Abstract
Soil salinity affects large areas of the world's cultivated land, causing significant reductions in crop yield. Despite the fact that most plants accumulate both sodium (Na+) and chloride (Cl–) ions in high concentrations in their shoot tissues when grown in saline soils, most research on salt tolerance in annual plants has focused on the toxic effects of Na+ accumulation. It has previously been suggested that Cl– toxicity may also be an important cause of growth reduction in barley plants. Here, the extent to which specific ion toxicities of Na+ and Cl– reduce the growth of barley grown in saline soils is shown under varying salinity treatments using four barley genotypes differing in their salt tolerance in solution and soil-based systems. High Na+, Cl–, and NaCl separately reduced the growth of barley, however, the reductions in growth and photosynthesis were greatest under NaCl stress and were mainly additive of the effects of Na+ and Cl– stress. The results demonstrated that Na+ and Cl– exclusion among barley genotypes are independent mechanisms and different genotypes expressed different combinations of the two mechanisms. High concentrations of Na+ reduced K+ and Ca2+ uptake and reduced photosynthesis mainly by reducing stomatal conductance. By comparison, high Cl– concentration reduced photosynthetic capacity due to non-stomatal effects: there was chlorophyll degradation, and a reduction in the actual quantum yield of PSII electron transport which was associated with both photochemical quenching and the efficiency of excitation energy capture. The results also showed that there are fundamental differences in salinity responses between soil and solution culture, and that the importance of the different mechanisms of salt damage varies according to the system under which the plants were grown.
Keywords: Barley, chloride, salinity, sodium, specific ion toxicity, tolerance
Introduction
The yield of grain crops over large areas of the world's farming land is limited by a number of physicochemical constraints in the subsoil including salinity and sodicity (Rengasamy, 2010). Attempts to develop viable management options to improve productivity of saline–sodic soils, such as irrigation and drainage, have met with minimal success to date. The use of breeding to develop better-adapted crops with improved physiological tolerance to saline–sodic soils offers a strategy for managing crop production on these soils. Improving salt tolerance of barley (Hordeum vulgare L.) has been of interest for a long time and has resulted in a considerable body of data from studies using physiological (Cramer et al., 1990; Munns and Rawson, 1999; Munns et al., 2000; Tavakkoli et al., 2010b), genetic (Mano and Takeda, 1997; Ellis et al., 2002), and cytogenetic (Forster et al., 1997) approaches.
The constituent cations of total soluble salts in soils are usually sodium (Na+), calcium (Ca2+), and magnesium (Mg2+) and the anions are chloride (Cl–), sulphate (SO42 −) and carbonate (including bicarbonate; CO32 − HCO3−). However, Na+ dominates the cations and Cl– the anions in the majority of saline soils to the extent that NaCl comprises from 50–80% of the total soluble salts (Rengasamy, 2010). Salt stress has 3-fold effects on plant growth: it reduces soil water potential leading to osmotic stress, it induces ion imbalance in cells, especially lower concentrations of K+, Ca2+, and NO3−, and it causes ion (Na+ and/or Cl–) toxicity. Since salt stress involves both osmotic and ionic stresses, growth suppression is directly related to the total concentration of soluble salts and osmotic potential of the soil solution. The detrimental effect is observed at the whole-plant level as the death of plants or a decrease in productivity (Munns and Tester, 2008). Therefore, understanding the mechanisms of tolerance to high soil concentration of NaCl is essential to improve crop salt tolerance.
For most plants to tolerate salinity, Na+ and Cl– uptake must be restricted while maintaining the uptake of macronutrients such as K+, NO3− and Ca2+) The mechanisms of Na+ and K+ transport in plants under salt stress has been extensively researched and reviewed (Amtmann and Sanders, 1998; Maathuis and Amtmann, 1999; Tester and Leigh, 2001; Tester and Davenport, 2003; Rodriguez-Navarro and Rubio, 2006; Apse and Blumwald, 2007; Shabala and Cuin, 2008). Reduced Na+ loading into the xylem is one of the main mechanisms of salinity tolerance and it is often considered one of the most crucial features of restricting Na+ accumulation in plant tissues (Tester and Davenport, 2003; Munns and Tester, 2008).
Given that the dominant salt in saline soils is NaCl, both Na+ and Cl– ions will occur in high concentrations. However, the contribution of Cl– to growth reduction under salt stress is less well understood than that of Na+ in broadacre crops. This reflects the fact that most research on salt tolerance in cereals has focused on Na+ with little regard to Cl– toxicity (Teakle and Tyerman, 2010). Both Na+ and Cl– should be given equal consideration since they are both metabolically toxic to plants if accumulated at high concentrations in the cytoplasm (Xu et al., 2000; White and Broadley, 2001; Tavakkoli et al., 2010a, b; Teakle and Tyerman, 2010). For some species, shoot or root concentrations of Na+, rather than Cl– are negatively correlated with salt tolerance (Kingsbury and Epstein, 1986; Kinraide, 1999; Lin and Kao, 2001), while there are many examples of species for which control of Cl– transport and Cl– exclusion from shoots is correlated with salt tolerance (Martin and Koebner, 1995; Luo et al., 2005; Islam et al., 2007; Teakle et al., 2007; Aydi et al., 2008; Tavakkoli et al., 2010a, b). There has also been some recent debate about the importance of soil Cl– and by implication plant Cl– uptake, as predictors of damage and yield loss at the field level. Based on analysis of a number of field trials, Dang et al. (2008) concluded that the Cl– concentration in the soil was more important to growth and yield reduction than Na+. They estimated the critical level (defined as the concentration that reduces the growth or yield by 10%) of subsoil Cl– concentration to be 490 mg Cl– kg−1 soil with Cl– concentration in the youngest mature leaf of bread wheat, durum wheat, and chickpea showing greater variability with increasing levels of subsoil constraints than Na+ concentration (Dang et al., 2006).
Research to determine Na+ toxicity relative to Cl− has largely been based on solution culture (Kingsbury and Epstein, 1986; Martin and Koebner, 1995; Luo et al., 2005; Slabu et al., 2009) and there has been little research to examine the responses to Na+ and Cl– toxicity in soil-based systems (Tavakkoli et al., 2010a). Given the inherent differences in plant growth interactions with solution and soil culture (Gregory et al., 2009; Tavakkoli et al., 2010b), a critical assessment of Na+ and Cl– ion toxicity in both systems is needed.
The aim of this study was critically to assess the extent to which Na+ and Cl– contribute to ion toxicity in barley and whether tolerant genotypes have a better ability to exclude Na+ and/or Cl–). Ion toxicity was assessed using a range of physiological techniques (tissue nutrient analysis, chlorophyll fluorescence, gas exchange) on selected barley varieties with different mechanisms of salt tolerance in both hydroponic and soil systems. It is argued that salt tolerance is related to the ability of a genotype to regulate both Na+ and Cl– transport in order to minimize cytoplasmic concentrations and to avoid toxicity.
Materials and methods
Barley varieties
The effects of Na+ and Cl– ion concentration on barley growth was assessed using four genotypes; Barque73, Clipper, Sahara, and Tadmor. In previous hydroponic experiments, Barque73 showed a higher salt tolerance compared with Tadmor (60% versus 42% relative growth at 150 mM NaCl) with similar Na+ concentration (2207 versus 2150 mmol kg−1 DW) but a superior ability to exclude Cl– (1341 versus 2440 mmol kg−1 DW) (E Tavakkoli, unpublished data). Clipper is a variety with an ability to exclude both Na+ and Cl– and Sahara accumulates Na+ and Cl– to high levels (Rivandi, 2009; Tavakkoli et al., 2010b). Barque73 and Tadmor were only used in Experiment 1 and all genotypes were used in subsequent experiments.
Growth conditions
Experiment 1. The separate effects of Na+ and Cl– ions at different concentrations on growth of barley in solution culture
A solution culture experiment was conducted to assess the effect of different concentrations of Na+ and Cl– ions on the growth of Barque73 and Tadmor. Ten concentrations (0, 10, 20, 40, 60, 80, 100, 120, 140, and 160 mM) of Na+-dominant and Cl–-dominant Hoagland's solutions were prepared by dissolving a mixture of Na+ salts (Na2SO4, Na2HPO4, and NaNO3) or a mixture of Cl– salts (CaCl2, MgCl2, and KCl) in Milli-Q water (conductivity ≥18.2 MΩ cm−1). All treatment solutions had a background of modified Hoagland's solution for nutrient supply, the composition of which (in mM) was: NH4NO3 (0.2), KNO3 (5), Ca (NO3)2 (2), MgSO4 (2), KH2PO4 (0.1), NaFe(III)-hydroxyethyl ethylenediamine triacetic acid (HEDTA) (0.05), H3BO3 (0.01), MnCl2 (0.005), ZnSO4 (0.005), CuSO4 (0.0005), and Na2MoO3 (0.0001). Solutions were changed every 10 d and the pH of the solution was monitored several times each week and maintained within a range of 6.0–6.5. The experiment was conducted in an air-conditioned growth chamber with day/night temperatures of approximately 25/18 °C. The intensity of photosynthetically active radiation was measured using a Li-Cor quantum sensor (model LI-1000, Li-Cor, Lincoln, NE, USA) and varied from 550 to 600 mmol m−2 s−1. At 10–12 d after germination, when the third leaf was beginning to appear, the Na+ and Cl– treatments were introduced in increments of 20 mM over 8 d. Supplementary Ca2+ (5 mM) as CaCl2 was added to the Na+ treatment to prevent Ca+2 deficiencies in plants (Genc et al., 2010).
The growth system of the plants was modified from Casey et al. (2003) and consisted of a polypropylene mesh supported at the bottom of a flanged tube (4 cm diameter×8 cm depth) which, in turn, formed the bottom of the tube. This assembly was inserted into a lid which rested on top of a PVC pot (10.4 cm diameter×32 cm depth) filled with nutrient solution. Each lid had a 0.5 cm drilled hole for a capillary air tube (see Supplementary Fig. S1 at JXB online). Uniformly sized seeds of each genotype were surface-sterilized in 70% ethanol for 1 min, followed by soaking in 3% sodium hypochlorite for 5 min, then rinsed three times with deionized water. Four seeds were placed on the mesh and, after the seedlings were established, they were thinned to one seedling. The tubes were then filled with cylindrical black polycarbonate pellets (approximately 2–4 mm long and 1–2 mm in diameter) to support the plant stem and prevent light penetration to the nutrient solution. At this stage the level of the solution was allowed to drop to about 1.5 cm below the mesh. The experimental design was a factorial, completely randomized design comprising 19 salt treatments (control and 9 rates Na+/Cl–)×two barley genotypes with three replicates.
