 25] and humans [18, 21–23] following ghrelin administration. Also, lean body mass increases following ghrelin mimetic administration [26, 27]. These effects, which reflect increases in muscle mass, are promising for cachexia treatment, as losses in body weight and sarcopenia are characteristic features of cachexia.
Stimulation of GH secretion
Ghrelin strongly stimulates GH secretion in humans [28–31], severalfold more potently than GHRH under similar circumstances. Furthermore, ghrelin and GHRH synergistically increases GH release [30]. Ghrelin might also play a role on GH release in a non-acute setting [32]. GH regulates IGF-I levels, promotes anabolism, and increases muscle strength [33, 34]. While GH enhances lipolysis, IGF-1 stimulates protein synthesis, myoblast differentiation, and muscle growth. Recombinant GH is currently approved by the U.S. Food and Drug Administration for use in HIV/AIDS wasting, parenteral nutrition-dependent short bowel syndrome, pediatric chronic kidney disease, and adult and pediatric GH-deficiency states [35]. Pharmacological doses of this agent, however, cause problematic side effects, such as dose-related arthralgias, carpal-tunnel syndrome, paresthesias, insulin resistance, sodium retention, and peripheral edema. In contrast, stimulation of GH production to supraphysiological levels following ghrelin administration has a paucity of side effects.
Ghrelin’s ability to increase circulating IGF-1 levels has been demonstrated in human studies of congestive heart failure (CHF) and COPD, in which 3-week ghrelin injections tended to increase IGF-1 levels [18, 36]. This effect is less evident as that seen for growth hormone secretagogues (GHS), such as MK-677 and anamorelin (RC-1291). Long-term treatment (6 months) with MK-677 in patients with hip fractures increased IGF-1 levels by 84% in comparison to 17% after placebo [37]. Anamorelin treatment induced impressive increases in food intake in a 12-week trial of cancer cachexia; post-treatment IGF-1 levels were 36.5 ng/mL after anamorelin treatment in comparison to 5.95 ng/mL after placebo. In a 6-day trial of healthy volunteers, post-treatment IGF-1 levels increased to greater than 60 ng/mL after anamorelin treatment in comparison to <0 ng/mL after placebo [38, 39]. In our study of patients undergoing total hip replacement for osteoarthritis, serum IGF levels changed significantly following eight daily ghrelin injections; the changes in post-treatment IGF-1 levels were 30.0 ng/mL for ghrelin in comparison to 5.6 ng/mL for placebo. These changes were not observed after 21 daily treatments. In males, however, serum IGF-1 levels after ghrelin treatment remained elevated in comparison to the placebo group. Thus, the timing and duration of ghrelin injection and the subjects receiving treatment (e.g., disease features and sex) may influence the effect of ghrelin on IGF-1 levels. In addition, serum levels of IGF-1, which is primarily produced by the liver, reflect the systemic effects of IGF-1. GH also induces the synthesis of IGF-I in non-hepatic tissues. The local (autocrine/paracrine) effects of IGF-1 may play distinct roles in various tissues, including muscle mass regulation [34, 40]. For instance, local muscle-restricted IGF-1 transgene expression accelerates the regeneration of injured skeletal muscle in mice, modulating inflammatory responses and limiting fibrosis [41].
Anti-inflammatory action
Evidence that ghrelin exerts anti-inflammatory actions has been accumulating. Ghrelin suppresses the production of proinflammatory cytokines, including IL-1β, IL-6, and TNF-α both in vitro [5, 42] and in vivo [43–45]. In clinical trials, daily administration of ghrelin for 3 weeks decreased inflammatory cytokine levels and neutrophil density in sputum from patients with chronic respiratory infections [46]. In contrast, ghrelin induces the anti-inflammatory cytokine IL-10 [43, 47].
Ghrelin inhibits the activation of nuclear factor-κB (NF-κB), a transcription factor known to control the production of multiple proinflammatory cytokines during inflammatory insults [42, 44, 47]. Although the molecular mechanisms and cellular targets mediating ghrelin inhibition of NF-κB activation remain to be determined, the vagus nerve may play an important role in the ghrelin-mediated inhibition of proinflammatory cytokine release [44, 48]. Cachexia and muscular wasting occur via protein degradation by the ubiquitin–proteasome pathway [49]. Two muscle-specific ubiquitin ligases, muscle RING-finger protein-1 (MuRF1) and atrogin-1/muscle atrophy F-box (MAFbx), are upregulated under catabolic conditions. NF-κB activation may regulate skeletal muscle proteasome expression and protein degradation. The elevations in MuRF1 and MAFbx expression seen in skeletal muscle after thermal injury, arthritis, and dexamethasone administration were normalized, attenuated, and prevented, respectively, by ghrelin or GHS administration [50–52]. IGF-1 prevents the expression of MuRF1 and MAFbx by inhibiting Forkhead box O transcription factors via stimulation of the phosphatidylinositol-3-kinase (PI3K)/Akt pathway. The IGF-1 receptor triggers activation of several intracellular kinases, including PI3K [53]. Thus, the effects of ghrelin on NF-κB activation and IGF-1 synthesis are favorable for minimizing inflammatory responses and sarcopenia in patients with cachexia.
