Abstract
The purpose of this study was to compare viable and nonviable bilabeled mesenchymal stem cells (MSCs) in arthritic joints with magnetic resonance imaging (MRI) and optical imaging (OI). MSCs were labeled with ferucarbotran and DiD. MRI and OI of bilabeled cells were compared with controls. Six rats with arthritis received intra-articular injections of bilabeled viable MSCs into the right knee and nonviable MSCs into the left knee. Animals underwent MRI and OI preinjection and at 4, 24, 48, and 72 hours postinjection. The results were analyzed with a mixed random effects model and Fisher probability. Bilabeled MSCs showed increased MRI and OI signals compared to unlabeled controls (p < .0001). After intra-articular injection, bilabeled MSCs caused significant T2 and T2* effect on MRI and fluorescence on OI up to 72 hours postinjection (p < .05). There was no significant difference between viable and nonviable MSC signal in the knee joints; however, some of the viable cells migrated to an adjacent inflamed ankle joint (p < .05). Immunohistochemistry confirmed viable MSCs in right knee and ankle joints and nonviable MSCs in the left knee. Viable and nonviable cells could not be differentiated with MRI or OI signal intensity but were differentiated based on their ability to migrate in vivo.
THERAPEUTIC CELL TRANSPLANTATION for rheumatoid arthritis (RA) and juvenile idiopathic arthritis are being investigated in both preclinical and clinical arenas. Hematopoietic stem cell (HSC) transplantation has proven feasible in both settings by providing support during aplasia and hematopoietic reconstitution, but the results have been hampered by reports of toxicity, transient responses, and late relapses. In contrast, mesenchymal stem cells (MSCs) remain a promising therapeutic option through their ability to differentiate and as immunomodulating or antiproliferative cells not requiring sustained engraftment for clinical benefit.1,2 However, murine models of arthritis are few and have been inconsistent in demonstrating improvement after MSC transplantation.3–6
Arthritis is a major preclinical focus within the field of molecular imaging, which can investigate through non-invasive real-time in vivo imaging the complex micro-environment and biologic pathways of arthritis, potentially clarifying the mechanism of disease. Magnetic resonance imaging (MRI) is considered the clinical modality of choice for the simple anatomic evaluation of arthritis but remains limited in identifying the acuity of disease and for therapeutic monitoring. The concordant use of molecular imaging offers the possibility of significantly enhancing MRI sensitivity.7,8 For example, cell labeling with contrast agents and imaging probes has enabled in vivo tracking and insight into temporal and spatial distribution in various models of disease.9,10 T cells, macrophages, and leukocytes have been labeled and tracked with nuclear medicine, optical imaging (OI), and MRI in animal models of arthritis.7,8 To date, evaluations of HSC or MSC migration in bone injury models, including joint pathology, have been limited.1,8,11,12
Bifunctional labeling of cells for dual-modality imaging enables a synergistic fusion of techniques, thus compensating for respective weaknesses and enhancing cell tracking. Labeling cells with radiotracers and fluorochromes or bioluminescent reporters is under way, whereby established nuclear imaging (single-photon computed tomography [SPECT] or positron emission tomography [PET]) is combined with OI. This allows improved depth penetration, three-dimensional resolution, and quantification. Similarly, the three-dimensional micrometer spatial resolution of MRI can be augmented by the high sensitivity of OI.8 Radiolabels offer high sensitivity and use small biologic molecules for labeling, such as iodine and fluorine, and therefore closely resemble physiologic compounds—an advantage over other modalities that use large molecules. OI and MRI labels are relatively lower in cost, have no radiation burden, and are stable over weeks.13 Translational imaging applications favor radiation-free diagnostic tests owing to the rising concern that cumulative radiation doses by radiographic tests place younger patients at risk for additional radiation-induced cancers later in life.
Exogenous MSC labeling with superparamagnetic iron oxide (SPIO) particles has been extensively investigated with MRI. SPIO particles are nontoxic and biodegradable and cause a significant decrease in the T2 and T2* relaxation rate. Recent in vitro studies have demonstrated that viable and nonviable cells could be differentiated based on the different signal effects of intracellular and extracellular iron oxide contrast agents.14,15 For OI, near-infrared (NIR) cyanine fluorochromes represent the most prominent and sensitive class of contrast agents. DiD, a carbocyanine dye, has previously demonstrated low cytotoxicity, high labeling efficiency, stability over time, and high resistance to intracellular transfer, but no studies have compared the appearance of viable and nonviable DiD-labeled cells.16
The bifunctional labeling of MSCs and transplantation into in vivo models of arthritis have not been evaluated with dual-modality imaging, which may enhance cell tracking and the evaluation of engraftment and/or treatment failure. In addition, the ability to differentiate viable versus nonviable MSCs in vivo based on imaging characteristics, like those described in vitro, could compound the former by depicting the effect of variables, including immunomodulating factors, differentiation factors, and genetic modification on cell viability. The purpose of this study was to bilabel human mesenchymal stem cells (hMSCs) with ferucarbotran and DiD and to compare the signal effect of viable and nonviable cells after local injection into arthritic joints with OI and MRI.
Material and Methods
The Committee on Animal Research at our institution approved this study, and it was performed in accordance with the National Institutes of Health guidelines for the humane care and use of laboratory animals as described in the Guide for the Care and Use of Laboratory Animals.17
Cells and Labeling Procedures
After institutional approval and donor consent, primary hMSCs were aspirated from bone marrow in the iliac crest. Bone marrow cells were plated and incubated for 12 hours. Nonadherent cells were removed, and the remaining cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) high glucose medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) (FMB, Hyclone, Logan, UT) and 1% penicillin-streptomycin at 37°C in a humidified 5% CO2 atmosphere. The culture medium was changed every third day. The cells were divided when 90% confluent and either redistributed to new culture flasks or used for experiments. All experiments were performed between passages 10 through 16.
The cells were labeled with ferucarbotran (Resovist, Bayer Schering AG, Berlin, Germany) and DiD (C67H103CIN2O3S, Molecular Probes, Eugene, OR). Ferucarbotran is a carboxydextran-coated second-generation SPIO particle. The ionic coat of ferucarbotran prevents the iron oxide core particles from aggregating, making it hydrophilic with a net negative charge and a mean diameter of 60 nm. The R1 and R2 relaxivities are 25 mM-1s-1 and 151 mM-1s-1.18 Labeling of hMSCs with ferucarbotran was achieved by combining 75 μL of ferucarbotran and 20 mL of serum-free DMEM, which was then added to 1.5 × × 106 adherent cells in pretreated 225 cm2 flasks at a concentration of 100 μg iron per mL medium. The cells were incubated for 2 hours and then supplemented with 4 mL of FBS to prevent cell death or differentiation. After incubation for another 12 hours, the cells were trypsinized with 0.05% trypsin–ethylenediaminetetraacetic acid (EDTA) and carefully washed three times with phosphate-buffered saline (PBS) (pH 7.4) by sedimentation (5 minutes, 400 rcf, 25°C).