Experiment 2. The separate effects of Na+ and Cl– ions at high concentration on barley growth using solution culture
A solution culture experiment was conducted to assess the relative importance of Na+ and Cl– ions to salt toxicity. The varieties, Barque73, Clipper, Sahara, and Tadmor were used because of their different sensitivities to salt stress and abilities to exclude Na+ and Cl– ions. A factorial experiment consisted of six different treatments: control, 120 mM NaCl, 120 mM NaCl+0.07 mM 4,4′-diisothiocyanostilbene-2,2′-disulphonic acid (DIDS), 120 mM Na+-gluconate, 120 mM Cl–-dominant, and 120 mM Na+-dominant Hoagland's solutions. The effect of Na+ independently of Cl– was examined in three ways: by using DIDS, which is a non-permeating amino acid that inhibits Cl– transport (Lin, 1981; Lin and Kao, 2001); by using Na+-gluconate because gluconate is an anion that is unable to permeate the cell membrane; and by using the Na+-Hoagland solution. The solutions were designed to have similar EC and osmotic potentials (Table 1). The growth conditions were identical to those of Experiment 1. The experimental design was a factorial completely randomized design comprising six treatments×four barley genotypes with three replicates.
Table 1.
The electrical conductivity and osmotic potential of hydroponic solutions used in Experiment 2
| Treatment | Solution EC (dS m−1) | Osmotic potential (MPa) |
| Control | 1.5 | –0.05 |
| NaCl | 11.5 | –0.85 |
| NaCl+DIDS | 11.4 | –0.84 |
| Na+-gluconate | 9.8 | –0.84 |
| Na+-dominant Hoagland | 11.4 | –0.84 |
| Cl–-dominant Hoagland | 11.8 | –0.86 |
Experiment 3. The separate effects of Na+ and Cl– ions at high concentration on barley growth using soil culture
An experiment was conducted using field soil to assess the effect of high concentrations of Na+ and Cl– ions on the growth of Barque73, Clipper, Sahara, and Tadmor. The A horizon (topsoil) soil of a sandy loam red Chromosol (Isbell, 1996) collected from Roseworthy (34.51 °S, 138.68 °E, South Australia) was used. Following collection, the soil was air-dried and ground to pass through a 5 mm sieve. A soil-water characteristic curve was determined using the pressure plate method (Klute, 1986) and the soil moisture content at field capacity (–10 kPa, equivalent to 37% w/w) was estimated.
The experiment was designed to compare growth in saline soils at similar soil electrical conductivities (EC) and soil osmotic potential (ΨO) but different ionic compositions. To achieve this, it compared the effects of 120 mmol kg−1 Na+ (applied as a range of sulphate, nitrate, and phosphate salts), 120 mmol kg−1 Cl– (applied as a range of calcium, magnesium, and potassium salts), and 120 mmol kg−1 NaCl at a similar EC in a soil-based system (Table 2). The Cl–, Na+, and NaCl-dominant saline soils were prepared by dissolving a mixture of Na+ salts (20 mM Na2SO4, 20 mM Na2HPO4, and 40 mM NaNO3), a mixture of Cl– salts (20 mM CaCl2, 20 mM MgCl2, and 40 mM KCl), and NaCl salt (120 mM NaCl), respectively, in milliQ H2O and spraying each solution on a 2 cm layer of soil to reach field capacity moisture content. Each soil was covered with plastic to control evaporation and left for 5 d at 25 °C to reach equilibrium, then mixed thoroughly and air-dried (Tavakkoli et al., 2010a). Basal fertilizer was thoroughly mixed with the soil at the following concentrations (in mg pot−1): NH4NO3 (380), KH2PO4 (229), CaCl2 (131), MgCl2 (332), CuCl2 (10.7), ZnCl2 (11), Na2MoO4 (6.84), and H3BO3 (15). All soils with basal fertilizer were wetted to field capacity with deionized water and allowed to equilibrate for 4 d at 25 °C. Samples of the four synthesized soils were moistened to field capacity (water potential at –10 kPa) and centrifuged at 4000 g for 30 min to extract the soil solution which was passed through 0.25 μm filter paper. Electrical conductivity, ΨO and ion concentrations of the solutions were measured (Table 2).
Table 2.
Osmotic potential (MPa), pH, electrical conductivity (dS m−1) and ionic concentration (mM) of the treatment soil solutions extracted at field capacity in Experiment 3.
| Ψo | pH | ECFC | Ca2+ | Mg+ | Na+ | K+ | P | S | Cl– | |
| Control | –0.01 | 7.8 | 1.2 | 9.0 | 2.7 | 1.9 | 1.9 | 0.12 | 4.2 | 1.9 |
| Na+-soil | –0.78 | 7.6 | 11.1 | 19.0 | 10.9 | 112.9 | 5.7 | 0.48 | 23.2 | 1.3 |
| Cl–-soil | –0.73 | 7.7 | 11.0 | 33.6 | 18.6 | 2.4 | 41.2 | 0.12 | 4.0 | 125.0 |
| NaCl-soil | –0.79 | 7.9 | 11.2 | 18.7 | 11.1 | 115.1 | 6.5 | 0.11 | 3.8 | 120.4 |
Values are means (n=2).
The plants were grown in pots, 10.4 cm in diameter and 32 cm deep in which there were two layers of soil; 2200 g of air-dried soil (subsoil) which contained the salt treatment with 800 g of untreated soil above (topsoil). Each layer was packed to a bulk density of 1.35 Mg m−3. The subsoil and topsoil were separated by a 3 cm layer of plastic beads (120 g) to prevent salt rising to the topsoil through capillarity action. The top 3 cm of the pot was also covered by plastic beads to minimize the water evaporation from the soil surface. Uniformly sized seeds of each genotype were surface-sterilized in 70% ethanol for 1 min, followed by soaking in 3% sodium hypochlorite for 5 min, then rinsed three times with deionized water. Four barley seeds were sown in each pot and thinned to one per pot after 5 d. The experiment was conducted under the same growth conditions as described in Experiment 1. The pots were weighed and watered to field capacity regularly and daily water use calculated. The experimental design was a factorial, completely randomized design comprising four treatments×four barley genotypes with three replicates, giving a total of 48 pots.
Measurements
Experiment 1
Plants were harvested after 49 d, the fresh and dry weights of the shoots were recorded, and the whole shoot moisture content was calculated. The shoots were digested in 40 ml of 1% HNO3 at 95 °C for 6 h in a 54-well HotBlock (Environmental Express, Mt Pleasant, South Carolina, USA). The concentration of Na+ and K+ in the digested samples was determined using a flame photometer (Model 420, Sherwood, Cambridge, UK). Chloride concentrations of the digested extracts were determined using a chloride analyser (Model 926, Sherwood Scientific, Cambridge, UK). Recovery of Na+, Cl–, and K+ from plant standards (Australasian Soil and Plant Analysis Council) were 94, 98, and 93%, respectively.
Experiments 2 and 3
Leaf chlorophyll content was measured using a hand-held SPAD 502 meter (Minolta, Osaka, Japan) in Experiment 3. Average SPAD chlorophyll readings were calculated from four measurements from the leaf tip to the leaf base of the fourth and the fifth leaf. An infrared, open gas exchange system LI-6400 (Li-Cor, Inc., Linclon, NE, USA) coupled with an integrated fluorescence chamber head (Li-6400-40 leaf chamber fluorometer; Li-Cor Inc., Nebraska, USA) was used to measure instantaneous gas exchange and chlorophyll fluorescence. All measurements were made on the fifth leaf, 4–5 h into a 9 h photoperiod on the day after the plants were rewatered to field capacity. The settings were chosen to match growth chamber conditions. Leaf temperature was maintained at 25 °C, light intensity was set at 800 μmol photons m−2 s−1 with a red/blue light source, and the CO2 concentration was set at 400 μmol mol−1. Leaf to air VPD was maintained at 1.1 kPa. The photosynthetic rate (A), stomatal conductance (gs), transpiration (T), and substomatal CO2 concentration (Ci) were recorded. Fluorescence parameters were estimated from the measurements of chlorophyll fluorescence on light-adapted leaves (Genty et al., 1989). The operating quantum efficiency of PSII photochemistry, ΦPSII, was calculated as (F′m − F′)/F′m. The efficiency of open PSII reaction centres (F′v/F′m) was calculated as (F′m − F′o)/F′m and the photochemical quenching (qP) factor was determined as (F′m − F′)/(F′m−F′o). The non-photochemical quenching (NPQ) was expressed as Fm/F′m−1 where F′m is the maximal fluorescence during a saturating flash of light >7 mmol m-2 s-1, and F′o is the minimal fluorescence of a momentarily darkened leaf.
To measure the osmotic potential (Ψs) of leaf sap, a disc of Whatman GF/B glass micro-fibre paper was placed in the barrel of a 2 ml plastic syringe so that it covered the outlet hole. A fresh leaf was then put in the barrel, the plunger was re-inserted, and the tip of the syringe was sealed with Blu-Tack®. The syringe was frozen in liquid nitrogen and stored until measurement. The frozen leaf was thawed to ambient temperature, the plunger and Blu-Tack were removed and the barrel of the syringe was placed in a 15 ml centrifuge tube, with its tip resting inside a 1.5 ml Eppendorf tube. After centrifugation at 2500 g for 10 min at 4 °C, the osmolality of a 10 μl sample collected in the Eppendorf tube was measured by a calibrated vapour pressure osmometer (Model 5520; Wescor, Inc., Utah, USA).
