Abstract
Background & Aims
Gallstones are common and contribute to morbidity and health-care costs, but their effects on mortality are unclear. We examined whether gallstone disease was associated with overall and cause-specific mortalities in a prospective national population-based sample.
Methods
We analyzed data from 14,228 participants in the third U.S. National Health and Nutrition Examination Survey (20–74 years old) who underwent gallbladder ultrasonography from 1988 to 1994. Gallstone disease was defined as ultrasound-documented gallstones or evidence of cholecystectomy. The underlying cause of death was identified from death certificates collected through 2006 (mean follow up=14.3 years). Mortality hazard ratios (HR) were calculated using Cox proportional hazards regression analysis, to adjust for multiple demographic and cardiovascular-disease risk factors.
Results
The prevalence of gallstones was 7.1% and of cholecystectomy was 5.3%. During a follow-up period of 18 years or more, the cumulative mortality was 16.5% from all causes (2,389 deaths), 6.7% from cardiovascular disease (886 deaths), and 4.9% from cancer (651 deaths). Participants with gallstone disease had higher all-cause mortality in age-adjusted (HR, 1.3; 95% confidence interval [CI], 1.2–1.5) and multivariate-adjusted analysis (HR, 1.3; 95% CI, 1.1–1.5). A similar increase was observed for cardiovascular disease mortality (multivariate-adjusted HR, 1.4; 95% CI, 1.2–1.7) and cancer mortality (multivariate-adjusted HR, 1.3; 95% CI, 0.98–1.8). Individuals with gallstones had a similar increase in risk of death as those with cholecystectomy (multivariate-adjusted HR, 1.1; 95% CI, 0.92–1.4).
Conclusions
In the U.S. population, persons with gallstone disease have increased mortality, overall, and mortalities from cardiovascular disease and cancer. This relationship was found for both ultrasound-diagnosed gallstones and cholecystectomy.
Keywords: gallstone disease, epidemiology, gallbladder, cholelithiasis
INTRODUCTION
Gallstones are common and greatly contribute to health care costs and patient morbidity, yet death directly due to complications of gallstones is rare today. Whether gallstone disease is associated with increased mortality is unclear. One study of American Pima Indians found increased overall mortality and cancer mortality among persons with gallstone disease.1 In this population-based survey with 20 years of follow-up, participants with gallstones, measured by oral cholecystography, or previous cholecystectomy had almost twice the risk of death as those with no gallstone disease. The risk of death from malignancy was over six and a half times as high with gallstone disease. However, the increased risk of overall mortality was not entirely explained by the higher cancer death rate with gallstone disease. Gallstones are considered to be the main risk factor for gallbladder cancer, however, this condition is rare.2 Gallstone disease has also been associated with extra-biliary malignancies.3, 4
An association of gallstones with cardiovascular disease has been suggested.5-8 Gallstone disease and cardiovascular disease are both common conditions and share a number of risk factors, particularly age, obesity, diabetes, and components of the metabolic syndrome. Cholesterol is the main component of the majority of gallstones in the U.S. as well as of atheroma. Whether the two conditions are found together more frequently than would be expected of two common conditions, and if so, whether this is the result of a common underlying cause or of a causal relationship between them is uncertain. Few studies have investigated the relationship of mortality with gallstone disease and, to our knowledge, none have done so in the general U.S. population.
Using death certificate data from the third National Health and Nutrition Examination Study (NHANES III), a prospective, population-based sample, we examined whether gallstone disease (gallstones or cholecystectomy) was associated with increased mortality overall and from specific causes. If an association was found, a second objective was to determine whether the relationship was similar for gallstones and cholecystectomy. The multitude of other variables collected in NHANES III allowed evaluation of potential confounders for mortality.
METHODS
NHANES III was conducted in the United States from 1988 through 1994 by the National Center for Health Statistics of the Centers for Disease Control and Prevention9 It consisted of a cross-sectional interview, examination, and laboratory data collected from a complex multistage, stratified, clustered probability sample representative of the civilian, noninstitutionalized population with oversampling of persons aged 60 years and older, African Americans, and Hispanics. The survey was approved by the Centers for Disease Control and Prevention Institutional Review Board, and all participants provided written informed consent to participate.
Of 18,738 sampled persons age 20-74 years, 14,645 (78%) were examined. We excluded participants who did not undergo a gallbladder ultrasound (n=351) or whose gallbladder lumen could not be adequately visualized on ultrasound (n=56) and those for whom mortality status was unknown (n=10). The analysis sample, therefore, consisted of 14,228 participants. Measures of insulin resistance have been shown to be related to gallstone disease,10 but were only available for a subgroup of participants who fasted prior to the examination. Therefore, a secondary analysis of 6,258 participants randomly assigned to be examined in the morning after an overnight fast excluded 840 who missed the morning examination or who fasted <8 or >24 hours.
Gallstone disease was defined as ultrasound-documented gallstones or evidence of a cholecystectomy (a right upper quadrant or epigastric scar and the absence of a gallbladder) by standard criteria.11 Based on videotaped recordings of ultrasound examinations, there was excellent agreement on gallbladder disease diagnosis between the ultrasonographer and reviewing radiologist (agreement of 99% with a kappa statistic of 0.97).
