Abstract
Positron emission tomography (PET) is one of the most rapidly growing areas of medical imaging, with many applications in the clinical management of patients with cancer. The principal goal of PET imaging is to visualize, characterize, and measure biological processes at the cellular, subcellular, and molecular levels in living subjects using noninvasive procedures. PET imaging takes advantage of the traditional diagnostic imaging techniques and introduces positron-emitting probes to determine the expression of indicative molecular targets at different stages of cancer progression. Although [18F]fluorodeoxyglucose ([18F]FDG)-PET has been widely utilized for staging and restaging of cancer, evaluation of response to treatment, differentiation of post-therapy alterations from residual or recurrent tumor, and assessment of prognosis, [18F]FDG is not a target-specific PET tracer. Over the last decade, numerous target-specific PET tracers have been developed and evaluated in preclinical and clinical studies. This review provides an overview of the current status and trends in the development of non-[18F]FDG PET probes in oncology and their application in the investigation of cancer biology.
Positron emission tomography (PET) is a nuclear imaging technique used to map biological and physiological processes in living subjects following the administration of radiolabeled tracers. Unlike conventional imaging modalities, such as magnetic resonance imaging (MRI) or computed tomography (CT), which mainly provide detailed anatomical images, PET can measure biochemical and physiological aberrations that occur prior to macroscopic anatomical signs of a disease, such as cancer. In PET, the radionuclide in the radiotracer decays and the resulting positrons subsequently annihilate on contact with electrons after travelling a short distance (~1 mm) within the body. Each annihilation produces two 511-keV photons in opposite trajectories and these two photons may be detected by the detectors surrounding the subject to precisely locate the source of the annihilation event. Subsequently, the “coincidence events” data can be processed by computers to reconstruct the spatial distribution of the radiotracers. Several positron-emitting radionuclides can be used in the development of successful PET radiotracer for research and clinical use. These radionuclides include, but are not limited to, 18F (Emax 635 keV, half-life [t1/2] 109.8 minutes), 11C (Emax 970 keV, t1/2 20.4 minutes), 15O (Emax 1.73 MeV, t1/2 2.04 minutes), 13N (Emax 1.30 MeV, t1/2 9.97 minutes), 64Cu (Emax 657 keV, t1/2 12.7 hour), 68Ga (Emax 1.90 MeV, t1/2 68.1 minutes), and 124I (Emax 2.13 MeV; 1.53 MeV; 808 keV, t1/2 4.2 days). 11C is an attractive and important positron-emitting isotope for labeling molecules of biological interest. Although the half-life of 11C is short (20.4 minutes) and multistep syntheses are not generally applicable for the radiosynthesis of 11C-containing molecules, a diverse array of reactions to introduce 11C into target molecules has been investigated and developed.1 Several nonconventional metallic isotopes with longer half-lives can be prepared in high yields in small biomedical cyclotrons facilitating delivery more easily than the delivery of short half-lived isotopes. For example, the availability of a 68Ga generator provides an opportunity to prepare PET radiotracers on site as needed. 64Cu, 86Y, and 124I are appropriate for labeling peptides and proteins. However, some metallic nuclides possess complex decay schemes. They usually decay with the emission of low β+-percentage branching (86Y, 33%; 66Ga, 57%), high β+-energy (86Y, 1.3 MeV; 66Ga, 4.2 MeV), as well as co-emission of a substantial amount of γ-radiation, all of which results in increased radiation dose to the patient. 18F appears to be an ideal radionuclide for routine PET imaging based on its almost perfect chemical and nuclear properties. As compared to other short-lived radionuclides, such as 11C, its half-life of 109.8 minutes is long enough to allow for time-consuming multi-step radiosyntheses, as well as imaging procedures to be extended over hours. In addition, 18F has a low β+-energy of 0.64 MeV, which promises a short positron linear range in tissue, leading to particularly high spatial resolution of up to 1 mm in PET images at lower radiation doses to patients.
[18F]-fluorodeoxyglucose ([18F]FDG) is the most widely used PET radiotracer in oncology. After administration of [18F]FDG into the body, it is taken up into various tissues and trapped intracellularly. In fact, increased glucose transport is associated with elevated glycolysis of the cancer cell and a corresponding increase in hexokinase activity. Tissues that metabolize glucose faster will accumulate more [18F]FDG. Therefore, cancer cells can be differentiated from benign tissues by their increased metabolism. Correspondingly, this uptake can be semiquantified on PET. A uniform distribution of [18F]FDG in the body would produce a standardized uptake value (SUV) of 1 and the amount of uptake in a lesion can, in itself, be used to predict the likelihood of malignancy. The important biological and physical properties of [18F]FDG result in its continued use in oncology. However, [18F]FDG is not a target-specific tracer and it cannot differentiate between cells that have a high metabolic rate associated with neoplasia, and those for which the increased metabolic rate is associated with other etiologies, such as infection or inflammation. In addition, many malignancies do not exhibit high metabolic rates and, thus, are not properly diagnosed by [18F]FDG.2-4
Over the past decade, alternative PET radiotracers to [18F]FDG that target specific biological process in cancer biology have been extensively explored. A basic understanding of molecular biology behind cancer is a prerequisite to successfully develop a target-specific PET imaging probe. Appropriate targets associated with the biological process in oncology need to be identified, selected, and even validated in a testing system. The development of a PET imaging probe is unlikely to be successful unless the molecular target is well defined and its biological role is well characterized. It is fortunate that the outcomes of biomedical research have yielded an enormous amount of information about the molecular events that take place during the development of cancer. With the explosion of biological data and information generated from various genomic, proteomic, and chemogenomic approaches, bioinformatics methods have become increasingly useful to extract or filter valuable targets by the combination of biological ideas with computer tools or statistical methods.5-9 In recent years, a variety of databases, such as Gene Expression Omnibus (GEO) (www.ncbi.nlm.nih.gov/geo); warehousing high-throughput microarray data of tumor expression profiles; and bioinformatics initiatives, such as Oncomine7,8 have been developed to accelerate the discovery of diagnostic markers for human diseases including cancer. With the availability of these biological data-mining tools, both the appropriate target linked to cancer disease and the location and distribution (intercellular, cell membrane, extracellular matrix, etc) of the molecular targets can be more readily identified than before. Currently, these identified targets include biological events such as angiogenesis, apoptosis, hypoxia, and tumor proliferation, or molecular biomarkers such as protein kinases, growth factor receptors, and specific overexpressed enzymes or receptors. In this review, we describe recent advances made in PET imaging of cancer biology with a focus on the best-studied biological targets and discuss trends in developing future PET imaging probes.
PET PROBES FOR IMAGING ANGIOGENESIS
Angiogenesis, the formation of new blood vessels from pre-existing vasculature, plays a vital role in tumor growth and metastatic spread.10 Tumor growth beyond a few millimeters in diameter requires an independent vasculature for the cellular supply of oxygen and nutrients and removal of waste products.11 Tumor angiogenesis is a complex, multi-step process that follows a characteristic sequence of events mediated and controlled by growth factors, cellular receptors, and adhesion molecules.12-14 In this process, five phases can be distinguished, including (1) endothelial cell activation, (2) basement membrane degradation, (3) endothelial cell migration, (4) vessel formation, and (5) angiogenic remodeling.15 Tumor angiogenesis starts when the tumor cells migrate along blood vessels. The rapid proliferation and accumulation of tumor cells create a gradient of high interstitial fluid pressure (IFP) within the tumor, and tumor cells compress the vessels to constrict the blood supply. Resulting from the localized, reduced perfusion, tumor cells become hypoxic and secrete growth factors, such as vascular endothelial growth factor (VEGF), the acidic and basic fibroblast growth factors (aFGF and bFGF), and platelet-derived endothelial cell growth factor (PD-ECGF),16 driven by hypoxia-inducible factor-1α (HIF-1α).17 Activated endothelial cells express the dimeric transmembrane integrin αvβ3, which interacts with extracellular matrix proteins and regulates migration of the endothelial cell through the extracellular matrix during vessel formation.18 The activated endothelial cells can secrete a number of proteolytic enzymes, such as members of the matrix metalloproteinase (MMPs) family, to degrade the matrix, facilitate cell invasion, and clear the way for angiogenesis. As for vessel formation, endothelial cells initially assemble as solid cords. Subsequently, the inner layer of endothelial cells undergoes apoptosis leading to the formation of the vessel lumen. Finally, the primary and immature vasculature undergoes extensive remodeling during which the vessels are stabilized through the recruitment of smooth muscle cells and pericytes. Biomarkers expressed uniquely in tumor angiogenesis are attractive targets for the development of tumor angiogenic diagnostics.
