Abstract
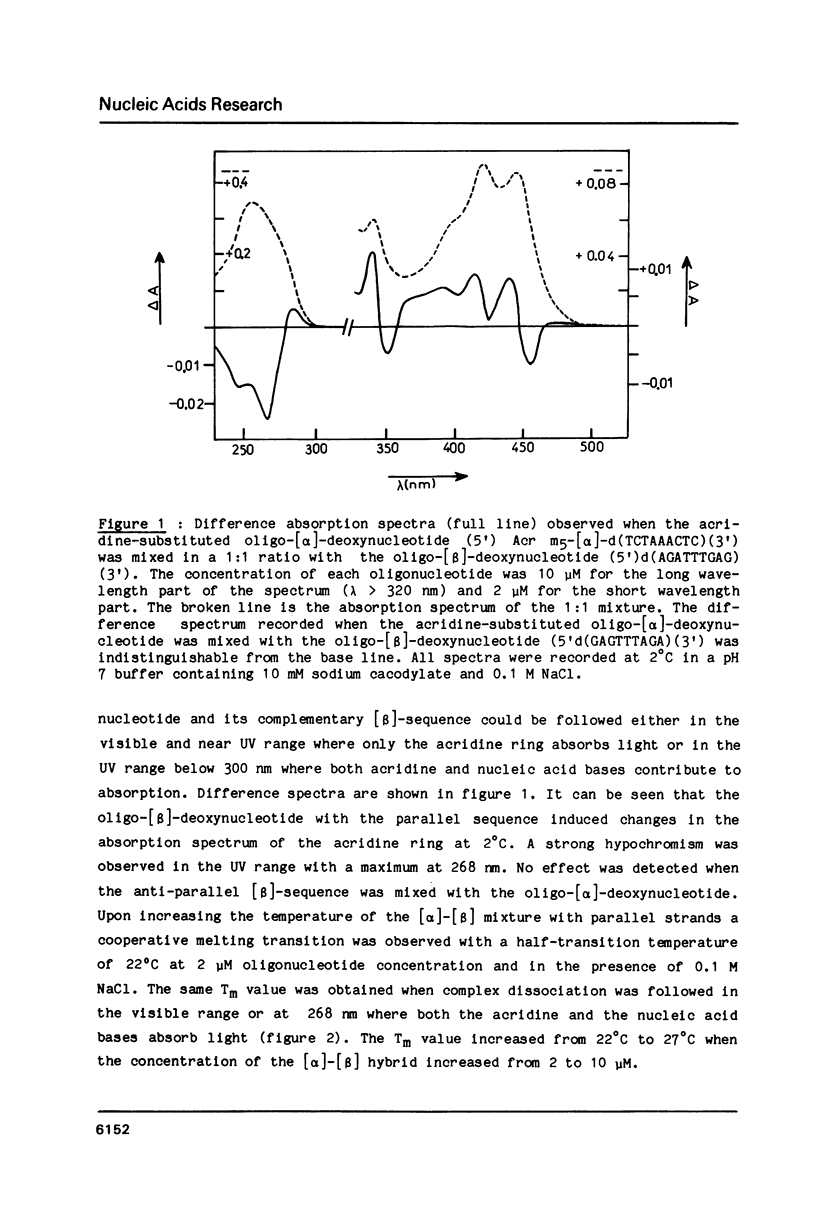
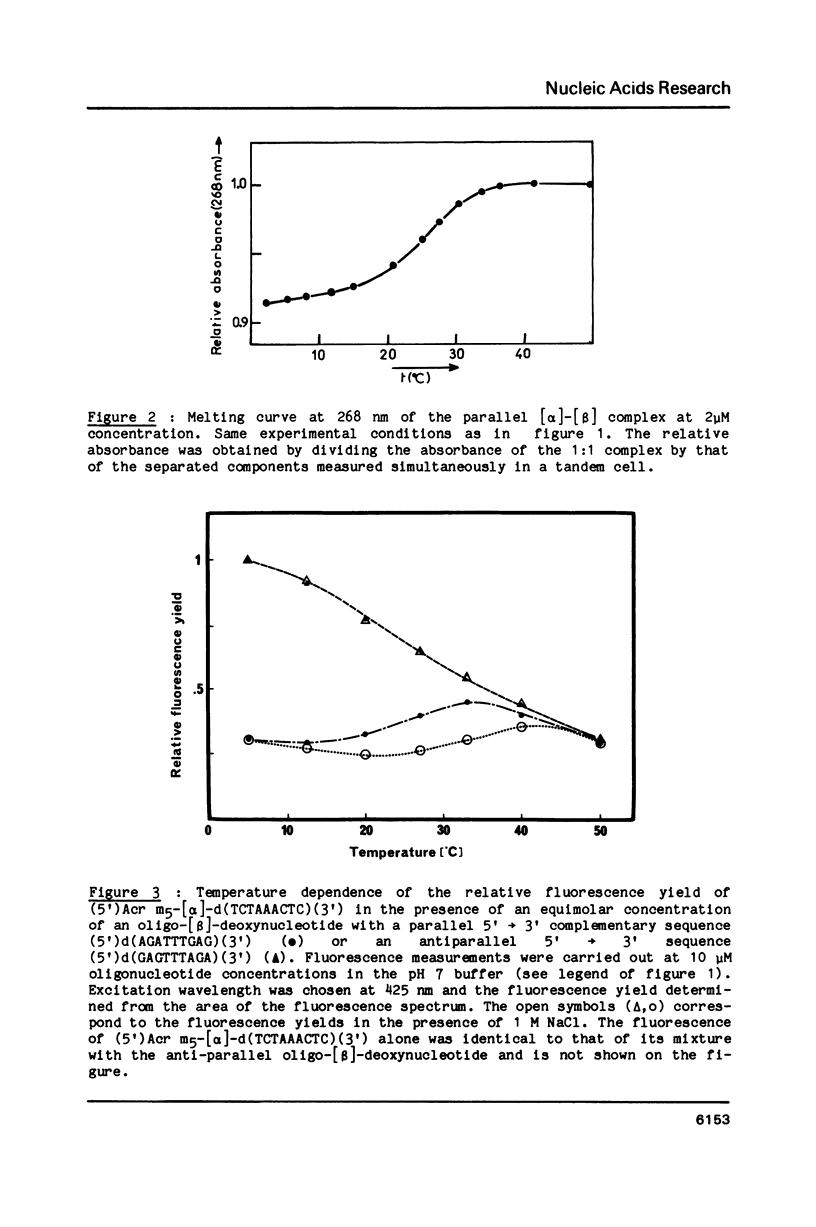
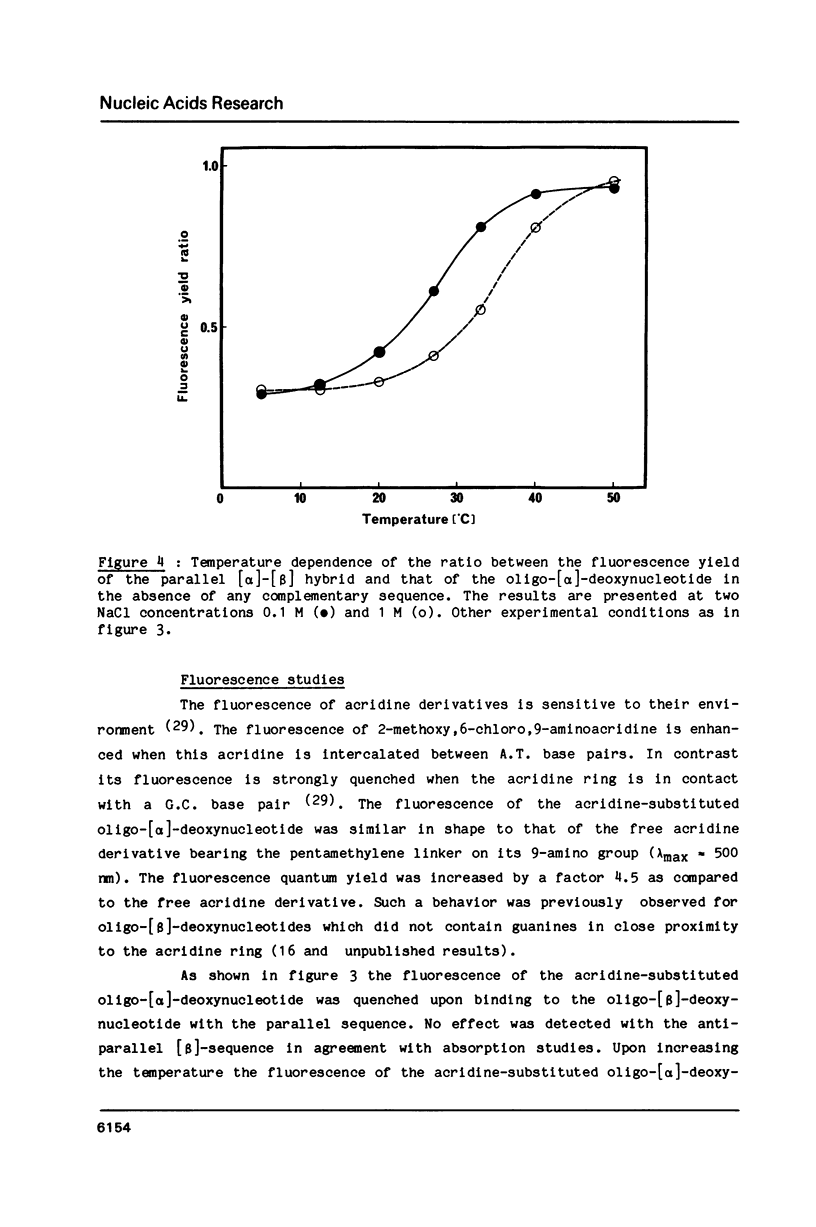
An oligo-[alpha]-deoxynucleotide of sequence (5')d(TCTAAACTC) (3') was synthesized using the alpha-anomers of deoxynucleosides and its 5'-phosphate was covalently linked to a 9-amino acridine derivative via a pentamethylene linker. Two oligo-[beta]-deoxynucleotides containing the complementary sequence in either the 5'----3' or the 3'----5' orientation were synthesized using natural [beta]-deoxynucleosides. Complex formation was investigated by absorption and fluorescence spectroscopies. No change in spectroscopic properties was detected with the anti-parallel [beta] sequence. Absorption changes were induced in the visible absorption band of the acridine derivative at 2 degrees C when the acridine-substituted oligo-[alpha]-deoxynucleotide was mixed in equimolecular amounts with the complementary [beta]-sequence in the parallel orientation. Hypochromism was observed in the UV range. The fluorescence of the acridine derivative was quenched by the guanine base present in the second position of the complementary sequence. Cooperative dissociation curves were observed and identical values of melting temperatures were obtained by absorption and fluorescence. An increase in salt concentration stabilized the complex with a delta Tm of 8 degrees C when NaCl concentration increased from 0.1 to 1 M. These results demonstrate that an oligo-[alpha]-deoxynucleotide covalently linked to an intercalating agent is able to form a double helix with an oligo-[beta]-deoxynucleotide. The two strands of this [alpha]-[beta] double helix adopt a parallel 5'----3' orientation. The acridine ring is able to intercalate between the first two base pairs on the 5'-side of the duplex structure.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asseline U., Delarue M., Lancelot G., Toulmé F., Thuong N. T., Montenay-Garestier T., Hélène C. Nucleic acid-binding molecules with high affinity and base sequence specificity: intercalating agents covalently linked to oligodeoxynucleotides. