Abstract
Background
There is recent evidence that inflammatory signals can modulate lymphatic vessel permeability, but current understanding of the mechanisms regulating lymphatic endothelial barrier function is limited. The objectives of this study were to 1) investigate whether inflammatory mediators that increase microvascular permeability also cause barrier dysfunction of lymphatic endothelial cell monolayers, and 2) determine the roles of signaling pathways that affect intercellular junctions and cell contraction in lymphatic endothelial barrier function.
Methods and Results
Transendothelial electrical resistance (TER) of confluent adult human microlymphatic endothelial cells of dermal origin (HMLEC-d) served as an indicator of lymphatic endothelial barrier function. Human umbilical vein endothelial cells (HUVEC) were used to model blood-tissue barrier function. The inflammatory mediators histamine and thrombin each caused a decrease in TER of HMLEC-d and HUVEC monolayers, with notable differences between the two cell types. Treatment with 8-Br-cAMP enhanced HMLEC-d barrier function, which limited histamine and thrombin-induced decreases in TER. Blockade of myosin light chain kinase (MLCK) with ML-7 did not affect histamine or thrombin-induced decreases in TER. Treatment with the Rho kinase (ROCK) inhibitor Y-27632 caused a decrease in HMLEC-d barrier function.
Conclusions
These data show that inflammatory mediators can cause lymphatic endothelial barrier dysfunction, although the responses are not identical to those seen with blood endothelial cells. ROCK and cAMP both promote lymphatic endothelial barrier function, however ROCK appears to also serve as a mediator of histamine and thrombin-induced barrier dysfunction.
Introduction
The lymphatic system is a multifunctional transport network that is important for maintaining normal immune function and fluid homeostasis. Severe impairment or absence of lymphatic function is the cause of lymphedema, a debilitating condition with no cure and limited treatment options.1 Lymphedema can be a hereditary disorder, classified as primary lymphedema, or may occur following damage to the lymphatic system as a result of infection, injury, or surgical procedures in which lymph nodes are removed. These are collectively known as secondary lymphedema.1 Currently there is a very limited understanding of the basic mechanisms underlying lymphatic function, as well as the complex interactions leading to development of lymphedema.
Initial lymphatics (also known as terminal lymphatics or lymphatic capillaries) are responsible for lymph formation, which is driven by interstitial fluid pressure.2 Once fluid enters the primary lymphatics, microscopic primary one-way valves between lymphatic endothelial cells prevent retrograde fluid movement.3,4 Lymph is then pumped to the lymph nodes by the combination of intrinsic pump activity of the downstream collecting lymphatics as well as extrinsic forces imposed by the tissues.5 After lymph has been filtered in the lymph nodes, it travels through postnodal collecting lymphatics that eventually coalesce into the thoracic duct and right lymphatic duct, which deliver the lymph to the venous circulation.5
The responsiveness of lymphatics to inflammatory stimuli, and how this may affect interstitial fluid transport as well as trafficking of antigens and immune cells to the lymph nodes is an emerging area of study. It has been known for some time that lymph is filtered within lymph nodes, where lymphocytes and other immune cells are recruited out of the lymph, along with a significant amount of fluid that is removed and returned to the central circulation.6,7 However, there is also newer evidence that fluid and macromolecular solutes can be filtered from the lumen of collecting lymphatics.8 In addition, leakage of tracers in the lymphatic lumen may occur in response to inflammatory conditions.3 Previous studies also show that lymphatic endothelial cells grown in culture can alter their barrier function in response to various stimuli.9,10
The mechanisms underlying inflammatory mediator-induced changes in lymphatic vessel permeability have not been extensively studied. However, given that lymphatic and venular endothelial cells have a common developmental origin,11 there are likely similarities with the mechanisms underlying permeability regulation in the capillary and venular endothelium. Here, fluids and solutes can cross the endothelial barrier by either a transcellular route involving channels, transporters, vesicle trafficking, and vesicular channels, or by a paracellular route that is regulated by factors that determine the characteristics of the space between cells.12 The paracellular route is thought to be the primary path for solute and fluid flux during inflammation-induced microvascular hyperpermeability.12 The amount of paracellular space available for macromolecular flux is determined by the combination of 1) the strength of junctions, due in large part to the conformation of junctional proteins like VE-cadherin, that are responsible for adhesions between endothelial cells, and 2) the stress on junctions imposed by the centripetal tension generated by the actin cytoskeleton in individual endothelial cells.13
Various intracellular signaling pathways control intercellular junctions between, and centripetal force within, endothelial cells. Several reports have identified cAMP as a mediator that promotes enhanced endothelial barrier function, through its action to promote enhanced VE-cadherin binding, leading to stable junctions between endothelial cells.14–16 The phosphorylation of myosin light chains (MLC), which promotes actin-myosin-mediated contraction, is responsible for the generation of centripetal tension within endothelial cells. MLC phosphorylation is mediated by MLC kinase (MLCK), which is known to mediate the microvascular hyperpermeability responses to neutrophils and soluble inflammatory mediators such as histamine or thrombin.13,17,18 In addition, MLC phosphorylation is regulated by the MLC phosphatase (MLCP). The RhoA-Rho kinase (ROCK) pathway can inhibit MLCP, and serves as an additional pathway to elicit elevated endothelial centripetal tension and microvascular hyperpermeability.19
The current study investigated how stimulation of lymphatic endothelial cells with inflammatory mediators affects their barrier function. Two well-described inflammatory mediators that increase microvascular permeability, thrombin and histamine, were used. Thrombin, acting on protease-activated receptor (PAR)-1, causes a profound increase in lung microvascular permeability when administered in vivo as well as a very robust hyperpermeability response in cultured blood endothelial cell models.20,21 Histamine, which acts on histamine receptors (H1–H4) also increases endothelial permeability13,22,23 and is known to modulate pump activity of collecting lymphatics.24,25 PAR-1 and H1 (the histamine receptor thought to be important for increasing microvascular permeability) are both Gq-coupled receptors, and their activation leads to elevated PLC activity, formation of IP3 and DAG, and downstream mobilization of Ca2+ from internal stores and PKCα activation, respectively.26–28 The elevated cytosolic Ca2+ increases levels of Ca2+-bound calmodulin which activates MLCK. In addition, PKCα can phosphorylate the Rho-GDP guanine nucleotide dissociation inhibitor (RhoGDI) causing enhanced RhoA/ROCK signaling.28 These and additional signaling pathways can also lead to the phosphorylation of various junctional proteins, facilitating opening of the paracellular space.27 In the current study, we tested whether MLCK or ROCK may have similar roles in lymphatic endothelial cells. We also investigated the potential role of cAMP to enhance barrier function of lymphatic endothelium. These studies were performed using adult human dermal microlymphatic endothelial cells (HMLEC-d).
