Abstract
Unravelling the mechanisms underlying desiccation tolerance is crucial in order to understand the position of algal species in the intertidal zone. The alga Porphyra columbina lives in the uppermost part of the rocky intertidal zones around the world and was selected as a model for this study. Naturally desiccated plants were collected during low tide and studied for morphological changes, oxidative burst induction, biomolecule oxidation, antioxidant responses, and photosynthetic status. Naturally hydrated plants collected during high tides were used for comparative purposes. In addition, changes induced by desiccation were assessed in vitro and the capacity to recover from desiccation was determined by rehydrating the fronds in seawater. The global results show that desiccation induces morphological and cellular alterations accompanied by a loss of ∼96% of the water content. Overproduction of reactive oxygen species (ROS) was induced by desiccation and two peaks of H2O2 were detected at 1 and 3 h of desiccation. However, during in vitro rehydration post-desiccation, the ROS quickly returned to the basal levels. At the biomolecular level, only a low production of oxidized proteins was recorded during desiccation, whereas the activity of diverse antioxidant enzymes increased. However, this activity diminished to near basal levels during rehydration. The photosynthetic efficiency (Fv/Fm) during desiccation declined by 94–96% of the values recorded in hydrated plants. This reduction was generated by the low levels of trapped energy flux per cross-section (TRo/CS), electron transport flux per CS (ETo/CS), and density of reaction centres (RC/SCo) as well as the chlorophyll content. The inverse pattern was observed for the levels of phycocyanin and phycoerythrin content. Fv/Fm and the photosynthetic indicators were restored to normal levels after only 5 min of rehydration. The results indicate that desiccation in P. columbina causes overproduction of ROS that is efficiently attenuated. The morphological and photosynthetic changes could be operating as tolerance mechanisms due to the fact that these responses principally prevent biomolecular alteration and cellular collapse. Thus, the activation of different physiological mechanisms helps to explain the high tolerance to desiccation of P. columbina and, at least in part, the position of this species at the highest level in the intertidal zone.
Keywords: Antioxidant metabolism, desiccation, oxidative stress, photosynthesis, Porphyra columbina, ROS
Introduction
In the intertidal zones of rocky beaches around the world, sessile benthic organisms are exposed to constantly fluctuating and extreme abiotic conditions and, as a result, intermittent intracellular oxidative stress conditions develop by the accumulation of reactive oxygen species (ROS) (Davison and Pearson, 1996; Mittler, 2002; Pinto et al., 2003). ROS are produced directly by excitation of O2 and subsequent formation of singlet oxygen, or by the transfer of one, two, or three electrons to O2, which results in the formation of superoxide radicals, hydrogen peroxide (H2O2), or hydroxyl radicals, respectively (Baker and Orlandi, 1995). Although some of these reactive species may function as important signal molecules that modify gene expression and modulate the activity of specific defence proteins (Mittler, 2002; Pitzschke et al., 2006), at high concentration all ROS can be extremely harmful. In this context, in a scenario where the production of ROS exceeds the buffering capacity of the antioxidant mechanisms, oxidation of proteins, lipids, polysaccharides, and nucleic acids takes place (e.g. Deighton et al., 1999; Rustérucci et al., 1999; Thoma et al., 2003).
The primary response of an organism to an oxidative stress condition includes the activation of antioxidant enzymes, and the use of water-soluble antioxidant compounds and lipid-soluble antioxidant molecules (Foyer et al., 1997; Noctor and Foyer, 1998; Smirnoff, 2000). Antioxidant enzymes include catalase (CAT) and ascorbate peroxidase (AP), which are able to reduce H2O2 to water (Foyer et al., 1997; Asada, 1999), and peroxiredoxins (PRXs), expressed mainly in the organelles (Tripathi et al., 2009) and involved in detoxification of H2O2 and alkyl hydroperoxides (Dietz et al., 2006). Antioxidant mechanisms are present in all living organisms, and have been studied with particular emphasis in host–pathogen interactions (Hammond-Kosack and Jones, 1996; Wojstazeck, 1997; Bolwell, 1999; Bolwell et al., 2002) and to clarify how organisms cope with extreme environmental conditions (Schraudner et al., 1998; Bowler and Fluhr, 2000; Tripathi et al., 2009).
Macroalgae are a fundamental part of marine food webs, since they are responsible for most of the coastal primary production (Lobban and Harrison, 1994). Adverse effects on macroalgae caused by natural or anthropogenic phenomena can directly or indirectly affect organisms at higher trophic levels and, finally, the integrity of entire ecosystems (e.g. Medina et al., 2005). Even though it is well known that intertidal algae are well adapted to constantly fluctuating and, in some cases, extreme environmental conditions, information on the mechanisms underlying that adaptation are poorly known. However, some studies have demonstrated that desiccation, temperature, irradiation, and excess of various metals cause oxidative stress in species such as Stictosiphonia arbuscula (Burritt et al., 2002), Fucus spp. (Collén and Davison, 1999a), Mastocarpus stellatus (Collén and Davison, 1999b), Scytosiphon lomentaria (Contreras et al., 2005, 2010), Ulva compressa (Ratkevicius et al., 2003; Contreras-Porcia et al., 2010), Chondrus crispus (Collén and Davison, 1999b; Collén et al., 2007), and Lessonia nigrescens (Contreras et al., 2009).
Desiccation is an important stress factor faced by living organisms because, as cells lose water, essential macromolecules are induced to form non-functional aggregates, and organelles collapse and, eventually, disintegrate (Alpert, 2006). Although mobile organisms actively avoid desiccation, others, such like some plants and seaweeds, are well adapted to significant water loss, displaying full physiological recovery during rehydration (Bohnert, 2000; Farrant, 2000; Alpert, 2006). Resurrection plants, for example, belong to a small number of angiosperms (e.g. Myrothamnus flabellifolius, Xerophyta viscose, and Sporobolus stapfianus) that tolerate water loss of up to 90% (Farrant, 2000; Alpert, 2006). Adaptation to desiccation, in general terms, is based on the ability of an organism to equilibrate its internal water potential with the dry environment, and on the ability to re-start normal functions after rehydration (Alpert, 2000). In comparison with vascular plants (Vicré et al., 2004a), the effects of desiccation and the molecular mechanisms involved in its tolerance are poorly understood in macroalgae. For example, in one of the few reports available, the activation of different antioxidant enzymes such as AP and glutathione reductase (GR) was recorded in S. arbuscula (Burritt et al., 2002) as a response to desiccation-mediated oxidative stress. To our knowledge, the remaining studies have focused on assessing the capacity to tolerate desiccation displayed by the photosynthetic apparatus in Porphyra, Gracilaria, Chondrus, and Ulva among others (Smith et al., 1986; Abe et al., 2001; Ji and Tanaka, 2002; Zou and Gao, 2002). So far, the only study using molecular approaches to unravel the responses to desiccation found that genes coding for photosynthetic and ribosomal proteins were up-regulated in Fucus vesiculosus (Pearson et al., 2001, 2010).
Red algae (Rhodophyta), the most ancient linage of photosynthetic eukaryotes (Baldauf et al., 2000; Butterfield, 2000;Yoon et al., 2004), are distributed globally and include several commercially important species. One of them, Porphyra columbina Montagne (Bangiales, Rhodophyta), occurs in Chile from 20°S to 54°S, is highly seasonal, and grows abundantly along the upper intertidal zone (Alveal, 1970; Santelices, 1989; Hoffman and Santelices, 1997; González and Santelices, 2003), where it may lose up to 80% of fresh weight during low tide (personal observations). This is consistent with studies which have found that other species of Porphyra also display high desiccation tolerance as compared with most seaweeds (Smith et al., 1986; Sibbald and Vidaver, 1987; Cabello-Pasini et al., 2000; Ji and Tanaka, 2002). However, the role of desiccation as a trigger in the establishment of an oxidative stress condition, the co-occurring physiological changes, and the mechanisms of tolerance themselves in Porphyra remain open issues.
Taking the above into consideration, P. columbina was selected as a model to address the relationship between desiccation and the cascade of events involving oxidative stress, cellular changes, and physiological and biochemical responses that may help to explain the high tolerance to water loss and the place this species occupies in the intertidal zone. The effect of desiccation on various indicators was assessed in plants naturally dehydrated during low tide and compared with those indicators in fully hydrated plants. In addition, the kinetics of the responses to desiccation were monitored in vitro, and recovery from desiccation-mediated oxidative stress was quantified in rehydrated plants post-desiccation.
Materials and methods
The effects of desiccation in P. columbina were studied by monitoring and recording the (i) morphological changes in the cells, (ii) establishment of an oxidative stress condition, (iii) oxidation of biomolecules, and (iv) antioxidant and photosynthetic responses. These effects were evaluated in plants naturally affected by desiccation during low tide and referred to as normal conditions (NC). Furthermore, the kinetics of desiccation were evaluated in vitro, and compared with those in plants kept hydrated in the laboratory (Controls; C). For these trials, dehydrated plants were collected along 100 m of coastline in Maitencillo (32° 39.5' S, 71° 26.6' W) after they had been air exposed for the entire period of low tide (∼4 h); hydrated fronds of the same species were collected at the same locality but during high tide. Dehydrated fronds were pooled, randomly separated in several replicates of 10–15 individuals each, blotted dry, and frozen on site in liquid nitrogen. In addition, several replicates were kept in plastic bags without seawater and transported to the laboratory in a cooler with ice-packs at 5–7 °C. Hydrated fronds collected for determination of several basal parameters were also pooled, divided into several replicates of 10–15 individuals each, blotted dry, and frozen on site in liquid nitrogen. Hydrated fronds for in vitro experiments were pooled and kept in plastic bags containing seawater and transported to the laboratory in a cooler with ice-packs at 5–7 °C. Once in the laboratory, these hydrated thalli were exhaustively rinsed with 0.45-μm-filtered seawater and cleaned using an ultrasound bath (Ultrasonic cleaner, Model 575T; Cortland, NJ, USA).