It is well documented that integrin αvβ3 is expressed on the cell membrane of various tumor cell types such as late stage glioblastoma, melanoma, ovarian cancer, breast cancer, and prostate cancer.19-21 The integrin αvβ3, which binds to arginine-glycine-aspartic acid (RGD)-containing components of the interstitial matrix such as vitronectin, fibronectin and thrombospondin,22,23 is significantly upregulated in tumor angiogenesis. Based on these findings, linear as well as cyclic RGD peptides were introduced and shown to have high binding affinity and selectivity for integrin αvβ3.24,25 The potential of RGD-containing peptides labeled with position-emitting radionuclides to serve as PET radiotracers has been investigated by several groups. First, the in vivo application of radioiodinated RGD peptides revealed the target receptor-specific tumor uptake but also predominant hepatobiliary elimination, resulting in high activity concentrations in the liver and small intestines.26 Consequently, several strategies to improve the pharmacokinetics of radiohalogenated peptides have been studied including conjugation with sugar moieties, hydrophilic amino acids, and polyethylene glycol (PEG).27-30
It was found that glycosylation on the lysine side chain of cyclic RGD peptides decreased lipophilicity and hepatic uptake.30 A glycopeptide based on cyclo(Arg-Gly-Asp-D-Phe-Lys), [18F]galacto-RGD, was then synthesized.31 [18F]galacto-RGD exhibited integrin αvβ3-specific tumor uptake with PET in an integrin-positive M21 melanoma xenograft model.32-34 Initial clinical trials in healthy volunteers and a limited number of cancer patients revealed that [18F]galacto-RGD could be safely administered to humans and was able to delineate certain lesions that are integrin-positive (Figure 1).32,34,35 Good tumor/background ratios with [18F]galacto-RGD PET, along with the immunohistochemistry results showing high vascular integrin αvβ3 expression, suggest that [18F]galacto-RGD PET might be used as a surrogate marker of angiogenesis.36 In addition, no obvious correlation was found between the tracer uptake of [18F]FDG and [18F]galacto-RGD in patients with various tumors, indicating that integrin αvβ3 expression and glucose metabolism are not closely correlated in tumor lesions and, thus, [18F]galacto-RGD and [18F]FDG can provide complementary information in cancer patients.37 Despite the successful translation of [18F]galacto-RGD into clinical trials, several key issues remain to be resolved, such as tumor-targeting efficacy and pharmacokinetics.38 Conjugation of PEG has been shown to improve many properties of peptides and proteins, including plasma stability, immunogenicity, and pharmacokinetics. 18F- and 64Cu-labeled RGD-containing peptides were studied and demonstrated an effect on the pharmacokinetics, tumor uptake and retention of the RGD peptides, which could be due to the nature of the lead structure and the size of the PEG moiety. The PEGylated RGD peptide 64Cu-DOTA-PEG3400-RGD showed lower uptake in liver and intestine with no effect on tumor uptake and retention as compared to a non-PEGylated ligand 64Cu-DOTA-RGD.27 In an experimental comparison, [18F]FB-PEG3400-RGD demonstrated significantly improved tumor retention relative to [18F]FB-RGD without compromising hepatic and renal clearance of activity.39
Figure 1.
Correlation of tracer accumulation and integrin αvβ3 expression. (A–C) A patient with a soft tissue sarcoma dorsal to the right knee joint. (A) The sagittal section of a [18F]Galacto-RGD PET acquired 170 minutes post-injection shows circular peripheral tracer uptake in the tumor with variable intensity and a maximum SUV of 10.0 at the apical-dorsal aspect of the tumor (arrow). (B) The image fusion of the [18F]Galacto-RGD PET and the corresponding CT scan after intravenous injection of contrast medium shows that the regions of intense tracer uptake correspond with the enhancing tumor wall, whereas the non-enhancing hypodense center of the tumor shows no tracer uptake. (C) Immunohistochemistry of a peripheral tumor section using the anti-αvβ3 monoclonal antibody LM609 demonstrates intense staining predominantly of tumor vasculature. (D–F) A patient with malignant melanoma and a lymph node metastasis in the right axilla. (D) The axial section of a [18F]Galacto-RGD PET acquired 140 minutes post-injection shows intense focal uptake in the lymph node (arrow). (E) Image fusion of the [18F]Galacto-RGD PET and the corresponding post-contrast CT. (F) Immunohistochemistry of the lymph node using the anti-αvβ3 monoclonal antibody LM609 demonstrates intense staining predominantly of tumor cells and also blood vessels. Reproduced from Haubner R, et al. Noninvasive visualization of the activated alphavbeta3 integrin in cancer patients by positron emission tomography and [18F]Galacto-RGD. PLoS Med 2005;2:e70.32
Additional strategies for improving pharmacokinetic behavior, as well as tumor uptake and the retention pattern of peptides with an RGD motif, include introduction of hydrophilic amino acids and multimerization of RGD. The rationale is that the interaction between integrin αvβ3 and RGD-containing extracellular matrix (ECM)-proteins involves multivalent binding sites with clustering of integrins. Thus, multimeric RGD peptides can act as more effective antagonists with better targeting capability and higher cellular uptake through the integrin-dependent binding.40 A series of multimeric RGD peptides labeled with 18F for PET imaging have been reported.41-46 The dimeric RGD peptide-based tracer, [18F]FB-E[c(RGDyK)]2 (abbreviated as 18F-FRGD2), showed predominant renal excretion and almost twice as much tumor uptake in the same animal model compared with the monomeric tracer [18F]FB-c(RGDyK).43,46 Tumor uptakes quantified by microPET scans in six tumor xenograft models correlated well with integrin αvβ3 expression level measured by sodium dodecyl sulfate polyacrylamide gel electropheresis (SDS-PAGE) autoradiography. The tetrameric RGD peptide-based tracer, [18F]FB-E[E[c(RGDfK)]2]2, showed significantly higher receptor binding affinity than the corresponding monomeric and dimeric RGD analogues and demonstrated rapid blood clearance, high metabolic stability, predominant renal excretion, and significant receptor-mediated tumor uptake, with good contrast in xenograft-bearing mice (Figure 2).45
Figure 2.
(A) Decay-corrected whole-body mice bearing U87MG tumor at 5, 15, 30, 60, 120, and 180 minutes after injection of 18F-FPRGD4 (3.7 MBq [100 mCi]). (B) Decay-corrected whole-body coronal microPET images of c-neu Oncomice at 30, 60, and 150 min (5-minute static image) after intravenous injection of 18F-FPRGD4. (C) Decay-corrected whole-body coronal microPET images of orthotopic MDA-MB-435 tumor-bearing mouse at 30, 60, and 150 minutes after intravenous injection of 18F-FPRGD4. (D) Decay-corrected whole-body coronal microPET images of DU-145 tumor-bearing mouse (5-minute static image) after intravenous injection of 18F-FPRGD4. (E) Coronal microPET images of a U87MG tumor bearing mouse at 30 and 60 minutes after co-injection of 18F-FPRGD4 and a blocking dose of c(RGDyK). Arrows indicate tumors in all cases. Reproduced from Wu Z, et al. microPET of tumor integrin alphavbeta3 expression using 18F-labeled PEGylated tetrameric RGD peptide (18F-FPRGD4). J Nucl Med 2007;48:1536–44.45
Radiolabeled nanoparticles represent a new class of probes that has enormous potential for research and clinical applications. Development of both inorganic and organic nanoparticles targeting integrin αvβ3 for PET imaging has been reported recently.47,48 Inorganic single-walled carbon nanotubes (SWNTs) coated noncovalently with PEG and labeled with cyclic RGD peptides and DOTA for 64Cu chelation have been achieved.48 PET imaging demonstrated that SWNTs were able to target integrin αvβ3-positive tumors in mice. Rapid renal excretion was not observed although the PEG chains were affixed to the SWNTs noncovalently, indicating that these functionalized SWNTs were quite stable in vivo. The additional photophysical properties of SWNTs allowed for ex vivo study by Raman spectroscopy, from which the tissue distributions were found to be in agreement with the in vivo PET and ex vivo biodistribution data. The unique property of a single nanoparticle makes it possible for multimodality imaging, such as PET/CT, PET/MRI, and PET/optical imaging.
VEGF, a key regulator in embryonic and somatic angiogenesis, plays a vital role in cancer processes.49,50 Among the VEGF gene family, VEGF-A is a homodimeric, disulfide-bound glycoprotein existing in at least seven isoforms, consisting of 121, 145, 148, 165, 183, 189, or 206 amino acid residues. The angiogenic actions of VEGF-A are mainly mediated through two endothelium specific receptor tyrosine kinases, VEGFR-1 (Flt-1) and VEGFR-2 (Flk-2/KDR).51 All of the VEGF-A isoforms bind to both VEGFR-1 and VEGFR-2, of which VEGFR-2 is the major mediator of the mitogenic, angiogenic, and permeability-enhancing effects of VEGF-A and plays an important role in tumor angiogenesis. Therefore, the development of VEGF- or VEGFR-targeted PET imaging probes could serve as a new paradigm for the assessment of anti-angiogenic therapeutics and for better understanding the role and expression profile of VEGF/VEGFR in angiogenesis-related cancer disease.
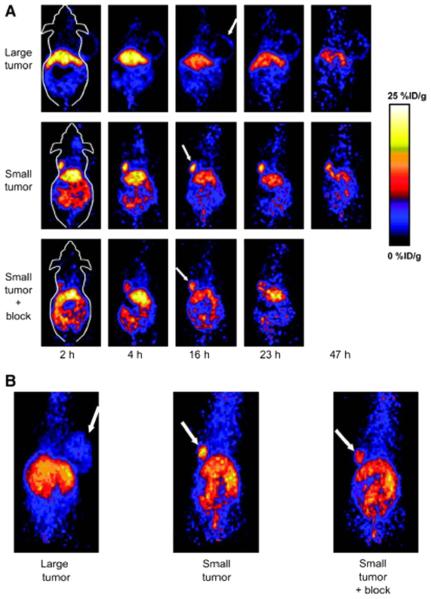
The first report on PET imaging of VEGFR expression used DOTA-VEGF121 labeled with 64Cu.52 DOTA-VEGF121 conjugation exhibited nanomolar receptor binding affinity in vitro, which is comparable to VEGF121. PET imaging revealed rapid, specific, and prominent uptake of [64Cu]DOTA-VEGF121 (10%~15% injected dose [ID]/g) in highly vascularized small U87MG tumors (60 mm3) with high VEGFR-2 expression but significantly lower and sporadic uptake (~3% ID/g) in large U87MG tumors (1,200 mm3) with low VEGFR-2 expression (Figure 3). Western blotting of tumor tissue lysate, immunofluorescence staining, and blocking studies with unlabeled VEGF121 confirmed that the tumor uptake is VEGFR-specific. This study demonstrated the dynamic nature of VEGFR expression during tumor progression in that even for the same tumor model, VEGFR expression level can be dramatically different at various stages. Successful demonstration of the ability of [64Cu]DOTA-VEGF121 to visualize VEGFR expression in vivo allows for clinical translation of this tracer to image tumor angiogenesis and to guide VEGFR-targeted cancer therapy.52 Further studies showed that the uptake of [64Cu]DOTA-VEGF121 in the tumor peaked when the tumor size was about 100 to 250 mm3. Both smaller and larger tumors showed lower tracer uptake, indicating high VEGFR-2 expression falls in a narrow range of tumor size.53 In another follow-up study, a VEGFR-specific fusion toxin VEGF121/rGel (composed of VEGF121 linked with a G4S tether to recombinant plant toxin gelonin) was used to treat orthotopic glioblastoma in a mouse model.54 Before initiation of treatment, PET imaging with [64Cu]-labeled VEGF121/rGel was performed to evaluate the tumor targeting efficacy and the pharmacokinetics. It was found that [64Cu]DOTA-VEGF121/rGel exhibited high tumor accumulation/retention and high tumor-to-background contrast up to 48 hours after injection in glioblastoma xenografts. Based on the in vivo pharmacokinetics of [64Cu]DOTA-VEGF121/rGel, VEGF121/rGel was administered every other day for the treatment of orthotopic U87MG glioblastomas. Histological analysis revealed specific tumor neovasculature damage after treatment with four doses of VEGF121/rGel.54 64Cu was also used to site-specifically label VEGF121 and it was found that PEGylation showed considerably prolonged blood clearance.55
Figure 3.
