Abstract
Paravalvular leaks are well-recognized sequelae of mechanical aortic valve implantation. Clinical manifestations include hemolysis, arrhythmias, and congestive heart failure. Frequently, patients who receive mechanical aortic valves are poor candidates for repeat valve surgery, and the reoperative mortality rate is high. Percutaneous intervention has recently become an alternative to surgery in selected patients. Herein, we describe the percutaneous closure of an aortic paravalvular leak in a 45-year-old man who had undergone 2 aortic valve replacements with mechanical valves. The patient, who was at high surgical risk due to comorbidities, underwent the implantation of 2 AMPLATZER devices with the use of real-time 2- and 3-dimensional transesophageal echocardiography. The early outcome of the procedure was favorable and without sequelae. To our knowledge, this is the 1st report of the closing of an aortic paravalvular leak with the use of 2 closure devices and real-time echocardiographic guidance.
Key words: Aortic valve insufficiency/complications/diagnosis/etiology; echocardiography, transesophageal; heart catheterization; heart valve diseases/therapy/ultrasonography; heart valve prosthesis/adverse effects/standards; postoperative complications/therapy/ultrasonography; prosthesis failure; risk assessment; risk factors; treatment outcome
Paravalvular leaks are well-recognized sequelae of mechanical aortic valve implantation. In 15% to 50% of cases, small paravalvular leaks are detected, but these are typically of minimal clinical significance.1 In 1% to 5% of cases, however, the leaks are associated with hemolysis, arrhythmias, or congestive heart failure.2–7 Patients who receive mechanical aortic valves can be poor candidates for repeat valve surgery: the reoperative mortality rate is as high as 15%.6 Palliative medical therapy is largely intended to alleviate the symptoms of congestive heart failure.
Recently, endovascular closure devices for structural and congenital heart disease have found an off-label use in the percutaneous repair of paravalvular leakage in selected high-risk surgical patients. In 64 reported cases, the overall short-term success rate was approximately 85%; repeat procedures were needed in approximately 25% of the patients. Over 70% of the described cases involved mitral paravalvular leaks.1–5 Herein, we describe the percutaneous treatment of an aortic paravalvular leak with the use of real-time echocardiography to guide the implantation of 2 closure devices.
Case Report
In January 2009, a 45-year-old man with a history of 2 aortic valve replacements was referred to our institution with progressive shortness of breath and a severe paravalvular leak. The patient had undergone aortic valve replacement with a mechanical valve at age 20 years for what he described as rheumatic disease. At age 43 years, he underwent repeat aortic valve replacement with a St. Jude mechanical valve after experiencing endocarditis and an embolic cerebrovascular accident with residual right-sided hemiparesis. During the 6 months after that surgery, he developed 2 subdural hematomas that required surgical evacuation and drainage in the setting of a supratherapeutic international normalized ratio due to his need for warfarin therapy. During the next 18 months, he continued to experience dyspnea from worsening heart failure that was caused by the valve disease. Due to his comorbidities, he was considered to be a high-risk surgical candidate for a 3rd aortic valve replacement, so he was referred to our institution for possible percutaneous closure of the paravalvular leak.
At presentation, the patient was cachectic and afebrile, with a blood pressure of 98/55 mmHg, a heart rate of 93 beats/min, a respiratory rate of 16 breaths/min, and an oxygen saturation of 100% on room air. He had mildly elevated jugular venous pressure, clear lungs on auscultation, and a high-pitched decrescendo murmur at the left sternal border in early diastole. Laboratory analysis showed no evidence of hemolysis, and 4 sets of blood cultures grew no bacteria. A transesophageal echocardiogram (TEE) revealed a cavity (1.2 × 1 cm at largest diameter) below the aortic valve ring with severe paravalvular leakage draining anteriorly into the left ventricle (Fig. 1).