In vitro experiments: kinetics of desiccation stress and recovery
Prior to desiccation experiments, hydrated plants were acclimated in filtered seawater during 12 h in a culture chamber at 12–14 °C, under 30–50 μm photon m−2 s−1 irradiance on a 12:12 light:dark cycle. Later, the plants were blotted dry and exposed to air in a growth chamber at 14–20 °C and 70–80 μm photon m−2 s−1 irradiance for 4 h. This period was used to mimic the daytime low tide faced by the plants in their habitat. Algal samples (three to five replicates of 20–30 fronds each) were collected after 0.5, 1, 2, 3, and 4 h of desiccation to evaluate several parameters. In addition, a subset of fronds dehydrated for 4 h were immediately rehydrated in 0.45-μm-filtered seawater to characterize the kinetics of recovery to oxidative stress caused by desiccation. Culture conditions were as those described above for the desiccation experiment. Finally, the rehydration period lasted for 4 h, and the tissue was collected after 0.5, 1, 2, 3, and 4 h.
Level of desiccation
The level of desiccation that P. columbina faces in its normal habitat (dehydrated plants collected from the field) and during the in vitro experiment, was expressed as relative water content (RWC%) following the formula
RWC=(Wf–Wd)×(Wf–Wdo)−1,
where Wf is the wet weight of fully hydrated fronds, Wd is the natural or in vitro dehydrated weight and Wdo is the dry weight determined after drying for 48 h at 80 °C. To obtain the RWC of plants dehydrated naturally, the tissue was weighed before collection, rehydrated for 2 h in filtered seawater, blotted dry, and weighed again. In this context, the RWC is the complement of desiccation and thus, a fully hydrated thallus has an RWC of 100% and a fully dehydrated thallus has an RWC near to 0%: decreasing RWC implies increasing desiccation level.
Morphological analyses
Hand-made cross-sections of naturally hydrated and dehydrated fronds, five of each category, in addition to in vitro dehydrated and rehydrated fronds, were used to characterize changes at light microscopy level. The hydrated sections were mounted in seawater and the dehydrated section in a synthetic, water-free resin (Permount™; Electron Microscopy Sciences, Washington, PA, USA) to prevent the rehydration of the samples during the analysis. Cell size in each category of fronds was defined as the average from 100 cell measurements. Images were recorded in a Nikon Optiphot II microscope (Nikon Corp., Tokyo, Japan) coupled to a digital recording system (CoolSNAP-Procf; Media Cybernetics, Silver Spring, MD, USA) and analysed using the Image Pro Plus Version 4.5 software (Media Cybernetics). Texture of the surface was visualized by scanning electron microscopy (SEM). Fragments of 5×5 mm of tissue from hydrated and naturally dehydrated fronds were fixed for 2 d in 0.22-μm-filtered seawater containing 3% glutaraldehyde. After rinsing to remove excess fixative solution, fragments were dehydrated using an ethanol series (10–100%), critical point dried, and coated with gold–palladium. The surface of the fragments was observed and photographed in a Jeol JSM-25-SII scanning electron microscope. Dehydration must be gentle and gradual in order to induce retraction and changes in texture as a result of dehydration by the alcohol rinses.
Transmission electron microscopy (TEM) analysis of hydrated fronds, in vitro dehydrated fronds, and fronds rehydrated for 4 h was carried out using triplicate samples. Tissue fragments were fixed in 0.22-μm-filtered seawater containing 3% glutaraldehyde and 1% p-formaldehyde for 3 weeks at 5 °C (Correa and McLachlan, 1991). Samples were post-fixed for 2 h at 5 °C in 0.05 M sodium cacodylate buffer (pH 7.8) with the addition of 2% OsO4 and 1% potassium hexacyanoferrate, dehydrated using an ethanol series (10–100%), and embedded in Spurr's resin (Spurr, 1969) for 1 week. Sections for TEM were stained with uranyl acetate–lead citrate and analysed using a Phillips Tecnai 12 electron microscope operated at 60 kV.
Quantification of ROS
Hydrogen peroxide was determined as described by Contreras et al. (2009) in hydrated and naturally dehydrated fronds by incubating 0.5–1 g fresh weight in 100 ml of 5 μM DCHF-DA (Calbiochem, San Diego, CA, USA), for 1 h at room temperature. In addition, the kinetics of H2O2 production were determined in fronds exposed to in vitro desiccation (0–4 h of desiccation) and rehydration (0–4 h of rehydration). Superoxide anions were measured as described by Contreras et al. (2009) by incubating fronds in 100 ml of 100 μM hydroethidine (HE) (Molecular Probes, Invitrogen, Eugene, OR, USA), for 1 h at room temperature.
Hydrogen peroxide and superoxide anions were visualized in situ by incubating frond fragments for 1 h at room temperature with 10 μM DCHF-DA and 100 μM HE, respectively. After rinsing several times in filtered seawater, the whole fragments were immediately observed in a Nikon Optiphot II fluorescence microscope with an emission filter of 459–490 nm.
Lipid peroxidation
Lipid peroxidation production was determined as the amount of thiobarbituric acid (T-BARS) reactive species (i.e. lipoperoxides) according to Ratkevicius et al. (2003).
Protein oxidation
Determination of oxidized proteins was based on the reaction of carbonyls resulting from free radical modification of proteins and 2,4-dinitrophenyl hydrazine (DNPH) according to Achary et al. (2008). Briefly, 2 g fresh weight of algal tissue were frozen in liquid nitrogen and homogenized in a pre-chilled mortar using a pestle. A total of 5 ml of 50 mM sodium buffer pH 7.0, containing 0.1 mM EDTA and 1% polyvinylpolypyrrolidone was added during the homogenization. The homogenate was centrifuged at 7400 g for 15 min at 4 °C and the supernatant stored at –20 °C. Two aliquots, each containing 1 mg of proteins, were mixed with an equal volume of 20% (w/v) trichloroacetic acid (TCA) and centrifuged at 7400 g for 15 min at 4 °C. The pellets were suspended with 300 μl of 2 N HCl with or without (blank) 10 mM DNPH and left for 1 h at room temperature. Samples were then precipitated with 500 μl of 20% TCA for 10 min at –20 °C, centrifuged at 7400 g for 15 min at 4 °C, and the supernatant discarded. After rinsing with 500 μl ethanol:ethyl acetate (1:1), the pellets were dissolved in 3 ml of 20 mM sodium phosphate buffer pH 6.8, containing 6 M guanidinium hydrochloride and centrifuged at 7400 g for 15 min at 4 °C. Finally, the carbonyl concentration was calculated from the difference in absorbance recorded at 380 nm for DNPH-treated and HCl-treated (blank) samples (ϵ=22 mM−1 cm−1) and expressed in μmol of DNPH incorporated per mg of protein.
Antioxidant enzyme activities
The protein extracts and the activities of the antioxidant enzymes were determined according to Contreras et al. (2005). However, some concentrations of substrates were modified, such as CAT (18 mM H2O2), AP (0.4 mM ASC and 20 mM H2O2), dehydroascorbate reductase (DHAR) [1 mM reduced glutathione and 500 μM dehydroascorbate (DHA)], and GR (0.5 mM oxidized glutathione and 0.15 mM NADPH). PRX activity was determined using thioredoxin (TRX) as reducing agent due to the type of PRX described in Porphyra (i.e. 2-Cys PRX; Reith and Munholland, 1995; Baier and Dietz, 1997). For that, TRX-dependent PRX activity was determined in 1 ml of reaction mixture containing 0.1 M phosphate buffer pH 7.0, 5 mM H2O2, 1 U TRX, 1 U thioredoxin reductase (TR), 0.15 mM NADPH, and 150 μg of protein extract. The decrease in absorbance at 340 nm due to NADPH consumption was monitored for 10 min and PRX activity was calculated using the extinction coefficient of NADPH (ϵ=6.3 mM−1 cm−1).
Photosynthetic activity and photosynthetic pigments
Maximal photosynthetic efficiency (Fv/Fm) was assessed in 5–10 fronds. Before recording the fluorescence, fronds were kept in plastic bags and dark acclimated with leaf clips for 30 min, sufficient to allow complete re-oxidation of the photosystem (PS) II reaction centres. The fluorescence emission rates were evaluated using a portable fluorometer (Plan Efficiency Analyser PEA; Hansatech Instruments Ltd, King's Lynn, UK) with a maximum light intensity of 2000 μmol m−2 s−1. In order to investigate the effect of desiccation on electron transport at the acceptor site of PS II, the JIP analysis (Strasser et al., 2000) was performed to determine the following indices: absorbed energy flux per cross-section (ABS/CS, expresses the total number of photons absorbed), trapped energy flux per CS (TRo/CS, describes the maximal rate by which an excitation is trapped), electron transport flux per CS (ETo/CS), dissipation energy per CS (DIo/CS), and density of reaction centres (RC/CSo). Finally, the content of chlorophyll a (Chl a), phycoerythrin (PE), and phycocyanin (PC) were determined as in Korbee et al. (2005) and expressed in mg g−1 dry tissue (DT).
Statistical analyses
The significance of the differences in all parameters tested were determined by two-way analysis of variance (ANOVA) followed by Tukey's multiple comparisons tests (T). Prior to the statistical analyses, data were checked for variance homogeneity using Levene and Anderson–Darling tests and for normal distribution using Kolmogorov–Smirnov and Bartlett tests (Zar, 1999). When data did not fulfill these requirements, standard data transformations were applied (Zar, 1999). Differences between mean values were considered to be significant at a probability of 5% (P <0.05) (Zar, 1999).