Data were collected at baseline, as previously described, on factors known or thought to be related to gallstone disease or mortality and included as covariates in multivariate analyses: age (years), sex, ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other), education (years; <12, 12, >12), cigarette smoking (never, former, <1 pack/day, ≥1 pack/day), alcohol drinking (never, former, <1 drink/day, 1-2 drinks/day, or >2 drinks/day), doctor-diagnosed diabetes, physical activity intensity (METs), caffeinated beverage consumption (mg of caffeine/day), body mass index (BMI; weight [kg]/height [m2]), waist and hip circumferences (cm), systolic and diastolic blood pressure (mm Hg), hemoglobin A1C (%), serum total and high-density lipoprotein (HDL) cholesterol concentrations (mg/dL), and C-reactive protein (mg/dL; 0-0.3, >0.3).10, 12-18 γ-glutamyltransferase (GGT) was added to the protocol after data collection began (n=10,442) and categorized as normal or elevated (>51 IU/L in men or >33 IU/L in women). Among a subgroup of participants who attended a morning examination after an overnight fast, concentrations of serum triglycerides (mg/dL), plasma glucose (mg/dL), and serum insulin (pmol/L) were measured; and an insulin resistance index using the homeostasis model assessment was determined.19
Participants were passively followed for mortality through December 31, 2006, using a probabilistic match that linked NHANES III participants with National Death Index records to ascertain vital status and cause of death. This matching methodology is well established and has been described in detail.20 The accuracy of the NHANES III-National Death Index matching methodology was high in a validation study that applied it to the NHANES I Epidemiologic Follow-up Study (96.1% of decedents and 99.4% of living participants were classified correctly).21 Persons not matched to a death record were considered to be alive through the end of follow-up. Mortality outcomes were based on death certificate underlying cause of death coded according to the International Classification of Diseases, Ninth Revision (ICD-9) for deaths occurring between 1988 and 1998, and according to the International Classification of Diseases, Tenth Revision (ICD-10) for deaths occurring between 1999 and 2006.20 Outcomes consisted of all-cause mortality and the following cause-specific mortality: complications of gallbladder disease, excluding gallbladder cancer (ICD-9 codes 574-576; ICD-10 codes K80.0-K80.8 and K81-K83), cardiovascular disease (ICD-9 codes 390-459; ICD-10 codes I00-I99), malignancy (ICD-9 codes 140-239; ICD-10 codes C00-D48), digestive disease (excluding dental, malignant, and infectious) (ICD-9 codes 530-537, 550-571 and 572.1-579; ICD-10 codes K20-K31, K40-K74.6, and K75.1-K93.8), diabetes mellitus (ICD-9 code 250; ICD-10 codes E10-E14), infectious disease (ICD-9 codes 001-139.8, 320-326, 460-466.1, 480-487.8, 540-543, 572.0, 590, 599.0, and 680-686; ICD-10 codes A00-B99, G00-G09, J00-J06, J20-J21, J09-J18, K35-K38, K75.0, N39.0, and L00-L08), and all other mortality.
Statistical analysis
Baseline characteristics were compared by gallstone disease status using a t test for continuous variables or a χ2 test for categorical variables. Age-adjusted baseline characteristics were compared using linear regression analysis (SUDAAN, PROC REGRESS, SUDAAN User=s Manual, Release 10.0, 2008; Research Triangle Institute, Research Triangle Park, NC) to calculate adjusted (least squares) mean estimates. Cumulative mortality during follow-up among persons with and without gallstone disease was calculated using Kaplan-Meier analysis. Hazard rate ratio (HR) estimates (relative risk) for mortality outcomes were calculated by Cox proportional hazard regression analysis (SUDAAN, PROC SURVIVAL, SUDAAN User=s Manual, Release 10.0, 2008; Research Triangle Institute, Research Triangle Park, NC) to control for effects of potential risk factors while taking into consideration varying lengths of follow-up. Time at risk was from the date of the NHANES III examination to the date of death or to December 31, 2006. For analyses of cause-specific mortality, participants who died of other causes were censored at the date of death. All factors met the proportional hazard assumption of a relatively constant risk ratio through examination of -log (-log) plots of survival versus time by categories.22 Interaction between sex or age and gallstone disease status was also evaluated by adding two-way interaction terms to unadjusted- and multivariate-adjusted models. Multivariate analyses excluded persons with missing values for any risk factor included in the model. A p-value of <0.05 was considered to indicate statistical significance. All analyses utilized sample weights that accounted for unequal selection probabilities and nonresponse. All variance calculations accounted for the design effects of the survey using Taylor series linearization.23
RESULTS
The prevalence (±SE) of gallstones was 7.1% (±0.38%) and cholecystectomy 5.3% (±0.29%). Because participants with gallstone disease tended to be considerably older than those without (mean 53.2 years versus 40.7 years, p<0.001), other baseline characteristics were compared adjusted for age (Table 1). In contrast to persons without gallstone disease, those with gallstone disease were more likely to be female, Mexican-American, diabetic, less educated and less physically active, and to have a higher BMI and prevalence of elevated C-reactive protein and GGT, and a lower HDL cholesterol and alcohol intake (Table 1). A higher waist-to-hip ratio, serum total cholesterol, systolic blood pressure, and proportion of former smokers among persons with gallstone disease was found in unadjusted analysis only (data not shown), while lower caffeine consumption was seen only with age-adjustment.
Table 1.
