Abstract
Dysregulation of the ubiquitin-proteasome system (UPS) has been implicated in a wide range of pathologies including cancer, neurodegeneration, and viral infection. Inhibiting the proteasome has been shown to be an effective therapeutic strategy in humans; yet toxicity with this target remains high. Ubiquitin Ligases (E3s) represent an alternative attractive therapeutic target in the UPS. Here we will discuss current platforms that report on E3 ligase activity and can detect E3 inhibitors, while underlining the advantages and disadvantages of each approach.
Keywords: Ubiquitin, ligase, E3, RING, HECT, high-throughput screening
Introduction
The conjugation of Ubiquitin (Ub) is an important regulatory mechanism that is widespread in many biological processes. Many diseases have been linked to this pathway making the associated enzymes particularly interesting for therapeutic targets. The UPS has been validated with the approval of the proteasome inhibitor bortezomib for the treatment of multiple myeloma; however, significant toxicities were seen during clinical trials suggesting the need for more selective targets that do not affect all substrates targeted for destruction in the cell (1–4). Ubiquitylation is temporally and spatially controlled with strict substrate specificity. To target a protein for degradation by the proteasome a poly-ubiquitin chain must be covalently attached to the protein at a lysine residue. It is the poly-ubiquitin chain that is recognized by the proteasome and results in the degradation of the attached protein. Three classes of enzyme, the ubiquitin-activating enzyme (E1), the ubiquitin-conjugating enzyme (E2), and the ubiquitin-protein ligase (E3) mediate the reaction. The E1 first activates a free ubiquitin in an ATP-dependent manner, forming a high-energy thioester bond between the E1's active-site cysteine and the carboxyl-terminus of ubiquitin. The activated ubiquitin is then transferred to an E2, forming a similar thioester linkage. Upon recognizing a specific substrate and recruiting an E2, the E3 ligase mediates the ubiquitin transfer from the E2 to the lysine residue(s) of the substrate. Mono-ubiquitylation, resulting in protein functionalization, is also possible with the corresponding E2 enzyme. Similar mechanisms can also be found in the ubiquitin-like (UBL) protein pathways, e.g.,Nedd8, Sumo, and ISG15 (reviewed in (5)).
Ubiquitin Ligases (E3s) represent an alternative target in the UPS that should yield compounds with toxicity profiles that are superior to that of a proteasome inhibitor (6, 7). There are two classes of E3, RING E3s, which act as scaffolds to bring the components of the ubiquitylation machinery together in close contact with the substrate and HECT E3s, which form an intermediate with Ub before transferring it to the substrate. To develop therapeutic agents that target E3s, it is important to have a readily quantifiable assay platform that is suitable for high-throughput screening (HTS). To date only one inhibitor of E3s has reached clinical trials (8), demonstrating that current assay technologies need to be improved to find compounds that translate from in vitro assay to the clinic. Here we will provide an overview of current E3 HTS approaches and discuss the advantages and disadvantages of each.
Druggability of Ubiquitin Ligases
The approval of the proteasome inhibitor bortezomib (velcade) for the treatment of multiple myeloma validated targeting of the ubiquitin-proteasome pathway (UPS) for the treatment of cancer. However, extended treatment with bortezomib is associated with toxicity and drug resistance, limiting its efficacy (1–4). E3 ligases represent an attractive drug target in the UPS because of their limited number of substrates and selective regulatory pathways. One of the most interesting E3 targets is the Skp1-Cul1-Roc1-Fbox Protein complex (SCF) (9). The cullin and cullin-like-family share a conserved Cullin Homology domain amongst the five human cullins (Cul1, Cul2, Cul3, Cul4A/Cul4B, and Cul5) and three cullin-like proteins (Apc2, Cul7, and Parc) that have been identified. All of the proteins in the cullin super-family bind a RING domain protein, ROC1, and together form the core of a scaffold that creates multi-subunit RING UBL ligases. The remainder of the scaffold is made up of adaptor proteins, F-box Proteins, which bind a large variety of substrates and allow the regulation of an extensive variety of cellular functions. Two therapeutically relevant F-box proteins that are targeted for drug discovery are Skp2 and β-TRCP because of the key roles they play in cell cycle progression (10, 11). However, to inhibit these proteins one must disrupt a protein-protein interaction, typically considered more difficult to target than an enzymatic catalytic site, but not impossible with recent advances in understanding these interactions (12). It is also important to consider that in a typical ubiquitylation reaction, not only is an E3 and an associated substrate present, but also E1 and E2 enzymes. This makes follow-up assays important for the deconvolution of any lead compounds in an HTS campaign to determine which enzyme is being affected.
