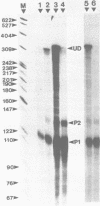
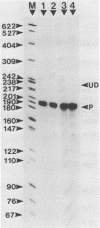
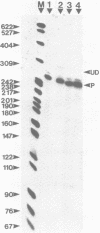
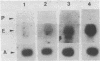
Abstract
The endo A gene encoding for an intermediate filament protein, a cytokeratin is usually expressed in epithelial cells. The regulation of this gene, probed by using cycloheximide, an inhibitor of protein synthesis was studied in various cell lines. The lines explored were undifferentiated embryonal carcinoma PCC4 cells which normally do not express endo A gene, PCC4 cells cultivated permanently at 31 degrees C (PCC4-31), which are epithelial-like cells derived by differentiation from PCC4 cells, but which do express endo A gene, TDM1 cells, an epithelial teratocarcinoma cell line, and 3T6 mouse fibroblasts. Treatment of undifferentiated PCC4 cells by cycloheximide led to transcriptional induction of the endo A gene, and the same effect was observed after this treatment in PCC4-31 cells. By contrast, cycloheximide did not induce endo A gene expression in 3T6 cells, and reduced the transcriptional activity of this gene in TDM1 cells. We conclude that a labile inhibitor (or several) blocks endo A gene expression in undifferentiated PCC4 cells. We suggest that in these cells, the expression of the endo A gene is regulated both positively and negatively, possibly by a cellular E1A-like activity, as we previously demonstrated it for Py virus (C. Crémisi and C. Babinet, 1986 J. Virol. 59; 761-763). We further suggest that negative regulatory factors involved in this regulation are absent in TDM cells and reduced in PCC4-31 cells.
Full text
PDF


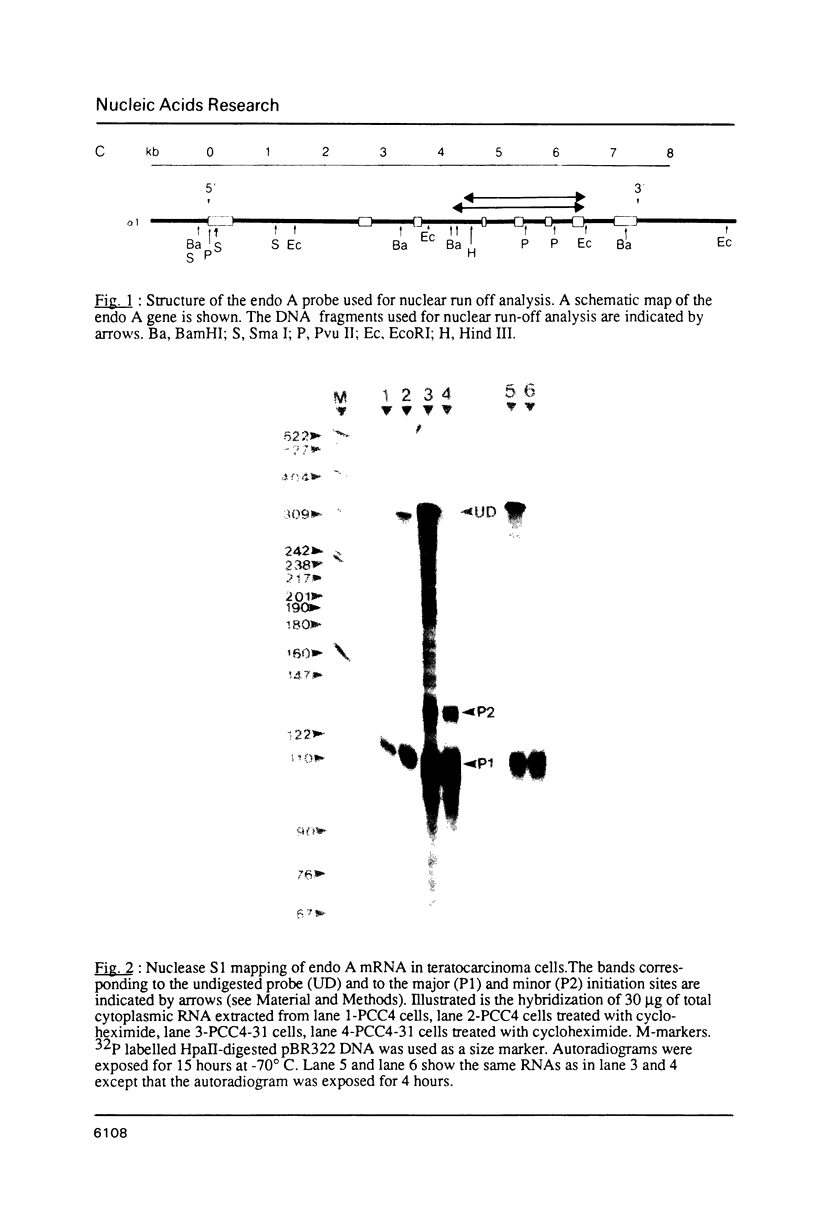


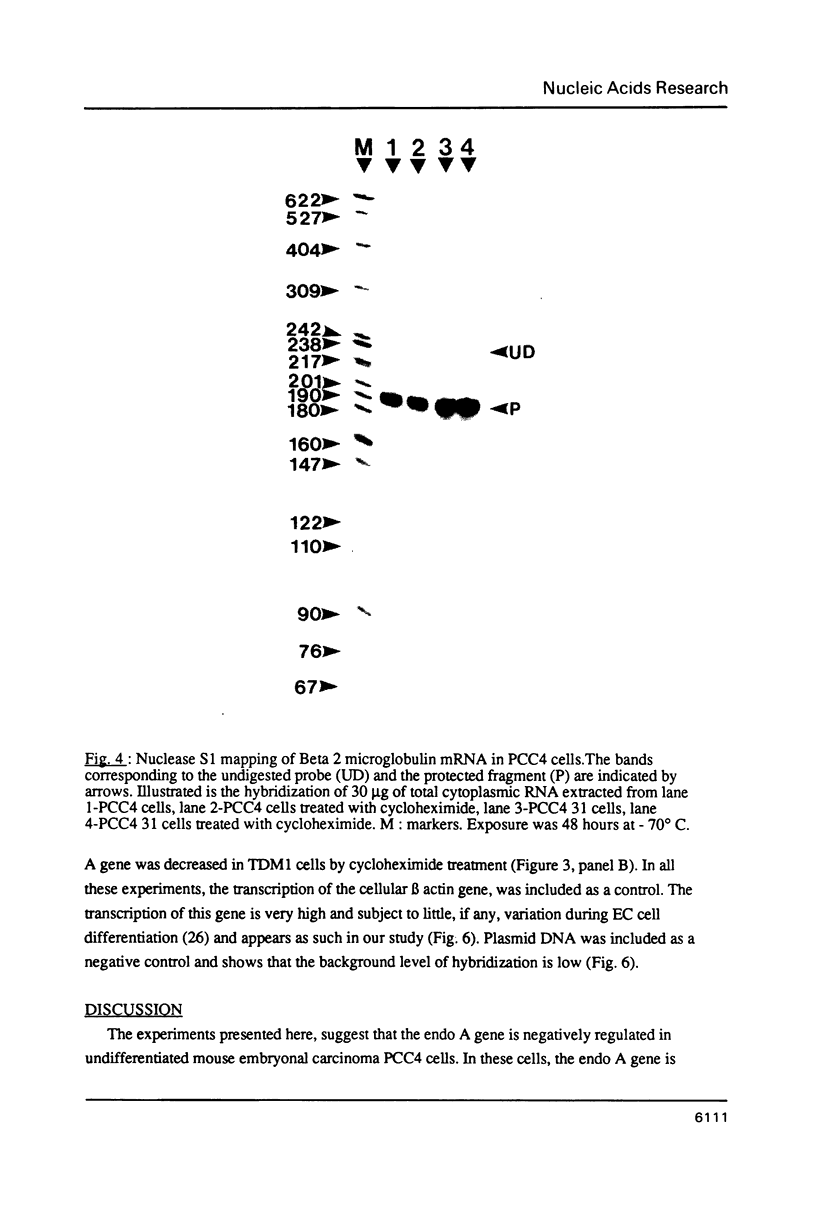
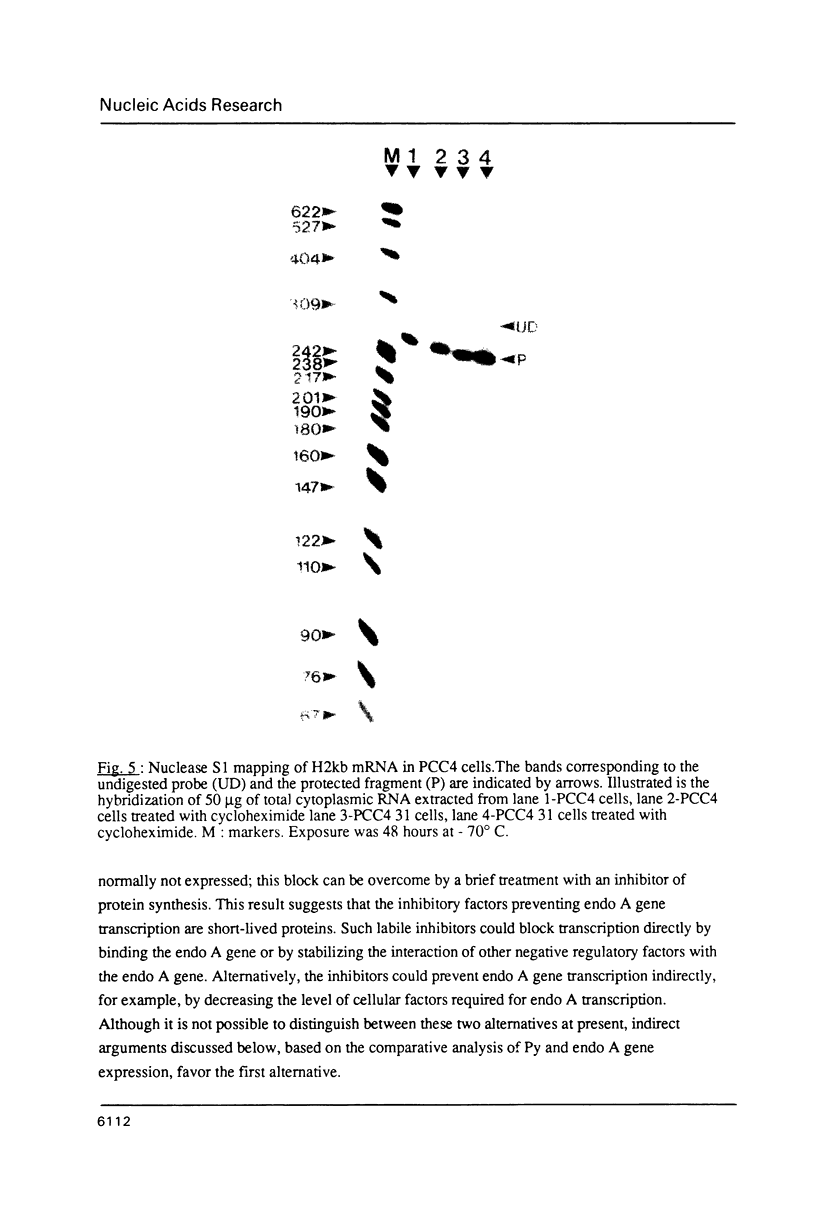




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borrelli E., Hen R., Chambon P. Adenovirus-2 E1A products repress enhancer-induced stimulation of transcription. Nature. 1984 Dec 13;312(5995):608–612. doi: 10.1038/312608a0. [DOI] [PubMed] [Google Scholar]
