Abstract
Cachexia affects up to two thirds of all cancer patients and is a significant cause of morbidity and mortality. It is a complex metabolic syndrome associated with the underlying illness and characterized by loss of skeletal muscle tissue with or without loss of fat mass. Cachexia’s other prominent clinical symptoms include anorexia, systemic inflammation, pediatric growth failure, and hypogonadism. The relationship between the symptoms of cancer cachexia and the underlying illness is unclear, and there is an urgent need for a better understanding of the pathophysiology of this syndrome. Normal Zn metabolism is often disrupted in cancer patients, but the possible effects of systemic Zn dyshomeostasis in cachexia have not been investigated. We propose that the acute phase response can mediate Zn redistribution and accumulation in skeletal muscle tissue and contribute to the activation of the ubiquitin–proteasome pathway that regulates protein catabolism. This chronic redistribution deprives Zn from other tissues and organs and compromises critical physiological functions in the body. The cardinal symptoms of Zn deficiency are anorexia, systemic inflammation, growth failure in children, and hypogonadism. These symptoms also prominently characterize cancer cachexia suggesting that the role of systemic Zn dyshomeostasis in cachexia should be investigated.
Keywords: Cachexia muscle wasting, Zinc, Systemic Zn dyshomeostasis, Anorexia, Systemic inflammation, Hypogonadism, Growth failure
Introduction
Cachexia affects up to two thirds of all cancer patients and is directly responsible for one fifth of all cancer-related deaths [1]. Muscle wasting is one of the most devastating, complex, and enigmatic aspects of cancer, but it is also common in other conditions such as chronic heart failure [2], chronic obstructive pulmonary disease [3], chronic kidney disease [4], chronic inflammation [5], severe trauma [6], AIDS [7], and sepsis [8]. The 2008 Cachexia Consensus Conference defined cachexia as “a complex metabolic syndrome associated with underlying illness and characterized by loss of muscle with or without loss of fat mass.” Other prominent clinical features of cachexia include anorexia, systemic inflammation, pediatric growth failure, and hypogonadism [9]. It is unclear how these symptoms are related to each other or to the underlying illness, and as Lainscak and colleagues recently noted, there is an urgent need for effective therapies and a precise definition of this common and deadly syndrome [10].
We propose that systemic Zn dyshomeostasis is a salient characteristic of cancer cachexia, and that Zn redistribution is mediated by the acute phase response (APR) as a host defense mechanism in response to infection, inflammation, trauma, or cancer. We hypothesize that chronic APR can induce significant Zn accumulation in skeletal muscle tissue resulting in ubiquitin–proteasome pathway mediated protein catabolism and functional systemic Zn deficiency associated with anorexia, inflammation, growth failure, and hypogonadism.
Zn is a critical trace element that has a broad range of vital catalytic and structural functions in all eukaryotic cells and higher organisms, and it is of exceptional biologic importance for humans [11]. As Maret points out, “Zn ions are essential for all forms of life. In humans, they have catalytic and structural functions in an estimated 3,000 zinc proteins” [12]. Zn homeostasis is often disturbed during cancer, and in certain malignancies Zn uptake appears to be an index of tumor viability [13]. Zn can also upregulate telomerase activity that is associated with the unlimited proliferation of cancer cells [14, 15]. However, Murakami and Hirano note that while tumors need Zn to survive and grow, “excess Zn may induce tumor cell apoptosis, although the sensitivities of the different types of tumors are likely to vary” [16]. Low Zn levels have been reported in cancer patients [17–22], but we are aware of only one clinical study that specifically examined the link between serum Zn levels and cancer-induced muscle wasting [23]. In 1989, Westin and colleagues showed in a small pilot study with 6 patients that cachectic subjects with head and neck cancer had significantly lower serum Zn levels compared to controls (p < 0.025). The results should be viewed with caution due to the small size of the cohort and the fact that the patients were alcoholics. Alcohol abuse has been associated with abnormal Zn metabolism. It is important to note that while Zn is commonly measured from serum, normal levels can be found in patients suffering from Zn dyshomeostasis due to Zn released from catabolic muscles, hemolysis, protein binding, hormone-mediated redistribution, and postprandial effects [23, 24]. Future studies should consider an alternative method suggested by Prasad and colleagues for determining Zn status in humans that is not affected by these processes [25].
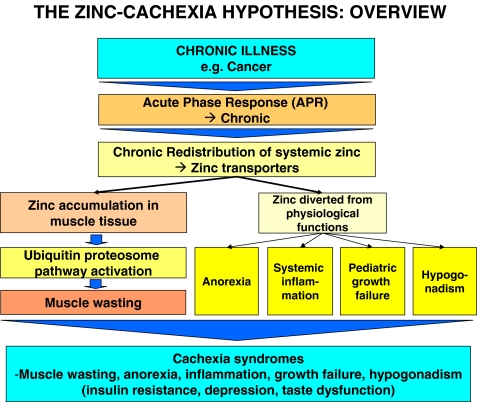
There are other important clinical mechanisms that can contribute to systemic Zn dyshomeostasis in cancer patients. These include low dietary intake of Zn, hypoalbuminemia, and fecal losses of Zn. The adult body contains 2–3 g of Zn [26], of which 57% is found in skeletal muscle and 29% in bone [27]. However, there is no functional reserve or store for Zn. The body uses a small rapidly exchangeable pool of Zn which is dependent on constant nutritional replenishment. The recommended daily allowance (RDA) for Zn is 15 mg [28], and low daily intake of Zn rich food stuffs such as shellfish, beef, lamb, veal, and poultry [29] can contribute to Zn deficiency in cancer patients. The majority (75–85%) of plasma Zn is bound to serum albumin [30], and hypoalbuminemia can compromise Zn transport. Finally, fecal losses of Zn due to gastrointestinal surgery associated with cancer can contribute to low Zn levels [31].
APR and Zn redistribution
We hypothesize that systemic Zn redistribution and dyshomeostasis play a central role in cancer cachexia. However, Zn metabolism is vital for human health and is normally tightly regulated. There is no systemic store for Zn [32], yet the body seems to be able to accommodate tenfold changes in Zn intake by adjusting the rate of absorption and excretion of the metal [27]. Considering how vigilantly physiological Zn homeostasis is maintained, we investigated if there is an identifiable mechanism that can initiate and sustain the redistribution of Zn in pathological situations.
APR is a host defense mechanism triggered in response to trauma, inflammation, infection, and cancer. Gabay and Kushner explain that “a large number of changes, distant from the site or sites of inflammation and involving many organ systems, may accompany inflammation [and] these systemic changes [are] referred to as the acute phase response, even though they accompany both acute and chronic inflammation” [33]. APR seems to play an important role in cancer cachexia and as Stephens, Skipworth, and Fearon note, “at the time of diagnosis, around half of all cancer patients will demonstrate an APR” [1]. The proportion of patients with APR increases with the progression of the disease, and in certain malignancies, the presence of APR is a significant predictor of survival [34].
One of the main functions of APR is the orchestrated acceleration of hepatic production of specific plasma proteins used during the defense response. To achieve this increased rate of protein production, APR initiates the hepatic amino acid uptake and the large increase in the synthesis of acute phase proteins by the liver [35]. The liver of cancer patients and tumor-bearing animals incorporates amino acids at a significantly (p < 0.025) higher rate compared to healthy controls [36]. The persistent hepatic synthesis of acute phase reactants may represent a nutritional sink that sucks amino acids mobilized from skeletal muscle tissue that is aggressively broken down during APR. Indeed, 2.6 g of muscle protein must be catabolized to produce 1 g of fibrinogen [1], one of the most important acute phase proteins [37]. The correlation between muscle wasting and increased hepatic protein synthesis is known, but the mechanism behind APR-mediated protein catabolism in skeletal muscle is poorly understood.
The redistribution of systemic Zn and hypozincemia are prominent characteristics of APR [33, 35, 38]. Clinical data indicate that APR-induced hypozincemia is in part due to internal Zn redistribution [39, 40], the liver being a major target [41]. However, Zn can also be redistributed into skeletal muscle during APR. A rat model of chronic heart failure that uses aldosterone to induce a persistent APR showed Zn65 uptake not only in the liver, but also in uninjured skeletal muscle where Zn65 increased nearly twofold after 1 week of aldosterone administration and remained 50% higher than controls at week 4 [42].