Age-adjusted baseline characteristics of participants by gallstone disease (GSD)* status
| Characteristic | No GSD (n=12,210) | GSD (n=2,018) | p-value† |
|---|---|---|---|
| Women (% ± SE) | 49.1 ± 0.50 | 69.5 ± 1.9 | <0.001 |
| Race-ethnicity (% ± SE) | |||
| Non-Hispanic white | 75.6 ± 1.3 | 75.7 ± 1.6 | 0.94 |
| Non-Hispanic black | 11.3 ± 0.64 | 9.2 ± 0.76 | 0.011 |
| Mexican American | 5.1 ± 0.43 | 7.4 ± 0.77 | <0.001 |
| Other | 8.0 ± 0.83 | 7.7 ± 1.1 | 0.65 |
| Education (years; % ± SE) | |||
| <12 | 22.9 ± 0.98 | 25.6 ± 1.6 | 0.048 |
| 12 | 33.9 ± 0.72 | 39.1 ± 1.6 | 0.001 |
| >12 | 43.2 ± 1.2 | 35.3 ± 1.9 | <0.001 |
| BMI (kg/m2; mean (SD)) | 26.2 (0.10) | 28.8 (0.38) | <0.001 |
| Waist-to-hip ratio (mean (SD)) | 90.6 (0.15) | 90.9 (0.38) | 0.48 |
| Glucose status abnormal (% ± SE)‡ | 5.7 ± 0.36 | 11.5 ± 1.4 | <0.001 |
| Serum total cholesterol (mg/dL; mean (SD)) | 204 (0.82) | 200 (1.0) | 0.014 |
| Serum HDL cholesterol (mg/dL; mean (SD)) | 50.8 (0.35) | 49.5 (0.56) | 0.031 |
| Systolic blood pressure (mm Hg; mean (SD)) | 121 (0.23) | 122 (0.59) | 0.19 |
| Diastolic blood pressure (mm Hg; mean (SD)) | 74.4 (0.19) | 73.2 (0.37) | <0.001 |
| Cigarette smoking (% ± SE) | |||
| Never | 44.9 ± 0.87 | 47.6 ± 1.7 | 0.16 |
| Former | 25.2 ± 0.55 | 24.1 ± 2.1 | 0.61 |
| <1 pack per day | 13.1 ± 0.51 | 12.1 ± 1.1 | 0.42 |
| ≥1 pack per day | 16.8 ± 0.72 | 16.3 ± 1.2 | 0.63 |
| Alcohol drinking (% ± SE) | |||
| Never | 11.7 ± 0.60 | 15.7 ± 1.6 | 0.012 |
| Former | 30.3 ± 0.93 | 41.1 ± 1.7 | <0.001 |
| <1 drink per day | 41.5 ± 1.1 | 34.2 ± 1.9 | <0.001 |
| 1-2 drinks per day | 9.4 ± 0.52 | 6.0 ± 0.90 | <0.001 |
| >2 drinks per day | 7.1 ± 0.43 | 3.1 ± 0.64 | <0.001 |
| Caffeine (mg/day; mean (SD))§ | 232 (5.7) | 210 (10.0) | 0.040 |
| Physical activity intensity (METs; mean (SD))∥ | 114 (3.3) | 91 (4.5) | <0.001 |
| C-reactive protein >0.3 mg/dL (% ± SE) | 23.5 ± 0.98 | 35.9 ± 1.9 | <0.001 |
| γ-glutamyltransferase elevated (% ± SE)¶ | 13.1 ± 0.61 | 20.6 ± 1.9 | <0.001 |
METs, ratio of work metabolic rate to resting metabolic rate.
Ultrasound-documented gallstones or evidence of a cholecystectomy.
From linear regression analysis.
Doctor-diagnosed diabetes or hemoglobin A1C ≥6.5% (95th percentile).
Sum of caffeine from regular coffee (137 mg/cup), regular tea (47 mg/cup), & regular or diet colas and sodas (46 mg/bottle or can) (Michels, J Natl Cancer Inst, 2005; 97:282-92).
Sum of the products of activity frequency in the previous month and an intensity rating (Ainsworth, Med Sci Sports Exerc, 1993; 25:71-80) for 9 common activities.
Subgroup with GGT measured (n=10,442); >51 IU/L in men or >33 IU/L in women.
The median follow-up among the 14,228 participants aged 20-74 years was 14.7 years (range, 0.025–18.1 years). The cumulative mortality from all causes was 16.5% (2,389 deaths) at 18 years of follow-up. The cause-specific cumulative mortality (underlying cause) was 6.7% (886 deaths) from cardiovascular disease, 4.9% (651 deaths) from cancer, 0.031% (5 deaths) from complications of gallbladder disease, 0.87% (115 deaths) from digestive disease (excluding dental, malignant and infectious), 0.54% (102 deaths) from diabetes mellitus, 1.1% (146 deaths) from infectious disease, and 3.5% (489 deaths) from all other causes. Only one death was attributed to gallbladder cancer which occurred in a participant with gallstones on ultrasound.