- Brûlet P., Babinet C., Kemler R., Jacob F. Monoclonal antibodies against trophectoderm-specific markers during mouse blastocyst formation. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4113–4117. doi: 10.1073/pnas.77.7.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremisi C., Babinet C. Negative regulation of early polyomavirus expression in mouse embryonal carcinoma cells. J Virol. 1986 Sep;59(3):761–763. doi: 10.1128/jvi.59.3.761-763.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremisi C., Duprey P. Spontaneous differentiation of murine teratocarcinoma stem cells at low temperature. J Cell Physiol. 1986 Nov;129(2):230–236. doi: 10.1002/jcp.1041290215. [DOI] [PubMed] [Google Scholar]
- Cremisi C. Effect of 5-azacytidine treatment on mouse embryonal carcinoma cells. J Cell Physiol. 1983 Aug;116(2):181–190. doi: 10.1002/jcp.1041160209. [DOI] [PubMed] [Google Scholar]
- Dandolo L., Vasseur M., Kress C., Aghion J., Blangy D. Temperature dependent expression of polyoma virus in murine embryonal carcinoma cells. J Cell Physiol. 1980 Oct;105(1):17–24. doi: 10.1002/jcp.1041050104. [DOI] [PubMed] [Google Scholar]
- Dony C., Kessel M., Gruss P. Post-transcriptional control of myc and p53 expression during differentiation of the embryonal carcinoma cell line F9. Nature. 1985 Oct 17;317(6038):636–639. doi: 10.1038/317636a0. [DOI] [PubMed] [Google Scholar]
- Duprey P., Morello D., Vasseur M., Babinet C., Condamine H., Brûlet P., Jacob F. Expression of the cytokeratin endo A gene during early mouse embryogenesis. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8535–8539. doi: 10.1073/pnas.82.24.8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. J., Kaufman M. H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981 Jul 9;292(5819):154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Goodbourn S., Burstein H., Maniatis T. The human beta-interferon gene enhancer is under negative control. Cell. 1986 May 23;45(4):601–610. doi: 10.1016/0092-8674(86)90292-8. [DOI] [PubMed] [Google Scholar]
