Abstract
The management of acute, severe cardiac valvular regurgitation requires expeditious multidisciplinary care. Although acute, severe valvular regurgitation can be a true surgical emergency, accurate diagnosis and subsequent treatment decisions require clinical acumen, appropriate imaging, and sound judgment. An accurate and timely diagnosis is essential for successful outcomes and requires appropriate expertise and a sufficiently high degree of suspicion in a variety of settings. Whereas cardiovascular collapse is the most obvious and common presentation of acute cardiac valvular regurgitation, findings may be subtle, and the clinical presentation can often be nonspecific. Consequently, other acute conditions such as sepsis, pneumonia, or nonvalvular heart failure may be mistaken for acute valvular regurgitation. In comparison with that of the right-sided valves, regurgitation of the left-sided valves is more common and has greater clinical impact. Therefore, this review focuses on acute regurgitation of the aortic and mitral valves.
Key words: Acute disease; aortic valve insufficiency/classification/complications/diagnosis/etiology/physiopathology/surgery/therapy; cardiology/standards; echocardiography; endocarditis, bacterial/physiopathology/therapy; heart valve diseases/diagnosis/etiology/physiopathology/surgery/therapy; heart valve prosthesis implantation/standards; mitral valve insufficiency/classification/complications/diagnosis/etiology/physiopathology/surgery/therapy; physical examination; prosthesis failure
The management of cardiac patients who have acute valvular regurgitation is largely anecdotal: no randomized trials have been conducted to guide practitioners. Much of the medical literature describes the experience of single or multiple centers and has not been driven by protocol. Despite these limitations, the available data are sufficient to provide overarching principles. These in turn have enabled the development of practice guidelines from the American College of Cardiology/American Heart Association (ACC/AHA) and the European Society of Cardiology (ESC). The data and guidelines emphasize the need for a high degree of clinical suspicion, the timely use of echocardiography, and, in most patients, definitive treatment with valve repair or replacement.1-4 Herein, we review acute left-sided cardiac valvular regurgitation and relevant considerations in regard to its management.
Causes of Acute Valvular Regurgitation
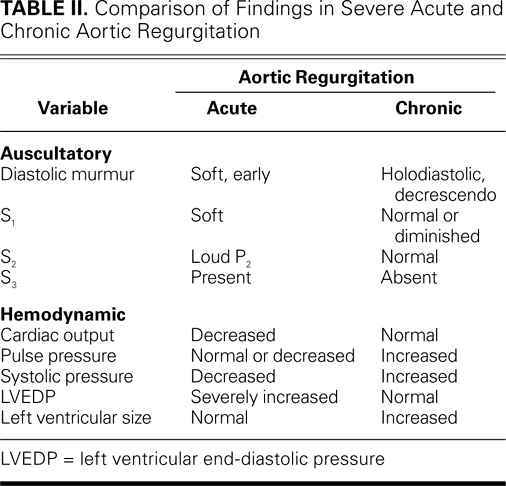
Acute valvular regurgitation can be caused by endocarditis, prosthetic valve dysfunction, chordal rupture, leaflet perforation, and iatrogenic factors (Table I). Acute exacerbation of chronic regurgitation can occur in coronary ischemia, accompany chordal rupture or leaflet perforation from endocarditis, or occur during valvular and nonvalvular percutaneous procedures. As the population of patients with prosthetic valves has increased, so have the rates of prosthetic valve endocarditis and dysfunction. Acute mechanical valve thrombosis and tears in bioprosthetic leaflets have become common. Paravalvular regurgitation can occur, particularly in patients with prosthetic valve endocarditis.5
TABLE I. Causes of Acute Valvular Regurgitation
Endocarditis is the most common cause of acute regurgitation in a native or prosthetic aortic valve. In a native valve, endocarditis can lead to leaflet perforation, leaflet prolapse due to annular destruction, or failure of coaptation because of vegetation. In a prosthetic valve, endocarditis can interfere with coaptation or lead to leaflet degradation or a paravalvular leak. Stanford Type A aortic dissection, whether from a connective-tissue disorder, a bicuspid aortic valve, or atherosclerotic disease, can present with acute aortic regurgitation. A rarer cause is blunt trauma, which can lead to leaflet rupture or to the rupture of a fenestration in an aortic leaflet.6,7
Acute mitral regurgitation can be classified as organic or functional. Organic causes, which can result in structural disruption of the valve, include leaflet perforation from endocarditis, chordal rupture in myxomatous valvular disease, and papillary muscle rupture due to myocardial infarction (MI). Prosthetic valve dysfunction because of endocarditis or paravalvular leakage is also considered to be organic. Functional mitral regurgitation results from abnormalities of the left ventricle (LV). The most common example is dilated cardiomyopathy, in which lateral displacement of the papillary muscles leads to leaflet tethering. In acute ischemia, an akinetic wall segment and papillary muscle dysfunction can impair mitral valve closure. In acute ischemia, mitral valve coaptation may be incomplete due to changes in mitral annular shape from posterior annular dyskinesia or circularization.8
The distinction between organic and functional causes is important. Organic causes frequently require surgical repair or valve replacement; conversely, functional causes may improve after the nonsurgical treatment of underlying myocardial ischemia, infarction, or cardiomyopathy.
Clinicians typically encounter chronic, rather than acute, functional mitral regurgitation. However, processes that result in a rapid decline in ventricular function can present with acute functional mitral regurgitation as a manifestation of acute heart failure, such as in Takotsubo cardiomyopathy (left apical ballooning) and peripartum cardiomyopathy.9–11 In Takotsubo cardiomyopathy, functional mitral regurgitation can result from systolic anterior motion of the mitral valve and obstruction of the LV outflow tract due to apical ballooning, with preserved basal ventricular function.11 The rapid onset of peripartum cardiomyopathy can include acute, severe mitral regurgitation. Leaflet inflammation and myocardial dysfunction from rheumatic carditis can cause acute mitral regurgitation, and some data suggest that the degree of valvular dysfunction influences outcomes.12 Acute rheumatic carditis is more prevalent in developing countries than in industrialized nations.13
The Pathophysiology of Acute Valvular Regurgitation
Acute and chronic valvular regurgitation differ dramatically in their presentation and required management. The pathophysiology and hemodynamic impact of chronic regurgitation are typically gradual and somewhat predictable. Chronic regurgitation of either the aortic or the mitral valve enables the LV to dilate and remodel, thereby maintaining forward stroke volume and cardiac output. Correspondingly, LV end-diastolic pressure (LVEDP) remains nearly normal unless coexisting factors impair diastolic filling. Only in a chronic, gradually decompensated state is ventricular remodeling unable to sustain perfusion.