The pro-inflammatory cytokines, in particular tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and interleukin-6 (IL-6) that are central to the induction of the APR [1], significantly reduce systemic Zn levels. The administration of TNF-α (p < 0.01; [43]), IL-6 (p < 0.001; [44]), and lipopolysaccharide (LPS; p < 0.0001; [39]) results in the significant decrease of serum Zn levels. The chronic administration of IL-1 to rats resulted in significantly reduced plasma Zn levels (<70 μg/dl vs. >110 μg/dl, p < 0.01), severely diminished appetite (p < 0.0001), increased protein breakdown and decreased synthesis (p < 0.05), and over a tenfold increase in IL-6 plasma levels (p < 0.001; [45]). IL-6 significantly (p < 0.01) upregulates the Zrt/IRT-like protein (ZIP) 14 Zn influx transporter that has a central role in inducing hypozincemia during APR [35]. The Zn transporters are divided into the ZIP influx and the Zn-transporter efflux transporter groups [46]. They move Zn into and out of cells and organelles and are responsible for Zn redistribution in pathological situations such as inflammation and cancer [47]. Key APR factors directly regulate Zn homeostasis, and chronic APR can lead to perpetual systemic Zn redistribution. These results also help to explain why cancer patients, who commonly demonstrate chronic APR, also suffer from systemic Zn dyshomeostasis.
Zn accumulation in muscle tissue
We suggest that systemic Zn dyshomeostasis observed in cancer is in part due to the accumulation of Zn in skeletal muscle tissue that constitutes ∼40% of human body mass (BM). Malignant growth disturbs the Zn metabolism in the body [16], but we know of only two pre-clinical studies that specifically examined the relationship between cancer-induced muscle wasting and Zn accumulation in muscle tissue. A 1987 fibrosarcoma study with rats showed that Zn is significantly accumulated into cachexic skeletal muscle tissue (p < 0.05; [48]). Zn levels in the tumor tissue doubled in the same 12-day period. We recently examined the role of Zn in the cachexia-inducing murine adenocarcinoma-16 (MAC-16) model and showed that Zn levels in skeletal muscle tissue correlate with muscle wasting [49]. The Zn concentration in the gastrocnemius muscle of mice with more than 20% weight loss was twice as high as that in controls (p < 0.05). The extracellular Zn chelating compound D-myo-inositol 1,2,6-triphosphate (Alpha trinositol, AT; Bioneris Ab; [50]), a polyanionic isomer of myo-inositol phosphate that forms a mononuclear 1:1 complex with Zn and binds the ion to phosphates P1 and P6 in its inositol ring structure [51], reduced Zn accumulation (p < 0.05) in skeletal muscle tissue while significantly attenuating muscle atrophy (p < 0.001; [49]). We have previously shown that AT attenuates both the loss of lean body mass (p < 0.001) and tumor growth (p < 0.01) in the MAC-16 model. The anti-cachexic effect of AT was not dependent on tumor suppression. [52]. Also another metal chelator, curcumin (diferuloylmethane), that binds Zn through its beta-diketone group and forms a mononuclear (1:1) complex with Zn [53], attenuates weight loss (p < 0.05) in the MAC-16 model [54].
A 1986 Lewis Lung Carcinoma (LLC) study showed that 21 days after tumor implantation Zn concentration in murine skeletal muscle tissue had doubled compared to the controls (p < 0.01). The study did not measure muscle wasting [55], but LLC is known to cause cachexia and is commonly used as an animal model for the syndrome [56–58]. Interestingly, a 1987 study on hamsters with muscular dystrophy showed an 82% increase in the skeletal muscle Zn levels compared to controls (p < 0.001; [59]). A recent study in mice with dystrophic muscle wasting reported similar results [60].
A recent clinical pilot study with 26 cancer cachexia patients showed that Zn progressively and significantly (p < 0.01) accumulates in the skeletal muscle tissue of cachexic patients. In patients with more than 9.5% weight loss, the Zn concentration in skeletal muscle tissue more than doubled compared to healthy controls (Siren et al., unpublished results). To our knowledge this is the first clinical measurement of Zn concentrations in cachexic skeletal muscle tissue. Considering that in healthy individuals, approximately 60% of total body Zn is found in skeletal muscle tissue [27], that constitutes ~40 % of total BM, the doubling of Zn levels in cachexic muscle indicates that these patients suffer from severe systemic Zn dyshomeostasis.
Cachexic muscle tissue can significantly accumulate Zn, and the clinical usefulness of this trace element as a diagnostic biomarker for muscle wasting should be investigated.
Zn and the ubiquitin–proteasome pathway
It is unclear if Zn accumulation in cachexic skeletal muscle is a cause or an effect of protein catabolism. However, the fact that Zn chelators can significantly attenuate muscle wasting in vivo and also attenuate both increased protein degradation and decreased protein synthesis induced by diverse cachexic factors in vitro suggests that the ion may be a causative factor in the catabolic process.
In cachexia, increased protein degradation and decreased protein synthesis occur simultaneously and result in muscle wasting. The activation of the ubiquitin–proteasome pathway seems to be essential for protein catabolism [61–63] and has been implicated in a variety of pathological conditions [64]. According to Tisdale, “studies in animal models of cancer cachexia, as well as in cancer patients, suggest that the ubiquitin–proteasome pathway plays the predominant role in the degradation of myofibrillar proteins, particularly in patients with a weight loss of >10%” [65]. Double-stranded RNA-dependent protein kinase (PKR) seems to play an important in role the activation of the ubiquitin–proteasome pathway. Phosphorylation of PKR leads to the induction of eukaryotic initiation factor 2α (eIF2α) phosphorylation resulting in depressed protein synthesis. PKR also activates IκB kinase, leading to the degradation of the inhibitors IκBa and IκBb and the concomitant release of nuclear factor-κB (NF-κB) that is a central regulator of protein degradation [66]. Eley and Tisdale conclude that the “activation of PKR may provide the link between the inhibition of protein synthesis and induction of muscle protein degradation, leading to muscle atrophy in response to diverse cellular stress in a range of conditions in addition to cancer cachexia, including HIV-AIDS, sepsis, burns, and weightlessness” [67].
AT is an extracellular Zn chelator that effectively attenuates both the increased protein degradation and decreased protein synthesis induced by TNF-α, TNF-α + interferon-gamma, LPS, proteolysis-inducing factor (PIF), and angiotensin II (Ang II; all p < 0.001; [68]). Tisdale notes that the activation of PKR is thought to be critical for both the depression protein synthesis and the increase in protein degradation, and that AT likely inhibits a common step leading to the activation of PKR. The effect of AT on protein degradation is accompanied by the attenuation of the increased expression and activity of the ubiquitin–proteasome pathway. AT completely attenuated the activation of PIF-induced phosphorylation of both PKR and eIF2α and the nuclear accumulation of NF-κB (all p < 0.001). AT also inhibited the activation of caspase-3 and -8 (p < 0.001), which are thought to lead to the activation of PKR. The ability of increasing concentrations Zn to reverse the attenuation by AT of the increased activity of the ubiquitin–proteasome pathway induced by PIF and Ang II, as well as the depression of protein synthesis induced by PIF (all p < 0.001), indicates that Zn may be involved in the signaling process.
Effects of systemic Zn dyshomeostasis
We argue that chronic functional Zn deficiency in specific tissues and organs contributes to the salient clinical symptoms of cancer cachexia such as anorexia, systemic inflammation, pediatric growth failure, and hypogonadism. Zn dyshomeostasis associated with cancer may have serious implications for systemic Zn metabolism. As King and colleagues note, “because plasma must provide Zn to all the tissues, maintaining relatively constant plasma Zn concentrations is essential to sustaining normal function and health” [27]. Physiological signs of Zn depletion are not evident until a drop in plasma Zn concentration occurs, but clinical symptoms manifest rapidly thereafter. Zn deficiency has been studied for nine decades and is characterized by symptoms that are strikingly similar to those commonly found in cachexia. Against this background, it is indeed surprising that the possible link between systemic Zn dyshomeostasis and cancer cachexia has not been investigated.
However, nutritional factors such as leucine and other branched chain amino acids, arginine, glutamine, polyunsaturated fatty acids [69], creatine [70], and cystine [71] have been studied in relation to cancer cachexia. These factors have limited metabolic functions, and they cannot account for the severe and multifaceted symptoms that saliently characterize cachexia.
Anorexia in cachexia
Anorexia, broadly defined as the loss of appetite or desire to eat, is common in cancer cachexia patients. However, anorexia appears to be a distinct syndrome as it does not cause loss of lean body mass by itself [72]. The relationship between cachexia and anorexia is unclear, but it has been argued that cancer anorexia may result from the signaling defects of orexigenic factors such as neuropeptide Y (NPY; [65]). There is a demonstrated decrease in hypothalamic NPY immunostaining in tumor-bearing rats [73], and in anorexic cancer patients, the mean NPY serum levels are significantly (p < 0.004) lower compared with healthy controls [74]. Zn deficiency is thought to induce anorexia by impairing the release of NPY from the terminals in the paraventicular nucleus of the hypothalamus that is required for receptor activation [75].
