Abstract
Urgent repair (within 48 hr after diagnosis) of posterior ventricular septal defect in the presence of cardiogenic shock, consequent to acute myocardial infarction, is associated with a very high mortality rate. The use of left ventricular mechanical support devices has the potential to impart hemodynamic stability and to delay surgical treatment until such time as scar tissue forms around the defect, sufficient to hold a suture patch.
From May 2004 through July 2007, 5 patients who were in cardiogenic shock as a consequence of acute posterior ventricular septal defect underwent early implantation of a transfemoral microaxial Impella® Recover® LP 5.0 Support System as mechanical support (bridge to surgery).
The mean duration of support by the left ventricular assist device was 14.4 ± 6 days. No one died during assistance. The device reduced left-to-right shunting, systolic pulmonary artery pressure, central venous pressure, and pulmonary capillary wedge pressure. Liver, kidney, and lung function improved, and the 30-day mortality rate was 40%.
Although this is a retrospective study of a very small patient population, without benefit of a control group, it is the first report of its kind. This initial experience using the Impella Recover 5.0 in cases of cardiogenic shock due to posterior ventricular septal defect suggests that this conservative approach is a feasible and safe way to improve hemodynamic conditions and delay surgery. Further clinical experience is needed to confirm these early results.
Key words: Heart-assist devices; heart rupture, post-infarction/surgery/therapy; heart septal defects, ventricular/complications/surgery/therapy; heart septum; hemodynamics; myocardial infarction/complications/therapy; retrospective study; shock, cardiogenic; treatment outcome
Ventricular septal defect (VSD) is a rare but catastrophic sequela of acute myocardial infarction (AMI), occurring in about 1% to 2% of all AMI patients. Among the acute VSDs, posterior defects are the most frequent, and their treatment can be challenging. In fact, both medical and surgical approaches carry a very high mortality rate: the 30-day mortality rate ranges from about 60% for those receiving surgical treatment up to 90% for medical treatment.1,2
Many surgical techniques have been devised to avoid complications arising from the fragility of the infarcted tissues. In 1982, Daggett and colleagues3 proposed passing pledgeted mattress sutures through the margins of the infarcted area (from endocardium to epicardium) and then through the patch affixed to the epicardial surface. David and co-authors4 described the endocardial repair of a posterior VSD by excluding the infarcted area. Despite the large experience of these centers, the results in terms of hospital death were not out of the ordinary; most deaths occurred in patients who had severe cardiac failure and posterior or right ventricular (RV) infarction. Other factors associated with a high mortality rate are early repair, posterior defects, advanced age, use of catecholamines, and hemodynamic instability.2,5,6 Patients who are unresponsive to medical treatment or to the intra-aortic balloon pump (IABP) require an aggressive surgical approach and experience the worst outcomes. The debate regarding the management of patients who have posterior VSDs that are unresponsive to medical treatment or IABPs is still open, because early surgical repair has not shown improvement in survival. However, the contention that these patients do not qualify for surgery is difficult to support, considering their relative youth and their often-uneventful medical histories. In the uncertain management of patients who have posterior VSDs unresponsive to medical treatment or an IABP, ventricular assist devices may offer a new intermediate treatment option; but the presence of an interventricular shunt and a large area of necrosis (frequently involving the apex of the heart, where the outflow cannula would be inserted) are major limitations in the use of left ventricular assist devices (LVADs). The purpose of our study was to determine if early use of LVADs can improve organ perfusion and hemodynamic conditions enough to enable a delay in surgery sufficient to produce fibrosis of the VSD ridge—thereby creating a stronger surface for patch suture and reducing the chance of dehiscence.
Patients and Methods
The Impella® Recover® LP 5.0 Support System (AbioMed, Inc.; Danvers, Mass) is an intravascular microaxial blood pump designed for short-term support. Because the device is a minimally invasive ventricular unloading catheter that is placed through the femoral artery via a small incision, it does not require an operating room and a cardiac surgeon. (Percutaneous implantation is possible only for the smaller Impella® Recover® LP 2.5.) The pump, which incorporates a rotor driven by an electric motor (Fig. 1), is placed through the aortic valve. It then aspirates blood from the left ventricle (LV) and pumps it into the ascending aorta (Fig. 2). The amount of flow depends on the rotation speed (maximum, 32,000 rpm) and on the pressure gradient between the aorta and the LV. The pressure detector, located on the head of the rotor, registers this gradient, thereby ensuring the correct setting of the device. The rotation speed (there are 9 speeds, ranging from P1 through P9) can be set on the control console in order to regulate the flow, which can reach 5.2 L/min at the maximum rotation speed.
