Abstract
Stable isotope signatures of Zn have shown great promise in elucidating changes in uptake and translocation mechanisms of this metal in plants during environmental changes. Here this potential was tested by investigating the effect of high Zn concentrations on the isotopic fractionation patterns of Phragmites australis (Cav.) Trin. ex Steud. Plants were grown for 40 d in a nutritive solution containing 3.2 μM (sufficient) or 2 mM (toxic) Zn. The Zn isotopic composition of roots, rhizomes, shoots, and leaves was analysed. Stems and leaves were sampled at different heights to evaluate the effect of long-distance transport on Zn fractionation. During Zn sufficiency, roots, rhizomes, and shoots were isotopically heavy (δ66ZnJMC Lyon=0.2‰) while the youngest leaves were isotopically light (–0.5‰). During Zn excess, roots were still isotopically heavier (δ66Zn=0.5‰) and the rest of the plant was isotopically light (up to –0.5‰). The enrichment of heavy isotopes at the roots was attributed to Zn uptake mediated by transporter proteins under Zn-sufficient conditions and to chelation and compartmentation in Zn excess. The isotopically lighter Zn in shoots and leaves is consistent with long-distance root to shoot transport. The tolerance response of P. australis increased the range of Zn fractionation within the plant and with respect to the environment.
Keywords: Isotope fractionation, MC-ICP-MS, metallomics, metals, nutrition, Phragmites australis, reed
Introduction
Increasing Zn environmental pollution has originated from several anthropogenic sources (Popovic et al., 2001; Konstantinou and Albanis, 2004; Mathur et al., 2005; Pruvot et al., 2006; Kong and White, 2010). Zn is a micronutrient essential for plants at trace levels, but high concentrations can be toxic (Marschner, 1995). Toxicity symptoms in plants include stunting, chlorosis, induced Fe deficiency, leaf folding, and stem splitting (Rosen et al., 1978; Davis and Parker, 1993).
In spite of the increasing concern about Zn pollution, the mechanisms of Zn uptake, transport, and tolerance remain poorly understood. In this scenario, a multicollector inductively coupled plasma mass spectrometer (MC-ICP-MS) appears to be a valuable tool to explore plant metallomics (von Blanckenburg et al., 2009). Plants discriminate the stable isotopes of a variety of elements, i.e. C, N, O, and S, a capacity that has been widely utilized to investigate the physiology and responses of plants to the environment (Monaghan et al., 1999; Yun and Ro, 2008; Cabrera-Bosquet, et al., 2009). MC-ICP-MS has allowed the research on stable isotopes to be extended to heavier elements such as Zn, opening up a field of new possibilities. The study of the isotopic fractionation of essential elements such as Cu, Fe, and Zn can make a substantial contribution to developing plant metallomics, by helping to unravel the mechanisms of uptake, distribution, and compartmentation of metabolically relevant metals.
Zn has four stable isotopes, 64Zn, 66Zn, 67Zn, and 68Zn. Their average relative abundances in naturally occurring Zn are 48.98, 27.81, 4.11, and 18.57%, respectively (Rosman and Taylor, 1998). Processes at equilibrium, such as adsorption to a surface or the formation of covalent bounds, favour the accumulation of the heavier isotopes in the reaction product, whereas kinetic processes such as diffusion-mediated transport discriminate against the heavy isotope (Criss, 1999; Rodushkin et al., 2004). Weiss et al. (2005) performed the first analyses of Zn isotopes in plants, and found that shoots were isotopically lighter with respect to roots, and roots isotopically heavier with respect to solution. They attributed these effects to root to shoot passive transport, cell wall binding of heavy Zn, or preferential diffusion of light Zn into root cells. Gélabert et al. (2006) reported the enrichment in heavy isotopes of Zn adsorbed to diatoms with respect to solution. John et al. (2007) showed that this was removed by washing the Zn adsorbed onto the diatom surface, and that desorbed cells were impoverished in 66Zn. The magnitude of fractionation changed with increasing Zn supply from –0.2‰ to –0.8‰, corresponding to the switch from high- to low-affinity Zn transport into the cell. Viers et al. (2007) studied several plant species in a pristine watershed, and found a significant fractionation between species and between plant organs of the same species, which they ascribed to root uptake from soil and translocation within the plants. The leaves of the tallest species had the most negative isotopic signatures, and they hypothesized a correlation between the length of the plants and the extent of Zn fractionation. This was confirmed by Moynier et al. (2009), who described lower δ66Znleaves in bamboo than in lentils. Bamboo leaves were also enriched in light isotopes as a function of the distance from the root. Finally, Arnold et al. (2010a) found that rice shoots were isotopically heavier in Zn deficiency, due to Zn uptake mediated by phytosiderophores.
These findings suggest that isotopes can be used: (i) to detect physiological responses to environmental changes (i.e. different amounts of available Zn) and (ii) to identify potential changes in uptake or transport mechanisms. However, current research on plants is focused on Zn isotopic discrimination under normal or Zn-deficient conditions. The use of isotopes is still needed to recognize the activation of tolerance mechanisms in response to high levels of Zn, for example extrusion, sequestration by metal-binding compounds, or subcellular compartmentation. The aim of this study was to demonstrate that the physiological mechanisms of response to toxic levels of Zn are able to discriminate between Zn isotopes.
Phragmites australis (Cav.) Trin. ex Steud. was chosen as model plant because it is tolerant to toxic Zn concentrations, and responds by accumulating excess Zn mainly in the roots and restricting its uptake and transport to the shoots (Weis and Weis, 2004). The specific objectives of this research were (i) to test whether the exposure to toxic Zn levels causes any alteration in the Zn fractionation pattern of P. australis; (ii) to check the hypothesis proposed by Moynier et al. (2009) and Viers et al. (2007) that there is a correlation between the height of leaves and the Zn isotopic fractionation; and (iii) to examine the usefulness of the technique to study the physiology of Zn toxicity.
Materials and methods
Plant material
Phragmites australis (Cav.) Trin. ex Steud plants were purchased from a local nursery (Bioriza, Breda, Spain). Plants were root-washed in tap water to remove the original peat–vermiculite substrate, weighed, and placed in a pure hydroponics system in individual pots. The nutritive solution comprised: 130.25 mg l−1 NO3–, 5.5 mg l−1 NH4+, 28.5 mg l−1 PO42–, 35.5 mg l−1 K+, 24.5 mg l−1 Ca2+, 4 mg l−1 Mg2+, 14.25 mg l−1 SO42–, 0.325 mg l−1 Fe, 0.240 mg l−1 Mn, 0.09 mg l−1 Zn, 0.030 mg l−1 B, 0.090 mg l−1 Cu, 0.028 mg l−1 Mo, and 0.005 mg l−1 Co. The pH was adjusted to 6.5. Plants were allowed to acclimate to hydroponics for 27 d, until they recovered a vigorous growth, and then were selected within a small range of fresh weight of 161.2±5.0 g (FW±SE; n=16). There were two Zn treatments: control (3.2 μM Zn), where plants were grown in the same nutritive solution as during acclimation, and Zn+ (2 mM Zn), where the nutritive solution was amended with ZnSO4·7H2O (Sigma-Aldrich, 99% ACS reagent) to reach the desired concentration. Eight plants per treatment were randomly distributed and grown under glasshouse conditions for 40 d (29 April to 13 July 2009). Previous research proved that this time span allows for enough Zn accumulation and fractionation (Weiss et al., 2005). The temperature was 23.1±0.3 °C (mean±SE), the relative humidity 53.6±1.3%, and the transmission of the greenhouse covers 51%. Nutritive solution was renewed every 3–4 d and deionized water was added daily to compensate the loss due to evaporation and transpiration.