Other actions
The role of ghrelin in stimulating gastric emptying and acid secretion is well-established [54]. This effect may ameliorate gastrointestinal symptoms in patients with anorexia–cachexia syndrome. Ghrelin also increases endogenous nitric oxide release [55, 56], which may influence the orexigenic and anti-inflammatory actions of ghrelin [57, 58]. These qualities may be important in the treatment of cachexia.
Plasma ghrelin levels in cachexia
Plasma ghrelin levels are elevated in cachectic conditions caused by a variety of underlying disorders [59–63]. Although this phenomenon has been called “ghrelin resistance,” these elevations may be a compensatory response reflecting the negative energy balance state. While there is usually an inverse relationship between plasma ghrelin levels and body mass index (BMI), no significant difference in ghrelin levels between normal subjects and cachectic patients after matching for BMI. In patients with end-stage renal disease (ESRD), conflicting results (i.e., increases [64–66], decreases [67], or no change [67, 68]) for circulating ghrelin concentrations have been reported [69]. Aygen et al. recently reported elevations in both ghrelin and des-acyl ghrelin in ESRD patients undergoing hemodialysis in comparison to age-matched healthy controls [65]. Iglesias et al. indicated that patients undergoing hemodialysis possessed similar ghrelin concentrations to the control group; only peritoneal dialysis patients exhibited significantly lower ghrelin concentrations at baseline than those found in patients on conservative management [67]. These conflicting results are due, at least in part, to cross-sectional studies using different ghrelin assays that compared patients with different residual renal functions, ages, genders, and nutritional status. Residual renal function may affect the metabolism and clearance of ghrelin. Longitudinal studies following patients with renal disease using ghrelin assays measuring both ghrelin and des-ghrelin are required to determine the pathophysiologic role of ghrelin in cachexia associated with ESRD. Post-hemodialysis serum ghrelin levels are significantly lower than pre-hemodialysis ghrelin levels, supporting the view that ghrelin is cleared by hemodialysis [65, 69, 70].
Clinical studies
Trials seeking to apply the effects of ghrelin to the treatment of cachexia have been expanding. These studies have sought to evaluate ghrelin as a treatment for patients with the cachexia associated with CHF, COPD, cancer, ERSD, etc. Cachexia, which manifests as excessive weight loss in the setting of an underlying chronic disease [71], is typically associated with anorexia as a major cause of weight loss. Weight loss and decreased appetite are the major causes of morbidity and mortality in patients with anorexia–cachexia syndrome. There is an immediate need for effective, well-tolerated treatments to stimulate appetite [72], prompting several trials to explore the application of ghrelin as a treatment for patients with cachexia.
CHF cachexia
Ghrelin induces a positive energy balance state through both GH-dependent and -independent mechanisms and has protective cardiovascular effects [73]. GH treatment may be especially useful in a subgroup of patients with cardiac cachexia [74]. Ghrelin stimulates food intake, induces adiposity, regulates the central nervous system to decrease sympathetic nerve outflow, and inhibits apoptosis of cardiomyocytes and endothelial cells in a GH-independent manner. Nagaya et al. investigated the effects of ghrelin on cardiac cachexia in patients with CHF [36] (Table 1). Daily administration of ghrelin for 3 weeks increased both food intake and body weight. This study also demonstrated improvements in patient exercise capacity, muscle wasting, and left ventricular function. Ghrelin treatment also resulted in significantly decreased plasma norepinephrine levels. Although this study was neither randomized nor placebo-controlled, the eight CHF patients who did not receive ghrelin (control group) were followed to rule out any time-course effects during hospitalization. None of the aforementioned parameters changed in patients with CHF who did not receive ghrelin therapy. Further studies will be necessary to identify the pathways involved in this ghrelin effect and to determine the best therapeutic strategies for ghrelin use to combat the wasting process found in cardiac cachexia [74]. Clinical trials are currently attempting to reproduce these data in a double-blind, placebo-controlled fashion.
Table 1.
Summary of clinical studies of ghrelin/GHS for cachexia therapy
| Diseases | Reference | Year | Study design | Ghrelin/GHS administration |
|---|---|---|---|---|
| CHF | [24] | 2004 | Open-label pilot study | Ghrelin, 2 μg/kg b.i.d. for 3 weeks, i.v. |
| COPD | [27] | 2005 | Open-label pilot study | Ghrelin, 2 μg/kg b.i.d. for 3 weeks, i.v. |
| Cancer cachexia | [76] | 2004 | Acute, randomized, placebo-controlled, cross-over study | Ghrelin, 5 pmol/kg/min, i.v., for >180 min |
| Cancer cachexia | [31] | 2007 | Randomized, placebo-controlled study | Anamorelin, 50 mg/day, oral, 12 weeks |
| Cancer cachexia | [77] | 2008 | Randomized, placebo-controlled, cross-over study | Ghrelin, 2 or 8 μg/kg, i.v., for 4 days, once a day |
| ESRD | [79] | 2005 | Acute, randomized, placebo-controlled, cross-over study | Ghrelin, 3.6 nmol/kg, s.c. |
| ESRD | [80] | 2009 | Randomized, placebo-controlled, cross-over study | Ghrelin, 12 μg/kg, s.c., for 1 wk, once a day |
COPD cachexia
Patients with COPD often exhibit some degree of cachexia [75], which is an independent risk factor for mortality in COPD; GH treatment increases muscle mass in such patients. COPD and CHF are both associated with multiple pathophysiological disturbances, including anemia and neurohormonal activation [76]. In COPD patients, ghrelin exhibits anti-inflammatory effects. Chronic respiratory infections, characterized by neutrophil-dominant airway inflammation, lead to end-stage cachexia [77]. The cytotoxicity of accumulated neutrophils against bronchial and alveolar epithelial cells induces a deterioration of pulmonary function in COPD, resulting in excess energy expenditure and weight loss in patients. Intravenous ghrelin treatment for 3 weeks reduced both neutrophil counts in sputum samples and the volume of sputum, suggesting suppression by ghrelin of excess neutrophilic influx [46].
