Abstract
The presence and role of melatonin in plants are still under debate owing to difficulties of identification and quantification. Accordingly, although it has been frequently proposed that melatonin acts as an antioxidant in phototrophic organisms, experimental data on its physiological role are scarce. This study describes the use of a rapid and simple new method for quantification of melatonin in the marine macroalga Ulva sp., organisms routinely exposed to tide-related environmental stresses and known for their high tolerance to abiotic conditions. The method was used here to show that exposure to oxidative stress-inducing environmental conditions (elevated temperature and heavy metals) induced a rise in melatonin level in the algae. Addition of exogenous melatonin alleviated the algae from cadmium-induced stress. Interestingly, although the algae were taken from a culture growing free floating and kept under constant photoperiod and water level, they exhibited a semi-lunar rhythm of melatonin levels that correlated with predicted spring tides. The correlation can probably be interpreted as reflecting preparation for predicted low tides, when the algae are exposed to increasing temperature, desiccation, and salinity, all known to induce oxidative stress. Given the simplicity of the described method it can easily be adapted for the study of melatonin in many other phototrophic organisms. These results provide, for the first time, experimental data that support both an antioxidant role for melatonin and its semi-lunar rhythm in macroalgae.
Keywords: antioxidant, heavy metals, macroalgae, melatonin, oxidative stress, phytomelatonin, semi-lunar rhythms, superoxide dismutase (SOD), TLC
Introduction
Melatonin (N-acetyl-5-methoxytryptamine; MLT) is a naturally occurring compound belonging to the indoleamines. It is involved in many biological activities in animals, among them sleep, temperature homeostasis, inhibition of parasite infection, development of tumors, and antioxidative activity (Tan et al., 2002; Rahman et al., 2005; Garcia et al., 2006; Yang et al., 2007).
Interestingly, MLT is found not only in animals but also in eukaryotic unicells, plants, algae, and prokaryotes (Reiter et al., 2001; Köhidai et al., 2002; Hardeland and Poeggeler, 2003; Iriti et al., 2006; Paredes et al., 2009; Posmyk and Janas, 2009). Its identification in and isolation from plants and algal sources is complicated and usually requires the use of sophisticated instrumentation, such as liquid chromatography–mass spectroscopy/mass spectroscopy (LC-MS/MS) and gas chromatography/mass spectroscopy (GC/MS), owing to the presence of similar indoles, the auxins (Cao et al., 2006). In line with its role in animal physiology, MLT in plants and algae appears to participate in the regulation of photoperiodic and rhythmic phenomena such as flowering (Kolař et al., 1997). It has been suggested that an additional important characteristic of MLT in plants and algae might be its ability to scavenge free radicals (Poeggeler et al., 2002; Tan et al., 2002; Hardeland et al., 2006; Reiter et al., 2007a). However, there is little experimental support for its function as an antioxidant in plants (Arnao and Hernandez-Ruiz, 2009a, b) and none in microalgae (Arnao and Hernandez-Ruiz, 2007; Posmyk and Janas, 2009), although numerous studies have demonstrated that MLT can scavenge radicals, both directly or by up-regulating the activity of antioxidant enzymes such as superoxide dismutase (SOD), catalase, and glutathione peroxidase (Rodriguez et al., 2004; Reiter et al., 2007b). Furthermore, addition of external MLT enhances germination under chilling stress (Posmyk et al., 2008a), a known cause of oxidative stress (Prasad et al., 1995). Such studies suggested that MLT could act as a protectant against environmentally induced oxidative stress and might be beneficial for organisms facing such stress.
A multitude of marine organisms inhabit intertidal zones. Recognized as among the harshest environments on earth, such zones are characterized by rhythmic stresses of dehydration. Temperature, solar radiation, salinity, and osmotic pressure all rise and fall in a recurring rhythm as the water level changes (Denny and Wethey, 2001). These stresses are likely to be followed by oxidative stress (Kalir and Poljakoff, 1981; Wundram et al., 1996; Zue, 2000; Kakinuma et al., 2001; Abrahamsson et al., 2003), suggesting that organisms exposed to such conditions could benefit from high levels of MLT at low tide. In the present study, the protective role of MLT and the relationship between MLT level and sea level were examined in the green macroalga Ulva sp., which inhabits intertidal zones.
Ulva spp. are macroalgae that are common in intertidal coastal zone habitats worldwide (Einav, 2004), although some naturally free-floating populations have been described (Malta et al., 1999; Yabe et al., 2009). Studies employing HPLC and ELISA have shown that these macroalgae contain MLT (Toury, 2005; Pape and Luning, 2006). Here, the physiological role of MLT in an Ulva sp. was investigated by a novel quantitative method that was developed and utilized in this study. The method was based on separation of ethanolic Ulva extract by thin layer chromatography (TLC) followed by a fluorescence quantification method to measure the concentration of MLT in the algal material. This procedure was used here to study the influence of environmental stressors on MLT content and to assess the semi-lunar rhythm as a ‘zeitgeber’ or environmental signal for MLT content in the Ulva sp.
Materials and methods
All chemicals used were obtained from Sigma-Aldrich unless otherwise stated.
Algal material
Samples of Ulva were obtained from a culture growing free floating in an aquarium in the Israel Oceanographic and Limnological Research Center in Haifa, Israel. The cultures, which had originally been collected from rocky habitats on the eastern Mediterranean shore between Haifa and Atlit, were growing in circulating natural seawater under dimmed natural sunlight (50% shade net). The exact Ulva species was not determined, and indeed is not easy to determine (Hayden and Waaland, 2004; Loughnane et al., 2008), but the algae were from the Ulva (and not the Enteromorpha) morphological type (Hayden et al., 2003; Einav, 2004). The Ulva sp. used was probably not the previously studied Ulva lactuca, as this species is practically unknown in the Mediterranean Sea (Einav, 2004).