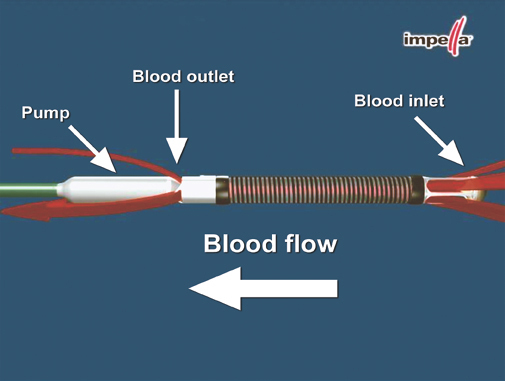
Fig. 1 Illustration shows the structure of the Impella and the direction of flow. (Photo courtesy of AbioMed)

Fig. 2 Illustration shows the Impella properly positioned across the aortic valve: the blood inlet is in the left ventricle, while the blood outlet is in the ascending aorta. (Photo courtesy of AbioMed)
The Impella Recover 5.0 is pushed through the aorta and into the LV under fluoroscopic guidance. Extracorporeal circulation is not required. As the device is positioned across the aortic valve, the microaxial pump is started at the minimum rotation speed, in order to avoid aortic regurgitation. Rotation speed can be regulated from the lowest speed to the highest, if necessary to avoid shunt inversion. Moreover, the speed should be regulated in accordance with the RV function: in the event of cavity dilation, a higher rotation speed optimizes unloading. Although the correct intraventricular position can be verified by the pressure gradient shown on the control console, transesophageal echocardiography (TEE), fluoroscopy, or both are mandatory during implantation.
To avoid clot formation, the impeller housing is flushed continuously with a hyper-osmolar solution (50 mL of 40% glucosate solution, plus 5,000 IU of heparin administered at about 5 mL/hr, depending upon activated partial thromboplastin time and the pressure of the delivery system). Systemic anticoagulation is not required, because the heparin included in the purge is enough to reach safe levels of activated partial thromboplastin time (>55 sec) or activated clotting time (>160 sec).
From May 2004 through July 2007, 13 patients were evaluated at our institution for acute VSD. Five of these patients (4 men and 1 woman; mean age, 61.8 ± 4.8 yr; age range, 58–68 yr) had developed a posterior VSD and cardiogenic shock within 48 hr after diagnosis of AMI. In all cases, the electrocardiogram had shown an inferior AMI, and there had been a significant increase of cardiac enzymes and severe RV dysfunction. In all cases, occlusion of the right coronary artery had caused a large inferior AMI and, afterwards, a posterior VSD. The VSD dimensions were considerable (range, 3 × 2.5 cm–3.5 × 4 cm), and they followed a serpiginous course: usually, they were far enough from the mitral annulus that a patch suture could be placed. In 1 case (patient 4), the VSD was so close to the mitral annulus that surgical closure was precluded and the patient had to receive a heart transplant. In 3 patients, there was widespread coronary artery disease. Mitral regurgitation was moderate to severe in 3 patients. All of the patients were admitted to our institution in cardiogenic shock, despite the use of IABPs and drugs. All of them had severe renal failure, with oliguria or anuria. One of them was also on mechanical ventilation. They underwent implantation of a transfemoral microaxial Impella Recover LP 5.0 device as a bridge to surgery. Preoperative data on the patients are listed in Table I.
TABLE I. Baseline Characteristics of the Patients
Patients' cases are set forth more completely below.