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3297–3301. doi: 10.1073/pnas.81.11.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asseline U., Nguyen T. T., Hélène C. Nouvelles substances à forte affinité spécifique pour des séquences d'acides nucléiques: oligodésoxynucléotides liés de façon covalente à un agent intercalant. C R Seances Acad Sci III. 1983;297(7):369–372. [PubMed] [Google Scholar]
- Asseline U., Toulme F., Thuong N. T., Delarue M., Montenay-Garestier T., Hélène C. Oligodeoxynucleotides covalently linked to intercalating dyes as base sequence-specific ligands. Influence of dye attachment site. EMBO J. 1984 Apr;3(4):795–800. doi: 10.1002/j.1460-2075.1984.tb01887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake K. R., Murakami A., Miller P. S. Inhibition of rabbit globin mRNA translation by sequence-specific oligodeoxyribonucleotides. Biochemistry. 1985 Oct 22;24(22):6132–6138. doi: 10.1021/bi00343a015. [DOI] [PubMed] [Google Scholar]
- Cazenave C., Loreau N., Thuong N. T., Toulmé J. J., Hélène C. Enzymatic amplification of translation inhibition of rabbit beta-globin mRNA mediated by anti-messenger oligodeoxynucleotides covalently linked to intercalating agents. Nucleic Acids Res. 1987 Jun 25;15(12):4717–4736. doi: 10.1093/nar/15.12.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R., Yanofsky C. A complementary DNA oligomer releases a transcription pause complex. J Biol Chem. 1983 Aug 10;258(15):9208–9212. [PubMed] [Google Scholar]
- Green P. J., Pines O., Inouye M. The role of antisense RNA in gene regulation. Annu Rev Biochem. 1986;55:569–597. doi: 10.1146/annurev.bi.55.070186.003033. [DOI] [PubMed] [Google Scholar]
- Hélène C., Montenay-Garestier T., Saison T., Takasugi M., Toulmé J. J., Asseline U., Lancelot G., Maurizot J. C., Toulmé F., Thuong N. T. Oligodeoxynucleotides covalently linked to intercalating agents: a new class of gene regulatory substances. Biochimie. 1985 Jul-Aug;67(7-8):777–783. doi: 10.1016/s0300-9084(85)80167-x. [DOI] [PubMed] [Google Scholar]