In acute regurgitation, remodeling does not occur, which leads to a cascade of events. Acute aortic and mitral regurgitation share several hemodynamic sequelae. In both, the effective forward stroke volume is decreased. In aortic regurgitation, the net forward flow is decreased because of retrograde diastolic runoff into the ventricle. In mitral regurgitation, a portion of the systolic ejection is diverted to the left atrium. In regurgitation of both valves, compensatory tachycardia may initially preserve cardiac output, due to reduced effective stroke volume; nonetheless, hypotension, end-organ failure, and other evidence of cardiogenic shock eventually develops. Left atrial and pulmonary capillary wedge pressures increase abruptly because of the noncompliant left atrium, and pulmonary edema develops—albeit by different mechanisms, depending upon which valve is involved. Pulmonary edema can also occur during an acute exacerbation of chronic regurgitation.
Aortic Valve Regurgitation
Several characteristics distinguish acute from chronic aortic regurgitation (Table II). In acute aortic regurgitation, normal ventricular size results in a marked increase in LVEDP that leads to impaired forward stroke volume, decreased systolic pressure, and narrow pulse pressure.14,15 Despite compensation due to the Frank-Starling mechanism, the ventricle functions on a steep pressure-volume curve because of a lack of chamber dilation. Existing disease processes that impair diastolic function, such as hypertension or aortic stenosis, can produce a more dramatic clinical presentation of acute aortic regurgitation. If acute regurgitation has resulted in shock, sympathetic activation and the renin-angiotensin cascade can increase systemic vascular resistance. The cascade continues with abnormal mitral valve function because of acute hemodynamic changes. Markedly elevated LVEDP can cause early mitral valve closure, and tachycardia can limit mitral inflow, further decreasing ventricular filling.16 Coronary ischemia can complicate acute aortic regurgitation: decreased diastolic coronary flow decreases myocardial perfusion, while elevated LVEDP and tachycardia increase myocardial oxygen demand. This supply-demand mismatch is worsened further if obstructive coronary lesions are present or if aortic dissection impairs coronary flow. Existing chronic aortic regurgitation and ventricular enlargement can blunt the hemodynamic impact of acutely worsened regurgitation. Furthermore, in chronic regurgitation, when LVEDP is relatively low, the additional stroke volume manifests itself as increased systolic pressure. Therefore, reliance on pulse pressure as an indicator of regurgitation could result in a substantial underestimation of the severity of acute aortic regurgitation. In chronic aortic regurgitation, increased systolic pressure also results in increased afterload, which is not seen in acute regurgitation due to the low stroke volume.
TABLE II. Comparison of Findings in Severe Acute and Chronic Aortic Regurgitation
Mitral Valve Regurgitation
Acute and chronic mitral valve regurgitation also have distinct characteristics (Table III). In acute mitral regurgitation, regurgitant volume in a normal-sized, noncompliant left atrium results in a marked increase in left atrial pressure2—the mechanism by which acute pulmonary edema develops. In comparison, left atrial size and compliance are increased in chronic mitral regurgitation, but left atrial pressures remain normal despite the regurgitant volume. These findings parallel the changes in the LV in aortic regurgitation. Coexisting conditions may affect a patient's tolerance of acutely increased left atrial and LV volume. A patient with a history of chronic mitral regurgitation and preserved ventricular function might tolerate the marked increase in volume better than would a patient with impaired ventricular function, whose heart would quickly decompensate upon the acute worsening of mitral regurgitation. For example, pulmonary edema associated with ST-elevation MI is often due to new functional mitral regurgitation and is an independent sign of poor prognosis.17 Pressure monitoring can reveal significant V waves in the chronic or acute condition, although V waves tend to be more pronounced in acute mitral regurgitation. As in acute aortic regurgitation, increased preload affords a degree of initial compensation; however, the inability of the LV and left atrium to accommodate the increased volume results in marked increases in LVEDP and left atrial pressure.
TABLE III. Comparison of Findings in Severe Acute and Chronic Mitral Regurgitation
The Clinical Presentation of Acute Valvular Regurgitation
The usual and most obvious symptoms of acute valvular regurgitation are dyspnea, hemodynamic instability, and shock (including weakness, dizziness, and altered mental status). Other symptoms, such as severe chest pain from aortic dissection or fever from endocarditis, may indicate the underlying cause. However, findings can be nonspecific. Some patients with acute mitral regurgitation present solely with new-onset dyspnea without evidence of impending cardiovascular collapse, and the erroneous diagnosis may be a noncardiogenic pulmonary process or heart failure that is due to a different cause. Tables II and III show that examination findings in valvular regurgitation can be subtle. For this reason, the examining physician can conclude that chronic regurgitation consequent to ventricular enlargement (such as in apical displacement) has not yet developed, and murmurs are frequently soft. Tachycardia (which increases cardiac output) and tachypnea (which compensates for pulmonary edema) can further hinder the detection of faint murmurs.
Auscultatory findings differ in acute and chronic regurgitation. In acute aortic regurgitation, one hears a faint early diastolic murmur due to the rapid equilibration of LV and aortic diastolic pressures, whereas in chronic aortic regurgitation, one hears a louder decrescendo diastolic murmur. Early closure of the mitral valve because of elevated LVEDP yields a soft S1, lack of aortic leaflet coaptation during valve closure yields a soft A2, and the P2 may be loud if pulmonary hypertension is present. The peripheral (Watson's water-hammer pulse) and central arterial signs (Corrigan's pulse) that are associated with chronic aortic regurgitation indicate increased pulsatility from increased stroke volume, high systolic peak pressure, and wide pulse pressure. These palpable pulses are typically not present in acute aortic regurgitation, due to the diminished stroke volume and decreased pulse pressure. In acute, severe mitral regurgitation, the rapid equilibration of ventricular and atrial pressures during systole yields a faint systolic murmur rather than the holosystolic murmur that is typical in chronic mitral regurgitation.
Relying solely upon physical examination findings can lead to inaccurate diagnoses of acute regurgitation and its severity. Additional testing is needed in order to determine appropriate treatment.