Plants were then thoroughly washed in tap water, bathed for 30 min in ice-cold 1 mM LaCl3 and 0.05 mM CaCl2 to remove adsorbed and apoplastically bound Zn (following Weiss et al., 2005), and rinsed in deionized water. The isotopic composition of absorbed Zn depends on the physicochemical characteristics of the solution and the adsorbent surface rather than on biologically regulated processes (Gélabert et al., 2006), and will not be considered in this study. The isotopic fractionation of Zn adsorbed on iron oxides or onto biological surfaces leads to the enrichment of the heavy isotopes (Pokrovsky et al., 2005; Gélabert et al., 2006; John et al., 2007). This approach was selected to allow for the comparison between studies, even with species that show no metal plaques.
Plant height was recorded and eight samples were collected from each plant: living roots (LR), dead roots (DR), rhizomes (RZ), low shoots (LS), low leaves (LL), high shoot (HS), high leaves (HL), and youngest leaves (YL). Stems were collected at distances from the root: between 5 cm and 12 cm for the low shoots and between 20 cm and 27 cm for the high shoots. Leaves growing at these two different height intervals were named low and high leaves, respectively. The last three leaves of each stem were labelled as the youngest leaves. Pairs of plants were pooled together. Fresh samples were oven-dried at 60 °C until constant weight, and ground with a ball mill.
Photosynthetic performance
The chlorophyll content and fluorescence and the gas exchange of leaves was measured 1–2 d before the end of the experiment. Chlorophyll content on a leaf area basis was obtained using a portable chlorophyll meter (SPAD-502 Minolta, Illinois, USA), following Krugh et al. (1994). This device provides an indexed relative chlorophyll content (IRCC) ranging from 0 to 99.9. Always the third fully developed leaves at 2.5 cm from the leaf base were measured on five representative pre-bloom leaves per plant.
Photosynthetic gas exchange and chlorophyll fluorescence were determined in the third last fully expanded leaf of each plant using a LI-COR 6400 Portable Photosynthesis System (LI-COR Inc., Lincoln, NE, USA), with a saturating light (photosynthetic photon flux density of 1200 μmol photons m−2 s−1), 400 μmol mol−1 of CO2, and an air temperature of 25.9±0.1 °C. Leaves were previously dark adapted for 30 min to measure the maximum quantum yield (Fv/Fm). The same leaves were then re-acclimated to environmental light to determine the relative quantum yield (Fv'/Fm'), quantum yield of photosystem II photochemistry (ΦPSII) (Genty et al., 1989), quantum yield of CO2 fixation (ΦCO2), electron transport rate (ETR, μmol m−2s−1), photochemical (qP) and non-photochemical quenching (qN, NPQ), light-saturated net CO2 assimilation rate (As, μmol CO2 m−2 s−1), stomatal conductance to water (gs, mol H2O m−2 s−1), intercellular CO2 concentration (Ci, μmol CO2 mol air−1), and transpiration rate (E, mmol H2O m−2 s−1).
Zn content
Plant samples were digested in two steps, first overnight at 90 °C in HNO3:H2O2 (1:1 v/v), then 0.5 ml of hydrofluoric acid was added and they were digested for 2 h at 90 °C. Digests were evaporated to dryness on a hotplate at 120 °C and the residues were re-dissolved in 3 ml of 7 M HCl. Each solution was split into three aliquots: 1 ml for Zn concentration measurements, 1 ml for Zn isotope analysis, and 1 ml for archive. The first aliquot was made up to 3.5 ml of 1 M HCl prior to concentration measurements on a Varian VISTA PRO (Palo Alto, CA, USA) ICP-AES (inductively coupled plasma atomic emission spectrometer), for which analytical errors were 0.4–5% of the measured values. Per each batch of 12 samples, a blank and a sample of either olive leaves (Olea europaea L., BCR-62), an aquatic plant (Lagarosiphon major [Ridl.] Moss, BCR-60), light sandy soil (BCR-142R), or lichen (BCR-482) certified reference material from the Community Bureau of Reference (BCR®) were processed in the same way and analysed (Table 1). Digestions were carried out in the clean laboratory of the Department of Earth Science and Engineering (Imperial College of London). Zn content determination was performed in the research facilities of the Natural History Museum (London, UK).
Table 1.
Zinc content of the standards used in ICP-AES analyses
| Sample type | Zn content (μg g−1) |
% Recovery | ||
| Certified | Measured | |||
| BCR-142R | Light sandy soil | 93±3 | 91±14 | 98 |
| BCR-482 | Lichen | 101±2 | 91±9 | 91 |
| BCR-60 | Lagarosiphon major (Ridl.) Moss | 313±8 | 309±13 | 98 |
| BCR-62 | Olea europaea L. | 16.0±0.7 | 13±3 | 82 |
Data are represented as means ±SE.
The bioconcentration factor for Zn (BCF) was calculated following Ali et al. (2004):
| (1) |
where [Znp] is the Zn concentration of the plant sample [μg g−1 dry weight (DW)] and [Zns] is the Zn concentration of the nutritive solution (μg ml−1).
Zn isotopic signature
Zinc isotopes were analysed on the second aliquot. An isotope spike enriched in 64Zn, 66Zn, and 67Zn was added to the sample aliquot to achieve a total of 1000 ng of Zn and a spike:sample mass ratio of 1 (Arnold et al., 2010b). Zn was separated from the matrix using anion exchange chromatography as detailed in Arnold et al. (2010a). Zinc fractions were re-dissolved in 0.1 M HNO3 for the subsequent isotope ratio analysis using a HR Nu Plasma MC-ICP-MS (Nu Instruments, Wrexham, UK).
Isotope ratios are reported in δ-notation:
| (2) |
where (66Zn/64Zn)sample is the isotope ratio of the sample and (66Zn/64Zn)JMC Lyon is the isotope ratio of the standard reference solution used—that is, JMC 3-0749L. Accuracy of the isotope measurements was assessed by the analysis of two in-house single element solutions (Romil Zn and London Zn) and two natural standard reference materials (rye grass BCR-281 and blend ore BCR-027). As shown in Table 2, data from this study agree within error with previously published values for the in-house standards, the rye grass BCR-281, and for the blend ore BCR-027 (Mason et al., 2004; Chapman et al., 2006; Peel et al., 2008; Arnold, 2009).
Table 2.
Isotopic signature of the standards used in this study
| Reference material | Publication | δ66ZnJMC Lyon | n |
| BCR-027 (blend ore) | Chapman et al. (2006) | 0.33±0.07 | 8 |
| Arnold (2009) | 0.23±0.06 | 4 | |
| This study | 0.34±0.08 | 9 | |
| BCR-281 (rye grass) | Arnold (2009) | 0.38±0.09 | 7 |
| This study | 0.5±0.1 | 5 | |
| Romil | Mason et al. (2004) | –9.01±0.08 | 6 |
| Weiss et al. (2007) | –8.98±0.07 | Unknown | |
| Arnold (2009) | –9.0±0.1 | Unknown | |
| This study | –9.1±0.1 | 12 | |
| London | Arnold (2009) | 0.08±0.04 | 10 |
| This study | 0.10±0.06 | 9 |
Samples were double-spiked and analysed by MC-ICP-MS (see Materials and methods), using the standard reference solution JMC 3-0749L. Data are compared with the literature. δ66Zn is expressed in ‰ and displayed as means ±2SD.