It is well known that Zn deficiency causes anorexia in many animal species [76, 77]. Chesters and Quarterman observed already 40 years ago that “a fall in food intake is highly characteristic of Zn deficiency” [78]. Young rats are very responsive to a Zn-deficient diet and exhibit decreased food intake within 3–5 days of Zn deprivation. Decreased appetite is the first visible sign of Zn deficiency, and it generally occurs in advance of other symptoms [79].
The correlation between anorexia and low systemic Zn levels has also been extensively studied in humans. Patients with eating disorders may develop Zn deficiency for a variety of reasons, such as low dietary intake of Zn, impaired Zn absorption, vomiting, diarrhea, and binging on low Zn foods [80]. Anorexia patients may also suffer from diminished absorption of dietary Zn [81]. A study with 30 hospitalized anorexic patients who had lost 34% of the height/age adjusted weight, found that the mean plasma Zn level was considerably lower compared to healthy controls (p < 0.01). Eight patients had plasma Zn levels below 60 μg/dl [82]. Another clinical study with 24 anorexic patients showed that 54% had biochemical evidence of Zn deficiency [83]. In several open trials, Zn supplementation has improved weight gain in anorexia patients [84–86]. In a randomized, double-blinded clinical study, the rate of increase in the body mass index (BMI) of the Zn supplemented group was twice that of the placebo group (p > 0.03) [87]. Still, the role of Zn deficiency in the onset and progression of anorexia is both unappreciated and underestimated [79]. Several authors advocate the use of Zn supplementation as a cheap, effective and safe treatment for anorexia [88–90].
We compared the serum Zn levels in anorexia patients with those of cancer patients to investigate the possible role of Zn dyshomeostasis in cancer-induced anorexia. Three clinical studies with anorexia patients who lost between 15% and 50% of their original body weight reported mean serum Zn levels between 71.9 μg/dl and 73.9 μg/dl [24, 82, 91]. Patients with a variety of malignancies (carcinoma of the bronchus, lung, breast prostate, bladder, cervix, gallbladder and mouth) reported mean serum Zn levels between 59.6 μg/dl and 77.2 μg/dl. The corresponding range in healthy controls was 95.5–99.3 μg/dl [18, 92, 93]. These results indicate that cancer patients can have serum Zn levels as low as or lower than patients with advanced anorexia.
We know of only one clinical study that specifically examined the relationship between cancer cachexia-induced anorexia and serum Zn levels [94]. The average weight of 10 small cell lung carcinoma patients declined from 81.7 to 74.1 kg during a 7 month period. The patients suffered from diminished appetite, and their mean caloric intake was 72% of the RDA. The mean serum Zn concentration in the study group was 71 μg/dl. The authors of the 1986 study characterize this level as low but normal, and conclude that Zn does not appear to be an anorexigenic factor. We suggest that this conclusion is incorrect, because studies in non-cancer anorexia patients show that anorexia is associated with Zn serum levels below 75 μg/dl. Cancer cachexia patients with anorexia may suffer from functional systemic Zn deficiency, and Zn should be evaluated as a possible clinical biomarker in these patients.
Systemic inflammation in cachexia
Immunodeficiency is associated with many types of malignancies, including head and neck, lung, esophagus and breast cancer, but the underlying mechanisms are poorly understood [95]. The correlation between systemic inflammation and cancer cachexia was first demonstrated by Simons and colleagues in 1999 [96]. Recently, Fearon noted that systemic inflammation, defined as C-reactive protein >10 mg/l, is a key feature of cancer cachexia [97]. The correlation between chronic systemic inflammation and progressive loss of lean body mass has been observed in several clinical studies [98].
Altered Zn metabolism may contribute to systemic inflammation observed in cancer cachexia because Zn homeostasis is critical for efficient immune function [99]. Haase and Rink observe that “zinc is essential for the immune system, and zinc deficiency affects multiple aspects of innate and adaptive immunity” [100]. Already mild forms of Zn deficiency adversely affect immunity [101], and Zn deficiency is constantly observed in clinical cases of chronic systemic inflammation [102]. Mouse models have demonstrated that 30 days of suboptimal intake of Zn can lead to 30–80% losses in the host’s immune defense capacity [103]. Patients with diminished systemic Zn levels show a diminished immune response and a far greater susceptibility to infection. Zn supplementation reduces both spontaneous inflammatory activity (p < 0.001) and defects in the termination of inflammatory activity in elderly subjects [104]. The results from the past three decades indicate that Zn deficiency diminishes antibody- and cell-mediated immune responses in both humans and animals [105–107].
Zn has a broad impact on key immunity mediators, such as enzymes, thymic peptides, and cytokines, and regulates lymphoid cell activation, proliferation, and apoptosis [108]. The activity of practically all immune cells is modulated by Zn in vitro and in vivo, and Zn affects the expression of hundreds of genes in immune cells. Inflammation disrupts Zn homeostasis on both a systemic and cell level, and Zn deficiency that is a secondary characteristic of many diseases may aggravate the underlying condition [109]. The integrity of the human immune system can be severely impaired by functional Zn deficiency, and chronic Zn dyshomeostasis deficiency may contribute to the systemic inflammation observed in cachexia patients.
Growth failure in cachexia
Growth failure is commonly observed in pediatric patients suffering from cancer cachexia [9, 110]. The specific reasons for cancer associated growth failure are unknown, but it has been suggested that the reasons are multifactorial and include increased metabolic rate and lipolysis, decreased nutrient intake, and changes in carbohydrate and protein metabolism [111, 112]. The metabolic changes associated with cancer cachexia are complex, and it is unlikely that one factor by itself could explain impaired growth in pediatric patients. However, we suggest that Zn redistribution and dyshomeostasis may contribute to growth failure in cachexic children.
Zn is essential to the function of a large number of macromolecules and for over 300 enzymic reactions [113], and as MacDonald notes, “the inhibition of growth is the cardinal symptom of zinc deficiency” [114]. Raulin demonstrated in 1869 that Zn is necessary for the growth of the fungus Aspergillus niger [115]. Since then, its critical role in the normal development of higher plants [116], animals [117–119], and humans [120] has been established. Zn deficiency adversely and seriously affects growth in animals and humans [121, 122]. Reduced energy intake is not the limiting factor in growth because force feeding a Zn-inadequate diet to animals fails to maintain growth [114]. Zn deficiency is often the result of insufficient dietary intake or poor absorption through the digestive tract. Not surprisingly, growth failure caused by Zn deficiency is recognized as a serious nutritional health issue in many developing countries [123].
Zn deficiency results in retarded growth in infants and children [124–126]. Already moderate Zn deficiency causes growth retardation and delayed puberty in adolescents [121]. Prenatal Zn supplementation correlates significantly (p < 0.001) with infant weight [127]. Children who suffered from stunted growth and who received Zn supplementation significantly (p < 0.001) increased in both height and weight compared to the placebo group [128]. Pediatric geophagia patients who suffered from marked growth failure had subnormal serum Zn levels compared to controls (73.9 μg/dl vs. 110.5 μg/dl; p < 0.001). The growth failure was significantly attenuated by Zn supplementation [129]. Interestingly, the mean serum Zn levels in the geophagic patients were comparable to those of patients with serious anorexia (71.9–73.9 μg/dl) and advanced cancer (59.6–77.2 μ/dl).
We propose that Zn dyshomeostasis may contribute to the growth failure in pediatric cancer patients. If functional Zn deficiency is verified as a causative factor in cachexic growth failure, it could provide clinicians with a novel biomarker and improve pediatric care.
Hypogonadism in cachexia
Hypogonadism refers to a defect of the gonads that results in the underproduction of testosterone and is common in cancer patients, especially in those suffering from weight loss. A recent clinical study noted that “hypogonadism is a frequent condition in patients with advanced, incurable cancer and is associated with negative mood, fatigue, and symptoms related to anorexia/cachexia” [130]. Chlebowski and Heber studied 44 patients with lung cancer, adenocarcinoma of the colon and rectum, and prostate carcinoma with proven metastatic spreads [131]. The mean ideal body weight of patients with low testosterone and low or normal luteinizing hormone was significantly lower (p < 0.05) than in patients with normal testosterone levels. The authors conclude that “hypogonadism is a relatively common biochemical abnormality in men with cancer, particularly in patients experiencing weight loss.”