- Goodfellow P. N. The use of cloned gene probes to study differentiation in teratocarcinomas. Cell Differ. 1984 Dec;15(2-4):257–267. doi: 10.1016/0045-6039(84)90083-6. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Rigby P. W., Lane D. P. Negative regulation of viral enhancers in undifferentiated embryonic stem cells. Cell. 1985 Sep;42(2):519–526. doi: 10.1016/0092-8674(85)90109-6. [DOI] [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hen R., Borrelli E., Chambon P. Repression of the immunoglobulin heavy chain enhancer by the adenovirus-2 E1A products. Science. 1985 Dec 20;230(4732):1391–1394. doi: 10.1126/science.2999984. [DOI] [PubMed] [Google Scholar]
- Herbomel P., Bourachot B., Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984 Dec;39(3 Pt 2):653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- Hogan B. L., Taylor A., Adamson E. Cell interactions modulate embryonal carcinoma cell differentiation into parietal or visceral endoderm. Nature. 1981 May 21;291(5812):235–237. doi: 10.1038/291235a0. [DOI] [PubMed] [Google Scholar]
- Imperiale M. J., Kao H. T., Feldman L. T., Nevins J. R., Strickland S. Common control of the heat shock gene and early adenovirus genes: evidence for a cellular E1A-like activity. Mol Cell Biol. 1984 May;4(5):867–874. doi: 10.1128/mcb.4.5.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly F., Condamine H. Tumor viruses and early mouse embryos. Biochim Biophys Acta. 1982 Apr 29;651(2-3):105–141. doi: 10.1016/0304-419X(82)90009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linney E., Donerly S. DNA fragments from F9 PyEC mutants increase expression of heterologous genes in transfected F9 cells. Cell. 1983 Dec;35(3 Pt 2):693–699. doi: 10.1016/0092-8674(83)90102-2. [DOI] [PubMed] [Google Scholar]
