Abstract
Background & Aims
The ability of the liver to regenerate hepatic mass is essential to withstanding liver injury. The process of liver regeneration is tightly regulated by distinct signaling cascades involving components of the innate immune system, cytokines, and growth factors. However, the role of the adaptive immune system in regulation of liver regeneration is not well-defined. The role of adaptive immune system in liver regeneration was investigated in lymphocyte-deficient mice and in conditional lymphotoxin-deficient mice.
Methods
A model of liver regeneration after 70% partial hepatectomy was used, followed by examination of liver pathology, survival, DNA synthesis, and cytokine expression.
Results
We found that mice deficient in T cells show a reduced capacity for liver regeneration following partial hepatectomy. Furthermore, surface lymphotoxin, provided by T cells, is critical for liver regeneration. Mice specifically deficient in T-cell lymphotoxin had increased liver damage and a reduced capacity to initiate DNA synthesis after partial hepatectomy. Transfer of splenocytes from wild-type but not lymphotoxin-deficient mice improved liver regeneration in T cell-deficient mice. We found that an agonistic antibody against the lymphotoxin β receptor was able to facilitate liver regeneration by reducing liver injury, increasing interleukin-6 production, hepatocyte DNA synthesis, and survival of lymphocyte-deficient (Rag) mice after partial hepatectomy.
Conclusions
The adaptive immune system directly regulates liver regeneration via a T cell-derived lymphotoxin axis, and pharmacological stimulation of lymphotoxin β receptor might represent a novel therapeutic approach to improve liver regeneration.
The liver has a unique ability to completely recover following massive injury. Understanding the mechanisms that control hepatocyte division and survival has broad implications in treatment of acute and chronic liver diseases as well as increasing the feasibility of split liver transplantation. The process of liver regeneration is orchestrated by distinct signaling cascades involving components of the innate immune system, cytokines, and growth factors.1–3 Mice deficient in C3/C5 components of complement,4 MyD88, a central adaptor of toll-like receptor signaling,5 or Kupffer cells6,7 are reported to display impaired liver regeneration. Cytokines, such as interleukin (IL)-6 and tumor necrosis factor (TNF), from liver tissues play an important role in the process of liver regeneration by controlling hepatocyte apoptosis and survival.1–3,8,9 Tumor necrosis factor receptor (TNFR) I-deficient mice or mice injected with anti-TNF antibodies show impaired liver regeneration.10,11 Activated Kupffer cells secrete TNF and IL-6, cytokines that promote proliferation and survival of hepatocytes after partial hepatectomy (PH),7,8,12 although some negative effects of Kupffer cells on liver regeneration have been reported.13
Currently, the role of the adaptive system in liver regeneration is scantily defined. Significant populations of classical CD4+ and CD8+ T cells, nonclassical T cells, and smaller populations of B cells are resident in the healthy liver.14,15 The coresidence of these lymphocytes in the liver may create a unique environment capable of regulating liver homeostasis. For example, B cells appear to promote liver fibrosis in a CCl4-induced injury model.16 Ultrastructural studies revealed that T cells can directly contact hepatocytes through a widely fenestrated endothelial barrier17; however, the physiological role of these interactions is not clear. Interactions of T cells with hepatocytes and endothelial cells influence T cell fate resulting in either T-cell activation or anergy and deletion.15,18 Recently, we reported on the reverse interaction, whereby T cells can directly influence hepatocyte function.19 LIGHT (TNFSF14) expressed on T cells modulates hepatic lipid metabolism through the lymphotoxin β receptor (LTβR) suggesting that T cells are actively involved in regulating hepatocyte physiology.19 However, the role of T cells and the adaptive immune system in regulating liver regeneration remains largely unexplored and is the focus of this study.
Lymphotoxin (LT) is a central member of the TNF family.20 The biologically active form of surface LT is a heterotrimeric membrane complex LTα1β2 that interacts with a distinct LTβR, whereas a soluble LTα3 homotrimer interacts with TNFRs.20,21 LT is expressed on lymphocytes, whereas LTβR is expressed on hepatocytes, stromal, epithelial, and myeloid cells but not lymphocytes.20–22 This pattern of expression suggests that LT functions at a critical interface between lymphocytes and the surrounding stromal and parenchymal cells. B cell-derived LT (B-LTβ) is primarily responsible for lymphoid organ maintenance and immune response.20,21,23 Unlike B-LTβ–/– mice, genetic inactivation of LT on T cells (T-LTβ–/– mice) results in normal development of secondary lymphoid organs and immune responses.24 The physiologic role of T cell-derived LT (T-LTβ) has not been defined.
The contribution of the LT axis in liver regeneration has only recently started to be appreciated.25 Inactivation of LTβR (LTβR–/–) or the LTα subunit (LTα–/–) in mice enhances mortality and liver injury after PH implicating LTβR signaling in liver regeneration.25 The soluble LTα3 homotrimer signaling through the TNFRI may explain why TNF receptor-deficient mice (TNFRI–/–) display severe defects in liver regeneration,10 whereas TNF liganddeficient mice (TNF–/–) regenerate liver normally after PH.26 Furthermore, mice deficient in both TNF and LTα subunit (TNF/LTα–/–) have abnormal liver regeneration after PH.27 We have not defined the role of surface LT (LTα1β2) because the inactivation of the LTα gene ablates both soluble LTα and LTα1β2. Additionally, LIGHT, another ligand for the LTβR, was implicated in regulation of liver mass because LIGHT transgenic mice develop hepatomegaly.25 Although we have explored LTβR's role in liver regeneration,25 it is not clear whether surface LT (LTα1β2) has any function in liver regeneration.
In the present study, we have specifically defined the role of surface LT (LTα1β2) and uncovered an unexpected and essential role of the adaptive immune system in liver regeneration. Furthermore, we demonstrate an unrecognized mechanism linking LTβR stimulation to IL-6 production and STAT3 phosphorylation. Based on our discovery, we have developed a new treatment strategy exploiting the LT axis as an augmenter of liver regeneration.
Materials and Methods
Mice
Six- to 10-week-old male C57Black/6, TCRβδ–/–, TCRδ–/–, Rag1–/–, CD4–/–, and CD8–/– mice were purchased from The Jackson Laboratory (Bar Harbor, ME). LTβR–/–, LTβ–/–, T-LTβ–/–, and B-LTβ–/– mice were maintained and genotyped as previously described.24,25 All mice used in this study were on C57Black/6 background, maintained at the University of Chicago or Johns Hopkins University, and handled according to guidelines by the National Institutes of Health and approved by the Institutional Animal Care and Use Committee.
Partial Hepatectomy and Treatments
The procedure was performed as previously described.25,28 Sham procedures consisted of anesthetized mice in which the peritoneum was opened and the liver is manipulated but not resected. Agonistic anti-LTβR antibody (ACH6, 75 μg) was injected intravenously in 200 μL of sterile phosphate-buffered saline (PBS) immediately after PH. Inhibitors of LTβR signaling, anti-LTβ blocking antibody (BBF6, 100 μg) or soluble LTβR fused to human Fc portion of IgG (LTβR-Ig, 100 μg), were injected intravenously 2 hours before PH. Hamster anti-keyhole limpet hemocyanin (HA4/8) antibody was injected as control in some experiments. BBF6, ACH6, and HA4/8 antibodies29 were kindly provided by Dr Browning, Biogen (Idec Inc, Cambridge, MA). The LTβR-Ig used in this study has been previously described.25
Other Materials and Methods
Other materials and methods are described in the supplementary materials (see supplementary materials online at www.gastrojournal.org). These include histology, BrdU labeling, TUNEL labeling, transaminase and cytokine analyses, cell transfers, real-time reverse-transcription polymerase chain reaction (RT-PCR), flow cytometry, and Western blotting.