In contrast to the RING E3s, the HECT E3s have intrinsic catalytic activity characterized by an active cysteine residue that forms a thioester with ubiquitin from an E2. This intermediate allows for the E3 to directly transfer ubiquitin to the substrate. Notably, this transfer requires a conformational change in the HECT domain (13). Altogether, the HECT E3s provide more desired characteristics for drug inhibition than RING E3s (6). Both classes of E3 are involved in numerous diseases (Table 1) and HTS campaigns to find inhibitors need to consider the advantages and disadvantages of each approach.
Table 1.
Ubiquitin ligases with published disease associations.
| Pathology | Ubiquitin Ligases |
|---|---|
| Cancer | CARP2 (23), hdm2 (7, 24), SCF (β-TrCP/Skp2/Rbx4/SAG) (11), BRCA1 (25),c-Cbl (26), CHIP (27), E6-AP (6), HACE1 (28), RNF5 (29), Pirh2 (30), pVHL (31) |
| Neurodegeneration | Parkin (32),TRIM11 (33), UCH-L1 (34), mahogunin (35), malin (36) |
| Metabolic diseases | Praja1 (37), MuRF1 (38), SCFAtrogin1 (39), |
| Immune diseases | Hrd1 (40),TRAF6 (47), SLIM (42),GRAIL (43), ITCH (44),AIRE (45), ROQUIN (46) |
| Viral infection | Nedd4 (47), TRIM (48) |
Current assay systems for Ubiquitin Ligases
Unbound Reaction Components
E3 ligases facilitate the covalent attachment of ubiquitin to a target substrate, which results in an increase in proximity of these two proteins. This action allows for the use of fluorescence resonance energy transfer (FRET), a commonly used technology for HTS. The principle of this assay relies on two individually fluorescently labeled proteins; one acts as the fluorescent protein donor while the other acts as the acceptor. When these proteins are brought into close proximity, energy is transferred between the donor and acceptor, wherein the acceptor emission can be detected upon donor excitation. In contrast, when the two proteins are dissociated, only donor emission is detectable following donor excitation. The ratio between donor and acceptor emission reports on the relative interaction between two populations of proteins.
Several groups have used FRET technology to screen for inhibitors of E3 autoubiquitylation and substrate ubiquitylation. Although different formats have been applied, the basic idea is the same. Ubiquitin is labeled with one of the FRET pairs, commonly the FRET donor Eu3+. When the substrate or E3, frequently labeled with the FRET acceptor allophycocyanin (APC), is ubiquitylated the FRET pairs are brought into close proximity and a shift towards APC's emission wavelength (665nm) is seen. An example of this assay is illustrated in Figure 1A (14). This technique has the advantage that all enzymes are free in solution to interact. This approach was used to detect MDM2 ubiquitylation of p53 by labeling p53 with Eu3+ and Ub with Cy5 (15). A different approach used a mixture of Ub labeled with either fluorescein or tetramethylrhodamine (TAMRA); when polyubiquitin chains are formed, the fluorescein-Ub acts as a donor for the TAMRA-Ub in the chain allowing for the detection of chain assembly (16).
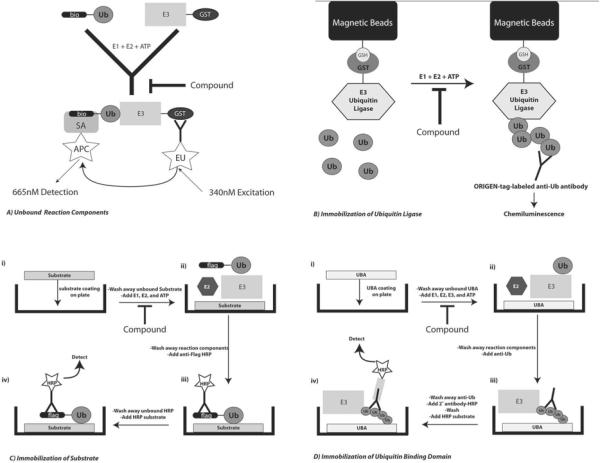
Figure 1.