Algal samples were washed with distilled water to remove dirt and epiphytes. The samples were then placed in aquaria 80×40×50 cm and cultivated in commercial sea salt medium (dried sea salts, Aqua Medic Reef salt at a final salt concentration of 4% w/v). NH4Cl and NaH2PO4 were added (final concentrations 1 mM and 0.1 mM, respectively) to avoid nitrogen or phosphate limitation (Israel et al., 2006). Algae were kept at a constant temperature of 23 °C and under long-day photoperiod (16L:8D) with the light going on at 06:00. A 500-W halogen lamp was used as a light source. Water was constantly ventilated by aquarium air pumps and air stones. These algae were used as a stock for the experiments.
Induction of stress conditions
All experiments were carried out in triplicate. After 3 weeks cultivation algae were transferred to new aquaria and exposed to the following different potential stress inducers in illuminated growth chambers: temperature (23 °C, 30 °C, or 37 °C); heavy metals: cadmium as CdCl2 (Cd2+; final concentrations: high, 1 mg l−1; medium, 0.01 mg l−1; low, 0); lead as PbCl2 (Pb2+ final concentrations: high, 5 mg l−1; medium, 0.05 mg l−1; low, 0): zinc as ZnCl2 (Zn2+ final concentration: high, 30 mg l−1; medium, 0.3 mg l−1; low, 0). All experiments were carried out in 2-l tanks that were ventilated as described above. When noted, exogenous MLT supplement (50 mg l−1 each time) was added together with the heavy metal and every 2 d thereafter until the end of the experiment. Each experiment lasted 7–10 d. Samples for analysis of MLT, chlorophyll, and SOD were taken as described.
Extraction of melatonin
Algal material was washed with double-distilled water and dried on a paper towel. Washed samples were ground in liquid nitrogen and the resulting powder was transferred to 1.5-ml centrifuge tubes (130 mg), each containing acid-treated glass beads (150–212 μm; Sigma), and 1.3 ml absolute ethanol. The tubes were covered with aluminum foil to avoid light-induced damage, vortexed for 30 min at room temperature, and centrifuged (10 000 g for 10 min at room temperature). Without touching the pellet, aliquots of supernatant (1 ml) were transferred to new 1.5-ml tubes and placed open in a dry block (100 °C) until the solvent had completely evaporated (∼45 min). The dry extract was suspended in 100 μl of ethanol (analytical grade) and kept at –20 °C until used for TLC separation.
Thin-layer chromatography
The TLC method described by Costantini and Paoli (1998), with some modifications, was used for MLT separation. Briefly, the resuspended ethanol extracts were loaded onto 0.2 mm thick silica gel TLC plates (7×7 cm, Alugram SIL G/UV254) and the plates were developed with a mixture of dichloromethane/methanol (95:5 v/v). Plates were visualized under 254 nm light (UVItec, LF-104.S, 2×4W) and compared with a sample pre-spiked with MLT standard to compensate for the ‘matrix effect’, i.e. the effect of the other extractants on the MLT retention factor. Bands corresponding to the MLT standard retention factor were scraped off with a razor blade, transferred into a new 1.5-ml tube and suspended in 1 ml of ethanol (HPLC grade). The tubes were centrifuged briefly to precipitate silica and the supernatant taken for fluorescence quantification.
Quantification of MLT by fluorescence measurement
Samples separated on the TLC plate as described above were placed in a quartz cuvette for fluorescence measurement (Fluoromax 4 fluorometer; Horiba Jobin Yvon). Samples were subjected to excitation at 280 nm and emission was recorded at 335 nm (Prozialeck et al., 1978). A calibration curve was constructed using MLT standard solution. When TLC separation was poor, the MLT standard solution was added to raise the baseline (50 μl of 0.1 ng ml−1). This reading was then subtracted from the peak height. Such ‘standard addition’ is a common practice used to solve matrix effects (Saxberg and Kowalski, 1979).
Gas chromatography/mass spectroscopy
For validation of the TLC method, a TLC sample extracted as described above was analysed by GC/MS (Agilent Technologies 5975 GC/MSD) according to the protocol published by Aboul-Enein et al. (1999). N-methyl-N-trimethylsilyltrifluoroacetamide was used as a derivatizing agent, following the manufacturer's instructions (Sigma), with ethyl acetate as solvent. A similarly treated MLT standard solution was used for comparison of the results.
Chlorophyll quantification
Chlorophyll was quantified using the ethanol extract. Briefly, algal ethanol extract was diluted 10-fold with ethanol and absorbance at 665 and at 443 nm was measured (PRIM spectrophotometer; Secomam). Concentrations of chlorophyll (Chl) a and b were calculated using the equation [μg Chl a ml−1=(13.7)(A665)–(5.76)(A443); μg Chl b ml−1=(25.8)(A443)–(7.6)(A665)] (Knudson et al., 1977; Ritchie, 2006).
Protein extraction
Proteins were extracted from algal samples using the CelLytic P Cell Lysis Reagent. Briefly, powdered algae (100 mg, plus 200 ml extraction reagent in a 2-ml tube) were soaked in ice-cold water, homogenized three times (30 s each time) in a Polytron PT1200 (Kinematica), and centrifuged (10 000 g, 10 min, room temperature). The supernatant, containing soluble proteins, was frozen at –20 °C until required for the activity assay.
SOD activity assay
SOD activity was assayed from the protein extract using a commercial kit (SOD Assay Kit-WST; Sigma) and read with an ELISA reader (PowerWave XS; Biotek), according to the manufacturer's instructions.