Statistical Analysis
Data are expressed as means ± SD. Statistical significance was determined by a 2-tailed Student t test (*P < .05, **P < .01, ***P < .001).
Results
T Cells Are Essential for Liver Regeneration
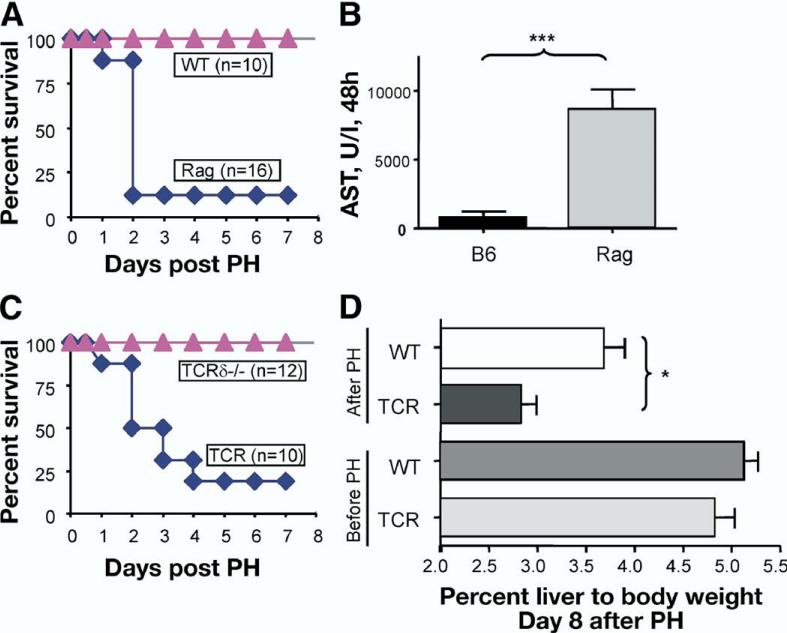
To define the role of the adaptive immune system in liver regeneration, we utilized Rag1–/– mice, which lack both T and B cells (Rag mice). To stimulate hepatocyte regeneration, we used the PH model in which approximately 70% of the rodent liver is surgically resected.28 We found that, whereas wild-type (WT) mice survived PH, most Rag mice died 48 hours post-PH (Figure 1A). To quantify liver damage, we measured ala-nine aminotransferase (ALT) and aspartate aminotransferase (AST) activity at 24 hours and 48 hours post-PH. Serum ALT and AST activity were markedly increased in Rag mice at 24 hours and 48 hours post-PH (Figure 1B and data not shown) suggesting increased liver damage in mice lacking T and B cells. Except for massive liver necrosis and apoptosis similar to that of LTβR–/– mice, no secondary organ pathology was present in Rag mice (data not shown). Collectively, these data suggest that Rag mice lacked sufficient regenerative capacity and died because of liver injury.
Figure 1.
T cells are essential for liver regeneration. Mice underwent 70% partial hepatectomy (PH), and survival was monitored for 8 days. Data represent 1 of 2 independent experiments. N = number of mice per group. (A) Rag mice show an increased mortality after PH compared with WT. (B) Increased liver damage in Rag mice after PH. Serum aspartate aminotransferase (AST) levels at 48 hours after PH. (C) T cell-deficient mice (TCRβδ–/–, designated TCR) show an increased mortality after PH. Mice with inactivation of TCRδ chain only (TCRδ–/–) show normal survival. (D) T cell-deficient mice show reduced ratio of liver to body mass at 8 days after PH.
To define a role of T cells in liver regeneration, we performed PH on mice lacking nearly all T cells (TCRβδ–/–, designated TCR). TCR mice displayed 75% mortality in the 8 days post-PH (Figure 1C). In contrast, TCRδ–/– mice, lacking only γδ T cells, regenerated liver normally (Figure 1C). Liver mass per body weight ratios were reduced in TCR mice at 8 days after PH (Figure 1D). These data suggest that the adaptive immune system, namely T cells, influences the process of liver regeneration.
Both CD8 and CD4 T Cells Are Required for Liver Regeneration
The liver contains resident classical T cells, among which CD8+ T cells are generally more abundant than CD4+ T cells.15,18 To define the role of particular cell types of the adaptive immune system, we first depleted CD8 and CD4 cells in WT mice using anti-CD8 and anti-CD4 antibodies. Mice depleted of CD8 or CD4 T cells showed an increased mortality post-PH (Figure 2A and B). Moreover, mice genetically deficient in CD8– or CD4– T cells each showed a similar increased mortality post-PH (Figure 2C and D). Histologic examination of the livers reveals notably larger areas of necrosis in CD8–/– mice at 48 hours post-PH compared with WT mice (Figure 2E). Quantifying DNA synthesis following PH included administering bromodeoxyuridine (BrdU), a thymidine analog, to the mice 2 hours pre-mortem (46 hours post-PH) and counting the number of BrdU-positive hepatocyte nuclei following postmortem immunohistochemical staining for BrdU (Figure 2E). Not only was there increased liver cell death in CD8–/– mice but also decreased DNA synthesis by hepatocytes (Figure 2E and F). The number of apoptotic cells in the liver was markedly increased in CD8–/– mice compared with WT mice (Figure 2E). These data indicate that CD8+ T cells as well as CD4+ T cells are important for the liver's ability to regenerate hepatic mass.
Figure 2.
Both CD8 and CD4 T cells are required for liver regeneration. (A and B) Mice depleted of CD8 or CD4 T cells show increased mortality after PH. Wild-type mice were injected with anti-CD8 or anti-CD4 antibody 24 hours prior to PH. (C and D) CD8–/– and CD4–/– mice show an increased mortality after PH. (E) CD8–/– mice display increased areas of liver necrosis and apoptosis and reduced DNA synthesis at 48 hours after PH. Liver sections were stained with H&E (left panel), anti-BrdU antibody (middle panel), and apoptotic cells using TUNEL protcol (right panel). Represents 1 of 2 independent experiments (5 mice per group). Original magnification, ×200. (F) Reduced DNA synthesis in CD8–/– mice at 48 hours after PH as determined by positive BrdU incorporation.
Adoptive Splenocyte Transfer Improves Liver Regeneration in T Cell-Deficient Mice
To define whether T cells are sufficient for restoring liver regeneration in TCR mice, splenocytes were adoptively transferred from WT mice to TCR mice 1 week pre-PH (Figure 3). This transfer significantly improved liver regeneration of TCR mice, as revealed by reduced liver damage and transaminase levels and increased DNA synthesis (Figure 3A–C). However, a similar transfer of T cells from LTα-deficient mice could not rescue liver regeneration as demonstrated by lack of DNA synthesis in TCR mice (Figure 3B and C). WT or LTα-deficient splenocytes repopulate the liver of TCR mice after transfer (see supplementary Table 1 online at www.gastrojournal.org), suggesting that impaired liver regeneration of TCR mice receiving LTα–/– splenocytes is not due to impaired migration of LT-deficient cells to the liver. These data support the notion that LTα-deficient splenocytes do not have an inherent ability to induce liver regeneration in TCR mice comparable with that of WT splenocytes.
Figure 3.