Results
Morphological and ultrastructural changes induced by desiccation
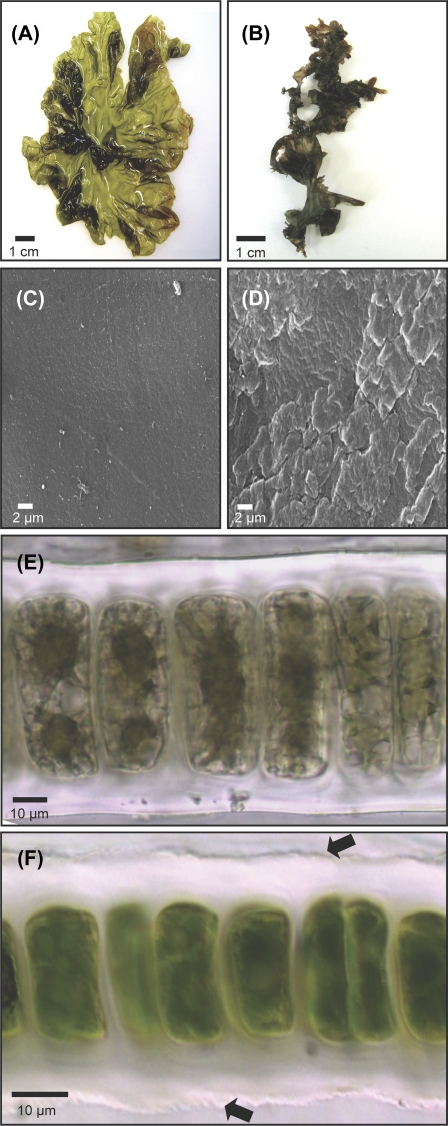
Morphological changes are normally experienced by P. columbina during the period of low tide desiccation (Fig. 1). Fully hydrated fronds are greenish red, expanded, and translucent (Fig. 1A), whereas dehydrated fronds become dark purple, tightly folded, stiff, and brittle (Fig. 1B). The contrasting macroscopic aspect of both types of frond was also reflected under the SEM, where the surface of hydrated fronds was smooth (Fig. 1C), whereas that of dehydrated fronds appeared wrinkled and bumpy (Fig. 1D). In cross-sections of hydrated fronds (Fig. 1E), cells appear rectangular to oval, 42–57 μm in length and 9–26 μm in width (Fig. 1E), whereas in dehydrated individuals (Fig. 1F) size decreased by 32–52% (20–29 μm in length and 6–18 μm in width). In addition, cells in dehydrated fronds (Fig. 1F) were characterized by a condensed and opaque cytoplasm accompanied by a darker homogeneous pigmentation in comparison with hydrated cells, where plastids and vacuoles were clearly recognizable.
Fig. 1.
Surface view of P. columbina fronds in the hydrated (A) and dehydrated (B) state. SEM of the surface of hydrated (C) and dehydrated (D) fronds. Cross-section of immature hydrated (E) and dehydrated (F) thalli showing the monostromatic structure of the fronds. Arrows show the irregular borders of the cell wall of dehydrated plants. (This figure is available in colour at JXB online.)
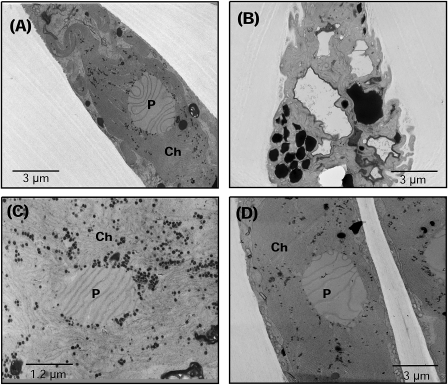
Desiccation also induced important changes in the fine structure of the cells (Fig. 2). In naturally hydrated plants, the cell structure appeared natural, with the central chloroplast (Fig. 2A) displaying a standard thylakoidal organization. In dehydrated plants, cells showed an irregular contour (Fig. 2B), a considerable folding of the plasma membrane that gave a convoluted aspect, blurred thylakoidal membranes, and an important accumulation of electron-dense bodies inside the chloroplast (Fig. 2C). However, the cellular structure modified as the result of desiccation was restored following rehydration (Fig. 2D).
Fig. 2.
Ultrastructure of P. columbina hydrated (A), dehydrated (B, C), and rehydrated (D). (A) General view of a cell with a central chloroplast (Ch) and pyrenoid (P) with clear thylakoidal lamellae. (B) Dehydrated cell with clear plasma membrane folding and the presence of electron-dense bodies in the chloroplast. (C) Blurred chloroplast from dehydrated plants. (D) Cell from rehydrated plants with the absence of plasmatic membrane folding like that observed in naturally hydrated fronds (A).
Desiccation degree
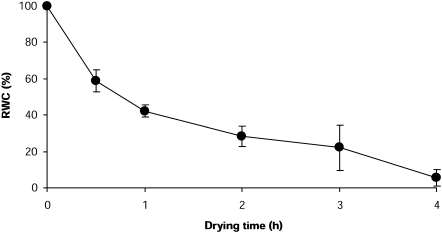
Plants exposed to desiccation during low tide displayed a low RWC of ∼4% (Table 1), the same (T=3.045, P=0.06) displayed by fronds exposed to in vitro desiccation for 4 h (Table 1). In both cases, water loss was close to 96% in comparison with fully hydrated plants. The in vitro desiccation kinetics showed that, although water loss was gradual, 40% is lost during the first 30 min and >50% is lost during the first hour of exposure to air (Fig. 3).
Table 1.
Basal values of RWC%, ROS (i.e. H2O2 and superoxide anions), lipoperoxide, and carbonyl content determined in naturally hydrated, naturally dehydrated, in vitro dehydrated, and in vitro rehydrated P. columbina plants
| Hydrated plants | Dehydrated plants in vivo/in vitro | Rehydrated plants | |
| RWC (%) | 100 | 3.55±2.8*/5.41±4.8* | 94.8±2.8ns |
| ROS | |||
| Hydrogen peroxide (nmol DCF g−1 FT) | 0.21±0.1 | 2.66±0.9*/2.44±0.6* | 0.47±0.1 ns |
| Superoxide anions (nmol 2OH-E g−1 FT) | 0.79±0.5 | 3.75±0.6*/4.05±0.6* | 1.52±0.3 ns |
| Lipid oxidation | |||
| Lipoperoxide (nmol g−1 DT) | 70.20±11.5 | 74.8±24.9 ns/72.4±13.3 ns | 68.3±5.8 ns |
| Protein oxidation | |||
| Carbonyl content (nmol mg−1 protein) | 0.26±0.1 | 1.16±0.3*/1.00±0.3* | 0.21±0.1 ns |
Each value is an average of three independent replicates±1 SD.
DCF, 2,4 dichlorofluoresceine; ns not significant.
*Significant (P<0.05) differences with values measured in hydrated plants.
Fig. 3.
Relative water content in P. columbina fronds exposed to in vitro desiccation for 4 h. Bars represent mean values of three independent replicates ±1 SD.
Over-induction of ROS by desiccation stress
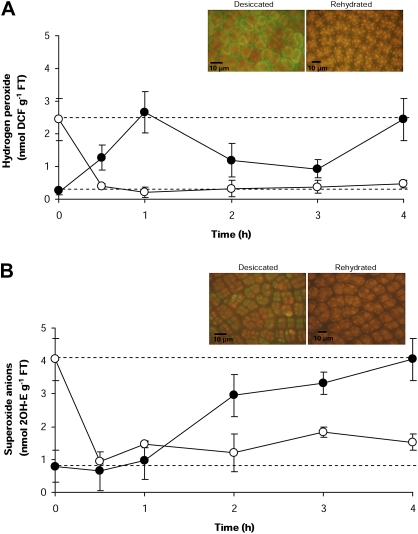
Desiccation induces the overproduction of H2O2 and superoxide anions (O2.–) (Table 1), and their reaction products displayed a patchy distribution on the surface of dehydrated fronds (Fig. 4). In both naturally dehydrated fronds and those dehydrated in vitro, H2O2 and O2.– reached levels 12- and 5-fold higher, respectively, than in hydrated fronds (Table 1). When hydrated individuals were exposed to in vitro desiccation, H2O2 levels reached a maximum after 1 h of air exposure, to then decline to close to basal levels (Fig. 4A). However, a second peak was recorded after 4 h of desiccation, reaching levels 12 times higher [2.4±0.6 nmol g−1 fresh tissue (FT)] than those recorded in hydrated fronds (0.2±0.09 nmol g−1 FT) (Fig. 4A). In contrast, the levels of O2.– increased gradually during desiccation, reaching a maximum level after 2 h of desiccation (Fig. 4B). During the remaining period of desiccation, these levels changed only marginally. Contrarily, in the rehydrated fronds, both types of ROS quickly returned to basal levels after 0.5 h of exposure to fresh seawater, from 2.4±0.6 to 0.5±0.12 nmol g−1 FT for H2O2 and from 4.05±0.64 to 1.52±0.25 nmol g−1 FT for O2.– (naturally hydrated compared with rehydrated H2O2 levels T=0.7, P=0.999 and for O2.– levels T=1.0, P=0.998).
Fig. 4.
Kinetics of ROS production in P. columbina fronds exposed to in vitro desiccation (filled circles) and rehydration (open circles). The upper discontinuous line represents the mean levels of ROS determined in naturally dehydrated plants and the low discontinuous line the mean concentration in naturally hydrated plants. The levels of H2O2 were detected using DCHF-DA (A) and superoxide anions (O2.–) using HE (B). Inserts show, by fluorescence microscopy, maximum production of H2O2 (1 h) and O2.– (2 h) in fronds exposed to desiccation and the absence of these ROS in rehydrated plants. Bars represent mean values of three independent replicates ±1 SD. (This figure is available in colour at JXB online.)