Statistical analysis
Statistical analysis was carried out with SPSS. Normality tests were performed for each experiment. The influence of treatment on MLT levels, SOD activity, and chlorophyll levels was analysed by one-way ANOVA and differences between means were analysed by Student's t-test. Correlations were analysed by Pearson's correlation coefficient test.
Results
Melatonin separation and quantification
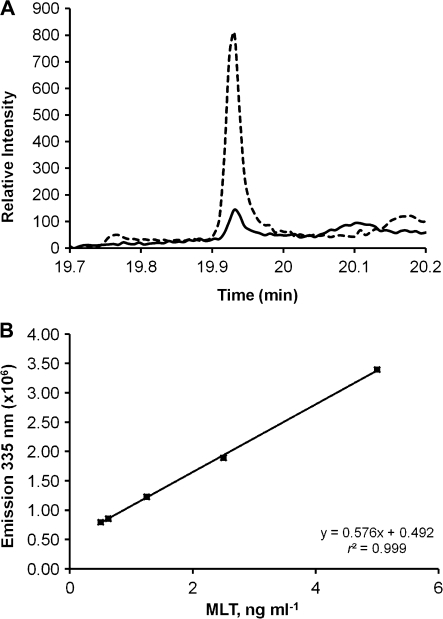
The methods used here for MLT extraction and separation were validated by comparing the GC/MS chromatograms and m/z spectra for Ulva sp. MLT with and without MLT standard spiking. Both samples yielded clear peaks with mean retention times of 19.92 min (Fig. 1A) and characteristic ions at m/z ratios of 73, 232, 245, and 304 (data not shown; Aboul-Enein et al., 1999). Quantification by fluorescence enabled detection of MLT down to 0.5 ng MLT ml−1 (Fig. 1B).
Fig. 1.
(A) GC/MS analysis of MLT standard sample (broken line) and of Ulva extract (continuous line) after separation by TLC. (B) MLT fluorescence calibration curve; excitation at 280 nm, emission at 335 nm.
Effect of temperature
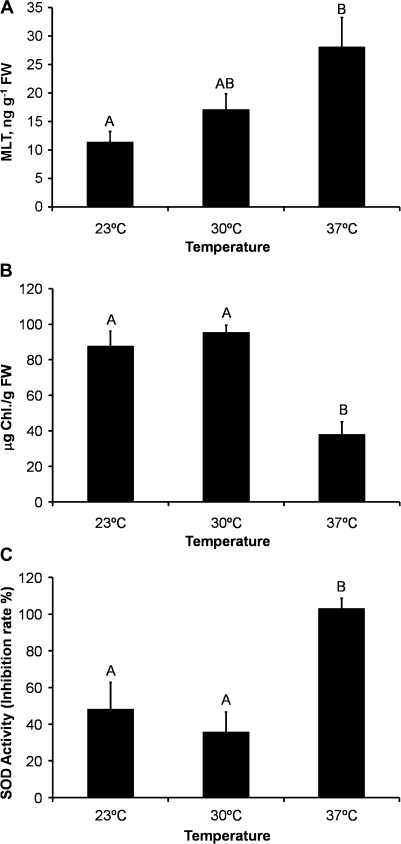
An increase in temperature had a significant positive effect on MLT levels [Pearson correlation: r=0.79, P<0.05; ANOVA: F(2, 6)=5.74, P<0.05] (Fig. 2A). Chlorophyll levels, on the other hand, remained constant at 23 °C and 30 °C but dropped dramatically at 37 °C [F(2, 6)=49.14, P<0.05; Fig. 2B]. SOD activity mirrored the chlorophyll content, showing a dramatic increase at 37 °C [F(2, 6)=10.65, P<0.05; Fig. 2C].
Fig. 2.
Effects of temperature on (A) MLT concentration in Ulva sp. tissue [r=0.79, P<0.05; F(2, 6)=5.74, P<0.05], (B) chlorophyll concentration [F(2, 6)=49.14, P<0.05], and (C) SOD enzymic activity [F(2, 6)=10.65, P<0.05]. All measurements were done after 10 d of treatment. FW, fresh weight. Data presented as mean of three measurements±standard error.