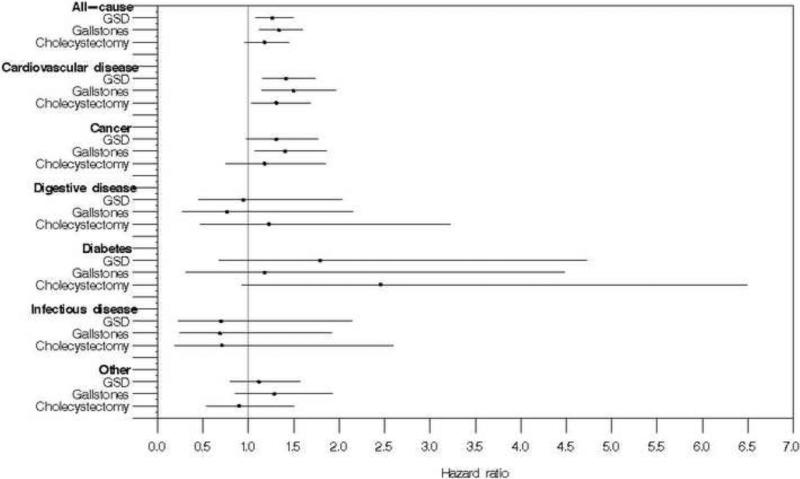
Over 18 years of follow-up, study participants with gallstone disease had higher unadjusted cumulative all-cause mortality than those without gallstone disease (Table 2). Gallstone disease was associated with a 30% higher all-cause mortality in both age-adjusted analysis (p<0.001) and multivariate analysis adjusted for potential demographic and numerous cardiovascular disease risk factors (HR=1.3, 95% CI=1.1-1.5, p=0.004) (Table 2, Figure 1). Mortality from cardiovascular disease was 50% higher among persons with gallstone disease in age-adjusted analysis (p<0.001) and was only mildly diminished in multivariate-adjusted analysis (HR=1.4, 95% CI=1.2-1.7, p=0.001) (Table 2, Figure 1). Cancer mortality was 30% higher with gallstone disease in both age-adjusted (p=0.042) and multivariate-adjusted analysis, although the latter did not reach statistical significance (HR=1.3, 95% CI=0.98-1.8, p=0.069) (Table 2, Figure 1). Of 651 cancer deaths, 150 were identified as digestive tract cancers (ICD-9 codes 150-159; ICD-10 codes C15-C19 and C20-C26) of which 58 were attributed to the colon (ICD-9 codes 153 and 154.0-154.1; ICD-10 codes C18-C20). Compared with overall cancer mortality, the multivariate-adjusted hazard ratios were higher for all digestive tract cancers (HR, 1.6; 95% CI, 0.84–2.9; p=0.16) and for colon cancer (HR, 1.5; 95% CI, 0.62–3.6; p=0.36), but neither reached statistical significance. Among other common cancers, gallstone disease was unrelated to mortality from cancers of the trachea/bronchus/lung (ICD-10 codes C33-C34; n=208; multivariate-adjusted HR, 0.98; 95% CI, 0.62–1.6; p=0.94), or from breast cancer (ICD-10 code C50; n=51; multivariate-adjusted HR, 1.1; 95% CI, 0.43–3.0; p=0.78).
Table 2.
Cumulative probability (unadjusted) over 18 years and age-adjusted hazard ratios and 95% confidence intervals for death (underlying cause) by gallstone disease* status in the United States, 1988–2006 (N=14,228)
| Mortality outcome Gallstone disease status | Number of deaths | Unadjusted cumulative mortality (%)† | Age-adjusted hazard ratio‡ | 95% C.I. | p-value |
|---|---|---|---|---|---|
| All-cause | |||||
| No gallstone disease | 1,758 | 13.8 | 1.0 | ||
| Gallstone disease | 631 | 36.3 | 1.3 | 1.2 - 1.5 | <0.001 |
| Gallstones | 372 | 35.6 | 1.4 | 1.2 - 1.6 | <0.001 |
| Cholecystectomy | 259 | 37.6 | 1.3 | 1.1 - 1.5 | 0.009 |
| Cardiovascular disease | |||||
| No gallstone disease | 639 | 5.2 | 1.0 | ||
| Gallstone disease | 247 | 18.2 | 1.5 | 1.3 - 1.8 | <0.001 |
| Gallstones | 149 | 18.8 | 1.6 | 1.3 - 2.1 | <0.001 |
| Cholecystectomy | 98 | 17.2 | 1.3 | 1.1 - 1.6 | 0.004 |
| Cancer | |||||
| No gallstone disease | 485 | 4.2 | 1.0 | ||
| Gallstone disease | 166 | 10.7 | 1.3 | 1.01 - 1.7 | 0.042 |
| Gallstones | 101 | 11.5 | 1.4 | 1.1 - 1.8 | 0.011 |
| Cholecystectomy | 65 | 9.4 | 1.2 | 0.79 - 1.7 | 0.44 |
| Digestive disease (excludes dental, malignant, and infectious) | |||||
| No gallstone disease | 78 | 0.74 | 1.0 | ||
| Gallstone disease | 37 | 2.0 | 1.2 | 0.72 - 2.0 | 0.47 |
| Gallstones | 18 | 1.4 | 0.81 | 0.36 - 1.8 | 0.60 |
| Cholecystectomy | 19 | 2.9 | 1.7 | 0.93 - 3.2 | 0.086 |
| Diabetes | |||||
| No gallstone disease | 64 | 0.31 | 1.0 | ||
| Gallstone disease | 38 | 2.6 | 2.6 | 1.3 - 5.3 | 0.008 |
| Gallstones | 16 | 1.1 | 1.6 | 0.56 - 4.3 | 0.39 |
| Cholecystectomy | 22 | 4.9 | 4.0 | 1.9 - 8.4 | <0.001 |
| Infectious disease | |||||
| No gallstone disease | 119 | 1.0 | 1.0 | ||
| Gallstone disease | 27 | 1.5 | 0.83 | 0.31 - 2.2 | 0.70 |
| Gallstones | 15 | 1.8 | 0.92 | 0.34 - 2.5 | 0.87 |
| Cholecystectomy | 12 | 1.2 | 0.70 | 0.20 - 2.4 | 0.57 |
| Other | |||||
| No gallstone disease | 373 | 3.1 | 1.0 | ||
| Gallstone disease | 116 | 7.4 | 1.1 | 0.83 - 1.5 | 0.44 |
| Gallstones | 73 | 6.4 | 1.2 | 0.82 - 1.7 | 0.37 |
| Cholecystectomy | 43 | 8.8 | 1.1 | 0.68 - 1.6 | 0.80 |
Ultrasound-documented gallstones or evidence of a cholecystectomy.