Patient 1. A 68-year-old man, 4 days after an inferior AMI, developed a large posterior VSD (3 × 4 cm) and severe mitral regurgitation. His hemodynamic condition was supported by dobutamine 7.5 μg/kg/min and adrenaline 0.1 μg/kg/min, and by IABP. The Impella was implanted on the 6th day after AMI, using a flow rate of between 2.1 and 3.8 L/min (P4); surgical repair was performed 23 days later. Mitral regurgitation improved after Impella implantation, and it was not corrected. The patient did not require mechanical ventilation. The Impella had to be replaced 12 days after the implantation because of high resistance to the purge solution. The patient died of RV dysfunction on the first postoperative day (POD).
Patient 2. A 58-year-old man with widespread coronary artery disease developed a posterior VSD (4 × 2.5 cm) on the 4th day after AMI. He was on mechanical ventilation, and his hemodynamic condition was supported by dobutamine 5 μg/kg/min and adrenaline 0.05 μg/kg/min, and by IABP. The Impella was implanted on the 6th day after AMI, using a flow rate of between 2.8 and 4.5 L/min (P8). Surgical repair and coronary artery bypass grafting (CABG) were performed 12 days later. The patient had an increase in indirect bilirubin level, probably related to hemolysis caused by the high speed of rotation (P8). The patient required blood-cell transfusion, and he died on the first POD due to tracheal laceration, most likely caused by the long duration of mechanical ventilation.
Patient 3. A 66-year-old man developed a posterior VSD (3.5 × 4 cm) on the 3rd day after AMI. His hemodynamic condition was supported by dobutamine 5 μg/kg/min and by IABP: the Impella was implanted immediately, using a flow rate of between 2.2 and 3.7 L/min (P5). Surgical repair was performed 11 days later. The patient did not require mechanical ventilation, and he was discharged from the intensive care unit (ICU) on the 5th POD and from the hospital on the 14th POD. He is still alive and has normal hepatic and renal function.
Patient 4. A 59-year-old man with severe multivessel disease had a posterior VSD (3 × 4 cm) and moderate mitral regurgitation on the 3rd day after AMI. His hemodynamic condition was supported by dobutamine 5 μg/kg/min and by IABP. His renal function was severely impaired, and he needed hemodialysis. One day after the VSD diagnosis, the Impella was implanted using a flow rate between 3 and 4.2 L/min (P7). The patient did not require mechanical ventilation. During Impella support, there was progressive improvement of renal and hepatic function (no more hemodialysis, from the 3rd day after Impella implantation), and about 8 days later he received a heart transplant. The patient was discharged from the ICU on the 10th POD and from the hospital on the 35th POD. He is still alive with normal hepatic and renal function.
Patient 5. A 58-year-old woman with multivessel disease developed a posterior VSD (3 × 2.5 cm) and moderate mitral regurgitation on the 2nd day after AMI. Her hemodynamic condition was supported by dobutamine 10 μg/kg/min, dopamine 5 μg/kg/min, and IABP. She was on mechanical ventilation. The day after the VSD diagnosis, the patient underwent Impella implantation using a flow rate of between 2.5 and 3.5 L/min (P5). Surgical repair, CABG, and mitral annuloplasty were performed 18 days later. The patient was extubated on the 5th POD. Her clinical condition improved, with recovery of hepatic and renal function. However, she developed an infection of the femoral wound, which was treated with antibiotics and wound débridement, followed by daily care with silver gauzes. She died in the ICU on the 42nd POD due to untreatable bleeding of the femoral artery.
In all patients, the diagnosis of posterior VSD was completed using echocardiography (transthoracic and TEE) and heart catheterization (left and right). The mean interval between AMI and VSD diagnosis was 3.2 ± 0.8 days. All of the patients were urgently admitted into the catheterization laboratory, where the Impella was implanted, and then they were moved to the ICU. Table II summarizes the ICU treatment and the surgical outcomes. A Swan-Ganz catheter was positioned to monitor the pulmonary artery pressures and the mixed venous oxygen saturation, and to calculate the left-to-right shunting. The position of the Impella tip was controlled and corrected by TEE every 2 days after the implantation. The Impella Recover system worked in all cases at flow rates between P3 and P8.