Adoptive splenocyte transfer improves liver regeneration in T cell-deficient mice. Fifty million splenocytes (Spl) from WT or LTα–/– mice were transferred to TCR mice 1 week prior to PH. Represents 1 of 2 independent experiments (5 mice per group). (A) ALT levels in serum at 48 hours after partial hepatectomy. (B) Increased DNA synthesis in T cell-deficient mice after transfer of splenocytes from WT but not from LTα-deficient mice. (C) Liver H&E sections at 48 hours after PH indicating the decreased injury in TCR mice reconstituted with WT but not LTα–/– splenocytes. Original magnification, ×400.
LT Expressed by T Cells Is Required for Liver Regeneration
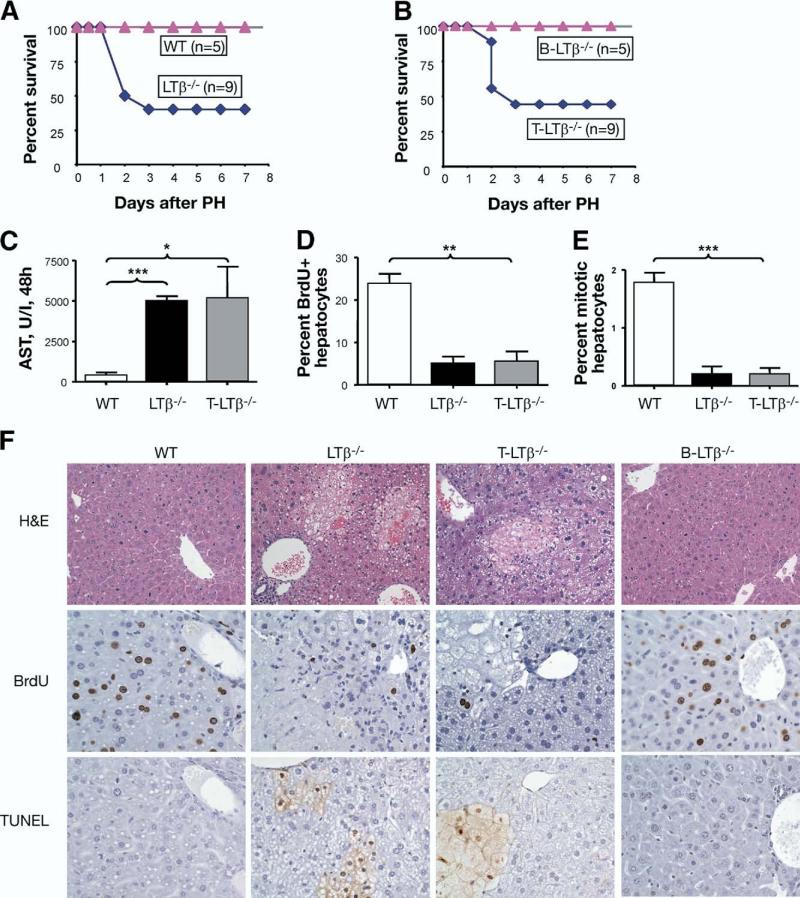
Impaired liver regeneration observed in LTα-deficient mice suggests the involvement of LT in liver regen eration.25 However, inactivation of LTα subunit prevents signaling through both TNFRI (via the soluble homotrimer) and LTβR (via the surface LTα1β2 complex). To specifically define the role of surface LTα1β2 in liver regeneration, we studied liver regeneration in LTβ–/– mice. Inactivation of the LTβ subunit prevents the formation of biologically active surface LTα1β2 complex while preserving the soluble LTα3 form. We found that LTβ–/– mice showed an increased mortality (50%) post-PH (Figure 4A). LTβ–/– mice had increased serum AST that coincided with enhanced areas of liver necrosis and apoptosis and reduced DNA synthesis and hepatocyte proliferation at 38 hours and 48 hours after PH (Figure 4C–F and see supplementary Figure 1 online at www.gastrojournal.org). To define further the effect of LT on liver injury, we analyzed the kinetics of ALT at 6 hours, 24 hours, and 36 hours after PH. We found that, in WT mice, ALT levels gradually reduced 36 hours after PH, whereas ALT levels of LTβ–/– mice remained elevated at all time points (see supplementary Figure 2 online at www.gastrojournal.org). These results suggest that membrane LT prevents liver injury and promotes hepatocyte survival after PH. A few LTβR–/– mice that survived PH were able to regenerate liver and showed no specific pathology at day 6 after PH (data not shown), suggesting the possibility of compensation by other LTβR-independent pathways.
Figure 4.
LT expressed by T cells is essential for liver regeneration. (A) Reduced survival of LTβ–/– mice after PH. (B) Increased mortality of T cell-specific LTβ-deficient mice (T-LTβ–/–) after PH. N = number of mice per group. (C) Increased liver damage of T-LTβ–/– mice after PH. AST levels in serum of indicated mice at 48 hours after partial hepatectomy are shown. Represents 1 of 2 independent experiments, 5 mice per group. (A–C) Represent 1 of 2 independent experiments. (D) Reduced DNA synthesis in LTβ–/– and T-LTβ–/– mice 48 hours after PH, analyzed by BrdU incorporation. (E) Reduced mitotic activity of LTβ–/– and T-LTβ–/– mice at 48 hours after PH. Dividing hepatocytes in metaphase were calculated from H&E-stained liver sections at 40 high-power fields. (F) Reduced DNA synthesis and increased areas of necrosis and apoptosis in T-LTβ–/– mice 48 hours after PH. Upper panels: H&E staining of liver sections. Original magnification, ×200. Middle panels: Frozen liver sections were stained with anti-BrdU antibody. Representative liver images are shown. Original magnification, ×400. Lower panels: Frozen liver sections were stained for apoptotic cells using TUNEL protocol. Original magnification, ×400.
Membrane LT is primarily expressed on B and T cells but not on stromal cells.20 To define whether membrane LT specifically expressed on T cells or B cells is required for liver regeneration, we next used a mouse model with genetic inactivation of surface LT on T cells (T-LTβ–/– mice) and B cells (B-LTβ–/– mice).24 T-LTβ–/– mice showed a consistently increased mortality post-PH, whereas B-LTβ–/– mice all survived PH similar to that of WT mice (Figure 4B). T-LTβ–/– mice displayed increased liver damage as analyzed by serum AST levels (Figure 4C) and by histologic examination (Figure 4F). The number of BrdU-positive hepatocytes and mitotic activity were significantly reduced in T-LTβ–/– mice compared with WT and B-LTβ–/– mice (Figure 4D–F). To define whether reduced DNA synthesis was associated with increased hepatocyte apoptosis, we used TUNEL staining. T-LTβ–/– mice showed an increased number of apoptotic hepatocytes compared with WT and B-LTβ–/– mice (Figure 4F), suggesting that LT production specifically by T cells may not only promote hepatocyte proliferation but also provide a protective effect by inhibiting apoptosis. These data strongly suggest that surface LTα1β2 produced by T cells is essential for liver regeneration.