The metabolic link between hypogonadism and cachexia is unresolved, but the critical role of Zn in gonad function and steroidogenesis is well known. Zn is essential for spermatogenesis and testosterone steroidogenesis [132], and hypogonadism is a classic symptom of Zn deficiency [133]. The first study linking Zn and testosterone metabolism by the prostate gland was published in 1971 [134]. Karaca and colleagues succinctly note that “hypogonadism is a major manifestation of Zn deficiency in both humans and animals” [135].
Several possible molecular mechanisms linking Zn and hypogonadism have been proposed. Hypogonadism associated with Zn deficiency seems to result from changes in testicular steroidogenesis or indirectly from Leydig cell failure [136]. A study with young rams showed that Zn deficiency completely blocked testicular growth [137]. The Zn-specific effect on gonad function is localized within the testis where it reduces the capacity to produce testosterone, leading to low intratesticular concentrations of testosterone, a critical factor for the growth, development, and function of the seminiferous tubules. This mechanism seems to be related to the biochemical disruption of Leydig cell function.
Zn can directly modulate serum testosterone levels, and in rats, Zn supplementation results in a considerable increase in testosterone levels (p < 0.05; [138]). In the clinical study on healthy males, testosterone concentrations declined significantly (p = 0.005) after 20 weeks on a Zn-deficient diet serum [139]. It is known that diminished levels of anabolic hormones, e.g., testosterone, can lead to the loss of skeletal muscle mass [97], indicating that Zn dyshomeostasis could exacerbate muscle wasting also through a secondary mechanism. Cancer cachexia patients with functional Zn deficiency may suffer from impaired steroidogenesis and gonad activity since Zn is critical for both functions.
Conclusion
Cachexia is a complex metabolic syndrome, and the debate regarding its definition is ongoing. In this paper, we have examined the link between cancer cachexia and systemic Zn redistribution. Zn dyshomeostasis has been associated with malignant growth, but the pathophysiological consequences of chronic Zn redistribution during cancer cachexia have been ignored. We suggest that Zn dyshomeostasis is associated with the salient symptoms of cachexia such as muscle catabolism, anorexia, inflammation, growth failure, and hypogonadism. To our knowledge, this is the first report implicating systemic Zn redistribution and dyshomeostasis as central causative mechanisms in cancer cachexia.
The APR plays an important role in cachexia, and it is known to disrupt the carefully maintained Zn homeostasis and to mediate the systemic redistribution of Zn. This redistribution is mediated by Zn transporters that are significantly upregulated by APR factors such as IL-6. The primary purpose of muscle protein catabolism associated with APR seems to be the supply of amino acids for the production of hepatic proteins deployed in the defense response. We suggest that Zn accumulation into skeletal muscle tissue may be associated with protein catabolism and that this link should be further investigated.
Cancer cachexia is characterized not only by muscle wasting, but also by anorexia, systemic inflammation, pediatric growth failure, and hypogonadism. Zn homeostasis is essential for the normal function of all these processes and functional Zn deficiency compromises them. We hypothesize that Zn is accumulated in certain tissues and deprived from others resulting in severe systemic Zn dyshomeostasis and that as a result, cachexia may require completely new therapeutic approaches. Skeletal muscle Zn levels may provide the first practical and cost-effective diagnostic biomarker for cachexia, and we urge research centers to measure the Zn levels in their cachexic muscle biopsy samples.
The Zn–cachexia hypothesis has parsimonious explanatory power, but clearly the correlation and causality of Zn dyshomeostasis in cancer cachexia need to be further investigated. However, it is well established that Zn metabolism is critical for normal health and function and that systemic and chronic Zn dyshomeostasis has serious pathophysiological implications.
Other cachexia symptoms, such as insulin resistance, depression, and taste dysfunction are also associated with Zn deficiency [140–142] and should be further explored in light of the zinc-cachexia hypothesis. The possible role of systemic Zn dyshomeostasis in other cachexia-inducing conditions such as AIDS (originally known as ‘Slim Disease’), chronic heart failure, chronic obstructive pulmonary disease, chronic kidney disease, rheumatoid arthritis, severe injury, chronic inflammation, and sepsis should also be investigated. We propose that systemic Zn redistribution and dyshomeostasis play a central and thus far unappreciated role in cancer cachexia.
Acknowledgments
The authors dedicate this work to the memory of Pirkko and Jorma Gallen-Kallela who made its completion possible. Matti and Pontus Siren have been supported in this work by the Paavo Nurmi Foundation (Helsinki, Finland). All authors of this manuscript comply with the guidelines of ethical authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle [143].
Conflict of interest
Siren PMA and Siren MJ are directors of Bioneris Ab. There are no other conflicts of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Stephens NA, Skipworth RJ, Fearon KC. Cachexia, survival and the acute phase response. Curr Opin Support Palliat Care. 2008;2:267–274. doi: 10.1097/SPC.0b013e3283186be2. [DOI] [PubMed] [Google Scholar]
- 2.von Haehling S, Lainscak M, Springer J, Anker SD. Cardiac cachexia: a systematic overview. Pharmacol Ther. 2009;121:227–252. doi: 10.1016/j.pharmthera.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Koehler F, Doehner W, Hoernig S, Witt C, Anker SD, John M. Anorexia in chronic obstructive pulmonary disease—association to cachexia and hormonal derangement. Int J Cardiol. 2007;119:83–89. doi: 10.1016/j.ijcard.2006.07.088. [DOI] [PubMed] [Google Scholar]
- 4.Cheung WW, Paik KH, Mak RH. Inflammation and cachexia in chronic kidney disease. Pediatr Nephrol. 2010;25:711–724. doi: 10.1007/s00467-009-1427-z. [DOI] [PubMed] [Google Scholar]
- 5.Delano MJ, Moldawer LL. The origins of cachexia in acute and chronic inflammatory diseases. Nutr Clin Pract. 2006;21:68–81. doi: 10.1177/011542650602100168. [DOI] [PubMed] [Google Scholar]
- 6.Tisdale MJ. The ubiquitin–proteasome pathway as a therapeutic target for muscle wasting. J Support Oncol. 2005;3:209–217. [PubMed] [Google Scholar]
- 7.Kotler DP. Nutritional alterations associated with HIV infection. J Acquir Immune Defic Syndr. 2000;25:S81–S87. doi: 10.1097/00042560-200010001-00013. [DOI] [PubMed] [Google Scholar]
- 8.Wray CJ, Mammen JM, Hasselgren PO. Catabolic response to stress and potential benefits of nutrition support. Nutrition. 2002;18:971–977. doi: 10.1016/s0899-9007(02)00985-1. [DOI] [PubMed] [Google Scholar]
- 9.Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, Marks D, Mitch WE, Muscaritoli M, Najand A, Ponikowski P, Rossi Fanelli F, Schambelan M, Schols A, Schuster M, Thomas D, Wolfe R, Anker SD. Cachexia: a new definition. Clin Nutr. 2008;27:793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Lainscak M, Filippatos GS, Gheorghiade M, Fonarow GC, Anker SD. Cachexia: common, deadly, with an urgent need for precise definition and new therapies. Am J Cardiol. 2008;101:8E–10E. doi: 10.1016/j.amjcard.2008.02.065. [DOI] [PubMed] [Google Scholar]
- 11.Hambidge KM, Krebs NF. Zinc deficiency: a special challenge. J Nutr. 2007;137:1101–1105. doi: 10.1093/jn/137.4.1101. [DOI] [PubMed] [Google Scholar]
- 12.Maret W. Molecular aspects of human cellular zinc homeostasis: redox control of zinc potentials and zinc signals. Biometals. 2009;22:149–157. doi: 10.1007/s10534-008-9186-z. [DOI] [PubMed] [Google Scholar]
- 13.Takeda A, Tamano H, Enomoto S, Oku N. Zinc-65 imaging of rat brain tumors. Cancer Res. 2001;61:5065–5069. [PubMed] [Google Scholar]
- 14.Nemoto K, Kondo Y, Himeno S, Suzuki Y, Hara S, Akimoto M, Imura N. Modulation of telomerase activity by zinc in human prostatic and renal cancer cells. Biochem Pharmacol. 2000;59:401–405. doi: 10.1016/s0006-2952(99)00334-2. [DOI] [PubMed] [Google Scholar]