The precision of the isotope measurements was estimated from replicate analysis of the BCR-281 standard (see Table 2). The typical error (expressed as 2σ standard deviation) was ±0.12‰. Procedural blank contributions were ∼4 ng of Zn. All mineral acids were sub-boiled in a quartz still and diluted using a 18 MΩ grade Millipore system (Bedford, MA, USA).
To assess the effect of the treatment on the distribution of isotopes across plant sections further, the fractionation between sections was calculated following Moynier et al. (2009) as:
where Δδ66Zni–j is the fractionation between sections i and j, and δ66Zni and δ66Znj are the isotopic signature of section i and j, respectively. The discrimination with respect to the growth medium was calculated according to the equation (Farquhar et al., 1989):
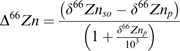 |
(3) |
where δ66Znso is the isotopic signature of the source, in this case the nutritive solution, and δ66Znp is the isotopic signature of the plant sample.
Statistical methods
Two-way analysis of variance (ANOVA) was carried out to evaluate the effect of plant section, Zn treatment, and their interaction with Zn concentration, and δ66Zn. Logarithmic transformation was performed when data did not meet the assumption of equal variances. To determine which groups were significantly different from each other, the post-hoc test that best separated the groups, either Student–Neuman–Keuls or Duncan, was selected. Student's t-test was chosen for mean comparisons between treatments for the photosynthetic parameters (As, gs, Ci, Fv/Fm, Fv'/Fm', ΦPSII, ΦCO2, ETR, qP, qN, NPQ, and E) and Zn isotopic fractionation between sections (Δδ66Zni–j). Pearson's correlation was employed to test whether there was a linear relationship between δ66Zn and photosynthetic performance. Statistical analyses were done with the software SPSS (Statistical Package for the Social Sciences) 2005 v14.0 for Windows. Sigma Plot software 2006 (v10.0) was used for graphic edition.
Results
Photosynthetic performance and growth
There was a substantial reduction of plant height and chlorophyll content due to Zn exposure (Table 3). The As, gs, and E decreased to 50% in Zn+ plants while no changes in Ci occurred. In the dark-adapted leaves of both treatments Fv/Fm remained stable. In contrast, Fv'/Fm', ΦPSII, ΦCO2, qP, and ETR showed a clear decrease in Zn+ light-adapted leaves. This was accompanied by an increase in qN and NPQ.
Table 3.
Effect of Zn levels on plant growth and photosynthetic traits of Phragmites australis
| Parameter | Control | Zn+ | t |
| Plant height (cm) | 106±4 | 79±3 | 5.8** |
| IRCC | 38.0±1.2 | 32.6±1.1 | 4.0** |
| As (μmol CO2 m−2 s−1) | 14±3 | 7.0±1.3 | 2.7* |
| gs (mol H2O m−2 s−1) | 0.18±0.05 | 0.08±0.01 | 2.4* |
| Ci (μmol CO2 mol air−1) | 246±7 | 246±11 | 0.0 |
| Fv/Fm | 0.80±0.01 | 0.79±0.01 | 0.8 |
| Fv'/Fm' | 0.46±0.02 | 0.37±0.01 | 4.2** |
| ΦPSII | 0.24±0.02 | 0.15±0.02 | 3.7** |
| ΦCO2 | 0.014±0.002 | 0.008±0.001 | 2.8* |
| qP | 0.52±0.02 | 0.39±0.04 | 2.6* |
| qN | 0.81±0.02 | 0.88±0.01 | –4.1** |
| NPQ | 2043±127 | 2652±141 | –3.1* |
| ETR (μmol m−2 s−1) | 122±9 | 75±9 | 3.7** |
| E (mmol H2O m−2 s−1) | 3.9±0.8 | 1.9±0.3 | 2.6* |
Plants were grown in 3.2 μM (controls) or 2 mM Zn (Zn+). Data represent means ±SE, where n=8 for plant height and IRCC (df=14), and n=6 for the rest of the parameters (df=10). The variable gs was log-transformed. The t-test value (t) is indicated as significant at P <0.05 (*) or P <0.01 (**).
As, light-saturated net CO2 assimilation rate; Ci: intercellular CO2 concentration; E, transpiration rate; ETR, electron transport rate; Fv/Fm, maximum quantum yield; Fv'/Fm', relative quantum yield; gs, stomatal conductance to water; IRCC, index of relative chlorophyll content; qN and NPQ, non-photochemical quenching; qP, photochemical quenching; ΦCO2, quantum yield of CO2 fixation; ΦPSII, quantum yield of PSII photochemistry.
Zn content
The Zn content of all plant sections increased with increasing Zn supply (Table 4). The Zn concentration of plant samples was higher than that of the growth solution, but the BCF was much reduced in Zn+ plants (Table 4). Plants grown at different Zn concentrations differed in the distribution pattern of Zn (and consequently BCF). In controls, living roots achieved the highest Zn levels, whereas dead roots had the lowest. In contrast, in Zn+ plants, dead and living roots achieved the highest levels, whereas leaves, shoots, and rhizomes contained little Zn in comparison. All Zn+ emerged sections had very similar Zn concentration except for the high leaves and youngest leaves, where it was lower.
Table 4.
Concentration of Zn achieved in different plant sections
| Plant section | Zn content (mg g–1) |
BCF |
||
| Controls | Zn+ | Controls | Zn+ | |
| Roots | ||||
| Living | 0.09±0.04 d | 12±6 h | 960±167 l | 93±19 p |
| Dead | 0.02±0.01 a | 14±7 h | 268±27 i | 105±27 n |
| Rhizomes | 0.02±0.01 a | 2.7±1.4 g | 274±46 i | 21±5 m |
| Shoots | ||||
| Low | 0.04±0.02 b | 3±2 g | 433±46 j | 25±3 o |
| High | 0.06±0.03 c | 2.3±1.1 g | 640±40 k | 17.2±1.3 p |
| Leaves | ||||
| Low | 0.05±0.03 b,c | 4±2 g | 583±79 j.k | 27±5 o |
| High | 0.04±0.02 b,c | 1.1±0.6 f | 490±48 j,k | 8.7±0.4 o |
| Youngest | 0.04±0.02 b | 0.5±0.2 e | 389±8 j | 3.7±0.2 o |
Plants were grown in 3.2 μM (controls) or 2 mM Zn (Zn+). Data represent means ±SE (n=4). The effect of plant section, Zn treatment, and their interaction was significant (P <0.001) according to two-way ANOVA (results not shown). Different letters indicate different groups according to Duncan post-hoc test on the log-transformed variables.
BCF, bioconcentration factor.
Zn isotopes
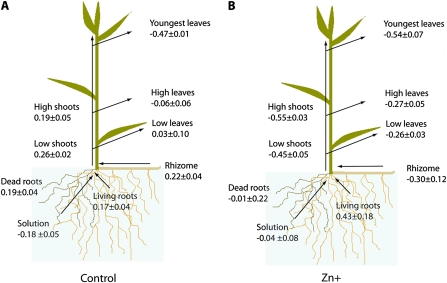
The δ66Zn varied between plant sections (Fig. 1). In the control experiment, only the youngest leaves were significantly different from the rest of the plant sections, showing a lighter isotopic signature. The shoots were slightly heavier than the leaves. The Zn+ treatment altered the fractionation pattern (Fig. 1). Shoots of Zn+ plants were lighter than the leaves, whereas the root samples were heavier than the shoots and the youngest leaves. However, only the rhizomes and the shoots were significantly different among treatments, and isotopically much lighter in Zn+ plants. The isotope signature of the youngest leaves was similar in both treatments, although the shoots of Zn+ plants were shorter. There was no significant difference detectable between low and high shoot or between low and high leaves in any of the treatments either.