Stimulation of LTβR Signaling Rescues Rag Mice After Partial Hepatectomy
Observing the inability of Rag mice to regenerate liver after PH (Figure 1) and the essential role of T-cell surface LTα1β2 in this process (Figure 4) prompted us to test whether we can develop a novel treatment through pharmacologic activation of LTβR signaling to improve liver regeneration. Agonistic anti-LTβR antibody (ACH6, 75 μg) injected intravenously into Rag mice immediately after PH reduced Rag mice mortality (Figure 5A), serum ALT levels at 24 hours (see supplementary Figure 3 online at www.gastrojournal.org), and serum ALT levels and necrotic areas in the liver at 48 hours after PH (Figure 5B and D). Liver mass as percent of body weight at day 8 post-PH increased significantly in TCR mice treated with ACH6 antibody (Figure 5C). These data suggest that, in the absence of endogenous T cells, the stimulation of LTβR signaling alone can sufficiently promote liver regeneration. Injection of anti-LTβR antibody to WT mice did not result in development of hepatitis or an increase in liver damage as assayed by ALT activity in serum and liver histology (data not shown). DNA synthesis in livers of treated Rag mice matched that found in WT (Figure 5D and E). Hepatocyte mitotic activity was significantly increased in Rag mice following anti-LTβR treatment (Figure 5F) and correlated with a reduction in the number of apoptotic cells (Figure 5D, lower panel). These results suggest that pharmacological stimulation of LTβR signaling can be a potential treatment to improve liver regeneration.
Figure 5.
Stimulation of LTβR signaling rescues Rag mice after partial hepatectomy. (A) Rag1–/– mice were injected IV with 75 μg of anti-LTβR agonistic antibody (ACH6) immediately after PH. Survival was monitored for 8 days. Represents 1 of 2 independent experiments. N = number of mice per group. (B) ALT activity in serum of anti-LTβR antibody treated mice at 48 hours after PH, 6 mice per group. WT mice were used as a control. (C) T cell-deficient mice (8 mice per group) received 75 μg of anti-LTβR agonistic antibody (ACH6) intravenously immediately after PH. Liver mass is shown as percent of body weight at day 8 after PH. (D) Representative images of H&E staining (upper panel), BrdU incorporation (middle panel), and apoptotic cells using TUNEL protocol (lower panel) at 48 hours after PH are shown. Original magnification, ×400. (E) The amount of DNA synthesis was determined using BrdU incorporation. WT mice were used as control. (F) Increased mitotic activity in livers of Rag mice treated with anti-LTβR agonistic antibody mice at 48 hours after PH. Dividing hepatocytes in metaphase were calculated from H&E-stained liver sections at 40 high-power fields. WT mice were used as control.
LTβR Signaling Controls IL-6 Pathway for Liver Regeneration
IL-6 plays an important role in the regulation of liver regeneration.8,9 IL-6 is rapidly activated after PH, reaching peak levels at 4–6 hours after PH.1,8,9 To define whether LTβR signaling is essential for IL-6 production after PH, we measured IL-6 levels in serum of WT mice treated with a soluble LTβR fusion protein (LTβR-Ig), which blocks LTβR signaling, and with anti-LTβ antibody, which blocks LTβ binding to the receptor (Figure 6A). We found that pretreatment with either LTβR-Ig fusion protein or anti-LTβ antibody significantly reduced IL-6 levels in serum of WT mice at 8 hours post-PH (Figure 6A).
Figure 6.
LTβR signaling regulates IL-6 pathway in liver regeneration. (A) Reduced IL-6 levels in serum of WT mice treated with LTβR inhibitors. Wild-type mice were treated with the LTβR fused to the human Fc portion of IgG (LTβR-Ig) or anti-LTβ antibody (100 μg) 2 hours before partial hepatectomy. (B) Increased IL-6 mRNA expression in livers of Rag mice after anti-LTβR stimulation. The levels of IL-6 in Rag mice treated with anti-LTβR agonistic antibody (ACH6, 75 μg) and control hamster antibody (H4/8, 75 μg) were measured in livers at 6 hours after PH by real-time RT-PCR (5 mice per group). (C) Increased IL-6 production in serum of Rag mice after anti-LTβR stimulation. IL-6 levels in Rag mice injected with anti-LTβR (ACH6, 75 μg) and control hamster antibody (H4/8, 75 μg) were measured in serum at 6 hours after PH by cytokine bead assay (BD Biosciences, San Jose, CA). (D) LIGHT and TNF synergistically induce IL-6 in a LTβR-dependent manner. WT mouse embryonic fibroblasts were incubated with DMEM and 0.3% FCS with the indicated concentrations of recombinant LIGHT and TNF for 24 hours. Supernatants were analyzed in triplicates for the presence of IL-6 by sandwich ELISA. LTβR-Ig (1 μg/mL) effectively blocked IL-6 expression induced by LIGHT and TNF. (E) Anti-LTβR stimulation promotes TNF production by macrophages. TNF levels were measured in supernatants of thioglycolate-elicited peritoneal macrophages stimulated in vitro with indicated doses of anti-LTβR antibody (ACH6) by cytokine bead assay (BD Biosciences) (F) Increased expression of TNF in livers of Rag mice after anti-LTβR stimulation. Increased TNF mRNA expression in livers of Rag mice after anti-LTβR stimulation. IL-6 levels in control untreated Rag and anti-LTβR agonistic antibody (ACH6, 75 μg) Rag-treated mice (5 mice per group) were measured in livers at 6 hours after partial hepatectomy by real-time RT-PCR. All panels represents 1 of 2 independent experiments.
We next examined whether stimulation of the LTβR could promote IL-6 production after PH. We measured IL-6 levels in Rag mice treated with agonistic anti-LTβR antibody (ACH6) compared with untreated Rag mice at 6 hours after PH (Figure 6B and C). This treatment improved survival of Rag mice after PH (Figure 5). IL-6 messenger RNA (mRNA) expression was up-regulated by 4-fold in the liver of ACH6-treated Rag mice compared with Rag mice treated with control hamster anti-keyhole limpet hemocyanin antibody (HA4/8) (Figure 6B). IL-6 protein levels in serum correlated well with IL-6 mRNA expression in the liver of these mice (Figure 6C). Altogether, these data support that the LTβR and LT interaction leads to IL-6 production in vivo and contributes to increased survival after PH.
We next wanted to test whether stimulation of LTβR signaling can directly activate IL-6 production in vitro. We stimulated mouse embryonic fibroblasts with recombinant LIGHT or anti-LTβR antibody and measured IL-6 levels using sandwich ELISA (Figure 6D). We found that stimulation of LTβR with recombinant LIGHT or anti-LTβR alone did not induce IL-6 production (Figure 6D and data not shown). TNF alone induced negligible IL-6 production. Remarkably, TNF and LIGHT combinations synergistically induced IL-6 production that was ameliorated by the presence of the LTβR-Ig fusion protein (Figure 6D). We further found that in vitro LTβR stimulation of peritoneal macrophages increased TNF production in a dose-dependent manner (Figure 6E). These data suggest that additional factors, such as TNF, are required for optimal expression of IL-6 after LTβR stimulation. To define whether LTβR stimulation can increase TNF production after PH, we measured TNF levels in liver of Rag mice treated with anti-LTβR agonistic antibody (Figure 6F). The expression of TNF was significantly increased in Rag mice treated with anti-LTβR antibody compared with untreated Rag mice (Figure 6F). We also demonstrated enhanced STAT3 phosphorylation in the liver 5 hours after anti-LTβR agonistic antibody treatment (see supplementary Figure 4 online at www.gastrojournal.org). These data indicate that LTβR-dependent activation of TNF could contribute to IL-6 production and STAT3 phosphorylation in vivo. Together, our data suggest that increased anti-LTβR signaling promotes IL-6 pathway after PH to support liver regeneration.