Materials and Methods
Materials
Cryopreserved adult human dermal microlymphatic endothelial cells (HMVEC-dLYAd; abbreviated herein as HMLEC-d), human umbilical vein endothelial cells (HUVEC), Clonetics microvascular endothelial growth medium (EGM-2MV), and endothelial basal medium (EBM) were purchased from Lonza (Chicago, IL). Thrombin (T4625) and histamine (H7250) were purchased from Sigma (St. Louis, MO). 8-Br-cAMP, ML-7, and Y-27632 were purchased from EMD-Calbiochem (Gibbstown, NJ).
Cell culture and determination of barrier function
HMLEC-d and HUVEC were routinely seeded on gelatin-coated cultureware and maintained in EGM-2MV in a 37°C, 5% CO2 humidified incubator. An Electrical Cell-Substrate Impedance Sensor (ECIS) Model 1600R (Applied Biophysics, Troy, NY) was used for barrier function measurements as previously described.9,10,19 Briefly, the cells were seeded at a density of 1.5 × 105 cells in 400 μl medium per well onto gelatin-coated arrays containing a single small gold electrode (250 μm diameter) and a large counter electrode in each well (8W1E, Applied Biophysics). The cells were allowed to attach overnight and formed a confluent monolayer. A 1 μA ac signal at 4 kHz was applied from an approximate constant current source, and the ECIS instrument monitored the voltage across the electrodes and its phase relative to the applied current, providing a report of total impedance. Treating the cell-electrode system as a series RC circuit, the ECIS 1600R converted the impedance data to resistance and capacitance of the cell monolayer, which represent barrier function and membrane capacitance, respectively. Transendothelial resistance (TER) is presented as an index of lymphatic endothelial barrier function.
Experimental protocols
After the overnight incubation, any HMLEC-d or HUVEC monolayer displaying a baseline TER of less than 5000 Ω was excluded from study. The medium was changed to EBM, and the cells were incubated at least 2 h prior to the experimental treatments. Thrombin was applied at 1 U/ml, a dose determined as optimal for eliciting HUVEC barrier function based on previous publications29 and preliminary experiments (data not shown). Histamine was applied at 10 μM, which typically causes a brief barrier dysfunction in HUVEC.30 To test the ability of cAMP-dependent pathways to block thrombin- or histamine-induced barrier function, cells were pretreated with 100 μM 8-Br-cAMP31,32 30 min prior to thrombin or histamine. The roles of MLCK and ROCK were tested by pretreating cells with ML-7 (10 μM)33 or Y-27632 (5 μM)19,34, respectively, prior to thrombin or histamine treatment.
Data analysis
For each experiment, a tracing of the mean TER vs. time, obtained from multiple electrodes is shown. The maximum changes in TER in response to various treatments were averaged and are presented as means ± S.E. The significance between groups was determined by t-tests, or one-way ANOVA followed by Tukey's multiple comparisons test where appropriate. Significance was accepted at P < 0.05.
Results
Characterization of histamine- and thrombin-induced lymphatic endothelial barrier dysfunction
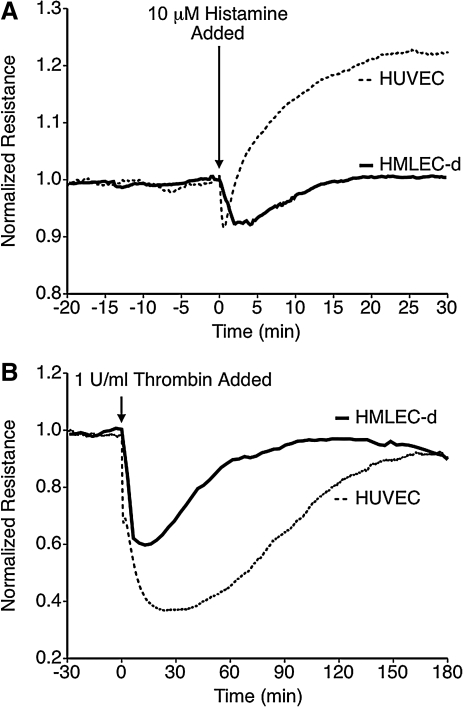
Histamine and thrombin have both been used to cause microvascular hyperpermeability in previous studies.13,20,35 The ability of these inflammatory mediators to cause lymphatic endothelial barrier dysfunction was tested using cultured HMLEC-d monolayers. In addition, a set of experiments with HUVEC was performed to compare the responses of lymphatic and blood endothelial cells (Fig. 1). Histamine treatment caused an equivalent decrease in TER of HMLEC-d and HUVEC (8.7 ± 2.4 % vs. 10.0 ± 1.4 % decrease, respectively), but the time courses of histamine-induced changes in TER were different in each cell type. For HMLEC-d, the initial drop was slightly slower than in HUVEC, and the recovery of barrier function was gradual, reaching the baseline TER level approximately 15 min after the addition of histamine. With HUVEC, the drop in TER was equivalent in magnitude to HMLEC-d but shorter in duration, usually lasting no longer than 2 min. This was then followed by an increase in TER above baseline (Fig. 1A), as has been previously reported.36
FIG. 1.