Biomolecules oxidized by desiccation stress
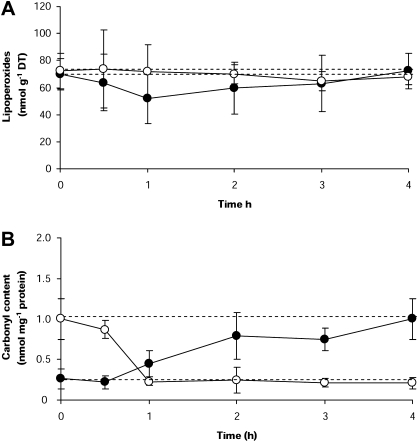
The levels of lipoperoxides recorded in dehydrated plants from the field did not significantly differ from those determined in either fully hydrated (T=0.36, P=0.983), in vitro dehydrated (T=0.998, P=0.17), or rehydrated fronds (T=0.15, P=0.998) (Table 1). In addition, the kinetics of lipoperoxides determined in desiccated and rehydrated plants (Fig. 5) followed the same pattern (F=0.1; P=0.959), remaining stable during the experiment (desiccation kinetic: F=0.45, P=0.851; rehydration kinetic: F=0.12, P=0.984).
Fig. 5.
Lipid peroxidation (A) and protein oxidation (B) in P. columbina fronds dehydrated in vitro (filled circles) and rehydrated (open circles). The upper discontinuous line represents the mean levels of lipoperoxides or carbonyl content determined in naturally dehydrated plants and the low discontinuous line the mean levels determined in naturally hydrated plants. Lipid peroxidation is expressed as the amount of lipoperoxides (nmol g−1 DT) measured, and protein oxidation as the amount of carbonyl content incorporated by protein (nmol mg−1 protein). Bars represent mean values of three independent replicates ±1 SD.
On the other hand, our results showed clear differences in the level of protein oxidation between hydrated and dehydrated plants (Table 1). In both dehydrated fronds from the field and fronds dehydrated in vitro for 4 h (T=0.91, P=0.80) protein oxidation reached levels 4-fold higher than the those recorded in hydrated plants (Table 1). During in vitro desiccation, protein oxidation levels reached their maximum after 2 h (T=3.63, P=0.043) (Fig. 5B). In contrast, protein oxidation levels quickly decreased when the fronds were rehydrated, returning to the levels of naturally hydrated plants (T=0.30, P=1.0) 1 h after exposure to fresh seawater (Fig. 5B).
Activation of antioxidant enzymes by desiccation stress
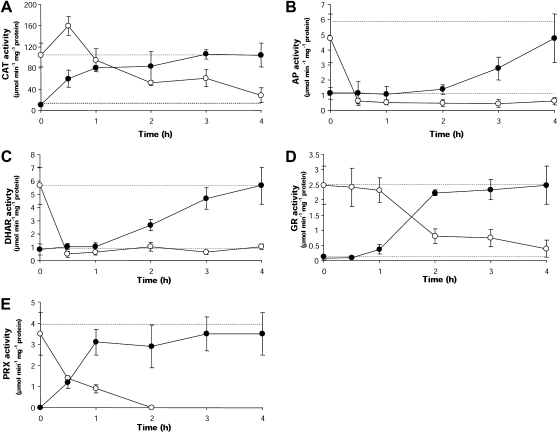
The activity of CAT, AP, DHAR, and GR increased in P. columbina dehydrated during low tide as well as in fronds dehydrated in vitro (Table 2, Fig. 6). These activities were 6–32 times higher than in hydrated plants. For example, GR activity varied from 0.082 μmol min−1 mg−1 protein in hydrated fronds to 2.5 μmol min−1 mg−1 protein in dehydrated fronds (naturally and in vitro). A TRX-dependent PRX activity of 3.5–4 μmol min−1 mg−1 protein was recorded in plants desiccated in vitro (Table 2). However, this activity was not detected in hydrated plants from the field or in 4 h in vitro rehydrated plants (Table 2). In spite of the general trend of enhanced enzymic activity, the patterns of response to desiccation varied according to the enzyme (Fig. 6). For example, CAT activity significantly increased (T=3.687, P=0.028) after only 0.5 h of desiccation with respect to the basal level recorded in hydrated plants (Fig. 6A). On the other hand, DHAR (Fig. 6C) and GR (Fig. 6D) activities increased significantly (DHAR: T=5.248, P=0.002; GR: T=14.85, P=0.0) after 2 h of desiccation. AP activity took longer (i.e. 3 h) to depart significantly from the normal level detected in hydrated fronds and it never reached the values displayed by dehydrated plants in nature (T=5.57; P=0.003) (Fig. 6B). PRX activity was detected at 0.5 h of desiccation, maintaining this activation during the whole desiccation period (Fig. 6E). During rehydration, the activity of all enzymes diminished down to the basal level recorded in naturally hydrated plants following enzyme-specific kinetics (Fig. 6). AP activity, however, dropped below the basal level (Fig. 6B) and never recovered the levels recorded in naturally hydrated plants (4 h rehydrated plants compared with naturally hydrated plants; T=1.620, P=0.042). In the case of PRX, the activity diminished drastically during the post-desiccation rehydration period and after 1 h rehydration it was no longer detected (Fig. 6E).
Table 2.
Activities of antioxidant enzymes determined in naturally hydrated, naturally dehydrated, in vitro dehydrated, and in vitro rehydrated P. columbina plants
| Enzyme (μmol min−1 mg−1 protein) | Hydrated plants | Dehydrated plants in vivo/in vitro | Rehydrated plants |
| CAT | 10.24±2.4 | 95.74±19.33*/104.54±22.3* | 28.63±13.7ns |
| AP | 1.12±0.4 | 6.29±1.5*/4.76±1.6* | 0.62±0.2* |
| DHAR | 0.82±0.4 | 5.52±1.7*/5.66±1.4* | 1.04±0.2 ns |
| GR | 0.082±0.007 | 2.51±0.9*/2.48±0.2* | 0.39±0.3 ns |
| PRX | n.d. | 3.98±0.6*/3.53±0.9* | n.d. |
Each value is an average of three independent replicates ±1 SD.
*Significant (P<0.05) differences with values measured in hydrated plants.
ns Not significant; n.d., not detected.
Fig. 6.
Kinetics of antioxidant enzyme activities in P. columbina fronds dehydrated in vitro (filled circles) and rehydrated (open circles). The upper discontinuous line represents the mean activities of the antioxidant enzymes determined in naturally dehydrated plants and the low discontinuous line the mean activities in naturally hydrated plants. Bars represent mean values of three independent replicates ±1 SD.
Photosynthetic efficiency and photosynthetic pigments
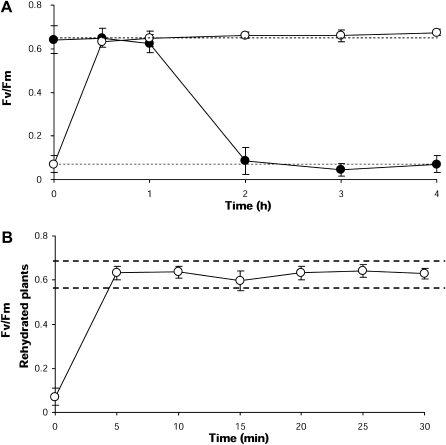
The maximum photosynthetic efficiency (Fv/Fm) in hydrated fronds was 0.689±0.03, whereas in fronds dehydrated during low tide and in those dehydrated in vitro values were <0.05 (94–96% decline; Table 3). Differences in Fv/Fm between natural and in vitro desiccation were not significant (T=3.25, P=0.054). When fronds were exposed to in vitro desiccation, the Fv/Fm values diminished gradually, reaching the lowest levels after 2 h of desiccation (Fig. 7A). In contrast, when fronds were rehydrated after the desiccation period, Fv/Fm was restored to maximum levels (∼0.680) after only 0.5 h of exposure to fresh seawater (Table 3, Fig. 7A). Earlier monitoring of changes in Fv/Fm demonstrated that recovery of this parameter occurred as quickly as 5 min after the beginning of rehydration (T=2.79, P=0.118; Fig. 7B).
Table 3.
Photosynthetic efficiency (Fv/Fm), energy flux indices, and concentration of photosynthetic pigments determined in naturally hydrated, naturally dehydrated, in vitro dehydrated, and in vitro rehydrated P. columbina plants
| Photosynthetic parameter | Hydrated plants | Dehydrated plants in vivo/in vitro | Rehydrated plants |
| Fv/Fm | 0.689±0.03 | 0.028±0.008*/0.043±0.016* | 0.674±0.014 ns |
| ABS/CS | 248.8±100.3 | 665.1±131.2*/719.5±536.7* | 245.6±135.2 ns |
| TRo/CS | 142.7±65.7 | 6.2±5.3*/32.9±27.9* | 160.8±91.4 ns |
| ETo/CS | 70.6±42.5 | 1.2±0.9*/7.2±6.9* | 78.5±51.1 ns |
| DIo/CS | 108.9±38.5 | 658.9±126.3*/623.3±498.1* | 80.8±44.8 ns |
| RC/SCo | 82.3±44.4 | 3.2±2.8*/19.3±16.9* | 97.4±63.4 ns |
| Chl a | 1.74±0.13 | 0.92±0.06*/0.97±0.11* | 1.86±0.23 ns |
| PE | 1.39±0.51 | 2.50±1.20*/2.53±1.11* | 1.28±0.58 ns |
| PC | 0.44±0.12 | 2.32±0.37*/2.28±0.39* | 0.40±0.12 ns |
Each value is an average of three independent replicates ±1 SD.
*Significant (P<0.05) differences with values measured in hydrated plants.
ns Not significant.