The ferucarbotran-labeled cell pellet was suspended and labeled with DiD, a lipophilic, cyanine NIR fluorochrome. DiD is characterized by a molecular weight of 1052.08 Da and excitation and emission maxima of 644 nm and 665 nm, respectively. Labeling with DiD was achieved by combining 5 μL of DiD and 1 mL of serum-free medium per 1.0 × 106 ferucarbotran-labeled cells. These cells were incubated for 20 minutes and then washed three times with PBS by sedimentation. All described labeling procedures, doses, and incubation times were chosen based on previously established protocols.16,19–21 Cells were counted using a hemocytometer (Neugebauer Chamber, Xenogen, Alameda, CA), and viability was tested by the trypan blue exclusion assay (Sigma-Aldrich, St. Louis, MO). The concentration of iron within the labeled hMSCs was determined by inductively coupled plasma atomic emission spectrometry (IRIS Advantage, Thermo Jarrell Ash, Franklin, MA). Representative samples of ferucarbotran-and DiD-labeled hMSCs were imaged using a Zeiss-LSM 510 confocal microscope (Carl Zeiss MicroImaging. Inc., Thornwood, NY) to confirm labeling and localization.
In Vitro Studies
The bilabeled hMSCs were suspended in 400 μL of isotonic ficoll solution at a density of 1.07 g/mL, which prevents cell sedimentation and death during imaging.14 We evaluated a decreasing concentration of labeled cells (1 × 106, 5 × 105, 2.5 × 105, 1.25 × 105, and 6.25 × 104) and unlabeled controls with OI and MRI directly after labeling; 2.5 × 105 bilabeled cells were cultured in vitro for 4, 24, 48, and 72 hours and 12 days to confirm sustained cell viability and OI and MRI signals. In addition, the OI and MRI signals of bilabeled cells were compared to the OI and MRI signals of single labeled (ferucarbotran or DiD alone) and unlabeled cells.
Apoptosis Induction
Mitomycin C, a classic inducer of P53-mediated apoptosis, was used to induce hMSC death by apoptosis according to the method described by Crowston and colleagues.22 To establish the incubation time that would maximize in vivo (posttransplantation) hMSC apoptosis, we incubated labeled and unlabeled controls with 0.5 mg of mitomycin C per mL DMEM for 1, 2, 4, 6, and 8 hours. The hMSCs were imaged with OI and MRI and tested for viability at 0 and 48 hours following mitomycin C incubation.22
Animals and Arthritis Induction
In vivo studies were performed in 11 (experimental n = 6, pilot study n = 5) 4- to 6-week-old female homozygous athymic nude rats (Harlan, Indianapolis, IN; 150–200 g), which prevented the need for myeloablation. The rats were fed a manganese-free diet (SsniffR/M-H, ssniff Spezialdiaeten GmbH, Soest, Germany) to decrease autofluorescence of the bowel. An antigen-induced arthritis was induced in the rats while under anesthesia by intraperitoneal (960 μL) and bilateral intra-articular knee injections (20 μL per knee) of 5.2 mg of PGPS10S (Fischer Scientific, Pittsburgh, PA) according to the method described by Simon and colleagues.14 The rats were observed daily for signs of arthritis (joint swelling and limping). The axial diameter of the ankle joints was measured with a caliper on the day of arthritis induction and daily thereafter. On day 3 postinjection, when a marked polyarthritis of the knee and ankle joints had developed, each rat was anesthetized (ketamine 50 mg/kg and xylazine 5 mg/kg) and received intra-articular injections of hMSCs, in 20 μL of serum-free DMEM, into their arthritic knee joints.
In the first pilot study, two rats received intra-articular injections of 250,000, 125,000, 62,500, or 31,250 hMSCs to determine the optimal cell number for maximal signal detection. Three rats were then investigated to exclude confounding variables: one rat received an intra-articular injection of DiD-labeled hMSCs, one rat received an intra-articular injection of ferucarbotran-labeled hMSCs, and one rat received an intra-articular injection of unlabeled hMSCs into the right knee joint. The left knee joints of these rats served as nontreated control joints.
Subsequently, six rats with antigen-induced arthritis received intra-articular injections of 250,000 bilabeled hMSCs in the right knee and 250,000 bilabeled hMSCs treated with mitomycin C. The hMSCs, which were injected in the left knee joint, had been incubated with 0.5 mg/mL mitomycin C for 6 hours according to the protocol described above. The mitomycin-treated hMSCs were viable on intra-articular injection.
All animals underwent OI and MRI studies preinjection and at 4, 24, 48, and 72 hours postinjection of hMSCs. Two animals underwent OI and MRI studies at 12 days postinjection.
Optical Imaging
All OI studies were performed using the IVIS 50 small-animal scanner (Xenogen, Alameda, CA) and Cy5.5 (excitation 615–665 nm and emission 695–770 nm) filter set. For in vitro studies, cell samples were placed in a nonfluorescing black container. The rats were anesthetized with isofluorane and placed in the light-tight heated (37°C) chamber. The animals were imaged in two positions at all time points: (1) anterior (facing the charge-coupled device camera) and (2) posterior. Identical illumination parameters (exposure time 2 seconds, lamp level high, filters Cy5.5 and Cy5.5 bkg, f/stop 2, field of view [FOV] 12, binning 4) were selected for each acquisition. Grayscale reference images were also obtained under low-level illumination. The reported fluorescent OI studies are in real time and unprocessed.
OI Analysis
Images were acquired and analyzed using Living Image 2.5 software (Xenogen) integrated with Igorpro (Wavemetrics, Lake Oswego, OR). Images were measured in units of average efficiency (fluorescent image is normalized by a stored reference image of the excitation light intensity; thus, images are unitless) and corrected for background signal. For all in vitro image analyses, regions of interest (ROI) were defined as the circular area of the cell samples. For all in vivo image analyses, peak signal ROI were defined as 50% of maximum signal intensity by the ROI function. Background signal (normal tissue) was also calculated for each image by the ROI function.
Magnetic Resonance Imaging
All studies were performed with a 3 T MRI scanner (Signa EXCITE HD 3 T, GE Medical Systems, Milwaukee, WI). The in vitro samples were immersed in a water bath (20°C) to diminish susceptibility artifacts and scanned with a circularly polarized quadrature knee coil (Clinical MR Solutions, Brookfield, WI). Pulse sequences comprised axial spin echo (SE) sequences with multiple repetition time (TR) (4,000, 1,000, 500, 250 ms) and echo time (TE) (60, 45, 30, 15 ms) values for measurement of T1 and T2 relaxation times. T2* axial gradient echo (GE) images were obtained with a flip angle of 30°, a TR of 500 ms, and multiple TE values (28.8, 14.4, 7.2, 3.7 ms) for measurement of T2* relaxation times. All sequences were single acquisitions with an FOV of 160 × 160 mm, a matrix of 256 × 196 pixels, a slice thickness of 5 mm, and a bandwidth of 15.63 Hz.