Effect of heavy metals and the protective effect of exogenous MLT
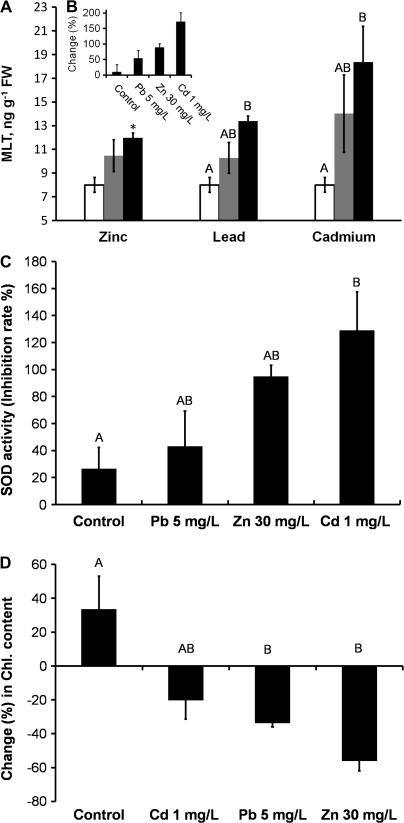
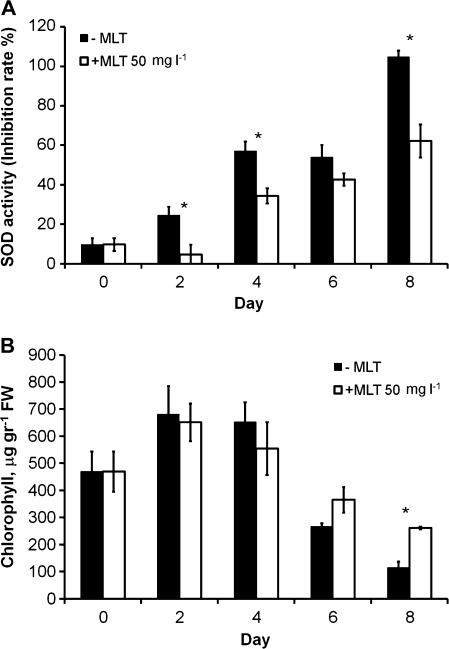
Maximal heavy metal concentrations were chosen according to Israeli governmental regulations for wastewater sea discharge and according to Markham et al. (1980). An increase in the dosage of heavy metals had a significant positive effect on MLT levels, with the effect especially marked in the case of cadmium [r=–0.69, P<0.05; F(2, 6)=9.3, P<0.05] (Fig. 3A). MLT levels reached 17 ng g−1 fresh weight (FW) when the algae were exposed to 1 mg ml−1 Cd, corresponding to an increase of 171% in MLT levels relative to levels at time zero (Fig. 3B). The effects induced by the other metals (Pb and Zn) were similar but smaller (Fig. 3A, B). SOD activity showed good correlation with MLT levels (Fig. 3C), while chlorophyll levels showed a reversed correlation, i.e. they dropped with a rise in metal concentration (Fig. 3D; see also Kumar et al., 2010). Interestingly, addition of exogenous MLT relieved some of the oxidative stress, as evidenced by a decrease in SOD activity (Fig. 4A) and an increase in chlorophyll levels (Fig. 4B). Somewhat analogous protection by MLT was demonstrated by Posmyk et al. (2008b) and by Tan et al. (2007a, b).
Fig. 3.
Effects of heavy metals on (A) MLT concentration after 8 d of treatment with different heavy metals: zinc (t4=3.2), lead [F(2, 6)=6.71, P<0.05], and cadmium [r=–0.69, P<0.05; F(2, 6)=9.3, P<0.05], at three different concentrations (empty bar, low; grey bar, medium; dark bar, high; see Materials and methods for concentrations). (B) Increase (as percent of zero time) in MLT concentration in the presence of high levels of zinc, lead, and cadmium as percentage above zero time (for simplicity only the highest concentrations are shown). (C) Increase (as percentage above zero time) in SOD enzymic activity [F(3, 8)=4.51, P<0.05] in the presence of zinc, lead, and cadmium (for simplicity only the highest concentrations are shown). (D) Change (as percentage above zero time) in chlorophyll content [F(3, 8)=7.94, P<0.05] in the presence of zinc, lead, and cadmium (for simplicity only the highest concentrations are shown). Data presented as mean of three measurements±standard error.
Fig. 4.
Protective effect of exogenous MLT (50 mg l−1 every 2 d) during 8 d of treatment with a high level of cadmium (1 mg l−1): (A) SOD activity (day 2, t4=5.22; day 4, t4=5.66; day 8, t4=8.22; P<0.05). (B) Chlorophyll content (day 8, t4 =–10.96; P<0.05). Data presented as mean of three measurements±standard error. Asterisks indicate statistically significant differences.
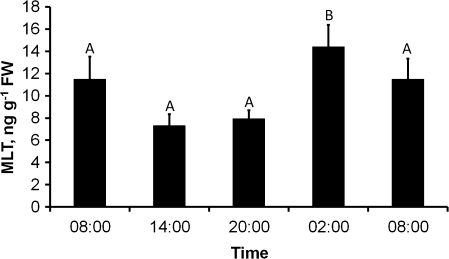
Light/dark cycle
When MLT levels were determined at different times during the light/dark cycle, they were found to be maximal at night and in the early morning (02:00 and 08:00 local time). Towards early afternoon (14:00) the levels dropped, rising again to a maximum the next night. These results demonstrated a significant influence of sampling time on MLT levels [F(3,24)=4.443, P<0.05] (Fig. 5).
Fig. 5.
Changes in Ulva MLT levels during a circadian long-photoperiod day (16L:8D) [F(3,24)=4.443, P<0.05].
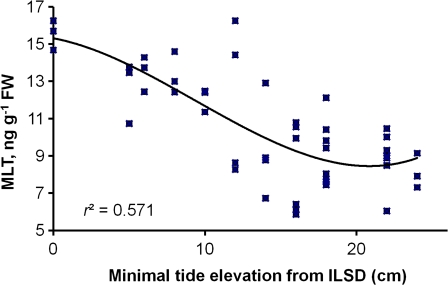
Semi-lunar rhythm of MLT levels
Over a period of months, small changes in MLT levels were observed. A plot of these results against tidal lunar changes revealed a strong correlation between MLT levels and a weekly record of minimal sea level, i.e. maximum MLT levels at spring tides (r=–0.66, P<0.01; Fig. 6).
Fig. 6.
Correlation between minimal sea level on the day before sampling and MLT levels (r=–0.66, P<0.01, n=49). Trend line is a third order polynomial. ILSD, Israel Land Survey Datum. (This figure is available in colour at JXB online.)
Discussion
Identification and quantification of MLT in plants and algae is a complicated task, and accordingly its physiological roles are an issue of ongoing research. Immunoassays, for example, tend to yield high rates of false-positive results owing to the existence of many other indoles, while various other methods require the use of sophisticated and expensive equipment such as LC-MS/MS or GC/MS.