A) Unbound Reaction Components. Biotin labeled Ub (bio-Ub) is mixed with GST-tagged E3, E1, E2 and ATP. anti-GST antibody labeled with Eu3+ and streptavidin-APC are then added and excited in the Eu wavelength and the fluorescence of APC is measured. B) Immobilization of Ubiquitin Ligases. GST-tagged E3 is bound to GSH coated magnetic beads. E1, E2, Ub, ATP and compounds are added to the reaction and measured by anti-Ub antibody labeled with the ORIGEN tag that gives a chemiluminescent response. C) Immobilization of Substrate. A high-binding plate is coated with the substrate, unbound substrate is washed off, and the E1, E2, FLAG-Ub, and E3 are added. After unbound proteins are removed anti-FLAG antibody conjugated to HRP is added to the well, washed and HRP substrate is added to detect a signal. D) UBA is immobilized to a high-binding plate. Unbound UBA is removed and E1, E2, E3, Ub, and ATP are added to the well. After the components are removed anti-Ub antibody is added, washed, and secondary antibody conjugated to HRP is added. HRP substrate is added after a final wash step.
In addition to the ubiquitylation of p53 discussed above (17), solution based FRET assays have been shown to work for autoubiquitylation for the E3s Rsc (14) and TRAF6 (18). However, there are some problems with this approach. First, the distance between the FRET pairs has significant impact upon the signal. The Förster R0 value (the distance that there is a 50% energy transfer between the pair) can be limiting as several E3s and substrates can easily span 70–80Å (19). One must take great care in choosing where the labeling is done and the situation can be further complicated by the fact that the substrate or E3 is not always labeled directly, but is frequently expressed with a fusion tag, which in turn binds an associated matrix that is labeled with APC. This further increases the distance between the FRET donor and acceptor and consequently decreases the signal that can be achieved in the assay. In many cases the components are no longer native during the assay, as they are pre-bound to a matrix in addition to a fusion tag. While these assays do not have wash steps, making the procedure and equipment required more accessible, it does open up the possibility for the compounds in the assay to cause background fluorescence and interference. To get around this problem the compounds must be kept at low concentrations, which might not be optimal for primary screening depending on the composition of the compound library.
An alternative approach has been used to detect inhibitors of a HECT E3, Rsc (14). First, a GST-Rsc ubiquitylation reaction was conducted using biotin-labeled Ub. After Rsc autoubiquitylation, the reaction was transferred to streptavidin coated plates. Following several wash steps, anti-GST Eu3+ antibody was added and detected after washing. An inhibitor would cause a decrease in the signal emitted by Eu3+ as less biotin-Ub-Rsc-GST would be bound in the well. Notably, this approach also requires not only multiple wash steps, but a transfer step as well, which decreases the throughput and increases the hardware requirements. The tags on the proteins could also cause interference. In a similar format, 125I-labeled ubiquitin has also been used, in which case quantification was performed by scintillation spectrometry (17). In these two approaches, the components are free during the reaction and are immobilized only after the reaction has been given time to run to completion.
Immobilization of E3s in HTS
Detecting autoubiquitylation of an E3 ubiquitin ligase is an established method for HTS campaigns to find inhibitors, in the hope that inhibition of autoubiquitylation would translate to inhibition of substrate ubiquitylation. Typically, an E3 is immobilized to a plate or a bead by non-specific interactions or affinity tags; the E3 can then undergo autoubiquitylation when the E1, E2, Ub, and ATP are present. Unbound reaction components are removed by washing steps and a pre-labeled Ub or Ub-antibody is used to quantify the amount of polyubiquitylated E3.
This approach is open to many different options for immobilization and detection. One of the best characterized assays of this type was developed by Meso-Scale Discovery (20) and utilizes the ORIGEN-tag-labeled anti-Ub antibody to produce an electrochemilumiescence signal that is read on an ORIGEN M-8 Analyzer (Figure 1B). In this case, GST-fused E3 is immobilized on GSH-magnetic beads followed by E3 autoubiquitylation. The M8 analyzer allows for the separation of bound from unbound Ub samples, significantly reducing background, and the amount of Ub is determined by chemiluminescence after the addition of antibody.
This approach utilizes a GST tag, which could interfere with potential compound binding sites. Also, immobilizing the E3 to a bead prior to the reaction may inhibit the ability of the E3 to function, particularly HECT E3s, which undergo significant conformational changes during the ubiquitylation process (13). While E3 autoubiquitylation could be an indicator of substrate ubiquitylation, it is not guaranteed and one must follow-up to confirm that substrate ubiquitylation is indeed inhibited.