Discussion
The significance of the adaptive immune system in liver regeneration has been largely ignored. Ultrastructural studies illuminated the direct access T cells have for contacting hepatocytes, but the functional consequence of their interaction remained unexplored. Our study reveals a novel function of T cells in the regulation of liver regeneration. We have established that LT expression by T cells is essential for liver regeneration and that stimulation of LTβR signaling induces IL-6 production and STAT3 phosphorylation, which together promote liver regeneration. Our findings suggest that the adaptive immune system directly regulates liver regeneration via a T cell-derived LT axis. Furthermore, we demonstrate that pharmacological stimulation of LTβR signaling may represent a novel therapeutic approach to improve liver regeneration.
Early evidence of the adaptive immune system's involvement in liver regeneration originated from observing the impaired liver regeneration of mice lacking mature T and B cells (Rag mice) and in T cell-deficient mice (Figures 1 and 5). We explored the role of T cells using 3 approaches: analysis of liver regeneration in mouse strains genetically deficient in various T-cell subsets, depletion of T-cell subsets from WT mice, and adoptive lymphocyte transfers. Interestingly, we found that both CD4+ and CD8+ T cells were important for liver regeneration (Figure 2). Our data elucidate a novel and essential role of T cells in liver regeneration.
Unlike many tissues, such as kidney or brain, the liver contains a large number of resident T cells that have access to hepatocytes through widely fenestrated endothelial cells without having to cross a basement membrane.15,17 The pool of T cells in the liver is highly diversified; unconventional γδ T, natural killer T (NKT) cells, CD8αα T cells, and regulatory T cells represent a substantial proportion of lymphoid cells.14,15 We found no defect in liver regeneration of TCRδ–/– mice, suggesting that γδ T cells are not essential for liver regeneration (Figure 1).
The role of NKT cells in liver regeneration is controversial; some reports provide evidence for positive effects of NKT cells,30 whereas others report negative effects of NKT cells on liver regeneration.31–33 The potential harmful effect of NKT cells may be attributed to inflammation promoted by activated NKT cells. The timing of NKT activation is important because the sensitivity of hepatocytes to TNF- and Fas-mediated apoptosis dramatically changes after PH.34 Fas agonistic antibody improved liver regeneration if injected immediately after PH but induced hepatocyte cell death if delivered before surgery.35 Similarly, activation of NKT cells with αGalCer improved liver regeneration if injected after PH30 but inhibited liver regeneration if delivered before surgery.31 Previous studies observed that CD4+ NKT cells increase their population inside the liver after PH, whereas the proportion of CD8+ T cells remains unchanged.36 This suggests that resident CD8+ T cells might directly create a favorable environment for liver regeneration.
Because Rag and TCR mice lack both T and NKT cells, there is a possibility that both cell types contribute to the increased susceptibility of these mice to PH. Lack of NKT cells in LT and LTβR KO mice makes it difficult to address whether lack of NKT cells or LT/LTβR signaling is affecting liver regeneration. Our study clearly demonstrates that the function of LT on T cells in liver regeneration is likely distinct from NKT cells because T-LTβ–/– mice show a defect in liver regeneration but not in NKT cell development (see supplementary Figure 5 online at www.gastrojournal.org).
LT is expressed on T, B, and NK cells.37 Analysis of mice with conditional inactivation of membrane LT on T or B cells revealed that LT expression by T cells is essential for liver regeneration (Figure 4). Although LT from B cells is important for the function of various lymphoid tissues, the role of LT on T cells was unclear.23,24 This study is the first example of a biologic role of LT produced by T cells in nonlymphoid organs.
We speculate 2 possibilities for the action of LT from T cells in liver regeneration: (1) T cells contact LTβR expressing hepatocytes through fenestrated basal endothelium to directly promote hepatocyte proliferation. (2) LT expressing T cells first activate stromal cells that secrete other cytokines essential for hepatocyte proliferation and prevention of apoptosis. The accessory role of intermediate resident stromal cell secretion of IL-6 and TNF cytokines for hepatocyte proliferation and survival has been documented.7,8,12 Our data linking LTβR signaling to IL-6 production has uncovered an important mechanism to promote hepatocyte survival after PH.
Augmenting liver regeneration has valuable therapeutic applications. Living donor liver transplantation is a remarkably effective lifesaving procedure for select patients with end-stage liver disease.38,39 Unfortunately, the need for a substantial graft volume to support life places healthy donors at a significant risk.38,39 Therapeutic interventions capable of enhancing liver regeneration will reduce risks for living donors. Additionally, the ability to expand hepatocyte numbers might also facilitate hepatocyte cellular transplants. Furthermore, enhancing liver regeneration following acute liver failure may allow for recovery of hepatic mass without the need for a liver transplant.
To develop a therapeutic approach to improve liver regeneration, we developed an approach based on stimulation of LTβR signaling immediately after PH. We showed that stimulation of LTβR signaling with an agonistic antibody dramatically improved liver regeneration in lymphocyte deficient mice. Our data suggest that the effect of LTβR signaling stimulation includes both improved hepatocyte proliferation and prevention of apoptosis. Increased IL-6 production and STAT3 phosphor-ylation observed after anti-LTβR stimulation likely contribute to the hepatoprotective effect of LTβR signaling after PH. In line with our data, previous studies using an in vitro system revealed that treatment of cultured human hepatocytes with LTβR ligand LIGHT protects against TNF-mediated apoptosis.40 Future studies will be required to define further whether the same signaling is also invoked in other models of regeneration caused by hepatocyte damage before this mechanism can be used to enhance liver regeneration in patients with liver disease. Collectively, our data suggest that the adaptive immune system directly regulates liver regeneration via a T cell-derived LT axis and that pharmacological stimulation of LTβR signaling may represent a novel therapeutic approach to improve liver regeneration.
Supplementary Material
Supplementary Materials and Methods
Histology, BrdU labeling, and TUNEL labeling
Tissue sections were fixed in 10% buffered formalin and processed either for routine H&E staining or BrdU and TUNEL immunohistochemical studies. TUNEL staining was performed on paraffin-embedded, formalinfixed tissue using the ApopTag Plus Peroxidase In Situ Apoptosis Detection kit (Chemicon, Madison, WI) according to the manufacturer's directions. BrdU solution (1 mg BrdU in phosphate-buffered saline [PBS]) was injected intraperitoneally 2 hours prior to analysis. DNA synthesis was measured by immunohistochemical staining of paraffin liver sections with anti-BrdU antibody. Percent of BrdU-positive hepatocytes nuclei and hepatocytes in metaphase among total hepatocytes nuclei was calculated from 1000 hepatocytes per mouse.
Transaminase Activity and Cytokines Analyses
Blood was collected by retroorbital puncture, following Institutional Animal Care and Use Committee approved procedures. Animals were killed with CO2 followed by cervical dislocation, and tissues were removed and flash-frozen in liquid N2 for biochemical analyses or fixed in 10% neutral-buffered formalin for histology. Serum transaminase activities were determined using Reflotron GPT (ALT) and GOT (AST) tabs and a Reflotron Plus Chemistry Analyzer (Roche Diagnostic Corporation, Indianapolis, IN) according to the manufacturer's instructions. Concentrations of IL-6 in sera were determined by Cytokine Bead Assay (BD Biosciences, San Jose, CA) or by R&D Systems ELISA following the manufacturer's recommendations.