MicroPET of [64Cu]DOTA-VEGF121 in U87MG tumor-bearing mice. (A) Serial microPET scans of large and small U87MG tumor-bearing mice injected intravenously with 5–10 MBq of [64Cu]DOTA-VEGF121. Mice injected with [64Cu]DOTA-VEGF121 30 minutes after injection of 100 μg VEGF121 are also shown (denoted as “Small tumor + block”). (B) Two-dimensional whole-body projection of the three mice shown in A at 16 hours after injection of [64Cu]DOTA-VEGF121. Tumors are indicated by arrows. Reproduced from Cai W, et al. PET of vascular endothelial growth factor receptor expression. J Nucl Med 2006;47:2048–56.52
VEGF-A isoforms bind to both VEGFR-1– and VEGFR-2– expressing 4T1 tumors.56 Because rodent kidneys expressed high levels of VEGFR-1, the renal uptake of [64Cu]DOTA-VEGFDEE was significantly lower than that of [64Cu]DOTA-VEGF121, indicating that VEGFDEE is superior to wild-type VEGF121 for imaging tumor angiogenesis.57 Future studies may include the development of more potent VEGFR-2-specific mutants and site-specific labeling of VEGF analog proteins with various radionuclides to improve imaging quality and result analysis.
PET PROBES FOR IMAGING HYPOXIA
The prevalence of hypoxic areas is a characteristic feature of locally malignant solid tumors. It has been found that up to 50% to 60% of locally advanced solid tumors may exhibit hypoxic and/or anoxic tissue areas that are heterogeneously distributed within the tumor mass. These hypoxic areas result from an imbalance between oxygen supply and consumption, which is caused by abnormal structure and function of the microvessels supplying the tumor, increased diffusion distances between the nutritive blood vessels and the tumor cells, and reduced oxygen transport capacity of the blood due to the presence of disease- or treatment-related anemia.58-60 Recent studies have demonstrated a clear relevance of this hypoxic microenvironment to tumor-associated metabolic alterations, which are tightly linked to the biology of the tumor.61-63 Therefore, noninvasive PET imaging for identification and quantitation of tumor hypoxia status could play a key role in predicting and monitoring treatment response.
Initial attempts to image hypoxia by PET mainly focused on radiolabeled 2-nitroimidazole analogues.64 These compounds are reduced into reactive intermediary metabolites by intracellular reductases in a process that is directly related to the level of oxygenation/hypoxia. Subsequently, these metabolites covalently bind to thiol groups of intracellular proteins and thereby accumulate within viable hypoxic cells. Several nitroimidazole compounds with different properties and radiolabeled with different PET radionuclides have been described,65,66 among which [18F]fluoromisonidazole ([18F]FMISO) is the most widely used PET hypoxia probe both in preclinical and clinical studies (Figure 4).67 For example, an attempt has been made to define the relationship between [18F]FMISO uptake and hypoxic fraction in a 36B10 glioma rat xenograft model. Although the relationship between classically defined radiobiologically hypoxic fraction and [18F]FMISO time-activity data remained to be clarified, [18F]FMISO retention provided useful correlations with the degree of hypoxia.68 In another study, [18F]FMISO uptake was compared with the exogenous hypoxia marker pimonidazole and the endogenous hypoxia marker carbonic anhydrase IX (CA IX) in a rhabdomyosarcoma rat xenograft model. A statistically significant correlation was obtained between the hypoxic volumes defined with [18F]FMISO PET and the volumes derived from the pimonidazole- and CA IX-stained tumor sections, indicating the value of [18F]FMISO PET to measure hypoxia.69 In clinical studies, tumor uptake of [18F]FDG and [18F]FMISO in patients with head and neck cancer has been compared. No correlation was found between [18F]FDG uptake and pO2 measurements, whereas an association between [18F]FMISO uptake and pO2 measurements existed.70,71 Further comparison of [18F]FDG and [18F]FMISO indicated that no correlation exists between these tracers, as both represent different tumor characteristics.72-74 In another study, the ability of pretherapy [18F]FMISO PET to predict survival was investigated in 73 patients with head and neck cancer, and pretreatment [18F]FMISO uptake proved to be an independent prognostic factor.75 In a recent study of eight patients with non-small cell lung cancer receiving chemotherapy and/or radiotherapy, changes in [18F]FMISO uptake measured early response to therapy and may predict freedom from disease as well as overall survival.76
Figure 4.
Structures of [18F]FMISO, [18F]FAZA, and [64Cu]ATSM-PET radiotracers for imaging tumor hypoxia.
In addition to [18F]FMISO, an 18F-labeled 2-nitroimidazole derivative, [18F]fluoroazomycin arabinoside ([18F] FAZA; Figure 4) displays enhanced in vivo stability to enzymatic cleavage.77 Two studies compared [18F]FMISO and [18F]FAZA in various tumor xenograft models and reported superior pharmacokinetics for [18F]FAZA over [18F]FMISO.77,78 In both studies, [18F]FAZA displayed higher tumor-to-background, tumor-to-muscle, and tumor-to-blood ratios due to its more rapid clearance from blood and nontarget tissues. A clinical study that included 18 patients with advanced squamous cell head and neck cancer evaluated the role of [18F]FAZA PET imaging to identify hypoxia in order to plan radiation treatment. It was concluded that radiation treatment planning and intensity-modulated radiotherapy based on [18F]FAZA uptake measurements are feasible.79
Another alternative PET probe for hypoxia imaging that holds great promise is based on a metal complex of radioactive copper with ATSM, diacetyl-bis(N4-methylthiosemicarbazone). Cu(II)-ATSM is a neutral lipophilic molecule, which is highly membrane permeable. It can undergo reduction by cellular reducing equivalents and can be converted to [Cu(I)-ATSM]−, which becomes entrapped in cells because of its negative charge when cells are hypoxic. A recent study concluded that 64Cu-ATSM (Figure 4) is a suitable PET hypoxia marker in most tumor types after comparing the autoradiographic distributions of 64Cu-ATSM with the hypoxia markers EF5, pimonidazole, and CA IX in R3230 mammary adenocarcinomas, fibrosarcomas, and 9L gliomas.80 A number of studies compared 64Cu-ATSM with [18F]FMISO and [18F]FDG in vivo. For example, it was shown that the regional distribution of [18F]FMISO at 2 hours correlates well with the distribution of 64Cu-ATSM at 10 minutes or 24 hours in 9L gliosarcoma tumors, whereas a poor correlation existed between 64Cu-ATSM and [18F]FDG at 10 minutes.81 A recent clinical study compared the image quality and tumor uptake of 60Cu-ATSM and 64Cu-ATSM in 10 patients with cervical carcinoma. The investigators concluded that 64Cu-ATSM was a safe radiopharmaceutical that can be used to obtain high quality images of tumor hypoxia in human cancers.82
PET PROBES FOR IMAGING APOPTOSIS
Apoptosis, or type I cell death, is the organized energy-dependent self disassembly of unneeded or senescent cells. When triggered by appropriate internal and/or external signals, these cells undergo preprogrammed cytoplasmic shrinkage, membrane blebbing, and budding off of intracellular contents carefully packaged into small membrane bound packets that are subsequently ingested by adjacent cells and phagocytes without provoking an inflammatory response.83 Initiation of apoptotic cell death leads to activation of a family of cysteine proteases (caspases) that act as central executioners of the apoptotic process.84 Radiation therapy as well as chemotherapy can induce apoptosis in tumor cells. Consequently, PET imaging that provides information on the rate and extent of apoptosis is of great interest in monitoring the efficacy of anticancer treatment.
Annexin V and its derivatives are extensively studied probes targeting apoptosis.85 Annexin V (molecular weight ≈ 36,000, 319 amino acid residues) is a member of the calcium and phospholipid binding family of annexin proteins that displays selective, nanomolar affinity toward phosphatidylserine (PS) residues.86,87 Induction of apoptosis results in rapid externalization of PS from the inner leaflet of the plasma membrane to its outer surface.88 Accordingly, annexin-mediated imaging of PS has been extensively investigated for identifying cells at the early stages of apoptosis. Annexin V and its derivatives have been radiolabeled with a wide variety of positron-emitting radionuclides including 18F, 11C, and 64Cu.89 One of the studies has used N-succinimidyl 4-fluorobenzoate to synthesize 18F-labeled annexin V.90 The fluorine labeled agent has lower uptake in the liver, spleen and kidney compared to HYNICannexin V. Another method involves site-specific derivatization with an 18F-maleimide labeled compound to mutant annexin V-117 or annexin V-128.91 However, both of these methods need more preclinical study before further development as imaging markers of apoptosis.