An open-label pilot study examined the ability of ghrelin to improve cachexia and functional capacity in patients with COPD; ghrelin was administered intravenously for 3 weeks to seven cachectic patients with COPD [18]. Repeated ghrelin administration significantly increased food intake, body weight, lean body mass, and peripheral and respiratory muscle strength. Ghrelin treatment ameliorated the exaggerated sympathetic nerve activity, as indicated by marked decreases in plasma norepinephrine levels. In cachectic patients with COPD, treatment with ghrelin improved appetite, body composition, muscle wasting, functional capacity, and sympathetic augmentation. Subsequently, another placebo-controlled trial demonstrated that ghrelin increased both appetite and body weight with an apparent dose-dependent trend towards improved physical performance (chair stand score) [78]. A larger clinical trial is currently being conducted to confirm these data in a double-blind, placebo-controlled fashion. Comparisons of this treatment to current standard medications will be required [76].
Cancer cachexia
Anorexia, frequently encountered in cancer patients, is one of the major causes of malnutrition and cachexia in this patient population. Ghrelin administration resulted in significant increases in weight and food intake in rodent models of cancer cachexia [79–81]. In all studies, ghrelin improved both food intake and weight gain in rodent models. DeBoer et al. determined that weight gain resulted from a reversal in the loss of lean body mass, a critical component of cachexia [81].
Several randomized, double-blind placebo-controlled trials have demonstrated the efficacy and safety of ghrelin or GHS in patients with cancer-associated cachexia [38, 82, 83]. Nearry et al. performed a randomized, placebo-controlled, cross-over clinical trial to determine if ghrelin could stimulate appetite in seven cancer patients with severe anorexia [82]. Ghrelin infusion resulted in a marked increase in energy intake in comparison to saline-treated controls; all patients in the study demonstrated increased food consumption. The meal appreciation score was also higher in ghrelin-treated individuals. Strasser et al. detailed a randomized, double-crossover, phase 1/2 study in patients with advanced cancer [83]. They infused a low or high dose of ghrelin or placebo before lunch daily for 4 days in each course. Nutritional intake or eating-related symptoms did not differ between the ghrelin- and placebo-treated groups. More patients, however, preferred ghrelin to placebo at the middle and end of study, although this finding was not dose-dependent. In contrast to the results of Neary et al., this study did not demonstrate any increases in food intake. As the patient characteristics and study designs were very different in the two studies, further investigation will be required. Garcia et al. performed a randomized, placebo-controlled trial over a 12-week period using subjects with a variety of cancer types (predominantly lung cancer). Anamorelin was infused, which produced an improvement in total body mass trending toward increased lean mass. Quality of life, however, was unchanged between the groups receiving anamorelin and placebo.
An important concern regarding the use of ghrelin in cancer cachexia is that ghrelin may increase growth factors, such as GH and IGF-1, to stimulate tumor growth. Additionally, ghrelin itself may have mitogenic potential. As far as we know, no in vivo data has examined the differences in tumor growth after ghrelin or GHS treatment. Long-term, large-scale clinical trials are required to determine if ghrelin treatment promotes tumor growth.
End-stage renal disease
ESRD is a chronic condition frequently associated with nutritional dysfunction [84]. This type of malnutrition is highly resistant to intervention and a major predictor of morbidity and mortality for patients on either peritoneal dialysis or hemodialysis. Wynne et al. sought to determine if a single injection of ghrelin could enhance food intake in patients with evidence of malnutrition receiving maintenance peritoneal dialysis [85]. Nine peritoneal dialysis patients exhibiting mild to moderate malnutrition administered either ghrelin or a saline placebo subcutaneously were examined in a randomized, double-blind, cross-over protocol. Ghrelin administration significantly increased mean absolute energy intake during the study meals and maintained nonsignificant increases observed in energy intake over the first 24 h without a subsequent rebound. This research group has subsequently sought to analyze the efficacy of repeated ghrelin administrations in malnourished dialysis patients [86]. They performed a double-blind randomized cross-over study of a week of daily subcutaneous ghrelin injections in a group of 12 malnourished dialysis patients. Ghrelin administration significantly increased appetite, with increases in energy intake noted at the first study meal. Persistence of this effect throughout the week was confirmed by food diaries and final study meals, indicating that daily ghrelin treatment achieved a sustained positive change in energy balance in malnourished dialysis patients. In support of this data, an animal study using a nephrectomized rat model of renal cachexia demonstrated that daily treatment for 2 weeks with ghrelin or two GHS agents (BIM-28125 and BIM-28131) resulted in increased food intake, improved lean body mass accrual, and decreased circulating inflammatory cytokines [25]. Long-term studies are needed to demonstrate the efficacy in improving appetite, weight gain, lean body mass, and quality of life.