Comparison of histamine and thrombin-induced barrier dysfunction in lymphatic and blood endothelial cell monolayers. (A) Histamine caused a decrease in TER in both HUVEC and HMLEC-d, but with different time courses. The time course of decreased TER in HUVEC typically lasted less than 2 min, followed by an elevation of TER above baseline. In HMLEC-d, histamine decreased TER to a similar magnitude, but the TER remained lower than baseline for approximately 15 min, with no subsequent elevation in TER above baseline. (B) Thrombin causes a rapid decrease in TER in both HMLEC-d and HUVEC; however, the magnitude of the change is less in HMLEC-d. Each tracing represents the average of N = 4 electrodes for each group.
Thrombin also decreased TER in both HMLEC-d and HUVEC (Fig. 1B). The response in HUVEC was similar as shown in previous reports.13,20,37 For HMLEC-d, the magnitude of thrombin-induced barrier dysfunction was less than with HUVEC (a 35.6 ± 27 % decrease for HMLEC-d versus 63.6 ± 1.5 % for HUVEC, P < 0.01). These data indicate that these inflammatory stimuli produce barrier dysfunction in both lymphatic and blood endothelial cells, however the magnitudes and time courses of the responses varies between the two cell types.
cAMP promotes enhanced lymphatic endothelial barrier function
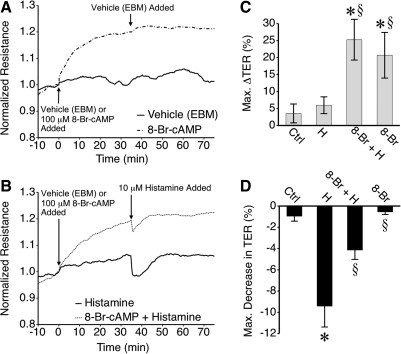
Application of the stable cAMP analog 8-Br-cAMP to HMLEC-d caused an increase in TER (Fig. 2). The gradual increase reached a plateau about 25–30 min after the addition of 8-Br-cAMP (Fig. 2A). TER changed very little upon treatment with vehicle. Upon stimulation with histamine, TER decreased to a lesser degree in 8-Br-cAMP-treated HMLEC-d than in those that received vehicle only (Fig. 2B). The maximum increases in TER elicited by 8-Br-cAMP were significant compared to cells treated with vehicle only (Fig. 2C), as was the inhibition of histamine-induced decreases in TER by 8-Br-cAMP (Fig. 2D).
FIG. 2.
Treatment with 8-Br-cAMP enhances lymphatic endothelial barrier function and attenuates histamine-induced barrier dysfunction. (A) Application of 8-Br-cAMP, but not vehicle (EBM) caused mean TER to rise and reach a new steady state. Subsequent application of the vehicle for histamine (EBM) did not affect TER. (B) In HMLEC-d pretreated with 8-Br-cAMP, the decrease in mean TER caused by histamine appeared attenuated. (C) The mean maximal increase in TER caused by 8-Br-cAMP within 30 min of treatment was significantly higher than vehicle. (D) 8-Br-cAMP also significantly attenuated the decrease in TER caused by histamine. *P < 0.05 vs. control (Ctrl). §P < 0.05 vs. histamine (H). Other abbreviations: 8-Br-cAMP (8-Br), 8-Br-cAMP + Histamine (8-Br + H). N = 4 for all groups.
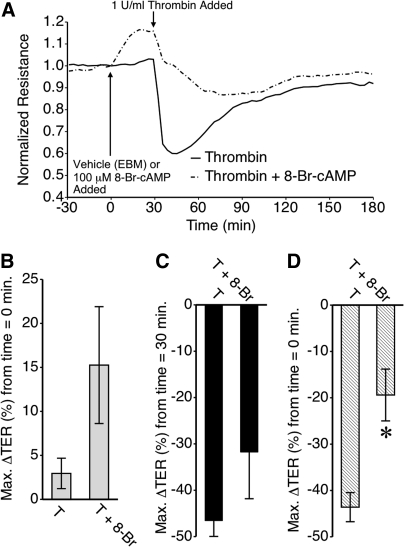
8-Br-cAMP also limited thrombin-induced barrier dysfunction, although this was due mainly to an elevated TER just prior to addition of thrombin (Fig. 3). HMLEC-d were again pretreated with 8-Br-cAMP or vehicle, and then thrombin was applied (Fig. 3A). 8-Br-cAMP increased TER (Fig 3B), although the change was not significantly different from vehicle-treated cells in this experiment (P = 0.09). When compared to the time point just prior to thrombin addition, the mean maximum decrease in TER elicited by thrombin was not significantly different between 8-Br-cAMP versus vehicle-treated cells (Fig. 3C). However, when compared to the time point just prior to 8-Br-cAMP, the overall decrease in TER following the application of thrombin was significantly attenuated in HMLEC-d pretreated with 8-Br-cAMP, compared to vehicle-treated cells (Fig. 3D). These data indicate that cAMP promotes barrier function of lymphatic endothelial cells.