- Kawasaki E. S. Quantitative hybridization-arrest of mRNA in Xenopus oocytes using single-stranded complementary DNA or oligonucleotide probes. Nucleic Acids Res. 1985 Jul 11;13(13):4991–5004. doi: 10.1093/nar/13.13.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancelot G., Asseline U., Thuong N. T., Hélène C. 31P NMR studies of the binding of the oligonucleotide (Ap)3A to an oligodeoxythymidylate covalently linked to an acridine derivative. J Biomol Struct Dyn. 1986 Apr;3(5):913–921. doi: 10.1080/07391102.1986.10508473. [DOI] [PubMed] [Google Scholar]
- Lancelot G., Asseline U., Thuong N. T., Hélène C. Proton and phosphorus nuclear magnetic resonance studies of an oligothymidylate covalently linked to an acridine derivative and of its binding to complementary sequences. Biochemistry. 1985 May 7;24(10):2521–2529. doi: 10.1021/bi00331a019. [DOI] [PubMed] [Google Scholar]
- Lancelot G., Thuong N. T. Nuclear magnetic resonance studies of complex formation between the oligonucleotide d(TATC) covalently linked to an acridine derivative and its complementary sequence d(GATA). Biochemistry. 1986 Sep 23;25(19):5357–5363. doi: 10.1021/bi00367a002. [DOI] [PubMed] [Google Scholar]
- Lemaitre M., Bayard B., Lebleu B. Specific antiviral activity of a poly(L-lysine)-conjugated oligodeoxyribonucleotide sequence complementary to vesicular stomatitis virus N protein mRNA initiation site. Proc Natl Acad Sci U S A. 1987 Feb;84(3):648–652. doi: 10.1073/pnas.84.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. S., Agris C. H., Aurelian L., Blake K. R., Murakami A., Reddy M. P., Spitz S. A., Ts'o P. O. Control of ribonucleic acid function by oligonucleoside methylphosphonates. Biochimie. 1985 Jul-Aug;67(7-8):769–776. doi: 10.1016/s0300-9084(85)80166-8. [DOI] [PubMed] [Google Scholar]