Diagnostic Testing
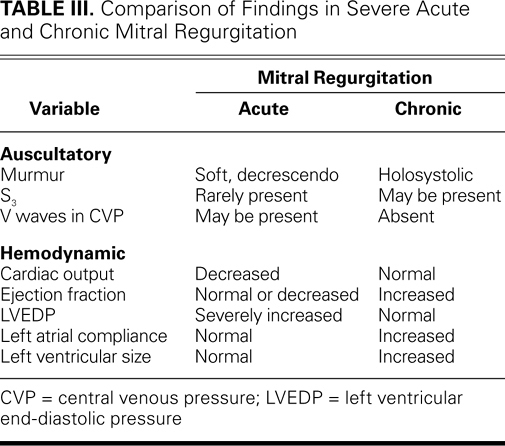
All patients in whom acute valvular regurgitation is suspected should undergo electrocardiography and chest radiography. Electrocardiograms typically reveal sinus tachycardia with nonspecific ST- and T-wave abnormalities. Ischemic ST changes can indicate regurgitation mediated by ischemia—coronary artery disease, or aortic dissection that affects the coronary ostia. Hemodynamic disturbances, such as very high LVEDP, can exacerbate coronary insufficiency. Chest radiographs typically show pulmonary edema and a left heart of normal size. Cardiomegaly can indicate the worsening of an existing valvular condition, and a widened mediastinum suggests aortic dissection. Rarely, acute mitral regurgitation directs regurgitant flow to a single pulmonary vein, and edema is seen most prominently in that lung segment (Fig. 1).18 This may be mistaken for pneumonia, particularly if the patient has endocarditis or is not severely ill. Measuring cardiac biomarkers aids the diagnosis of acute coronary syndromes and ischemia: determining the level of B-type natriuretic peptide can help distinguish cardiac causes from noncardiac causes of dyspnea and shock.
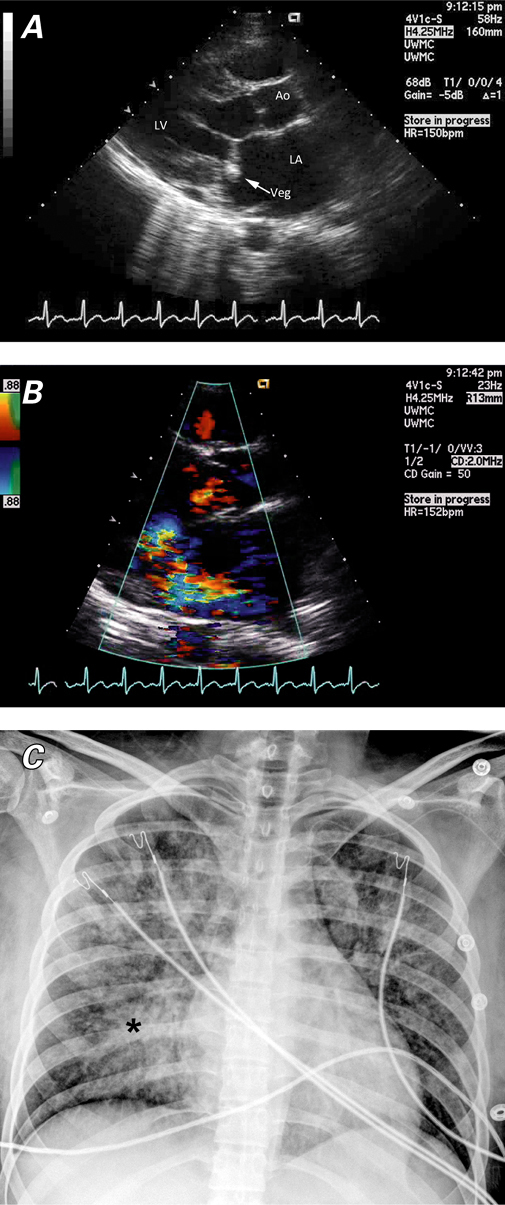
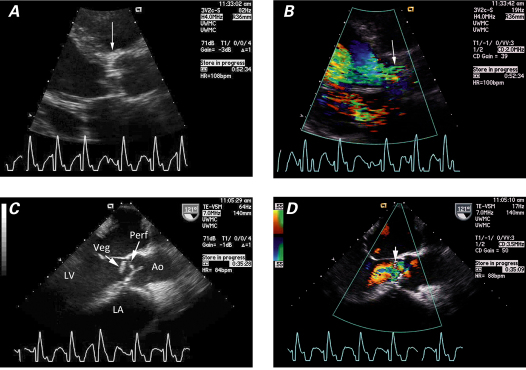
Fig. 1 Transthoracic echocardiograms (parasternal long-axis views) show mitral valve endocarditis. A ) A large vegetation is attached to the anterior leaflet, and B ) color-flow Doppler shows a posteriorly directed, eccentric jet of mitral regurgitation. C ) Chest radiograph shows a heart of normal size. Differential pulmonary edema (asterisk) between the right and left lung fields is consistent with an eccentric jet. Ao = aorta; LA = left atrium; LV = left ventricle; Veg = vegetation
Echocardiography is essential in the diagnosis of acute valvular dysfunction (Table IV). Severe aortic or mitral regurgitation, normal LV size, and normal or hyperdynamic ventricular function all point to an acute process.19 Quantitative measures are more useful in chronic regurgitation than in acute regurgitation. The estimation of effective regurgitant orifice area and regurgitant volume can be inaccurate in acute regurgitation (particularly in the presence of tachycardia) and depends upon dynamic loading conditions.20,21 Echocardiography typically reveals the mechanism of regurgitation, such as aortic dissection or ruptured mitral chordae tendineae (Fig. 2). In patients whose chronic regurgitation has led to ventricular enlargement, the acuteness of regurgitation is difficult to evaluate unless a clinical examination is performed and comparisons with prior studies are made.