Of the molecular biochemical alterations that occur during apoptosis, activation of caspases, particularly caspase-3, is the most attractive for developing specific in vivo PET imaging probes. Radiolabeled caspase-3 substrates and inhibitors, and monoclonal antibodies targeted to human annexin V have been used as alternative approaches for apoptosis imaging. Development of non-peptidyl caspase-3 PET probes has been mainly focused on the 5-pyrrolidinylsulfonyl isatin class of caspase-3 inhibitors. The dicarbonyl function of isatins covalently binds to the cysteine residue of the active site of caspase-3. Recently, a library of isatin-5 sulfonamides has been designed and [18F]ICMT-11 was selected for further evaluation on the basis of subnanomolar affinity for activated capsase-3, high metabolic stability, and facile radiolabeling.92 The studies demonstrated that [18F]ICMT-11 binds to a range of drug-induced apoptotic cancer cells in vitro and to 38C13 murine lymphoma xenografts in vivo by up to twofold at 24 hours post-treatment compared to vehicle treatment (Figure 5). In addition, the results showed the increased signal intensity in tumors after drug treatment, detected by whole body in vivo PET imaging, was associated with increased apoptosis. [18F]ICMT-11 as a caspase-3 specific PET imaging radiotracer that has the potential for the assessment of tumor apoptosis in anticancer drug development and the monitoring of early responses to therapy. Overall, the isatin-based PET probes are promising for imaging caspase-3; however, the studies are limited to several animal models without any published human data. The specificity of this class of tracers in vivo also is unclear.
Figure 5.
[18F]ICMT-11 PET imaging analysis. 38C13 xenograft-bearing mice were treated with 100 mg/kg cyclophosphamide CPA (n = 3) or vehicle (n = 3) for 24 hours and subsequently subjected to 60 minutes dynamic [18F]ICMT-11 PET imaging. (A) [18F]ICMT-11 PET images of two representative 38C13 xenograft-bearing mice treated with CPA or vehicle. White arrowheads indicate the tumor. (B) Tumor and blood were removed after the scan and analyzed for [18F]ICMT-11 tissue uptake. (C) The tumor time versus radioactivity curve (TAC). (D) Semiquantitative imaging variables extracted from the TAC. Data are mean ± SEM. Reproduced from Nguyen QD, et al. Positron emission tomography imaging of drug-induced tumor apoptosis with a caspase-3/7 specific [18F]-labeled isatin sulfonamide. Proc Natl Acad Sci U S A 2009;106:16375–80.92
PET PROBES FOR IMAGING TUMOR CELL PROLIFERATION
The proliferative activity of cells is one of the hallmarks of malignant tumors, and the majority of anticancer drugs are designed to inhibit cell proliferation. PET imaging for proliferation will help to detect proliferative changes in tumors occurring during various therapeutic interventions. [11C]-thymidine was the first PET radiotracer successfully used to evaluate proliferative activity in various tumors.93 Thymidine is a native nucleoside, which is incorporated into deoxyribonucleic acid (DNA). It is taken up into cells by the nucleoside transporters located in the cell membrane. Once inside the cell, thymidine is phosphorylated to thymidine monophosphate by the cytosolic enzyme thymidine kinase 1 (TK1). Since TK1 is overexpressed in various tumors, there is preferential uptake of radiolabeled thymidine by malignant tumors as compared with normal tissues. However, the short 20-minute half-life of 11C and its rapid metabolism by thymidine phosphorylase make it difficult to use 11C-thymidine in daily clinical practice. A series of PET probes that were subsequently developed to address these limitations include the [18F]-labeled analogs, 3′-deoxy-3′-[18F]fluorothymidine ([18F]FLT) and 1-(2′-deoxy-2′-[18F]fluoro-β-d-arabinofuranosyl) thymine ([18F]FMAU) (Figure 6).94
Figure 6.
Structures of [18F]FLT and [18F]FMAU–PET radiotracers for imaging tumor cell proliferation.
[18F]FLT is taken up by cells and phosphorylated by TK1 with subsequent trapping within the cell.95 Intracellular [18F]FLT retention can thus be used as a measure of cellular TK1 activity. FLT monophosphate can be phosphorylated by thymidylate kinase to FLT diphosphonate and subsequently into FLT triphosphonate by nucleotide diphosphate kinase. FLT triphosphonate cannot be incorporated into the growing DNA chain as the 3′-position is substituted by 18F. Since [18F]FLT triphosphonate cannot be incorporated into DNA, [18F]FLT is only an indirect measure of cell proliferation. Several reports have been published describing the use of PET imaging of [18F]FLT uptake as a surrogate marker for early in vivo quantitative imaging of drug-induced changes in cell proliferation. In one example, PET imaging with [18F]FLT was used for noninvasive measurement of the biological activity of a novel histone deacetylase inhibitor (LAQ824).96 Histone deacetylase inhibitors have been shown to cause growth inhibition of cancer cells in vitro and in animal models.97 Treatment of mice bearing HCT116 colon carcinoma xenografts with LAQ824 resulted in a dose-dependent decrease in [18F]FLT tumor uptake. Dose-dependent decreases of tumor TK1 and protein levels were also observed with LAQ824 pretreatment. Another study evaluated treatment response with [18F]FLT in 14 patients with newly diagnosed primary or metastatic breast cancer.98 All patients underwent [18F]FLT and [18F]FDG scans before chemotherapy or antihormonal therapy, 2 weeks after termination of the first treatment cycle and after the end of treatment or after 1 year if treatment had not yet been terminated. The treatment-related changes observed with the two radiotracers were consistent. However, changes in serum tumor marker levels were more strongly associated with tumor [18F]FLT uptake than with [18F]FDG uptake. In addition, the [18F]FLT uptake at 2 weeks correlated well with the long-term efficacy of the treatment. The main limitations of this study were the small number of patients and the use of different treatment regimens. Kenny et al evaluated treatment response in 13 patients (17 lesions) with stage II–IV breast cancer and concluded that [18F]FLT-PET scans can detect changes in breast cancer proliferation at 1 week after initiation of a standard combination chemotherapy regimen of 5-fluorouracil, epirubicin, and cyclophosphamide.99 In addition, the authors also showed the reproducibility of [18F]FLT-PET in serial studies. Despite the encouraging results of preclinical studies and a few clinical studies, several limitations of [18F]FLT should be taken into account before a conclusion is drawn as to its ability to measure therapy response. Recent publications have demonstrated that the metabolism of [18F]FLT is complex, especially in the context of evaluation of treatment response. Kenny et al showed heterogeneous [18F]FLT tumor uptake in primary and metastatic breast cancer.100 In addition, [18F]FLT may be less sensitive in tumors with low TK1 expression levels. Therefore, it is unclear whether the sensitivity of [18F]FLT imaging of primary and metastatic tumor lesions is sufficient for reliable assessment of therapy response. Moreover, high physiological uptake of [18F]FLT in bone marrow and liver will pose a problem in the assessment of therapy response of disease involving these organs.
FMAU has been developed for cell proliferation imaging with labeling either in the 5-methyl group of the pyrimidine base with 11C or at the 2′-fluoro position of the sugar with 18F.101 Preclinical studies have shown that FMAU retention in tumors and nontumor tissues with rapid cell turnover (eg, marrow and small intestine) reflects incorporation into DNA.102,103 FMAU is highly resistant to catabolism in both animals and humans, with the injected compound dominating time-activity curves in blood during the first hour after injection.102,103 Preliminary clinical studies have shown tumor uptake of 11C- or 18F-FMAU in a variety of cancers104,105 to be comparable to that seen in human studies with [18F]FLT.106,107 In humans, 11C- or 18F-FMAU has relatively higher liver, kidney, and myocardial uptake yet much lower marrow uptake and rate of urinary excretion than [18F]FLT, suggesting a potential role for FMAU in cases requiring assessment of bone metastasis or the pelvic region. Another potential advantage is that, in part because of its rapid blood clearance, 11C- or 18F-FMAU uptake in tumors reaches a plateau by about 10 minutes after bolus injection of the radiotracer. Overall, because [18F]FMAU can be incorporated into DNA, it appears to be a highly promising PET tracer that can directly measure cell proliferation. It would be of great interest to define the role of [18F]FMAU-PET imaging in the evaluation of anticancer treatment response in preclinical and clinical studies in the near future.
PET PROBES FOR IMAGING EPIDERMAL GROWTH FACTOR RECEPTORS
Epidermal growth factor receptor (EGFR) is associated with a well-characterized proto-oncogene that has been shown to promote tumor progression in several solid cancers.108 EGFR is a member of the structurally related ErbB family of receptor tyrosine kinases.109 The EGF family includes four receptors: HER1 (ErbB1), HER2 (ErbB2), HER3 (ErbB3), and HER4 (ErbB4).110 Activation of EGFR contributes to several tumorigenic mechanisms, and in many tumors, overexpression of EGFR may act as a prognostic indicator, predicting poor survival or more advanced disease stage.111 Currently, monoclonal antibodies (mAbs), which block the binding of EGF to the extracellular ligand-binding domain of the receptor, and small tyrosine kinase inhibitors have shown promise from a therapeutic standpoint as well as for PET imaging.
Erbitux (Cetuximab; ImClone LLC, New York, NY) was the first mAb targeted against EGFR that was approved by the US Food and Drug Administration (FDA) for the treatment of patients with EGFR-expressing metastatic colorectal carcinoma. Cetuximab binds to the extracellular domain of EGFR with nanomolar affinity, similar to that of the natural ligand, EGF.112 We reported a study of PET imaging with a 64Cu-labeled DOTA-cetuximab conjugate (64Cu-DOTA-cetuximab) in seven xenograft tumor mouse models.113 The overall uptake of 64Cu-labeled DOTA-cetuximab was similar in major organs and tissues in all seven of the tested tumor models (U87MG human glioblastoma, PC-3 human prostate carcinoma, CT-26 murine colorectal carcinoma, HCT-8, HCT-116, SW620 human colorectal carcinoma, and MDA-MB-435 human breast cancer). Tumor uptake determined by PET imaging showed a good correlation with EGFR expression levels as measured by Western blot analysis. High uptake of the tracer was observed for the high EGFR expression U87MG and PC-3 tumors (13.2% ID/g and 12.8% ID/g, respectively) at 48 hours post-injection. Panitumumab (ABX-EGF) is another mAb targeted against EGFR. We recently performed small-animal PET studies with 64Cu-labeled panitumumab in xenografts derived from three cell lines of human head and neck squamous cell carcinoma (HNSCC) (Figure 7).114 Nude mice bearing HNSCC tumors with different levels of EGFR expression were imaged with small-animal PET using 64Cu-DOTA-panitumumab. The antibody distribution in the tumors was confirmed by ex vivo immunostaining. CD31 immunostaining and an Evans blue assay were also performed to assess the tumor vascular density and permeability. The results showed that UM-SCC-22B tumors with the lowest EGFR protein expression had the highest 64Cu-DOTA-panitumumab accumulation, whereas SQB20 tumors with the highest EGFR expression showed the lowest 64Cu-DOTA-panitumumab accumulation. Ex vivo staining demonstrated that SQB20 cells still had extremely high EGFR expression after forming tumors in nude mice, indicating that the low uptake of 64Cu-DOTA-panitumumab in SQB20 tumors was not due to the loss of EGFR expression. The results from CD31 immunostaining and Evans blue permeability assay suggest that the low vessel density, poor vascular permeability, and binding site barrier are likely responsible for the overall low tumor uptake of the highly EGFR-expressing SQB20 tumors. The results from this study provide evidence for the lack of an observed correlation between therapeutic efficacy of cetuximab and panitumumab and EGFR expression level as determined by immunohistochemistry or fluorescent in situ hybridization and may shed new light on the complications of anti-EGFR mAb therapy for HNSCC and other malignancies.