TABLE II. Intensive Care Unit Status during LVAD Assistance, and Surgical Outcome
Statistical Analysis
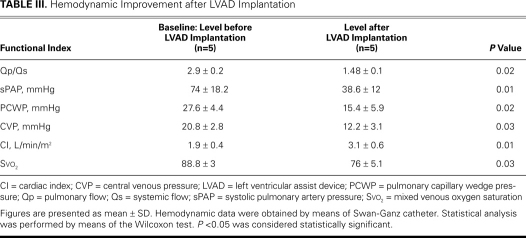
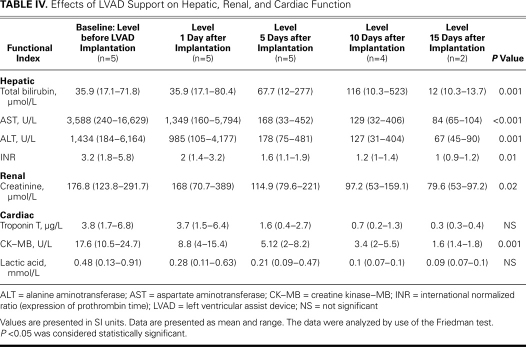
All the data were analyzed using nonparametric statistics because of the very small number of patients considered. The Wilcoxon test was used for Table III, comparing the hemodynamic data before and after Impella implantation. The Friedman test was used for Table IV, showing the improvement in multiorgan function after Impella implantation. A P value <0.05 was considered statistically significant.
TABLE III. Hemodynamic Improvement after LVAD Implantation
TABLE IV. Effects of LVAD Support on Hepatic, Renal, and Cardiac Function
Results
After Impella implantation, all the patients had full recovery of organ function, as shown by improvement in clinical condition, and all patients were bridged to surgery. The mean duration of LVAD support was 14.4 ± 6 days (range, 8–23 d), during which time there were no deaths. No thromboembolic or bleeding event was recorded during LVAD assistance. In only 1 case was the Impella repositioned (patient 1). Significant hemolysis (indirect bilirubin, >2 mg/dL; reticulocytes, >100 ×109/L; and low haptoglobin levels) was reported in 1 patient, who required red blood cell transfusion. All the hemodynamic values improved within the first 24 hours of Impella support, as shown in Table III. There was a significant reduction of the left-to-right shunting (Qp/Qs) in all cases, with significant reduction of venous oxygen saturation. Of note, there was no instance of paradoxical embolism, because we strongly avoided flow inversion through the VSD. Accordingly, the systolic pulmonary artery, central venous, and pulmonary capillary wedge pressures decreased significantly. A striking improvement of the mean cardiac index was also noted. These positive changes were maintained throughout the period of assistance. Table IV summarizes the effects of the Impella on hepatic, renal, and cardiac function. All patients had preoperative renal dysfunction (low urine output or anuria, with increases in blood urea nitrogen and serum creatinine levels), but normal urine output resumed in 4 patients. One patient required hemodialysis before implantation and until the 3rd POD. Mean serum creatinine levels dropped during mechanical support (from 176.8 µmol/L to 79.6 µmol/L 15 days after implantation; P=0.02). All patients had severe hepatic dysfunction because of severe right-heart failure, but an important decrease of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels was noted in all patients during mechanical support (ALT from 1,434 to 67 U/L 15 days after implantation, P=0.001; AST from 3,588 U/L to 84 U/L 15 days after implantation; P<0.001). The levels of total bilirubin decreased in all patients but one (from 35.9 µmol/L to 12 µmol/L 15 days after implantation; P=0.001). In patient number 2, there was significant hemolysis.
Prothrombin time (expressed as international normalized ratio) decreased significantly (from 3.2 to 1, 15 days after implantation; P=0.01). Cardiac enzymes had already dropped from the first day after microaxial pump implantation: troponin T from 3.8 µg/L to 0.3 µg/L 15 days after implantation, P=not significant (NS); and creatine kinase–MB from 17.6 U/L to 1.6 U/L 15 days after implantation, P=0.001. The CK–MB values before implantation were substantially more than 10% of total CK, but had returned to normal range (<10%) 15 days after implantation. Lactic acid levels were reduced because of the improvement of systemic perfusion (from 0.48 mmol/L to 0.09 mmol/L 15 days after implantation, P=NS).