For in vivo MRI studies, the rats were anesthetized (ketamine 50 mg/kg; xylazine 5 mg/kg) and then placed supine within a circularly polarized Mayo BCID wrist coil (Mayo Foundation, Rochester, MN) and shielded to preserve body temperature. Sagittal MRIs of the knee joints were obtained using a T1-weighted SE sequence (TR 500 ms, TE 15 ms, bandwidth (BW) 15.63 Hz, FOV 12 cm, matrix 256 × 196, 2 acquisitions), a T2-weighted SE sequence (2000/15/15.63/12/256 × 196/2), and a T2*-weighted GE sequence (500/14/15.63/12/256 × 196/1, alpha 30).
MRI Analysis
MRIs of the in vitro samples were analyzed on a SUN SPARC workstation (Sun Microsystems, Mountain View, CA) using a self-written Interactive Data Language program (Research Systems, Boulder, CO). T1, T2, and T2* maps of the samples were calculated from multiecho sequences assuming a monoexponential signal decay using a nonlinear function least-square curve fitting on a pixel-by-pixel basis. Care was taken to analyze only data points with signal intensities significantly above the noise level. T1, T2, and T2* relaxation times of cell solutions were derived by ROI measurements of the test samples on these maps. R1, R2, and R2* relaxation rates were determined as 1/T1, 1/T2, and 1/T2*. Differences in relaxation rates (ΔR1, ΔR2, and ΔR2*) between bilabeled and unlabeled control samples were calculated.
T1-, T2-, and T2*-weighted MRIs of rat knee and ankle joints were evaluated by two radiologists in consensus using a score where 0 = no change in signal within the joints, 1 = minimal signal effect, and 2 = marked signal effect.
Organ Harvest, Tissue Processing, Histology, and Analyses
After the last imaging study, the rats were sacrificed and the knee and ankle joints were harvested, placed in tissue holders, and frozen at −80°C to obtain frozen sections for fluorescence microscopy. Specimens were then placed in 10% paraformaldehyde and decalcified in a solution of Easy Cut Decal (DCECDGAL, American Master Tech, Lodi, CA) for 245 minutes. To minimize the chance of overdecalcification, the specimens were checked every 15 minutes. The specimens were bisected parasagittally, dehydrated through graded alcohol washes, paraffin embedded, and sectioned into 5 μm transverse slices. For evaluation of cell morphology, sections were stained with standard hematoxylin and eosin (H&E). For evaluation of DiD, the sections underwent counterstaining of the cell nuclei with diamidino-2-phenylinole (DAPI; BIOMOL Research Laboratories, Plymouth Meeting, PA; dilution 1:10,000; incubation time 1 minute). For evaluation of hMSCs within the tissues, the section underwent immunostaining with CD44, which is a known marker for hMSCs.
Statistical Analyses
A statistician at our institution performed the statistical analyses. Data were displayed as means ± standard deviations of n independent measurements. Statistical significance was assigned for p values < .05. Statistical analyses of all in vitro and in vivo OI studies were performed using a mixed effects model that includes least square means and supporting p values for both fixed and random effects. Analyses of all in vitro MRI studies were done with a paired t-test.
The effect size of the contrast enhancement for in vivo MRI studies was determined using Fisher probability. For enhanced in vivo comparison between OI and MRI, Fisher probability was also applied to the same in vivo OI results.
Results
In Vitro Studies
The trypan blue test revealed a cell viability of 98% (SD 3.8%) before and 97% (SD 2.6%) after the labeling procedure at 0, 4, 24, 48, and 72 hours and 12 days. Fluorescent microscopy demonstrated uptake of ferucarbotran in secondary lysosomes of the cell cytoplasm and integration of DiD in the cell membrane (Figure 1).
Figure 1.
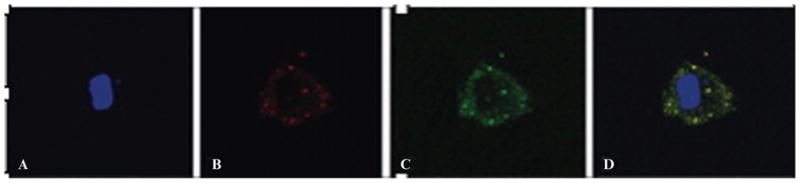
High-power (40×) fluorescent image of a ferucarbotran- and DiD-labeled human mesenchymal stem cell (hMSC). A, The blue channel demonstrates the cell’s nucleus counterstained with DAPI. B, The red channel demonstrates DiD located within the cell membrane. C, The green channel demonstrates ferucarbotran located within the cytoplasm with the antidextran counterstain. D, A fusion image (blue, red, and green channels) of the double-labeled hMSC. Scale: 10 μm.
The bilabeled cells showed a marked fluorescent signal with OI and a marked T2 and T2* effect with MRI, whereas unlabeled controls showed only minimal signal with either imaging modality (Figure 2, A, B, and D). Quantitative analyses revealed a significantly increased fluorescence signal of bilabeled cells compared to unlabeled controls at 0, 4, 24, 48, and 72 hours and 12 days (p < .0001) and a significant (p < .05) decrease in T2 and T2* relaxation rates of bilabeled cells compared to unlabeled controls. This T2 effect (hypointense signal of bilabeled hMSCs) was also apparent on T1-weighted sequences. The bilabeled cell dilution series (range 62,500 to 1 × 106) demonstrated a nonlinear increase in fluorescent signal with increasing cell concentration (p < .0001) that reached a plateau between 5 × 105 and 1 × 106 cells (see Figure 2, A and B, and Figure 3). Likewise, the T2 and T2* effect increased nonlinearly (see Figure 2D and Figure 3).
Figure 2.