TABLE IV. Echocardiographic Findings in Acute Valvular Regurgitation
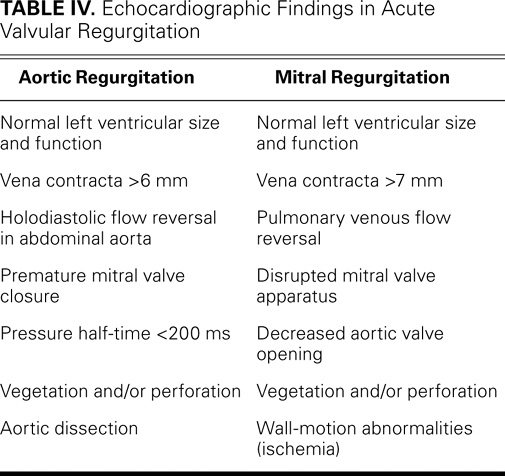
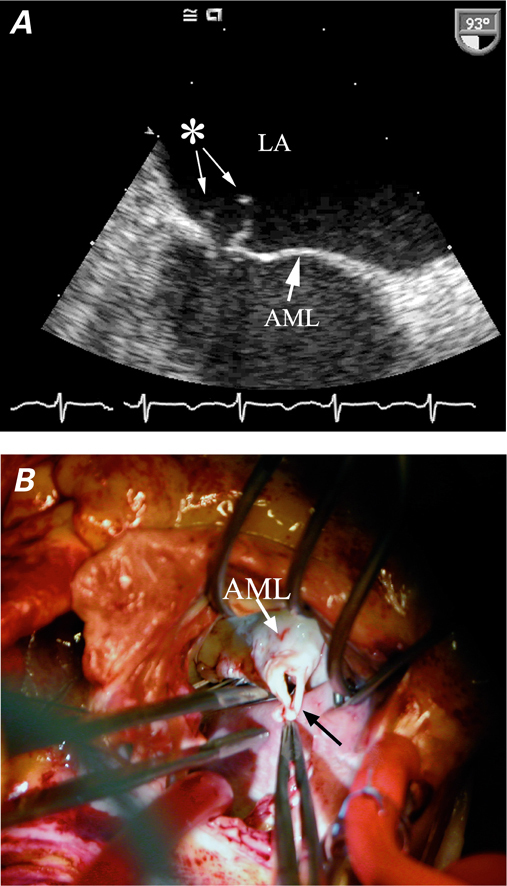
Fig. 2 A ) Transesophageal echocardiogram shows a flail anterior mitral leaflet (AML) and ruptured chordae tendineae (asterisk). B ) Intraoperative photograph of ruptured chordae (black arrow) from the AML. Photograph courtesy of Don Oxorn, MD. LA = left atrium
The use of color-flow Doppler echocardiography can lead to an underestimation of the severity of regurgitation, particularly if the regurgitant jet is eccentric. If a transthoracic study is not conclusive, transesophageal echocardiography (TEE) can reveal the mechanism and severity of the regurgitation. In prosthetic valve dysfunction, TEE can also show artifacts. Interpreting the results of TEE is often crucial to the planning of operative repair, such as the identification of leaflet or annular involvement. During planning for the use of aortic homografts or a Ross repair, TEE data on annular size are indispensable. However, if TEE results will not change the decision to perform surgery, the images can be obtained in the operating room.
Left-heart catheterization is not routinely indicated in the preoperative evaluation of patients with acute valvular regurgitation. Catheterization can be considered when electrocardiographic changes suggest acute ischemia. Patients with acute mitral regurgitation from ischemia or papillary muscle rupture may benefit from revascularization along with mitral valve repair or replacement. Conversely, patients whose mechanisms of regurgitation are nonischemic (such as mitral regurgitation due to chordal rupture or aortic regurgitation) may poorly tolerate the contrast medium and the time needed for catheterization. Patients with aortic dissection rarely require cardiac catheterization, and the procedure may worsen the dissection. When the aortic dissection is diagnosed by use of echocardiography, cross-sectional imaging can fully reveal the dissection anatomy; however, in most instances, cross-sectional imaging leads to the diagnosis before echocardiography does.22
Because the morbidity and mortality rates of acute severe regurgitation are high if it is untreated, rapid diagnosis is crucial. Definitive treatment should not be delayed.
Medical Therapy
Aortic Valve Regurgitation. The treatment for acute aortic regurgitation is aortic valve repair or replacement. Medical therapy can stabilize the patient's condition before surgery, but it is not a substitute for surgery.23,24 Vasodilators such as sodium nitroprusside can be used to improve forward flow, and inotropic agents such as dobutamine can improve the patient's cardiac output. Intra-aortic balloon pumps (IABPs) tend to be contraindicated in acute aortic regurgitation, because balloon inflation during diastole may impair LV hemodynamics. Balloon use is specifically contraindicated in cases of aortic dissection.
Mitral Valve Regurgitation. Organic causes of mitral regurgitation are managed similarly to those of acute aortic regurgitation. Supportive therapy can provide patients with stability and resuscitation en route to the operating room. The use of IABPs can be beneficial. In organic mitral regurgitation, the fundamental abnormality is structural and therefore requires surgical intervention. In functional mitral regurgitation, medical therapy may be sufficient to avoid immediate operative intervention; however, surgery should be considered if therapy fails.
In patients with acute functional mitral regurgitation, therapy is directed at restoring blood flow to the territory at risk. Severe mitral regurgitation affects 7% of patients who experience cardiogenic shock.25 Acute mitral regurgitation in these patients is a sign of a poor prognosis: an observed mortality rate of 55% improved to only 39% in patients who were selected for emergency surgery.25 The SHOCK trial determined that early revascularization improved outcomes at 6 months in patients with cardiogenic shock and acute MI. Upon further analysis, the prognostic importance of mitral regurgitation to the short- and long-term survival of such patients was inversely related to the degree of regurgitation.26 This suggests that aggressive treatment with early revascularization may improve survival in patients with severe mitral regurgitation after acute MI.27 Therefore, if revascularization is needed, the presence of severe mitral regurgitation should prompt surgical intervention.
Acute mitral regurgitation in the presence of acute cardiomyopathy or decompensated heart failure can respond to aggressive heart-failure therapy. The use of IABPs is effective in patients with acute functional mitral regurgitation of any cause, and IABPs are particularly useful in patients with underlying myocardial ischemia or cardiomyopathy. Although the role of LV assist devices has not been well studied in acute mitral regurgitation, these devices may be used in cases of decompensated heart failure that does not respond to medical therapy.