Water use was determined as the difference between the water added to the pots to replace that lost and that retained in the plant (due to growth). Transpiration efficiency (TE=A/T) was determined by measurements of A and T on single leaves and water use efficiency (WUE) was determined by gravimetric determinations of growth and water loss on whole plants. Plants were harvested 49 d after sowing, 1 d after watering to field capacity. Fresh weight was measured before plants were dried at 70 °C for 72 h and dry weights were recorded. Shoot moisture content was estimated from the difference between fresh weight and dry weight. The nutrient concentration of the whole shoot was measured after digestion in nitric acid/hydrogen peroxide in a closed vessel (50 ml polypropylene centrifuge tube) using a hot block digestion system. Elemental analyses of plant samples were performed using inductively coupled plasma-optical emission spectrometry (Zarcinas et al., 1987). Chloride concentrations of the digested extracts were determined using a chloride analyser (Model 926, Sherwood Scientific, Cambridge, UK).
Statistical analysis
Statistical analyses were performed using R 2.7.1 (R Development Core Team, 2006). Data for growth, ion content, and moisture content were analysed using ANOVA to determine if significant differences were present among means. Differences among the mean values were assessed by Least Significant Differences (LSD0.05). Relationships between individual variables were examined using simple linear correlations and regressions which were performed using SigmaPlot (version 10).
Results
Experiment 1. The effect of different Na+ and Cl– ion concentrations on plant biomass
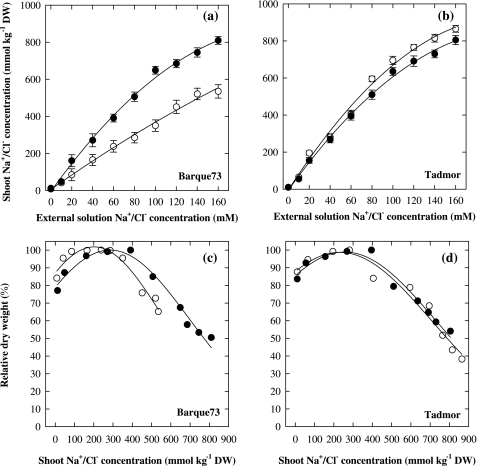
The increase in shoot Na+ concentration as a function of external Na+ concentration followed a curvilinear pattern in both genotypes (Fig. 1a, b). In Na+-dominant Hoagland's solution, maximum growth and production of shoot dry weight was attained at a Na+ tissue concentration of 300–400 mmol kg−1 DW in both genotypes (Fig. 1c, d). The Cl– tissue concentration of Tadmor followed a very similar pattern to its Na+ uptake and accumulated to similar concentrations, whereas in Barque73 the Cl– concentration increased linearly with an increase in the Cl– concentration of the external solution and was always less than the Na+ concentrations. In Cl–-dominant Hoagland's solutions, the growth of Barque73 reached maximum at a whole shoot concentration of 165–285 mmol kg−1 DW beyond which growth declined (Fig. 1c). In Tadmor, there was an increase in shoot dry weight up to tissue Cl– concentration of 195–280 mmol kg−1 DW, and growth declined sharply beyond this concentration (Fig. 1d). Barque73 accumulated up to 35% less shoot Cl– concentration compared with Tadmor. At the highest external Cl– concentration, dry weight of Barque73 was reduced by 35% with a tissue Cl– concentration of 530 mmol kg−1 DW, whereas the dry weight of Tadmor was reduced by 61% with a tissue Cl– concentration of 865 mmol kg−1 DW (Fig. 1c, d).
Fig. 1.
The whole shoot concentration of Na+ (black circles) and Cl– (open circles) of Barque73 and Tadmor as a function of the external concentration of Na+ and Cl– in hydroponic solution (a, b) and the relationship between relative shoot dry matter (%) and shoot Na+ and Cl– concentration. The dry matter weight of Barque73 and Tadmor in the control treatment was 4.45 g and 4.59 g, respectively. Bars indicate the standard error of the means. Fitted curves for (a) and (b) are derived from linear or exponential regressions, while the curves in (c) and (d) are the best fit of gaussian equation (three parameters). Values are means (n=3).
Experiment 2. The separate effects of Na+ and Cl– ions at high concentration on barley growth in solution culture
Whole shoot ion concentration and leaf osmotic potential
For plants grown in the control treatment, tissue Na+ concentration was 4–10 mmol kg−1 DW and no difference was apparent among the four genotypes. The Na+ concentration in all varieties increased with increasing concentration of Na+ in treatment solutions (Table 3). Sahara had the highest Na+ concentration of all the genotypes (∼1040 mmol kg−1 DW). The Na+ concentration in shoots of Clipper was about 5% less than Sahara, while Barque73 and Tadmor had the lowest Na+ concentration (∼720 mmol kg−1 DW) (Table 3) which is consistent with previous hydroponic screening of these varieties (E Tavakkoli, unpublished data). Potassium concentrations were not significantly affected by NaCl and Na+ treatments and did not differ significantly among the four varieties but were about 10% higher in Cl–-dominant Hoagland's solutions due to using KCl to prepare this salt solution. The K+:Na+ ratio never fell below 1.1, which is the ratio considered critical for maintaining metabolic activity (Munns, 1985). Chloride concentrations in the shoot tissue of all genotypes were 10–15 mmol kg-1 DW in the control and 2 mmol kg−1 DW in the Na+-dominant Hoagland's solutions and the Na+-gluconate treatments. The concentration increased significantly when grown under Cl–-dominant Hoagland's solutions and NaCl treatments, but the responses of genotypes differed significantly (Genotype×Treatment interaction, P <0.001). Consistent with our previous results, Barque73 showed greater Cl– exclusion (relative to Na+) whereas the shoot Cl– concentration of the other three genotypes increased to levels similar to their shoot Na+ concentration. Treatments with NaCl in the presence of DIDS resulted in about 75–83% reduction in shoot Cl– concentrations, but did not affect Na+ concentrations (Table 3).
Table 3.
The leaf osmotic potential (MPa) and whole plant shoot concentrations of Na+, K+, and Cl– (mmol kg−1 DW) of four genotypes of barley grown on solutions of NaCl (120 mM) in the presence and absence of DIDS (0.07 mM), Na+-gluconate (120 mM), Na+-Hoagland (120 mM), and Cl–-Hoagland (120 mM) for 49 d
| Leaf Ψs | Na+ | K+ | Cl– | |
| Barque73 | ||||
| Control | –1.04 | 4 | 1133 | 10 |
| NaCl | –2.46 | 780 | 1130 | 495 |
| NaCl+DIDS | –2.40 | 714 | 1120 | 128 |
| Na+-gluconate | –2.34 | 710 | 1133 | 2 |
| Na+-Hoagland | –2.31 | 722 | 1140 | 2 |
| Cl–-Hoagland | –2.35 | 5 | 1242 | 503 |
| Clipper | ||||
| Control | –1.06 | 4 | 1145 | 12 |
| NaCl | –2.55 | 974 | 1128 | 1139 |
| NaCl+DIDS | –2.52 | 985 | 1139 | 155 |
| Na+-gluconate | –2.45 | 979 | 1122 | 2 |
| Na+-Hoagland | –2.47 | 971 | 1133 | 2 |
| Cl–-Hoagland | –2.46 | 2 | 1253 | 1011 |
| Sahara | ||||
| Control | –1.03 | 10 | 1132 | 15 |
| NaCl | –2.11 | 1040 | 1123 | 1299 |
| NaCl+DIDS | –2.10 | 1042 | 1121 | 209 |
| Na+-gluconate | –2.04 | 1039 | 1123 | 2 |
| Na+-Hoagland | –2.09 | 1051 | 1129 | 2 |
| Cl–-Hoagland | –2.04 | 8 | 1267 | 1066 |
| Tadmor | ||||
| Control | –1.06 | 5 | 1148 | 11 |
| NaCl | –2.54 | 791 | 1122 | 759 |
| NaCl+DIDS | –2.45 | 727 | 1131 | 225 |
| Na+-gluconate | –2.49 | 715 | 1149 | 3 |
| Na+-Hoagland | –2.50 | 730 | 1133 | 2 |
| Cl–-Hoagland | –2.55 | 4 | 1263 | 751 |
| LSD0.05 (Genotype×Treatment) | 0.07 | 15.7 | 24.3 | 10.2 |
Values are means (n=3).
Plant biomass and water relations
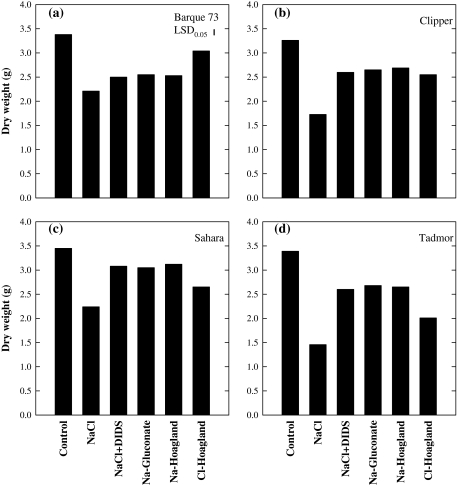
The four genotypes did not differ significantly in shoot dry weight when grown in non-saline solution for 49 d. Increased levels of salts in the solution reduced shoot dry weights of all the varieties significantly but the responses of varieties differed (Fig. 2). In Barque73 the shoot dry weight was reduced by 24% in Na+ (NaCl+DIDS, Na+-gluconate, and Na+-Hoagland) and 10% in Cl– treatments (Cl–-Hoagland), respectively, however, the reduction due to NaCl was about 34%. The dry weight of Clipper was reduced by 18%, 23%, and 47% in Na+, Cl–, and NaCl treatments, respectively. Sahara had a similar salt tolerance to Barque73 in the NaCl treatment but the growth reduction in Na+ treatments was 10%, whereas it was reduced by 23% in the Cl– treatment. Tadmor had the lowest shoot dry weight under NaCl salinity compared with the other three (43%). It showed similar shoot dry weight under high Na+ concentration as Barque73, however, the growth reduction under Cl– treatments was 40% indicating a significant effect of Cl– to shoot dry weight depression. Adding DIDS to NaCl increased the shoot dry weight of plants by 10%, 45%, 37%, and 62% in Barque73, Clipper, Sahara, and Tadmor, respectively, compared with NaCl in the absence of DIDS (Fig. 2). The pattern of growth of four genotypes in Na+-gluconate was very similar to that obtained in Na+-dominant Hoagland's solution. Leaf osmotic potential also decreased 2-fold in the saline treatments compared to control (Table 3). The reduction in Ψs was similar in Na+, Cl–, and NaCl treatments. Sahara had significantly higher Ψs compared with the other three genotypes.