Fig. 1.
Isotopic signature of the studied plant sections compared with solutions. Plants were supplied with 3.2 μM (control, A) or 2 mM Zn (Zn+, B). Data represent means ±SE (n=3). δ66Zn is expressed in ‰. (This figure is available in colour at JXB online.)
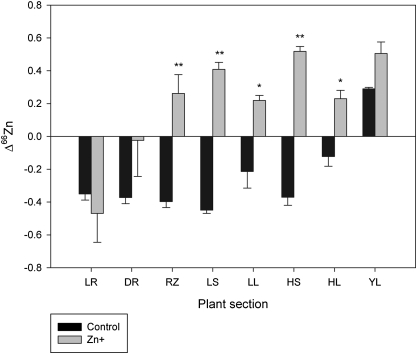
The Δδ66Zni–j was calculated between adjacent sections and between the roots and the youngest leaves or the high shoots (Table 5). The Δδ66Znleaves–shoots as well as Δδ66ZnRZ–LR and Δδ66ZnHS–LR were significantly affected by Zn+ treatment. In contrast, high Zn did not modify the Δδ66Zn between stem or leaf samples collected at different heights. Also there was no influence of the treatments on the fractionation between the roots and the youngest leaves. In agreement with the previous results, the controls discriminated against the light isotope except in the youngest leaves, whereas the Zn+ discriminated in favour of the lighter isotope except in the roots (Fig. 2). Both treatments differed significantly in the Δ66Zn of rhizomes, shoots, and leaves. Plants caused the enrichment in heavy isotopes of the nutritive solution, which was more evident in high Zn solutions.
Table 5.
Fractionation between plant sections
| Sample | Δδ66Zni–j |
t (df) | |
| Control | Zn+ | ||
| DR–LR | 0.02±0.03 | –0.5±0.2 | 1.91 (2.1) |
| RZ–DR | 0.03±0.01 | –0.3±0.2 | 2.03 (4) |
| LS–RZ | 0.05±0.04 | –0.2±0.1 | 1.40 (4) |
| LL–LS | –0.2±0.1 | 0.19±0.07 | –3.15 (4)* |
| HS–LL | 0.2±0.1 | –0.30±0.01 | 3.50 (4)* |
| HL–HS | –0.25±0.05 | 0.29±0.08 | –5.68 (4)** |
| YL–HL | –0.41±0.05 | –0.28±0.03 | –2.27 (4) |
| RZ–LR | 0.05±0.04 | –0.7±0.3 | 3.11 (4)* |
| HS–LS | –0.08±0.06 | –0.11±0.07 | 0.33 (4) |
| HL–LL | –0.09±0.08 | –0.01±0.08 | –0.68 (4) |
| YL–LR | –0.64±0.05 | –1.0±0.1 | 2.73 (4) |
| HS–LR | 0.02±0.08 | –1.0±0.2 | 4.57 (4)* |
Fractionation was calculated as Δδ66Zni-–j=δ66Znj–δ66Zni. Data represent means ±SE (n=3). The t-test value (t) is indicated as significant at P <0.05 (*) or P <0.01 (**).
LR, living roots; DR, dead roots; RZ, rhizomes; LS, low shoots; LL, low leaves; HS, high shoots; HL, high leaves; YL, youngest leaves.
Fig. 2.
Isotopic discrimination of the studied plant sections with respect to nutritive solutions. Plants were supplied with 3.2 μM (control) or 2 mM (Zn+) Zn. Data represent means ±SE (n=3). The Δ66Zn is expressed in ‰ and was calculated as Δ66Zn=(δ66Znsource–δ66Znsample)/(1+δ66Znsample/103). The t-test value (t) is indicated as significant at P <0.05 (*) or P <0.01 (**). LR, living roots; DR, dead roots; RZ, rhizomes; LS, low shoots; LL, low leaves; HS, high shoots; HL, high leaves; YL, youngest leaves.
Correlation between δ66Zn and plant height or photosynthetic parameters
Plant height showed a strong and positive linear correlation with δ66Zn of high shoot (r=0.972, P=0.001) and low shoot (r=0.929, P=0.007), and a weaker one with rhizome (r=0.813, P=0.049). The correlations of plant height with the rest of plant sections were not significant. The gas exchange and chlorophyll fluorescence were correlated with δ66Zn of high leaves where measurements were performed in both control and Zn+ plants. The relationships found to be significant are shown in Table 6. The results are consistent with the effect of the treatments on the photosynthetic performance parameters seen above. High values of δ66Zn in high leaves (as shown by controls) were associated with a higher gs, ΦPSII, and ΦCO2, and with a lower NPQ.
Table 6.
Pearson's correlation between δ66Zn and some photosynthetic performance parameters
| δ66Zn | gs | Fv′/Fm′ | ΦCO2 | qN | NPQ |
| r | 0.921* | 0.921* | 0.883* | –0.944* | –0.974** |
| Significance (bilateral) | 0.027 | 0.026 | 0.047 | 0.016 | 0.005 |
| n | 5 | 5 | 5 | 5 | 5 |
The δ66Zn was measured in high leaves. The correlation coefficient (r) is indicated as significant at P <0.05 (*) or P <0.01 (**).
Fv′/Fm′, relative quantum yield; gs. stomatal conductance to water; qN and NPQ, non-photochemical quenching; ΦCO2, the quantum yield of CO2 fixation.
Finally, the concentration of Zn showed a negative linear correlation with δ66Zn, which was strong in the sections low shoots (r= –0.964, P=0.002) and high shoots (r= –0.971, P=0.001), and weaker in high leaves (r= –0.828, P=0.042).
Discussion
Photosynthetic performance and growth
The results showed a clear toxic effect of Zn+ treatment on P. australis: growth, photosynthesis, and gas exchange were impaired. Thus the Zn+ fractionation data are representative of Zn-stressed plants. Chlorophyll fluorescence and gas exchange data are examined to discuss the possible causes of the As decrement. In Zn+ plants, gs and E decreased to 50%, indicating a strong inhibition of stomatal opening. A limited gas exchange can affect As by restricting the uptake of both C from the atmosphere and nutrients from the growth solution. In the present experiment, Zn+ plants did not show a reduction of Ci. Hence CO2 availability was not the limiting factor for As in Zn+ plants, because the C demand for assimilation was lower. Nevertheless, chlorophyll fluorescence was unchanged in dark-adapted leaves, where Fv/Fm remained stable, showing that PSII was functional. Only when leaves were transferred to the light, did Fv'/Fm', ΦPSII, and ΦCO2 show a clear decrease in Zn+ plants. This was accompanied by a reduction in qP and ETR, and an increase in qN and NPQ. All these data put together suggest that whereas PSII remained mainly unaffected, Zn impaired the efficiency of electron transport downstream, causing PSII to become easily saturated by light. This explains the slow C assimilation and the decrease of ΦCO2. Therefore, the present data suggest that the inhibition of transpiration was not the direct cause of the reduced C fixation. Zinc has been reported to inhibit or damage almost every point of the photosynthetic apparatus, i.e. chlorophyll synthesis, PSII, the oxygen-evolving complex, the plastoquinone pool, PSI, and Rubisco (Prasad, 2004). Many of these effects could cause the observed decreased photosynthetic performance. In addition, stomatal closure could reduce nutrient uptake. Deficiency of N can lead to an indirect impairment of the photosynthetic apparatus, and limit As. This is consistent with the decrease in total chlorophyll content indicated by decreased IRCC figures. Decreased N content has also been associated in the literature with the inhibition of Rubisco and the dark phase of photosynthesis (Ciompi et al., 1996).