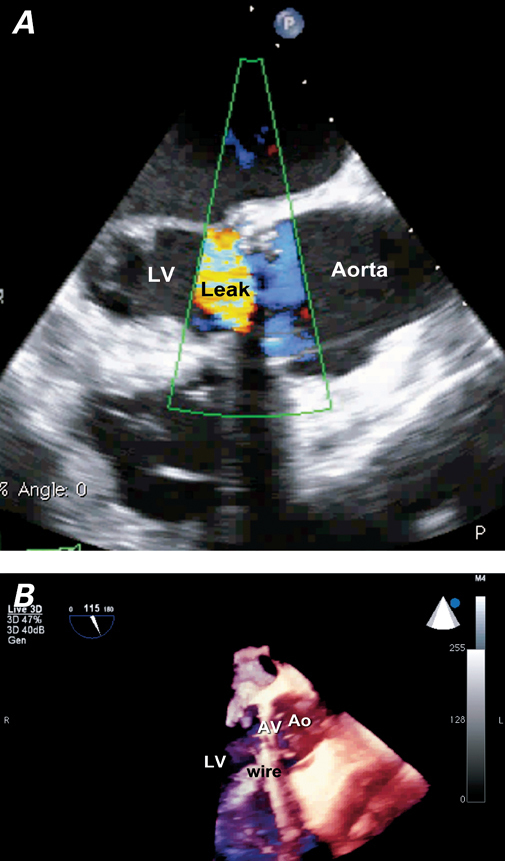
Fig. 1 Transesophageal echocardiography shows a severe paravalvular leak, which communicates with a cavity (1.2 × 1 cm in largest diameter) below the aortic valve ring anteriorly and drains into the left ventricle (LV). A ) Two-dimensional image with color-flow Doppler shows the superiorly directed leak. B ) Three-dimensional image reveals a cavity below the aortic valve anteriorly. Ao = aorta; AV = aortic valve
The patient was started on diuretic therapy and prophylactic antibiotics. After a multidisciplinary discussion that included cardiologists, cardiothoracic surgeons, primary-care physicians, and the patient, it was concluded that reoperation posed an excessive risk (Society of Thoracic Surgeons operative risk score, >30%).8
The patient agreed to undergo percutaneous closure of the paravalvular leak. The procedure was performed while he was under general anesthesia. Fluoroscopy and real-time 2-dimensional (2D) and 3-dimensional (3D) TEE were used along with right-heart catheterization that revealed normal right- and left-sided filling pressures and normal cardiac output—positive results of the diuretic therapy. Coronary angiography showed no significant coronary artery disease; however, the native left anterior descending coronary artery (LAD) was small, and a larger, anomalous LAD branch arose from the proximal right coronary artery and did not take an intra-arterial course (Fig. 2). Aortography confirmed grade 4 aortic regurgitation through a paravalvular leak.
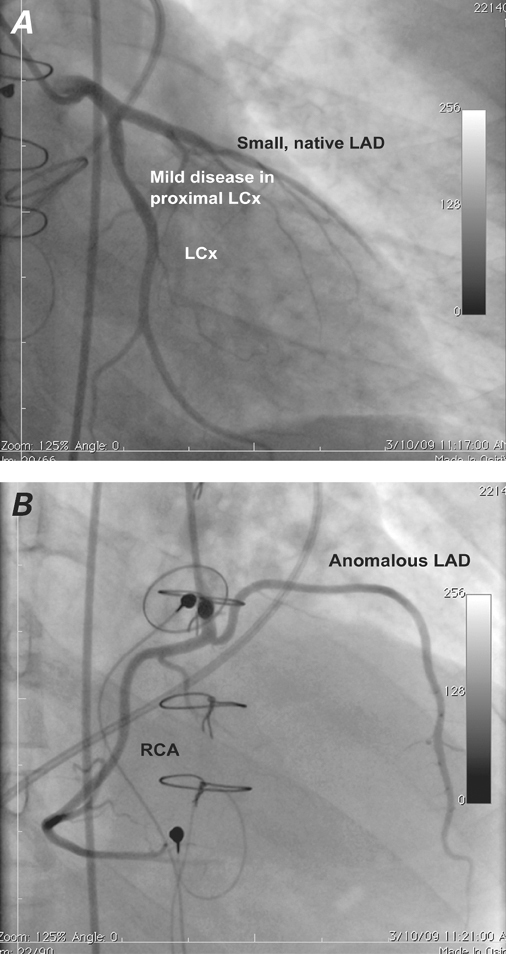
Fig. 2 Coronary angiograms (left anterior oblique views) show no significant coronary artery disease. Also shown are A ) the bifurcation of the left main coronary artery into the left circumflex coronary artery (LCx) and the small, native left anterior descending coronary artery (LAD); and B ) the large, anomalous LAD branch that arises from the proximal right coronary artery (RCA).