Others
Ghrelin treatment has also been applied to other causes of anorexia, sarcopenia, and emaciation, including anorexia nervosa [87], functional dyspepsia [88], aging [21], post-gastrectomy anorexia [22], esophagectomy [23], chemotherapy [89, 90], and thermal injury [91]. In addition, the effects of ghrelin mimetics were examined in age-dependent sarcopenia [21, 32]. These applications certainly provide additional insight into the successful treatment of cachexia, a wasting syndrome developing in the setting of a variety of chronic illnesses.
Conclusion
Ghrelin exhibits anti-cachectic effects in a number of animal and human studies. Ghrelin treatment is safe and well-tolerated. Several larger-scale clinical trials are currently attempting to reproduce these effects for the treatment of cachexia, including that associated with CHF, cancer, COPD, and ESRD. Long-term, large-scale trials are eagerly awaited to determine if ghrelin is an effective therapy for cachexia.
Acknowledgments
Research in the authors’ laboratory was supported in part by funds from the Ministry of Education, Culture, Sports, Science and Technology of Japan; the Ministry of Health, Labour and Welfare of Japan; the Program for the Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NIBIO); the Mitui Sumitomo Insurance Welfare Foundation; and the Tokyo Biochemical Research Foundation.
The authors of this manuscript certify that they comply with the Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle [92].
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 2.van der Lely AJ, Tschop M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev. 2004;25:426–457. doi: 10.1210/er.2002-0029. [DOI] [PubMed] [Google Scholar]
- 3.Korbonits M, Goldstone AP, Gueorguiev M, Grossman AB. Ghrelin—a hormone with multiple functions. Front Neuroendocrinol. 2004;25:27–68. doi: 10.1016/j.yfrne.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 5.Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, et al. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114:57–66. doi: 10.1172/JCI21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dembinski A, Warzecha Z, Ceranowicz P, Tomaszewska R, Stachura J, Konturek SJ, et al. Ghrelin attenuates the development of acute pancreatitis in rat. J Physiol Pharmacol. 2003;54:561–573. [PubMed] [Google Scholar]
- 7.Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Cummings DE. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav. 2006;89:71–84. doi: 10.1016/j.physbeh.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 9.Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology. 2007;132:2116–2130. doi: 10.1053/j.gastro.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 10.Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–4328. doi: 10.1210/en.141.11.4325. [DOI] [PubMed] [Google Scholar]
- 11.Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992. doi: 10.1210/jc.86.12.5992. [DOI] [PubMed] [Google Scholar]
- 12.Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and Agouti-related protein mRNA levels and body weight in rats. Diabetes. 2001;50:2438–2443. doi: 10.2337/diabetes.50.11.2438. [DOI] [PubMed] [Google Scholar]
- 13.Shintani M, Ogawa Y, Ebihara K, Aizawa-Abe M, Miyanaga F, Takaya K, et al. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes. 2001;50:227–232. doi: 10.2337/diabetes.50.2.227. [DOI] [PubMed] [Google Scholar]
- 14.Chen HY, Trumbauer ME, Chen AS, Weingarth DT, Adams JR, Frazier EG, et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145:2607–2612. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- 15.Toshinai K, Date Y, Murakami N, Shimada M, Mondal MS, Shimbara T, et al. Ghrelin-induced food intake is mediated via the orexin pathway. Endocrinology. 2003;144:1506–1512. doi: 10.1210/en.2002-220788. [DOI] [PubMed] [Google Scholar]
- 16.Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 17.Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50:2540–2547. doi: 10.2337/diabetes.50.11.2540. [DOI] [PubMed] [Google Scholar]
- 18.Nagaya N, Itoh T, Murakami S, Oya H, Uematsu M, Miyatake K, et al. Treatment of cachexia with ghrelin in patients with COPD. Chest. 2005;128:1187–1193. doi: 10.1378/chest.128.3.1187. [DOI] [PubMed] [Google Scholar]