The strong positive correlation of δ66ZnHL with and gs, ΦPSII, and ΦCO2, and the negative correlation with NPQ indicates that δ66ZnHL could be an interesting parameter to assess the inhibition of photosynthesis due to the toxic effect of excess Zn. Nevertheless, the results must be treated with caution due to the low number of replicates.
Zn content
Phragmites australis has innate tolerance to Zn and other metals (Ye et al., 1997). The lower BCF, the accumulation of Zn in roots, and the limitation to Zn export to the green tissues comprise an avoidance response that confers increased tolerance to Zn excess (Denny and Wilkins, 1987; Maestri et al., 2010). The higher BCF of dead roots in Zn+ is consistent with the use of root senescence to release Zn, an excretion mechanism of tolerant plants (Duarte et al., 2010). The Zn levels achieved by leaves and shoots are far from reaching the 1% Zn in leaf dry matter generally accepted as the threshold to reach Zn hyperaccumulation (Verbruggen et al., 2009). The Zn concentrations of the different tissues (12–14 mg g−1 DW in roots, 2–3 mg g−1 DW in shoots, and 0.5–3 mg g−1 DW in leaves) were comparable with those found in the study of Jiang and Wang (2008) (14 mg g−1 DW in roots, 0.95 mg g−1 DW in shoots, and 1.5 mg g−1 DW in leaves), using the same species and Zn supply. The small discrepancies in the Zn content of shoots and leaves can be easily explained, as in the present study sampling was done at specific heights instead of taking samples representative of the whole stem.
Zn isotopes
Mechanisms explaining the isotopic fractionation pattern under normal Zn supply
Under Zn-sufficient conditions, all the plant tissues except the youngest leaves are enriched in the heavier isotopes compared with the nutrient solution. The δ66Zn of the youngest leaves is isotopically lighter than the rest of the sections. Mature leaves are slightly lighter isotopically than roots and shoots. These observations are in line with field observations of Viers and co-workers (2007). They found that only Megaphrynium macrostachyum (Benth.) Milne-Redh among the four species analysed showed a significant fractionation between root and shoot. The most negative δ66Zn values measured throughout the plant were found in leaves (Viers et al., 2007; Moynier et al., 2009). In contrast, different degrees of root to shoot fractionation were described in crops such as tomato, lettuce, and rice under different experimental conditions (Weiss et al., 2005; Arnold et al., 2010a). This suggests that the mechanisms of Zn uptake and transport are highly species specific and conditioned by the physiological status of the plant. Here, it is proposed that the isotopic distribution of controls comes from the combination of two processes: (i) the enrichment in heavy isotopes generated by Zn uptake in roots and (ii) the enrichment in light isotopes during the long-distance transport of free Zn ions in the plant.
The observed pattern is consistent with the uptake of Zn by root cells facilitated by transmembrane transporters, as previously suggested by Weiss et al. (2005). Various members of the ZIP family of proteins (zinc–iron permeases) are located on the plasmatic membrane and facilitate Zn uptake (Grotz and Guerinot, 2006). Alternatively, the chelation of Zn by ligands and its subsequent transport in the complexed form could cause the observed enrichment in the heavier isotopes. This explanation can be rejected because Zn is mostly taken up and transported as Zn2+ (Marschner, 1995). Other mechanisms favouring the heavy isotopes are in disagreement with the observations reported here, such as adsorption onto the root surface, binding to the cell walls, and compartmentation in cell organelles. All of them imply the retention of the heavier isotopes in the roots, preventing its transport to other reservoirs. The δ66Znroot would be more positive than the rest of the plant, in disagreement with the present results. The protocol used to remove the root-adsorbed and apoplastically bound Zn was thus apparently efficient. The obtained data (δ66Znroot=0.18‰) are in line with previous experiments (Weiss et al., 2005), where a similar root desorption protocol was used for tomato, rice, and lettuce (δ66Znroot=0.15, 0.15, and 0.2‰, respectively).
The youngest leaves of controls were more negative than the rest of the plant. The transport of Zn2+ along the shoot has been suggested as the cause for the enrichment in light isotopes of shoots and leaves with height (Moynier et al., 2009), in agreement with the present results. There was a positive correlation between plant height and δ66Zn of transporting tissues, as previously suggested by Viers et al. (2007). The correlation was stronger as the samples were higher (high shoots >low shoots >rhizomes). The fractionation between low and high leaves was not statistically significant in this experiment (Δδ66ZnHL–LL= –0.090‰). However, the results are consistent with the small distance that separates the samples. The fractionation per distance was of –0.005‰ cm−1, very similar to –0.006‰ cm−1 calculated from Moynier et al. (2009) in bamboo. In the same direction, the leaves of controls were slightly lighter than the shoots at the same height, probably due to the translocation of Zn from the shoot along the leaves. Thus, the present data are consistent with an enrichment of lighter isotopes with distance from the root, but this can only be assessed if there is enough separation between samples.
The isotopic fractionation pattern reflects the tolerance response to high Zn concentrations
The protective mechanisms activated by plants under high Zn stress disrupt the Zn uptake, accumulation, distribution, and transport routes, which translated into a completely different fractionation pattern in this experiment.
There is little information about the regulation of ZIP transporters under excess Zn in plants. However, experiments in yeast demonstrated that ZRT1 is inactivated by high Zn supply (Gitan et al., 1998), limiting Zn influx into the cell. The activity of the transporters is probably inhibited in Zn+ plants, as shown by the decreased BCF. Thus, it is considered that Zn uptake mediated by transporters is not the cause of the enrichment in heavy isotopes of Zn+ roots.
Excess Zn is mainly accumulated in roots and localized in cell walls, intercellular spaces, and vacuoles (Heumann, 2002; Li et al., 2006; Jian and Wang, 2008). In the present experiment, δ66Zn was less negative in roots than in the rest of the organs, and Zn translocation from root to shoot was lower in Zn+ plants. This indicates that heavy Zn is effectively retained in roots, and the isotopically lighter sap is transported to the above-ground tissues. The youngest leaves of Zn+ plants have a δ66Zn similar to that of controls, even if their shoots were shorter and Zn was transported a shorter distance (106.0±3.5 cm for controls, 78.6±3.1 for Zn+, means±SE, n=4). In the opinion of the authors, this is because the xylem sap of Zn+ plants had a more negative δ66Zn from the root than that of controls.