The method developed in this study offers a reliable, simple, and rapid means of extraction and quantification of MLT from the green macroalga Ulva by a TLC and fluorescence-based procedure. The identity of MLT in the expected fractions was verified by GC/MS. The quantities of MLT obtained, as well as the reproducibility of the method, were in good agreement with published reports of phytomelatonin quantification (Murch et al., 1997; Harumi and Matsushima, 2000; Paredes et al., 2009; Posmyk and Janas, 2009). The new method was used here to quantify the levels of MLT in intertidal zone green macroalgae (Ulva sp.) under different environmental conditions.
Increase in SOD activity and decrease in chlorophyll content, both well-known indicators of oxidative stress (Lagriffoul et al., 1998; Sandalio et al., 2001; Parida and Das, 2005; Kumar et al., 2010), were used to measure oxidative stress in this study.
Many organisms that inhabit intertidal zones are inevitably exposed to a combination of oxidative stress initiators (e.g. high radiation, salinity, and desiccation). Consequently, Ulva spp. are known to adapt to a wide range of abiotic oxidative stress conditions, including high temperature (these organisms can reportedly sustain temperatures from –5 °C to 40 °C; see Kamermans et al., 1998 and Einav, 2004), low water content (Einav, 2004), high salinity (Einav, 2004), and the presence of heavy metals (Markham et al., 1980; Villares et al., 2001), but to date no data have been published as to the mechanism underlying this capability. This study demonstrated that MLT probably participates in this adaptation, and showed that recognized sources of oxidative stress such as high temperatures (Abrahamsson et al., 2003) and heavy metals (Lagriffoul et al., 1998; Schützendübel and Polle, 2002) cause a rise in MLT levels, and that exogenous MLT can relieve cadmium stress. MLT levels increased as temperatures rose from 23 °C to 37 °C (P<0.05) in good agreement with the known optimal photosynthesis and growth temperature for Ulva spp. (25–30 °C; Kakinuma et al., 2001; Einav and Israel, 2007). Interestingly, SOD activity increased and chlorophyll content dropped only at the highest temperature (37 °C) with very little change between 23 °C and 30 °C, suggesting that MLT provides a major, and maybe even the primary, line of defence in Ulva, probably thanks to the low costs of producing a small molecule and the MLT high radical-scavenging efficiency.
MLT levels reportedly follow a circadian rhythm in Chenopodium rubrum (Kolař et al., 1997) and in Eichhornia crassipes (Tan et al., 2007b). MLT levels in our Ulva sample during daylight hours were lower than in the dark. This could be due to a circadian rhythm-related enzymic activation during the night as demonstrated in higher organisms, MLT disassembly pursuant to exposure to light (Andrisano et al., 1999), or increased metabolic activity during the night caused by increased rates of growth and mitotic division (Titlyanov et al., 1996; Kalita et al., 2007; also see Britz, 1976 and Britz and Briggs, 1976 for other circadian behaviour in Ulva). Interestingly, although the Ulva culture used in this study was growing free floating, it was possible to demonstrate a semi-lunar rhythm in its MLT levels. This rhythmic character fitted well with the intertidal zone habitat of the Ulva spp. At the intertidal zone sea level reach a global minimum in a reciprocal 2-week cycle when the earth, moon, and sun are approximately aligned, and the tidal effects of the sun and the moon reinforce one another, resulting in spring tide. At these times radiation, desiccation, salinity, and temperature rise to a maximum leading to an increase in oxidative stress. Given the rhythmicity of this stress, it would be beneficial for a tidal zone organism such as Ulva to increase and decrease antioxidant mechanisms in advance. This study indeed showed that MLT levels follow a semi-lunar rhythm. As clearly demonstrated in the results, there was a significant negative correlation between sea levels (spring or neap tide) and MLT levels that preceded the stress-inducing changes (Fig. 6), even though the algae showing this rhythmic behaviour were not exposed to actual changes in day length or water level at the time of the experiments.
That Ulva sp. harbour semi-lunar rhythms is not a new discovery. In 1947 Gilbert Smith reported a semi-lunar rhythm when Ulva spp. release their gametes (Smith, 1947). Similar rhythms have been described in other tidal-zone organisms (Berry, 1986; Palmer, 2000; Naylor, 2001; Togashi and Cox, 2001). The correlation between MLT level and sea level might also be related to reproductive pathways, as seen in many organisms including mammals (Balik et al., 2004), birds (Ubuka et al., 2005), and plants (Paredes et al., 2009).
In summary, this study resulted in the first experimental demonstration of both the antioxidant role of MLT and its semi-lunar rhythm in macroalgae. The findings suggest that MLT might be a major antioxidant in many intertidal organisms.
Acknowledgments
We thank Professor Ido Izthaki for consultations on statistical analysis, the Israel Oceanographic and Limnological Research Institute for the algal material, and Ms Ailie Marx for her helpful comments.