Pulmonary function recovered in all patients. Only 1 patient required mechanical ventilation during support, and the ventilation had been started before Impella implantation. Long duration of mechanical ventilation in this patient caused serious tracheomalacia, which ultimately was responsible for his death. During LVAD assistance, we used only vasodilator drugs like nitroprusside and nitroglycerin to reduce the afterload. Table II summarizes the ICU management and surgical treatment. Postoperative TEE revealed no residual VSD and no mitral regurgitation. Two of the 5 patients died after surgical repair, yielding a 30-day mortality rate of 40%. Only 1 of these patients died of a cardiac cause (RV failure on the first POD). The other death (in patient 2) was caused by postoperative tracheal laceration due to tracheomalacia.
Discussion
Acute VSDs occur in about 2% of patients after AMI. The size of the defect determines the degree of the shunting, the extent of hemodynamic compromise, and therefore the likelihood of survival. Posterior VSDs carry a particularly bad prognosis.7 The higher mortality rate is thought to be secondary to the associated infarction of a large portion of the RV and to the complex large VSDs that occur with basal ruptures.8 Despite recent advances in surgical techniques, the correction of the basal portion of the septum remains technically very challenging. Emergency surgical repair of the VSD is thought to be the only possible approach in patients who have pulmonary edema or cardiogenic shock. The use of an IABP is usually not enough to reverse or stabilize this subset of patients, and delay in surgery is likely to induce multiorgan failure.9 All septal perforations are exposed to sheer forces and to the removal of necrotic tissue by macrophages—so the rupture site can expand, resulting in sudden hemodynamic collapse, even in patients with normal LV function who are clinically stable.10 Despite all modern advances in cardiac procedures, surgery has a poor prognosis,1 and medical treatment is absolutely inapplicable (30-day mortality rate in the GUSTO-I trial, 94%). Two major factors render surgical repair unsuccessful: the necrotic borders of the VSD are unsuitable for suturing and the cardiogenic shock worsens due to the AMI. New surgical strategies are required in this situation. At present, the gold standard is still IABP support. Counterpulsation reduces LV afterload, thereby increasing cardiac output and decreasing the left-to-right shunting, as reported by Gold and colleagues in 1973.11 In addition, IABP support is associated both with decreased myocardial oxygen consumption and with improved myocardial and peripheral organ perfusion. Although counterpulsation produces an overall improvement in the patient's condition, it cannot achieve complete hemodynamic stability.12 Peak improvement occurs within 24 hours, and no further benefit has been observed during prolonged balloon pumping.13 Pharmacologic therapy with inotropic agents and diuretics should be instituted promptly. The addition of vasodilators (for example, sodium nitroprusside or intravenous nitroglycerin) makes good theoretical sense, because it can decrease the left-to-right shunting associated with the mechanical defect, and thus increase cardiac output. However, these effects are often associated with a marked fall in mean arterial blood pressure and with reduced coronary perfusion, which are both poorly tolerated in these critically ill patients.
Since 2005, in all cases of posterior VSD with early cardiogenic shock, we have used the Impella Recover 5.0 system together with traditional IABP, in order to stabilize the patient and delay surgery. The interval between Impella implantation and surgery has ranged from 8 to 23 days; the objective of the delay in surgery was of course to strengthen the infarcted area with newly formed fibrotic tissue suitable for surgical sutures. Other advantages of our overall approach are reduction of left-to-right shunting, increase of cardiac output, and recovery of the stunned myocardium. For these reasons, the option of immediate surgery with subsequent Impella support was not considered.
We did not include patients with anterior VSDs in this study: anterior defects are usually smaller than posterior and, for that reason, cause less interventricular shunting. Moreover, patients with anterior VSDs are usually more stable, so IABPs and inotropic agents are enough for hemodynamic stabilization and delay of surgery. The risks of Impella insertion into the anterior VSD and of subsequent tissue damage and embolization are too high in these patients. Prompt surgical treatment of anterior VSD (unlike posterior VSD) usually offers acceptable results.