- 19.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 20.Dornonville de la Cour C, Lindqvist A, Egecioglu E, Tung YC, Surve V, Ohlsson C, et al. Ghrelin treatment reverses the reduction in weight gain and body fat in gastrectomised mice. Gut. 2005;54:907–913. doi: 10.1136/gut.2004.058578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akamizu T, Iwakura H, Ariyasu H, Murayama T, Sumi E, Teramukai S, et al. Effects of ghrelin treatment on patients undergoing total hip replacement for osteoarthritis: different outcomes from studies in patients with cardiac and pulmonary cachexia. J Am Geriatr Soc. 2008;56:2363–2365. doi: 10.1111/j.1532-5415.2008.02031.x. [DOI] [PubMed] [Google Scholar]
- 22.Adachi S, Takiguchi S, Okada K, Yamamoto K, Yamasaki M, Miyata H, et al. Effects of ghrelin administration after total gastrectomy: a prospective, randomized, placebo-controlled phase II study. Gastroenterology. 2010;138:1312–1320. doi: 10.1053/j.gastro.2009.12.058. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto K, Takiguchi S, Miyata H, Adachi S, Hiura Y, Yamasaki M, Nakajima K, Fujiwara Y, Mori M, Kangawa K, Doki Y. Randomized phase II study of clinical effects of ghrelin after esophagectomy with gastric tube reconstruction. Surgery. 2010;148:31–38. doi: 10.1016/j.surg.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 24.Ariyasu H, Iwakura H, Yamada G, Nakao K, Kangawa K, Akamizu T. Efficacy of ghrelin as a therapeutic approach for age-related physiological changes. Endocrinology. 2008;149:3722–3728. doi: 10.1210/en.2007-1650. [DOI] [PubMed] [Google Scholar]
- 25.Deboer MD, Zhu X, Levasseur PR, Inui A, Hu Z, Han G, et al. Ghrelin treatment of chronic kidney disease: improvements in lean body mass and cytokine profile. Endocrinology. 2008;149:827–835. doi: 10.1210/en.2007-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Svensson J, Lonn L, Jansson JO, Murphy G, Wyss D, Krupa D, et al. Two-month treatment of obese subjects with the oral growth hormone (GH) secretagogue MK-677 increases GH secretion, fat-free mass, and energy expenditure. J Clin Endocrinol Metab. 1998;83:362–369. doi: 10.1210/jc.83.2.362. [DOI] [PubMed] [Google Scholar]
- 27.Nass R, Pezzoli SS, Oliveri MC, Patrie JT, Harrell FE, Jr, Clasey JL, et al. Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: a randomized trial. Ann Intern Med. 2008;149:601–611. doi: 10.7326/0003-4819-149-9-200811040-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takaya K, Ariyasu H, Kanamoto N, Iwakura H, Yoshimoto A, Harada M, et al. Ghrelin strongly stimulates growth hormone release in humans. J Clin Endocrinol Metab. 2000;85:4908–4911. doi: 10.1210/jc.85.12.4908. [DOI] [PubMed] [Google Scholar]
- 29.Arvat E, Maccario M, Di Vito L, Broglio F, Benso A, Gottero C, et al. Endocrine activities of ghrelin, a natural growth hormone secretagogue (GHS), in humans: comparison and interactions with hexarelin, a nonnatural peptidyl GHS, and GH-releasing hormone. J Clin Endocrinol Metab. 2001;86:1169–1174. doi: 10.1210/jc.86.3.1169. [DOI] [PubMed] [Google Scholar]
- 30.Hataya Y, Akamizu T, Takaya K, Kanamoto N, Ariyasu H, Saijo M, et al. A low dose of ghrelin stimulates growth hormone (GH) release synergistically with GH-releasing hormone in humans. J Clin Endocrinol Metab. 2001;86:4552. doi: 10.1210/jc.86.9.4552. [DOI] [PubMed] [Google Scholar]
- 31.Akamizu T, Takaya K, Irako T, Hosoda H, Teramukai S, Matsuyama A, et al. Pharmacokinetics, safety, and endocrine and appetite effects of ghrelin administration in young healthy subjects. Eur J Endocrinol. 2004;150:447–455. doi: 10.1530/eje.0.1500447. [DOI] [PubMed] [Google Scholar]
- 32.Nass R, Farhy LS, Liu J, Prudom CE, Johnson ML, Veldhuis P, et al. Evidence for acyl-ghrelin modulation of growth hormone release in the fed state. J Clin Endocrinol Metab. 2008;93:1988–1994. doi: 10.1210/jc.2007-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gibney J, Healy ML, Sonksen PH. The growth hormone/insulin-like growth factor-I axis in exercise and sport. Endocr Rev. 2007;28:603–624. doi: 10.1210/er.2006-0052. [DOI] [PubMed] [Google Scholar]
- 34.Velloso CP. Regulation of muscle mass by growth hormone and IGF-I. Br J Pharmacol. 2008;154:557–568. doi: 10.1038/bjp.2008.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gullett NP, Hebbar G, Ziegler TR. Update on clinical trials of growth factors and anabolic steroids in cachexia and wasting. Am J Clin Nutr. 2010;91:1143 S–1147. doi: 10.3945/ajcn.2010.28608E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagaya N, Moriya J, Yasumura Y, Uematsu M, Ono F, Shimizu W, et al. Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation. 2004;110:3674–3679. doi: 10.1161/01.CIR.0000149746.62908.BB. [DOI] [PubMed] [Google Scholar]