- 15.Cipriano C, Tesei S, Malavolta M, Giacconi R, Muti E, Costarelli L, Piacenza F, Pierpaoli S, Galeazzi R, Blasco M, Vera E, Canela A, Lattanzio F, Mocchegiani E. Accumulation of cells with short telomeres is associated with impaired zinc homeostasis and inflammation in old hypertensive participants. J Gerontol A Biol Sci Med Sci. 2009;64:745–751. doi: 10.1093/gerona/glp048. [DOI] [PubMed] [Google Scholar]
- 16.Murakami M, Hirano T. Intracellular zinc homeostasis and zinc signaling. Cancer Sci. 2008;99:1515–1522. doi: 10.1111/j.1349-7006.2008.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Memon AU, Kazi TG, Afridi HI, Jamali MK, Arain MB, Jalbani N, Syed N. Evaluation of zinc status in whole blood and scalp hair of female cancer patients. Clin Chim Acta. 2007;379:66–70. doi: 10.1016/j.cca.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Gupta SK, Singh SP, Shukla VK. Copper, zinc, and Cu/Zn ratio in carcinoma of the gallbladder. J Surg Oncol. 2005;91:204–208. doi: 10.1002/jso.20306. [DOI] [PubMed] [Google Scholar]
- 19.Büntzel J, Bruns F, Glatzel M, Garayev A, Mücke R, Kisters K, Schäfer U, Schönekaes K, Micke O. Zinc concentrations in serum during head and neck cancer progression. Anticancer Res. 2007;27:1941–1943. [PubMed] [Google Scholar]
- 20.Kopański Z, Piekoszewski W, Habiniak J, Wojewoda T, Wojewoda A, Schlegel-Zawadzka M, Sibiga W. The clinical value of the determinations in the serum of zinc concentration in women with breast cancer. Folia Histochem Cytobiol. 2001;39:84–86. [PubMed] [Google Scholar]
- 21.Mazdak H, Yazdekhasti F, Movahedian A, Mirkheshti N, Shafieian M. The comparative study of serum iron, copper, and zinc levels between bladder cancer patients and a control group. Int Urol Nephrol. 2010;42:89–93. doi: 10.1007/s11255-009-9583-4. [DOI] [PubMed] [Google Scholar]
- 22.Zuo XL, Chen JM, Zhou X, Li XZ, Mei GY. Levels of selenium, zinc, copper, and antioxidant enzyme activity in patients with leukemia. Biol Trace Elem Res. 2006;114:41–53. doi: 10.1385/BTER:114:1:41. [DOI] [PubMed] [Google Scholar]
- 23.Westin T, Ahlbom E, Johansson E, Sandström B, Karlberg I, Edström S. Circulating levels of selenium and zinc in relation to nutritional status in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 1989;115:1079–1082. doi: 10.1001/archotol.1989.01860330069019. [DOI] [PubMed] [Google Scholar]
- 24.Katz RL, Keen CL, Litt IF, Hurley LS, Kellams-Harrison KM, Glader LJ. Zinc deficiency in anorexia nervosa. J Adolesc Health Care. 1987;8:400–406. doi: 10.1016/0197-0070(87)90227-0. [DOI] [PubMed] [Google Scholar]
- 25.Prasad AS, Bao B, Beck FW, Sarkar FH. Correction of interleukin-2 gene expression by in vitro zinc addition to mononuclear cells from zinc-deficient human subjects: a specific test for zinc deficiency in humans. Transl Res. 2006;148:325–333. doi: 10.1016/j.trsl.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Maret W, Sandstead HH. Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol. 2006;20:3–18. doi: 10.1016/j.jtemb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 27.King JC, Shames DM, Woodhouse LR. Zinc homeostasis in humans. J Nutr. 2000;130:1360S–1366S. doi: 10.1093/jn/130.5.1360S. [DOI] [PubMed] [Google Scholar]
- 28.Igic PG, Lee E, Harper W, Roach KW. Toxic effects associated with consumption of zinc. Mayo Clin Proc. 2002;77:713–716. doi: 10.4065/77.7.713. [DOI] [PubMed] [Google Scholar]
- 29.U.S. Department of Agriculture, Agricultural Research Service. USDA Nutrient Database for Standard Reference, Release 14. Available: http://www.nal.usda.gov/fnic/foodcomp/search/
- 30.Lu J, Stewart AJ, Sadler PJ, Pinheiro TJ, Blindauer CA. Albumin as a zinc carrier: properties of its high-affinity zinc-binding site. Biochem Soc Trans. 2008;36:1317–1321. doi: 10.1042/BST0361317. [DOI] [PubMed] [Google Scholar]
- 31.Armstrong T, Walters E, Varshney S, Johnson CD. Deficiencies of micronutrients, altered bowel function, and quality of life during late follow-up after pancreaticoduodenectomy for malignancy. Pancreatology. 2002;2:528–534. doi: 10.1159/000066095. [DOI] [PubMed] [Google Scholar]
- 32.Berg JM, Shi Y. The galvanization of biology: a growing appreciation for the roles of zinc. Science. 1996;271:1081–1085. doi: 10.1126/science.271.5252.1081. [DOI] [PubMed] [Google Scholar]
- 33.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 34.Falconer JS, Fearon KC, Ross JA, Elton R, Wigmore SJ, Garden OJ, Carter DC. Acute-phase protein response and survival duration of patients with pancreatic cancer. Cancer. 1995;75:2077–2082. doi: 10.1002/1097-0142(19950415)75:8<2077::aid-cncr2820750808>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 35.Liuzzi JP, Lichten LA, Rivera S, Blanchard RK, Aydemir TB, Knutson MD, Ganz T, Cousins RJ. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci USA. 2005;102:6843–6848. [Google Scholar]
- 36.Lundholm K, Ekman L, Edström S, Karlberg I, Jagenburg R, Scherstén T. Protein synthesis in liver tissue under the influence of a methylcholanthrene-induced sarcoma in mice. Cancer Res. 1979;39:4657–4661. [PubMed] [Google Scholar]
- 37.Preston T, Slater C, McMillan DC, Falconer JS, Shenkin A, Fearon KC. Fibrinogen synthesis is elevated in fasting cancer patients with an acute phase response. J Nutr. 1998;128:1355–1360. doi: 10.1093/jn/128.8.1355. [DOI] [PubMed] [Google Scholar]
- 38.Moshage H. Cytokines and the hepatic acute phase response. J Pathol. 1997;181:257–266. doi: 10.1002/(SICI)1096-9896(199703)181:3<257::AID-PATH756>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 39.Gaetke LM, McClain CJ, Talwalkar RT, Shedlofsky SI. Effects of endotoxin on zinc metabolism in human volunteers. Am J Physiol. 1997;272:E952–E956. doi: 10.1152/ajpendo.1997.272.6.E952. [DOI] [PubMed] [Google Scholar]
- 40.Boosalis MG, Solem LD, McCall JT, Ahrenholz DH, McClain CJ. Serum zinc response in thermal injury. J Am Coll Nutr. 1988;7:69–76. doi: 10.1080/07315724.1988.10720222. [DOI] [PubMed] [Google Scholar]
- 41.Cousins RJ. Absorption, transport, and hepatic metabolism of copper and zinc: special reference to metallothionein and ceruloplasmin. Physiol Rev. 1985;65:238–309. doi: 10.1152/physrev.1985.65.2.238. [DOI] [PubMed] [Google Scholar]
- 42.Selektor Y, Parker RB, Sun Y, Bhattacharya ZW, SK WKT. Tissue 65Zinc translocation in a rat model of chronic aldosteronism. J Cardiovasc Pharmacol. 2008;51:359–364. doi: 10.1097/FJC.0b013e318165b96e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bibby DC, Grimble RF. Temperature and metabolic changes in rats after various doses of tumour necrosis factor alpha. J Physiol. 1989;410:367–380. doi: 10.1113/jphysiol.1989.sp017538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakata Y, Morimoto A, Long NC, Murakami N. Fever and acute-phase response induced in rabbits by intravenous and intracerebroventricular injection of interleukin-6. Cytokine. 1991;3:199–203. doi: 10.1016/1043-4666(91)90017-8. [DOI] [PubMed] [Google Scholar]
- 45.Ling PR, Schwartz JH, Jeevanandam M, Gauldie J, Bistrian BR. Metabolic changes in rats during a continuous infusion of recombinant interleukin-1. Am J Physiol. 1996;270:E305–E312. doi: 10.1152/ajpendo.1996.270.2.E305. [DOI] [PubMed] [Google Scholar]
- 46.Farquharson MJ, Al-Ebraheem A, Geraki K, Leek R, Jubb A, Harris AL. Zinc presence in invasive ductal carcinoma of the breast and its correlation with oestrogen receptor status. Phys Med Biol. 2009;54:4213–4223. doi: 10.1088/0031-9155/54/13/016. [DOI] [PubMed] [Google Scholar]
- 47.Lichten LA, Cousins RJ. Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr. 2009;29:153–176. doi: 10.1146/annurev-nutr-033009-083312. [DOI] [PubMed] [Google Scholar]
- 48.Larsson S, Karlberg I, Selin E, Daneryd P, Peterson HI. Trace element changes in serum and skeletal muscle compared to tumour tissue in sarcoma-bearing rats. In Vivo. 1987;1:131–140. [PubMed] [Google Scholar]