Estimated using Kaplan-Meier analysis.
Estimated using Cox proportional hazard regression analysis.
Figure 1.
Multivariate-adjusted hazard ratios and 95% confidence intervals for death (underlying cause) among participants with gallstone disease (GSD; gallstones or cholecystectomy), gallstones, and cholecystectomy in the United States, 1988-2006
Diabetes mortality was over two and a half times more frequent with gallstone disease in age-adjusted analysis (p=0.008) (Table 2). Gallstone disease was strongly associated with diabetes at baseline. In a model adjusting for multiple factors, but not baseline glucose status, an increased risk remained, but no longer reached statistical significance (p=0.23) (Table 2, Figure 1). Glucose status could not be included in the multivariate-adjusted model because of the relatively small number of diabetes deaths and the strong association of diabetes mortality with abnormal glucose status. In a model adjusting for only baseline glucose status, gallstone disease remained associated with higher diabetes mortality (HR, 2.4; 95% CI, 1.2–5.1; p=0.018). Gallstone disease was unrelated to mortality from digestive disease, infectious disease, or all other causes in both age-adjusted and multivariate-adjusted analyses (Table 2, Figure 1). Of 115 deaths from digestive diseases, 47 were attributed to chronic liver disease (ICD-10 codes, K70, K73, and K74). Compared with mortality from all digestive diseases, the multivariate-adjusted hazard ratio for chronic liver disease mortality was higher (HR, 1.4; 95% CI, 0.36–5.4; p=0.62), but did not reach statistical significance. Among the subgroup on whom GGT was measured, adding it to multivariate-adjusted models had limited effect on relationships with mortality from all-causes (HR, 1.2; 95% CI, 1.01–1.5; p=0.040), cardiovascular disease (HR, 1.4; 95% CI, 1.05–1.9; p=0.023), and cancer (HR, 1.2; 95% CI, 0.85–1.6; p=0.32).
When ultrasound-diagnosed gallstones and cholecystectomy were examined separately in a model in which cholecystectomy, rather than no gallstone disease, was the reference group, participants with ultrasound-diagnosed gallstones had a similar risk of death as those with cholecystectomy (multivariate-adjusted HR, 1.1; 95% CI, 0.92-1.4). When ultrasound-diagnosed gallstones and cholecystectomy were examined separately in comparison to no gallstone disease, unadjusted cumulative mortality rates for all causes, cardiovascular disease, and cancer were higher with both gallstones and cholecystectomy (Table 2). All-cause mortality was increased both for participants with gallstones and with cholecystectomy in both age-adjusted and multivariate-adjusted analyses (Table 2, Figure 1, Figure 2). Results were statistically significant for gallstones in both analyses and for cholecystectomy in age-adjusted analysis. Cardiovascular disease mortality was statistically significantly increased both with gallstones and with cholecystectomy in both age-adjusted and multivariate-adjusted analyses (Table 2, Figure 1). Cancer mortality was increased both with gallstones and with cholecystectomy in both age-adjusted and multivariate-adjusted analyses (Table 2, Figure 1). Results were statistically significant for gallstones. Diabetes mortality was statistically significantly increased with cholecystectomy, but not gallstones in age-adjusted analysis only (Table 2, Figure 1). Neither gallstones nor cholecystectomy was related to mortality from digestive disease, infectious disease or all other causes (Table 2, Figure 1).
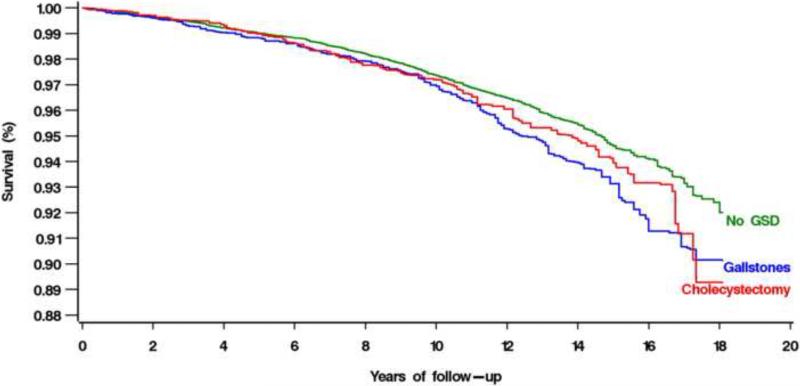
Figure 2.
Multivariate-adjusted cumulative all-cause mortality among participants with no gallstone disease (GSD), gallstones, and cholecystectomy in the United States, 1988-2006
Because gallstone disease prevalence differs between men and women and by age, we tested for interaction of gallstone disease status and sex or age on mortality outcomes by adding interaction terms for gallstone disease status and sex or age individually to the models. Interaction was not present with either sex or age for overall, cardiovascular disease or cancer mortality (p>0.05). A secondary analysis was conducted among a random subgroup of 6,258 participants examined in the morning after an overnight fast, adjusting for insulin resistance index in place of glucose status and for serum triglyceride concentrations, in addition to the factors adjusted for in the main analysis. There was little change in the relationship of gallstone disease with mortality outcomes with adjustment for these additional factors (Table 3).
Table 3.