- 37.Bach MA, Rockwood K, Zetterberg C, Thamsborg G, Hebert R, Devogelaer JP, et al. The effects of MK-0677, an oral growth hormone secretagogue, in patients with hip fracture. J Am Geriatr Soc. 2004;52:516–523. doi: 10.1111/j.1532-5415.2004.52156.x. [DOI] [PubMed] [Google Scholar]
- 38.Garcia JM, Graham C, Kumor KWP. A Phase II, randomized, placebo-controlled, double blind study of the efficacy and safety of RC-1291 for the treatment of cancer-cachexia [abstract] J Clin Oncol. 2007;25:S25. doi: 10.1200/JCO.2007.12.2341. [DOI] [Google Scholar]
- 39.Garcia JM, Polvino WJ. Pharmacodynamic hormonal effects of anamorelin, a novel oral ghrelin mimetic and growth hormone secretagogue in healthy volunteers. Growth Horm IGF Res. 2009;19:267–273. doi: 10.1016/j.ghir.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 40.LeRoith D. Clinical relevance of systemic and local IGF-I: lessons from animal models. Pediatr Endocrinol Rev. 2008;5(Suppl 2):739–743. [PubMed] [Google Scholar]
- 41.Pelosi L, Giacinti C, Nardis C, Borsellino G, Rizzuto E, Nicoletti C, et al. Local expression of IGF-1 accelerates muscle regeneration by rapidly modulating inflammatory cytokines and chemokines. FASEB J. 2007;21:1393–1402. doi: 10.1096/fj.06-7690com. [DOI] [PubMed] [Google Scholar]
- 42.Li WG, Gavrila D, Liu X, Wang L, Gunnlaugsson S, Stoll LL, et al. Ghrelin inhibits proinflammatory responses and nuclear factor-kappaB activation in human endothelial cells. Circulation. 2004;109:2221–2226. doi: 10.1161/01.CIR.0000127956.43874.F2. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez-Rey E, Chorny A, Delgado M. Therapeutic action of ghrelin in a mouse model of colitis. Gastroenterology. 2006;130:1707–1720. doi: 10.1053/j.gastro.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 44.Wu R, Dong W, Zhou M, Zhang F, Marini CP, Ravikumar TS, et al. Ghrelin attenuates sepsis-induced acute lung injury and mortality in rats. Am J Respir Crit Care Med. 2007;176:805–813. doi: 10.1164/rccm.200604-511OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Theil MM, Miyake S, Mizuno M, Tomi C, Croxford JL, Hosoda H, et al. Suppression of experimental autoimmune encephalomyelitis by ghrelin. J Immunol. 2009;183:2859–2866. doi: 10.4049/jimmunol.0803362. [DOI] [PubMed] [Google Scholar]
- 46.Kodama T, Ashitani J, Matsumoto N, Kangawa K, Nakazato M. Ghrelin treatment suppresses neutrophil-dominant inflammation in airways of patients with chronic respiratory infection. Pulm Pharmacol Ther. 2008;21:774–779. doi: 10.1016/j.pupt.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 47.Waseem T, Duxbury M, Ito H, Ashley SW, Robinson MK. Exogenous ghrelin modulates release of pro-inflammatory and anti-inflammatory cytokines in LPS-stimulated macrophages through distinct signaling pathways. Surgery. 2008;143:334–342. doi: 10.1016/j.surg.2007.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jackman RW, Kandarian SC. The molecular basis of skeletal muscle atrophy. Am J Physiol Cell Physiol. 2004;287:C834–C843. doi: 10.1152/ajpcell.00579.2003. [DOI] [PubMed] [Google Scholar]
- 50.Balasubramaniam A, Joshi R, Su C, Friend LA, Sheriff S, Kagan RJ, et al. Ghrelin inhibits skeletal muscle protein breakdown in rats with thermal injury through normalizing elevated expression of E3 ubiquitin ligases MuRF1 and MAFbx. Am J Physiol Regul Integr Comp Physiol. 2009;296:R893–R901. doi: 10.1152/ajpregu.00015.2008. [DOI] [PubMed] [Google Scholar]
- 51.Sheriff S, Joshi R, Friend LA, James JH, Balasubramaniam A. Ghrelin receptor agonist, GHRP-2, attenuates burn injury-induced MuRF-1 and MAFbx expression and muscle proteolysis in rats. Peptides. 2009;30:1909–1913. doi: 10.1016/j.peptides.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 52.Yamamoto D, Ikeshita N, Matsubara T, Tasaki H, Herningtyas EH, Toda K, et al. GHRP-2, a GHS-R agonist, directly acts on myocytes to attenuate the dexamethasone-induced expressions of muscle-specific ubiquitin ligases, Atrogin-1 and MuRF1. Life Sci. 2008;82:460–466. doi: 10.1016/j.lfs.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 53.Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14:395–403. doi: 10.1016/S1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- 54.Peeters TL. Central and peripheral mechanisms by which ghrelin regulates gut motility. J Physiol Pharmacol. 2003;54(Suppl 4):95–103. [PubMed] [Google Scholar]
- 55.Sibilia V, Rindi G, Pagani F, Rapetti D, Locatelli V, Torsello A, et al. Ghrelin protects against ethanol-induced gastric ulcers in rats: studies on the mechanisms of action. Endocrinology. 2003;144:353–359. doi: 10.1210/en.2002-220756. [DOI] [PubMed] [Google Scholar]