Glossary
Abbreviations
- GC/MS
gas chromatography/mass spectroscopy
- LC-MS/MS
liquid chromatography–mass spectroscopy/mass spectroscopy
- MLT
melatonin N-acetyl-5-methoxytryptamine
- SOD
superoxide dismutase
- TLC
thin layer chromatography
References
- Aboul-Enein HY, Doneanu C, Covaci A. Capillary GC/MS determination of melatonin in several pharmaceutical tablet formulations. Biomedical Chromatography. 1999;13:24–26. doi: 10.1002/(SICI)1099-0801(199902)13:1<24::AID-BMC765>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Abrahamsson K, Choo KS, Pedersén M, Johansson G, Snoeijs P. Effects of temperature on the production of hydrogen peroxide and volatile halocarbons by brackish-water algae. Phytochemistry. 2003;64:725–734. doi: 10.1016/s0031-9422(03)00419-9. [DOI] [PubMed] [Google Scholar]
- Andrisano V, Bertucci C, Battaglia A, Cavrini V. Photostability of drugs: photodegradation of melatonin and its determination in commercial formulations. Journal of Pharmaceutical and Biomedical Analysis. 1999;23:15–23. doi: 10.1016/s0731-7085(00)00259-4. [DOI] [PubMed] [Google Scholar]
- Arneo MB, Hernández-Ruiz J. Melatonin in plants: more studies are necessary. Plant Signaling and Behavior. 2007;2:381–382. doi: 10.4161/psb.2.5.4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arneo MB, Hernández-Ruiz J. Chemical stress by different agents affects the melatonin content of barley roots. Journal of Pineal Research. 2009a;46:295–299. doi: 10.1111/j.1600-079X.2008.00660.x. [DOI] [PubMed] [Google Scholar]
- Arneo MB, Hernández-Ruiz J. Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. Journal of Pineal Research. 2009b;46:58–63. doi: 10.1111/j.1600-079X.2008.00625.x. [DOI] [PubMed] [Google Scholar]
- Balík A, Kretschmannová K, Mazna P, Svobodová I, Zemková H. Melatonin action in neonatal gonadotrophs. Physiological Research. 53. Suppl. 2004;1:S153–S166. [PubMed] [Google Scholar]
- Berry AJ. Semi-lunar and lunar spawning periodicity in some tropical littorinid gastropods. Journal of Molluscan Studies. 1986;52:144–149. [Google Scholar]
- Britz SJ. Automatic monitoring of a circadian rhythm of change in light transmittance in Ulva. Plant Physiology. 1976;58:17–21. doi: 10.1104/pp.58.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britz SJ, Briggs WR. Circadian rhythms of chloroplast orientation and photosynthetic capacity in Ulva. Plant Physiology. 1976;58:22–27. doi: 10.1104/pp.58.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Murch SJ, O'Brien R, Saxena PK. Rapid method for accurate analysis of melatonin, serotonin and auxin in plant samples using liquid chromatography–tandem mass spectrometry. Journal of Chromatography A. 2006;1134:333–337. doi: 10.1016/j.chroma.2006.09.079. [DOI] [PubMed] [Google Scholar]
- Costantini A, Paoli F. Melatonin: quantitative analysis in pharmaceutical oral dosage forms using thin-layer chromatography (TLC) densitometry. Il Farmaco. 1998;53:443–447. [Google Scholar]
- Denny M, Wethey D. Physical processes that generate patterns in marine communities. In: Bertness MD, Gaines SD, Hay ME, editors. Marine community ecology. Sunderland: Sinauer Associates; 2001. pp. 3–37. [Google Scholar]
- Einav R. Israel: Bar Ilan University Academic Press Hebrew; 2004. Seaweeds of Eastern Mediterrane an coast. [Google Scholar]
- Einav R, Israel A. Seaweeds on the abrasion platforms of the intertidal zone of eastern Mediterranean shores. In: Seckbach J, editor. Algae and cyanobacteria in extreme environments. Cellular origin, life in extreme habitats and astrobiology series. Vol. 11. New York: Springer; 2007. pp. 193–207. [Google Scholar]
- Garcia-Santos G, Antolin I, Herrera F, Martin V, Rodriguez-Blanco J, del Pilar Carrera M, Rodriguez C. Melatonin induces apoptosis in human neuroblastoma cancer cells. Journal of Pineal Research. 2006;41:130–135. doi: 10.1111/j.1600-079X.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- Hardeland R, Pandi-Perumal SR, Cardinali DP. Molecules in focus: melatonin. International Journal of Biochemistry and Cell Biology. 2006;38:313–316. doi: 10.1016/j.biocel.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Hardeland R, Poeggeler B. Non-vertebrate melatonin. Journal of Pineal Research. 2003;34:233–241. doi: 10.1034/j.1600-079x.2003.00040.x. [DOI] [PubMed] [Google Scholar]
- Harumi T, Matsushima S. Separation and assay methods for melatonin and its precursors. Journal of Chromatography B. 2000;747:95–110. doi: 10.1016/s0378-4347(00)00064-5. [DOI] [PubMed] [Google Scholar]
- Hayden HS, Blomster J, Maggs C, Silva P, Stanhope M, Waaland RJ. Linnaeus was right all along: Ulva and Enteromorpha are not distinct genera. European Journal of Phycology. 2003;38:277–294. [Google Scholar]
- Hayden HS, Waaland RJ. A molecular systematic study of Ulva (Ulvaceae, Ulvales) from the northern Pacific. Phycologia. 2004;43:364–382. [Google Scholar]
- Iriti M, Rossoni M, Faoro F. Melatonin content in grape: myth or panacea? Journal of the Science of Food and Agriculture. 2006;86:1432–1438. [Google Scholar]
- Israel A, Levy I, Friedlander M. Experimental tank cultivation of Porphyra in Israel. Journal of Applied Phycology. 2006;18:235–240. [Google Scholar]