Intraprocedural TEE also indicated paravalvular leakage between the aorta and the left ventricular outflow tract through a cavity in the right anterior cusp of the aortic valve. The cavity was crossed with use of a 6F multipurpose catheter and a 0.035-in Terumo Glidewire® (Terumo Medical Corporation; Somerset, NJ). Selective angiography through the defect confirmed the presence of a complex paravalvular fistula to a large cavity below the valve ring, with drainage into the left ventricle (Fig. 3). The use of real-time 2D and 3D TEE along with standard angiography enabled us to estimate the size of the paravalvular leak and thus to attempt closure with a 10-mm AMPLATZER® Vascular Plug II (AGA Medical Corporation; Plymouth, Minn).
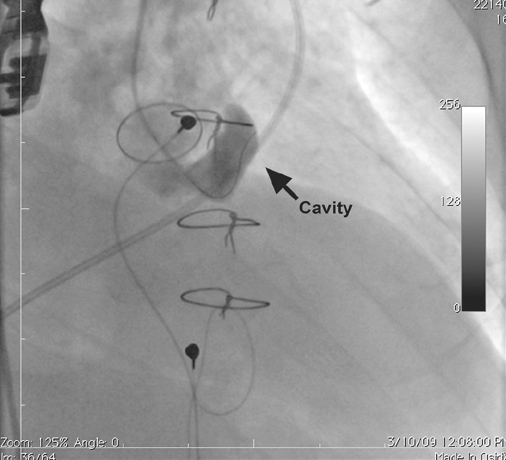
Fig. 3 Selective angiography through the defect shows a complex paravalvular fistula to an inferior cavity below the aortic valve ring. A 6F multipurpose catheter has entered the cavity.
The plug was positioned. However, before its deployment, repeat angiography and TEE showed a substantial residual leak adjacent to the plug, suggesting that the plug was too small. It was removed without difficulty. A 7F delivery sheath was inserted, and, due to its more favorable design with 2 concentric discs, a 6-mm AMPLATZER® Muscular VSD Occluder (AGA Medical) was deployed accurately and easily. However, repeat angiography and TEE revealed a residual leak (Fig. 4).
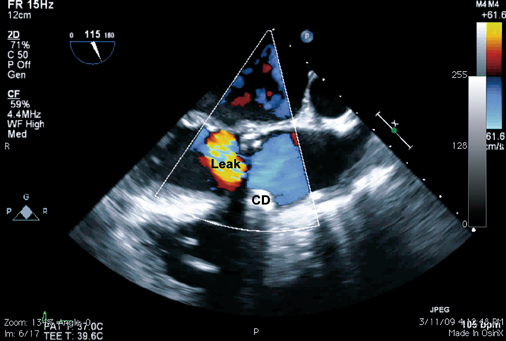
Fig. 4 Two-dimensional transesophageal echocardiogram shows a residual paravalvular leak after deployment of the 1st closure device (CD), a 6-mm AMPLATZER® Muscular VSD Occluder. A high-velocity regurgitant jet is seen superiorly.
We decided to cross the area adjacent to the Amplatzer VSD occluder to deploy a 2nd closure device. The course of the Glidewire was verified by means of 3D TEE. The crossing was challenging, because the Glidewire repeatedly prolapsed into the subaortic cavity. A Quick-Cross® 0.035-in guiding catheter (The Spectranetics Corporation; Colorado Springs, Colo) was successfully positioned over the Glidewire. The Glidewire was then exchanged for a stiffer 0.035-in Rosen wire, and the delivery of a 6F multipurpose catheter enabled the placement of a 10-mm Amplatzer Vascular Plug II adjacent to the Amplatzer VSD occluder. Echocardiography showed excellent device positioning and complete closure of the paravalvular leak (Fig. 5). Angiography showed excellent positioning, no interference with the adjacent valve leaflets, and no evidence of regurgitation. Coronary angiography revealed no impingement on the coronary arteries.
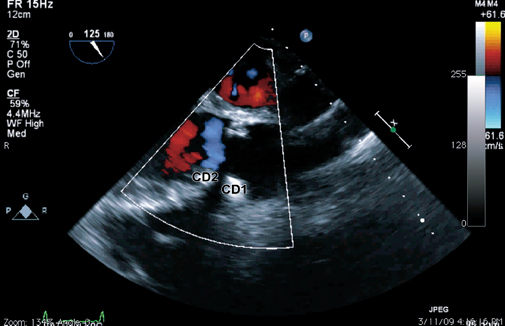
Fig. 5 Two-dimensional transesophageal echocardiogram (at 115°) shows the closure of the paravalvular leak after a 10-mm AMPLATZER® Vascular Plug II (CD2) was deployed near the 6-mm AMPLATZER Muscular VSD Occluder (CD1).