- Morvan F., Rayner B., Imbach J. L., Chang D. K., Lown J. W. alpha-DNA. I. Synthesis, characterization by high field 1H-NMR, and base-pairing properties of the unnatural hexadeoxyribonucleotide alpha-[d(CpCpTpTpCpC)] with its complement beta-[d(GpGpApApGpG)]. Nucleic Acids Res. 1986 Jun 25;14(12):5019–5035. doi: 10.1093/nar/14.12.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. C., Aurelian L., Reddy M. P., Miller P. S., Ts'o P. O. Antiviral effect of an oligo(nucleoside methylphosphonate) complementary to the splice junction of herpes simplex virus type 1 immediate early pre-mRNAs 4 and 5. Proc Natl Acad Sci U S A. 1986 May;83(9):2787–2791. doi: 10.1073/pnas.83.9.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séquin U. Nucleosides and nucleotides. Part 7. Four dithymidine monophosphates with different anomeric configurations, their synthesis and behaviour towards phosphodiesterases. Helv Chim Acta. 1974;57(1):68–81. doi: 10.1002/hlca.19740570108. [DOI] [PubMed] [Google Scholar]
- Thuong N. T., Chassignol M., Lancelot G., Mayer R., Hartmann B., Leng M., Hélène C. Synthesis and structural studies of a self-complementary decadeoxynucleotide d(AATTGCAATT). I.-Synthesis and chemical characterization of the decanucleotide. Biochimie. 1981 Oct;63(10):775–784. doi: 10.1016/s0300-9084(81)80037-5. [DOI] [PubMed] [Google Scholar]
- Toulmé J. J., Krisch H. M., Loreau N., Thuong N. T., Hélène C. Specific inhibition of mRNA translation by complementary oligonucleotides covalently linked to intercalating agents. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1227–1231. doi: 10.1073/pnas.83.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T., Saneyoshi M. Synthetic nucleosides and nucleotides. XXI. On the synthesis and biological evaluations of 2'-deoxy-alpha-D-ribofuranosyl nucleosides and nucleotides. Chem Pharm Bull (Tokyo) 1984 Apr;32(4):1441–1450. doi: 10.1248/cpb.32.1441. [DOI] [PubMed] [Google Scholar]
- Zamecnik P. C., Goodchild J., Taguchi Y., Sarin P. S. Inhibition of replication and expression of human T-cell lymphotropic virus type III in cultured cells by exogenous synthetic oligonucleotides complementary to viral RNA. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4143–4146. doi: 10.1073/pnas.83.12.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]