- 49.Russell ST, Siren PM, Siren MJ, Tisdale M. The role of zinc in the anti-tumour and anti-cachectic activity of D-myo-inositol 1, 2, 6-triphosphate. Br J Cancer. 2010;102:833–836. doi: 10.1038/sj.bjc.6605562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Claxson A, Morris C, Blake D, Sirén M, Halliwell B, Gustafsson T, Löfkvist B, Bergelin I. The anti-inflammatory effects of D-myo-inositol-1.2.6-trisphosphate (PP56) on animal models of inflammation. Agents Actions. 1990;29:68–70. doi: 10.1007/BF01964724. [DOI] [PubMed] [Google Scholar]
- 51.Felemez M, Spiess B. Investigation of the ternary D-myo-inositol 1, 2, 6 tris(phosphate)-spermine-Zn2+ system in solution. J Inorg Biochem. 2001;84:107–111. doi: 10.1016/s0162-0134(00)00220-8. [DOI] [PubMed] [Google Scholar]
- 52.Russell ST, Siren PM, Siren MJ, Tisdale M. Attenuation of skeletal muscle atrophy in cancer cachexia by D-myo-inositol 1, 2, 6-triphosphate. Cancer Chemother Pharmacol. 2009;64:517–527. doi: 10.1007/s00280-008-0899-z. [DOI] [PubMed] [Google Scholar]
- 53.Mei X, Luo X, Xu S, Xu D, Zheng Y, Xu S, Lv J. Gastroprotective effects of a new zinc(II)-curcumin complex against pylorus-ligature-induced gastric ulcer in rats. Chem Biol Interact. 2009;181:316–321. doi: 10.1016/j.cbi.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 54.Siddiqui RA, Hassan S, Harvey KA, Rasool T, Das T, Mukerji P, DeMichele S. Attenuation of proteolysis and muscle wasting by curcumin c3 complex in MAC16 colon tumour-bearing mice. Br J Nutr. 2009;102:967–975. doi: 10.1017/S0007114509345250. [DOI] [PubMed] [Google Scholar]
- 55.Frank AS, Schauble MK, Preiss IL. Trace element profiles in murine Lewis lung carcinoma by radioisotope-induced X-ray fluorescence. Am J Pathol. 1986;122:421–432. [PMC free article] [PubMed] [Google Scholar]
- 56.Busquets S, Fuster G, Ametller E, Olivan M, Figueras M, Costelli P, Carbó N, Argilés JM, López-Soriano FJ. Resveratrol does not ameliorate muscle wasting in different types of cancer cachexia models. Clin Nutr. 2007;26:239–244. doi: 10.1016/j.clnu.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 57.McCarthy DO, Graves E. Conjugated linoleic acid preserves muscle mass in mice bearing the Lewis lung carcinoma, but not the B16 melanoma. Res Nurs Health. 2006;29:98–104. doi: 10.1002/nur.20115. [DOI] [PubMed] [Google Scholar]
- 58.Markison S, Foster AC, Chen C, Brookhart GB, Hesse A, Hoare SR, Fleck BA, Brown BT, Marks DL. The regulation of feeding and metabolic rate and the prevention of murine cancer cachexia with a small-molecule melanocortin-4 receptor antagonist. Endocrinology. 2005;146:2766–2773. doi: 10.1210/en.2005-0142. [DOI] [PubMed] [Google Scholar]
- 59.Crawford AJ, Bhattacharya SK. Excessive intracellular zinc accumulation in cardiac and skeletal muscles of dystrophic hamsters. Exp Neurol. 1987;95:265–276. doi: 10.1016/0014-4886(87)90137-3. [DOI] [PubMed] [Google Scholar]
- 60.Muñoz-Delgado E, Morote-García JC, Romero-Romero R, López-García I, Hernández-Córdoba M. Determination of zinc in tissues of normal and dystrophic mice using electrothermal atomic absorption spectrometry and slurry sampling. Anal Biochem. 2006;348:64–68. doi: 10.1016/j.ab.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 61.Hersko A, Ciechanover A, Heller H, Haas AL, Rose IA. Proposed role of ATO in protein breakdown: conjugation of protein with multiple chains of the polypeptide of ATP-dependent proteolysis. Proc Natl Acad Sci USA. 1980;77:1783–1786. doi: 10.1073/pnas.77.4.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- 63.Mitch WE, Goldberg AL. Mechanisms of muscle wasting. The role of the ubiquitin–proteasome pathway. N Engl J Med. 1996;335:1897–1905. doi: 10.1056/NEJM199612193352507. [DOI] [PubMed] [Google Scholar]
- 64.Breen HB, Espat NJ. The ubiquitin–proteasome proteolysis pathway: potential target for disease intervention. J Parenter Enteral Nutr. 2004;28:272–277. doi: 10.1177/0148607104028004272. [DOI] [PubMed] [Google Scholar]
- 65.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89:381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- 66.Zamanian-Daryoush M, Mogensen TH, DiDonato JA, Williams BR. NF-kappaB activation by double-stranded-RNA-activated protein kinase (PKR) is mediated through NF-kappaB-inducing kinase and IkappaB kinase. Mol Cell Biol. 2000;20:1278–1290. doi: 10.1128/mcb.20.4.1278-1290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eley HL, Tisdale MJ. Skeletal muscle atrophy, a link between depression of protein synthesis and increase in degradation. J Biol Chem. 2007;282:7087–7097. doi: 10.1074/jbc.M610378200. [DOI] [PubMed] [Google Scholar]
- 68.Siren RST, PM SMJ, Tisdale MJ. Mechanism of attenuation of protein loss in murine C(2)C(12) myotubes by d-myo-inositol 1, 2, 6-triphosphate. Exp Cell Res. 2010;316:286–295. doi: 10.1016/j.yexcr.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 69.Siddiqui R, Pandya D, Harvey K, Zaloga GP. Nutrition modulation of cachexia/proteolysis. Nutr Clin Pract. 2006;21:155–167. doi: 10.1177/0115426506021002155. [DOI] [PubMed] [Google Scholar]
- 70.Sakkas GK, Schambelan M, Mulligan K. Can the use of creatine supplementation attenuate muscle loss in cachexia and wasting? Curr Opin Clin Nutr Metab Care. 2009;12:623–627. doi: 10.1097/MCO.0b013e328331de63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dröge W, Hack V, Breitkreutz R, Holm E, Shubinsky G, Schmid E, Galter D. Role of cysteine and glutathione in signal transduction, immunopathology and cachexia. Biofactors. 1998;8:97–102. doi: 10.1002/biof.5520080117. [DOI] [PubMed] [Google Scholar]
- 72.Argiles JM, López-Soriano FJ, Busquets S. Novel approaches to the treatment of cachexia. Drug Discov Today. 2008;13:73–78. doi: 10.1016/j.drudis.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 73.Davis MP, Dreicer R, Walsh D, Lagman R, LeGrand SB. Appetite and cancer-associated anorexia: a review. J Clin Oncol. 2004;22:1510–1517. doi: 10.1200/JCO.2004.03.103. [DOI] [PubMed] [Google Scholar]
- 74.Jatoi A, Loprinzi CL, Sloan JA, Klee GG, Windschitl HE. Neuropeptide Y, leptin, and cholecystokinin 8 in patients with advanced cancer and anorexia: a North Central Cancer Treatment Group exploratory investigation. Cancer. 2001;92:629–633. doi: 10.1002/1097-0142(20010801)92:3<629::aid-cncr1363>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 75.Levenson CW. Zinc regulation of food intake: new insights on the role of neuropeptide Y. Nutr Rev. 2003;61:247–249. doi: 10.1301/nr.2003.jul.247-249. [DOI] [PubMed] [Google Scholar]
- 76.Jing MY, Sun JY, Weng XY. Insights on zinc regulation of food intake and macronutrient selection. Biol Trace Elem Res. 2007;115:187–194. doi: 10.1007/BF02686029. [DOI] [PubMed] [Google Scholar]
- 77.McClain CJ, Kasarkis EJ, Jr, Allen JJ. Functional consequences of zinc deficiency. Prog Food Nutr Sci. 1985;9:185–226. [PubMed] [Google Scholar]
- 78.Chesters JK, Quarterman J. Effects of zinc deficiency on food intake and feeding patterns of rats. Br J Nutr. 1970;24:1061–1069. doi: 10.1079/bjn19700109. [DOI] [PubMed] [Google Scholar]
- 79.Shay NF, Mangian HF. Neurobiology of zinc-influenced eating behavior. J Nutr. 2000;130:1493S–1499S. doi: 10.1093/jn/130.5.1493S. [DOI] [PubMed] [Google Scholar]
- 80.Bakan R, Birmingham CL, Aeberhardt L, Goldner EM. Dietary zinc intake of vegetarian and nonvegetarian patients with anorexia nervosa. Int J Eat Disord. 1993;13:229–233. doi: 10.1002/1098-108x(199303)13:2<229::aid-eat2260130211>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 81.Dinsmore WW, Alderdice JT, McMaster D, Adams CE, Love AH. Zinc absorption in anorexia nervosa. Lancet. 1985;1:1041–1042. doi: 10.1016/s0140-6736(85)91637-x. [DOI] [PubMed] [Google Scholar]
- 82.Casper RC, Kirschner B, Sandstead HH, Jacob RA, Davis JM. An evaluation of trace metals, vitamins, and taste function in anorexia nervosa. Am J Clin Nutr. 1980;33:1801–1808. doi: 10.1093/ajcn/33.8.1801. [DOI] [PubMed] [Google Scholar]
- 83.Humphries L, Vivian B, Stuart M, McClain CJ. Zinc deficiency and eating disorders. J Clin Psychiatry. 1989;50:456–459. [PubMed] [Google Scholar]
- 84.Bryce-Smith D, Simpson R. Case of anorexia nervosa responding to zinc sulphate. Lancet. 1984;2:350. doi: 10.1016/s0140-6736(84)92717-x. [DOI] [PubMed] [Google Scholar]
- 85.Humphries L, McClain MP, Vivian B, Cunnup L, McClain CJ (1990) Anorexia nervosa, zinc supplementation and weight gain. In: Anderson, H (ed.) Biology of feast and famine: relevance to eating disorders symposium on nutrition research. (abs.)