Valvular thrombosis that results in acute regurgitation sometimes responds to thrombolytic therapy. However, in patients whose prosthetic valve dysfunction is severe and symptomatic, the ACC/AHA and ESC guidelines recommend surgery first. Thrombolytic agents should be reserved to treat patients who have confirmed valvular thrombosis when surgery is risky or unavailable, or when the thrombus burden is small and the patient has few symptoms.1–3
Surgical Treatment of Acute Aortic Valve Regurgitation
Anesthetic Considerations. Inducing anesthesia in a critically ill patient who has acute aortic regurgitation can be a challenge. Low diastolic pressure, tachycardia, and increased wall stress can impair coronary flow. If anesthesia further reduces blood pressure, coronary ischemia can exacerbate the patient's unstable condition. Therefore, the anesthesiologist should try to prevent tachycardia or hypotension during intubation and the induction of anesthesia. Hemodynamic monitoring is also crucial, and in many cases monitoring lines will have been placed before the patient's arrival in the operating room. The appropriate use of inotropic drugs, vasoconstrictors, sedatives, and anesthetic agents can increase the chances of optimal outcomes. The surgical team should be present during induction, ready to initiate support if the patient experiences cardiopulmonary collapse.
Surgical Considerations. Surgical options depend upon the pathophysiology and anatomy of the valve and the expected long-term outcome of correcting the acute aortic regurgitation. Myocardial protection is crucial, because the incompetent aortic valve and any associated aortic root disorder makes the antegrade delivery of cardioplegic solution ineffective or even dangerous. The reliable placement of a coronary sinus cannula for retrograde delivery of cardioplegic solution is essential. Necessity may justify placing bicaval cannulae and directly intubating the coronary sinus. Then, after the aortic valve and root have been properly evaluated, adjuvant cardioplegic solution can be delivered from a handheld apparatus through the coronary ostia. A LV vent placed through the right superior pulmonary vein may aid ventricular decompression and diastolic arrest. The value of other protective adjuncts, such as topical cooling or systemic hypothermia on cardiopulmonary bypass (CPB), is unclear.
Aortic Valve Endocarditis
Aortic valve endocarditis can prevent leaflet coaptation, lead to leaflet erosion, or affect the aortic annulus—singly or in combination (Fig. 3). During planning for surgery, directly evaluating the aortic annulus by use of intraoperative TEE is important. Annular integrity can be compromised by abscess formation and endocarditic erosion. In cases of annular involvement, the use of a homograft is typically recommended. Although aortic homografts are susceptible to prosthetic valve endocarditis, the additional tissue included with the homograft (the LV outflow tract and the anterior leaflet of the mitral valve) is sometimes essential for successful aortic root reconstruction. When endocarditis is limited to the valve leaflets, procedures beyond leaflet excision and aortic valve replacement are not indicated. Because the rates of infection are similar between mechanical and bioprosthetic replacement valves, after native-valve endocarditis, either type is suitable.28
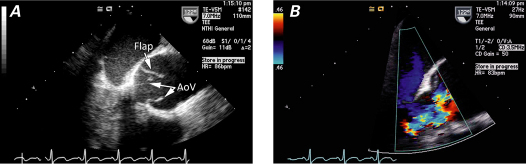
Fig. 3 Transthoracic echocardiograms (parasternal long-axis views) of aortic valve endocarditis show A ) the aortic valve, which is thickened and distorted by the vegetation (arrow), and B ) severe aortic regurgitation (arrow), in color-flow Doppler. Transesophageal echocardiograms (intraoperative midesophageal views) of the aortic valve show C ) a clear subvalvular vegetation (Veg) and the suggestion of perforation (Perf), and D ) findings that appear to be consistent with a perforation of the leaflet (arrow), in color-flow Doppler. Ao = aorta; LA = left atrium; LV = left ventricle
Some centers use a pulmonary autograft (the Ross procedure), particularly in children. However, as in elective aortic valve surgery, the benefits and risks of the Ross repair are debatable. In theory, a durable aortic valve replacement will grow with a child and not require future intervention. On the other hand, “single-valve disease” becomes “double-valve disease,” and data suggest that the new aortic valve requires replacement in 10% to 20% of patients within 10 years of the operation. Thus, the long-term outcomes may offset the theoretical benefit.29–31 Some patients cannot tolerate additional CPB time, so Ross repair is limited to a specific patient population in centers that have had extensive surgical experience. Finally, depending upon the clinical status of the patient and the condition of the aortic root, a 2-staged approach may be beneficial: simple valve replacement, followed by root reconstruction after the patient has recovered from multisystem organ failure.
Aortic Dissection
Stanford Type A dissection can lead to aortic insufficiency through various pathophysiologic processes, and the cause dictates the treatment (Fig. 4). When an aortic valve is trileaflet, reconstructing the aortic root with valve resuspension and replacing the ascending aorta with an interpositional graft can frequently correct valvular regurgitation. This is accomplished by reestablishing the commissural heights at the sinotubular junction and reattaching the sinuses of Valsalva to the aortic wall. When an aortic valve is bicuspid or when there is an aneurysm of the ascending aorta, correction can be achieved by performing a modified Bentall procedure, using a valved conduit with direct coronary reimplantation. A study of aortic root replacement with the use of mechanical conduits and aortic allografts revealed no significant difference in 1- and 5-year survival rates on the basis of conduit choice, including in patients with aortic dissection.32 Aortic root remodeling surgery (the Yacoub procedure) and aortic root reimplantation (the David procedures) have also been used successfully in aortic dissection. However, coronary ischemia can occur due to coronary artery involvement from aortic dissection or technical problems with coronary artery reimplantation, and surgical expertise is necessary for optimal outcomes.
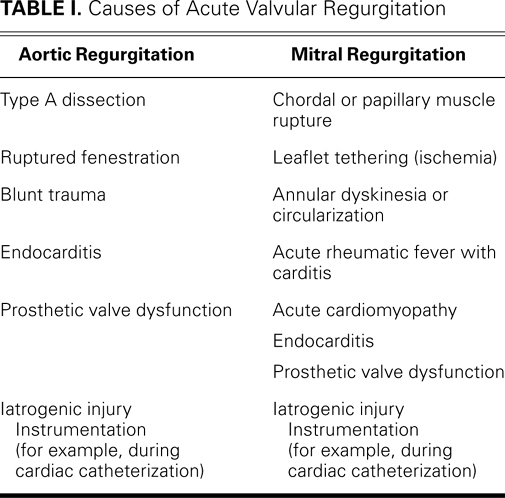
Fig. 4 Aortic dissection. Transesophageal echocardiograms (midesophageal long-axis views) of the aortic valve show A ) a flap in the aortic root with apparently normal aortic valve leaflets (AoV), and B ) severe aortic regurgitation, in color-flow Doppler.