Fig. 2.
Effects of NaCl (120 mM) in the presence and absence of DIDS (0.07 mM), Na+-gluconate (120 mM), Na+-dominated Hoagland (120 mM), and Cl–-dominated Hoagland (120 mM) on shoot dry weight of (a) Barque73, (b) Clipper, (c) Sahara, and (d) Tadmor grown for 49 d. Values are averages (n=3). LSD0.05=0.089 and CV=2.1%.
Experiment 3. The effects of Na+ and Cl– ions at high concentration on barley growth in saline soil
Soil properties
The Na+-treated and NaCl-treated soils had comparable levels of Na+ in the soil solution and the Cl–-treated and NaCl-treated had similar concentrations of Cl– (Table 2). The use of different salt solutions in the four treatments resulted in some variation in the concentrations of Ca2+, Mg2+, K+, P, and S. However, in all cases, the variation in plant nutrient concentrations was much lower than those in the soil solution (Tables 2, 4) and all were within the physiologically normal range for barley (Reuter and Robinson, 1997). While the effects of this variation in the other cations and anions on the growth of the plants in the different salt treatment can not be discounted completely, the much greater range in the final concentrations of Na+ and Cl– in the plants means that the responses to salt that were observed in barley were largely due to the effects of Na+, Cl–, and NaCl.
Table 4.
The shoot dry matter (g), water use efficiency (WUE, g l−1), leaf osmotic potential (MPa), and whole plant shoot concentration of Ca2+, Mg2+, Na+, K+, P, S, and Cl– (mmol kg−1DW) of four genotypes of barley grown on soils treated with Na+, Cl–, and NaCl salts for 49 d. Values are means (n=3).
| Dry weight | WUE | Leaf Ψs | Ca2+ | Mg2+ | Na+ | K+ | K+/Na+ | P | S | Cl– | |
| Barque73 | |||||||||||
| Control | 3.805 | 1.46 | –0.25 | 294 | 189 | 85 | 980 | 11.5 | 189 | 58 | 165 |
| Na+-soil | 3.010 | 1.47 | –0.75 | 144 | 155 | 688 | 648 | 0.94 | 210 | 95 | 162 |
| Cl–-soil | 3.415 | 1.46 | –0.72 | 335 | 214 | 95 | 1189 | 12.5 | 185 | 52 | 359 |
| NaCl-soil | 2.645 | 1.45 | –0.77 | 156 | 160 | 685 | 650 | 0.95 | 150 | 51 | 367 |
| Clipper | |||||||||||
| Control | 3.603 | 1.38 | –0.30 | 285 | 186 | 90 | 915 | 10.1 | 193 | 58 | 146 |
| Na+-soil | 2.755 | 1.35 | –0.87 | 148 | 125 | 450 | 373 | 0.82 | 209 | 91 | 148 |
| Cl–-soil | 2.555 | 1.33 | –0.89 | 364 | 211 | 106 | 1184 | 11.1 | 196 | 51 | 411 |
| NaCl-soil | 1.975 | 1.37 | –0.91 | 162 | 120 | 457 | 355 | 0.77 | 211 | 58 | 487 |
| Sahara | |||||||||||
| Control | 3.504 | 1.33 | –0.21 | 304 | 191 | 90 | 995 | 11.0 | 201 | 58 | 167 |
| Na+-soil | 3.111 | 1.33 | –0.59 | 253 | 160 | 750 | 881 | 1.1 | 220 | 89 | 153 |
| Cl–-soil | 2.715 | 1.29 | –0.57 | 397 | 215 | 110 | 1385 | 12.5 | 208 | 51 | 1100 |
| NaCl-soil | 2.338 | 1.29 | –0.60 | 279 | 167 | 757 | 885 | 1.1 | 196 | 53 | 1112 |
| Tadmor | |||||||||||
| Control | 3.903 | 1.46 | –0.31 | 279 | 187 | 84 | 955 | 11.3 | 190 | 57 | 141 |
| Na+-soil | 3.115 | 1.45 | –0.84 | 149 | 112 | 670 | 657 | 0.98 | 208 | 79 | 153 |
| Cl–-soil | 2.125 | 1.44 | –0.80 | 351 | 208 | 102 | 1188 | 11.6 | 193 | 50 | 980 |
| NaCl-soil | 1.333 | 1.27 | –0.85 | 132 | 116 | 675 | 651 | 0.96 | 150 | 52 | 999 |
| LSD0.05(Genotype×Treatment) | 0.167 | n.s | 0.022 | 13.8 | 7.1 | 27.1 | 29.1 | 0.58 | 7.2 | 5.5 | 15.5 |
Whole shoot ion concentration
For plants in the control treatments, the mean tissue Na+ concentration was 88 mmol kg−1 DW and there was no significant difference among the four genotypes (Table 4). As soil Na+ concentration increased, so too did tissue Na+ concentration in all genotypes. Large genotypic variation was observed in shoot Na+ concentration: Clipper had the lowest Na+ concentration (450 mmol kg−1) whereas Sahara had the highest (750 mmol kg−1), and Tadmor and Barque73 were intermediate with shoot Na+ concentration of about 685 mmol kg−1 DW. The concentration of shoot Cl– increased in Cl– and NaCl soils, and the Cl– concentrations were consistently greater than the tissue Na+ concentrations in control and NaCl treatments with the exception of Barque73 grown under NaCl. The responses of the four genotypes differed significantly (Genotype×Treatment interaction, P <0.001) (Table 4). The Cl– concentrations in Tadmor and Sahara were over 2–3 times greater than Barque73 and Clipper. Potassium concentration of all genotypes decreased significantly when exposed to Na+ or NaCl. Sahara consistently maintained a higher concentration of K+ and a much higher K+:Na+ ratio (>1.1), whereas this ratio fell below 1.1 for the other varieties, with Clipper having the lowest ratio (Table 4). This negative effect of Na+ on K+ was indicated between treatments within each genotype, however, among the genotypes there is a positive relationship between Na+ and K+. In other words, genotypes that exclude Na+ also seem to have lower K+, and the competitive relationship operates within a genotype but not between genotypes. Changes in calcium and magnesium tissue concentration followed a very similar pattern as K+, and it decreased in all genotypes when grown in Na+ and NaCl-dominant soils.
Plant biomass and water use
Increases in Na+, Cl– or NaCl concentration of the soil solution significantly (P <0.05) reduced shoot dry weight in all genotypes but the genotypic responses were different (Table 4). The shoot dry matter of Barque73, Tadmor, and Clipper was reduced by up to 20–25% in the Na+-dominant soil, whereas the reduction in Sahara was only 11%. The biomass reduction under Cl–-dominant soil was 10%, 30%, 22%, and 45% in Barque73, Clipper, Sahara, and Tadmor, respectively. The biomass reductions due to NaCl salinity reflected the additive effects of Na and Cl: they ranged from 30% in Barque73 and Sahara to 45% and 65% in Clipper and Tadmor, respectively.
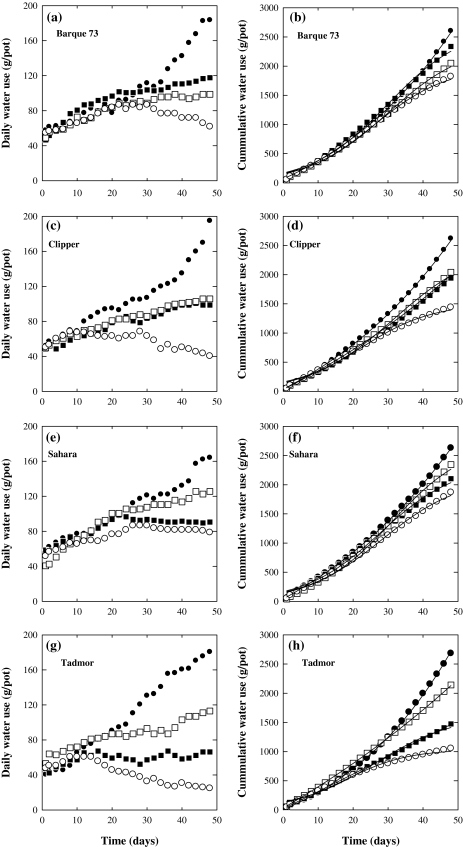
Daily water use increased consistently throughout the experiment in the control treatments of all four genotypes (Fig. 3). Salinity reduced daily water use with the largest reduction occurring in NaCl treatments. The greater sensitivity of Tadmor to salt is evident as is the greater effect of Cl– on water use. Similar to shoot dry weights (Table 4), the reduction in total water use due to the presence of NaCl was greater than that due to Na+ and Cl– individually, and was equal to the additive effect of the two. The cumulative water use at the final measurement can be used to quantify the relative effects of Na+, Cl–, and NaCl to total plant water uptake and, consequently, growth. Total water use in Barque73 in the Na+ and Cl– and NaCl treatments was reduced by 21%, 10%, and 30%, respectively, whereas in Tadmor the reductions were 20%, 45%, and 60%, respectively (Fig. 3b, d). The cumulative water use of Clipper was reduced by 22% and 25% by Na+ and Cl– treatments, respectively, and a 45% reduction occurred under NaCl. Sahara was more sensitive to Cl– than Na+ as indicated by a 20% reduction in cumulative water use under Cl– treatment compared with a 10% reduction under Na+ treatment (Fig. 3f). Leaf osmotic potential also decreased in the salt treatments compared with the control. Sahara and Barque73 had inherently higher Ψs than Tadmor and Clipper in the control (Table 4). Sahara maintained a significantly higher Ψs than the other varieties under salt stress.
Fig. 3.
The daily changes of water use (a, c, e, g) and cumulative water use (b, d, f, h) of four genotypes of barley under different levels of soil salinity generated from Cl–-salts (black squares), Na+-salts (open squares) or NaCl-salt (open circles) compared with the control (black circles) treatments. A sigmoidal curve fitted to the data of cumulative water use. Values are averages (n=3).