Immobilization of E3 Substrates in HTS
One way to be sure that a substrate is being ubiquitylated is to immobilize the substrate prior to a reaction and monitor for its ubiquitylation state. One group has carried out this approach in an ELISA based format using 1536 well plates (21) (Figure 1C). High-binding assay plates were incubated with substrate solution for 3h at room temperature. After the unbound substrate was removed, the E1 and E2 enzymes were added to the plate with ATP and FLAG-Ub to pre-charge the E2 with Ub and decrease the chance of finding E1 or E2 inhibitors. The compound and E3 ligase were then added and after the reaction was run for 1h the plates were washed to stop the reaction. Anti-FLAG HRP was incubated on the plates for 30min, removed, and HRP substrate was added. A compound that inhibited substrate ubiquitylation would result in a decrease in HRP reaction product.
Monitoring substrate ubiquitylation gives the greatest chance of finding a highly specific inhibitor with the lowest predicted toxicity profile. This is especially true for the SCF family of ubiquitin ligases, which have numerous adapters and substrates. The major disadvantage of this approach is immobilizing the substrate and not knowing how the substrate is bound to the plate. Native sites for compound interaction could be blocked based on the substrate's orientation after binding to the plate, making it important to determine the exact site on the substrate that is being targeted for ubiquitylation to confirm it is mimicking a native assay.
Immobilization of Ubiquitin Binding Domain
An alternative approach to all of the techniques described above is to exclusively capture poly-ubiquitylated proteins from a reaction (22). This format could be applied to substrate ubiquitylation, E3 autoubiquitylation, or potentially even an E3-independent E2 poly-ubiquitin chain. As shown in Figure 1D, all reaction components are free in solution and are only bound once a poly-ubiquitin chain is formed which is subsequently recognized by an immobilized ubiquitin binding domain. The proteins do not have to have any kind of protein-tag or direct labeling allowing for truly native protein interactions. This allows for an exploration of chemical space that is most similar to that encountered in a cell. The first compounds discovered using this approach are in early stage preclinical development.
Future of Ubiquitin Ligase Screening and Potential Therapies
Several ubiquitin ligases have been deemed to be good therapeutic targets (Table 1) and the first small-molecule inhibitors targeting an E3, in this case MDM2, are now in clinic trials (8). E3 HTS assays are difficult due to the number of functional proteins and enzymes that are required and frequently require deconvolution to eliminate the E1 and E2 inhibitors. However, the promise for increased specificity and reduced toxicity over proteasome inhibitors and the recent entry of compounds into clinical trials is drawing more attention to these potential therapeutic targets from the pharmaceutical industry. With the recent introduction of the ubiquitin binding HTS assay that utilizes all native proteins, as opposed to labeled or immobilized targets that have been used previously, we predict that the likelihood of obtaining E3 inhibitors with improved potency and selectivity is increased.
Abbreviations
- (HTS)
high-throughput screening
- (Ub)
Ubiquitin
- (UBL)
Ubiquitin-like
- (UPS)
Ubiquitin Proteasome System
- (SCF)
Skp1-Cul1-Roc-Fbox Protein complex
- (E1)
ubiquitin-activating enzyme
- (E2)
ubiquitin-conjugating enzyme
- (E3)
ubiquitin-protein ligase
- (FRET)
fluorescence resonance energy transfer
- (APC)
allophycocyanin
- (TAMRA)
tetramethylrhodamine
- (UBA)