Trauma or Ruptured Fenestration
Leaflet disruption can occur consequent to trauma or ruptured fenestration. Anatomy permitting, the leaflets can be successfully repaired—most commonly by patching with autologous pericardium. Other pericardial substitutes have also been used. Nonetheless, valve replacement is the safest choice for most surgeons. In blunt chest trauma, aortic valve regurgitation most often occurs because of leaflet perforation, dissection, or loss of commissural suspension.33 The choice of repair, replacement, or reconstruction depends upon the surgical anatomy.
Surgical Treatment of Acute Mitral Valve Regurgitation
Anesthetic Considerations. Patients with acute mitral regurgitation can experience acute cardiopulmonary collapse at any time; however, the instability of their condition may not be as clear as it is in patients with acute aortic regurgitation. The surgical team should be present during the induction of anesthesia, in case CPB is urgently needed. Acute mitral regurgitation from profound, ongoing myocardial ischemia often requires additional anesthetic techniques that will optimize myocardial oxygen supply and demand, preload and afterload, and coronary perfusion pressure. The preoperative placement of an IABP can improve coronary perfusion and mitigate the effects of anesthetic induction.
Surgical Considerations. Decisions in regard to the treatment of acute mitral regurgitation are strongly influenced by the cause of the regurgitation. In 1 series, the causes were acute MI in 45% of cases, degenerative valvular disease in 26%, and infective endocarditis in 28%. Although the technical treatment of the mitral valve may be similar, each cause requires a different approach and has a different outcome.22 Furthermore, unlike aortic regurgitation—wherein repair is rarely possible—mitral regurgitation can be treated by means of repair or replacement.
Adequate surgical exposure of the mitral valve is crucial. Exposure is often difficult when regurgitation is acute and the left atrium is not dilated; therefore, surgery must be approached carefully. Expeditious use of CPB, optimal myocardial protection, and excellent valve exposure yield the best results. The intraoperative approach to the mitral valve is also crucial: a variety of techniques should be considered, particularly when endocarditis affects multiple structures or the annulus, or when extensive annular reconstruction is needed. Approaches include a lateral Sondergaard incision in the groove in the left atrium, transatrial incisions, left atrial dome incisions, or transection of the superior vena cava with a more anterolateral approach to the left atrium. This last is particularly useful when endocarditis is noticeably destructive and more extensive annular reconstruction is required. Although median sternotomy is typical, experienced surgeons can execute less invasive approaches.
Mitral valve repair is almost always preferable to replacement. Therapeutic options, technical feasibility, and surgical priorities depend upon cause, acute pathophysiology, underlying pathology, and comorbidities. If the initial repair is unsatisfactory, further CPB and either repeat repair or valve replacement is necessary. Repair should therefore be undertaken when procedural success is likely, because a hemodynamically unstable patient might not be able to tolerate additional CPB. Influential factors include papillary muscle rupture; chordal rupture; annular dilation; hibernating, stunned, or infarcted myocardium; and the severity of coronary artery disease. Repair techniques include ring annuloplasty, annular reconstruction with pericardium, leaflet resection, leaflet reconstruction with pericardium, chordal replacement, vegetation excision, and edge-to-edge (Alfieri) approximation. Valve repair is more usual when acute annular dilation is the cause, such as in ischemia, or when the pathology involves the posterior leaflet only, such as in myxomatous valve disease or endocarditis with or without annular dilation. In annular involvement with infection or in anterior leaflet involvement with degenerative disease, the ability to repair the mitral valve dramatically decreases.
Having to replace a mitral valve after an unsuccessful repair should not be viewed as a failure. Patients with acute regurgitation are less likely to tolerate repeat exploration and repair. The loss of tolerance is more pronounced after a complex, time-consuming repair attempt, as in cases involving tissue destruction due to endocarditis. Frequently, annular involvement precludes simple repair with predictably good initial results.
In valve replacement, adequate débridement can avoid periprosthetic leaks due to inadequate visualization, poor tissue quality, or an undrained abscess. Preservation of the posterior and anterior chordae tendineae is ideal, because the LV has not adapted as in chronic regurgitation. Either a mechanical valve or a bioprosthesis can be used in cases of degenerative, ischemic, or infectious disease. Mechanical prostheses are often preferred because of their durability, low profile, and ease of insertion.
Ischemic Mitral Regurgitation
Ischemic mitral regurgitation is caused by wall-motion abnormalities that in turn cause tethering, papillary muscle dysfunction, or annular dilation. In myocardial ischemia, revascularization is mandatory. Cardiac catheterization is important in the evaluation and stabilization of patients who present with an acute coronary syndrome, with or without cardiogenic shock. Careful review of the preoperative coronary angiograms is essential during preparation for coronary artery bypass grafting (CABG). In patients who are hemodynamically unstable, too much time may be needed to prepare the internal mammary artery, and CABG might be best completed with the use of venous conduits. In addition, myocardial protection in the presence of acute MI is challenging. Either antegrade or retrograde delivery of cardioplegic solution is feasible; antegrade delivery through the newly placed venous conduits can increase myocardial protection. In contrast, particularly when ischemia is acute, the metabolically enhanced, slow induction of warm-blood cardioplegic solution can avoid systolic contracture of the LV (“stone heart” syndrome).
Controlled and potentially prolonged reperfusion at the conclusion of the operation may enable the recovery of myocardial function. For the same purpose, intra-aortic balloon counterpulsation is typically maintained for at least 24 hours postoperatively. Papillary muscle rupture, a rare complication of MI, occurs in 1% to 3% of MIs and is associated with a mortality rate of 80% with medical therapy alone.34,35 Historically, operative mortality rates were as high as 67%, and patients were frequently denied surgery for this reason. With the addition of CABG to mitral valve replacement or repair, the operative mortality rate is now less than 10%.36
Mitral Valve Endocarditis
Acute mitral valve regurgitation consequent to endocarditis requires operative débridement and reconstruction. The therapeutic approach is driven by the principle that all sites of active infection must be excised. The decision to repair the leaflet, to replace the chordae or the entire valve, or to reconstruct the annulus is best made after débridement. It can be especially challenging to reconstruct the fibrous trigone of the heart, which involves the annular support of both the aortic and mitral valves. The tissues are edematous and friable, and even the most experienced surgeon may find it difficult to achieve a 3-dimensional view. The use of aortic homografts may be optimal when both the mitral and aortic annuli show active infection or abscess. Repeat mitral valve surgery, such as in prosthetic valve endocarditis, poses a unique challenge. Even gentle manipulation of the mitral annulus, coupled with ongoing infection and previous scarring, can result in atrioventricular disruption. This catastrophic event is high-risk and must be anticipated.