Chlorophyll content, gas exchange, and chlorophyll fluorescence parameters
The leaf chlorophyll content of all genotypes was increased in Na+-dominant soil (15–19%. whereas it was reduced by between 20% and 36% in Clipper, Sahara, and Tadmor in Cl–-dominant and NaCl-dominant soils and remained unchanged in Barque73 (Table 5). The gas exchange parameters of all genotypes decreased in all salt treatments. The rate of photosynthesis (A) of plants grown in Na+-dominant soil decreased by 20–25% in Barque73, Clipper, and Tadmor, and by 10% in Sahara (Table 5). In Cl–-dominant soil A was reduced by 40% in Tadmor, 23% in Clipper and Sahara, and 10% in Barque73. The greatest reduction in A occurred in the NaCl treatment and followed a similar pattern to shoot dry weight and cumulative plant water use. Clipper and Tadmor had significantly lower A than Barque73 and Sahara in the Na+ and NaCl treatments. Stomatal conductance (gs) of all genotypes followed a similar pattern of reduction to A. The relative intercellular CO2 concentration (Ci/Ca) of all genotypes decreased by 2.5–4% when grown in the Na+ treatment. This value decreased by only 6% in Barque73 under Cl– treatment, but there was a larger reduction of Ci/Ca in Tadmor (40%), Sahara (22%), and Clipper (16%). The changes of Ci/Ca under NaCl salinity was similar or just slightly less than Cl– treatment which indicates the reduction of Ci/Ca under salinity was driven by Cl– concentration (Table 5).
Table 5.
Changes in leaf chlorophyll content (SPAD unit), CO2 assimilation rate (A, μmol CO2 m−2 s−1), stomatal conductance (gs, mol m−2 s−1), partial pressures of CO2 in inside of leaf and in the air (Ci:Ca), transpiration (T, mmol m−2 s−1), and transpiration efficiency (TE, μmol CO2 mmol−1 H2O) of four varieties of barley grown in control conditions or Cl–-dominant soil, Na+-dominant soil, and NaCl-dominant soil. Plants were grown for 49 d and values are from the last measurement at day 45. Values are means (n=3).
| Chlorophyll content | A | gs | Ci:Ca | T | TE | |
| Barque73 | ||||||
| Control | 39.0 | 21.78 | 0.83 | 0.78 | 4.86 | 4.48 |
| Na+-soil | 44.1 | 17.40 | 0.61 | 0.75 | 3.45 | 5.04 |
| Cl–-soil | 38.5 | 19.55 | 0.75 | 0.76 | 4.15 | 4.71 |
| NaCl-soil | 38.0 | 14.40 | 0.53 | 0.70 | 3.22 | 4.47 |
| Clipper | ||||||
| Control | 38.7 | 20.24 | 0.80 | 0.74 | 4.51 | 4.49 |
| Na+-soil | 46.1 | 16.25 | 0.60 | 0.71 | 3.35 | 4.85 |
| Cl–-soil | 30.5 | 15.51 | 0.61 | 0.58 | 3.46 | 4.48 |
| NaCl-soil | 33.7 | 11.14 | 0.41 | 0.55 | 2.60 | 4.28 |
| Sahara | ||||||
| Control | 41.0 | 21.23 | 0.85 | 0.77 | 4.73 | 4.49 |
| Na+-soil | 46.0 | 18.91 | 0.76 | 0.75 | 3.95 | 4.79 |
| Cl–-soil | 32.5 | 16.29 | 0.65 | 0.64 | 3.61 | 4.51 |
| NaCl-soil | 35.3 | 14.62 | 0.56 | 0.65 | 2.89 | 5.06 |
| Tadmor | ||||||
| Control | 39.3 | 20.70 | 0.83 | 0.74 | 4.62 | 4.48 |
| Na+-soil | 44.7 | 15.87 | 0.58 | 0.71 | 3.06 | 5.18 |
| Cl–-soil | 25.7 | 12.19 | 0.47 | 0.44 | 2.50 | 4.88 |
| NaCl-soil | 27.3 | 8.18 | 0.31 | 0.45 | 1.73 | 4.74 |
| LSD0.05 (Genotype×Treatment) | 2.36 | 0.800 | 0.045 | 0.040 | 0.095 | 0.255 |
The barley varieties used in this study showed some significant differences in the instantaneous rates of net transpiration (T) by single leaves which followed a similar pattern to changes in the A. The transpiration efficiencies (TE) generated from these data on individual leaves therefore varied little (Table 5): the difference between the maximum and minimum values of the TE for the different cultivars was about 15% of the maximum (P <0.05). There was a significant increase in TE under Na+ treatments in all genotypes and under Cl– only in Tadmor, however, the TE of Barque73 and Clipper was not affected by NaCl treatment while it increased in Sahara and Tadmor (P <0.05). The varieties also differed significantly in their water-use efficiencies (WUE) when these were estimated for whole plants (Table 4). The WUE was correlated with the growth among the four varieties, however, Na+, Cl–, and NaCl treatments did not significantly affect WUE.
The Cl– and NaCl salts had a marked effect on the four key fluorescence parameters measured but the effects varied with genotypes (Table 6). The presence of Cl– had a larger effect than Na+ in all varieties. Values for the efficiency of light harvesting of PSII (F′v/F′m) of Clipper and Sahara were reduced by 20–25% in the Cl– treatment and by 40% in Tadmor; the reduction in Barque73 was smaller, but still significant (P <0.05). A similar pattern was observed for qP and ΦPSII under both treatments. Significant relationships between A and both (F′v/F′m) (r=0.89) and ΦPSII (r=0.92) were found for Clipper, Sahara, and Tadmor, indicating that these parameters may not be independent or are co-regulated (Tables 5, 6). As the proportion of PSII reaction centres that remained open (qP) was reduced in Clipper, Sahara, and Tadmor, the portion of fluorescence quenching associated with thermal energy dissipation (NPQ) increased significantly, but the effect was larger in Tadmor. These values changed similarly for Barque73 under Cl– and NaCl treatments, but the changes are smaller in magnitude (Table 6).
Table 6.
Changes in fluorescence parameters: efficiency of light harvesting (F′v/F′m), actual quantum yield of PSII electron transport (ФPSII), photochemical quenching (qP) and non-photochemical quenching (NPQ) of intact leaves of four varieties of barley grown in control conditions or Cl–-dominant soil, Na+-dominant soil, and NaCl-dominant soil. Plants were grown for 49 d and values are from the last measurement at day 45. Values are means (n=3).
| (F′v/F′m) | ΦPSII | qP | NPQ | |
| Barque73 | ||||
| Control | 0.75 | 0.56 | 0.72 | 0.32 |
| Na+-soil | 0.73 | 0.55 | 0.70 | 0.33 |
| Cl–-soil | 0.70 | 0.50 | 0.65 | 0.36 |
| NaCl-soil | 0.71 | 0.51 | 0.62 | 0.35 |
| Clipper | ||||
| Control | 0.76 | 0.56 | 0.69 | 0.32 |
| Na+-soil | 0.74 | 0.51 | 0.68 | 0.34 |
| Cl–-soil | 0.60 | 0.42 | 0.52 | 0.38 |
| NaCl-soil | 0.59 | 0.41 | 0.50 | 0.38 |
| Sahara | ||||
| Control | 0.75 | 0.56 | 0.74 | 0.33 |
| Na+-soil | 0.73 | 0.54 | 0.75 | 0.37 |
| Cl–-soil | 0.57 | 0.41 | 0.58 | 0.39 |
| NaCl-soil | 0.56 | 0.40 | 0.55 | 0.40 |
| Tadmor | ||||
| Control | 0.76 | 0.55 | 0.73 | 0.32 |
| Na+-soil | 0.72 | 0.53 | 0.74 | 0.33 |
| Cl–-soil | 0.41 | 0.35 | 0.45 | 0.52 |
| NaCl-soil | 0.40 | 0.33 | 0.46 | 0.58 |
| LSD0.05 (Genotype×Treatment) | 0.057 | 0.037 | 0.044 | 0.022 |
Discussion
The work reported here is one of the few studies to have attempted to separate the toxic effects of Na+ and Cl– and their individual and combined contributions to salt tolerance at the whole plant level. Previous studies comparing the effects of Na+ and Cl− in cereals grown under salinity stress have mainly used short-term hydroponic experiments with a single salt of Na+ or Cl– and one or two genotypes (Kingsbury and Epstein, 1986; Lin and Kao, 2001; Tsai et al., 2004; Luo et al., 2005). This has produced only equivocal results because of the difficulty in changing the external concentration of one ion versus another without changing the osmotic pressure of the external solution (Munns and Tester, 2008) or the rate of uptake and activity of other ions (Cramer et al., 1986). Also, given the fact that the most convincing approach to test the toxicity of Na+ versus Cl− is by comparing genotypes differing in their salt tolerance and ion uptake (Munns and Tester, 2008; Teakle and Tyerman, 2010), there is a need to integrate the use of suitable genetic material and treatments with a mixture of the Na+ and Cl– salts to improve our understanding in this area. In this study, the Na+-dominant and Cl–-dominant Hoagland's solutions were designed to give equimolar concentrations of the Na+ and Cl– ions generated from various salts of Na+ and Cl– to avoid increasing particular counter-anions/counter-cations. Using barley varieties with known genetic variation in salinity tolerance and in Na+ and Cl– uptake also assisted in distinguishing the toxic effects of Na+ from Cl–. As the responses to salinity in hydroponics and soil may also be fundamentally different (Tavakkoli et al., 2010b), a soil-based experiment was designed to simulate the responses in field-grown plants. The method employed here maintained a constant EC and ΨO, but used a combination of different salts to produce soils enhanced with Na+, Cl–, and NaCl. It is essential to maintain the same ΨO over the treatments because changes in soil salt composition also affects soil ΨO and therefore plant growth. Comparisons of the effects of Na+ and Cl– on growth were possible due to the use of soils with similar EC and ΨO but different concentrations and combinations of Na+ and Cl–. Despite the utility of this approach, the method inevitably led to differences in the concentrations of the other balancing ions (Ca2+, Mg2+, K+, S, and P) in the soil solution (Table 2). These concentration differences may potentially affect the responses since the Ca2+ and K+ availability can change with salinity responses (Genc et al., 2010). However, the variation in concentrations of these ions in the plant tissue was considerably less that that measured in the soil solution (Table 4). As a result, the buffering capacity of the soil may have, in part, affected the activity of the ions in solution and may have reduced their potential interactions with Na+ and Cl– ions on salt tolerance (Khasawneh and Copeland, 1973).