FIG. 3.
Effect of 8-Br-cAMP on thrombin-induced lymphatic endothelial barrier dysfunction. (A) Time-course of thrombin-induced changes in mean HMLEC-d TER in cells pretreated with 8-Br-cAMP or vehicle (EBM). (B) 8-Br-cAMP caused an increase in TER but in this experiment the maximal change elicited by 8-Br-cAMP was not significantly different from vehicle (P = 0.09). (C) Pretreatment of HMLEC-d with 8-Br-cAMP (8-Br) did not significantly affect that maximum decrease in TER elicited by thrombin (T) when compared to the time point just prior to the thrombin addition. (D) The overall change in TER compared to time = 0 min (just before addition of 8-Br-cAMP) was significantly attenuated in cells treated with both 8-Br-cAMP and thrombin compared to thrombin alone. N = 8 for both groups.
MLCK and thrombin-induced lymphatic endothelial barrier dysfunction
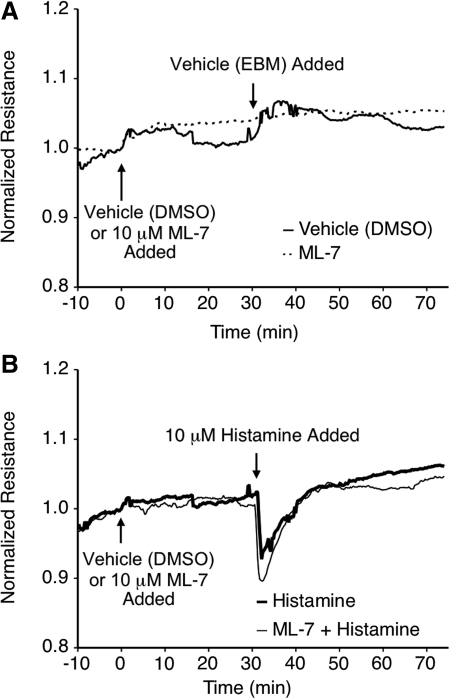
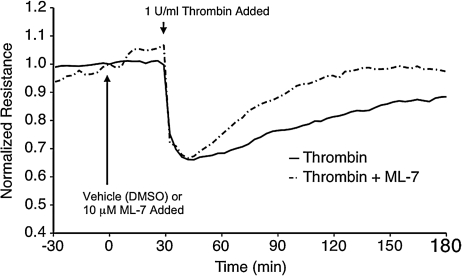
Several reports have indicated that endothelial cell contraction promotes elevated permeability.13,19,30,34,38 MLCK phosphorylates the regulatory sites on MLC allowing actin–myosin-mediated cell contraction and the development of centripetal force in endothelial cells.13 We tested the role of MLCK in histamine-induced lymphatic endothelial barrier dysfunction using the pharmacological inhibitor ML-7 (Fig. 4). Application of 10 μM ML-7 or its vehicle control (equivalent volume of EBM containing DMSO, resulting in 0.01% DMSO final concentration) to HMLEC-d monolayers did not cause any significant change in TER (Fig. 4A). In addition, ML-7 pretreatment did not significantly alter the time-course of lymphatic endothelial barrier dysfunction elicited by histamine (Fig. 4B). ML-7 also did not affect the magnitude of thrombin-induced barrier dysfunction in HMLEC-d (Fig. 5). However, the recovery of barrier function in cells pretreated with ML-7 progressed more quickly than cells pretreated with vehicle (DMSO) only. ML-7-treated cells reached baseline TER approximately 70 min after thrombin treatment, while cells not receiving ML-7 took much longer (Fig. 5).
FIG. 4.
Blockade of MLCK with ML-7 does not significantly affect the time course of histamine-induced lymphatic endothelial barrier dysfunction. (A) Application of ML-7 or vehicle (DMSO) did not significantly alter mean TER. Subsequent application of the vehicle used for histamine (EBM) also did not elicit any significant changes. (B) Pretreatment of the HMLEC-d with ML-7 did not alter the time course of histamine-induced changes in mean TER. N = 4 for each group.
FIG. 5.
Inhibition of MLCK with ML-7 does not significantly affect thrombin-induced lymphatic endothelial barrier dysfunction. HMLEC-d were pretreated with ML-7 or vehicle (equivalent volume, final concentration of 0.01% DMSO) prior to thrombin treatment. The tracings represent the mean TER of N = 4 electrodes for each group.
ROCK and endothelial barrier integrity
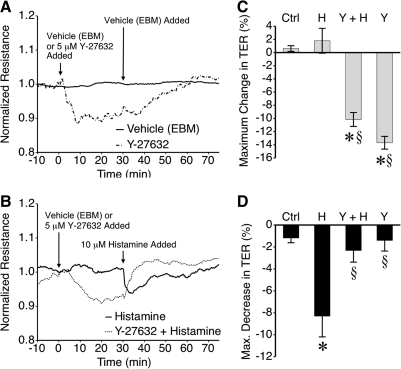
ROCK has also been shown to promote endothelial cell contraction by phosphorylating the MLCP regulatory subunit MYPT-1 and inhibiting MLCP activity.39 The role of ROCK in barrier function was tested using the selective ROCK inhibitor Y-27632 (Fig. 6). Interestingly, treatment of HMLEC-d with Y-27632, but not vehicle (an equivalent volume of EBM), caused a significant decrease in TER lasting approximately 40 min. before gradually returning to baseline (Figs. 6A and 6C). When histamine was applied after Y-27632, the histamine-induced decrease in TER was abolished and instead there was an upward increase in TER (Figs. 6B and 6D).