Figure 7.
(A) Small-animal PET images of HNSCC tumor-bearing nude mice at different time points after intravenous injection of 64Cu-DOTA-panitumumab (n = 4 per group). Decay-corrected transaxial images at different time points are shown, and tumors are indicated by arrowheads. For UM-SCC-22B and SAS tumors, scale ranged from 0% ID/g to 30% ID/g, and for SQB20 tumors, scale ranged from 0% ID/g to 15% ID/g for optimal visualization. (B) HNSCC tumor uptake levels of 64Cu-DOTA-panitumumab and 64Cu-DOTA-IgG at 20 hours after injection quantified from small-animal PET scans (n = 4). 22B = UM-SCC-22B. *P <.05. Reproduced with permission from Niu G, et al. PET of EGFR antibody distribution in head and neck squamous cell carcinoma models. J Nucl Med. 2009;50:1116–23.114
PET imaging of HER1 or HER2 has also been reported by using 68Ga radionuclide. A 68Ga-labeled, recombinant human EGF DOTA conjugate (68Ga-DOTA-hEGF) was used for HER1 imaging.115 In vitro studies with 68Ga-DOTA-hEGF conducted on EGFR-expressing cell lines, U343 glioma and A431 cervical carcinoma, demonstrated high binding affinity, rapid internalization of radiotracer and good retention. Biodistribution studies in mice bearing A431 tumor xenografts showed a tumor-to-blood ratio of 4.5 at 30 minutes post-injection (2.7% ID/g in tumor). Tumor was clearly visualized by PET imaging in a tumor-bearing mouse. In another study, a 68Ga-labeled F(ab′)2 fragment of herceptin (68Ga-DOTA-F(ab′)2-herceptin) was used to image HER2 downregulation after heat shock protein (Hsp90) inhibition.116 PET imaging was conducted on mice bearing BT474 breast tumor xenografts with 68Ga-DOTAF(ab′)2-herceptin and [18F]FDG before and after treatment with the Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin (17-AAG). A significant decrease of HER2 expression was seen within 24 hours of 17-AAG treatment with 68Ga-DOTA-F(ab′)2-herceptin imaging. In contrast, tumor uptake of [18F]FDG remained unchanged. The authors concluded that PET imaging of the HER2 radiotracer, using 68Ga-DOTA-F(ab′)2-herceptin, is superior to [18F]FDG imaging for evaluating tumor response to 17-AAG therapy.
PET PROBES FOR IMAGING SOMATOSTATIN RECEPTORS
Somatostatin exists in two isoforms: a short peptide having 14 amino acids, and a second peptide with 28 amino acids, both of which bind with high affinity to five receptor subtypes.117 A majority of malignant tumors, such as neuroendocrine tumor (NET), small cell lung cancer, breast tumor, and malignant lymphoma, overexpress multiple somatostatin receptor subtypes, of which the somatostatin receptor 2 (sst2) subtype is predominantly expressed.118
Somatostatin has a short plasma half-life (~2 minutes), making it unsuitable for PET probe development.119 However, octreotide (OC), an eight-amino acid analog of somatostatin, has a longer plasma half-life of 1.7 hours and higher metabolic stability than somatostatin.120 Subsequently, OC has been conjugated with various metal chelators and labeled with 64Cu or 68Ga for PET imaging to evaluate somatostatin receptor-positive tumors in rodent models and humans. In one study, 64Cu-TETA-OC was used as a PET imaging agent in humans and compared with 111In-DTPA-OC single-photon emission computed tomography (SPECT) imaging.121 64Cu-TETA-OC PET imaged more tumors in two patients as compared to 111In-DTPA-OC SPECT. Overall, 64Cu-TETA-OC PET showed better sensitivity for imaging neuroendocrine tumors, partially due to the greater sensitivity of PET over SPECT. In another study involving 84 patients, a new PET somatostatin analog, 68Ga-D-Phe1-Tyr3-octreotide DOTA conjugate (68Ga-DOTA-TOC), was used for NET imaging and compared to 111In-DOTA-TOC SPECT and 99mTc-HYNIC-TOC SPECT.122 CT imaging was also performed on each patient. Comparison of the three imaging modalities revealed accuracy for PET imaging of 96%, which was significantly higher than that of CT (63%) and SPECT (58%). In addition, the 68Ga-DOTA-TOC imaging results were true-positive in 32 of the patients whose SPECT results were false-negative. Moreover, PET detected more metastatic tumors (lymph node, bone, and liver) than SPECT, thus permitting more accurate disease staging. The investigators concluded that PET imaging with 68Ga-DOTA-TOC in conjunction with CT was superior to SPECT in the clinical diagnosis of NET.
CONCLUSION AND PERSPECTIVES
PET imaging has rapidly gained importance in preclinical and clinical applications for cancer diagnosis and treatment. PET imaging can provide a whole-body readout in an intact system, which is much more relevant and reliable than in vitro/ex vivo studies; decrease the workload and accelerate the drug development process; provide more statistically accurate results through longitudinal studies that can be performed in the same subject; facilitate lesion detection in cancer patients and patient stratification; and assess individualized anti-cancer treatment and dose accuracy.52,123 Noninvasive PET imaging of molecular markers in oncology can allow for much earlier diagnosis, earlier treatment, better prognosis, and improved staging and management.
A considerable variety of targeting agents (small molecules, peptides, peptidomimetics, antibodies, and nanoparticles) conjugated with various PET radionuclides have been applied for imaging biological events, as well as biomarkers involved in cancer biology. PET will likely be advanced on a wide scale in patients because of its high sensitivity, and less toxicity issues of PET imaging tracers as compared to MRI or ultrasound imaging probes. However, it is unlikely that a single parameter target structure or imaging technique will be used for the assessment of tumor biology in the future. Comprehensive information has to be acquired by multimodality imaging, which will allow for evaluation of the cancer cascade in its full complexity. It is expected that the new generation of clinical PET/CT and microPET/ microCT, as well as PET/MRI and microPET/microMRI will play a major role in molecular imaging of tumor biology for the years to come.
Additionally, to further improve imaging of the tumor process at the molecular level, it is necessary to identify new cancer-related targets and corresponding target-specific agents and to optimize currently available imaging probes. A better understanding of the physiological and pathological changes during tumor progression will be critical for new target identification. Optimization of available PET imaging probes can be achieved in several aspects. First, oligomerization (homo or hetero) and multimerization of the targeting ligand (typically peptide) can improve the binding affinity, as well as tissue retention likely due to the polyvalency effect.124 Second, site-specific labeling may be advantageous relative to random labeling in terms of retaining binding affinity and functional activity.125 Third, incorporation of an appropriate linker between the targeting ligand and the labeling moiety may result in favorable pharmacokinetic properties. Glycosylation, PEGylation, and various other linkers have been shown to improve the imaging quality. Fourth, development of new strategies to improve the labeling yield (most applicable to 18F-based radiotracers) is critical for future clinical studies. Finally, the development of multifunctionalized nanomaterials for multimodality imaging is an area of active investigation, yet the mechanism of accumulation and clearance must be further investigated.
To foster the continued discovery and development of target-specific PET imaging agents for cancer, cooperative efforts are needed from cellular/molecular biologists to identify and validate novel cancer imaging targets, chemists/radiochemists to synthesize and characterize the PET imaging probes, and engineers/medical physicists/mathematicians to develop high sensitivity/high resolution imaging devices/hybrid instruments and better image reconstruction algorithms. With the development of new PET tracers, clinical translation will be critical for achieving the maximum benefit from PET imaging probes with better targeting efficacy and desirable pharmacokinetics. Wider availability of PET scanners dedicated to small animal imaging studies as well as the exploratory investigational new drug (IND) mechanism proposed by the FDA to allow faster firstin-human studies will hasten the development of PET imaging probes from bench to bedside. It is expected that, in the foreseeable future, PET imaging will be routinely applied in anti-cancer clinical settings and thus pave the way toward personalized cancer medicine.
Acknowledgment
The authors would like to acknowledge the intramural research program at the National Institute of Biomedical Imaging and Bioengineering for financial support.