Growing barley under NaCl stress reduced the growth of barley in hydroponics and in soil with significant differences among genotypes in the sensitivity to salt stress. However, in both growth media, the reduction in growth was caused by the additive effect of the reductions due to Na+ and Cl–. Moreover, the responses to Na+ and Cl– stress were independent and so similar levels of tolerance to NaCl could be achieved by different combinations of responses to Na+ and Cl–. This result is clearly at odds with previous studies that have dismissed Cl– toxicity as a contributing factor to salt damage (Kingsbury and Epstein, 1986; Kinraide, 1999; Lin and Kao, 2001; Tsai et al., 2004). However, it is important to consider that these previous experiments either used sole counter-anions such as 121 mM nitrate (Kingsbury and Epstein, 1986), which at high concentrations can be phytotoxic (Chen et al., 2004), or were short-term studies lasting less than a week (Kinraide, 1999). The two-phase model of salt injury (Munns et al., 1995) suggests that specific ionic toxicity builds up over a longer period. Thus, a relationship which may arise under short-term studies may not necessarily be valid for long-term responses. Moreover, in the study by Lin and Kao (2001) where 50 mM Na+-gluconate was compared with 50, 100, and 150 mM NaCl, the interpretation of the effects of Na+ and Cl– was confounded by differences in the EC of the solutions as well as the ion concentrations. The contribution of Cl– toxicity to the effects of salt stress observed in the current experiments lends support to the arguments that high soil Cl– can be an important limitation to the growth and yield of crops (Dang et al., 2008, 2010).
The concentrations of Na+ and Cl– increased in barley plants exposed to salt (Fig. 1; Tables 3, 4), with tissue Cl– concentrations of soil-grown plants generally exceeding those of Na+ (Table 4). Increases in plant ion concentrations occur with all plants exposed to NaCl (Flowers et al., 2010) but there are few, if any, reliable estimates of the critical concentrations for Na+ and Cl– toxicity (Boursier et al., 1987; Reuter and Robinson, 1997). When grown under Na+-dominant Hoagland's solutions, the dry weight of both genotypes started to decline at a tissue Na+ concentration of >400 mmol kg−1 DW, however, Barque73 and Tadmor differed in their response to Cl– treatments. The tissue Cl– concentration of Barque73 increased up to 60 mM external Cl– concentration, after which plant growth declined, which corresponded to concentrations above 250–300 mmol Cl–. This is similar to the pattern of salt response that was proposed by Maas and Hoffman (1977). On the other hand, a greater decline in the growth of Tadmor with higher tissue Cl– concentration compared to Barque73 strongly suggests that the poor ability of Tadmor to exclude the Cl– from shoots reduces its growth. Although a few studies in the past have shown the importance of partitioning of Cl– in the leaf sheath (Boursier et al., 1987; Boursier and Läuchli, 1989) and maintenance of low Cl– concentrations in mesophyll cells of leaf blades of barley seedlings under salt stress (Huang and Van Steveninck, 1989), to our knowledge, this is the first report on a significant contribution of Cl– exclusion to the salt tolerance of barley at the whole plant level. The other interesting point of Fig. 1 is that barley plants responded positively to Na+ and Cl– at very low concentrations (up to 40 mM in external solution), which is the range found in the non-saline soil (Table 4).
A comparison between Barque73 and Tadmor with similar shoot Na+ concentrations but different levels of Cl– accumulation (Table 3) in Cl–-dominant Hoagland's solution clearly showed that maintenance of low Cl– concentration in Barque73 contributed to its higher Cl–-salt-tolerance (92%), whereas growth of Tadmor was reduced by 45%. The growth of Clipper was reduced by Na+ and Cl– treatments to a similar extent, whereas Sahara showed a slightly higher sensitivity to high Cl– compared with Na+. Together, these results strongly suggest that high concentrations of Na+ and Cl– were contributing to a growth reduction under saline treatments. It was indicated that an ability of a variety to exclude Cl– can be very important for improving salt tolerance similar to that showed for Barque73.
Maintenance of high K+ concentrations in salt-tolerant genotypes may be one of the mechanisms underlying their superior salt tolerance (Maathuis and Amtmann, 1999; Britto et al., 2010; Tester and Davenport, 2003), but the expression of this trait in this study differed between hydroponics and soil systems. A significant reduction in plant K+ occurred with increasing Na+ concentration under Na+ and NaCl treatments in soil but, in the hydroponic experiment, the concentrations of Na+ and K+ were independent of one another (Tables 3, 4). Although barley exhibited a high selectivity for K+ (Table 4) similar to that found in highly salt-tolerant species (Ball et al., 1987), the amount of K+ in leaf tissues declined with increasing salinity as Na+ increased. As Na+ cannot replace the specific functions of K+ required for protein synthesis (Leigh and Wyn Jones, 1984), it is very likely that changes in growth and photosynthetic capacity in association with increasing salinity in the present study are related to salinity-induced changes in K+ uptake by roots, and concomitantly related to the K+ concentration in the cytoplasm and chloroplasts. It is also important to note that the competitive relationship between Na+ and K+ operates within a genotype, but not between genotypes, and so it could not be extrapolated to salt tolerance among genotypes, an aspect that is frequently overlooked.
The effect of salinity on photosynthesis is complex. The positive relationship between stomatal conductance and net photosynthesis indicates that the primary limiting factor for net photosynthesis upon exposure to salinity is stomatal closure. Photosynthesis can be further limited by non-optimal metabolic conditions caused by ion toxicity or ion imbalances (Table 5). The main cause for the non-stomatal limitation of photosynthesis seems to be an accumulation of the toxic ions in leaf cells (Cramer, 1992; Greenway and Munns, 1980; Huang and Van Steveninck, 1989). The Na+ and Cl– accumulation showed a high correlation with the progressive decrease in growth and most of the photosynthetic characteristics studied (Table 5). Under high Na+ concentration, A was mostly limited by gs which suggests that the reduction in assimilation of CO2 was due to stomatal factors. Because stressed plants require high concentrations of K+ in epidermal and guard cells for the regulation of stomata opening and closure, salt-induced K+ loss leads to a closing of stomata (Chow et al., 1990). The lack of stomatal closure causes high transpiration and loss of water. Humble and Raschke (1971) indicated that K+ is the specific ion involved in stomatal opening. Shabala and Cuin (2008) also concluded that K+ distribution in the leaf apoplast is one of the important factors determining stomatal patchiness and heterogeneity in leaf photosynthesis. Therefore, one of the reasons that Sahara was less affected by Na+ may be due to its high K+ concentration and greater control of stomatal regulations under such stress compared with other genotypes. However a large reduction of Ci:Ca in parallel to gs under Cl– and NaCl in Clipper, Sahara, and Tadmor suggests specific damaging effects of Cl– on photosynthetic machinery in addition to stomatal limitations (Table 5).
Long-term inhibition of plant growth by salinity is associated with the appearance of leaf chlorosis or necrosis (Strogonov, 1974). Chlorosis also develops from chloride injuries (Kozlowski, 1997). A significant decline in leaf chlorophyll content with increasing leaf Cl– concentration was observed in Clipper, Sahara, and Tadmor. However, plants grown under Na+-dominant soil not only showed no reduction but a slight increase in chlorophyll concentration (Table 5). Therefore, it may be assumed that high concentrations of Na+ are not the primary reason for the degradation of chlorophyll under NaCl stress, and the decreased chlorophyll concentration may be induced by increased Cl– concentrations.
A significant correlation between reduced leaf chlorophyll content and the parameters of chlorophyll fluorescence with increasing Cl– concentration but not Na+ was found (Tables 5, 6). Moreover, while a significant reduction in (F′v/F′m), ΦPSII and qP of Tadmor, Clipper, and Sahara under Cl– and NaCl stress was indicated, Barque73 (the Cl–-excluding variety) maintained a higher capacity of the PSII system (Table 6). Not only does this finding suggest that a high concentration of Cl– is damaging the photosynthetic machinery, but also that Cl– exclusion is an important mechanism under saline conditions where Cl– concentrations are high. The decrease of Ci:Ca along with stomatal closure seems more likely to cause losses in PSII efficiency, probably by reducing the CO2 availability for photosynthesis. The decrease of Ci:Ca (Table 5) with declining gs, reduced F′v/F′m which is often associated with unrepaired damage to PSII rather than just with reduced operating efficiency (Lawson et al., 2002). The limited availability of CO2 would be likely to depress the amounts of electron-accepting NADP+ as the carbon reduction cycle slowed (Else et al., 2009). The quantum yield of PSII in the light results from the fraction of open centres that perform photochemistry (qP) and its reduction is indicative of a down-regulation of leaf photochemistry to match the reduced carbon acquisition under high Cl– and NaCl accumulation (i.e. photo-inhibition as well as salt-induced photo-damage).
Despite the fact that it was frequently stated that photosynthetic capacity is weakly correlated with the amounts of PSII reaction centres, and even less correlated with the PSI reaction centres (Jiang et al., 2006a, b; Linger and Brüggemann, 1999; Netondo et al., 2004), our results demonstrate that ΦPSII is highly correlated with A and gs. Similar results were obtained only in two other studies: in a study of grape leaves subjected to progressive drought (Flexas et al., 2002) and one examining barley plants under saline treatments (Jiang et al., 2006b). This study has provided estimates of the separate contributions of Na+ and Cl– to the effects of salt stress on yield of PSII electron transport. A reduced quantum yield, as found in the present experiment (Table 6) may result from a structural impact of high Cl– concentration on PSII. Salinity has been concluded to affect reaction centres of PSII either directly (Masojidek and Hall, 1992) or via an accelerated senescence (Kurra-Hotta et al., 1987). A structural change of PSII, its immediate surroundings or both is suggested by the increase of NPQ in plants grown at high salt concentration (Table 6). The rise in NPQ may also reflect the fact that reduced CO2 assimilation decreases demand for products of electron transport, and thus increases thermal dissipation of light energy (James et al., 2002). A possible effect of Cl– on plant cell function has been suggested by the sensitivity of leaf RuBP carboxylase levels, and therefore rate of assimilation of CO2, to leaf Cl– level in Prunus sp. (Ziska et al., 1990), which correlates with the known inhibitory effect of Cl– on protein and enzyme synthesis (Wyn Jones and Pollard, 1983; Gimmler et al., 1984). Thus it is certainly conceivable that excess Cl– ions in the cytoplasm could act to reduce growth and photosynthesis of the plant. If these effects were combined with those generated by the loss of K+ and Ca2+ homoeostasis, a substantial loss of plant viability could result.