- 86.Safai-Kutti S. Oral zinc supplementation in anorexia nervosa. Acta Psychiatr Scand Suppl. 1990;82:14–17. [PubMed] [Google Scholar]
- 87.Birmingham CL, Goldner EM, Bakan R. Controlled trial of zinc supplementation in anorexia nervosa. Int J Eat Disord. 1994;15:251–255. [PubMed] [Google Scholar]
- 88.Castro J, Deulofeu R, Gila A, Puig J, Toro J. Persistence of nutritional deficiencies after short-term weight recovery in adolescents with anorexia nervosa. Int J Eat Disord. 2004;35:169–178. doi: 10.1002/eat.10249. [DOI] [PubMed] [Google Scholar]
- 89.Su JC, Birmingham CL. Zinc supplementation in the treatment of anorexia nervosa. Eat Weight Disord. 2002;7:20–22. doi: 10.1007/BF03354425. [DOI] [PubMed] [Google Scholar]
- 90.Birmingham CL, Gritzner S. How does zinc supplementation benefit anorexia nervosa? Eat Weight Disord. 2006;11:e109–e111. doi: 10.1007/BF03327573. [DOI] [PubMed] [Google Scholar]
- 91.Casper RC, Kirschner B, Jacob RA. Zinc and copper status in anorexia nervosa. [proceedings] Psychopharmacol Bull. 1978;14:53–55. [PubMed] [Google Scholar]
- 92.Goel T, Sankhwar SN. Comparative study of zinc levels in benign and malignant lesions of the prostate. Scand J Urol Nephrol. 2006;40:108–112. doi: 10.1080/00365590500368922. [DOI] [PubMed] [Google Scholar]
- 93.Davies IJ, Musa M, Dormandy TL. Measurements of plasma zinc in malignant disease. J Clin Pathol. 1968;21:363–365. [PMC free article] [PubMed] [Google Scholar]
- 94.Lindsey AM, Piper BF. Anorexia, serum zinc, and immunologic response in small cell lung cancer patients receiving chemotherapy and prophylactic cranial radiotherapy. Nutr Cancer. 1986;8:231–238. doi: 10.1080/01635588609513899. [DOI] [PubMed] [Google Scholar]
- 95.Hadden JW. Immunodeficiency and cancer: prospects for correction. Int Immunopharmacol. 2003;3:1061–1071. doi: 10.1016/S1567-5769(03)00060-2. [DOI] [PubMed] [Google Scholar]
- 96.Simons JP, Schols AM, Buurman WA, Wouters EF. Weight loss and low body cell mass in males with lung cancer: relationship with systemic inflammation, acute-phase response, resting energy expenditure, and catabolic and anabolic hormones. Clin Sci (Lond) 1999;97:215–223. [PubMed] [Google Scholar]
- 97.Fearon KC. Cancer cachexia: developing multimodal therapy for a multidimensional problem. Eur J Cancer. 2008;44:1124–1132. doi: 10.1016/j.ejca.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 98.McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12:223–226. doi: 10.1097/MCO.0b013e32832a7902. [DOI] [PubMed] [Google Scholar]
- 99.Rink L, Haase H. Zinc homeostasis and immunity. Trends Immunol. 2007;28:1–4. doi: 10.1016/j.it.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 100.Haase H, Rink L. The immune system and the impact of zinc during aging. Immun Ageing. 2009;6:9. doi: 10.1186/1742-4933-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bao B, Prasad A, Beck FW, Suneja A, Sarkar F. Toxic effect of zinc on NF-kappaB, IL-2, IL-2 receptor alpha, and TNF-alpha in HUT-78 (Th(0)) cells. Toxicol Lett. 2006;166:222–228. doi: 10.1016/j.toxlet.2006.07.306. [DOI] [PubMed] [Google Scholar]
- 102.Vasto S, Mocchegiani E, Malavolta M, Cuppari I, Listì F, Nuzzo D, Ditta V, Candore G, Caruso C. Zinc and inflammatory/immune response in aging. Ann NY Acad Sci. 2007;1100:111–122. doi: 10.1196/annals.1395.009. [DOI] [PubMed] [Google Scholar]
- 103.Cook-Mills JM, Fraker PJ. Functional capacity of the residual lymphocytes from zinc-deficient adult mice. Br J Nutr. 1993;69:835–848. doi: 10.1079/bjn19930084. [DOI] [PubMed] [Google Scholar]
- 104.Kahmann L, Uciechowski P, Warmuth S, Plümäkers B, Gressner AM, Malavolta M, Mocchegiani E, Rink L. Zinc supplementation in the elderly reduces spontaneous inflammatory cytokine release and restores T cell functions. Rejuvenation Res. 2008;11:227–237. doi: 10.1089/rej.2007.0613. [DOI] [PubMed] [Google Scholar]
- 105.Fraker PJ, King LE, Laakko T, Vollmer TL. The dynamic link between the integrity of the immune system and zinc status. J Nutr. 2000;130:1399S–1406S. doi: 10.1093/jn/130.5.1399S. [DOI] [PubMed] [Google Scholar]
- 106.Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr. 1998;68:447S–463S. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- 107.Prasad AS. Zinc: role in immunity, oxidative stress and chronic inflammation. Curr Opin Clin Nutr Metab Care. 2009;12:646–652. doi: 10.1097/MCO.0b013e3283312956. [DOI] [PubMed] [Google Scholar]
- 108.Dardenne M. Zinc and immune function. Eur J Clin Nutr. 2002;56:S20–S23. doi: 10.1038/sj.ejcn.1601479. [DOI] [PubMed] [Google Scholar]
- 109.Haase H, Rink L. Functional significance of zinc-related signalling pathways in immune cells. Annu Rev Nutr. 2009;29:133–152. doi: 10.1146/annurev-nutr-080508-141119. [DOI] [PubMed] [Google Scholar]
- 110.Meacham LR, Mason PW, Sullivan KM. Auxologic and biochemical characterization of the three phases of growth failure in pediatric patients with brain tumors. J Pediatr Endocrinol Metab. 2004;17:711–717. doi: 10.1515/jpem.2004.17.5.711. [DOI] [PubMed] [Google Scholar]
- 111.Andrassy RJ, Chwals WJ. Nutritional support of the pediatric oncology patient. Nutrition. 1998;14:124–129. doi: 10.1016/s0899-9007(97)00225-6. [DOI] [PubMed] [Google Scholar]
- 112.DeWys WD. Pathophysiology of cancer cachexia: current understanding and areas for future research. Cancer Res. 1982;42:721s–726s. [PubMed] [Google Scholar]
- 113.Tapiero H, Tew KD. Trace elements in human physiology and pathology: zinc and metallothioneins. Biomed Pharmacother. 2003;57:399–411. doi: 10.1016/s0753-3322(03)00081-7. [DOI] [PubMed] [Google Scholar]
- 114.MacDonald RS. The role of zinc in growth and cell proliferation. J Nutr. 2000;130:1500S–1508S. doi: 10.1093/jn/130.5.1500S. [DOI] [PubMed] [Google Scholar]
- 115.Raulin J. Etudes cliniques sur la vegetation. Ann Sci Nat Bot Biol Veg. 1869;11:93. [Google Scholar]