When examined in detail, all the known mechanisms for Zn sequestration in roots are likely to select the heavy isotopes. Zinc probably forms covalent bounds with carboxyl and hydroxyl groups of pectin and with hydroxyl groups of cellulose in the cell walls (Straczek et al., 2008), and precipitates with insoluble phosphates or silicon in the apoplast (Neumann and zur Nieden, 2001; Straczek et al., 2008). In the cell, Zn binds to various ligands and is stored in subcellular organelles to keep Zn2+ low in the cytosol. Zinc is transferred into the vacuoles by metal tolerance proteins (MTPs) localized to the tonoplast (Blaudez et al., 2003; Dräger et al., 2004; Desbrosses-Fonrouge et al., 2005; Arrivault et al., 2006; Gustin et al., 2009). The tonoplast transporter AtZIF1 is also involved in Zn sequestration, probably by transporting either organic Zn ligands or Zn–ligand complexes into the vacuole (Haydon and Cobbett, 2007b). Different authors expect Zn to be chelated in the vacuoles by various ligands such as organic acids (OAs), proteins, and phytate (Van Steveninck et al., 1987; Salt et al., 1999; Tennstedt et al., 2009). The best candidates for Zn ligands in the vacuole are OAs such as citrate and malate, which are the most abundant metal ligands in plants and accumulate mainly in the vacuoles, as do excess metals. In agreement with this, the optimal stability of OA–metal complexes is achieved at vacuolar pH (Haydon and Cobett, 2007a). Besides, metal-binding peptides and proteins have been described to chelate Zn. Phytochelatins (PCs) are glutathione oligomers synthesized in response to metals, that chelate and detoxify Cd and As (Jabeen et al., 2009). Recent advances established that Zn promotes the synthesis of PCs, which are essential for Zn detoxification and contribute to Zn accumulation (Tennstedt et al., 2009). Cd complexed with PCs is pumped and sequestered into the vacuole (Salt et al., 1995; Cobbett and Goldsbrough, 2002). It is probable that PC–Zn complexes follow the same route, but direct evidence is lacking. Metallothioneins are cysteine-rich low molecular weight proteins found in plants, animals, and fungi, and are able to chelate Zn and many other metals. They are involved in Zn homeostasis and/or tolerance, but their exact function is as yet unknown (Rodríguez-Llorente et al., 2010). Finally, phytate is a P storage molecule that can bind to Zn as a mechanism for Zn storage or immobilization. Phytate–Zn complexes are found in roots (Van Steveninck et al., 1987, 1993; Terzano et al., 2008) and in seeds (Otegui et al., 2002; Rodrigues-Filho et al., 2005), either compartmentalized in the vacuoles or forming insoluble precipitates.
All three processes, Zn binding to cell walls, precipitation in intercellular spaces, and sequestration in the vacuole, are mass dependent and thus expected to favour the heavy isotope. It is difficult from the present design to tell which process was chiefly responsible for the enrichment in heavy isotopes of Zn+ roots. The youngest leaves of Zn+ were more negative than the rest of the leaves. Similarly to controls, the fractionation between low and high leaves was not statistically significant in this experiment (Δδ66ZnHL–LL= –0.011‰). The calculated Δδ66ZnHL–LL obtained from the linear regression of Zn+ leaves (Fig. 3) is of –0.090‰, very different from that observed but similar to that of controls. This provides evidence for the restriction of long-distance transport under toxic Zn levels. Both linear regressions for controls and Zn+ have a very similar slope, but the Zn+ plot is biased to the negative side. The youngest leaves of Zn+ have a δ66Zn similar to controls, in spite of the plants being shorter. The correlation between plant height and the intensity of Zn fractionation in leaves proposed by Viers et al. (2007) can thus be modified by the Zn status.
The enrichment in heavy isotopes of the nutritive solutions with time is in agreement with plants taking up Zn preferably by bulk flow, favouring the light isotopes, and with the higher biomass of above-ground tissues in this species (Ye et al., 1997). Also the Zn+ solution was more enriched in heavy isotopes than the control solution, as expected from the discrimination pattern observed for each treatment.
Conclusions
It has been proved that the study of Zn isotopes has great potential for investigation of the mechanisms of tolerance to Zn excess in plants. It was demonstrated that P. australis is able to discriminate Zn isotopes, and that the magnitude and sign of the resultant fractionation depends on Zn status and organ. It was shown that under Zn-sufficient levels, roots and shoots are enriched in the heavier Zn isotopes as compared with the source (δ66Zn=0.2 ‰), and the youngest leaves are impoverished (–0.5‰), whilst under Zn excess roots are enriched in the heavy isotopes (0.5‰) and the rest of the plant is isotopically lighter (up to –0.5‰). It has also been shown that Zn uptake by plants causes the enrichment in heavy isotopes of the nutritive solutions, which was stronger in Zn+ treatment (Δδ66Zncontrol=0.3‰, Δδ66ZnZn+=0.6‰). In conclusion, the tolerance response of P. australis increased the range of Zn fractionation within the plant and with respect to the environment.
An outline of the fractionation mechanisms compatible with the observed response was also provided. The enrichment in heavy isotopes of the roots was attributed to Zn uptake under Zn-sufficient conditions and to chelation and compartmentation in Zn excess. The enrichment in light isotopes of shoots and leaves is consistent with long-distance root to shoot transport, in agreement with the observations by Viers et al. (2007) and Moynier et al. (2009). Further research needs to be conducted to confirm these hypotheses and establish what molecules or processes are responsible for the described pattern.
Acknowledgments
This work was performed thanks to financial support from Imperial College of London, and Universitat de Barcelona via an APIF travel grant. We thank Barry Coles (Imperial College), Stanislav Strekopytov (Natural History Museum), and Josep Matas (Universitat de Barcelona) for technical assistance. CC wishes to thank Tim Arnold for sharing his experience with isotope analyses on plants so generously.
Glossary
Abbreviations
- As
light-saturated net CO2 assimilation rate
- BCF
bioconcentration factor
- Ci
intercellular CO2 concentration
- DR
dead roots
- E
transpiration rate
- ETR
electron transport rate
- Fv/Fm
maximum quantum yield
- Fv′/Fm′
relative quantum yield
- gs
stomatal conductance
- HL
high leaves
- HS
high shoots
- ICP-AES
inductively coupled plasma atomic emission spectrometer
- IRCC
index of relative chlorophyll content
- LL
low leaves
- LR
living roots
- LS
low shoots
- MC-ICP-MS
multicollector inductively coupled plasma mass spectrometer
- qN
NPQ
- non-photochemical quenching
- qP
photochemical quenching
- RZ
rhizomes
- YL
youngest leaves
- ΦPSII
quantum yield of PSII photochemistry
- ΦCO2
quantum yield of CO2 fixation
- Δ66Zn
isotopic discrimination with respect to the growth medium
- δ66Zn
isotopic signature
- Δδ66Zni-j
isotopic fractionation between sections i and j
References
- Ali NA, Ater M, Sunahara GI, Robidoux PY. Phytotoxicity and bioaccumulation of copper and chromium using barley (Hordeum vulgare L.) in spiked artificial and natural forest soils. Ecotoxicology and Environmental Safety. 2004;57:363–374. doi: 10.1016/S0147-6513(03)00074-5. [DOI] [PubMed] [Google Scholar]