FIG. 6.
Blockade of ROCK with Y-27632 caused lymphatic endothelial barrier dysfunction and also limited histamine-induced barrier dysfunction. (A) Application of the ROCK inhibitor Y-27632 decreased mean HMLEC-d TER, compared to vehicle alone (EBM). Also shown in (A) is the time course of TER changes after application of the vehicle control for histamine. (B) Application of histamine reduced mean TER in the vehicle group, but caused no further decrease in TER in the Y-27632 group. (C) The maximal decrease in TER caused by Y-27632 was significant compared to vehicle (evaluated within 30 min of application). (D) Histamine significantly decreased TER, but this was blocked by Y-27632 pretreatment. *P < 0.05 vs. control (Ctrl). §P < 0.05 vs. histamine (H). Other abbreviations: Y-27632 (Y), Y-27632 + Histamine (Y + H). N = 4 for all groups.
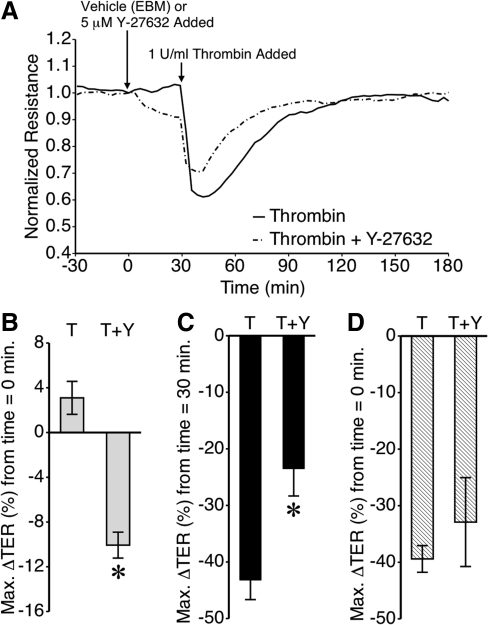
Pretreatment of HMLEC-d with Y-27632 also attenuated thrombin-induced barrier dysfunction (Fig. 7). After a Y-27632-induced decrease in TER, the thrombin-induced decrease in TER was attenuated, resulting in an overall drop in TER that was somewhat less pronounced than seen in cells treated with thrombin only (Fig. 7A). Again, the decrease in TER elicited by Y-27632 was significant (Fig. 7B). The thrombin-induced decrease in TER, relative to the time point just prior to the addition of thrombin, was significantly less in Y-27632-treated HMLEC-d than in vehicle only-treated cells (Fig. 7C). However, when compared to the time point just before the addition of Y-27632 or vehicle, the overall change was not significant between the two groups. These data with Y-27632 show that ROCK is an important mediator of lymphatic endothelial cell barrier integrity.
FIG. 7.
Effect of ROCK blockade with Y-27632 on thrombin-induced lymphatic endothelial barrier function. (A) Time course of thrombin-induced changes in mean TER, with or without Y-27632 pretreatment. (B) Y-27632 causes a significant decrease in HMLEC-d TER within 30 min of treatment. (C) The mean maximum thrombin-induced decrease in TER, compared to the TER just prior to adding thrombin, was significantly lower in the cells pretreated Y-27632. (D) The overall decrease in TER after both Y-27632 and thrombin was not significantly different from thrombin alone. *P < 0.05 vs. thrombin alone. N = 6 for the thrombin group and N = 4 for the thrombin + Y-27632 group.
Discussion
We have demonstrated that the inflammatory mediators histamine and thrombin cause a decrease in barrier function of cultured lymphatic endothelial cells, a response previously only observed with blood endothelial cells. Although there were some similarities between HMLEC-d and HUVEC, there were notable differences. For example, HMLEC-d displayed a longer time course of histamine-induced barrier dysfunction in than HUVEC. HMLEC-d also had a less pronounced thrombin-induced decrease in TER compared to HUVEC monolayers. These data suggest that the signaling and regulatory mechanisms that determine barrier function are not identical in endothelial cells of lymphatic and blood vessel origin. We also provide evidence that ROCK and cAMP are important for normal maintenance of barrier function in lymphatic endothelial cell monolayers. In addition, we show that MLCK activity is not necessary to achieve the maximal decreases in lymphatic endothelial barrier function elicited by histamine or thrombin.