ubiquitin binding domain
References
- 1.Chauhan D, Singh A, Brahmandam M, Podar K, Hideshima T, Richardson P, Munshi N, Palladino MA, Anderson KC. Combination of proteasome inhibitors bortezomib and NPI-0052 trigger in vivo synergistic cytotoxicity in multiple myeloma. Blood. 2008;111:1654–64. doi: 10.1182/blood-2007-08-105601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daviet L, Colland F. Targeting ubiquitin specific proteases for drug discovery. Biochimie. 2008;90:270–83. doi: 10.1016/j.biochi.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Kane RC, Farrell AT, Sridhara R, Pazdur R. United States Food and Drug Administration approval summary: bortezomib for the treatment of progressive multiple myeloma after one prior therapy. Clin Cancer Res. 2006;12:2955–60. doi: 10.1158/1078-0432.CCR-06-0170. [DOI] [PubMed] [Google Scholar]
- 4.Kane RC, Dagher R, Farrell A, Ko CW, Sridhara R, Justice R, Pazdur R. Bortezomib for the treatment of mantle cell lymphoma. Clin Cancer Res. 2007;13:5291–4. doi: 10.1158/1078-0432.CCR-07-0871. [DOI] [PubMed] [Google Scholar]
- 5.Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–33. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- 6.Bernassola F, Karin M, Ciechanover A, Melino G. The HECT family of E3 ubiquitin ligases: multiple players in cancer development. Cancer Cell. 2008;14:10–21. doi: 10.1016/j.ccr.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Sun Y. Targeting E3 ubiquitin ligases for cancer therapy. Cancer Biol Ther. 2003;2:623–9. [PubMed] [Google Scholar]
- 8.Patel S, Player MR. Small-molecule inhibitors of the p53-HDM2 interaction for the treatment of cancer. Expert Opin Investig Drugs. 2008;17:1865–82. doi: 10.1517/13543780802493366. [DOI] [PubMed] [Google Scholar]
- 9.Cardozo T, Abagyan R. Druggability of SCF ubiquitin ligase-protein interfaces. Methods Enzymol. 2005;399:634–53. doi: 10.1016/S0076-6879(05)99042-3. [DOI] [PubMed] [Google Scholar]
- 10.Reed SI. The ubiquitin-proteasome pathway in cell cycle control. Results Probl Cell Differ. 2006;42:147–81. doi: 10.1007/b136681. [DOI] [PubMed] [Google Scholar]
- 11.Nakayama KI, Nakayama K. Ubiquitin ligases: cell-cycle control and cancer. Nat Rev Cancer. 2006;6:369–81. doi: 10.1038/nrc1881. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Ruiz D, Gohlke H. Targeting protein-protein interactions with small molecules: challenges and perspectives for computational binding epitope detection and ligand finding. Curr Med Chem. 2006;13:2607–25. doi: 10.2174/092986706778201530. [DOI] [PubMed] [Google Scholar]
- 13.Zheng N. A closer look of the HECTic ubiquitin ligases. Structure. 2003;11:5–6. doi: 10.1016/s0969-2126(02)00940-1. [DOI] [PubMed] [Google Scholar]
- 14.Boisclair MD, McClure C, Josiah S, Glass S, Bottomley S, Kamerkar S, Hemmila I. Development of a ubiquitin transfer assay for high throughput screening by fluorescence resonance energy transfer. J Biomol Screen. 2000;5:319–28. doi: 10.1177/108705710000500503. [DOI] [PubMed] [Google Scholar]
- 15.Murray MF, Jurewicz AJ, Martin JD, Ho TF, Zhang H, Johanson KO, Kirkpatrick RB, Ma J, Lor LA, Thrall SH, Schwartz B. A high-throughput screen measuring ubiquitination of p53 by human mdm2. J Biomol Screen. 2007;12:1050–8. doi: 10.1177/1087057107308556. [DOI] [PubMed] [Google Scholar]
- 16.Gururaja TL, Pray TR, Lowe R, Dong G, Huang J, Daniel-Issakani S, Payan DG. A homogeneous FRET assay system for multiubiquitin chain assembly and disassembly. Methods Enzymol. 2005;399:663–82. doi: 10.1016/S0076-6879(05)99044-7. [DOI] [PubMed] [Google Scholar]
- 17.Yabuki N, Watanabe S, Kudoh T, Nihira S, Miyamato C. Application of homogeneous time-resolved fluorescence (HTRFTM) to monitor poly-ubiquitination of wild-type p53. Comb Chem High Throughput Screen. 1999;2:279–87. [PubMed] [Google Scholar]