The patient was treated with heparin throughout the procedure to prevent bleeding. Afterwards, he received ongoing warfarin therapy and was discharged from the hospital after 5 days with no sequelae. A follow-up cardiac computed tomogram was obtained to define the anomalous LAD and to obtain a 3D image showing the relationship between the 2 closure devices. The anomalous LAD coursed in front of the pulmonary artery with no impingement, and no reconstructive surgery was thought to be necessary. The patient was scheduled to return to the clinic in 6 to 8 weeks for repeat echocardiography, and for evaluations at 3, 6, and 12 months thereafter; however, he attended no appointments and was lost to follow-up.
Discussion
The percutaneous repair of paravalvular leaks is feasible and may be the treatment of choice in selected patients who are at high operative risk.2-5 To our knowledge, this is the 1st reported case in which 2 closure devices were deployed consecutively under real-time 2D and 3D TEE guidance to close an aortic paravalvular leak.
Previously reported sequelae of percutaneous repair include device dislodgment, impingement upon the mechanical valve struts, infection, bleeding, cardiac tamponade, stroke, and death.1–7,9 Although the data are sparse in regard to procedural sequelae, the reported mortality rate is less than 2%. No reports of short- and long-term follow-up have been published.
Many questions and controversies surround the largely investigational approach of percutaneous repair, including appropriate follow-up care, the optimal timing of repeat imaging, antibiotic prophylaxis, and antiplatelet therapy. Antibiotic prophylaxis is recommended for all patients who have received mechanical replacement valves. Our patient resumed warfarin therapy; however, in view of his history of subdural hematomas, we opted not to add aspirin or clopidogrel to his regimen.
In view of the risks of repeat valve replacement, we believe that cardiothoracic surgeons and interventional cardiologists should collaborate in the evaluation of percutaneous treatment for a high-risk patient who has morbidities, and that real-time TEE should be used for intraprocedural guidance. Using fluoroscopy and TEE for optimal device positioning and for real-time evaluation of efficacy is essential to an optimal procedural outcome.
References
- 1.Bhindi R, Bull S, Schrale RG, Wilson N, Ormerod OJ. Surgery insight: percutaneous treatment of prosthetic paravalvular leaks. Nat Clin Pract Cardiovasc Med 2008;5(3):140–7. [DOI] [PubMed]
- 2.Pate GE, Al Zubaidi A, Chandavimol M, Thompson CR, Munt BI, Webb JG. Percutaneous closure of prosthetic paravalvular leaks: case series and review. Catheter Cardiovasc Interv 2006;68(4):528–33. [DOI] [PubMed]
- 3.Webb JG, Pate GE, Munt BI. Percutaneous closure of an aortic prosthetic paravalvular leak with an Amplatzer duct occluder. Catheter Cardiovasc Interv 2005;65(1):69–72. [DOI] [PubMed]
- 4.Sorajja P, Cabalka AK, Hagler DJ, Reeder GS, Chandrasekaran K, Cetta F, Rihal CS. Successful percutaneous repair of perivalvular prosthetic regurgitation. Catheter Cardiovasc Interv 2007;70(6):815–23. [DOI] [PubMed]
- 5.Dussaillant GR, Romero L, Ramirez A, Sepulveda L. Successful percutaneous closure of paraprosthetic aorto-right ventricular leak using the Amplatzer duct occluder. Catheter Cardiovasc Interv 2006;67(6):976–80. [DOI] [PubMed]
- 6.Echevarria JR, Bernal JM, Rabasa JM, Morales D, Revilla Y, Revuelta JM. Reoperation for bioprosthetic valve dysfunction. A decade of clinical experience. Eur J Cardiothorac Surg 1991; 5(10):523–7. [DOI] [PubMed]
- 7.Zoghbi WA, Chambers JB, Dumesnil JG, Foster E, Gottdiener JS, Grayburn PA, et al. Recommendations for evaluation of prosthetic valves with echocardiography and Doppler ultrasound: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J Am Soc Echocardiogr 2009;22(9):975–1014. [DOI] [PubMed]
- 8.STS Risk Calculator [Internet]. Chicago: The Society of Thoracic Surgeons; 2010 [cited 2010 Dec 2]. Available from: http://www.sts.org/sections/stsnationaldatabase/riskcalculator/.
- 9.Bairaktaris A, Haas NA, Seifert D, Schaeffler R, Koertke H, Schenk S, Koerfer R. Pitfalls in catheter-based interventions to treat paravalvular leaks. J Thorac Cardiovasc Surg 2008;136 (4):1076–7. [DOI] [PubMed]