Chl a, PE, PC, in mg g−1 DT.
Fig. 7.
Kinetics of photosynthetic efficiency (Fv/Fm) in P. columbina fronds dehydrated in vitro (filled circles) and rehydrated (open circles). (A) The upper discontinuous line represents the mean levels of Fv/Fm determined in naturally hydrated plants and the low discontinuous line the mean levels of Fv/Fm in naturally dehydrated plants. (B) Recovery of Fv/Fm values after a short exposure to fresh seawater; the broken line indicates the range of Fv/Fm values in naturally hydrated plants. Bars represent mean values of three independent replicates ±1 SD.
Other photosynthetic indices determined in dehydrated fronds changed significantly in relation to those in hydrated fronds (Table 3). The ABS/CS and DIo/CS indices increased ∼3- and 6-fold in comparison with basal levels in naturally and in vitro dehydrated plants, respectively. By the contrary, the TRo/CS, ETo/CS, and RC/SCo indices decreased 80–96%; however, when fronds were rehydrated for 5 min, these indices returned to levels recorded in fronds naturally hydrated (Table 3).
In both naturally and in vitro dehydrated fronds Chl a decreased by 44–47% in relation to its concentration in hydrated fronds (Table 3). PE and PC content, on the other hand, increased 2- and 5-fold, respectively, in relation to the content recorded in hydrated plants (Table 3). Finally, Chl a, PE, and PC levels during rehydration were restored to the same levels as those registered in hydrated fronds (Table 3).
Discussion
The results demonstrate that desiccation generated by low tide induces, in P. columbina, morphological changes in the cells, accompanied by biochemical and physiological responses to the stressful condition. Our results also demonstrate the highly plastic nature of these changes, as all quickly reverted during high tide.
Among the changes observed in P. columbina during desiccation, cellular alterations were the most noteworthy. Nevertheless, these alterations have been shown in other biological systems exposed to higher levels of desiccation. For example, in the case of the resurrection plants Craterostigma wilmsii (Vicré et al., 2004a) and M. flabellifolius (Moore et al., 2006), considerable plasma membrane and cell wall folding accompanied by significant changes in polysaccharide content occurs during desiccation. Similar to our findings, a significant reduction in cell volume was reported when the resurrections plants C. wilmsii and M. flabellifolius are dried (Farrant et al., 2003; Moore et al., 2006). It has been hypothesized that reduction in cell size is due to the folding of the cell wall induced by decreased water content. In Porphyra, water content was reduced by 96%, similar to what has been recorded in resurrection plants undergoing desiccation (Le and McQueen-Mason, 2006). Based on the above, it has been proposed that cell wall folding is a cellular strategy that prevents the plasmalemma from tearing away from the cell wall during desiccation, ensuring cell integrity (Vicré et al., 2004b). In fact, in the case of Porphyra, when plants are rehydrated, cells return quickly to their original volume without apparent damage. Additional strategies are used by resurrection plants to attenuate the effect of water loss, including the storage of proteins, lipids, and particularly carbohydrates (Hoekstra et al., 2001; Le and McQueen-Mason, 2006). In Porphyra, the high accumulation of carbohydrates recorded in dehydrated plants (i.e. 4.8 mg g−1 versus 10.2 mg g−1 in dehydrated fronds) might represent evidence of a similar strategy. Thus, P. columbina could be part of the resurrection plant group in terms of mechanisms of tolerance to desiccation.
The induction of ROS production during desiccation was demonstrated by the high levels of ROS measured in dehydrated plants in comparison with naturally hydrated fronds. The production of ROS during desiccation has also been reported in the alga Scytosiphon arbuscula (Burritt et al., 2002) and in the vascular plant Atrichum androgynum (Mayaba et al., 2002). In spite of different experimental strategies used in the few studies reporting on the relationship between desiccation and ROS, the results agree with those found in P. columbina in the sense that the high levels of ROS quickly induced during desiccation also return rapidly to the basal level during rehydration. However, the source(s) of ROS during desiccation in P. columbina is (are) not clear as is known for other conditions triggering oxidative stress (Hammond-Kosack and Jones, 1996). Theoretically, ROS bursts could be induced by the low water potential. In fact, water dissipation during desiccation could generate electronic alterations, which in turn could facilitate ROS production (Baker and Orlandi, 1995). In addition, the biphasic increase in intracellular H2O2 has been determined in algae undergoing oxidative stress, where the production is exclusively organellar (see e.g. González et al., 2010). In vascular plants under water stress, ROS production has been detected in apoplast, xylem vessels, chloroplasts, and mitochondria (Mittler et al., 2004; Hu et al., 2006; Toldi et al., 2009). Therefore, it is possible to suggest that desiccation in P. columbina could induce organellar ROS production just as already reported in other algae and land plants. However, this hypothesis needs be experimentally tested in order to unequivocally identify the specific source of ROS.
Tolerance to desiccation could be, at least in part, related to the capacity of the plants to buffer the excess ROS and, as a consequence, attenuate the oxidative conditions within the cell and minimize the damage known to be caused by ROS excess. For example, in individuals of S. arbuscula sensitive to desiccation, ROS and lipoperoxide production was higher than in resistant individuals (Burrit et al., 2002). More efficient attenuation of ROS production in resistant individuals could be due to higher activity of antioxidant enzymes (Burrit et al., 2002). In fact, antioxidant activities have also been shown to increase in resurrection plants during desiccation (Kranner et al., 2002; Le and McQueen-Mason, 2006; Moore et al., 2009), which has been used to explain the high tolerance of these plants to desiccation stress. In conjunction with this, the activities of all antioxidant enzymes (CAT, AP, DHAR, and GR) tested in P. columbina increased significantly during desiccation. The involvement of these enzymes has been observed in other algal species exposed to different abiotic stresses that induce overproduction of ROS (Collén and Davison, 1999a; Ratkevicius et al., 2003; Shiu and Lee, 2005; Contreras et al., 2005, 2009). For example, tolerance to copper in Scytosiphon lomentaria and Ulva compressa is mainly due to attenuation of oxidative stress by antioxidant enzymes (Ratkevicius et al., 2003, Contreras et al., 2005, 2009), accompanied by overexpression of the corresponding genes/proteins (Contreras et al. 2010; Contreras-Porcia et al., 2010). Consistent with these results, the low tolerance to the same metal in sensitive species such as the kelp L. nigrescens, is caused by inactivation of the antioxidant enzymes (Contreras et al., 2009). In land plants, to prevent organellar damage, ROS can be scavenged by the action of antioxidant enzymes that are expressed differently in the various cellular compartments, such as the PRXs, whose activity is located mainly in chloroplasts and mitochondria (Triparthi et al., 2009). The TRX-dependent PRX (2-Cys) activity determined in this study during desiccation is probably induced in the chloroplast, known for this type of PRX (i.e. 2-Cys PRX) in Porphyra (Reith and Munholland, 1995). In addition, it is important to mention that during oxidative stress, chloroplastic ROS could interact with fatty acids inducing the formation of fatty acid hydroperoxides (Blokhina et al., 2003). In this context, the potential formation of fatty acid hydroperoxides could be reduced by PRX activity (Baier and Dietz, 1997) attenuating membrane damage to the chloroplast. Thus, in algae, activation of the antioxidant enzymic system seems essential for attenuating oxidative stress.
The TRX-dependent PRX activity, observed during desiccation was undetectable in hydrated plants. The lack of detectable activity in the hydrated plants could be explained by the low ROS levels measured in these plants. This enzyme is activated when the H2O2 levels in the cell rise (Dietz et al., 2006). Thus, it is expected that only when plants are exposed to desiccation, does the overproduction of H2O2 trigger PRX activation. It is important to highlight that, to date, PRX activation has only been reported in algae exposed to metals (Contreras et al. 2010; Contreras-Porcia et al., 2010). In this sense, the additional PRX activation in Porphyra under desiccation supports the hypothesis that, in algae, this enzyme could represent a conserved mechanism of tolerance to oxidative stress, assisting mainly in the organellar attenuation of ROS and lipid peroxidation. Finally, the results of the antioxidant activation of antioxidant enzymes due to desiccation help to explain the attenuation of ROS and the oxidation of low-weight biomolecules (i.e. lipoperoxides and carbonyls) as determined during the desiccation event. However, it is important to highlight that other tolerance mechanisms could be involved, such as the utilization of antioxidant compounds as polyphenols, ascorbate, or glutathione due to the importance of these compounds in ROS attenuation (Blokhina et al., 2003).
At the physiological level, the measurement of photosynthetic efficiency (Fv/Fm) is an effective parameter to assess the photosynthetic status of a plant under oxidative stress conditions (Maxwell and Johnson, 2000; Millan-Almaraz et al., 2009). One adaptation mechanism of photosynthetic alteration produced by an oxidative stress condition is photoinhibition, which is a reversible high-light-induced reduction of the photosynthetic quantum yield due to the down-regulation of PS II, where the energy is dissipated thermally (Hader et al., 2002). For example, under normal solar radiation the photosynthetic quantum yield of P. columbina and Porphyra leucosticta decreases (Figueroa et al., 1997; Hader et al., 2002) as well as the pigment content (e.g chlorophyll level). However, this situation is reversed quickly in dim light, where proteins and compounds photosynthetic are resynthesized. In the alga Mastocarpus papillatus undergoing desiccation there is a net decrease in photosynthesis, which recovers upon re-immersion (Bell, 1993). This mechanism is often found in resurrection plants (Farrant et al., 2003) and algal species adapted to high light conditions (Hader et al., 2002; Skene, 2004; Gómez et al., 2005) and differences can be detected even in ecotypes located at different heights in the intertidal zone (Varela et al., 2006; Gylle et al., 2009). Consistent with the above, Porphyra plants undergoing desiccation during low tide displayed an Fv/Fm significantly diminished, but quickly (i.e. 5 min) returned to normal after re-immersion, explained by down-regulation of PS II. However, in algal species such as U. compressa, L. nigrescens, and Gelidium rex, better adapted to the lower levels of the intertidal zone, the photosynthetic quantum yield is never restored when exposed to desiccation, levels the same as those perfectly tolerated by P. columbina (Contreras-Porcia unpublished data).