However, the use of the Impella in patients with posterior VSDs requires very close monitoring in the ICU and frequent echocardiographic follow-up in order to check the direction of flow. Ideally, the rotation speed (P1–P8) should be adjusted to achieve the highest reduction of left-to-right shunting, thereby avoiding flow inversion. However, in regulating the rotation speed, one must bear in mind multiple factors: not only left-to-right shunting, but adequate cardiac output, adequate RV unloading, and the maintenance of an activated clotting time longer than 160 to 180 sec, to avoid thromboembolic complications. For these reasons, rotation speed needs to be adjusted for each patient. Moreover, one should set the rotation speed at the lowest level that meets performance objectives, in order to avoid hemolysis. On the basis of our clinical experience, we prefer to avoid high rotation speeds.
Meyns and colleagues14 have already reported their experience using the precursor of the Impella system—an earlier axial-flow device, the Hemopump® Cardiac Assist System (Medtronic; Minneapolis, Minn)—as a bridge to transplantation in 2 patients with postinfarction VSD. Both patients experienced fatal pump failure during support. Examination of 1 device at autopsy disclosed necrotic material that was clogging the catheter system, but no neurologic or thromboembolic complications occurred during the use of the Hemopump. The authors did not report the location of the VSD. The new Impella system is smaller and less obtrusive than was the old Hemopump. We have already published our initial experience15 with the use of Impella in acute VSD and have discussed the usefulness of this device in these very complicated cases.16 Some other investigators have become interested in the use of percutaneous devices in acute VSD and have reported interesting results17: the most important limitation is that all of these papers are case reports. In this current paper, we support the theoretical discussion of Impella and acute VSD by showing our clinical data. In our experience with 5 cases of posterior VSD after AMI, the Impella Recover system enabled stable maintenance of the patients for several days. After removing the device, we found no evidence of technical problems, such as necrotic material in the catheter system. Nor did our patients experience neurologic complications, such as stroke or transient ischemic attack. We believe that the posterior and basal location of these VSDs interferes very minimally with the suction of the catheter tip: the microaxial pump still functions normally, without aspiration of necrotic material. Jeppsson and colleagues2 and Agnihotri and colleagues18 report that advanced age, critical status, use of catecholamines, early repair, and posterior rupture are independent predictors of 30-day death. In Jeppsson's experience, the total 30-day mortality rate for posterior VSD was 51% vs 30% for anterior VSD (P=0.004); this rose to 68% for posterior VSD operated upon within 48 hours (with an urgent-repair odds ratio of 4.2).2 In our experience, the 30-day hospital mortality rate in this difficult subset of VSD patients was 40%.
Limitations
We readily acknowledge that this is a retrospective study of a very small patient population, without benefit of a control group for comparison. Nevertheless, this very preliminary experience has value as the 1st report of the use of a microaxial Impella pump in posterior VSD presenting as cardiogenic shock.
Conclusion
Our study suggests the feasibility of using this new approach to enable hemodynamic and clinical improvement, to delay surgery until the infarct scar can form (in particular, the ridges of the VSD), and to reverse renal and hepatic impairment. This should lower the hospital mortality rate in a difficult subset of patients. Moreover, Impella implantation does not require a cardiac surgeon and can be performed in any catheterization laboratory. The consequent early stabilization should increase the number of patients who are suitable for surgical repair. Early Impella implantation can prevent multiorgan dysfunction and stabilizes the patient for 3 weeks (for 8–23 d, in our patients)—long enough for the formation of scar tissue adequate to hold the patch suture. However, further clinical experience is needed to confirm these early results.