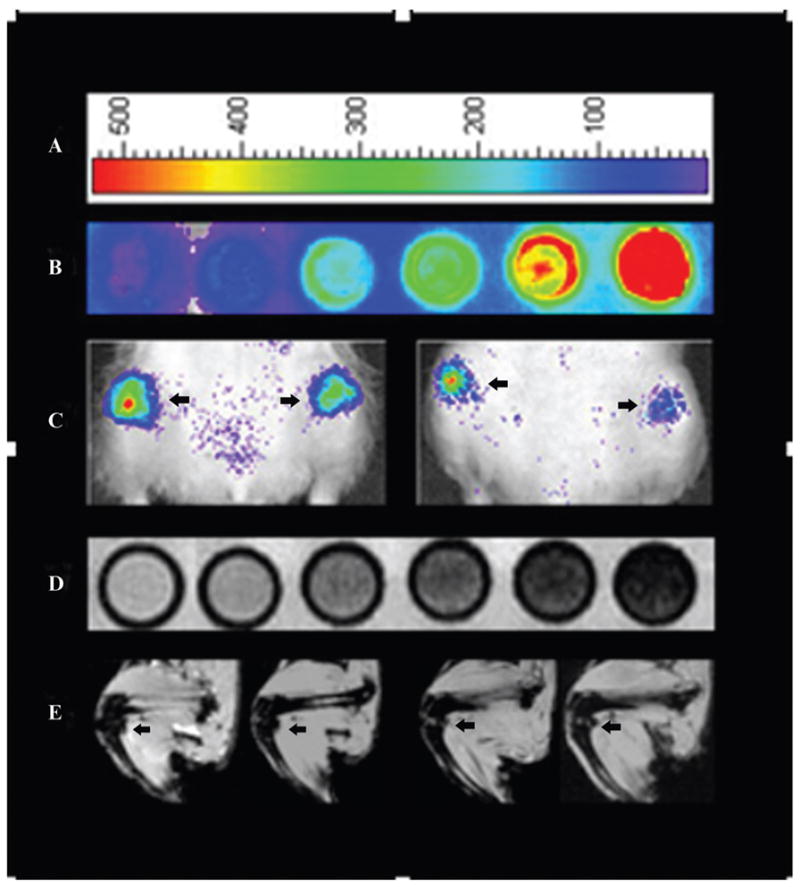
A, Optical image (OI) representative color scale. B, OI of in vitro bilabeled human mesenchymal stem cells (hMSCs) in increasing cell concentration from left to right: control, 62,500, 125,000, 250,000, 500,000, and 1,000,000. C, OI of intra-articular bilabeled hMSCs in decreasing cell concentration from left to right, as defined by an arrow pointing to each joint: 250,000, 125,000, 62,500, and 31,250. D, T2* magnetic resonance imaging (MRI) of in vitro bilabeled hMSCs in increasing cell concentration from left to right: control, 62,500, 125,000, 250,000, 500,000, and 1,000,000. E, T2* MRI of in vivo bilabeled hMSCs in decreasing cell concentration from left to right, as defined by an arrow pointing to each joint: 250,000, 125,000, 62,500, and 31,250.
Figure 3.
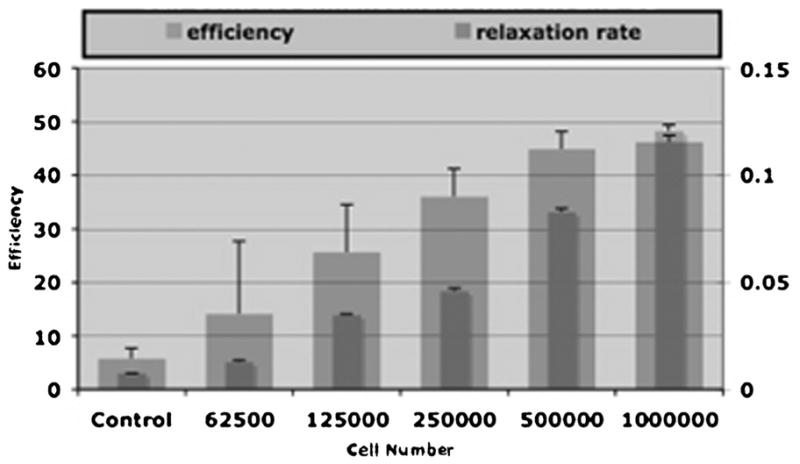
Mean signal intensities and standard deviations of increasing cell numbers of bilabeled human mesenchymal cells measured by region of interest with optical imaging (OI) (efficiency) and magnetic resonance imaging (T2* relaxation rate). There was a significant increase in OI signal with increasing cell concentration (p < .05). There was a significant decrease in the T2* relaxation rate with increasing cell concentration (p < .05).
Comparisons between bilabeled cells and single contrast agent–labeled cells (DiD or ferucarbotran alone) showed no impairment or significant difference in signal yield (p > .05). hMSCs labeled with DiD alone did not reveal any significant contrast effect on MRI compared to unlabeled cells (p > .05), and hMSCs labeled with ferucarbotran alone did not reveal a significantly different fluorescent signal compared to unlabeled controls on OI (Cy5.5 filter set) (p > .05).
Apoptosis Induction
Mitomycin C induced cell death by apoptosis, as determined by the trypan blue assay. The viability of the bilabeled and unlabeled hMSCs at 0 and 48 hours was dependent on the incubation time. The 6-hour mitomycin C incubation time resulted in 98% and 10% bilabeled hMSC viability at 0 and 48 hours, respectively. The 8-hour mitomycin C incubation time resulted in 80% and 5% bilabeled hMSC viability at 0 and 48 hours, respectively. The 1-, 2-, and 4-hour incubation times demonstrated a significantly lower percentage of apoptosis than that observed after the 6- and 8-hour incubation times. The 6-hour mitomycin C incubation time was used for the in vivo studies to maximize posttransplantation apoptosis.
In Vivo Studies
All 11 animals developed polyarthritis in both knee and ankle joints 2 to 3 days after antigen induction with clinical signs (swelling and limping) and increasing ankle diameter of 5 to 7 mm compared to baseline measurements.
Optical Imaging
Anterior and posterior OI baseline images of the lower extremities before hMSC injection revealed minimal autofluorescence of the knee and ankle joints, which was at least two folds of magnitude less than any subsequently observed contrast agent–induced signal. The joints that did not receive any intra-articular injection revealed similar minimal autofluorescence that did not change up to 72 hours postinjection.
Pilot Study
Directly after intra-articular injection of different quantities of bilabeled hMSCs into arthritic knee joints, the knee joints showed a significant fluorescent signal compared to precontrast internal control images, which increased with cell number (p < .0001) (Figure 2C). The fluorescent signal from the bilabeled hMSCs was not significantly different from that of DiD-labeled hMSCs in vivo (p > .05). Ferucarbotran-labeled and unlabeled cells were not detectable within the joints with OI.
Experimental Cohort
Knee Joints
The fluorescent signals of both viable and apoptotic bilabeled hMSCs were significantly increased on all postinjection images compared to preinjection images up to 72 hours postinjection (p = .0022 for each time point).
The fluorescent signal intensity of the right knees (ferucarbotran-DiD hMSCs) and the left knees (ferucarbotran-DiD hMSCs + mitomycin) were not significantly different up to 72 hours postinjection (p > .05) (Figure 4).
Figure 4.