- Arnold T. PhD Thesis. London: Imperial College London; 2009. The isotope biogeochemistry of Zn and Fe. [Google Scholar]
- Arnold T, Kirk GJD, Wissuwa M, Frei M, Zhao FJ, Mason TFD, Weiss DJ. Evidence for the mechanisms of zinc uptake by rice using isotope fractionation. Plant, Cell and Environment. 2010a;33:370–381. doi: 10.1111/j.1365-3040.2009.02085.x. [DOI] [PubMed] [Google Scholar]
- Arnold T, Schönbächler M, Rehkämper M, Dong S, Zhao FJ, Kirk GJD, Coles BJ, Weiss DJ. Measurement of zinc stable isotope ratios in biogeochemical matrices by double-spike MC-ICPMS and determination of the isotope ratio pool available for plants from soil. Analytical and Bioanalytical Chemistry. 2010b;398:3115–3125. doi: 10.1007/s00216-010-4231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrivault S, Senger T, Krämer U. The Arabidopsis metal tolerance protein AtMTP3 maintains metal homeostasis by mediating Zn exclusion from the shoot under Fe deficiency and Zn oversupply. The Plant Journal. 2006;46:861–879. doi: 10.1111/j.1365-313X.2006.02746.x. [DOI] [PubMed] [Google Scholar]
- Blaudez D, Kohler A, Martin F, Sanders D, Chalot M. Poplar metal tolerance protein 1 confers zinc tolerance and is an oligomeric vacuolar zinc transporter with an essential leucine zipper motif. The Plant Cell. 2003;15:2911–2928. doi: 10.1105/tpc.017541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Bosquet L, Sánchez C, Araus JL. Oxygen isotope enrichment (Δ18O) reflects yield potential and drought resistance in maize. Plant, Cell and Environment. 2009;32:1487–1499. doi: 10.1111/j.1365-3040.2009.02013.x. [DOI] [PubMed] [Google Scholar]
- Chapman J, Mason TFD, Weiss DJ, Coles BJ, Wilkinson JJ. Chemical separation and isotopic variations of Cu and Zn from five geological reference materials. Geostandards and Geoanalytical Research. 2006;30:5–16. [Google Scholar]
- Ciompi S, Gentili E, Guidi L, Soldatini GF. The effect of nitrogen deficiency on leaf gas exchange and chlorophyll fluorescence parameters in sunflower. Plant Science. 1996;118:177–184. [Google Scholar]
- Cobbett C, Goldsbrough P. Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annual Review of Plant Biology. 2002;53:159–182. doi: 10.1146/annurev.arplant.53.100301.135154. [DOI] [PubMed] [Google Scholar]
- Criss R. Principles of stable isotope distribution. New York: Oxford University Press; 1999. [Google Scholar]
- Davis JG, Parker MB. Zinc toxicity symptom development and partitioning of biomass and zinc in peanut plants. Journal of Plant Nutrition. 1993;16:2353–2369. [Google Scholar]
- Denny HJ, Wilkins D. Zinc tolerance in Betula spp. I. Effect of external concentration of zinc on growth and uptake. New Phytologist. 1987;106:517–514. [Google Scholar]
- Desbrosses-Fonrouge AG, Voigt K, Schroeder A, Arrivault S, Thomine S, Krämer U. Arabidopsis thaliana MTP1 is a Zn transporter in the vacuolar membrane, which mediates Zn detoxification and drives leaf Zn accumulation. FEBS Letters. 2005;579:S4165–S4174. doi: 10.1016/j.febslet.2005.06.046. [DOI] [PubMed] [Google Scholar]
- Dräger DB, Desbrosses-Fonrouge AG, Krach C, Chardonnens AN, Meyer RC, Saumitou-Laprade P, Krämer U. Two genes encoding Arabidopsis halleri MTP1 metal transport proteins co-segregate with zinc tolerance and account for high MTP1 transcript levels. The Plant Journal. 2004;39:425–439. doi: 10.1111/j.1365-313X.2004.02143.x. [DOI] [PubMed] [Google Scholar]
- Duarte B, Caetano M, Almeida PR, Vale C, Caçador I. Accumulation and biological cycling of heavy metal in four salt marsh species, from Tagus estuary (Portugal) Environmental Pollution. 2010;158:1661–1668. doi: 10.1016/j.envpol.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT. Carbon isotope discrimination and photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology. 1989;40:503–537. [Google Scholar]
- Genty B, Briantais JM, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica and Biophysica Acta. 1989;990:87–92. [Google Scholar]
- Gélabert A, Pokrovsky OS, Viers J, Schott J, Boudou A, Feurtet-Mazel A. Interaction between zinc and freshwater and marine diatom species: surface complexation and Zn isotope fractionation. Geochimica and Cosmochimica Acta. 2006;70:839–857. [Google Scholar]
- Gitan RS, Luo H, Rodgers J, Broderius M, Eide D. Zinc-induced inactivation of the yeast ZRT1 zinc transporter occurs through endocytosis and vacuolar degradation. Journal of Biological Chemistry. 1998;273:28617–28624. doi: 10.1074/jbc.273.44.28617. [DOI] [PubMed] [Google Scholar]
- Grotz N, Guerinot ML. Molecular aspects of Cu, Fe and Zn homeostasis in plants. Biochimica et Biophysica Acta. 2006;1763:595–608. doi: 10.1016/j.bbamcr.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Gustin JL, Loureiro ME, Kim D, Na G, Tikhonova M, Salt DE. MTP1-dependent Zn sequestration into shoot vacuoles suggests dual roles in Zn tolerance and accumulation in Zn-hyperaccumulating plants. The Plant Journal. 2009;57:1116–1127. doi: 10.1111/j.1365-313X.2008.03754.x. [DOI] [PubMed] [Google Scholar]
- Haydon MJ, Cobbett CS. Transporters of ligands for essential metal ions in plants. New Phytologist. 2007a;174:499–506. doi: 10.1111/j.1469-8137.2007.02051.x. [DOI] [PubMed] [Google Scholar]
- Haydon MJ, Cobbett CS. A novel major facilitator superfamily protein at the tonoplast influences zinc tolerance and accumulation in Arabidopsis. Plant Physiology. 2007b;143:1705–1719. doi: 10.1104/pp.106.092015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heumann HG. Ultrastructural localization of zinc in zinc-tolerant Armeria maritima ssp. halleri by autometallography. Journal of Plant Physiology. 2002;159:191–203. [Google Scholar]
- Jabeen R, Ahmad A, Iqbal M. Phytoremediation of heavy metals: physiological and molecular mechanisms. Botanical Reviews. 2009;75:339–364. [Google Scholar]
- Jiang X, Wang C. Zinc distribution and zinc-binding forms in Phragmites australis under zinc pollution. Journal of Plant Physiology. 2008;165:697–704. doi: 10.1016/j.jplph.2007.05.011. [DOI] [PubMed] [Google Scholar]
- John SG, Geis RW, Saito MA, Boyle EA. Zinc isotope fractionation during high-affinity and low-affinity zinc transport by the marine diatom Thalassiosira oceanica. Limnology and Oceanography. 2007;52:2710–2714. [Google Scholar]
- Kong G, White R. Toward cleaner production of hot dip galvanizing industry in China. Journal of Cleaner Production. 2010;18:1092–1099. [Google Scholar]
- Konstantinou IK, Albanis TA. Worldwide occurrence and effects of antifouling paint booster biocides in the aquatic environment: a review. Environment International. 2004;30:235–248. doi: 10.1016/S0160-4120(03)00176-4. [DOI] [PubMed] [Google Scholar]
- Krugh B, Bickham L, Miles D. The solid-state chlorophyll meter: a novel instrument for rapidly and accurately determining the chlorophyll concentrations in seedling leaves. Maize Genetics and Cooperation Newsletter. 1994;68:25–27. [Google Scholar]
- Li TQ, Yang XE, Yang JY, He ZL. Zn accumulation and subcellular distribution in the Zn hyperaccumulator Sedum alfredii Hance. Pedosphere. 2006;16:616–623. [Google Scholar]
- Maestri E, Marmiroli M, Visioli G, Marmiroli N. Metal tolerance and hyperaccumulation: costs and trade-offs between traits and environment. Environmental and Experimental Botany. 2010;68:1–13. [Google Scholar]