During desiccation, the levels of Chl a determined in P. columbina were lower than in rehydrated plants. Contrarily, the levels of PE and PC presented an opposite pattern. Although these results could be considered as ‘abnormal’, they agree with the mechanism of down-regulation of PS II (Strasser et al., 2000; Farrant et al., 2003). Even though carbon fixation could be inhibited during desiccation, the electron flow might continue, and the energy could be transferred from photo-excited chlorophyll pigments to 3O2, forming singlet oxygen (1O2), where superoxide and H2O2 would be produced (Kranner et al., 2002). However, in a situation where the photosynthesis process is inhibited by the down-regulation of PS II and where the energy is canalized to accessory pigments such as PC and PE, ROS production could be controlled. In this context, the low levels of chlorophyll of the PS, detected in P. columbina under desiccation could prevent chloroplastic ROS production. This situation was clearly demonstrated since during desiccation ROS were attenuated and no structural alteration in the chloroplast was detected. In fact, chloroplastic alterations are observed in algal species that are unable to attenuate an oxidative stress condition (Contreras et al., 2009).
In addition to the results mentioned above, the JIP analysis, done to assess the effect of desiccation on electron transport at the acceptor site of PS II, demonstrated that desiccation induces changes in energy fluxes in the photosynthetic apparatus of P. columbina. The total light energy flux absorbed (ABS) significantly increased during desiccation; however, this energy was mainly dissipated (DIo). These changes were accompanied by an incomplete supply of energy to the reaction centres (RC) of the PS as the trapping energy (TRo) decreased as did the number of RC. This reduction was reflected in a low electron transport flux (ETo) involved in CO2 fixation. The high dissipated energy level indicates that part of the energy could be transformed into heat or transferred to another system due mainly to photoinhibition of PS II, in order to prevent ROS production (Strasser et al., 2000; Force et al., 2003). These changes in the photosynthetic apparatus have been observed in different organisms affected by diverse abiotic factors such as high illumination, UV, or temperature (Aguilera et al., 2002; Force et al., 2003; Ferrante and Maggiore, 2007) where photoinhibition was identified by an increase in the effective dissipation and by attenuation of electronic transport (Strasser et al., 2000; Hader et al., 2002). In this context, during desiccation in P. columbina PS II is down-regulated as a tolerance mechanism to oxidative stress, such as the activation of the antioxidant system.
Other mechanisms of tolerance to oxidative stress in P. columbina at chloroplastic level could be based on the biliproteins PE and PC, which could act by dissipating, as heat, the excess energy available during desiccation, preventing the overproduction of ROS, and directly eliminating ROS. This is indirectly supported by the study of Romay et al. (2003), who described antioxidant functions for these biliproteins. These phycobiliproteins are small proteins that constitute the phycobilisomes, macromolecular protein complexes that serve as light harvesting complexes for the photosynthetic apparatus and impede ROS production (Tandeau de Marsac, 2003). When light harvesting is not balanced by energy utilization and dissipation, toxic radicals can form, leading to oxidative damage (Vranová et al., 2002). In addition, it has been demonstrated that PC is able to directly scavenge different types of ROS and reduce lipid peroxidation (Romay et al., 2003). Thus, different ROS attenuating pathways could be operating during the desiccation process, extending even to the level of photosynthetic pigments.
Finally, the results obtained in this study indicate that, in P. columbina, several mechanisms (i.e. down-regulation of PS II, accumulation of phycobiliproteins, and activation of the antioxidant system) act coordinately to reduce the oxidative stress generated by desiccation. It is important to highlight that these mechanisms are necessary during rehydration to re-establish in the tissues the physiological conditions of naturally hydrated fronds. This is consistent with our observation that the naturally hydrated physiological state of P. columbina is quickly restored during re-immersion, which explains its capacity to flourish, as no other seaweed does, at the highest level of the intertidal zone. Comparatively, species inhabiting a low position in the intertidal zone are far more sensitive to desiccation, as overproduction of ROS is not attenuated during rehydration (Contreras-Porcia unpublished data). In this context, we can hypothesize that oxidative stress induced by tide-related desiccation, could be an important factor shaping the patterns of vertical zonation at the intertidal zone. On the other hand, zonation patterns of intertidal species, and the mechanisms underlying these patterns, suggest that factors such as grazing, competition, and physiological adaptations to abiotic environmental factors other than desiccation, are also involved in modulating the distribution of seaweeds at the intertidal zone and, therefore, must not be underestimated (e.g. Lubchenco, 1980; Moreno and Jaramillo, 1983; Davison and Pearson, 1996; van Tamelen, 1996; Hays, 2007; Shafer et al., 2007).
Conclusion
The results obtained in this study demonstrate that desiccation generated during low tide induces, in P. columbina, an oxidative stress condition, and morphological and physiological alterations. The activation of different physiological mechanisms helps to explain the high tolerance to desiccation of this species. In this context, in vitro rehydration experiments demonstrated the rapid capacity of the species to recover from oxidative stress, and provide the functional basis that helps to explain, at least in part, the position of this species at the highest intertidal zone.
Acknowledgments
This work was supported by FONDECYT 11085019 to L.C. Additional funding comes from FONDAP 1501-0001 funded by CONICYT to the Center for Advanced Studies in Ecology and Biodiversity (CASEB) Program 7 and ICA to J.C. We are especially grateful to Aníbal Contreras and Rosa Flores for field assistance.
Glossary
Abbreviations
- AP
ascorbate peroxidase
- CAT
catalase
- Chl a
chlorophyll a
- DCFH-DA
2,4 dichlorofluoresceine diacetate
- DCF
2,4 dichlorofluoresceine
- DHA
dehydroascorbate
- DHAR
dehydroascorbate reductase
- DNPH
2,4-dinitrophenyl hydrazine
- Fv/Fm
photosynthetic efficiency
- GR
glutathione reductase
- HE
hydroethidine
- PE
phycoerythrin
- PC
phycocyanin
- PRX
peroxiredoxin
- PS II
photosystem II
- ROS
reactive oxygen species
- RWC
relative water content
- SEM
scanning electron microscopy
- TEM
transmission electron microscopy
- TR
thioredoxin reductase
- TRX
thioredoxin
References
- Abe S, Kurashima A, Yokohama Y, Tanaka J. The cellular ability of desiccation tolerance in japanese intertidal seaweeds. Botanica Marina. 2001;44:125–131. [Google Scholar]
- Achary VMM, Jena S, Panda KK, Panda BB. Aluminium induced oxidative stress and DNA damage in root cells of Allium cepa L. Ecotoxicology and Environmental Safety. 2008;70:300–310. doi: 10.1016/j.ecoenv.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Aguilera J, Bischof K, Karsten U, Hanelt D, Wiencke C. Seasonal variation in ecophysiological patterns in macroalgae from an Arctic fjord. II. Pigment accumulation and biochemical defense systems against high light stress. Marine Biology. 2002;140:1087–1095. [Google Scholar]
- Alpert P. The discovery, scope, and puzzle of desiccation tolerance in plants. Plant Ecology. 2000;151:5–17. [Google Scholar]
- Alpert P. Constraints of tolerance, why are desiccation-tolerant organisms so small or rare? Journal of Experimental Biology. 2006;209:1575–1584. doi: 10.1242/jeb.02179. [DOI] [PubMed] [Google Scholar]
- Alveal K. Estudios ficoecológicos en la región costera de Valparaíso. Revista de Biología Marina. 1970;14:7–88. [Google Scholar]
- Asada K. The water-water cycle in chloroplasts, scavenging of active oxygens and dissipation of excess photons. Annual Review of Plant Physiology and Plant Molecular Biology. 1999;50:601–639. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- Baier M, Dietz J. The plant 2-Cys peroxiredoxin BAS1 is a nuclear-encoded chloroplast protein, its expressional regulation, phylogenetic origin, and implications for its specific physiological function in plants. The Plant Journal. 1997;12:179–190. doi: 10.1046/j.1365-313x.1997.12010179.x. [DOI] [PubMed] [Google Scholar]
- Baker CJ, Orlandi EW. Active oxygen in plant pathogenesis. Annual Review of Phytopathology. 1995;33:299–321. doi: 10.1146/annurev.py.33.090195.001503. [DOI] [PubMed] [Google Scholar]
- Baldauf S, Roger AJ, Wenk-Siefert I, Doolittle WF. A kingdom-level phylogeny of eukaryotes based on combined protein data. Science. 2000;3:972–977. doi: 10.1126/science.290.5493.972. [DOI] [PubMed] [Google Scholar]