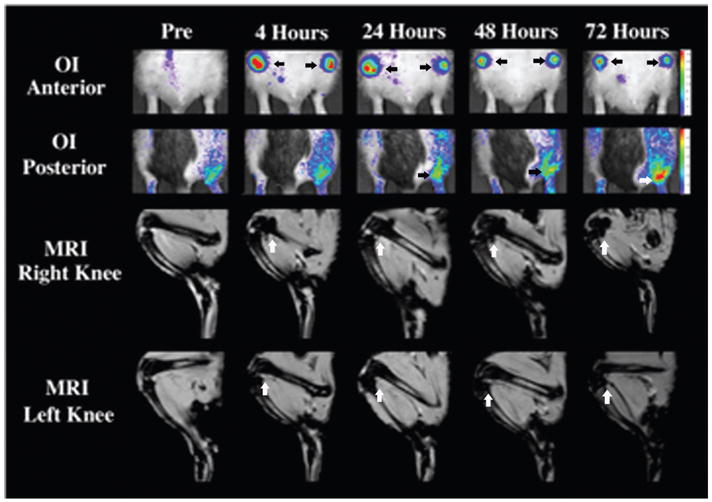
Representative optical images (OI) and magnetic resonance images (MRI) (T2*) scan of the lower extremities of one rat with antigen-induced arthritis that received intra-articular injections of ferucarbotran- and DiD-labeled human mesenchymal stem cells in the right knee and double-labeled cells treated with mitomycin C in the left knee. All time points are included, and an arrow defines relevant joints. OI anterior demonstrates the fluorescent signal of the knees postinjection up to 72 hours, which was not significantly different between the right and the left. OI posterior demonstrates the increased fluorescent signal of the right ankle joint between 24 and 72 hours postinjection, revealing migration of the double-labeled cells. T2* MRI of the knee joints demonstrates a significantly decreased signal at all time points postinjection up to 72 hours, which was not significantly different between the right and left joints.
Ankle Joints
Four animals showed a significantly increased fluorescent signal in the right ankle at 24 (p = .05), 48 (p = .045), and 72 hours (p = .004) postinjection compared to preinjection and background data. No corresponding effect was noted in the left ankle (p > .05) (see Figure 4).
Magnetic Resonance Imaging
Baseline MRI of the lower extremities before hMSC injection revealed markedly swollen knee joints with periarticular edema and moderate joint effusions. The ankle joints were more difficult to evaluate owing to partial-volume effects but also showed some soft tissue swelling and periarticular edema (see Figure 4).
Pilot Study
Directly after intra-articular injection of different quantities of bilabeled hMSCs, the knee joints showed areas of significantly decreased signal intensity on T2- and T2*-weighted MRI (p = .0022 for all cell quantities) compared to preinjection images (Figure 2E). The signal was similar, but the area and extent of signal abnormalities decreased with cell quantity (see Figure 2E). The MRI signals from the bilabeled hMSCs were not significantly different from that of ferucarbotran-labeled hMSCs (p > .05). DiD-labeled and unlabeled hMSCs were not detectable within the joints with MRI.
Experimental Cohort
Knee Joints
The MRI signal of the right (ferucarbotran-DiD hMSCs) and left (ferucarbotran-DiD hMSCs + mitomycin C) knees demonstrated significant focal signal loss in all postinjection scans compared to preinjection scans up to 72 hours postinjection (p = .0022 for both knee joints). The signal was not significantly different up to 72 hours postinjection (p > .05). Areas of focally decreased signal were detected in the suprapatellar and posterior joint recessus. The MRI signal intensities of the right and left knees were not significantly different at any time point postinjection. The MRI signal persisted in the two animals studied up to 12 days postinjection (see Figure 4 and Figure 5).
Figure 5.
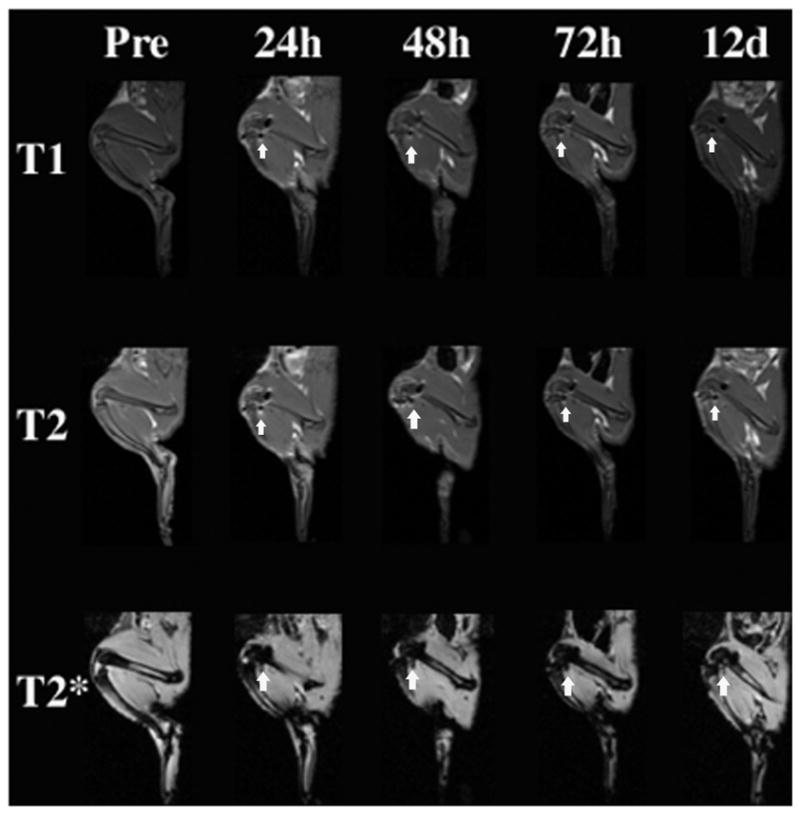
Representative magnetic resonance images of the T1, T2, and T2* sequences used to image the right and left knee joints in vivo. The decreased T2 and T2* signal of bilabeled cells was visible within the knee joints at all time points postinjection up to 12 days, as defined by arrows. The T2 effect was also apparent on T1-weighted sequences within the knee joints at all time points postinjection up to 12 days, as defined by arrows.
Ankle Joints
There was no definite signal change in the right or left ankle joints. However, evaluation of these joints was limited owing to their small size, potential partial-volume effects, and susceptibility artifacts from surrounding air (see Figure 4 and Figure 5).
Histology
H&E stain of all knee and ankle joints demonstrated features consistent with arthritis (Figure 6, A and D). Both knee joints demonstrated a population of cells that demonstrated cytoplasmic positivity for CD44, demonstrating the presence of hMSCs (Figure 6, (1), (2), B, and C). The presence of hMSCs in the synovium corresponds to the significant signal on OI and MRI. The right ankle joints that demonstrated a significant signal on OI beginning at 24 hours postinjection also had a population of cells that demonstrated cytoplasmic positivity for CD44, confirming that viable bilabeled hMSCs migrate in vivo (Figure 6, E and F).
Figure 6.