Clinical Outcomes
Broadly applicable clinical outcomes in acute valvular regurgitation are difficult to ascertain, because the patient population is diverse. The causes of acute valvular regurgitation and the times to intervention vary greatly, the complexity of intraoperative repair is unique to each patient, and the patients tend to be ill before and after surgery; therefore, outcomes depend upon multiple factors. Despite differences in the incidence and prevalence of each disease, selection of patients, the era of reporting, and surgeons' experience, data are available for certain disease entities. Recently reported outcomes of endocarditis in a multinational patient cohort37 emphasized the high mortality rate of the disease, despite modern medical and surgical therapy. Although mortality rates have decreased over the years, the 30-day mortality rate was 15% to 20% in that study.37 In another series, 48% of patients underwent surgery; more deaths occurred in those with pulmonary edema, but fewer occurred in those who underwent surgery compared with medical management alone.38 The SHOCK trial described how well early revascularization improved long-term outcomes in patients with acute mitral regurgitation and acute MI.27 In a 2005 study of Stanford Type A dissection, the mortality rate was 31.4% in patients in unstable condition who underwent surgery (the study did not distinguish between patients with and without valvular disease).39 Cardiogenic shock increases the risk of poor outcomes; accordingly, operative intervention before the onset of shock is a means of improving the outcomes. The cause of acute regurgitation also influences outcomes, as described in a recent report: the overall 30-day mortality rate was 22.5%, with the best outcomes in patients who had degenerative disease.22
Conclusion
Acute cardiac valvular regurgitation is a surgical emergency that requires accurate diagnosis and rapid intervention for an optimal outcome. Because examination findings in acute regurgitation differ from and are often more subtle than those in chronic regurgitation, the diagnosis is often missed when a patient presents with dyspnea and shock. A high degree of suspicion and early echocardiography are important in rapid diagnosis, and surgical treatment should be performed as promptly as possible. Although surgical mortality rates remain high, operative treatment remains the standard of care and provides the best chance for a successful outcome.
References
- 1.Bonow RO, Carabello BA, Chatterjee K, de Leon AC Jr, Faxon DP, Freed MD, et al. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients with Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2008;118(15):e523–661. [DOI] [PubMed]
- 2.American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, Bonow RO, Carabello BA, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients with Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons [published errata appear in Circulation 2007;115(15):e409 and Circulation 2010;121(23):e443]. Circulation 2006;114(5):e84–231. [DOI] [PubMed]
- 3.Vahanian A, Baumgartner H, Bax J, Butchart E, Dion R, Filippatos G, et al. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J 2007;28(2):230–68. [DOI] [PubMed]
- 4.Stout KK, Verrier ED. Acute valvular regurgitation. Circulation 2009;119(25):3232–41. [DOI] [PubMed]
- 5.Mohammadi S, Baillot R, Voisine P, Mathieu P, Dagenais F. Structural deterioration of the Freestyle aortic valve: mode of presentation and mechanisms. J Thorac Cardiovasc Surg 2006;132(2):401–6. [DOI] [PubMed]
- 6.Kan CD, Yang YJ. Traumatic aortic and mitral valve injury following blunt chest injury with a variable clinical course. Heart 2005;91(5):568–70. [DOI] [PMC free article] [PubMed]
- 7.Akiyama K, Hirota J, Taniyasu N, Maisawa K, Kobayashi Y, Tsuda M. Pathogenetic significance of myxomatous degeneration in fenestration-related massive aortic regurgitation. Circ J 2004;68(5):439–43. [DOI] [PubMed]
- 8.Glasson JR, Komeda M, Daughters GT, Bolger AF, Karlsson MO, Foppiano LE, et al. Early systolic mitral leaflet “loitering” during acute ischemic mitral regurgitation. J Thorac Cardiovasc Surg 1998;116(2):193–205. [DOI] [PubMed]
- 9.Parodi G, Del Pace S, Salvadori C, Carrabba N, Olivotto I, Gensini GF; Tuscany Registry of Tako-Tsubo Cardiomyopathy. Left ventricular apical ballooning syndrome as a novel cause of acute mitral regurgitation. J Am Coll Cardiol 2007; 50(7):647–9. [DOI] [PubMed]
- 10.Chandrasegaram MD, Celermajer DS, Wilson MK. Apical ballooning syndrome complicated by acute severe mitral regurgitation with left ventricular outflow obstruction–case report. J Cardiothorac Surg 2007;2:14. [DOI] [PMC free article] [PubMed]
- 11.Brunetti ND, Ieva R, Rossi G, Barone N, De Gennaro L, Pellegrino PL, et al. Ventricular outflow tract obstruction, systolic anterior motion and acute mitral regurgitation in Tako-Tsubo syndrome. Int J Cardiol 2008;127(3):e152–7. [DOI] [PubMed]
- 12.Gentles TL, Colan SD, Wilson NJ, Biosa R, Neutze JM. Left ventricular mechanics during and after acute rheumatic fever: contractile dysfunction is closely related to valve regurgitation. J Am Coll Cardiol 2001;37(1):201–7. [DOI] [PubMed]
- 13.Veasy LG, Tani LY. A new look at acute rheumatic mitral regurgitation. Cardiol Young 2005;15(6):568–77. [DOI] [PubMed]