REFERENCES
- 1.Miller PW, Long NJ, Vilar R, Gee AD. Synthesis of 11C, 18F, 15O, and 13N radiolabels for positron emission tomography. Angew Chem Int Ed Engl. 2008;47:8998–9033. doi: 10.1002/anie.200800222. [DOI] [PubMed] [Google Scholar]
- 2.Kayani I, Groves AM. 18F-fluorodeoxyglucose PET/CT in cancer imaging. Clin Med. 2006;6:240–4. doi: 10.7861/clinmedicine.6-3-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelloff GJ, Hoffman JM, Johnson B, et al. Progress and promise of FDG-PET imaging for cancer patient management and oncologic drug development. Clin Cancer Res. 2005;11:2785–808. doi: 10.1158/1078-0432.CCR-04-2626. [DOI] [PubMed] [Google Scholar]
- 4.Rohren EM, Turkington TG, Coleman RE. Clinical applications of PET in oncology. Radiology. 2004;231:305–32. doi: 10.1148/radiol.2312021185. [DOI] [PubMed] [Google Scholar]
- 5.Chen YP, Chen F. Identifying targets for drug discovery using bioinformatics. Expert Opin Ther Targets. 2008;12:383–9. doi: 10.1517/14728222.12.4.383. [DOI] [PubMed] [Google Scholar]
- 6.Klee EW, Finlay JA, McDonald C, et al. Bioinformatics methods for prioritizing serum biomarker candidates. Clin Chem. 2006;52:2162–4. doi: 10.1373/clinchem.2006.072868. [DOI] [PubMed] [Google Scholar]
- 7.Rhodes DR, Chinnaiyan AM. Bioinformatics strategies for translating genome-wide expression analyses into clinically useful cancer markers. Ann N Y Acad Sci. 2004;1020:32–40. doi: 10.1196/annals.1310.005. [DOI] [PubMed] [Google Scholar]
- 8.Rhodes DR, Kalyana-Sundaram S, Mahavisno V, et al. Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia. 2007;9:166–80. doi: 10.1593/neo.07112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Y, Adelstein SJ, Kassis AI. Target discovery from data mining approaches. Drug Discov Today. 2009;14:147–54. doi: 10.1016/j.drudis.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–60. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 11.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–10. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 12.Ellis LM, Liu W, Ahmad SA, et al. Overview of angiogenesis: biologic implications for antiangiogenic therapy. Semin Oncol. 2001;28:94–104. doi: 10.1016/s0093-7754(01)90287-8. [DOI] [PubMed] [Google Scholar]
- 13.Kuwano M, Fukushi J, Okamoto M, et al. Angiogenesis factors. Intern Med. 2001;40:565–72. doi: 10.2169/internalmedicine.40.565. [DOI] [PubMed] [Google Scholar]
- 14.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–8. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 15.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–95. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen M. Angiogenic factors as tumor markers. Invest New Drugs. 1997;15:29–37. doi: 10.1023/a:1005766511385. [DOI] [PubMed] [Google Scholar]
- 17.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–57. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 18.Eliceiri BP, Cheresh DA. The role of alphav integrins during angiogenesis. Mol Med. 1998;4:741–50. [PMC free article] [PubMed] [Google Scholar]
- 19.Cai W, Chen X. Anti-angiogenic cancer therapy based on integrin alphavbeta3 antagonism. Anticancer Agents Med Chem. 2006;6:407–28. doi: 10.2174/187152006778226530. [DOI] [PubMed] [Google Scholar]
- 20.Jin H, Varner J. Integrins: roles in cancer development and as treatment targets. Br J Cancer. 2004;90:561–5. doi: 10.1038/sj.bjc.6601576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizejewski GJ. Role of integrins in cancer: survey of expression patterns. Proc Soc Exp Biol Med. 1999;222:124–38. doi: 10.1177/153537029922200203. [DOI] [PubMed] [Google Scholar]
- 22.Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238:491–7. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 23.Xiong JP, Stehle T, Zhang R, et al. Crystal structure of the extracellular segment of integrin alpha Vbeta3 in complex with an Arg-Gly-Asp ligand. Science. 2002;296:151–5. doi: 10.1126/science.1069040. [DOI] [PubMed] [Google Scholar]
- 24.Aumailley M, Gurrath M, Muller G, Calvete J, Timpl R, Kessler H. Arg-Gly-Asp constrained within cyclic pentapeptides. Strong and selective inhibitors of cell adhesion to vitronectin and laminin fragment P1. FEBS Lett. 1991;291:50–4. doi: 10.1016/0014-5793(91)81101-d. [DOI] [PubMed] [Google Scholar]
- 25.Haubner R, Finsinger D, Kessler H. Stereoisomeric peptide libraries and peptidomimetics for designing selective inhibitors of the αvβ3 integrin for a new cancer therapy. Angew Chem Int Ed Engl. 1997;36:1374–89. [Google Scholar]
- 26.Haubner R, Wester HJ, Reuning U, et al. Radiolabeled alpha(v)beta3 integrin antagonists: a new class of tracers for tumor targeting. J Nucl Med. 1999;40:1061–71. [PubMed] [Google Scholar]
- 27.Chen X, Hou Y, Tohme M, et al. Pegylated Arg-Gly-Asp peptide: 64Cu labeling and PET imaging of brain tumor alphavbeta3-integrin expression. J Nucl Med. 2004;45:1776–83. [PubMed] [Google Scholar]
- 28.Harris JM, Martin NE, Modi M. Pegylation: a novel process for modifying pharmacokinetics. Clin Pharmacokinet. 2001;40:539–51. doi: 10.2165/00003088-200140070-00005. [DOI] [PubMed] [Google Scholar]
- 29.Haubner R. Alphavbeta3-integrin imaging: a new approach to characterise angiogenesis?? Eur J Nucl Med Mol Imaging. 2006;33(Suppl 1):54–63. doi: 10.1007/s00259-006-0136-0. [DOI] [PubMed] [Google Scholar]
- 30.Haubner R, Wester HJ, Burkhart F, et al. Glycosylated RGD-containing peptides: tracer for tumor targeting and angiogenesis imaging with improved biokinetics. J Nucl Med. 2001;42:326–36. [PubMed] [Google Scholar]
- 31.Haubner R, Kuhnast B, Mang C, et al. [18F]Galacto-RGD: synthesis, radiolabeling, metabolic stability, and radiation dose estimates. Bioconjug Chem. 2004;15:61–9. doi: 10.1021/bc034170n. [DOI] [PubMed] [Google Scholar]
- 32.Haubner R, Weber WA, Beer AJ, et al. Noninvasive visualization of the activated alphavbeta3 integrin in cancer patients by positron emission tomography and [18F]Galacto-RGD. PLoS Med. 2005;2:e70. doi: 10.1371/journal.pmed.0020070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haubner R, Wester HJ, Weber WA, et al. Noninvasive imaging of alpha(v)beta3 integrin expression using 18F-labeled RGD-containing glycopeptide and positron emission tomography. Cancer Res. 2001;61:1781–5. [PubMed] [Google Scholar]
- 34.Beer AJ, Haubner R, Wolf I, et al. PET-based human dosimetry of 18F-galacto-RGD, a new radiotracer for imaging alpha v beta3 expression. J Nucl Med. 2006;47:763–9. [PubMed] [Google Scholar]
- 35.Beer AJ, Haubner R, Goebel M, et al. Biodistribution and pharmacokinetics of the alphavbeta3-selective tracer 18F-galacto-RGD in cancer patients. J Nucl Med. 2005;46:1333–41. [PubMed] [Google Scholar]
- 36.Beer AJ, Grosu AL, Carlsen J, et al. [18F]galacto-RGD positron emission tomography for imaging of alphav-beta3 expression on the neovasculature in patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13:6610–6. doi: 10.1158/1078-0432.CCR-07-0528. [DOI] [PubMed] [Google Scholar]
- 37.Beer AJ, Lorenzen S, Metz S, et al. Comparison of integrin alphaVbeta3 expression and glucose metabolism in primary and metastatic lesions in cancer patients: a PET study using 18F-galacto-RGD and 18F-FDG. J Nucl Med. 2008;49:22–9. doi: 10.2967/jnumed.107.045864. [DOI] [PubMed] [Google Scholar]
- 38.Haubner R, Decristoforo C. Radiolabelled RGD peptides and peptidomimetics for tumour targeting. Front Biosci. 2009;14:872–86. doi: 10.2741/3283. [DOI] [PubMed] [Google Scholar]
- 39.Chen X, Park R, Hou Y, et al. MicroPET imaging of brain tumor angiogenesis with 18F-labeled PEGylated RGD peptide. Eur J Nucl Med Mol Imaging. 2004;31:1081–9. doi: 10.1007/s00259-003-1452-2. [DOI] [PubMed] [Google Scholar]
- 40.Boturyn D, Coll JL, Garanger E, Favrot MC, Dumy P. Template assembled cyclopeptides as multimeric system for integrin targeting and endocytosis. J Am Chem Soc. 2004;126:5730–9. doi: 10.1021/ja049926n. [DOI] [PubMed] [Google Scholar]
- 41.Chen X, Liu S, Hou Y, et al. MicroPET imaging of breast cancer alphav-integrin expression with 64Cu-labeled dimeric RGD peptides. Mol Imaging Biol. 2004;6:350–9. doi: 10.1016/j.mibio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 42.Chen X, Park R, Tohme M, Shahinian AH, Bading JR, Conti PS. MicroPET and autoradiographic imaging of breast cancer alpha v-integrin expression using 18F- and 64Cu-labeled RGD peptide. Bioconjug Chem. 2004;15:41–9. doi: 10.1021/bc0300403. [DOI] [PubMed] [Google Scholar]
- 43.Chen X, Tohme M, Park R, Hou Y, Bading JR, Conti PS. Micro-PET imaging of alphavbeta3-integrin expression with 18F-labeled dimeric RGD peptide. Mol Imaging. 2004;3:96–104. doi: 10.1162/15353500200404109. [DOI] [PubMed] [Google Scholar]