Cumulative probability (unadjusted) over 18 years and age- and multivariate-adjusted hazard ratios and 95% confidence intervals for death (underlying cause) by gallstone disease (GSD)* status among persons examined in the morning after an overnight fast in the United States, 1988 - 2006
| Mortality outcome GSD status | Number of deaths | Unadjusted cumulative mortality (%)† | Age-adjusted (n=6,258) |
Multivariate-adjusted‡ (n=5,569) |
Multivariate-adjusted§ (n=5,535) |
|||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio∥ | 95% C.I. | Hazard ratio∥ | 95% C.I. | Hazard ratio∥ | 95% C.I. | |||
| All-cause | ||||||||
| No GSD | 739 | 13.1 | 1.0 | 1.0 | 1.0 | |||
| GSD | 257 | 36.6 | 1.4 | 1.1 - 1.6 | 1.4 | 1.1 - 1.8 | 1.4 | 1.1 - 1.8 |
| Cardiovascular disease | ||||||||
| No GSD | 280 | 4.8 | 1.0 | 1.0 | 1.0 | |||
| GSD | 96 | 17.4 | 1.4 | 0.90 - 2.1 | 1.3 | 0.87 - 2.0 | 1.3 | 0.86 - 2.0 |
| Cancer | ||||||||
| No GSD | 216 | 4.0 | 1.0 | 1.0 | 1.0 | |||
| GSD | 83 | 13.2 | 1.6 | 1.1 - 2.4 | 1.8 | 1.2 - 2.8 | 1.8 | 1.1 - 2.8 |
| Digestive disease (excludes dental, malignant, and infectious) | ||||||||
| No GSD | 32 | 0.51 | 1.0 | 1.0 | 1.0 | |||
| GSD | 16 | 1.8 | 1.6 | 0.72 – 3.4 | 1.4 | 0.58 – 3.3 | 1.4 | 0.57 - 3.2 |
| Diabetes | ||||||||
| No GSD | 13 | 0.15 | 1.0 | 1.0 | 1.0 | |||
| GSD | 9 | 1.1 | 2.9 | 0.79 - 10.4 | NC | -- | NC | -- |
| Infectious disease | ||||||||
| No GSD | 47 | 1.2 | 1.0 | 1.0 | 1.0 | |||
| GSD | 8 | 1.1 | 0.65 | 0.13 - 3.2 | NC | -- | NC | -- |
| Other | ||||||||
| No GSD | 151 | 3.1 | 1.0 | 1.0 | 1.0 | |||
| GSD | 45 | 8.0 | 0.99 | 0.62 - 1.6 | 0.96 | 0.56 - 1.6 | 0.93 | 0.56 - 1.6 |
NC=no convergence to hazard ratio estimate
Ultrasound-documented gallstones or evidence of a cholecystectomy.
Estimated using Kaplan-Meier analysis.
Adjusted for age, sex, race-ethnicity, education, BMI, waist-to-hip ratio, glucose status (doctor-diagnosed diabetes, hemoglobin A1C >=6.5% (95th percentile)), total serum cholesterol, HDL cholesterol, smoking, drinking, caffeine, physical activity, C-reactive protein, systolic blood pressure, and diastolic blood pressure.
Adjusted for factors in previous column (excluding glucose status) plus fasting triglycerides and HOMA-IR.
Estimated using Cox proportional hazard regression analysis.
DISCUSSION
The main finding of this study was an association of gallstone disease with overall, cardiovascular disease, and cancer mortality in a large, national, population-based, prospective study. For overall and cardiovascular disease mortality, this was a consistent finding in both age-adjusted and multivariate-adjusted analyses. For cancer mortality, the strength of the relationship was unchanged, but did not reach statistical significance in multivariate-adjusted analysis. Similar to our results, a study of Pima Indians found increased overall mortality among persons with gallstone disease.1 In contrast to our study, cardiovascular disease mortality was not increased, while cancer mortality was over 6 times as high with gallstone disease. However, cardiovascular disease was uncommon among that American Indian population at the time of the study, while gallbladder cancer was common. In the general U.S. population gallbladder cancer is rare.24 Gallstone disease has also been associated with extra-biliary malignancies. In a Swedish case-control study, cholelithiasis or cholecystectomy at autopsy was more than twice as prevalent among younger women who died of cancer compared with those dying from other causes; no relationship was found among men or older women.3 Abdominal cancer patients had a higher frequency of previous cholecystectomy compared with the general population in a Finish study.4 In contrast, among residents of Rochester, MN, the incidence of non-gallbladder gastrointestinal malignancies was no greater with gallstones and cholecystectomy than in the general population.25
An association of gallstones with cardiovascular disease has been suggested by previous work, however, these studies examined incident cardiovascular disease rather than mortality and only one was prospective. In a Mexican cross-sectional study of persons undergoing routine employee physicals, those with cardiovascular disease diagnosed by stress test had almost three times the odds of ultrasound-diagnosed gallstones in multivariate-adjusted analysis.5 In a case-control study in the UK general population, ischemic heart disease was associated with a 30% higher odds of symptomatic gallbladder disease after adjustment for potential confounders.6 In a case-control study of gallbladder disease and breast cancer in Washington County, MD, an incidental finding was a strong association of gallbladder disease with a history of a myocardial infarction.7 An independent association between gallstones and coronary heart disease was demonstrated prospectively in the Framingham Study in the 1980s.8 During 26 years of follow-up, the risk of a first coronary disease event was doubled with a previous cholecystectomy after adjusting for coronary disease risk factors, but the increased risk was limited to men.