References
- 1.Crenshaw BS, Granger CB, Birnbaum Y, Pieper KS, Morris DC, Kleiman NS, et al. Risk factors, angiographic patterns, and outcomes in patients with ventricular septal defect complicating acute myocardial infarction. GUSTO-I (Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries) Trial Investigators. Circulation 2000;101(1):27–32. [DOI] [PubMed]
- 2.Jeppsson A, Liden H, Johnsson P, Hartford M, Radegran K. Surgical repair of post infarction ventricular septal defects: a national experience. Eur J Cardiothorac Surg 2005;27(2):216–21. [DOI] [PubMed]
- 3.Daggett WM, Buckley MJ, Akins CW, Leinbach RC, Gold HK, Block PC, Austen WG. Improved results of surgical management of postinfarction ventricular septal rupture. Ann Surg 1982;196(3):269–77. [DOI] [PMC free article] [PubMed]
- 4.David TE, Dale L, Sun Z. Postinfarction ventricular septal rupture: repair by endocardial patch with infarct exclusion. J Thorac Cardiovasc Surg 1995;110(5):1315–22. [DOI] [PubMed]
- 5.Cerin G, Di Donato M, Dimulescu D, Montericcio V, Menicanti L, Frigiola A, De Ambroggi L. Surgical treatment of ventricular septal defect complicating acute myocardial infarction. Experience of a north Italian referral hospital. Cardiovasc Surg 2003;11(2):149–54. [DOI] [PubMed]
- 6.Di Summa M, Actis Dato GM, Centofanti P, Fortunato G, Patane F, Di Rosa E, et al. Ventricular septal rupture after a myocardial infarction: clinical features and long term survival. J Cardiovasc Surg (Torino) 1997;38(6):589–93. [PubMed]
- 7.Moore CA, Nygaard TW, Kaiser DL, Cooper AA, Gibson RS. Postinfarction ventricular septal rupture: the importance of location of infarction and right ventricular function in determining survival. Circulation 1986;74(1):45–55. [DOI] [PubMed]
- 8.Bolooki H. Emergency cardiac procedures in patients in cardiogenic shock due to complications of coronary artery disease. Circulation 1989;79(6 Pt 2):I137–48. [PubMed]
- 9.Seyfarth M, Sibbing D, Bauer I, Frohlich G, Bott-Flugel L, Byrne R, et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol 2008;52(19):1584–8. [DOI] [PubMed]
- 10.Topaz O, Taylor AL. Interventricular septal rupture complicating acute myocardial infarction: from pathophysiologic features to the role of invasive and noninvasive diagnostic modalities in current management. Am J Med 1992;93(6):683–8. [DOI] [PubMed]
- 11.Gold HK, Leinbach RC, Sanders CA, Buckley MJ, Mundth ED, Austen WG. Intraaortic balloon pumping for ventricular septal defect or mitral regurgitation complicating acute myocardial infarction. Circulation 1973;47(6):1191–6. [DOI] [PubMed]
- 12.Montoya A. Ventricular septal rupture secondary to acute myocardial infarction. In: Pifarre R, editor. Cardiac surgery: acute myocardial infarction and its complications. Philadelphia: Hanley & Belfus; 1992. p. 159.
- 13.Scanlon PJ, Montoya A, Johnson SA, McKeever LS, Sullivan HJ, Bakhos M, Pifarre R. Urgent surgery for ventricular septal rupture complicating acute myocardial infarction. Circulation 1985;72(3 Pt 2):II185–90. [PubMed]
- 14.Meyns B, Vanermen H, Vanhaecke J, Sergeant P, Daenen W, Flameng W. Hemopump fails as bridge to transplantation in postinfarction ventricular septal defect. J Heart Lung Transplant 1994;13(6):1133–7. [PubMed]
- 15.Patane F, Zingarelli E, Sansone F, Rinaldi M. Acute ventricular septal defect treated with an Impella recovery as a ‘bridge therapy’ to heart transplantation. Interact Cardiovasc Thorac Surg 2007;6(6):818–9. [DOI] [PubMed]
- 16.Patane F, Centofanti P, Zingarelli E, Sansone F, La Torre M. Potential role of the Impella Recover left ventricular assist device in the management of postinfarct ventricular septal defect. J Thorac Cardiovasc Surg 2009;137(5):1288–9. [DOI] [PubMed]
- 17.Gregoric ID, Bieniarz MC, Arora H, Frazier OH, Kar B, Loyalka P. Percutaneous ventricular assist device support in a patient with a postinfarction ventricular septal defect. Tex Heart Inst J 2008;35(1):46–9. [PMC free article] [PubMed]
- 18.Agnihotri AK, Madsen JC, Daggett WM Jr. Surgical treatment of complications of acute myocardial infarction: posterior ventricular septal defect and free wall rupture. In: Cohn LH, editor. Cardiac surgery in the adult. 3rd ed. New York: McGraw-Hill Medical; 2008. p. 753–83.