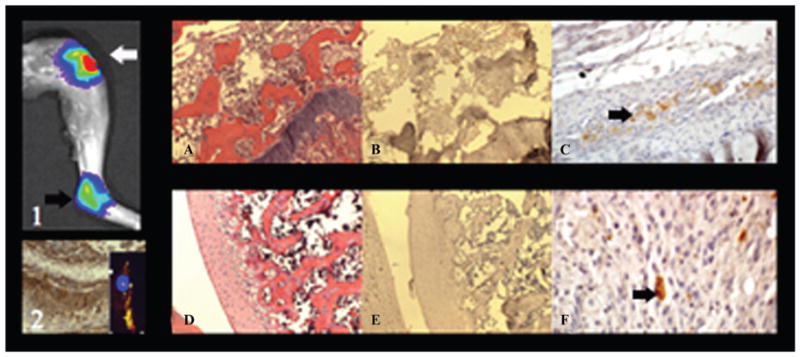
(1) Postmortem optical imaging of the right knee and ankle joint demonstrates a significant fluorescent signal and illustrates the described in vivo migration of viable bilabeled human mesenchymal stem cells (hMSCs). (2) Positive CD44 control sample from the immunohistochemistry kit and inset of a bilabeled hMSC fluorescent microscopy image. A, Hematoxylin-eosin stain of the right knee confirms arthritis with inflammatory cells and a markedly thickened synovium (× 10 original magnification). B, Corresponding CD44 stain confirms CD44-positive hMSCs in the arthritic synovium (× 10 original magnification). C, Corresponding image at a higher magnification (× 40 original magnification) demonstrates, at a single cell level, the CD44-positive hMSCs within the right knee joint, as defined by the arrow. D, Hematoxylin-eosin stain of the right ankle joint confirms arthritis with inflammatory cells (× 10 original magnification). E, Corresponding CD44 stain confirms scattered CD44-positive hMSCs within the right ankle joint (× 10 original magnification). F, Scattered CD44-positive hMSCs, as defined by the arrow (× 60 original magnification).
Discussion
To our knowledge, this is the first imaging study to evaluate bilabeled hMSCs in an arthritis model over time with OI and MRI. Our major findings are that labeling of hMSCs with ferucarbotran and DiD provides effective cell depiction with OI and MRI in vitro and in vivo. The OI and MRI contrast effect increased nonlinearly with cell concentration. There was no significant difference in the sustained contrast effect on OI or MRI between viable and nonviable hMSCs up to 72 hours postinjection. However, viable and nonviable hMSCs could be differentiated based on their migration/homing pattern in vivo because only viable cells migrated to the ipsilateral arthritic ankle joints as demonstrated with OI and confirmed with histology.
The domain of exogenous bifunctional labeling of stem cells for in vivo tracking remains very limited.
There are three approaches to bifunctional cell labeling for cell depiction with OI and MRI:
Several investigators have attached a fluorescent dye to an MRI contrast agent, thereby creating one contrast agent molecule that can be depicted with both OI and MRI. Examples of such bifunctional contrast agents, being investigated in vitro, include gadolinium-based DO3A-ethylamine–derived agents,23 gadofluorine M attached to a Cy dye,24 arginyl peptides cross-linked to nanoparticles and attached to Cy5.5, and lanthanide chelators (diethylenetriaminepentaacetic acid [DTPA] and tetraazacyclododecane-tetraacetic acid [DOTA]) bound to gadolinium and covalently attached to fluorescent and multifunctional nanoprobes.25 Bifunctional probes that have been used for in vivo cell tracking studies include gadophrin-2, gadofluorine-Cy3, and nanoparticles with attached fluorochromes.26 The disadvantage of these probes is that their optimal concentration for depiction with MRI is usually much higher compared to their optimal concentration for OI. Thus, internalized concentrations of these agents for cell labeling purposes usually represent a suboptimal compromise between the desired concentrations for the two imaging modalities.
Other investigators have introduced MRI contrast agents into genetically modified cells with intrinsic fluorescence (eg, green fluorescent protein [GFP] expression). This method was mainly used to correlate MRI data of transplanted cells with direct depiction of the same cells on histopathologic specimens.27,28 The disadvantage of this technique is in the translation for clinical application as the cells are genetically engineered. Further, OI studies using GFP-expressing cells have poor tissue penetration owing to the increased excitation and emission maxima compared to NIR labels.29
Yet other investigators achieved bifunctional cell labeling by the internalization of separate fluorescent and MRI contrast agents within their target cells. This approach has the advantage that labeling can be optimized for both OI and MRI. This approach has been applied for in vivo cell tracking with MRI and postmortem correlation with fluorescent microscopy.13,30–32 The approach has been used for neural stem cells (NSCs) labeled with PKH26 (fluorescent dye) and SPIO particles,13 NSC labeled with gadolinium rhodamine dextran,30,32 and MSCs labeled with magnetic silica nanoparticles.31
To date, no studies have used bifunctional fluorescent iron oxide labels for in vivo dual-modality imaging of MSCs, allowing us to establish proof of concept. This study was the first to demonstrate the detection and stable signal of bilabeled hMSCs with OI and MRI over several days. Synergistic imaging combined the single-cell sensitivity of OI, resulting in the detection of viable bilabeled hMSC migration to the right ankle joint. The high spatial and anatomic resolution of MRI allowed us to visualize the intra-articular hMSC distribution.
We chose ferucarbotran, a clinically applicable iron oxide–based contrast agent (ie, approved for clinical use in Europe and Japan), because its size and physiochemical surface properties allow for efficient internalization into stem cells by simple incubation without transfection agents. It is compartmentalized within secondary lysosomes, where it is slowly metabolized over time.33,34 Other SPIO particles would provide the same sensitivity for cell detection with MRI but require more complicated labeling procedures for stem cells. Another alternative would be gadolinium chelates, which have the advantage of providing T1 contrast (positive contrast) depending on the environment and image weighting27 instead of the described decrease in T2 signal seen with iron oxides that could be confused with bleeding or air. However, the sensitivity of gadolinium chelates is several magnitudes lower than that of iron oxides.
We chose DiD because it is an NIR probe and effectively labels cells by simple incubation. It localizes to a different cell compartment, the cell membrane, than ferucarbotran, thereby minimizing interferences between the two labels. In addition, DiD has demonstrated low cytotoxicity, high labeling efficiency, and high resistance to intracellular transfer.14 Research on exogenous NIR fluorescent probes is extremely broad. Numerous alternative fluorescent agents can be used for double labeling; however, cyanine dyes such as DiD represent the most prominent class and have proven effective for in vivo cell tracking with OI.29,35,36
The effect of OI and MRI contrast agents on cell properties and environment is imperative for the progression of preclinical research but remains to be entirely addressed.27 Potential impairments in the viability of labeled stem cells compared to nonlabeled controls have been reported to be dependent on the type of cells being investigated, the type of contrast agent, and the contrast agent dose, concentration, and incubation time.29 Iron oxide particles are biocompatible, and the viability of iron oxide–labeled MSCs appears to be most related to the internalized iron load. When labeled at less than 10 pg/cell, iron oxides appear to be slowly incorporated into the regular iron metabolism and do not change the physiology of the cells.15,37–42 Likewise, cyanine dyes could cause a dose-dependent toxic effect to the nucleus.43 The ferucarbotran and DiD concentrations in our MSCs did not show any effect on cell viability, as determined by the relatively simple trypan blue exclusion test.