- Bell EC. Photosynthetic response to temperature and desiccation of the intertidal alga. Mastocarpus papillatus. Marine Biology. 1993;117:337–346. [Google Scholar]
- Blokhina O, Virolainen E, Fagerstedt KW. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Annals of Botany. 2003;91:179–194. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert HJ. What makes desiccation tolerable? Genome Biology. 2000;2 doi: 10.1186/gb-2000-1-2-reviews1010. 1010.1–1010.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell GP. Role of active oxygen species and NO in plant defense responses. Current Opinion in Plant Biology. 1999;2:287–294. doi: 10.1016/S1369-5266(99)80051-X. [DOI] [PubMed] [Google Scholar]
- Bolwell GP, Bindschedler LV, Blee KA, Butt VS, Davies DR, Gardner SL, Gerrish C, Minibayeva F. The apoplastic oxidative burst in response to biotic stress in plants, a three-component system. Journal of Experimental Botany. 2002;53:1367–1376. [PubMed] [Google Scholar]
- Bowler C, Fluhr R. The role of calcium and activated oxygen as signals for controlling cross tolerance. Trends in Plant Science. 2000;5:241–246. doi: 10.1016/s1360-1385(00)01628-9. [DOI] [PubMed] [Google Scholar]
- Burritt DJ, Larkindale J, Hurd K. Antioxidant metabolism in the intertidal red seaweed Stictosiphonia arbuscula following desiccation. Planta. 2002;215:829–838. doi: 10.1007/s00425-002-0805-6. [DOI] [PubMed] [Google Scholar]
- Butterfield NJ. Bangiomorpha pubescens n. gen., n. sp.: implications for the evolution of sex, multicellularity, and the Mesoproterozoic/Neoproterozoic radiation of eukaryotes. Paleobiology. 2000;26:386–404. [Google Scholar]
- Cabello-Pasini A, Diaz-Martín MA, Muñiz-Salazar R, Zertuche-Gonzalez JA, Pacheco-Ruiz I. Effect of temperature and desiccation on the photosynthetic performance of Porphyra perforata. Journal of Phycology. 2000;36:10–14. [Google Scholar]
- Collén J, Davidson IR. Reactive oxygen metabolism in intertidal Fucus spp. (Phaeophyceae) Journal of Phycology. 1999a;35:62–69. [Google Scholar]
- Collén J, Davidson IR. Stress tolerance and reactive oxygen metabolism in the intertidal red seaweeds Mastocarpus stellatus and Chondrus crispus. Plant, Cell and Environment. 1999b;22:1143–1151. [Google Scholar]
- Collén J, Guisle-Marsollier I, Léger JJ, Boyen C. Response of the transcriptome of the intertidal red seaweed Chondrus crispus to controlled and natural stresses. New Phytology. 2007;176:45–55. doi: 10.1111/j.1469-8137.2007.02152.x. [DOI] [PubMed] [Google Scholar]
- Contreras L, Mella D, Moenne A, Correa JA. Differential responses to copper-induced oxidative stress in the marine macroalgae Lessonia nigrescens and Scytosiphon lomentaria (Phaeophyceae) Aquatic Toxicology. 2009;94:94–102. doi: 10.1016/j.aquatox.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Contreras L, Moenne A, Correa JA. Antioxidant responses in Scytosiphon lomentaria (Phaeophyceae) inhabiting copper-enriched coastal environments. Journal of Phycology. 2005;41:1184–1195. [Google Scholar]
- Contreras L, Moenne A, Gaillard F, Potin P, Correa JA. Proteomic analysis and identification of copper stress-regulated proteins in the marine alga Scytosiphon gracilis (Phaeophyceae) Aquatic Toxicology. 2010;96:85–89. doi: 10.1016/j.aquatox.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Contreras-Porcia L, González A, Vergara E, Medina C, Correa JA, Moenne A. Identification of copper-induces genes in the marine alga Ulva compressa (Chlorophyta) Marine Biotechnology. 2010 doi: 10.1007/s10126-010-9325-8. in press. doi: 10.1007/s10126-010-9325-8. [DOI] [PubMed] [Google Scholar]
- Correa JA, McLachlan JL. Endophytic algae of Chondrus crispus (Rodophyta). III. Host-specificity. Journal of Phycology. 1991;27:448–459. [Google Scholar]
- Davison IR, Pearson GA. Environmental stress in intertidal seaweeds. Journal of Phycology. 1996;32:197–211. [Google Scholar]
- Deighton N, Muckenshnabel B, Goodman BA, Williamson W. Lipid peroxidation and the oxidative burst associated with infection of Capsicum annum by. Botrytis cinerae. The Plant Journal. 1999;20:485–492. doi: 10.1046/j.1365-313x.1999.00622.x. [DOI] [PubMed] [Google Scholar]
- Dietz KJ, Jacob S, Oelze ML, Laxa M, Tognetti V, Nunes de Miranda SM, Baire M, Finkenmeier I. The function of peroxiredoxins in plant organelle redox metabolism. Journal of Experimental Botany. 2006;57:1697–1709. doi: 10.1093/jxb/erj160. [DOI] [PubMed] [Google Scholar]
- Farrant JM. A comparison of mechanisms of desiccation tolerance among three angiosperm resurrection. Plant Ecology. 2000;151:29–39. [Google Scholar]
- Farrant JM, Vander willigen C, Loffell DA, Bartsch S, Whittaker A. An investigation into the role of light during desiccation of three angiosperm resurrection plants. Plant, Cell and Environment. 2003;26:1275–1286. [Google Scholar]
- Ferrante A, Maggiore T. Chlorophyll a fluorescence measurements to evaluate storage time and temperature of Valeriana leafy vegetables. Postharvest Biology and Technology. 2007;45:73–80. [Google Scholar]
- Figueroa F, Salles S, Aguilera J, Jiménes C, Mercado J, Vinegla B, Flores-Moya A, Altamirano M. Effects of solar radiation on photoinhibition and pigmentation in the red alga. Porphyra leucosticta. Marine Ecology Progress Series. 1997;151:81–90. [Google Scholar]
- Force L, Critchley Ch, van Rensen JJS. New fluorescence parameters for monitoring photosynthesis in plants. The effect of illumination on the fluorescence parameters of the JIP-test. Photosynthesis Research. 2003;78:17–33. doi: 10.1023/A:1026012116709. [DOI] [PubMed] [Google Scholar]
- Foyer CH, López-Delgado H, Dat JF, Scott IM. Hydrogen peroxide-and glutathione-associated mechanisms of acclimatory stress tolerance and signaling. Physiologia Plantarum. 1997;100:241–254. [Google Scholar]
- Gómez I, Figueroa FL, Huovinen P, Ulloa N, Morales V. Photosynthesis of the red alga Gracilaria chilensis under natural solar radiation in an estuary in southern Chile. Aquaculture. 2005;244:369–382. [Google Scholar]
- González A, Santelices B. Proceedings of the 17th International seaweed symposium. New York: Oxford University Press Inc; 2003. A re-examination of the potential use of central Chilean Porphyra (Bangiales, Rhodopyta) for human consumption. [Google Scholar]
- González A, Vera J, Castro J, Denté G, Mellado M, Morales B, Correa JA, Moenne A. Co-ocurring increases of calcium and organellar reactive oxygen species determine differential activation of antioxidant and defense enzymes in Ulva compressa (Chlorophyta) exposed to copper excess. Plant, Cell and Environment. 2010;33:1627–1640. doi: 10.1111/j.1365-3040.2010.02169.x. [DOI] [PubMed] [Google Scholar]
- Gylle AM, Nygard Ch, Ekelund NGA. Desiccation and salinity effects on marine and brackish Fucus vesiculosus L. (Phaeophyceae) Phycologia. 2009;48:156–164. [Google Scholar]
- Hader D, Lebert M, Sinha RP, Barbieri ES, Helbling EW. Role of protective and repair mechanisms in the inhibition of photosynthesis in marine macroalgae. Photochemical and Photobiological Sciences. 2002;1:809–814. doi: 10.1039/b206152j. [DOI] [PubMed] [Google Scholar]
- Hammond-Kosack KE, Jones JDG. Resistance gene-dependent plant defense responses. The Plant Cell. 1996;8:1773–1791. doi: 10.1105/tpc.8.10.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays CG. Adaptive phenotypic differentiation across the intertidal gradient in the alga Silvetia compressa. Ecology. 2007;88:149–157. doi: 10.1890/0012-9658(2007)88[149:apdati]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Hoekstra FA, Golovina EA, Buitink J. Mechanisms of plant desiccation tolerance. Trends in Plant Science. 2001;6:431–438. doi: 10.1016/s1360-1385(01)02052-0. [DOI] [PubMed] [Google Scholar]
- Hoffmann AJ, Santelices B. Flora marina de Chile central. Ediciones Universidad Católica de Chile, 434 pp. 1997 [Google Scholar]
- Hu X, Zhang A, Zhang J, Jiang M. Abscisic acid is a key inducer of hydrogen peroxide production in leaves of maize plants exposed to water stress. Plant and Cell Physiology. 2006; 47:1484–1495. doi: 10.1093/pcp/pcl014. [DOI] [PubMed] [Google Scholar]
- Ji Y, Tanaka J. Effect of desiccation on the photosynthesis of seaweeds from the intertidal zone in Honshu, Japan. Phycological Research. 2002;50:145–153. [Google Scholar]
- Korbee N, Figueroa FL, Aguilera J. Effect of Light quality on the accumulation of photosynthetic pigments, proteins and mycosporine-like amino acids in the red alga Porphyra leucosticta (Bangiales, Rhodophyta) Journal of Photochemistry and Photobiology. 2005;80:71–78. doi: 10.1016/j.jphotobiol.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Kranner I, Beckett RP, Wornik S, Zorn M, Pfeifhofer HW. Revival of a resurrection plant correlates with its antioxidant status. The Plant Journal. 2002;31:13–24. doi: 10.1046/j.1365-313x.2002.01329.x. [DOI] [PubMed] [Google Scholar]
- Le T-N, McQueen-Mason SJ. Desiccation-tolerant plants in dry environments. Reviews in Environmental Science and Biotechnology. 2006;5:269–279. [Google Scholar]