- 18.Hong CA, Swearingen E, Mallari R, Gao X, Cao Z, North A, Young SW, Huang SG. Development of a high throughput time-resolved fluorescence resonance energy transfer assay for TRAF6 ubiquitin polymerization. Assay Drug Dev Technol. 2003;1:175–80. doi: 10.1089/154065803321537890. [DOI] [PubMed] [Google Scholar]
- 19.Zheng N, Schulman BA, Song L, Miller JJ, Jeffrey PD, Wang P, Chu C, Koepp DM, Elledge SJ, Pagano M, Conaway RC, Conaway JW, Harper JW, Pavletich NP. Structure of the Cul1-Rbx1-Skp1-F boxSkp2 SCF ubiquitin ligase complex. Nature. 2002;416:703–9. doi: 10.1038/416703a. [DOI] [PubMed] [Google Scholar]
- 20.Davydov IV, Woods D, Safiran YJ, Oberoi P, Fearnhead HO, Fang S, Jensen JP, Weissman AM, Kenten JH, Vousden KH. Assay for ubiquitin ligase activity: high-throughput screen for inhibitors of HDM2. J Biomol Screen. 2004;9:695–703. doi: 10.1177/1087057104267956. [DOI] [PubMed] [Google Scholar]
- 21.Cassaday J, Shah T, Murray J, O'Donnell GT, Kornienko O, Strulovici B, Ferrer M, Zuck P. Miniaturization and automation of an ubiquitin ligase cascade enzyme-linked immunosorbent assay in 1,536-well format. Assay Drug Dev Technol. 2007;5:493–500. doi: 10.1089/adt.2007.076. [DOI] [PubMed] [Google Scholar]
- 22.Marblestone JG, K. K, Eddins MJ, Leach CA, Butt S, Nicholson B, Mattern MR. Cold Spring Harbor Laboratory Meetings 2009, The Ubiquitin Family. Cold Spring Harbor; NY, USA: 2009. [Google Scholar]
- 23.Yang W, Dicker DT, Chen J, El-Deiry WS. CARPs enhance p53 turnover by degrading 14-3-3sigma and stabilizing MDM2. Cell Cycle. 2008;7:670–82. doi: 10.4161/cc.7.5.5701. [DOI] [PubMed] [Google Scholar]
- 24.Jiang BH, Liu LZ. PI3K/PTEN signaling in tumorigenesis and angiogenesis. Biochim Biophys Acta. 2008;1784:150–8. doi: 10.1016/j.bbapap.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Boulton SJ. Cellular functions of the BRCA tumour-suppressor proteins. Biochem Soc Trans. 2006;34:633–45. doi: 10.1042/BST0340633. [DOI] [PubMed] [Google Scholar]
- 26.Swaminathan G, Tsygankov AY. The Cbl family proteins: ring leaders in regulation of cell signaling. J Cell Physiol. 2006;209:21–43. doi: 10.1002/jcp.20694. [DOI] [PubMed] [Google Scholar]
- 27.Schulman BA, Chen ZJ. Protein ubiquitination: CHIPping away the symmetry. Mol Cell. 2005;20:653–5. doi: 10.1016/j.molcel.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 28.Hibi K, Sakata M, Sakuraba K, Shirahata A, Goto T, Mizukami H, Saito M, Ishibashi K, Kigawa G, Nemoto H, Sanada Y. Aberrant methylation of the HACE1 gene is frequently detected in advanced colorectal cancer. Anticancer Res. 2008;28:1581–4. [PubMed] [Google Scholar]
- 29.Bromberg KD, Kluger HM, Delaunay A, Abbas S, DiVito KA, Krajewski S, Ronai Z. Increased expression of the E3 ubiquitin ligase RNF5 is associated with decreased survival in breast cancer. Cancer Res. 2007;67:8172–9. doi: 10.1158/0008-5472.CAN-07-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheng Y, Laister RC, Lemak A, Wu B, Tai E, Duan S, Lukin J, Sunnerhagen M, Srisailam S, Karra M, Benchimol S, Arrowsmith CH. Molecular basis of Pirh2-mediated p53 ubiquitylation. Nat Struct Mol Biol. 2008;15:1334–42. doi: 10.1038/nsmb.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rathmell WK, Chen S. VHL inactivation in renal cell carcinoma: implications for diagnosis, prognosis and treatment. Expert Rev Anticancer Ther. 2008;8:63–73. doi: 10.1586/14737140.8.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura T, Lipton SA. Cell death: protein misfolding and neurodegenerative diseases. Apoptosis. 2009;14:455–68. doi: 10.1007/s10495-008-0301-y. [DOI] [PubMed] [Google Scholar]