Cell Purification and Transfers
Intrahepatic lymphocytes were purified from the liver by pressing the liver through a steel mesh (No. 200) into PBS, centrifuged at 800g for 5 minutes with the resulting pellet suspended in a 35% Percoll-PBS-heparin (100 U/mL) solution, and centrifuged at 800g for 20 minutes at room temperature. The pellet of mononuclear cells was cleared of RBC with a 5-minute osmotic lysis (0.15 mol/L NH4Cl, 1 mmol/L KHCO3, 0.1 mmol/L Na2-EDTA, pH 7.3) and washed twice in PBS. Lymphocytes were stained with antibodies (BD Biosciences) and ana lyzed by flow cytometry (FACSCanto; BD Biosciences). For adoptive transfer experiments, 100 million total splenocytes cleared of RBC were transferred to 300-rad irradiated TCR–/– mice 1 week prior to partial hepatectomy. Cellular depletions were accomplished with anti-CD4 (GK1.5) or anti-CD8 (PK136). Two hundred micrograms of depleting antibody was injected 2 days prior to partial hepatectomy in some experiments.
Real-Time Polymerase Chain Reaction
Total RNA was extracted by RNeasy mini kit from Qiagen. For complementary DNA (cDNA) synthesis, RNA were digested with DNase I and reverse transcribed using random primers with AMV Reverse Transcriptase (Promega). The concentration of the target gene was determined using the comparative CT (threshold cycle number at a cross point between amplification plot and threshold) method and normalized to hypoxanthineguanine phosporybosyltransferase (HPRT) and β-actin. cDNA were amplified using Taqman or Power Sybr Green PCR master mix (Applied Biosystems, Foster City, CA) and run on ABI 7300 cycler (Applied Biosystems). Polymerase chain reaction primers and probes used are as follows: for IL-6, forward 5′-ACAAGTCGGAGGCTTAATTACACAT, reverse 5′-AATCAGAATTGCCATTGCACAA, probe 5′-FAM-TTCTCTGGGAAATCGTGGAAATGAGAAAAGA-TAMRA; for TNF, forward 5′-ACGGCATGGATCTCAAAGAC, reverse 5′-AGATAGCAAATCGGCTGACG; for HPRT, forward 5′-TGAAGAGCTACTGTAATGATCAGTCAAC, reverse 5′-AGCAAGCTTGCAACCTTAACCA, 5′-FAM-TGCTTTCCCTGGTTAAGCAGTACAGCCC-TAMRA; for β-actin, forward 5′-AGAGGGAAATCGTGCGTGAC, reverse, CAATAGTGATGACCTGGCCGT, probe, 5′-FAM-CACTGCCGCATCCTCCCTCTCCC-TAMRA.
Western Blots
For protein extraction, liver homogenates were prepared using NP-40 lysis buffer (20 mmol/L Hepes, pH 8.0; 350 mmol/L NaCl; 20% glycerol; 1% NP-40; 1 mmol/L MgCl2; 0.2 mmol/L EGTA, pH 8.0) supplemented with protease inhibitors (Roche Diagnostic Corporation). After 10% SDS-PAGE proteins were transferred and probed with antibodies directed at STAT3 and p-705 STAT3 (Santa Cruz Biotechnology Corp, Santa Cruz, CA) and developed using enhanced chemiluminescence (ECL; Amersham Biosciences, Piscataway, NJ).
Supplementary Table 1. Cell Numbers in Liver of T Cell-Deficient Mice After Adoptive Splenocyte Transfer
Supplementary Figure 1. Reduced DNA synthesis in LTβ–/– mice at 38 hours after PH. (A) Representative images of H&E (upper panel) and BrdU incorporation (lower panel) at 38 hours after PH are shown. Original magnification, ×400. (B) The amount of DNA synthesis was determined using BrdU incorporation at 38 hours after PH. N = 5 mice per group. ***P < .001.
Supplementary Figure 2. Kinetics of liver inury after partial hepatectomy. Serum ALT levels of WT and LTβ–/– mice were measured at 6 hours, 24 hours, and 36 hours after PH. LTβ–/– mice show an increased liver injury during entire time period, whereas WT mice recover from liver damage at 36 hours after PH. N = 5 mice per group. *P < .05.
Supplementary Figure 3. Stimulation of LTβR signaling reduces liver injury of Rag mice 24 hours after PH. Rag1–/– mice were injected IV with 75 μg of anti-LTβR agonistic antibody (ACH6) immediately after PH. ALT activity in serum of anti-LTβR antibody treated mice at 24 hours after PH, 6 mice per group. *P < .05.
Supplementary Figure 4. Stimulation of LTβR signaling induces STAT3 phosphorylation after PH. Rag1–/– mice were injected IV with 75 μg of anti-LTβR agonistic antibody (ACH6) or vehicle (untreated) immediately after PH. Liver tissue was harvest 0 or 5 hours after PH for Western blot analysis. Liver tissue was homogenized, run on SDS-PAGE, transferred to nitrocellulose membrane, and probed with antibodies for total STAT3 and phosphorylation at tyrosine residue 705 of STAT3.
Supplementary Figure 5. T-LTβ–/– mice have normal NKT cell numbers in the liver. Mononuclear cells were purified from the livers of naïve WT and T-LTβ–/– mice and NKT cells visualized by flow cytometry with CD1d tetramers and antibodies to TCRβ, NK1.1, and CD4.
Acknowledgments
The authors disclose the following: Supported by grants from National Institutes of Health AI062026, CA115540, and DK58897 (to Y.-X.F.) and DK067187 and DK081417 (to R.A.A.); the Digestive Disease Research Core Center of the University of Chicago DK42086, and American Heart Association 0730419Z (to A.V.T.); NIH training grant T32HL007237 (to E.P.K.); and by DFG SFB633 (to S.A.N.).
Abbreviations used in this paper
- B-LTβ
B cell-derived LT
- LTβR
lymphotoxin β receptor
- PH
partial hepatectomy
- LT
lymphotoxin
- LIGHT
TNF superfamily member 14 (TNFSF14)
- T-LTβ
T cell-derived LT
Footnotes
The authors thank Klaus Pfeffer for providing LTβR–/– mice.
Y.-X.F. and R.A.A. contributed equally to this paper.
Supplementary Data
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at doi: 10.1053/j.gastro.2008.09.015.