- Lobban Ch.S, Harrison PJ . Seaweed ecology and physiology. Cambridge: Cambridge University Press; 1994. 366 pp. [Google Scholar]
- Lubchenco J. Algal zonation in the New England rocky intertidal community: an experimental analysis. Ecology. 1980;61:333–344. [Google Scholar]
- Maxwell K, Johnson GN. Chlorophyll fluorescence—a practical guide. Journal of Experimental Botany. 2000;51:659–668. doi: 10.1093/jxb/51.345.659. [DOI] [PubMed] [Google Scholar]
- Mayaba N, Minibayeva F, Beckett RP. An oxidative burst of hydrogen peroxide during rehydration following desiccation in the moss. Atrichum androgynum. New Phytology. 2002;155:275–283. [Google Scholar]
- Medina M, Andrade S, Faugeron S, Mella D, Correa J. Biodiversity of rocky intertidal benthic communities associated whit copper mine tailing discharge in northern Chile. Marine Pollution Bulletin. 2005;50:396–406. doi: 10.1016/j.marpolbul.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Millan-Almaraz JR, Guevara-Gonzalez RG, Romero-Troncoso R, Osornio-Rios RA, Torres-Pacheco I. Advantages and disadvantages on photosynthesis measurement techniques: A review. African Journal of Biotechnology. 2009;8:7340–7349. [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends in Plant Science. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Moore JP, Nguema-Ona E, Chevalier L, Lindsey GG, Brandt WF, Lerouge P, Farrant JM, Driouich A. Response of the leaf cell wall to desiccation in the resurrection plant Myrothamnus flabellifolius. Plant Physiology. 2006;141:651–662. doi: 10.1104/pp.106.077701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JP, Le NT, Brandt WF, Driouich A, Farrant JM. Towards a systems-based understanding of plant desiccation tolerance. Trends in Plant Science. 2009;14:110–117. doi: 10.1016/j.tplants.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Moreno C, Jaramillo E. The role of grazers in the zonation of the intertidal macroalgae of the Chilean coast. Oikos. 1983;41:73–76. [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione, keeping active oxygen under control. Annual Review of Plant Physiology. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Pearson G, Serrao E, Cancela L. Suppression subtractive hybridization for studying gene expression during aerial exposure and desiccation in fucoid algae. European Journal of Phycology. 2001;36:359–366. [Google Scholar]
- Pearson G, Hoarau G, Lago-Leston A, Coyer JA, Kube M, Reinhardt R, Henckel K, Serrao ETA, Corre E, Olsen JL. An expressed sequence tag analysis of the intertidal Brown seaweed Fucus serrratus (L.) and F. vesiculosus (L.) (Heterokontophyra, Phaeophyceae) in response to abiotic stressors. Marine Biotechnology. 2010;12:195–213. doi: 10.1007/s10126-009-9208-z. [DOI] [PubMed] [Google Scholar]
- Pinto E, Sigaud-Kutner TCS, Leitao MA, Okamoto OK, Morse D, Colepicolo P. Heavy metal-induced oxidative stress in algae. Journal of Phycology. 2003;39:1008–1018. [Google Scholar]
- Pitzschke A, Forzani C, Hirt H. Reactive oxygen species signaling in plants. Antioxidants and Redox Signaling. 2006;8:1757–1764. doi: 10.1089/ars.2006.8.1757. [DOI] [PubMed] [Google Scholar]
- Ratkevicius N, Correa JA, Moenne A. Copper accumulation, synthesis of ascorbate and activation of ascorbate peroxidase in Enteromorpha compressa (L.) Grev. (Chlorophyta) from heavy-metal enriched environments in northern Chile. Plant, Cell and Environment. 2003;26:1599–1608. [Google Scholar]
- Reith M, Munholland J. Complete nucleotide sequence of the Porphyra purpurea chloroplast. Plant Molecular Biology Reporter. 1995;13:333–335. [Google Scholar]
- Romay Ch, González R, Ledón N, Remirez D, Rimbau V. C-phycocyanin, a biliprotein with antioxidant, anti-Inflammatory and neuroprotective effects. Current Protein and Peptide Science. 2003;4:207–216. doi: 10.2174/1389203033487216. [DOI] [PubMed] [Google Scholar]
- Rustérucci C, Montillet JC, Agnel JP, Battesti C, Alonso B, Knoll A, Bessoule JJ, Etienne P, Blein JP, Triantaphylèdes C. Involvement of lypoxigenase-dependent production of fatty acid hydroperoxides in the development of the hypersensitive cell death induced by criptogein on tobacco leaves. Plant Physiology. 1999;11:885–891. doi: 10.1074/jbc.274.51.36446. [DOI] [PubMed] [Google Scholar]
- Santelices B. Utilización y Diversidad. Pontificia Universidad Católica de Chile; 1989. Algas marinas de Chile. Distribución Ecológica. [Google Scholar]
- Schraudner M, Moeder W, Wiese C, Van camp W, Inzé D, Langebartels C, Sandermann H. Ozone-induced oxidative burst in the ozone-biomonitor plant, tobacco Bel W3. The Plant Journal. 1998;16:235–245. doi: 10.1046/j.1365-313x.1998.00294.x. [DOI] [PubMed] [Google Scholar]
- Shafer DJ, Sherman TD, Wyllie-Echeverria S. Does desiccation tolerance control the vertical distribution of intertidal seagrasses? Aquatic Botany. 2007;87:161–166. [Google Scholar]
- Shiu Ch, Lee T. Ultraviolet-B-induced oxidative stress and responses of the ascorbate-glutathione cycle in a marine macroalgae Ulva fasciata. Journal of Experimental Botany. 2005;56:2851–2865. doi: 10.1093/jxb/eri277. [DOI] [PubMed] [Google Scholar]
- Sibbald PR, Vidaver W. Photosystem I-mediated regulation of water splitting in the red alga. Porphyra sanjuanensis. Plant Physiology. 1987;84:1373–1377. doi: 10.1104/pp.84.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene KR. Key differences in photosynthetic characteristics of nine species of intertidal macroalgae are related to their position on the shore. Canadian Journal of Botany. 2004;82:177–184. [Google Scholar]
- Smirnoff N. Ascorbic acid, metabolism and functions of a multi-facetted molecule. Current Opinion in Plant Biology. 2000;3:229–235. [PubMed] [Google Scholar]
- Smith CM, Satoh K, Fork DV. The effect of osmotic tissue dehydration and air drying on morphology and energy transfer in two species of Porphyra. Plant Physiology. 1986;80:843–847. doi: 10.1104/pp.80.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr AR. A low-viscosity epoxy resin embedding medium for electron microscopy. Journal of Ultrastructural Research. 1969;26:31–42. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Strasser A, Srivastava A, Tsimilli-Michael M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Yannu M, Pathre U, Mohanty P, editors. Probing photosynthesis: mechanisms, regulation and adaptation. London: Taylor and Francis; 2000. pp. 445–483. [Google Scholar]
- Tandeau de Marsac N. Phycobiliproteins and phycobilisomes, the early observation. Photobiological Research. 2003;76:197–205. doi: 10.1023/A:1024954911473. [DOI] [PubMed] [Google Scholar]
- Thoma I, Loeffler C, Sinha AK, Gupta M, Krishke M, Steffan B, Roitsh T, Mueller MJ. Cyclopentenone isoprostanes induced by reactive oxygen species trigger defense gene activation and phytoalexin accumulation in plants. The Plant Journal. 2003;34:363–375. doi: 10.1046/j.1365-313x.2003.01730.x. [DOI] [PubMed] [Google Scholar]
- Toldi O, Tuba Z, Scott P. Vegetative desiccation tolerance: is it a goldmine for bioengineering crops? Plant Science. 2009;176:187–199. [Google Scholar]
- Tripathi BN, Bhatt I, Dietz K-J. Peroxiredoxins, a less studied component of hydrogen peroxide detoxification in photosynthetic organisms. Protoplasma. 2009;235:3–15. doi: 10.1007/s00709-009-0032-0. [DOI] [PubMed] [Google Scholar]
- Van Tamelen PG. Algal zonation in tidepools, experimental evaluation of the roles of physical disturbance, herbivory and competition. Journal of Experimental Marine Biology and Ecology. 1996;201:197–231. [Google Scholar]
- Varela D, Santelices B, Correa JA, Arroyo MK. Spatial and temporal variation of photosynthesis in intertidal Mazzaella laminarioides (Bory) Fredericq (Rhodophyta, Gigartinales) Journal of Applied Phycology. 2006;18:827–838. [Google Scholar]
- Vicré M, Farrant JM, Driouich A. Insights into the cellular mechanisms of desiccation tolerance among angiosperms resurrection plant species. Plant, Cell and Environment. 2004a;27:1329–1340. [Google Scholar]
- Vicré M, Lerouxel O, Farrant J, Lerouge P, Driouich A. Composition and desiccation-induced alterations of the cell wall in the resurrection plant. Craterostigma wilmsii. Physiologia Plantarum. 2004b;120:229–239. doi: 10.1111/j.0031-9317.2004.0234.x. [DOI] [PubMed] [Google Scholar]
- Vranová E, Inzé D, Van Breusegen F. Signal transduction during oxidative stress. Journal of Experimental Botany. 2002;53:1227–1236. [PubMed] [Google Scholar]
- Wojstazseck P. Oxidative burst, an early plant response to pathogen infection. Biochemical Journal. 1997;322:681–692. doi: 10.1042/bj3220681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HS, Hackett JD, Ciniglia C, Pinto G, Bhattacharya D. A molecular timeline for the origin of photosynthetic eukaryotes. Molecular Biology and Evolution. 2004;21:809–818. doi: 10.1093/molbev/msh075. [DOI] [PubMed] [Google Scholar]
- Zar J. Biostatistical analysis. Englewood Cliffs: Prentice Hall; 1999. [Google Scholar]
- Zou D, Gao K. Effects of desiccation and CO2 concentration on emersed photosynthesis in Porphyra haitanensis (Bangiales, Rhodophyta), a species farmed in China. European Journal of Phycology. 2002;37:587–592. [Google Scholar]