- 33.Niikura T, Hashimoto Y, Tajima H, Ishizaka M, Yamagishi Y, Kawasumi M, Nawa M, Terashita K, Aiso S, Nishimoto I. A tripartite motif protein TRIM11 binds and destabilizes Humanin, a neuroprotective peptide against Alzheimer's disease-relevant insults. Eur J Neurosci. 2003;17:1150–8. doi: 10.1046/j.1460-9568.2003.02553.x. [DOI] [PubMed] [Google Scholar]
- 34.Gong B, Leznik E. The role of ubiquitin C-terminal hydrolase L1 in neurodegenerative disorders. Drug News Perspect. 2007;20:365–70. doi: 10.1358/dnp.2007.20.6.1138160. [DOI] [PubMed] [Google Scholar]
- 35.Whatley BR, Li L, Chin LS. The ubiquitin-proteasome system in spongiform degenerative disorders. Biochim Biophys Acta. 2008;1782:700–12. doi: 10.1016/j.bbadis.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garyali P, Siwach P, Singh PK, Puri R, Mittal S, Sengupta S, Parihar R, Ganesh S. The malin-laforin complex suppresses the cellular toxicity of misfolded proteins by promoting their degradation through the ubiquitinproteasome system. Hum Mol Genet. 2009;18:688–700. doi: 10.1093/hmg/ddn398. [DOI] [PubMed] [Google Scholar]
- 37.Sasaki A, Masuda Y, Iwai K, Ikeda K, Watanabe K. A RING finger protein Praja1 regulates Dlx5-dependent transcription through its ubiquitin ligase activity for the Dlx/Msx-interacting MAGE/Necdin family protein, Dlxin-1. J Biol Chem. 2002;277:22541–6. doi: 10.1074/jbc.M109728200. [DOI] [PubMed] [Google Scholar]
- 38.Murton AJ, Constantin D, Greenhaff PL. The involvement of the ubiquitin proteasome system in human skeletal muscle remodelling and atrophy. Biochim Biophys Acta. 2008;1782:730–43. doi: 10.1016/j.bbadis.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 39.Willis MS, Patterson C. Into the heart: the emerging role of the ubiquitin-proteasome system. J Mol Cell Cardiol. 2006;41:567–79. doi: 10.1016/j.yjmcc.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 40.Yamasaki S, Yagishita N, Tsuchimochi K, Nishioka K, Nakajima T. Rheumatoid arthritis as a hyper-endoplasmic-reticulum-associated degradation disease. Arthritis Res Ther. 2005;7:181–6. doi: 10.1186/ar1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hostager BS. Roles of TRAF6 in CD40 signaling. Immunol Res. 2007;39:105–14. doi: 10.1007/s12026-007-0082-3. [DOI] [PubMed] [Google Scholar]
- 42.Ungureanu D, Silvennoinen O. SLIM trims STATs: ubiquitin E3 ligases provide insights for specificity in the regulation of cytokine signaling. Sci STKE. 2005;2005:pe49. doi: 10.1126/stke.3042005pe49. [DOI] [PubMed] [Google Scholar]
- 43.Gomez-Martin D, Diaz-Zamudio M, Alcocer-Varela J. Ubiquitination system and autoimmunity: the bridge towards the modulation of the immune response. Autoimmun Rev. 2008;7:284–90. doi: 10.1016/j.autrev.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 44.Melino G, Gallagher E, Aqeilan RI, Knight R, Peschiaroli A, Rossi M, Scialpi F, Malatesta M, Zocchi L, Browne G, Ciechanover A, Bernassola F. Itch: a HECT-type E3 ligase regulating immunity, skin and cancer. Cell Death Differ. 2008;15:1103–12. doi: 10.1038/cdd.2008.60. [DOI] [PubMed] [Google Scholar]
- 45.Villasenor J, Benoist C, Mathis D. AIRE and APECED: molecular insights into an autoimmune disease. Immunol Rev. 2005;204:156–64. doi: 10.1111/j.0105-2896.2005.00246.x. [DOI] [PubMed] [Google Scholar]
- 46.Lin AE, Mak TW. The role of E3 ligases in autoimmunity and the regulation of autoreactive T cells. Curr Opin Immunol. 2007;19:665–73. doi: 10.1016/j.coi.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 47.Freed EO. Viral late domains. J Virol. 2002;76:4679–87. doi: 10.1128/JVI.76.10.4679-4687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meroni G, Diez-Roux G. TRIM/RBCC, a novel class of `single protein RING finger' E3 ubiquitin ligases. Bioessays. 2005;27:1147–57. doi: 10.1002/bies.20304. [DOI] [PubMed] [Google Scholar]