In the current study, reasons for the increase in mortality, especially cardiovascular mortality, related to gallstones are uncertain. Controlling for the major cardiovascular disease risk factors had little effect on the mortality rate ratio. Nevertheless, it is likely that mechanisms of gallbladder disease share features in common with mechanisms of cardiovascular disease and other common causes of death. The formation of cholesterol gallstones requires dysregulation of biliary lipid secretion and cholesterol supersaturation of bile and is accelerated by the presence of pro-nucleating factors and gallbladder hypomotility.26 It is the hepatic processing of lipids that seems most likely to be a common pathway for development of both gallstones and cardiovascular disease. It is also possible that gallbladder hypomotility, as is seen in diabetes and other causes of autonomic neuropathy, might be a marker for systemic motor and neuropathic abnormalities. Among other features potentially in common with gallstones and increased mortality are insulin resistance and fatty liver.27-28 Although we adjusted for HOMA-IR, it is at best an indirect measure of insulin resistance. For fatty liver, the study had no marker other than GGT levels.
In the current study, mortality from diabetes was increased among persons with gallstone disease in age-adjusted analysis, but in multivariate analysis no longer reached statistical significance. Because both gallstone disease and diabetes mortality were strongly associated with abnormal baseline glucose status, these results are difficult to interpret. Mortality from digestive disease, infectious disease, and all other causes was unrelated to gallstone disease in either age-adjusted or multivariate-adjusted analyses.
The second aim of the current study was to determine if risk of death was similar for participants with gallstones or cholecystectomy. In fact, mortality was nearly the same (Figure 2). Thus there was no evidence of added long-term increased risk of mortality following cholecystectomy, apart from having had gallstones. This argues against the absence of a gallbladder being responsible for increased mortality and suggests that some factor involved in gallstone formation may be the cause. This is a potentially important observation, given that cholecystectomy is the most frequently performed abdominal surgical procedure in the United States and is performed on millions of persons worldwide each year.29
A particular strength of the current study was mortality follow-up among study participants based on gallstone disease determined by ultrasonography. We are unaware of any other population-based study that included such a large number of participants with asymptomatic gallstones.11 A limitation was the lack of validation of cause of death. Although ascertainment of vital status using the National Death Index is very high, assigning cause of death based on death certificate diagnoses may lead to misclassification. Such inaccuracies might have resulted in misclassification of some participants, but they should not have led to biased results. The fact that all-cause mortality and certain cause-specific mortality were increased for gallstone disease argues against a strong effect for misclassification for common causes of death. Secondly, because participants were not reevaluated for gallstone disease during follow-up, some may have developed gallstones or undergone a cholecystectomy leading to misclassification. Consequently, the increased mortality we found may underestimate the true relationship of gallstone disease with mortality. These limitations are balanced by the benefits of a large, national, population-based sample, particularly the avoidance of ascertainment bias that occurs in clinical studies of selected patients and the ability to generalize the results to the U.S. population.
In conclusion, in the U.S. population, persons with gallstone disease had increased overall, cardiovascular disease, and cancer mortality. This relationship was found with both ultrasound-diagnosed gallstones and cholecystectomy and was largely unexplained by multiple demographic and cardiovascular disease risk factors. Further study is needed to determine whether increased mortality results from some unmeasured factor responsible for gallstone development.
ACKNOWLEDGMENTS
The National Center for Health Statistics (NCHS) was the source for the NHANES III Linked Mortality Files. All analyses, interpretations, and conclusions are those of the authors and not NCHS. The authors thank Negasi Beyene for assistance in using the NCHS Research Data Center, Tempie Shearon, Lead Research Area Specialist, Kidney Epidemiology and Cost Center, University of Michigan for assistance with programming for creation of the survival curve figures, Danita Byrd for programming assistance with SAS Graph software, and Zhongyu Fang for assistance creating the forest plot figure.
This work was supported by a contract from the National Institute of Diabetes and Digestive and Kidney Diseases (HHSN267200700001G).
Abbreviations
- BMI
body mass index
- CI
confidence ratio
- GGT
γ-glutamyltransferase
- GSD
gallstone disease
- HDL
high-density lipoprotein cholesterol
- HR
hazard ratio
- ICD
International Classification of Diseases
- NHANES
National Health and Nutrition Examination Survey
- SD
standard deviation
- SE
standard error
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest.
Contributor Information
Constance E. Ruhl, Social & Scientific Systems, Inc. 8757 Georgia Avenue, 12th floor Silver Spring, MD 20910 301-628-3272 (phone) 301-628-3201 (fax) cruhl@s-3.com.