- Kakinuma M, Shibahara N, Ikeda H, Maegawa M, Amano H. Thermal stress responses of a sterile mutant of Ulva pertusa(Chlorophyta) Fisheries Science. 2001;67:287–294. [Google Scholar]
- Kalir A, Poljakoff A. Changes in activity of malate dehydrogenase, catalase, peroxidase and superoxide dismutase in leaves of Halimione portulacoides (L.) Aellen exposed to high sodium chloride concentrations. Annals of Botany. 1981;47:75–85. [Google Scholar]
- Kalita TL, Titlyanova TV, Titlyanov EA. New rhythmic changes in mitosis and growth in low differentiated green and red marine macroalgae. Russian Journal of Marine Biology. 2007;33:207–212. [Google Scholar]
- Kamermans P, Malta E, Verschuure JM, Lentz FL, Schrijvers L. Role of cold resistance and burial for winter survival and spring initiation of an Ulva spp. (Chlorophyta) bloom in a eutrophic lagoon (Veerse Meer lagoon, The Netherlands) Marine Biology. 1998;131:45–51. [Google Scholar]
- Knudson LL, Tibbitts TW, Edwards GE. Measurement of ozone injury by determination of leaf chlorophyll concentration. Plant Physiology. 1977;60:606–608. doi: 10.1104/pp.60.4.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhidai L, Vakkuri O, Keresztesi M, Leppäluoto J, Csaba G. Melatonin in the unicellular Tetrahymena pyriformis: effects of different lighting conditions. Cell Biochemistry and Function. 2002;20:269–272. doi: 10.1002/cbf.973. [DOI] [PubMed] [Google Scholar]
- Kolař J, Machačkova I, Eder J, Prinsen E, Dongen WV, Onckelen HV, Illnerova H. Melatonin occurrence and daily rhythm in Chenopodium rubrum. Phytochemistry. 1997;44:1407–1413. [Google Scholar]
- Kumar M, Kumari P, Gupta V, Anisha PA, Reddy CR, Jha B. Differential responses to cadmium induced oxidative stress in marine macroalga Ulva lactuca (Ulvales, Chlorophyta) Biometals. 2010;23:315–325. doi: 10.1007/s10534-010-9290-8. [DOI] [PubMed] [Google Scholar]
- Lagriffoul A, Mocquot B, Mench M, Vangronsveld J. Cadmium toxicity effects on growth, mineral and chlorophyll contents and activities of stress related enzymes in young maize plants (Zea mays L.) Plant and Soil. 1998;200:241–250. [Google Scholar]
- Loughnane CJ, McIvor LM, Rindi F, Stengel DB, Guiry MD. Morphology, rbcL phylogeny and distribution of distromatic Ulva (Ulvophyceae, Chlorophyta) in Ireland and southern Britain. Phycologia. 2008;47:416–429. [Google Scholar]
- Malta E-J, Drausma SGA, Kamermans P. Free-floating Ulva in the southwest Netherlands: species or morphotypes? A morphological, molecular and ecological comparison. European Journal of Phycology. 1999;34:443–454. [Google Scholar]
- Manchester LC, Tan DX, Reiter RJ, Park W, Monis K, Qi W. High levels of melatonin in the seeds of edible plants: possible function in germ tissue protection. Life Science. 2000;67:3023–3029. doi: 10.1016/s0024-3205(00)00896-1. [DOI] [PubMed] [Google Scholar]
- Markham JW, Kremer BP, Sperling K-R. Cadmium effects on growth and physiology of Ulva lactuca. Helgoland Marine Research. 1980;33:103–110. [Google Scholar]
- Murch SJ, Simmons CB, Saxena PK. Melatonin in Feverfew and other medicinal plants. Lancet. 1997;350:1598–1599. doi: 10.1016/S0140-6736(05)64014-7. [DOI] [PubMed] [Google Scholar]
- Naylor E. Marine animal behavior in relation to lunar phase. Earth Moon Planets. 2001;85–86:291–302. [Google Scholar]
- Palmer JD. The clocks controlling the tide-associated rhythms of intertidal animals. BioEssays. 2000;22:32–37. doi: 10.1002/(SICI)1521-1878(200001)22:1<32::AID-BIES7>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Pape C, Luning K. Quantification of melatonin in phototrophic organisms. Journal of Pineal Research. 2006;41:157–165. doi: 10.1111/j.1600-079X.2006.00348.x. [DOI] [PubMed] [Google Scholar]
- Paredes SD, Korkmaz A, Manchester LC, Tan DX, Reiter RJ. Phytomelatonin: a review. Journal of Experimental Botany. 2009;60:57–69. doi: 10.1093/jxb/ern284. [DOI] [PubMed] [Google Scholar]
- Parida AK, Das AB. Salt tolerance and salinity effects on plants: a review. Ecotoxicology and Environmental Safety. 2005;60:324–349. doi: 10.1016/j.ecoenv.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Poeggeler B, Thuermann S, Dose A, Schoenke M, Burkhardt S, Hardeland R. Melatonin's unique radical scavenging properties – roles of its functional substituents as revealed by a comparison with its structural analogs. Journal of Pineal Research. 2002;33:20–30. doi: 10.1034/j.1600-079x.2002.01873.x. [DOI] [PubMed] [Google Scholar]
- Posmyk MM, Bałabusta M, Wieczorek M, Sliwinska E, Janas KM. Melatonin applied to cucumber (Cucumis sativus L.) seeds improves germination during chilling stress. Journal of Pineal Research. 2008a;46:214–223. doi: 10.1111/j.1600-079X.2008.00652.x. [DOI] [PubMed] [Google Scholar]
- Posmyk MM, Janas KM. Melatonin in plants. Acta Physiologiae Plantarum. 2009;31:1–11. [Google Scholar]
- Posmyk MM, Kuran H, Marciniak K, Janas KM. Presowing seed treatment with melatonin protects red cabbage seedlings against toxic copper ion concentrations. Journal of Pineal Research. 2008b;45:24–31. doi: 10.1111/j.1600-079X.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- Prasad TK, Anderson MD, Martin BA, Stewart CR. Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. The Plant Cell. 1995;6:65–74. doi: 10.1105/tpc.6.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prozialeck WC, Boehme DH, Vogel WH. The fluorometric determination of 5-methoxytryptamine in mammalian tissues and fluids. Journal of Neurochemistry. 1978;30:1471–1477. doi: 10.1111/j.1471-4159.1978.tb10480.x. [DOI] [PubMed] [Google Scholar]