Recent studies reported different MRI signal intensities in vitro of viable versus nonviable iron oxide–labeled MSC whereby the intracellular iron oxides in viable hMSCs provided a significantly decreased T2 signal compared to released iron oxides from MSCs that had undergone apoptosis.15 With this study, we evaluated if the same effect could be observed in vivo. Our results showed that the cell’s functional fate could not be characterized with OI or MRI based on the signal characteristics in vivo. An explanation for the observed difference is that cell death can occur via (1) cell necrosis, which is associated with cell lysis and release of iron oxides, or (2) apoptosis, which is associated with fragmentation but not lysis (ie, no release of iron oxides). The in vitro studies were done using a model of cell necrosis, whereas our experiments used a model of cell apoptosis.15 Given that iron oxides are not released in the latter, their T2 effect is expected to change less than in the necrosis model. The heterogeneous distribution of iron oxide–labeled MSCs within inflamed joints of variable volume and variable effusion may create additional confounding factors, which may mask subtle differences in T2 effects between iron oxide–labeled viable and apoptotic cells. However, we were able to differentiate viable and nonviable cells based on their physiology in vivo: viable cells migrated to adjacent joints, whereas apoptotic cells did not. The identification of apoptotic and dead stem cells with noninvasive diagnostic studies would have important implications for improvement and management of stem cell transplants. Thus, other strategies to identify dead stem cells are being explored, such as intrinsic contrast reporters of gene expression and smart probes that “turn on” with expression of a particular enzyme associated with cell death.39 However, modification of a cell’s genetic profile through the introduction of transgenes is an inherent risk and would not be a clinically feasible option. Thus, further studies are needed to define the normal physiology of stem cell homing and engraftment as well as clinically applicable diagnostic techniques to detect deviations from this “normal” process.
The labeling techniques that are used with OI and MRI were founded on radionuclide methods (eg, 111In-oxine, 111In-tropolonate, and 99mTc-HMPAO).44,45 Radionuclide labeling can be done by either simple incubation or transfection depending on the desired probe. Various agents, including 99mTc, 67Ga, and 111In, have been used to successfully label and track cells for the evaluation of RA. The advantages of radiotracers include sensitive tissue penetration, easy signal quantification, high spatial resolution, and rapidity. It is the only imaging modality that has been used to track cells in humans. However, the equipment is complex and expensive, the probes are radioactive and can only be produced at certain times, and their half-life is less than that defined for OI and MRI.8 Nevertheless, novel agents such as 99mTc-HMPAO have successfully been used to track cells in arthritic models and offer distinct improvements, including relatively low cost, ease of use, low radiation burden, general accessibility, and high sensitivity.46 Of note, no nuclear medicine studies have characterized stem cell viability before and after exposure to radiotracers.
We are aware of several limitations in our work. Our model of immune-mediated arthritis was transient and evolved over time, with the disease most severe at 7 days postinduction (as defined by daily quantitative joint measurements), and slowly resolved thereafter, thus precluding extended observations. The chosen disease model was to establish an effective method and proof of concept; however, the evaluation of normal controls would have provided additional information. The number of rats investigated was limited to the minimal defined number according to power calculations. Known limitations exist with OI-based cell tracking, including limited depth of penetration, limited quantification, and poor spatial resolution owing to scatter.47 The bifunctional label requires two steps of incubation with contrast agents. Although both labeling techniques take place by simple incubation, their combination results in increased cell loss compared to a single labeling procedure. The internalized contrast agents undergo a dilution effect with proliferation, thus leading to an inherent decrease in signal over time. However, our studies show that we obtained significant OI and MRI signal above-baseline levels for several days. Of note, a recent study from our group demonstrated that ferucarbotran-DiD labeling of hMSCs does not interfere with morphologic differentiation into chondrocytes, but bilabeled cells do exhibit significantly less glycosaminoglycans (GAG) production compared to unlabeled cells.48 No other studies have investigated the differentiation of bilabeled cells in vitro or in vivo. The majority of reports on the differentiation capacity of contrast agent–labeled stem cells focus on MRI contrast agents and have used iron oxide–based particles over gadolinium-based chelates.38,42 Thus, further studies have to evaluate the differentiation potential of bifunctional labels. Finally, additional histopathologic correlation demonstrating colocalization of the CD44 marker with the bilabel (ferucarbotran and DiD) would have further strengthened our results. Nevertheless, prior experiments have shown that DiD is not released from the hMSCs in vivo up to 72 hours postinjection and that free DiD provides a very low fluorescent signal with OI in vivo and is rapidly eliminated via renal excretion.12
Double labeling of cells for visualization with OI and MRI has potential clinical applications. MRI is established as the imaging modality of choice for musculoskeletal disease but is not sensitive or specific in the diagnosis or therapeutic monitoring of arthritis. OI, a newer imaging modality, is gradually being used in the clinical setting and is feasible for imaging small joints transcutaneously and larger joints intra-articularly. Thus double labeling of cells can be seen as complementary and an extension of current clinical practice.7,8 Specifically, double labeling would allow a clinician to monitor the effectiveness of transplantation through long-term MRI follow-up studies of cells and consequently assist in determining the therapeutic benefit and prognosis. This would also provide earlier insight into the need to make appropriate changes if the therapy is not effective. OI could be used for visualization of cells during transplantation (eg, with arthroscopy) and as a rapid and inexpensive means of documenting cell location posttransplantation. Further, preclinical double labeling studies also have indirect benefits to clinical practice in that animal models allow us to perform studies that are difficult, if not impossible, in humans. They also allow us to facilitate imaging technology improvements that can be eventually applied to human beings.29,45
Conclusion
The combination of ferucarbotran and DiD provides an easy, sensitive, and stable label for stem cells, allowing accurate and repetitive detection of MSCs in arthritic joints with MRI and OI. Viable and nonviable cells could not be differentiated based on signal intensity in arthritic joints but based on their ability to migrate in vivo. Bifunctional imaging is a technique that could potentially improve the evaluation of novel stem cell treatments and understanding of biologic determinants in stem cell therapy and inflammatory diseases.
Acknowledgments
We thank Hubert Kim, MD, PhD (orthopedic surgeon, director of the Cartilage Repair and Regeneration Center at the University of California, San Francisco Orthopedic Center), for providing hMSCs for this project; Margaret Mayes, BA, MS (Department of Pathology and Laboratory Medicine at the University of California, San Francisco), for processing specimens for histopathologic analyses; Guido Piontek, PhD (Division of Neuropathology, Institute of Pathology, Technical University of Munich, Germany), for processing specimens for histopathologic analyses; and Alison Sutton, BA, MBA (graphic designer, Montreal, QC), for assistance in preparing images for the manuscript.