- 116.Sommer AL, Lipman CB. Evidence on the indispensable nature of zinc and boron for higher green plants. Plant Physiol. 1926;1:231–249. doi: 10.1104/pp.1.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Todd WR, Elvehjem CA, Hart EB. Zinc in the nutrition of the rat. Am J Physiol. 1933;107:146–156. [Google Scholar]
- 118.Tucker HF, Salmon WD. Parakeratosis or zinc deficiency disease in the pig. Proc Soc Exp Biol Med. 1955;88:613–616. doi: 10.3181/00379727-88-21670. [DOI] [PubMed] [Google Scholar]
- 119.Ott EA, Smith WH, Stob M, Beeson WM. Zinc deficiency syndrome in the young lamb. J Nutr. 1964;82:41–50. doi: 10.1093/jn/82.1.41. [DOI] [PubMed] [Google Scholar]
- 120.Prasad AS, Miale A, Jr, Farid Z, Sandstead HH, Schulert AR, Darby WJ. Biochemical studies on dwarfism, hypogonadism, and anemia. Arch Intern Med. 1963;111:407–428. doi: 10.1001/archinte.1963.03620280007003. [DOI] [PubMed] [Google Scholar]
- 121.Prasad AS. Clinical, endocrinological and biochemical effects of zinc deficiency. Clin Endocrinol Metab. 1985;14:567–589. doi: 10.1016/s0300-595x(85)80007-4. [DOI] [PubMed] [Google Scholar]
- 122.Jameson S. Zinc nutrition and human pregnancy, in progress in clinical and biological research, volume 129. In: Prasad AS, Cavdar AO, Brewer GJ, Aggett PJ, editors. Zinc deficiency in human subjects. New York: Alan R Liss; 1983. p. 63. [PubMed] [Google Scholar]
- 123.Wapnir P. Zinc deficiency, malnutrition and the gastrointestinal tract. J Nutr. 2000;130:1388S–1392S. doi: 10.1093/jn/130.5.1388S. [DOI] [PubMed] [Google Scholar]
- 124.Díaz-Gómez NM, Doménech E, Barroso F, Castells S, Cortabarria C, Jiménez A. The effect of zinc supplementation on linear growth, body composition, and growth factors in preterm infants. Pediatrics. 2003;111:1002–1009. doi: 10.1542/peds.111.5.1002. [DOI] [PubMed] [Google Scholar]
- 125.Salgueiro MJ, Zubillaga MB, Lysionek AE, Caro RA, Weill R, Boccio JR. The role of zinc in the growth and development of children. Nutrition. 2002;18:510–519. doi: 10.1016/s0899-9007(01)00812-7. [DOI] [PubMed] [Google Scholar]
- 126.Nishi Y. Zinc and growth. J Am Coll Nutr. 1996;15:340–344. doi: 10.1080/07315724.1996.10718608. [DOI] [PubMed] [Google Scholar]
- 127.Iannotti LL, Zavaleta N, Leon Z, Shankar AH, Caulfield LE. Maternal zinc supplementation and growth in Peruvian infants. Am J Clin Nutr. 2008;88:154–160. doi: 10.1093/ajcn/88.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.West UM, CE HJ, Deurenberg P, Hautvast JG. Zinc supplementation and stunted infants in Ethiopia: a randomised controlled trial. Lancet. 2000;355:2021–2026. doi: 10.1016/S0140-6736(00)02348-5. [DOI] [PubMed] [Google Scholar]
- 129.Cavdar AO, Arcasoy A, Cin S, Babacan E, Gözdasoglu S. Geophagia in Turkey: iron and zinc deficiency, iron and zinc absorption studies and response to treatment with zinc in geophagia cases, in progress in clinical and biological research, volume 129. In: Prasad AS, Cavdar AO, Brewer GJ, Aggett PJ, editors. Zinc deficiency in human subjects. New York: Alan R Liss; 1983. p. 71. [PubMed] [Google Scholar]
- 130.Strasser F, Palmer JL, Schover LR, Yusuf SW, Pisters K, Vassilopoulou-Sellin R, DeGracia B, Willey JS, Bruera E. The impact of hypogonadism and autonomic dysfunction on fatigue, emotional function, and sexual desire in male patients with advanced cancer: a pilot study. Cancer. 2006;107:2949–2957. doi: 10.1002/cncr.22339. [DOI] [PubMed] [Google Scholar]
- 131.Chlebowski RT, Heber D. Hypogonadism in male patients with metastatic cancer prior to chemotherapy. Cancer Res. 1982;42:2495–2498. [PubMed] [Google Scholar]
- 132.Prasad AS. Zinc deficiency in human subjects. Prog Clin Biol Res. 1983;129:1–33. [PubMed] [Google Scholar]
- 133.McClain CJ, Gavaler JS, Van Thiel DH. Hypogonadism in the zinc-deficient rat: localization of the functional abnormalities. J Lab Clin Med. 1984;104:1007–1015. [PubMed] [Google Scholar]
- 134.Grant JK, Minguell J, Taylor P, Weiss M. A possible role of zinc in the metabolism of testosterone by the prostate gland. Biochem J. 1971;125:21P. doi: 10.1042/bj1250021pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Karaca Z, Tanriverdi F, Kurtoglu S, Tokalioglu S, Unluhizarci K, Kelestimur F. Pubertal arrest due to Zn deficiency: the effect of zinc supplementation. Hormones (Athens) 2007;6:71–74. [PubMed] [Google Scholar]
- 136.Hamdi SA, Nassif OI, Ardawi MS. Effect of marginal or severe dietary zinc deficiency on testicular development and functions of the rat. Arch Androl. 1997;38:243–253. doi: 10.3109/01485019708994883. [DOI] [PubMed] [Google Scholar]
- 137.Martin GB, White CL, Markey CM, Blackberry MA. Effects of dietary zinc deficiency on the reproductive system of young male sheep: testicular growth and the secretion of inhibin and testosterone. J Reprod Fertil. 1994;101:87–96. doi: 10.1530/jrf.0.1010087. [DOI] [PubMed] [Google Scholar]
- 138.Kaya O, Gokdemir K, Kilic M, Baltaci AK. Zinc supplementation in rats subjected to acute swimming exercise: its effect on testosterone levels and relation with lactate. Neuro Endocrinol Lett. 2006;27:267–270. [PubMed] [Google Scholar]
- 139.Prasad AS, Mantzoros CS, Beck FW, Hess JW, Brewer GJ. Zinc status and serum testosterone levels of healthy adults. Nutrition. 1996;12:344–348. doi: 10.1016/s0899-9007(96)80058-x. [DOI] [PubMed] [Google Scholar]
- 140.Haase H, Maret W. Protein tyrosine phosphatases as targets of the combined insulinomimetic effects of zinc and oxidants. Biometals. 2005;18:333–338. doi: 10.1007/s10534-005-3707-9. [DOI] [PubMed] [Google Scholar]
- 141.Levenson CW. Zinc: the new antidepressant? Nutr Rev. 2006;64:39–42. doi: 10.1111/j.1753-4887.2006.tb00171.x. [DOI] [PubMed] [Google Scholar]
- 142.Heyneman CA. Zinc deficiency and taste disorders. Ann Pharcother. 1996;30:186–187. doi: 10.1177/106002809603000215. [DOI] [PubMed] [Google Scholar]
- 143.von Haehling S, Morley JE, Coats AJS, Anker SD (2010) Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle 1. doi:10.1007/s13539-010-0003-5 [DOI] [PMC free article] [PubMed]