- Rahman MA, Azuma Y, Fukunaga H, Murakami T, Sugi K, Fukushi H, Miura K, Suzuki H, Shirai M. Serotonin and melatonin, neurohormones for homeostasis, as novel inhibitors of infections by the intracellular parasite Chlamydia. Journal of Antimicrobial Chemotherapy. 2005;56:861–868. doi: 10.1093/jac/dki331. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Burkhardt S, Manchester LC. Melatonin in plants. Nutrition Reviews. 2001;59:286–90. doi: 10.1111/j.1753-4887.2001.tb07018.x. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Manchester LC, Simopoulos AP, Maldonadoa MD, Flores LJ, Terrona MP. Melatonin in edible plants (phytomelatonin): identification, concentrations, bioavailability and proposed functions. World Review of Nutrition and Dietetics. 2007a;97:211–230. doi: 10.1159/000097917. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Terron MP, Flores LJ, Czarnocki Z. Melatonin and its metabolites: new findings regarding their production and their radical scavenging actions. Acta Biochimica Polonica. 2007b;54:1–9. [PubMed] [Google Scholar]
- Ritchie RJ. Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynthesis Research. 2006;89:27–41. doi: 10.1007/s11120-006-9065-9. [DOI] [PubMed] [Google Scholar]
- Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, Reiter RJ. Regulation of antioxidant enzymes: a significant role for melatonin. Journal of Pineal Research. 2004;36:1–9. doi: 10.1046/j.1600-079x.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- Sandalio LM, Dalurzo HC, Gómez M, Romero-Puertas MC, del Río LA. Cadmium-induced changes in the growth and oxidative metabolism of pea plants. Journal of Experimental Botany. 2001;52:2115–2126. doi: 10.1093/jexbot/52.364.2115. [DOI] [PubMed] [Google Scholar]
- Saxberg BEH, Kowalski BR. Generalized standard addition method. Analytical Chemistry. 1979;51:1031–1038. [Google Scholar]
- Schützendübel A, Polle A. Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. Journal of Experimental Botany. 2002;53:1351–1365. [PubMed] [Google Scholar]
- Smith GM. On the reproduction of some pacific coast species of Ulva. American Journal of Botany. 1947;34:80–87. [PubMed] [Google Scholar]
- Tan DX, Manchester LC, Halton P, Reiter RJ. doi: 10.4161/psb.2.6.4639. 2007a. Phytoremediative capacity of plants enriched with melatonin. Plant Signaling and Behavior 2, 514–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan DX, Manchester LC, Mascio P, Martinez GR, Prado FM, Reiter RJ. doi: 10.1096/fj.06-7745com. 2007b. Novel rhythms of N1-acetyl-N2-formyl-5-methoxykynuramine and its precursor melatonin in water hyacinth: importance for phytoremediation. Federation of European Microbiological Societies Journal 21, 1724–1729. [DOI] [PubMed] [Google Scholar]
- Tan DX, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, Sainz RM, Mayo JC, Kohen R, Allegra M, Hardeland R. Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Current Topics in Medicinal Chemistry. 2002;2:181–197. doi: 10.2174/1568026023394443. [DOI] [PubMed] [Google Scholar]
- Titlyanov EA, Titlyanova TV, Luning K. Diurnal and circadian periodicity of mitosis and growth in marine macroalgae. II. The green alga Ulva pseudocurvata. European Journal of Phycology. 1996;31:181–188. [Google Scholar]
- Togashi T, Cox PA. Tidal-linked synchrony of gamete release in the marine green alga, Monostroma angicava Kjellman. Journal of Experimental Marine Biology and Ecology. 2001;264:117–131. [Google Scholar]
- Toury N. MSc Thesis. University of Haifa; 2005. Seasonal and daily changes of melatonin levels in plants – does it play the role of an antioxidant? Israel. [Google Scholar]
- Ubuka T, Bentley GE, Ukena K, Wingfield JC, Tsutsui K. Melatonin induces the expression of gonadotropin-inhibitory hormone in the avian brain. Proceedings of the National Academy of Science, USA. 2005;102:3052–3057. doi: 10.1073/pnas.0403840102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villares R, Puente X, Carballeira A. Ulva and Enteromorpha as indicators of heavy metal pollution. Hydrobiologia. 2001;462:221–232. [Google Scholar]
- Wundram M, Selmara D, Bahadirb M. The Chlamydomonas test: a new phytotoxicity test based on the inhibition of algal photosynthesis enables the assessment of hazardous leachates from waste disposals in salt mines. Chemosphere. 1996;32:1623–1631. [Google Scholar]
- Yabe T, Ishii Y, Amano Y, Koga T, Hayashi S, Nohara S, Tatsumoto H. Green tide formed by free-floating Ulva spp. at Yatsu tidal flat, Japan. Limnology. 2009;10:239–245. [Google Scholar]
- Yang QH, Xu JN, Xu RK, Pang SF. Antiproliferative effects of melatonin on the growth of rat pituitary prolactin-secreting tumor cells in vitro. Journal of Pineal Research. 2007;42:172–179. doi: 10.1111/j.1600-079X.2006.00403.x. [DOI] [PubMed] [Google Scholar]
- Zhu JK. Salt and drought stress signal transduction in plants. Annual Review of Plant Biology. 2000;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]