Most of the studies described in the literature about the effects of salinity on the growth of cereals have used hydroponic systems to assess the importance of different mechanisms of salt tolerance and/or to develop selection criteria to improve salt tolerance. However, in this study, some important differences were observed between solution and soil culture which must be taken into account for future research. The maintenance of high K+ concentrations in salt-tolerant genotypes is one of the mechanisms underlying their salt tolerance, but the expression of this trait differed between hydroponics and soil systems. In the hydroponic experiment, the concentrations of Na+ and K+ were independent of one another (Table 3). By contrast, in the soil experiment a significant K+ and Na+ discrimination was found. In addition, genetic differences in Na+ exclusion and Na+:K+ discrimination between genotypes were not expressed in hydroponics. In soil, however, genetic differences in Na+ exclusion were expressed (Tables 3, 4). The leaf osmotic potential of plants grown under the hydroponic system was significantly lower than those grown in soil, which reflects the large difference between the two cultures in terms of the rate of ion uptake by plants (Tables 3, 4). Much of the research has, in the past, involved suddenly exposing plants to such high concentrations of NaCl (>100 mM) so as to cause osmotic shock rather than osmotic stress which induces major trauma that rarely if ever occurs in nature (Passioura 2010). An attempt was made to overcome the trauma of osmotic shock by increasing the concentration of salt gradually, in several small steps over a few days rather than in one large step (see the Materials and methods), but even this may uncover little useful genetic variation in salt tolerance. The important point is that it can take weeks for such variation to become evident in soil and especially under field conditions, and soil-grown plants will have more time to adapt to the salt concentration than plants in hydroponic systems (Passioura 2010). This is of a particular importance for adaptation mechanisms such as osmotic adjustment, which requires the uptake of ions and the formation of compatible solutes (Vetterlein et al. 2004).
Conclusion
The results of this study clearly indicated that the high Cl– and Na+ concentrations of saline soils can be a major cause of a reduction in barley growth. The greater reduction in growth under NaCl treatment compared with Na+ and Cl– separately, suggests that high concentrations of Na+ and Cl– have an additive and/or interactive effect. The results demonstrated that Na+ and Cl– exclusion are independent mechanisms, and different genotypes expressed different combinations of the two mechanisms. High Na+ interferes with K+ and Ca2+ nutrition and stomatal regulation, while high Cl– concentration reduces photosynthetic capacity due to chlorophyll degradation. This study also demonstrated that solution culture may not allow differences in salt tolerance between genotypes to be discerned and the physiological responses are not the same when the same materials were grown in soil. The results of this work provided support for further studies to examine both Na+ and Cl– ions in parallel, which is of crucial importance for the understanding of salt damage and for the manipulation of salt tolerance.
Supplementary Material
Acknowledgments
We thank Waite Analytical Services (The University of Adelaide) for their help with the elemental analyses and Dr G Lyons, Dr J Emms, and Dr J Smith for useful comments on the manuscript. This work was supported by a grant from the Grains Research and Development Corporation to ET and by the University of Adelaide.
References
- Amtmann A, Sanders D. Mechanisms of Na uptake by plant cells. Advances in Botanical Research. 1998;29:75–112. [Google Scholar]
- Apse MP, Blumwald E. Na+ transport in plants. FEBS Letters. 2007;581:2247–2254. doi: 10.1016/j.febslet.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Aydi S, Sassi S, Abdelly C. Growth, nitrogen fixation and ion distribution in Medicago truncatula subjected to salt stress. Plant and Soil. 2008;312:59–67. [Google Scholar]
- Ball MC, Chow WS, Anderson JM. Salinity-induced potassium deficiency causes loss of functional photosystem II in leaves of the grey mangrove, Avicennia marina, through depletion of the atrazine-binding polypeptide. Australian Journal of Plant Physiology. 1987;14:351–361. [Google Scholar]
- Boursier P, Läuchli A. Mechanisms of chloride partitioning in the leaves of salt-stressed Sorghum bicolor L. Physiologia Plantarum. 1989;77:537–544. [Google Scholar]
- Boursier P, Lynch J, Lauchli A, Epstein E. Chloride partitioning in leaves of salt-stressed sorghum, maize, wheat and barley. Australian Journal of Plant Physiology. 1987;14:463–473. [Google Scholar]
- Britto DT, Ebrahimi-Ardebili S, Hamam AM, Coskun D, Kronzucker HJ. 42K analysis of sodium-induced potassium efflux in barley: mechanism and relevance to salt tolerance. New Phytologist. 2010;186:373–384. doi: 10.1111/j.1469-8137.2009.03169.x. [DOI] [PubMed] [Google Scholar]
- Casey WH, Kinrade SD, Knight CTG, Rains DW, Epstein E. Aqueous silicate complexes in wheat, Triticum aestivum L. Plant, Cell and Environment. 2003;27:51–54. [Google Scholar]
- Chen BM, Wang ZH, Li SX, Wang GX, Song HX, Wang XN. Effects of nitrate supply on plant growth, nitrate accumulation, metabolic nitrate concentration and nitrate reductase activity in three leafy vegetables. Plant Science. 2004;167:635–643. [Google Scholar]
- Chow WS, Marilyn C, Ball C, Anderson JM. Growth and photosynthetic responses of spinach to salinity: implictions of K+ nutrition for salt tolerance. Australian Journal of Plant Physiology. 1990;17:563–578. [Google Scholar]
- Cramer G, Lauchli A, Epstein E. Effects of NaCl and CaCl2 on ion activities in complex nutrient solutions and root growth of cotton. Plant Physiology. 1986;81:792–797. doi: 10.1104/pp.81.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer GR. Kinetics of maize leaf elongation. 2. Responses of a Na+-excluding cultivar and a Na+-including cultivar to varying Na+/Ca2+ salinities. Journal of Experimental Botany. 1992;43:857–864. [Google Scholar]
- Cramer GR, Epstein E, Läuchli A. Effects of sodium, potassium and calcium on salt-stressed barley. I. Growth analysis. Physiologia Plantarum. 1990;80:83–88. [Google Scholar]
- Dang YP, Dalal RC, Buck SR, et al. Diagnosis, extent, impacts, and management of subsoil constraints in the northern grains cropping region of Australia. Australian Journal of Soil Research. 2010;48:105–119. [Google Scholar]
- Dang YP, Dalal RC, Mayer DG, et al. High subsoil chloride concentrations reduce soil water extraction and crop yield on Vertisols in north-eastern Australia. Australian Journal of Agricultural Research. 2008;59:321–330. [Google Scholar]
- Dang YP, Dalal RC, Routley R, Schwenke GD, Daniells I. Subsoil constraints to grain production in the cropping soils of the north-eastern region of Australia: an overview. Australian Journal of Experimental Agriculture. 2006;46:19–35. [Google Scholar]
- Ellis RP, Forster BP, Gordon DC, et al. Phenotype/genotype associations for yield and salt tolerance in a barley mapping population segregating for two dwarfing genes. Journal of Experimental Botany. 2002;53:1163–1176. doi: 10.1093/jexbot/53.371.1163. [DOI] [PubMed] [Google Scholar]
- Else MA, Janowiak F, Atkinson CJ, Jackson MB. Root signals and stomatal closure in relation to photosynthesis, chlorophyll a fluorescence and adventitious rooting of flooded tomato plants. Annals of Botany. 2009;103:313–323. doi: 10.1093/aob/mcn208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flexas J, Bota J, Escalona JM, Sampol B, Medrano H. Effects of drought on photosynthesis in grapevines under field conditions: an evaluation of stomatal and mesophyll limitations. Functional Plant Biology. 2002;29:461–471. doi: 10.1071/PP01119. [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Gaur PM, Gowda CLL, et al. Salt sensitivity in chickpea. Plant, Cell and Environment. 2010;33:490–509. doi: 10.1111/j.1365-3040.2009.02051.x. [DOI] [PubMed] [Google Scholar]
- Forster BP, Russell JR, Ellis RP, et al. Locating genotypes and genes for abiotic stress tolerance in barley: a strategy using maps, markers and the wild species. New Phytologist. 1997;137:141–147. [Google Scholar]
- Genc Y, Tester M, Mcdonald GK. Calcium requirement of wheat in saline and non-saline conditions. Plant and Soil. 2010;327:331–345. [Google Scholar]
- Genty B, Briantais JM, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta. 1989;990:87–92. [Google Scholar]
- Gimmler H, Kaaden R, Kirchiner U, Weyand A. The chloride sensitivity of Dunaliella parva enzymes. Zeitschrift für Pflanzenphysiologie. 1984;114:131–150. [Google Scholar]
- Greenway H, Munns R. Mechanisms of salt tolerance in nonhalophytes. Annual Review of Plant Physiology. 1980;31:149–190. [Google Scholar]
- Gregory PJ, Bengough AG, Grinev D, Schmidt S, Thomas WTB, Wojciechowski T, Young IM. Root phenomics of crops: opportunities and challenges. Functional Plant Biology. 2009;36:922–929. doi: 10.1071/FP09150. [DOI] [PubMed] [Google Scholar]
- Huang CX, Van Steveninck RFM. Maintenance of low Cl– concentrations in mesophyll cells of leaf blades of barley seedlings exposed to salt stress. Plant Physiology. 1989;90:1440–1443. doi: 10.1104/pp.90.4.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humble GD, Raschke K. Stomatal opening quantitatively related to potassium transport. Plant Physiology. 1971;48:447–453. doi: 10.1104/pp.48.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbell RF. The Australian soil classification. Melbourne: CSIRO; 1996. [Google Scholar]