- 14.Reimold SC, Maier SE, Fleischmann KE, Khatri M, Piwnica-Worms D, Kikinis R, Lee RT. Dynamic nature of the aortic regurgitant orifice area during diastole in patients with chronic aortic regurgitation. Circulation 1994;89(5):2085–92. [DOI] [PubMed]
- 15.Mann T, McLaurin L, Grossman W, Craige E. Assessing the hemodynamic severity of acute aortic regurgitation due to infective endocarditis. N Engl J Med 1975;293(3):108–13. [DOI] [PubMed]
- 16.Eusebio J, Louie EK, Edwards LC 3rd, Loeb HS, Scanlon PJ. Alterations in transmitral flow dynamics in patients with early mitral valve closure and aortic regurgitation. Am Heart J 1994;128(5):941–7. [DOI] [PubMed]
- 17.Figueras J, Pena C, Soler-Soler J. Thirty day prognosis of patients with acute pulmonary oedema complicating acute coronary syndromes. Heart 2005;91(7):889–93. [DOI] [PMC free article] [PubMed]
- 18.D'Aloia A, Faggiano P, Brentana L, Boldini A, Procopio R, Racheli M, Dei Cas L. A difficult diagnosis: right unilateral cardiogenic pulmonary edema. Usefulness of biochemical markers of heart failure for the correct diagnosis. Ital Heart J 2005;6(9):771–4. [PubMed]
- 19.Jeresaty RM. Left ventricular function in acute non-ischaemic mitral regurgitation. Eur Heart J 1991;12 Suppl B:19–21. [DOI] [PubMed]
- 20.Reimold SC, Byrne JG, Caguioa ES, Lee CC, Laurence RG, Peigh PS, et al. Load dependence of the effective regurgitant orifice area in a sheep model of aortic regurgitation. J Am Coll Cardiol 1991;18(4):1085–90. [DOI] [PubMed]
- 21.Wisenbaugh T, Berk M, Essop R, Middlemost S, Sareli P. Effect of mitral regurgitation and volume loading on pressure half-time before and after balloon valvotomy in mitral stenosis. Am J Cardiol 1991;67(2):162–8. [DOI] [PubMed]
- 22.Lorusso R, Gelsomino S, De Cicco G, Beghi C, Russo C, De Bonis M, et al. Mitral valve surgery in emergency for severe acute regurgitation: analysis of postoperative results from a multicentre study. Eur J Cardiothorac Surg 2008;33(4):573–82. [DOI] [PubMed]
- 23.Evangelista A, Tornos P, Sambola A, Permayer-Miralda G. Role of vasodilators in regurgitant valve disease. Curr Treat Options Cardiovasc Med 2006;8(6):428–34. [DOI] [PubMed]
- 24.Bekeredjian R, Grayburn PA. Valvular heart disease: aortic regurgitation. Circulation 2005;112(1):125–34. [DOI] [PubMed]
- 25.Thompson CR, Buller CE, Sleeper LA, Antonelli TA, Webb JG, Jaber WA, et al. Cardiogenic shock due to acute severe mitral regurgitation complicating acute myocardial infarction: a report from the SHOCK Trial Registry. SHould we emergently revascularize Occluded Coronaries in cardiogenic shocK? J Am Coll Cardiol 2000;36(3 Suppl A):1104–9. [DOI] [PubMed]
- 26.Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med 1999;341 (9):625–34. [DOI] [PubMed]
- 27.Picard MH, Davidoff R, Sleeper LA, Mendes LA, Thompson CR, Dzavik V, et al. Echocardiographic predictors of survival and response to early revascularization in cardiogenic shock. Circulation 2003;107(2):279–84. [DOI] [PubMed]
- 28.Moon MR, Miller DC, Moore KA, Oyer PE, Mitchell RS, Robbins RC, et al. Treatment of endocarditis with valve replacement: the question of tissue versus mechanical prosthesis. Ann Thorac Surg 2001;71(4):1164–71. [DOI] [PubMed]
- 29.Pasquali SK, Shera D, Wernovsky G, Cohen MS, Tabbutt S, Nicolson S, et al. Midterm outcomes and predictors of reintervention after the Ross procedure in infants, children, and young adults. J Thorac Cardiovasc Surg 2007;133(4):893–9. [DOI] [PubMed]
- 30.Elkins RC, Thompson DM, Lane MM, Elkins CC, Peyton MD. Ross operation: 16-year experience. J Thorac Cardiovasc Surg 2008;136(3):623–30, 630.e1–5. [DOI] [PubMed]
- 31.de Kerchove L, Rubay J, Pasquet A, Poncelet A, Ovaert C, Pirotte M, et al. Ross operation in the adult: long-term outcomes after root replacement and inclusion techniques. Ann Thorac Surg 2009;87(1):95–102. [DOI] [PubMed]
- 32.Lima B, Hughes GC, Lemaire A, Jaggers J, Glower DD, Wolfe WG. Short-term and intermediate-term outcomes of aortic root replacement with St. Jude mechanical conduits and aortic allografts. Ann Thorac Surg 2006;82(2):579–85. [DOI] [PubMed]
- 33.Symbas PJ, Horsley WS, Symbas PN. Rupture of the ascending aorta caused by blunt trauma. Ann Thorac Surg 1998;66 (1):113–7. [DOI] [PubMed]
- 34.Wei JY, Hutchins GM, Bulkley BH. Papillary muscle rupture in fatal acute myocardial infarction: a potentially treatable form of cardiogenic shock. Ann Intern Med 1979;90(2):149–52. [DOI] [PubMed]
- 35.Kishon Y, Oh JK, Schaff HV, Mullany CJ, Tajik AJ, Gersh BJ. Mitral valve operation in postinfarction rupture of a papillary muscle: immediate results and long-term follow-up of 22 patients. Mayo Clin Proc 1992;67(11):1023–30. [DOI] [PubMed]
- 36.Russo A, Suri RM, Grigioni F, Roger VL, Oh JK, Mahoney DW, et al. Clinical outcome after surgical correction of mitral regurgitation due to papillary muscle rupture. Circulation 2008;118(15):1528–34. [DOI] [PubMed]
- 37.Murdoch DR, Corey GR, Hoen B, Miro JM, Fowler VG Jr, Bayer AS, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 2009;169(5):463–73. [DOI] [PMC free article] [PubMed]
- 38.Hoen B, Alla F, Selton-Suty C, Beguinot I, Bouvet A, Briancon S, et al. Changing profile of infective endocarditis: results of a 1-year survey in France. JAMA 2002;288(1):75–81. [DOI] [PubMed]
- 39.Trimarchi S, Nienaber CA, Rampoldi V, Myrmel T, Suzuki T, Mehta RH, et al. Contemporary results of surgery in acute type A aortic dissection: The International Registry of Acute Aortic Dissection experience. J Thorac Cardiovasc Surg 2005; 129(1):112–22. [DOI] [PubMed]