Inflammatory mediators such as histamine have previously been shown to directly modulate contractile activity of collecting lymphatics.24,40,41 However, to our knowledge this is the first study of how histamine and thrombin affect lymphatic endothelial barrier function. One study has shown that experimental induction of inflammation with the combination of fMLP and platelet activating factor causes leakage of large solutes (Quantum Dots) from initial lymphatics in skeletal muscle.3 Another recent study has characterized the permeability of rat mesenteric collecting lymphatics to albumin,8 although changes in collecting lymphatic permeability to inflammatory mediators have not yet been investigated. Our findings show that histamine and thrombin can compromise barrier function of the lymphatic endothelium. Considering that leakage of solutes from lymph could limit the clearance of inflammatory mediators, this type of leakage may represent a feed forward mechanism to maximize the strength of an inflammatory response. Feed forward mechanisms typically have the potential to turn into a vicious cycle, and conceivably the ultimate result in this case could be lymphedema. It is worth noting that anti-inflammatory therapy has recently been shown to ameliorate experimentally-induced mouse tail lymphedema.42
Recognizing the well-defined role of cAMP in enhancing VE-cadherin mediated endothelial adhesions and promoting microvascular barrier function,14,15 the ability of exogenous 8-Br-cAMP to inhibit histamine and thrombin-induced barrier dysfunction in lymphatic endothelial cells was tested. The results, showing that 8-Br-cAMP enhances barrier function and because of this, limits histamine and thrombin-induced barrier dysfunction, are similar to observations in microvessels and blood endothelial cell models.14,15 The data are also concordant with another study in which cultured lymphatic endothelial cell tubes generated in vitro had significantly decreased permeability following application of dibutyrl-cAMP.16 In that study, the tubes grown without dibutyryl-cAMP had discontinuous VE-cadherin labeling at junctions, while 80 or 400 μM dibutyryl-cAMP caused continuous labeling of VE-cadherin at cell–cell junctions.16 Thus, cAMP appears to be a universal barrier-enhancing signal in both lymphatic and blood endothelial cells.
Considering the similarities and differences in the responses of HUVEC and HMLEC-d to thrombin and histamine, it is conceivable that the basic skeleton signaling pathways of the overall mechanism may be shared between the cell types. However, variation in the expression or activity of individual signaling molecules could account for the different magnitudes and time courses of the responses. We evaluated the roles MLCK or ROCK, two key signaling molecules that promote MLC phosphorylation in this paradigm, in HMLEC-d. In previous studies with blood endothelial cells, MLCK and ROCK both increased endothelial cell centripetal tension, leading to barrier dysfunction.13,19,30,38 In the current study, MLCK had no effect on the histamine-induced decrease in TER in HMLEC-d. This differs from previous observations with HUVEC, in which ML-7 pretreatment inhibited histamine barrier dysfunction.43 On the other hand, the finding that ML-7 did not alter the initial thrombin-induced decrease in HMLEC-d TER, but did hasten the recovery of TER, is similar to the response reported for HUVEC.13 These data suggest that while thrombin-induced MLCK activation may have equal importance between HUVEC and HMLEC-d, MLCK does not have a prominent role in histamine-induced hyperpermeability of lymphatic endothelium.
In our studies of ROCK signaling, we observed the unanticipated result that inhibition of ROCK decreased TER of lymphatic endothelial cells. This differs from previous observations in HUVEC and bovine coronary venular endothelial cells, in which ROCK inhibitors had no effect on baseline permeability.19,29,34,44 One study reported enhanced barrier function following ROCK inhibition in bovine aortic endothelial cells.45 However, in agreement with previous studies using blood endothelial cells,19,29,34,44 inhibition of ROCK blocked further decreases in TER after histamine treatment and attenuated the thrombin-induced reduction in TER. Interestingly, the HMLEC-d response to histamine after Y-27632 pretreatment was an abrupt increase in TER and a return to baseline. Rho small GTPases strongly influence each other's activity, and ROCK is one downstream mediator of RhoA. It is possible that other Rho small GTPases such as Rac1, which our laboratory recently reported can increase lymphatic endothelial TER,9 may be responsible for the observed response to histamine in the absence of ROCK activity.
In summary, the data demonstrate that inflammatory mediators known to produce microvascular hyperpermeability can also disrupt barrier function of lymphatic endothelial cells. It is possible that inflammation-induced increases in permeability of lymphatic vessels may represent a feed-forward mechanism to maximize the strength of a local inflammatory response. Additional studies, including those investigating lymphatic permeability in vivo during inflammation, will be of importance to developing a detailed understanding of the complex mechanisms underlying lymphatic function under both normal and disease conditions.
Footnotes
Supported by NIH Grant P20 RR018766 and a grant from the American Heart Association.
Acknowledgments
This work was supported by grants from the National Institutes of Health (P20 RR018766) and the American Heart Association (0835386N).
Author Disclosure Statement
The author has no conflicts of interest or financial ties to report.