References
- 1.Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 2.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 3.Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strey CW, Markiewski M, Mastellos D, et al. The proinflammatory mediators C3a and C5a are essential for liver regeneration. J Exp Med. 2003;198:913–923. doi: 10.1084/jem.20030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seki E, Tsutsui H, Iimuro Y, et al. Contribution of Toll-like receptor/myeloid differentiation factor 88 signaling to murine liver regeneration. Hepatology. 2005;41:443–450. doi: 10.1002/hep.20603. [DOI] [PubMed] [Google Scholar]
- 6.Meijer C, Wiezer MJ, Diehl AM, et al. Kupffer cell depletion by CI2MDP-liposomes alters hepatic cytokine expression and delays liver regeneration after partial hepatectomy. Liver. 2000;20:66–77. doi: 10.1034/j.1600-0676.2000.020001066.x. [DOI] [PubMed] [Google Scholar]
- 7.Abshagen K, Eipel C, Kalff JC, et al. Loss of NF-κB activation in Kupffer cell-depleted mice impairs liver regeneration after partial hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1570–G1577. doi: 10.1152/ajpgi.00399.2006. [DOI] [PubMed] [Google Scholar]
- 8.Cressman DE, Greenbaum LE, DeAngelis RA, et al. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379–1383. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- 9.Blindenbacher A, Wang X, Langer I, et al. Interleukin 6 is important for survival after partial hepatectomy in mice. Hepatology. 2003;38:674–682. doi: 10.1053/jhep.2003.50378. [DOI] [PubMed] [Google Scholar]
- 10.Yamada Y, Kirillova I, Peschon JJ, et al. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci U S A. 1997;94:1441–1446. doi: 10.1073/pnas.94.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akerman P, Cote P, Yang SQ, et al. Antibodies to tumor necrosis factor-α inhibit liver regeneration after partial hepatectomy. Am J Physiol. 1992;263:G579–G585. doi: 10.1152/ajpgi.1992.263.4.G579. [DOI] [PubMed] [Google Scholar]
- 12.Iwai M, Cui TX, Kitamura H, et al. Increased secretion of tumour necrosis factor and interleukin 6 from isolated, perfused liver of rats after partial hepatectomy. Cytokine. 2001;13:60–64. doi: 10.1006/cyto.2000.0797. [DOI] [PubMed] [Google Scholar]
- 13.Rai RM, Yang SQ, McClain C, et al. Kupffer cell depletion by gadolinium chloride enhances liver regeneration after partial hepatectomy in rats. Am J Physiol. 1996;270:G909–G918. doi: 10.1152/ajpgi.1996.270.6.G909. [DOI] [PubMed] [Google Scholar]
- 14.Emoto M, Kaufmann SH. Liver NKT cells: an account of heterogeneity. Trends Immunol. 2003;24:364–369. doi: 10.1016/s1471-4906(03)00162-5. [DOI] [PubMed] [Google Scholar]
- 15.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:S54–S62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 16.Novobrantseva TI, Majeau GR, Amatucci A, et al. Attenuated liver fibrosis in the absence of B cells. J Clin Invest. 2005;115:3072–3082. doi: 10.1172/JCI24798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warren A, Le Couteur DG, Fraser R, et al. T lymphocytes interact with hepatocytes through fenestrations in murine liver sinusoidal endothelial cells. Hepatology. 2006;44:1182–1190. doi: 10.1002/hep.21378. [DOI] [PubMed] [Google Scholar]
- 18.Crispe IN, Giannandrea M, Klein I, et al. Cellular and molecular mechanisms of liver tolerance. Immunol Rev. 2006;213:101–118. doi: 10.1111/j.1600-065X.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 19.Lo JC, Wang Y, Tumanov AV, et al. Lymphotoxin β receptor-dependent control of lipid homeostasis. Science. 2007;316:285–288. doi: 10.1126/science.1137221. [DOI] [PubMed] [Google Scholar]
- 20.Ware CF. Network communications: lymphotoxins, LIGHT, and TNF. Annu Rev Immunol. 2005;23:787–819. doi: 10.1146/annurev.immunol.23.021704.115719. [DOI] [PubMed] [Google Scholar]
- 21.Tumanov AV, Christiansen PA, Fu Y- X. The role of lymphotoxin receptor signaling in diseases. Curr Mol Med. 2007;7:567–578. doi: 10.2174/156652407781695701. [DOI] [PubMed] [Google Scholar]
- 22.Browning JL, French LE. Visualization of lymphotoxin-β and lymphotoxin-β receptor expression in mouse embryos. J Immunol. 2002;168:5079–5087. doi: 10.4049/jimmunol.168.10.5079. [DOI] [PubMed] [Google Scholar]
- 23.Fu YX, Chaplin DD. Development and maturation of secondary lymphoid tissues. Annu Rev Immunol. 1999;17:399–433. doi: 10.1146/annurev.immunol.17.1.399. [DOI] [PubMed] [Google Scholar]
- 24.Tumanov A, Kuprash D, Lagarkova M, et al. Distinct role of surface lymphotoxin expressed by B cells in the organization of secondary lymphoid tissues. Immunity. 2002;17:239–250. doi: 10.1016/s1074-7613(02)00397-7. [DOI] [PubMed] [Google Scholar]
- 25.Anders RA, Subudhi SK, Wang J, et al. Contribution of the lymphotoxin β receptor to liver regeneration. J.Immunol. 2005;175:1295–300. doi: 10.4049/jimmunol.175.2.1295. [DOI] [PubMed] [Google Scholar]
- 26.Fujita J, Marino MW, Wada H, et al. Effect of TNF gene depletion on liver regeneration after partial hepatectomy in mice. Surgery. 2001;129:48–54. doi: 10.1067/msy.2001.109120. [DOI] [PubMed] [Google Scholar]
- 27.Knight B, Yeoh GC. TNF/LTα double knockout mice display abnormal inflammatory and regenerative responses to acute and chronic liver injury. Cell Tissue Res. 2005;319:61–70. doi: 10.1007/s00441-004-1003-6. [DOI] [PubMed] [Google Scholar]
- 28.Greene AK, Puder M. Partial hepatectomy in the mouse: technique and perioperative management. J Invest Surg. 2003;16:99–102. [PubMed] [Google Scholar]
- 29.Rennert PD, James D, Mackay F, et al. Lymph node genesis is induced by signaling through the lymphotoxin β receptor. Immunity. 1998;9:71–79. doi: 10.1016/s1074-7613(00)80589-0. [DOI] [PubMed] [Google Scholar]
- 30.Nakashima H, Inui T, Habu Y, et al. Activation of mouse natural killer T cells accelerates liver regeneration after partial hepatectomy. Gastroenterology. 2006;131:1573–1583. doi: 10.1053/j.gastro.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 31.Ito H, Ando K, Nakayama T, et al. Role of Vα 14 NKT cells in the development of impaired liver regeneration in vivo. Hepatology. 2003;38:1116–1124. doi: 10.1053/jhep.2003.50471. [DOI] [PubMed] [Google Scholar]
- 32.Huang W, Dong Z, Wei H, et al. Selective elimination of hepatic natural killer T cells with concanavalin A improves liver regeneration in mice. Liver Int. 2006;26:339–345. doi: 10.1111/j.1478-3231.2005.01221.x. [DOI] [PubMed] [Google Scholar]
- 33.Dong Z, Zhang J, Sun R, et al. Impairment of liver regeneration correlates with activated hepatic NKT cells in HBV transgenic mice. Hepatology. 2007;45:1400–1412. doi: 10.1002/hep.21597. [DOI] [PubMed] [Google Scholar]
- 34.Takehara T, Hayashi N, Mita E, et al. Delayed Fas-mediated hepatocyte apoptosis during liver regeneration in mice: hepato-protective role of TNF α. Hepatology. 1998;27:1643–1651. doi: 10.1002/hep.510270625. [DOI] [PubMed] [Google Scholar]
- 35.Desbarats J, Newell MK. Fas engagement accelerates liver regeneration after partial hepatectomy. Nat Med. 2000;6:920–923. doi: 10.1038/78688. [DOI] [PubMed] [Google Scholar]
- 36.Minagawa M, Oya H, Yamamoto S, et al. Intensive expansion of natural killer T cells in the early phase of hepatocyte regeneration after partial hepatectomy in mice and its association with sympathetic nerve activation. Hepatology. 2000;31:907–915. doi: 10.1053/he.2000.5850. [DOI] [PubMed] [Google Scholar]
- 37.Ware CF, VanArsdale TL, Crowe PD, et al. The ligands and receptors of the lymphotoxin system. Curr Top Microbiol Immunol. 1995;198:175. doi: 10.1007/978-3-642-79414-8_11. [DOI] [PubMed] [Google Scholar]
- 38.Kulkarni S, Malago M, Cronin DC II. Living donor liver transplantation for pediatric and adult recipients. Nat Clin Pract Gastroenterol Hepatol. 2006;3:149–157. doi: 10.1038/ncpgasthep0437. [DOI] [PubMed] [Google Scholar]
- 39.Clavien PA, Petrowsky H, DeOliveira ML, et al. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545–1559. doi: 10.1056/NEJMra065156. [DOI] [PubMed] [Google Scholar]
- 40.Matsui H, Hikichi Y, Tsuji I, et al. LIGHT, a member of the tumor necrosis factor ligand superfamily, prevents tumor necrosis factor-α-mediated human primary hepatocyte apoptosis, but not Fas-mediated apoptosis. J Biol Chem. 2002;277:50054–50061. doi: 10.1074/jbc.M206562200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials and Methods
Histology, BrdU labeling, and TUNEL labeling
Tissue sections were fixed in 10% buffered formalin and processed either for routine H&E staining or BrdU and TUNEL immunohistochemical studies. TUNEL staining was performed on paraffin-embedded, formalinfixed tissue using the ApopTag Plus Peroxidase In Situ Apoptosis Detection kit (Chemicon, Madison, WI) according to the manufacturer's directions. BrdU solution (1 mg BrdU in phosphate-buffered saline [PBS]) was injected intraperitoneally 2 hours prior to analysis. DNA synthesis was measured by immunohistochemical staining of paraffin liver sections with anti-BrdU antibody. Percent of BrdU-positive hepatocytes nuclei and hepatocytes in metaphase among total hepatocytes nuclei was calculated from 1000 hepatocytes per mouse.