- Martin G. R. Teratocarcinomas and mammalian embryogenesis. Science. 1980 Aug 15;209(4458):768–776. doi: 10.1126/science.6250214. [DOI] [PubMed] [Google Scholar]
- McBurney M. W., Jones-Villeneuve E. M., Edwards M. K., Anderson P. J. Control of muscle and neuronal differentiation in a cultured embryonal carcinoma cell line. Nature. 1982 Sep 9;299(5879):165–167. doi: 10.1038/299165a0. [DOI] [PubMed] [Google Scholar]
- Morello D., Duprey P., Israel A., Babinet C. Asynchronous regulation of mouse H-2D and beta-2 microglobulin RNA transcripts. Immunogenetics. 1985;22(5):441–452. doi: 10.1007/BF00418090. [DOI] [PubMed] [Google Scholar]
- Morello D., Moore G., Salmon A. M., Yaniv M., Babinet C. Studies on the expression of an H-2K/human growth hormone fusion gene in giant transgenic mice. EMBO J. 1986 Aug;5(8):1877–1883. doi: 10.1002/j.1460-2075.1986.tb04439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. Induction of the synthesis of a 70,000 dalton mammalian heat shock protein by the adenovirus E1A gene product. Cell. 1982 Jul;29(3):913–919. doi: 10.1016/0092-8674(82)90453-6. [DOI] [PubMed] [Google Scholar]
- Oshima R. G. Developmental expression of murine extra-embryonic endodermal cytoskeletal proteins. J Biol Chem. 1982 Apr 10;257(7):3414–3421. [PubMed] [Google Scholar]
- Paulin D., Perreau J., Jakob H., Jacob F., Yaniv M. Tropomyosin synthesis accompanies formation of actin filaments in embryonal carcinoma cells induced to differentiate by hexamethylene bisacetamide. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1891–1895. doi: 10.1073/pnas.76.4.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A., Wright S., Cedar H., Flavell R., Grosveld F. Regulated expression of an introduced MHC H-2K bm1 gene in murine embryonal carcinoma cells. Nature. 1984 Aug 2;310(5976):415–418. doi: 10.1038/310415a0. [DOI] [PubMed] [Google Scholar]
- Rosenthal A., Wright S., Quade K., Gallimore P., Cedar H., Grosveld F. Increased MHC H-2K gene transcription in cultured mouse embryo cells after adenovirus infection. Nature. 1985 Jun 13;315(6020):579–581. doi: 10.1038/315579a0. [DOI] [PubMed] [Google Scholar]