References
- 1.Rockson SG. The unique biology of lymphatic edema. Lymphat Res Biol. 2009;7:97–100. doi: 10.1089/lrb.2009.7202. [DOI] [PubMed] [Google Scholar]
- 2.Granger DN. Korthuis RJ. Kvietys PR. Tso P. Intestinal microvascular exchange during lipid absorption. Am J Physiol. 1988;255:G690–695. doi: 10.1152/ajpgi.1988.255.5.G690. [DOI] [PubMed] [Google Scholar]
- 3.Lynch PM. Delano FA. Schmid–Schonbein GW. The primary valves in the initial lymphatics during inflammation. Lymphat Res Biol. 2007;5:3–10. doi: 10.1089/lrb.2007.5102. [DOI] [PubMed] [Google Scholar]
- 4.Schmid–Schonbein GW. The second valve system in lymphatics. Lymphat Res Biol. 2003;1:25–29. doi: 10.1089/15396850360495664. ; discussion 29–31. [DOI] [PubMed] [Google Scholar]
- 5.Zawieja DC. Contractile physiology of lymphatics. Lymphat Res Biol. 2009;7:87–96. doi: 10.1089/lrb.2009.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adair TH. Moffatt DS. Paulsen AW. Guyton AC. Quantitation of changes in lymph protein concentration during lymph node transit. Am J Physiol. 1982;243:H351–359. doi: 10.1152/ajpheart.1982.243.3.H351. [DOI] [PubMed] [Google Scholar]
- 7.Brace RA. Taylor AE. Guyton AC. Time course of lymph protein concentration in the dog. Microvasc Res. 1977;14:243–249. doi: 10.1016/0026-2862(77)90023-1. [DOI] [PubMed] [Google Scholar]
- 8.Scallan JP. Huxley VH. In vivo determination of collecting lymphatic vessel permeability to albumin: A role for lymphatics in exchange. J Physiol. 2010;588:243–254. doi: 10.1113/jphysiol.2009.179622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breslin JW. Kurtz KM. Lymphatic endothelial cells adapt their barrier function in response to changes in shear stress. Lymphat Res Biol. 2009;7:229–237. doi: 10.1089/lrb.2009.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breslin JW. Yuan SY. Wu MH. VEGF-C alters barrier function of cultured lymphatic endothelial cells through a VEGFR-3-dependent mechanism. Lymphat Res Biol. 2007;5:105–113. doi: 10.1089/lrb.2007.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srinivasan RS. Dillard ME. Lagutin OV. Lin FJ. Tsai S. Tsai MJ. Samokhvalov IM. Oliver G. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. 2007;21:2422–2432. doi: 10.1101/gad.1588407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duran WN. Sanchez FA. Breslin JW. Microcirculatory Exchange Function. In: RF Tuma., editor; WN Duran., editor; K Ley., editor. Handbook of Physiology: Microcirculation. 2nd. San Diego, CA: Academic Press–Elsevier; 2008. pp. 81–124. [Google Scholar]
- 13.Moy AB. Van Engelenhoven J. Bodmer J. Kamath J. Keese C. Giaever I. Shasby S. Shasby DM. Histamine and thrombin modulate endothelial focal adhesion through centripetal and centrifugal forces. J Clin Invest. 1996;97:1020–1027. doi: 10.1172/JCI118493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waschke J. Drenckhahn D. Adamson RH. Barth H. Curry FE. cAMP protects endothelial barrier functions by preventing Rac-1 inhibition. Am J Physiol Heart Circ Physiol. 2004;287:H2427–2433. doi: 10.1152/ajpheart.00556.2004. [DOI] [PubMed] [Google Scholar]
- 15.He P. Zeng M. Curry FE. Dominant role of cAMP in regulation of microvessel permeability. Am J Physiol Heart Circ Physiol. 2000;278:H1124–1133. doi: 10.1152/ajpheart.2000.278.4.H1124. [DOI] [PubMed] [Google Scholar]
- 16.Price GM. Chrobak KM. Tien J. Effect of cyclic AMP on barrier function of human lymphatic microvascular tubes. Microvasc Res. 2008;76:46–51. doi: 10.1016/j.mvr.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia JG. Davis HW. Patterson CE. Regulation of endothelial cell gap formation and barrier dysfunction: Role of myosin light chain phosphorylation. J Cell Physiol. 1995;163:510–522. doi: 10.1002/jcp.1041630311. [DOI] [PubMed] [Google Scholar]
- 18.Yuan SY. Wu MH. Ustinova EE. Guo M. Tinsley JH. De Lanerolle P. Xu W. Myosin light chain phosphorylation in neutrophil-stimulated coronary microvascular leakage. Circ Res. 2002;90:1214–1221. doi: 10.1161/01.res.0000020402.73609.f1. [DOI] [PubMed] [Google Scholar]
- 19.Breslin JW. Sun H. Xu W. Rodarte C. Moy AB. Wu MH. Yuan SY. Involvement of ROCK-mediated endothelial tension development in neutrophil-stimulated microvascular leakage. Am J Physiol Heart Circ Physiol. 2006;290:H741–750. doi: 10.1152/ajpheart.00238.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta D. Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 21.Dudek SM. Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91:1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- 22.Huang Q. Yuan Y. Interaction of PKC and NOS in signal transduction of microvascular hyperpermeability. Am J Physiol. 1997;273:H2442–2451. doi: 10.1152/ajpheart.1997.273.5.H2442. [DOI] [PubMed] [Google Scholar]
- 23.Guo M. Breslin JW. Wu MH. Gottardi CJ. Yuan SY. VE-cadherin and beta-catenin binding dynamics during histamine-induced endothelial hyperpermeability. Am J Physiol Cell Physiol. 2008;294:C977–984. doi: 10.1152/ajpcell.90607.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox JL. von der Weid PY. Effects of histamine on the contractile and electrical activity in isolated lymphatic vessels of the guinea-pig mesentery. Br J Pharmacol. 2002;136:1210–1218. doi: 10.1038/sj.bjp.0704820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plaku KJ. von der Weid PY. Mast cell degranulation alters lymphatic contractile activity through action of histamine. Microcirculation. 2006;13:219–227. doi: 10.1080/10739680600556902. [DOI] [PubMed] [Google Scholar]