Transaminase Activity and Cytokines Analyses
Blood was collected by retroorbital puncture, following Institutional Animal Care and Use Committee approved procedures. Animals were killed with CO2 followed by cervical dislocation, and tissues were removed and flash-frozen in liquid N2 for biochemical analyses or fixed in 10% neutral-buffered formalin for histology. Serum transaminase activities were determined using Reflotron GPT (ALT) and GOT (AST) tabs and a Reflotron Plus Chemistry Analyzer (Roche Diagnostic Corporation, Indianapolis, IN) according to the manufacturer's instructions. Concentrations of IL-6 in sera were determined by Cytokine Bead Assay (BD Biosciences, San Jose, CA) or by R&D Systems ELISA following the manufacturer's recommendations.
Cell Purification and Transfers
Intrahepatic lymphocytes were purified from the liver by pressing the liver through a steel mesh (No. 200) into PBS, centrifuged at 800g for 5 minutes with the resulting pellet suspended in a 35% Percoll-PBS-heparin (100 U/mL) solution, and centrifuged at 800g for 20 minutes at room temperature. The pellet of mononuclear cells was cleared of RBC with a 5-minute osmotic lysis (0.15 mol/L NH4Cl, 1 mmol/L KHCO3, 0.1 mmol/L Na2-EDTA, pH 7.3) and washed twice in PBS. Lymphocytes were stained with antibodies (BD Biosciences) and ana lyzed by flow cytometry (FACSCanto; BD Biosciences). For adoptive transfer experiments, 100 million total splenocytes cleared of RBC were transferred to 300-rad irradiated TCR–/– mice 1 week prior to partial hepatectomy. Cellular depletions were accomplished with anti-CD4 (GK1.5) or anti-CD8 (PK136). Two hundred micrograms of depleting antibody was injected 2 days prior to partial hepatectomy in some experiments.
Real-Time Polymerase Chain Reaction
Total RNA was extracted by RNeasy mini kit from Qiagen. For complementary DNA (cDNA) synthesis, RNA were digested with DNase I and reverse transcribed using random primers with AMV Reverse Transcriptase (Promega). The concentration of the target gene was determined using the comparative CT (threshold cycle number at a cross point between amplification plot and threshold) method and normalized to hypoxanthineguanine phosporybosyltransferase (HPRT) and β-actin. cDNA were amplified using Taqman or Power Sybr Green PCR master mix (Applied Biosystems, Foster City, CA) and run on ABI 7300 cycler (Applied Biosystems). Polymerase chain reaction primers and probes used are as follows: for IL-6, forward 5′-ACAAGTCGGAGGCTTAATTACACAT, reverse 5′-AATCAGAATTGCCATTGCACAA, probe 5′-FAM-TTCTCTGGGAAATCGTGGAAATGAGAAAAGA-TAMRA; for TNF, forward 5′-ACGGCATGGATCTCAAAGAC, reverse 5′-AGATAGCAAATCGGCTGACG; for HPRT, forward 5′-TGAAGAGCTACTGTAATGATCAGTCAAC, reverse 5′-AGCAAGCTTGCAACCTTAACCA, 5′-FAM-TGCTTTCCCTGGTTAAGCAGTACAGCCC-TAMRA; for β-actin, forward 5′-AGAGGGAAATCGTGCGTGAC, reverse, CAATAGTGATGACCTGGCCGT, probe, 5′-FAM-CACTGCCGCATCCTCCCTCTCCC-TAMRA.
Western Blots
For protein extraction, liver homogenates were prepared using NP-40 lysis buffer (20 mmol/L Hepes, pH 8.0; 350 mmol/L NaCl; 20% glycerol; 1% NP-40; 1 mmol/L MgCl2; 0.2 mmol/L EGTA, pH 8.0) supplemented with protease inhibitors (Roche Diagnostic Corporation). After 10% SDS-PAGE proteins were transferred and probed with antibodies directed at STAT3 and p-705 STAT3 (Santa Cruz Biotechnology Corp, Santa Cruz, CA) and developed using enhanced chemiluminescence (ECL; Amersham Biosciences, Piscataway, NJ).
Supplementary Table 1. Cell Numbers in Liver of T Cell-Deficient Mice After Adoptive Splenocyte Transfer
Supplementary Figure 1. Reduced DNA synthesis in LTβ–/– mice at 38 hours after PH. (A) Representative images of H&E (upper panel) and BrdU incorporation (lower panel) at 38 hours after PH are shown. Original magnification, ×400. (B) The amount of DNA synthesis was determined using BrdU incorporation at 38 hours after PH. N = 5 mice per group. ***P < .001.
Supplementary Figure 2. Kinetics of liver inury after partial hepatectomy. Serum ALT levels of WT and LTβ–/– mice were measured at 6 hours, 24 hours, and 36 hours after PH. LTβ–/– mice show an increased liver injury during entire time period, whereas WT mice recover from liver damage at 36 hours after PH. N = 5 mice per group. *P < .05.
Supplementary Figure 3. Stimulation of LTβR signaling reduces liver injury of Rag mice 24 hours after PH. Rag1–/– mice were injected IV with 75 μg of anti-LTβR agonistic antibody (ACH6) immediately after PH. ALT activity in serum of anti-LTβR antibody treated mice at 24 hours after PH, 6 mice per group. *P < .05.
Supplementary Figure 4. Stimulation of LTβR signaling induces STAT3 phosphorylation after PH. Rag1–/– mice were injected IV with 75 μg of anti-LTβR agonistic antibody (ACH6) or vehicle (untreated) immediately after PH. Liver tissue was harvest 0 or 5 hours after PH for Western blot analysis. Liver tissue was homogenized, run on SDS-PAGE, transferred to nitrocellulose membrane, and probed with antibodies for total STAT3 and phosphorylation at tyrosine residue 705 of STAT3.
Supplementary Figure 5. T-LTβ–/– mice have normal NKT cell numbers in the liver. Mononuclear cells were purified from the livers of naïve WT and T-LTβ–/– mice and NKT cells visualized by flow cytometry with CD1d tetramers and antibodies to TCRβ, NK1.1, and CD4.