- 56.Xu X, Jhun BS, Ha CH, Jin ZG. Molecular mechanisms of ghrelin-mediated endothelial nitric oxide synthase activation. Endocrinology. 2008;149:4183–4192. doi: 10.1210/en.2008-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morley JE, Farr SA. Cachexia and neuropeptide Y. Nutrition. 2008;24:815–819. doi: 10.1016/j.nut.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 58.Konturek PC, Brzozowski T, Engel M, Burnat G, Gaca P, Kwiecien S, et al. Ghrelin ameliorates colonic inflammation. Role of nitric oxide and sensory nerves. J Physiol Pharmacol. 2009;60:41–47. [PubMed] [Google Scholar]
- 59.Nagaya N, Uematsu M, Kojima M, Date Y, Nakazato M, Okumura H, et al. Elevated circulating level of ghrelin in cachexia associated with chronic heart failure: relationships between ghrelin and anabolic/catabolic factors. Circulation. 2001;104:2034–2038. doi: 10.1161/hc4201.097836. [DOI] [PubMed] [Google Scholar]
- 60.Itoh T, Nagaya N, Yoshikawa M, Fukuoka A, Takenaka H, Shimizu Y, et al. Elevated plasma ghrelin level in underweight patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:879–882. doi: 10.1164/rccm.200310-1404OC. [DOI] [PubMed] [Google Scholar]
- 61.Garcia JM, Garcia-Touza M, Hijazi RA, Taffet G, Epner D, Mann D, et al. Active ghrelin levels and active to total ghrelin ratio in cancer-induced cachexia. J Clin Endocrinol Metab. 2005;90:2920–2926. doi: 10.1210/jc.2004-1788. [DOI] [PubMed] [Google Scholar]
- 62.Shimizu Y, Nagaya N, Isobe T, Imazu M, Okumura H, Hosoda H, et al. Increased plasma ghrelin level in lung cancer cachexia. Clin Cancer Res. 2003;9:774–778. [PubMed] [Google Scholar]
- 63.Kerem M, Ferahkose Z, Yilmaz UT, Pasaoglu H, Ofluoglu E, Bedirli A, et al. Adipokines and ghrelin in gastric cancer cachexia. World J Gastroenterol. 2008;14:3633–3641. doi: 10.3748/wjg.14.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perez-Fontan M, Cordido F, Rodriguez-Carmona A, Peteiro J, Garcia-Naveiro R, Garcia-Buela J. Plasma ghrelin levels in patients undergoing haemodialysis and peritoneal dialysis. Nephrol Dial Transplant. 2004;19:2095–2100. doi: 10.1093/ndt/gfh313. [DOI] [PubMed] [Google Scholar]
- 65.Aygen B, Dogukan A, Dursun FE, Aydin S, Kilic N, Sahpaz F, et al. Ghrelin and obestatin levels in end-stage renal disease. J Int Med Res. 2009;37:757–765. doi: 10.1177/147323000903700319. [DOI] [PubMed] [Google Scholar]
- 66.Rodriguez Ayala E, Pecoits-Filho R, Heimburger O, Lindholm B, Nordfors L, Stenvinkel P. Associations between plasma ghrelin levels and body composition in end-stage renal disease: a longitudinal study. Nephrol Dial Transplant. 2004;19:421–426. doi: 10.1093/ndt/gfg559. [DOI] [PubMed] [Google Scholar]
- 67.Iglesias P, Diez JJ, Fernandez-Reyes MJ, Codoceo R, Alvarez-Fidalgo P, Bajo MA, et al. Serum ghrelin concentrations in patients with chronic renal failure undergoing dialysis. Clin Endocrinol (Oxf) 2006;64:68–73. doi: 10.1111/j.1365-2265.2005.02418.x. [DOI] [PubMed] [Google Scholar]
- 68.Szczepanska M, Szprynger K, Mazur B, Zwolinska D, Kilis-Pstrusinska K, Makulska I. Plasma ghrelin levels in children with chronic renal failure on peritoneal dialysis. Perit Dial Int. 2007;27:61–66. [PubMed] [Google Scholar]
- 69.Mak RH, Cheung W, Purnell J. Ghrelin in chronic kidney disease: too much or too little? Perit Dial Int. 2007;27:51–55. [PubMed] [Google Scholar]
- 70.Jarkovska Z, Hodkova M, Sazamova M, Rosicka M, Dusilova-Sulkova S, Marek J, et al. Plasma levels of active and total ghrelin in renal failure: a relationship with GH/IGF-I axis. Growth Horm IGF Res. 2005;15:369–376. doi: 10.1016/j.ghir.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 71.Morley J, Thomas D, MG W. Cachexia: pathophysiology and clinical relevance. Am J Clin Nutr. 2006;83:735–743. doi: 10.1093/ajcn/83.4.735. [DOI] [PubMed] [Google Scholar]
- 72.Cummings DE, Foster-Schubert KE, Overduin J. Ghrelin and energy balance: focus on current controversies. Curr Drug Targets. 2005;6:153–169. doi: 10.2174/1389450053174569. [DOI] [PubMed] [Google Scholar]
- 73.Nagaya N, Kangawa K. Therapeutic potential of ghrelin in the treatment of heart failure. Drugs. 2006;66:439–448. doi: 10.2165/00003495-200666040-00004. [DOI] [PubMed] [Google Scholar]
- 74.Akashi YJ, Springer J, Anker SD. Cachexia in chronic heart failure: prognostic implications and novel therapeutic approaches. Curr Heart Fail Rep. 2005;2:198–203. doi: 10.1007/BF02696650. [DOI] [PubMed] [Google Scholar]