- 26.Malik AB. Lo SK. Thrombin-endothelial interactions: Role in lung vascular permeability. Mol Aspects Med. 1985;8:515–554. doi: 10.1016/0098-2997(85)90013-5. [DOI] [PubMed] [Google Scholar]
- 27.Kumar P. Shen Q. Pivetti CD. Lee ES. Wu MH. Yuan SY. Molecular mechanisms of endothelial hyperpermeability: Implications in inflammation. Expert Rev Mol Med. 2009;11:e19. doi: 10.1017/S1462399409001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tiruppathi C. Ahmmed GU. Vogel SM. Malik AB. Ca2+ signaling, TRP channels, and endothelial permeability. Microcirculation. 2006;13 doi: 10.1080/10739680600930347. 693-7-08. [DOI] [PubMed] [Google Scholar]
- 29.van Nieuw Amerongen GP. van Delft S. Vermeer MA. Collard JG. van Hinsbergh VW. Activation of RhoA by thrombin in endothelial hyperpermeability: Role of Rho kinase and protein tyrosine kinases. Circ Res. 2000;87:335–340. doi: 10.1161/01.res.87.4.335. [DOI] [PubMed] [Google Scholar]
- 30.Moy AB. Blackwell K. Kamath A. Differential effects of histamine and thrombin on endothelial barrier function through actin-myosin tension. Am J Physiol Heart Circ Physiol. 2002;282:H21–29. doi: 10.1152/ajpheart.2002.282.1.H21. [DOI] [PubMed] [Google Scholar]
- 31.Birukova AA. Burdette D. Moldobaeva N. Xing J. Fu P. Birukov KG. Rac GTPase is a hub for protein kinase A and Epac signaling in endothelial barrier protection by cAMP. Microvasc Res. 2009;79:128–138. doi: 10.1016/j.mvr.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tiruppathi C. Malik AB. Del Vecchio PJ. Keese CR. Giaever I. Electrical method for detection of endothelial cell shape change in real time: assessment of endothelial barrier function. Proc Natl Acad Sci USA. 1992;89:7919–7923. doi: 10.1073/pnas.89.17.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan Y. Huang Q. Wu HM. Myosin light chain phosphorylation: Modulation of basal and agonist-stimulated venular permeability. Am J Physiol. 1997;272:H1437–1443. doi: 10.1152/ajpheart.1997.272.3.H1437. [DOI] [PubMed] [Google Scholar]
- 34.Breslin JW. Yuan SY. Involvement of RhoA and Rho kinase in neutrophil-stimulated endothelial hyperpermeability. Am J Physiol Heart Circ Physiol. 2004;286:H1057–1062. doi: 10.1152/ajpheart.00841.2003. [DOI] [PubMed] [Google Scholar]
- 35.Yuan SY. Signal transduction pathways in enhanced microvascular permeability. Microcirculation. 2000;7:395–403. [PubMed] [Google Scholar]
- 36.Moy AB. Winter M. Kamath A. Blackwell K. Reyes G. Giaever I. Keese C. Shasby DM. Histamine alters endothelial barrier function at cell-cell and cell-matrix sites. Am J Physiol Lung Cell Mol Physiol. 2000;278:L888–898. doi: 10.1152/ajplung.2000.278.5.L888. [DOI] [PubMed] [Google Scholar]
- 37.Birukova AA. Adyshev D. Gorshkov B. Bokoch GM. Birukov KG. Verin AD. GEF-H1 is involved in agonist-induced human pulmonary endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2006;290:L540–548. doi: 10.1152/ajplung.00259.2005. [DOI] [PubMed] [Google Scholar]
- 38.Moy AB. Bodmer JE. Blackwell K. Shasby S. Shasby DM. cAMP protects endothelial barrier function independent of inhibiting MLC20-dependent tension development. Am J Physiol. 1998;274:L1024–1029. doi: 10.1152/ajplung.1998.274.6.L1024. [DOI] [PubMed] [Google Scholar]
- 39.Matsumura F. Hartshorne DJ. Myosin phosphatase target subunit: Many roles in cell function. Biochem Biophys Res Commun. 2008;369:149–156. doi: 10.1016/j.bbrc.2007.12.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferguson MK. Shahinian HK. Michelassi F. Lymphatic smooth muscle responses to leukotrienes, histamine and platelet activating factor. J Surg Res. 1988;44:172–177. doi: 10.1016/0022-4804(88)90046-7. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe N. Kawai Y. Ohhashi T. Dual effects of histamine on spontaneous activity in isolated bovine mesenteric lymphatics. Microvasc Res. 1988;36:239–249. doi: 10.1016/0026-2862(88)90025-8. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura K. Radhakrishnan K. Wong YM. Rockson SG. Anti-inflammatory pharmacotherapy with ketoprofen ameliorates experimental lymphatic vascular insufficiency in mice. PLoS One. 2009;4:e8380. doi: 10.1371/journal.pone.0008380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Nieuw Amerongen GP. Draijer R. Vermeer MA. van Hinsbergh VW. Transient and prolonged increase in endothelial permeability induced by histamine and thrombin: Role of protein kinases, calcium, and RhoA. Circ Res. 1998;83:1115–1123. doi: 10.1161/01.res.83.11.1115. [DOI] [PubMed] [Google Scholar]
- 44.Wojciak–Stothard B. Potempa S. Eichholtz T. Ridley AJ. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J Cell Sci. 2001;114:1343–1355. doi: 10.1242/jcs.114.7.1343. [DOI] [PubMed] [Google Scholar]
- 45.Carbajal JM. Gratrix ML. Yu CH. Schaeffer RC., Jr. ROCK mediates thrombin's endothelial barrier dysfunction. Am J Physiol Cell Physiol. 2000;279:C195–204. doi: 10.1152/ajpcell.2000.279.1.C195. [DOI] [PubMed] [Google Scholar]