- 44.Dijkgraaf I, Liu S, Kruijtzer JA, et al. Effects of linker variation on the in vitro and in vivo characteristics of an 111In-labeled RGD peptide. Nucl Med Biol. 2007;34:29–35. doi: 10.1016/j.nucmedbio.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 45.Wu Z, Li ZB, Chen K, Cai W, He L, Chin FT, et al. microPET of tumor integrin alphavbeta3 expression using 18F-labeled PEGylated tetrameric RGD peptide (18F-FPRGD4) J Nucl Med. 2007;48:1536–44. doi: 10.2967/jnumed.107.040816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang X, Xiong Z, Wu Y, et al. Quantitative PET imaging of tumor integrin alphavbeta3 expression with 18F-FRGD2. J Nucl Med. 2006;47:113–21. [PMC free article] [PubMed] [Google Scholar]
- 47.Almutairi A, Rossin R, Shokeen M, et al. Biodegradable dendritic positron-emitting nanoprobes for the noninvasive imaging of angiogenesis. Proc Natl Acad Sci U S A. 2009;106:685–90. doi: 10.1073/pnas.0811757106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Z, Cai W, He L, et al. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat Nanotechnol. 2007;2:47–52. doi: 10.1038/nnano.2006.170. [DOI] [PubMed] [Google Scholar]
- 49.Broumas AR, Pollard RE, Bloch SH, Wisner ER, Griffey S, Ferrara KW. Contrast-enhanced computed tomography and ultrasound for the evaluation of tumor blood flow. Invest Radiol. 2005;40:134–47. doi: 10.1097/01.rli.0000152833.35744.7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 51.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–27. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 52.Cai W, Rao J, Gambhir SS, Chen X. How molecular imaging is speeding up antiangiogenic drug development. Mol Cancer Ther. 2006;5:2624–33. doi: 10.1158/1535-7163.MCT-06-0395. [DOI] [PubMed] [Google Scholar]
- 53.Chen K, Cai W, Li ZB, Wang H, Chen X. Quantitative PET imaging of VEGF receptor expression. Mol Imaging Biol. 2009;11:15–22. doi: 10.1007/s11307-008-0172-1. [DOI] [PubMed] [Google Scholar]
- 54.Hsu AR, Cai W, Veeravagu A, et al. Multimodality molecular imaging of glioblastoma growth inhibition with vasculature-targeting fusion toxin VEGF121/rGel. J Nucl Med. 2007;48:445–54. [PubMed] [Google Scholar]
- 55.Backer MV, Levashova Z, Patel V, et al. Molecular imaging of VEGF receptors in angiogenic vasculature with single-chain VEGF-based probes. Nat Med. 2007;13:504–9. doi: 10.1038/nm1522. [DOI] [PubMed] [Google Scholar]
- 56.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 57.Wang H, Cai W, Chen K, et al. A new PET tracer specific for vascular endothelial growth factor receptor 2. Eur J Nucl Med Mol Imaging. 2007;34:2001–10. doi: 10.1007/s00259-007-0524-0. [DOI] [PubMed] [Google Scholar]
- 58.Vaupel P, Harrison L. Tumor hypoxia: causative factors, compensatory mechanisms, and cellular response. Oncologist. 2004;9(Suppl 5):4–9. doi: 10.1634/theoncologist.9-90005-4. [DOI] [PubMed] [Google Scholar]
- 59.Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–39. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 60.Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–76. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 61.Fyles AW, Milosevic M, Wong R, et al. Oxygenation predicts radiation response and survival in patients with cervix cancer. Radiother Oncol. 1998;48:149–56. doi: 10.1016/s0167-8140(98)00044-9. [DOI] [PubMed] [Google Scholar]
- 62.Nordsmark M, Bentzen SM, Rudat V, et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol. 2005;77:18–24. doi: 10.1016/j.radonc.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 63.Brizel DM, Scully SP, Harrelson JM, et al. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996;56:941–3. [PubMed] [Google Scholar]
- 64.Chapman JD. Hypoxic sensitizers—implications for radiation therapy. N Engl J Med. 1979;301:1429–32. doi: 10.1056/NEJM197912273012606. [DOI] [PubMed] [Google Scholar]
- 65.Lee ST, Scott AM. Hypoxia positron emission tomography imaging with 18F-fluoromisonidazole. Semin Nucl Med. 2007;37:451–61. doi: 10.1053/j.semnuclmed.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 66.Krohn KA, Link JM, Mason RP. Molecular imaging of hypoxia. J Nucl Med. 2008;49(Suppl 2):129S–48S. doi: 10.2967/jnumed.107.045914. [DOI] [PubMed] [Google Scholar]
- 67.Mees G, Dierckx R, Vangestel C, Van de Wiele C. Molecular imaging of hypoxia with radiolabelled agents. Eur J Nucl Med Mol Imaging. 2009;36:1674–86. doi: 10.1007/s00259-009-1195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rasey JS, Casciari JJ, Hofstrand PD, Muzi M, Graham MM, Chin LK. Determining hypoxic fraction in a rat glioma by uptake of radiolabeled fluoromisonidazole. Radiat Res. 2000;153:84–92. doi: 10.1667/0033-7587(2000)153[0084:dhfiar]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 69.Dubois L, Landuyt W, Haustermans K, Dupont P, Bormans G, Vermaelen P, et al. Evaluation of hypoxia in an experimental rat tumour model by [(18)F]fluoromisonidazole PET and immunohistochemistry. Br J Cancer. 2004;91:1947–54. doi: 10.1038/sj.bjc.6602219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zimny M, Gagel B, DiMartino E, et al. FDG—a marker of tumour hypoxia? A comparison with [18F]fluoromisonidazole and pO2-polarography in metastatic head and neck cancer. Eur J Nucl Med Mol Imaging. 2006;33:1426–31. doi: 10.1007/s00259-006-0175-6. [DOI] [PubMed] [Google Scholar]
- 71.Gagel B, Piroth M, Pinkawa M, et al. pO polarography, contrast enhanced color duplex sonography (CDS), [18F] fluoromisonidazole and [18F] fluorodeoxyglucose positron emission tomography: validated methods for the evaluation of therapy-relevant tumor oxygenation or only bricks in the puzzle of tumor hypoxia?? BMC Cancer. 2007;7:113. doi: 10.1186/1471-2407-7-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rajendran JG, Wilson DC, Conrad EU, et al. [(18)F]FMISO and [(18)F]FDG PET imaging in soft tissue sarcomas: correlation of hypoxia, metabolism and VEGF expression. Eur J Nucl Med Mol Imaging. 2003;30:695–704. doi: 10.1007/s00259-002-1096-7. [DOI] [PubMed] [Google Scholar]
- 73.Cherk MH, Foo SS, Poon AM, et al. Lack of correlation of hypoxic cell fraction and angiogenesis with glucose metabolic rate in non-small cell lung cancer assessed by 18F-fluoromisonidazole and 18F-FDG PET. J Nucl Med. 2006;47:1921–6. [PubMed] [Google Scholar]
- 74.Cher LM, Murone C, Lawrentschuk N, et al. Correlation of hypoxic cell fraction and angiogenesis with glucose metabolic rate in gliomas using 18F-fluoromisonidazole, 18F-FDG PET, and immunohistochemical studies. J Nucl Med. 2006;47:410–8. [PubMed] [Google Scholar]
- 75.Rajendran JG, Schwartz DL, O'Sullivan J, et al. Tumor hypoxia imaging with [F-18] fluoromisonidazole positron emission tomography in head and neck cancer. Clin Cancer Res. 2006;12:5435–41. doi: 10.1158/1078-0432.CCR-05-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gagel B, Reinartz P, Demirel C, et al. [18F] fluoromisonidazole and [18F] fluorodeoxyglucose positron emission tomography in response evaluation after chemo-/radiotherapy of non-small-cell lung cancer: a feasibility study. BMC Cancer. 2006;6:51. doi: 10.1186/1471-2407-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Piert M, Machulla HJ, Picchio M, et al. Hypoxia-specific tumor imaging with 18F-fluoroazomycin arabinoside. J Nucl Med. 2005;46:106–13. [PubMed] [Google Scholar]
- 78.Reischl G, Dorow DS, Cullinane C, et al. Imaging of tumor hypoxia with [124I]IAZA in comparison with [18F]FMISO and [18F]FAZA—first small animal PET results. J Pharm Pharm Sci. 2007;10:203–11. [PubMed] [Google Scholar]
- 79.Grosu AL, Souvatzoglou M, Roper B, et al. Hypoxia imaging with FAZA-PET and theoretical considerations with regard to dose painting for individualization of radiotherapy in patients with head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;69:541–51. doi: 10.1016/j.ijrobp.2007.05.079. [DOI] [PubMed] [Google Scholar]
- 80.Yuan H, Schroeder T, Bowsher JE, Hedlund LW, Wong T, Dewhirst MW. Intertumoral differences in hypoxia selectivity of the PET imaging agent 64Cu(II)-diacetylbis(N4-methylthiosemicarbazone) J Nucl Med. 2006;47:989–98. [PubMed] [Google Scholar]
- 81.Dence CS, Ponde DE, Welch MJ, Lewis JS. Autoradio-graphic and small-animal PET comparisons between (18)F-FMISO, (18)F-FDG, (18)F-FLT and the hypoxic selective (64)Cu-ATSM in a rodent model of cancer. Nucl Med Biol. 2008;35:713–20. doi: 10.1016/j.nucmedbio.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lewis JS, Laforest R, Dehdashti F, Grigsby PW, Welch MJ, Siegel BA. An imaging comparison of 64Cu-ATSM and 60Cu-ATSM in cancer of the uterine cervix. J Nucl Med. 2008;49:1177–82. doi: 10.2967/jnumed.108.051326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blankenberg FG. In vivo imaging of apoptosis. Cancer Biol Ther. 2008;7:1525–32. doi: 10.4161/cbt.7.10.6934. [DOI] [PubMed] [Google Scholar]
- 84.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–6. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 85.Blankenberg FG. Recent advances in the imaging of programmed cell death. Curr Pharm Des. 2004;10:1457–67. doi: 10.2174/1381612043384790. [DOI] [PubMed] [Google Scholar]
- 86.Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–20. [PubMed] [Google Scholar]