- Marschner H. Mineral nutrition of higher plants. 2nd edn. London: Academic Press; 1995. [Google Scholar]
- Mason TFD, Weiss DJ, Horstwood M, Parrish RR, Russell SS, Mullaneb E, Coles BJ. High precision Cu and Zn isotope analysis by plasma source mass spectrometry. Part 2: correcting for mass bias discrimination effects. Journal of Analytical Atomic Spectrometry. 2004;19:218–226. [Google Scholar]
- Mathur N, Bhatnagar P, Nagar P, Bijarnia MK. Mutagenicity assessment of effluents from textile/dye industries of Sanganer, Jaipur (India): a case study. Ecotoxicology and Environmental Safety. 2005;61:105–113. doi: 10.1016/j.ecoenv.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Monaghan J, Scrimgeour C, Stein W, Zhao F, Evans E. Sulphur accumulation and redistribution in wheat (Triticum aestivum): a study using stable sulphur isotope ratios as a tracer system. Plant, Cell and Environment. 1999;22:831–839. [Google Scholar]
- Moynier F, Pichat S, Pons ML, Fike D, Balter V, Albarède F. Isotopic fractionation and transport mechanisms of Zn in plants. Chemical Geology. 2009;267:125–130. [Google Scholar]
- Neumann D, zur Nieden U. Silicon and heavy metal tolerance of higher plants. Phytochemistry. 2001;56:685–692. doi: 10.1016/s0031-9422(00)00472-6. [DOI] [PubMed] [Google Scholar]
- Otegui MS, Capp R, Staehelin LA. Developing seeds of Arabidopsis store different minerals in two types of vacuoles and in the endoplasmic reticulum. The Plant Cell. 2002;14:1311–1327. doi: 10.1105/tpc.010486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel K, Weiss DJ, Chapman J, Arnold T, Coles BJ. A simple combined sample-standard bracketing and inter-element correction procedure for accurate mass bias correction and precise Zn and Cu isotope ratio measurements. Journal of Analytical Atomic Spectrometry. 2008;23:103–110. [Google Scholar]
- Pokrovsky OS, Viers J, Freydier R. Zinc stable isotope fractionation during its adsorption on oxides and hydroxides. Journal of Colloid and Interface Science. 2005;291:192–200. doi: 10.1016/j.jcis.2005.04.079. [DOI] [PubMed] [Google Scholar]
- Popovic A, Djordjevic D, Polic P. Trace and major element pollution originating from coal ash suspension and transport processes. Environment International. 2001;26:251–255. doi: 10.1016/s0160-4120(00)00114-8. [DOI] [PubMed] [Google Scholar]
- Prasad MNV. Heavy metal stress in plants: from biomolecules to ecosystems. Berlin: Springer-Verlag; 2004. [Google Scholar]
- Pruvot C, Douay F, Herve F, Waterlot C. Heavy metals in soil, crops and grass as a source of human exposure in the former mining areas. Journal of Soils and Sediments. 2006;6:215–220. [Google Scholar]
- Rodrigues-Filho UP, Vaz J, Felicissimo MP, et al. Heterometallic manganese/zinc–phytate complex as a model compound for metal storage in wheat grains. Journal of Inorganic Biochemistry. 2005;99:1973–1982. doi: 10.1016/j.jinorgbio.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Llorente ID, Pérez-Palacios P, Doukkali B, Caviedes MA, Pajuelo E. Expression of the seed-specific metallothionein mt4a in plant vegetative tissues increases Cu and Zn tolerance. Plant Science. 2010;178:327–332. [Google Scholar]
- Rodushkin I, Engstrom E, Stenberg A, Baxter DC. Determination of low-abundance elements at ultra-trace levels in urine and serum by inductively coupled plasma-sector field mass spectrometry. Analytical and Bioanalytical Chemistry. 2004;380:247–257. doi: 10.1007/s00216-004-2742-7. [DOI] [PubMed] [Google Scholar]
- Rosen J, Pike C, Golden M, Freeman J. Zinc toxicity in corn as a result of a geochemical anomaly. Plant and Soil. 1978;50:151–159. [Google Scholar]
- Rosman KJR, Taylor PDP. Isotopic compositions of the elements 1997. Pure and Applied Chemistry. 1998;70:217–236. [Google Scholar]
- Salt DE, Blaylock M, Kumar NP, Dushenkov V, Ensley BD, Chet I, Raskin I. Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Bio-Technology. 1995;13:468–474. doi: 10.1038/nbt0595-468. [DOI] [PubMed] [Google Scholar]
- Salt DE, Prince RC, Baker AJ, Raskin I, Pickering IJ. Zinc ligands in the metal hyperaccumulator Thlaspi caerulescens as determined using X-ray absorption spectroscopy. Environmental Science and Technology. 1999;33:713–717. [Google Scholar]
- Straczek A, Sarret G, Manceau A, Hinsinger P, Geoffroy N, Jaillard B. Zinc distribution and speciation in roots of various genotypes of tobacco exposed to Zn. Environmental and Experimental Botany. 2008;63:80–90. [Google Scholar]
- Tennstedt P, Peisker D, Bottcher C, Trampczynska A, Clemens S. Phytochelatin synthesis is essential for the detoxification of excess zinc and contributes significantly to the accumulation of zinc. Plant Physiology. 2009;149:938–948. doi: 10.1104/pp.108.127472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzano R, Al Chami Z, Vekemans B, Janssens K, Miano T, Ruggiero P. Zinc distribution and speciation within rocket plants (Eruca vesicaria L. Cavalieri) grown on a polluted soil amended with compost as determined by XRF microtomography and micro-XANES. Journal of Agricultural and Food Chemistry. 2008;56:3222–3231. doi: 10.1021/jf073304e. [DOI] [PubMed] [Google Scholar]
- Van Steveninck R, Babare A, Fernando D, Van Steveninck R. The binding of zinc in root cells of crop plants by phytic acid. Plant and Soil. 1993;155–156:525–528. [Google Scholar]
- Van Steveninck RFM, Van Steveninck ME, Fernando DR, Godbold DL, Horst WJ, Marschner H. Identification of zinc-containing globules in roots of a zinc-tolerant ecotype of Deschampsia caespitosa. Journal of Plant Nutrition. 1987;10:1239–1246. [Google Scholar]
- Verbruggen N, Hermans C, Schat H. Molecular mechanisms of metal hyperaccumulation in plants. New Phytologist. 2009;181:759–776. doi: 10.1111/j.1469-8137.2008.02748.x. [DOI] [PubMed] [Google Scholar]
- Viers J, Oliva P, Nonell A, Gélabert A, Sonke JE, Freydier R, Gainville R, Dupré B. Evidence of Zn isotopic fractionation in a soil–plant system of a pristine tropical watershed (Nsimi, Cameroon) Chemical Geology. 2007;239:124–137. [Google Scholar]
- Von Blanckenburg F, von Wiren N, Guelke M, Weiss DJ, Bullen TD. Fractionation of metal stable isotopes by higher plants. Elements. 2009;5:375–380. [Google Scholar]
- Weis JS, Weis P. Metal uptake, transport and release by wetland plants: implications for phytoremediation and restoration. Environment International. 2004;30:685–700. doi: 10.1016/j.envint.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Weiss DJ, Mason TFD, Zhao FJ, Kirk GJD, Coles BJ, Horstwood MSA. Isotopic discrimination of zinc in higher plants. New Phytologist. 2005;165:703–710. doi: 10.1111/j.1469-8137.2004.01307.x. [DOI] [PubMed] [Google Scholar]
- Weiss DJ, Rausch N, Mason TFD, Coles BJ, Wilkinson JJ, Ukonmaanaho L, Arnold T, Nieminen TM. Atmospheric deposition and isotope biogeochemistry of zinc in ombrotrophic peat. Geochimica and Cosmochimica Acta. 2007;71:3498–3517. [Google Scholar]
- Ye ZH, Baker AJM, Wong MH, Willis AJ. Zinc, lead and cadmium tolerance, uptake and accumulation by the common reed, Phragmites australis (Cav.) Trin. ex Steudel. Annals of Botany. 1997;80:363–370. [Google Scholar]
- Yun SI, Ro H- M. Stable C and N isotopes: a tool to interpret interacting environmental stresses on soil and plant. Journal of Applied Biology and Chemistry. 2008;51:262–271. [Google Scholar]