- Schrier P. I., Bernards R., Vaessen R. T., Houweling A., van der Eb A. J. Expression of class I major histocompatibility antigens switched off by highly oncogenic adenovirus 12 in transformed rat cells. 1983 Oct 27-Nov 2Nature. 305(5937):771–775. doi: 10.1038/305771a0. [DOI] [PubMed] [Google Scholar]
- Sleigh M. J., Lockett T. J. SV40 enhancer activation during retinoic acid-induced differentiation of F9 embryonal carcinoma cells. EMBO J. 1985 Dec 30;4(13B):3831–3837. doi: 10.1002/j.1460-2075.1985.tb04155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R., Ziff E. B. HeLa cell beta-tubulin gene transcription is stimulated by adenovirus 5 in parallel with viral early genes by an E1a-dependent mechanism. Mol Cell Biol. 1984 Dec;4(12):2792–2801. doi: 10.1128/mcb.4.12.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland S., Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978 Oct;15(2):393–403. doi: 10.1016/0092-8674(78)90008-9. [DOI] [PubMed] [Google Scholar]
- Vasseur M., Duprey P., Brûlet P., Jacob F. One gene and one pseudogene for the cytokeratin endo A. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1155–1159. doi: 10.1073/pnas.82.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velcich A., Ziff E. Adenovirus E1a proteins repress transcription from the SV40 early promoter. Cell. 1985 Mar;40(3):705–716. doi: 10.1016/0092-8674(85)90219-3. [DOI] [PubMed] [Google Scholar]
- Wang S. Y., LaRosa G. J., Gudas L. J. Molecular cloning of gene sequences transcriptionally regulated by retinoic acid and dibutyryl cyclic AMP in cultured mouse teratocarcinoma cells. Dev Biol. 1985 Jan;107(1):75–86. doi: 10.1016/0012-1606(85)90377-x. [DOI] [PubMed] [Google Scholar]
- Weintraub H. Assembly and propagation of repressed and depressed chromosomal states. Cell. 1985 Oct;42(3):705–711. doi: 10.1016/0092-8674(85)90267-3. [DOI] [PubMed] [Google Scholar]