- 87.Boersma HH, Kietselaer BL, Stolk LM, et al. Past, present, and future of annexin A5: from protein discovery to clinical applications. J Nucl Med. 2005;46:2035–50. [PubMed] [Google Scholar]
- 88.Corsten MF, Hofstra L, Narula J, Reutelingsperger CP. Counting heads in the war against cancer: defining the role of annexin A5 imaging in cancer treatment and surveillance. Cancer Res. 2006;66:1255–60. doi: 10.1158/0008-5472.CAN-05-3000. [DOI] [PubMed] [Google Scholar]
- 89.Lahorte CM, Vanderheyden JL, Steinmetz N, Van de Wiele C, Dierckx RA, Slegers G. Apoptosis-detecting radioligands: current state of the art and future perspectives. Eur J Nucl Med Mol Imaging. 2004;31:887–919. doi: 10.1007/s00259-004-1555-4. [DOI] [PubMed] [Google Scholar]
- 90.Murakami Y, Takamatsu H, Taki J, et al. 18F-labelled annexin V: a PET tracer for apoptosis imaging. Eur J Nucl Med Mol Imaging. 2004;31:469–74. doi: 10.1007/s00259-003-1378-8. [DOI] [PubMed] [Google Scholar]
- 91.Li X, Link JM, Stekhova S, et al. Site-specific labeling of annexin V with F-18 for apoptosis imaging. Bioconjug Chem. 2008;19:1684–8. doi: 10.1021/bc800164d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nguyen QD, Smith G, Glaser M, Perumal M, Arstad E, Aboagye EO. Positron emission tomography imaging of drug-induced tumor apoptosis with a caspase-3/7 specific [18F]-labeled isatin sulfonamide. Proc Natl Acad Sci U S A. 2009;106:16375–80. doi: 10.1073/pnas.0901310106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wells P, Gunn RN, Alison M, et al. Assessment of proliferation in vivo using 2-[(11)C]thymidine positron emission tomography in advanced intra-abdominal malignancies. Cancer Res. 2002;62:5698–702. [PubMed] [Google Scholar]
- 94.Shields AF. Positron emission tomography measurement of tumor metabolism and growth: its expanding role in oncology. Mol Imaging Biol. 2006;8:141–50. doi: 10.1007/s11307-006-0039-2. [DOI] [PubMed] [Google Scholar]
- 95.Shields AF, Grierson JR, Dohmen BM, et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med. 1998;4:1334–6. doi: 10.1038/3337. [DOI] [PubMed] [Google Scholar]
- 96.Leyton J, Alao JP, Da Costa M, et al. In vivo biological activity of the histone deacetylase inhibitor LAQ824 is detectable with 3′-deoxy-3′-[18F]fluorothymidine positron emission tomography. Cancer Res. 2006;66:7621–9. doi: 10.1158/0008-5472.CAN-05-3962. [DOI] [PubMed] [Google Scholar]
- 97.McLaughlin F, La Thangue NB. Histone deacetylase inhibitors open new doors in cancer therapy. Biochem Pharmacol. 2004;68:1139–44. doi: 10.1016/j.bcp.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 98.Pio BS, Park CK, Pietras R, et al. Usefulness of 3′-[F-18]fluoro-3′-deoxythymidine with positron emission tomography in predicting breast cancer response to therapy. Mol Imaging Biol. 2006;8:36–42. doi: 10.1007/s11307-005-0029-9. [DOI] [PubMed] [Google Scholar]
- 99.Kenny L, Coombes RC, Vigushin DM, Al-Nahhas A, Shousha S, Aboagye EO. Imaging early changes in proliferation at 1 week post chemotherapy: a pilot study in breast cancer patients with 3′-deoxy-3′-[18F]fluorothymidine positron emission tomography. Eur J Nucl Med Mol Imaging. 2007;34:1339–47. doi: 10.1007/s00259-007-0379-4. [DOI] [PubMed] [Google Scholar]
- 100.Kenny LM, Vigushin DM, Al-Nahhas A, et al. Quantification of cellular proliferation in tumor and normal tissues of patients with breast cancer by [18F]fluorothymidine-positron emission tomography imaging: evaluation of analytical methods. Cancer Res. 2005;65:10104–12. doi: 10.1158/0008-5472.CAN-04-4297. [DOI] [PubMed] [Google Scholar]
- 101.Conti PS, Alauddin MM, Fissekis JR, Schmall B, Watanabe KA. Synthesis of 2′-fluoro-5-[11C]-methyl-1-beta-D-arabinofuranosyluracil ([11C]-FMAU): a potential nucleoside analog for in vivo study of cellular proliferation with PET. Nucl Med Biol. 1995;22:783–9. doi: 10.1016/0969-8051(95)00017-r. [DOI] [PubMed] [Google Scholar]
- 102.Bading JR, Shahinian AH, Vail A, et al. Pharmacokinetics of the thymidine analog 2′-fluoro-5-methyl-1-beta-D-arabinofuranosyluracil (FMAU) in tumor-bearing rats. Nucl Med Biol. 2004;31:407–18. doi: 10.1016/j.nucmedbio.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 103.Sun H, Mangner TJ, Collins JM, Muzik O, Douglas K, Shields AF. Imaging DNA synthesis in vivo with 18F-FMAU and PET. J Nucl Med. 2005;46:292–6. [PubMed] [Google Scholar]
- 104.Conti PS, Bading JR, Mouton PP, et al. In vivo measurement of cell proliferation in canine brain tumor using C-11-labeled FMAU and PET. Nucl Med Biol. 2008;35:131–41. doi: 10.1016/j.nucmedbio.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 105.Sun H, Sloan A, Mangner TJ, et al. Imaging DNA synthesis with [18F]FMAU and positron emission tomography in patients with cancer. Eur J Nucl Med Mol Imaging. 2005;32:15–22. doi: 10.1007/s00259-004-1713-8. [DOI] [PubMed] [Google Scholar]
- 106.Buck AK, Bommer M, Stilgenbauer S, et al. Molecular imaging of proliferation in malignant lymphoma. Cancer Res. 2006;66:11055–61. doi: 10.1158/0008-5472.CAN-06-1955. [DOI] [PubMed] [Google Scholar]
- 107.Muzi M, Spence AM, O'Sullivan F, et al. Kinetic analysis of 3′-deoxy-3′-18F-fluorothymidine in patients with gliomas. J Nucl Med. 2006;47:1612–21. [PubMed] [Google Scholar]
- 108.Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 109.Matheny KE, Barbieri CE, Sniezek JC, Arteaga CL, Pietenpol JA. Inhibition of epidermal growth factor receptor signaling decreases p63 expression in head and neck squamous carcinoma cells. Laryngoscope. 2003;113:936–9. doi: 10.1097/00005537-200306000-00004. [DOI] [PubMed] [Google Scholar]
- 110.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–54. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 111.Laskin JJ, Sandler AB. Epidermal growth factor receptor: a promising target in solid tumours. Cancer Treat Rev. 2004;30:1–17. doi: 10.1016/j.ctrv.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 112.Fan Z, Masui H, Altas I, Mendelsohn J. Blockade of epidermal growth factor receptor function by bivalent and monovalent fragments of 225 anti-epidermal growth factor receptor monoclonal antibodies. Cancer Res. 1993;53:4322–8. [PubMed] [Google Scholar]
- 113.Cai W, Chen K, He L, Cao Q, Koong A, Chen X. Quantitative PET of EGFR expression in xenograft-bearing mice using 64Cu-labeled cetuximab, a chimeric anti-EGFR monoclonal antibody. Eur J Nucl Med Mol Imaging. 2007;34:850–8. doi: 10.1007/s00259-006-0361-6. [DOI] [PubMed] [Google Scholar]
- 114.Niu G, Li Z, Xie J, Le QT, Chen X. PET of EGFR antibody distribution in head and neck squamous cell carcinoma models. J Nucl Med. 2009;50:1116–23. doi: 10.2967/jnumed.109.061820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Velikyan I, Sundberg AL, Lindhe O, et al. Preparation and evaluation of (68)Ga-DOTA-hEGF for visualization of EGFR expression in malignant tumors. J Nucl Med. 2005;46:1881–8. [PubMed] [Google Scholar]
- 116.Smith-Jones PM, Solit D, Afroze F, Rosen N, Larson SM. Early tumor response to Hsp90 therapy using HER2 PET: comparison with 18F-FDG PET. J Nucl Med. 2006;47:793–6. [PMC free article] [PubMed] [Google Scholar]
- 117.Bodei L, Paganelli G, Mariani G. Receptor radionuclide therapy of tumors: a road from basic research to clinical applications. J Nucl Med. 2006;47:375–7. [PubMed] [Google Scholar]
- 118.Reubi JC, Waser B. Concomitant expression of several peptide receptors in neuroendocrine tumours: molecular basis for in vivo multireceptor tumour targeting. Eur J Nucl Med Mol Imaging. 2003;30:781–93. doi: 10.1007/s00259-003-1184-3. [DOI] [PubMed] [Google Scholar]
- 119.Scarpignato C, Pelosini I. Somatostatin analogs for cancer treatment and diagnosis: an overview. Chemotherapy. 2001;47(Suppl 2):1–29. doi: 10.1159/000049157. [DOI] [PubMed] [Google Scholar]
- 120.Forrer F, Valkema R, Kwekkeboom DJ, de Jong M, Krenning EP. Neuroendocrine tumors. Peptide receptor radionuclide therapy. Best Pract Res Clin Endocrinol Metab. 2007;21:111–29. doi: 10.1016/j.beem.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 121.Anderson CJ, Dehdashti F, Cutler PD, et al. 64Cu-TETA-octreotide as a PET imaging agent for patients with neuroendocrine tumors. J Nucl Med. 2001;42:213–21. [PubMed] [Google Scholar]
- 122.Gabriel M, Decristoforo C, Kendler D, et al. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007;48:508–18. doi: 10.2967/jnumed.106.035667. [DOI] [PubMed] [Google Scholar]
- 123.Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17:545–80. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- 124.Li ZB, Wu Z, Chen K, Ryu EK, Chen X. 18F-labeled BBN-RGD heterodimer for prostate cancer imaging. J Nucl Med. 2008;49:453–61. doi: 10.2967/jnumed.107.048009. [DOI] [PubMed] [Google Scholar]
- 125.Rodriguez-Porcel M, Cai W, Gheysens O, et al. Imaging of VEGF receptor in a rat myocardial infarction model using PET. J Nucl Med. 2008;49:667–73. doi: 10.2967/jnumed.107.040576. [DOI] [PMC free article] [PubMed] [Google Scholar]