- 75.Nagaya N, Kojima M, Kangawa K. Ghrelin, a novel growth hormone-releasing peptide, in the treatment of cardiopulmonary-associated cachexia. Intern Med. 2006;45:127–134. doi: 10.2169/internalmedicine.45.1402. [DOI] [PubMed] [Google Scholar]
- 76.Lainscak M, Andreas S, Scanlon PD, Somers VK, Anker SD. Ghrelin and neurohumoral antagonists in the treatment of cachexia associated with cardiopulmonary disease. Intern Med. 2006;45:837. doi: 10.2169/internalmedicine.45.1867. [DOI] [PubMed] [Google Scholar]
- 77.Ashitani J, Matsumoto N, Nakazato M. Ghrelin and its therapeutic potential for cachectic patients. Peptides. 2009;30:1951–1956. doi: 10.1016/j.peptides.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 78.Gertner JM, Oo C (2009) Performance improvement in COPD cachexia with SUN11031 (a synthetic human ghrelin) in a placebo controlled trial [abstract]. The 5th cachexia conference, Barcelona, 2009, p 143
- 79.Hanada T, Toshinai K, Kajimura N, Nara-Ashizawa N, Tsukada T, Hayashi Y, et al. Anti-cachectic effect of ghrelin in nude mice bearing human melanoma cells. Biochem Biophys Res Commun. 2003;301:275–279. doi: 10.1016/S0006-291X(02)03028-0. [DOI] [PubMed] [Google Scholar]
- 80.Wang W, Andersson M, Iresjo BM, Lonnroth C, Lundholm K. Effects of ghrelin on anorexia in tumor-bearing mice with eicosanoid-related cachexia. Int J Oncol. 2006;28:1393–1400. [PubMed] [Google Scholar]
- 81.DeBoer MD, Zhu XX, Levasseur P, Meguid MM, Suzuki S, Inui A, et al. Ghrelin treatment causes increased food intake and retention of lean body mass in a rat model of cancer cachexia. Endocrinology. 2007;148:3004–3012. doi: 10.1210/en.2007-0016. [DOI] [PubMed] [Google Scholar]
- 82.Neary NM, Small CJ, Wren AM, Lee JL, Druce MR, Palmieri C, et al. Ghrelin increases energy intake in cancer patients with impaired appetite: acute, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89:2832–2836. doi: 10.1210/jc.2003-031768. [DOI] [PubMed] [Google Scholar]
- 83.Strasser F, Lutz TA, Maeder MT, Thuerlimann B, Bueche D, Tschop M, et al. Safety, tolerability and pharmacokinetics of intravenous ghrelin for cancer-related anorexia/cachexia: a randomised, placebo-controlled, double-blind, double-crossover study. Br J Cancer. 2008;98:300–308. doi: 10.1038/sj.bjc.6604148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bossola M, Tazza L, Giungi S, Luciani G. Anorexia in hemodialysis patients: an update. Kidney Int. 2006;70:417–422. doi: 10.1038/sj.ki.5001572. [DOI] [PubMed] [Google Scholar]
- 85.Wynne K, Giannitsopoulou K, Small CJ, Patterson M, Frost G, Ghatei MA, et al. Subcutaneous ghrelin enhances acute food intake in malnourished patients who receive maintenance peritoneal dialysis: a randomized, placebo-controlled trial. J Am Soc Nephrol. 2005;16:2111–2118. doi: 10.1681/ASN.2005010039. [DOI] [PubMed] [Google Scholar]
- 86.Ashby DR, Ford HE, Wynne KJ, Wren AM, Murphy KG, Busbridge M, et al. Sustained appetite improvement in malnourished dialysis patients by daily ghrelin treatment. Kidney Int. 2009;76:199–206. doi: 10.1038/ki.2009.114. [DOI] [PubMed] [Google Scholar]
- 87.Hotta M, Ohwada R, Akamizu T, Shibasaki T, Takano K, Kangawa K. Ghrelin increases hunger and food intake in patients with restricting-type anorexia nervosa: a pilot study. Endocr J. 2009;56:1119–1128. doi: 10.1507/endocrj.K09E-168. [DOI] [PubMed] [Google Scholar]
- 88.Akamizu T, Iwakura H, Ariyasu H, Hosoda H, Murayama T, Yokode M, et al. Repeated administration of ghrelin to patients with functional dyspepsia: its effects on food intake and appetite. Eur J Endocrinol. 2008;158:491–498. doi: 10.1530/EJE-07-0768. [DOI] [PubMed] [Google Scholar]
- 89.Liu YL, Malik NM, Sanger GJ, Andrews PL. Ghrelin alleviates cancer chemotherapy-associated dyspepsia in rodents. Cancer Chemother Pharmacol. 2006;58:326–333. doi: 10.1007/s00280-005-0179-0. [DOI] [PubMed] [Google Scholar]
- 90.Garcia JM, Cata JP, Dougherty PM, Smith RG. Ghrelin prevents cisplatin-induced mechanical hyperalgesia and cachexia. Endocrinology. 2008;149:455–460. doi: 10.1210/en.2007-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Balasubramaniam A, Wood S, Joshi R, Su C, Friend LA, Sheriff S, et al. Ghrelin stimulates food intake and growth hormone release in rats with thermal injury: synthesis of ghrelin. Peptides. 2006;27:1624–1631. doi: 10.1016/j.peptides.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 92.von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle 2010;1:7–8. [DOI] [PMC free article] [PubMed]