- Islam S, Malik AI, Islam AKMR, Colmer TD. Salt tolerance in a Hordeum marinum–Triticum aestivum amphiploid, and its parents. Journal of Experimental Botany. 2007;58:1219–1229. doi: 10.1093/jxb/erl293. [DOI] [PubMed] [Google Scholar]
- James R, Rivelli AR, Munns R, Caemmere SV. Factors affecting CO2 assimilation, leaf injury and growth in salt-stressed durum wheat. Functional Plant Biology. 2002;29:1393–1403. doi: 10.1071/FP02069. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Roche D, Monaco TA, Durham S. Gas exchange, chlorophyll fluorescence parameters and carbon isotope discrimination of 14 barley genetic lines in response to salinity. Field Crops Research. 2006a;96:269–278. [Google Scholar]
- Jiang Q, Roche D, Monaco TA, Hole D. Stomatal conductance is a key parameter to assess limitations to photosynthesis and growth potential in barley genotypes. Plant Biology. 2006b;8:515–521. doi: 10.1055/s-2006-923964. [DOI] [PubMed] [Google Scholar]
- Khasawneh FE, Copeland JP. Cotton root growth and uptake of nutrients: relation of phosphorus uptake to quantity, intensity, and buffering capacity. Soil Science Society of America Journal. 1973;37:250–254. [Google Scholar]
- Kingsbury R, Epstein E. Salt sensitivity in wheat. A case for specific ion toxicity. Plant Physiology. 1986;80:651–654. doi: 10.1104/pp.80.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide TB. Interactions among Ca2+, Na+, and K+in salinity toxicity: quantitative resolution of multiple toxic and ameliorative effects. Journal of Experimental Botany. 1999;50:1495–1505. [Google Scholar]
- Klute A. Water retention: laboratory methods. In: Klute A, editor. Methods of soil analysis. 1986. Part 1. Agronomy Monographs 9. Madison, WI: ASA and SSSA, 635–662. [Google Scholar]
- Kozlowski TT. Responses of woody plants to flooding and salinity. Tree Physiology Monographs. 1997;1:1–29. [Google Scholar]
- Kurra-Hotta M, Satoh K, Katoh S. Relationship between photosynthesis and chlorophyll content during leaf senescence of rice seedlings. Plant and Cell Physiology. 1987;28:1321–1329. [Google Scholar]
- Lawson T, Oxborough K, Morison JIL, Baker NR. Responses of photosynthetic electron transport in stomatal guard cells and mesophyll cells in intact leaves to light, CO2 and humidity. Plant Physiology. 2002;128:52–56. [PMC free article] [PubMed] [Google Scholar]
- Leigh RA, Wyn Jones RG. A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New Phytologist. 1984;97:1–13. [Google Scholar]
- Lin CC, Kao CH. Relative importance of Na+, Cl−, and abscisic acid in NaCl-induced inhibition of root growth of rice seedlings. Plant and Soil. 2001;237:165–171. [Google Scholar]
- Lin W. Inhibition of anion transport in corn root protoplast. Plant Physiology. 1981;68:435–438. doi: 10.1104/pp.68.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linger P, Brüggemann W. Correlations between chlorophyll fluorescence quenching parameters and photosynthesis in a segregating Lycopersicon esculentum L. peruvianum population as measured under constant conditions. Photosynthesis Research. 1999;61:145–156. [Google Scholar]
- Luo Q, Yu B, Liu Y. Differential sensitivity to chloride and sodium ions in seedlings of Glycine max and G. soja under NaCl stress. Journal of Plant Physiology. 2005;162:1003–1012. doi: 10.1016/j.jplph.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Maas EV, Hoffman GJ. Crop salt tolerance-current assessment. Journal of Irrigation and Drainage Engineering. 1977;103:115–134. [Google Scholar]
- Maathuis FJM, Amtmann A. K+ nutrition and Na+ toxicity: the basis of cellular K+/Na+ ratios. Annals of Botany. 1999;84:123–133. [Google Scholar]
- Mano Y, Takeda K. Mapping quantitative trait loci for salt tolerance at germination and the seedling stage in barley (Hordeum vulgare L.) Euphytica. 1997;94:263–272. [Google Scholar]
- Martin P, Koebner R. Sodium and chloride ions contribute synergistically to salt toxicity in wheat. Biologia Plantarum. 1995;37:265–271. [Google Scholar]
- Masojidek J, Hall DO. Salinity and drought stress are amplified by high irradiance in sorghum. Photosynthetica. 1992;27:159–171. [Google Scholar]
- Munns R. Na+, K+, and Cl– in xylem sap flowing to shoots of NaCl-treated barley. Journal of Experimental Botany. 1985;36:1032–1042. [Google Scholar]
- Munns R, Guo JM, Passioura JB, Cramer GR. Leaf water status controls day-time but not daily rates of leaf expansion in salt-treated barley. Australian Journal of Plant Physiology. 2000;27:949–957. [Google Scholar]
- Munns R, Rawson HM. Effect of salinity on salt accumulation and reproductive development in the apical meristem of wheat and barley. Australian Journal of Plant Physiology. 1999;26:459–464. [Google Scholar]
- Munns R, Schachtman DP, Condon AG. The significance of a two-phase growth response to salinity in wheat and barley. Australian Journal of Agricultural Research. 1995;22:561–569. [Google Scholar]
- Munns R, Tester M. Mechanisms of salinity tolerance. Annual Review of Plant Biology. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Netondo GW, Onyango JC, Beck E. Sorghum and salinity. II. Gas exchange and chlorophyll fluorescence of sorghum under salt stress. Crop Science. 2004;44:806–811. [Google Scholar]
- Passioura JB. Scaling up: the essence of effective agricultural research. Functional Plant Biology. 2010;37:585–591. [Google Scholar]
- R Development Core Team . A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2006. [Google Scholar]
- Rengasamy P. Soil processes affecting crop production in salt-affected soils. Australian Journal of Soil Research. 2010;37:613–620. [Google Scholar]
- Reuter DJ, Robinson JB, editors. Plant analysis. An interpretation manual. 2nd edn. Melbourne: CSIRO Publishing; 1997. [Google Scholar]
- Rivandi A. Toward map-based cloning of Na+ exclusion gene from barley (Hordeum vulgare L.) The University of Adelaide; 2009. [Google Scholar]
- Rodriguez-Navarro A, Rubio F. High-affinity potassium and sodium transport systems in plants. Journal of Experimental Botany. 2006;57:1149–1160. doi: 10.1093/jxb/erj068. [DOI] [PubMed] [Google Scholar]
- Shabala S, Cuin TA. Potassium transport and plant salt tolerance. Physiologia Plantarum. 2008;133:651–669. doi: 10.1111/j.1399-3054.2007.01008.x. [DOI] [PubMed] [Google Scholar]
- Slabu C, Zörb C, Steffens D, Schubert S. Is salt stress of faba bean (Vicia faba) caused by Na+or Cl– toxicity? Journal of Plant Nutrition and Soil Science. 2009;172:644–650. [Google Scholar]
- Strogonov BP. Structure and function of plant cell in saline habitats. New York, Toronto: Wiley; 1974. [Google Scholar]
- Tavakkoli E, Rengasamy P, Mcdonald GK. High concentrations of Na+ and Cl– ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress. Journal of Experimental Botany. 2010a;61:4449–4459. doi: 10.1093/jxb/erq251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakkoli E, Rengasamy P, Mcdonald GK. The response of barley to salinity stress differs between hydroponics and soil systems. Functional Plant Biology. 2010b;37:621–633. [Google Scholar]
- Teakle NL, Flowers TJ, Real D, Colmer TD. Lotus tenuis tolerates the interactive effects of salinity and waterlogging by ‘excluding’ Na+ and Cl– from the xylem. Journal of Experimental Botany. 2007;58:2169–2180. doi: 10.1093/jxb/erm102. [DOI] [PubMed] [Google Scholar]
- Teakle NL, Tyerman SD. Mechanisms of Cl– transport contributing to salt tolerance. Plant, Cell and Environment. 2010;33:566–589. doi: 10.1111/j.1365-3040.2009.02060.x. [DOI] [PubMed] [Google Scholar]
- Tester M, Davenport R. Na+ tolerance and Na+ transport in higher plants. Annals of Botany. 2003;91:503–527. doi: 10.1093/aob/mcg058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tester M, Leigh RA. Partitioning of nutrient transport processes in roots. Journal of Experimental Botany. 2001;52:445–457. doi: 10.1093/jexbot/52.suppl_1.445. [DOI] [PubMed] [Google Scholar]
- Tsai YC, Hong C-Y, Liu L-F, Kao H. Relative importnace of Na+ and Cl– in NaCl-induced antioxidant systems in roots of rice seedlings. Physiologia Plantarum. 2004;122:86–94. [Google Scholar]
- Vetterlein D, Kuhn K, Schubert S, Jahn R. Consequences of sodium exclusion on the osmotic potential in the rhizosphere–comparison of two maize cultivars differing in Na+ uptake. Plant Nutrition and Soil Science. 2004;167:337–344. [Google Scholar]
- White PJ, Broadley MR. Chloride in soils and its uptake and movement within the plant: a review. Annals of Botany. 2001;88:967–988. [Google Scholar]
- Wyn Jones RG, Pollard A. Proteins, enzymes and inorganic ions. In: Lauchli A, Bieleski RL, editors. Encyclopaedia of plant physiology (New series) Inorganic plant nutrition. Heidelberg: Springer Verlag; 1983. pp. 528–562. [Google Scholar]
- Xu G, Magen H, Tarchitzky J, Kafkafi U. Advances in chloride nutrition of plants. Advances in Agronomy. 2000;68:97–150. [Google Scholar]
- Zarcinas BA, Cartwright B, Spouncer LR. Nitric acid digestion and multi-element analysis of plant material by inductively coupled plasma spectrometry. Communications in Soil Science and Plant Analysis. 1987;18:131–146. [Google Scholar]
- Ziska LH, Seemann JR, DeJong TM. Salinity induced limitations on photosynthesis in Prunus salicina, a deciduous tree species. Plant Physiology. 1990;93:864–870. doi: 10.1104/pp.93.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.