James E. Everhart, National Institute of Diabetes and Digestive and Kidney Diseases National Institutes of Health Department of Health and Human Services 2 Democracy Plaza, Room 655 6707 Democracy Boulevard MSC 5450 Bethesda, MD 20892-5450
REFERENCES
- 1.Grimaldi CH, Nelson RG, Pettitt DJ, et al. Increased mortality with gallstone disease: results of a 20-year population-based survey in Pima Indians. Ann Intern Med. 1993;118:185–190. doi: 10.7326/0003-4819-118-3-199302010-00005. [DOI] [PubMed] [Google Scholar]
- 2.Sanders G, Kingsnorth AN. Gallstones. Bmj. 2007;335:295–299. doi: 10.1136/bmj.39267.452257.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lowenfels AB, Domellof L, Lindstrom CG, et al. Cholelithiasis, cholecystectomy, and cancer: a case-control study in Sweden. Gastroenterology. 1982;83:672–676. [PubMed] [Google Scholar]
- 4.Hyvarinen H, Partanen S. Association of cholecystectomy with abdominal cancers. Hepatogastroenterology. 1987;34:280–284. [PubMed] [Google Scholar]
- 5.Mendez-Sanchez N, Bahena-Aponte J, Chavez-Tapia NC, et al. Strong association between gallstones and cardiovascular disease. Am J Gastroenterol. 2005;100:827–830. doi: 10.1111/j.1572-0241.2005.41214.x. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Perez A, Garcia Rodriguez LA. Gallbladder disease in the general population: association with cardiovascular morbidity and therapy. Pharmacoepidemiol Drug Saf. 2007;16:524–531. doi: 10.1002/pds.1346. [DOI] [PubMed] [Google Scholar]
- 7.Wysowski DK, Goldberg EL, Comstock GW, et al. A study of a possible association between breast cancer and gallbladder disease. Am J Epidemiol. 1986;123:532–543. doi: 10.1093/oxfordjournals.aje.a114268. [DOI] [PubMed] [Google Scholar]
- 8.Bortnichak EA, Freeman DH, Jr., Ostfeld AM, et al. The association between cholesterol cholelithiasis and coronary heart disease in Framingham, Massachusetts. Am J Epidemiol. 1985;121:19–30. doi: 10.1093/oxfordjournals.aje.a113978. [DOI] [PubMed] [Google Scholar]
- 9.Plan and operation of the Third National Health and Nutrition Examination Survey, 1988-94 (DHHS Publication No. (PHS) 94-1308) National Center for Health Statistics; 1994. [PubMed] [Google Scholar]
- 10.Ruhl CE, Everhart JE. Association of diabetes, serum insulin, and C-peptide with gallbladder disease. Hepatology. 2000;31:299–303. doi: 10.1002/hep.510310206. [DOI] [PubMed] [Google Scholar]
- 11.Everhart JE, Khare M, Hill M, et al. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology. 1999;117:632–639. doi: 10.1016/s0016-5085(99)70456-7. [DOI] [PubMed] [Google Scholar]
- 12.Gunter EW, Lewis BG, Koncikowski SM. Laboratory procedures used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988-1994. US Department of Health and Human Services, Center for Disease Control and Prevention, National Center for Environmental Health, National Center for Health Statistics; 1996. [Google Scholar]
- 13.National Health and Nutrition Examination Survey III; Body Measurements (Anthropometry) Westat; Rockville, MD: 1988. [Google Scholar]
- 14.National Health and Nutrition Examination Survey III Cycle 2: pulse and blood pressure procedures for household inteviewers. Westat; 1989. [Google Scholar]
- 15.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Michels KB, Willett WC, Fuchs CS, et al. Coffee, tea, and caffeine consumption and incidence of colon and rectal cancer. J Natl Cancer Inst. 2005;97:282–292. doi: 10.1093/jnci/dji039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruhl CE, Everhart JE. Association of coffee consumption with gallbladder disease. Am J Epidemiol. 2000;152:1034–1038. doi: 10.1093/aje/152.11.1034. [DOI] [PubMed] [Google Scholar]
- 18.Ruhl CE, Everhart JE. Elevated serum alanine aminotransferase and gamma-glutamyltransferase and mortality in the United States population. Gastroenterology. 2009;136:477–485. e11. doi: 10.1053/j.gastro.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 20.National Center for Health Statistics . Office of Analysis and Epidemiology, The Third National Health and Nutrition Examination Survey (NHANES III) Linked Mortality File, Mortality follow-up through 2006: Matching Methodology May 2009. Hyattsville; Maryland: [January 26, 2010]. (Available at the following address: http://www.cdc.gov/nchs/data/datalinkage/matching_methodology_nhanes3_final.pdf). [Google Scholar]
- 21.Menke A, Muntner P, Batuman V, et al. Blood lead below 0.48 micromol/L (10 microg/dL) and mortality among US adults. Circulation. 2006;114:1388–1394. doi: 10.1161/CIRCULATIONAHA.106.628321. [DOI] [PubMed] [Google Scholar]
- 22.Kleinbaum DG. Survival Analysis: A Self-Learning Text. Springer; New York, NY: 1996. [Google Scholar]
- 23.Breslow NE, Day NE. Statistical methods in cancer research: the design and analysis of cohort studies. International Agency for Research on Cancer; Lyon, France: 1987. pp. 48–79. [PubMed] [Google Scholar]
- 24.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134–1144. doi: 10.1053/j.gastro.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 25.Maringhini A, Moreau JA, Melton LJ, 3rd, et al. Gallstones, gallbladder cancer, and other gastrointestinal malignancies. An epidemiologic study in Rochester, Minnesota. Ann Intern Med. 1987;107:30–35. doi: 10.7326/0003-4819-107-1-30. [DOI] [PubMed] [Google Scholar]
- 26.Portincasa P, Moschetta A, Palasciano G. Cholesterol gallstone disease. Lancet. 2006;368:230–9. doi: 10.1016/S0140-6736(06)69044-2. [DOI] [PubMed] [Google Scholar]
- 27.Jornayvaz FR, Samuel VT, Shulman GI. The role of muscle insulin resistance in the pathogenesis of atherogenic dyslipidemia and nonalcoholic fatty liver disease associated with the metabolic syndrome. Annu Rev Nutr. 2010;30:273–90. doi: 10.1146/annurev.nutr.012809.104726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341–50. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 29.Owings MF, Kozak LJ. Vital Health Stat 13(139) National Center for Health Statistics; 1998. [July 26, 2010]. Ambulatory and inpatient procedures in the United States, 1996. (Available at the following address: http://www.cdc.gov/nchs/data/hdasd/13_139t9.pdf). [PubMed] [Google Scholar]