Footnotes
Financial disclosure of authors: This work was supported, in part, by a seed grant from the Society for Pediatric Radiology and by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant number NIH R01AR054458).
Financial disclosure of reviewers: None reported.
References
- 1.van Laar JM, Tyndall A. Adult stem cells in the treatment of autoimmune diseases. Rheumatology (Oxford) 2006;45:1187–93. doi: 10.1093/rheumatology/kel158. [DOI] [PubMed] [Google Scholar]
- 2.De Kleer IM, Brinkman DM, Ferster A, et al. Autologous stem cell transplantation for refractory juvenile idiopathic arthritis: analysis of clinical effects, mortality, and transplant related morbidity. Ann Rheum Dis. 2004;63:1318–26. doi: 10.1136/ard.2003.017798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Djouad F, Fritz V, Apparailly F, et al. Reversal of the immunosuppressive properties of mesenchymal stem cells by tumor necrosis factor alpha in collagen-induced arthritis. Arthritis Rheum. 2005;52:1595–603. doi: 10.1002/art.21012. [DOI] [PubMed] [Google Scholar]
- 4.Stoop R, Albrecht D, Gaissmaier C, et al. Comparison of marker gene expression in chondrocytes from patients receiving autologous chondrocyte transplantation versus osteoarthritis patients. Arthritis Res Ther. 2007;9:R60. doi: 10.1186/ar2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kostura L, Kraitchman DL, Mackay AM, et al. Feridex labeling of mesenchymal stem cells inhibits chondrogenesis but not adipogenesis or osteogenesis. NMR Biomed. 2004;17:513–7. doi: 10.1002/nbm.925. [DOI] [PubMed] [Google Scholar]
- 6.Jorgensen C, Gordeladze J, Noel D. Tissue engineering through autologous mesenchymal stem cells. Curr Opin Biotechnol. 2004;15:406–10. doi: 10.1016/j.copbio.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Biswal S. Molecular imaging of musculoskeletal diseases. Semin Musculoskelet Radiol. 2003;7:317–50. doi: 10.1055/s-2004-815679. [DOI] [PubMed] [Google Scholar]
- 8.Mayer-Kuckuk P, Boskey AL. Molecular imaging promotes progress in orthopedic research. Bone. 2006;39:965–77. doi: 10.1016/j.bone.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Frangioni JV, Hajjar RJ. In vivo tracking of stem cells for clinical trials in cardiovascular disease. Circulation. 2004;110:3378–83. doi: 10.1161/01.CIR.0000149840.46523.FC. [DOI] [PubMed] [Google Scholar]
- 10.Koransky ML, Ip TK, Wu S, et al. In vivo monitoring of myoblast transplantation into rat myocardium. J Heart Lung Transplant. 2001;20:188–9. doi: 10.1016/s1053-2498(00)00392-2. [DOI] [PubMed] [Google Scholar]
- 11.Gheysens O, Lin S, Cao F, et al. Noninvasive evaluation of immunosuppressive drug efficacy on acute donor cell survival. Mol Imaging Biol. 2006;8:163–70. doi: 10.1007/s11307-006-0038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutton EJ, Boddington SE, Nedopil AJ, et al. An optical imaging method to monitor stem cell migration in a model of immune-mediated arthritis. Opt Express. 2009;17:24403–13. doi: 10.1364/OE.17.024403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flexman JA, Minoshima S, Kim Y, Cross DJ. Magneto-optical labeling of fetal neural stem cells for in vivo MRI tracking. Conf Proc IEEE Eng Med Biol Soc. 2006;1:5631–4. doi: 10.1109/IEMBS.2006.259982. [DOI] [PubMed] [Google Scholar]
- 14.Simon GH, Bauer J, Saborovski O, et al. T1 and T2 relaxivity of intracellular and extracellular USPIO at 1.5T and 3T clinical MR scanning. Eur Radiol. 2006;16:738–45. doi: 10.1007/s00330-005-0031-2. [DOI] [PubMed] [Google Scholar]
- 15.Henning TD, Wendland MF, Golovko D, et al. Relaxation effects of ferucarbotran-labeled mesenchymal stem cells at 1.5T and 3T: discrimination of viable from lysed cells. Magn Reason Med. 2009;62:324–32. doi: 10.1002/mrm.22011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon GH, Daldrup-Link HE, Kau J, et al. Optical imaging of experimental arthritis using allogeneic leukocytes labeled with a near-infrared fluorescent probe. Eur J Nucl Med Mol Imaging. 2006;33:998–1006. doi: 10.1007/s00259-006-0081-y. [DOI] [PubMed] [Google Scholar]
- 17.Guide for the care and use of laboratory animals. 28. Vol. 25. Bethesda (MD): National Institutes of Health; 1996. [Google Scholar]
- 18.Wang YX, Hussain SM, Krestin GP. Superparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR imaging. Eur Radiol. 2001;11:2319–31. doi: 10.1007/s003300100908. [DOI] [PubMed] [Google Scholar]
- 19.Daldrup-Link HE, Meier R, Rudelius M, et al. In vivo tracking of genetically engineered, anti-HER2/neu directed natural killer cells to HER2/neu positive mammary tumors with magnetic resonance imaging. Eur Radiol. 2005;15:4–13. doi: 10.1007/s00330-004-2526-7. [DOI] [PubMed] [Google Scholar]
- 20.Metz S, Bonaterra G, Rudelius M, et al. Capacity of human monocytes to phagocytose approved iron oxide MR contrast agents in vitro. Eur Radiol. 2004;14:1851–8. doi: 10.1007/s00330-004-2405-2. [DOI] [PubMed] [Google Scholar]
- 21.Rudelius M, Daldrup-Link HE, Heinzmann U, et al. Highly efficient paramagnetic labelling of embryonic and neuronal stem cells. Eur J Nucl Med Mol Imaging. 2003;30:1038–44. doi: 10.1007/s00259-002-1110-0. [DOI] [PubMed] [Google Scholar]
- 22.Crowston JG, Akbar AN, Constable PH, et al. Antimetabolite-induced apoptosis in Tenon’s capsule fibroblasts. Invest Ophthalmol Vis Sci. 1998;39:449–54. [PubMed] [Google Scholar]
- 23.Mishra A, Pfeuffer J, Mishra R, et al. A new class of Gd-based DO3A-ethylamine-derived targeted contrast agents for MR and optical imaging. Bioconjug Chem. 2006;17:773–80. doi: 10.1021/bc050295b. [DOI] [PubMed] [Google Scholar]
- 24.Henning TD, Saborowski O, Golovko D, et al. Cell labeling with the positive MR contrast agent gadofluorine M. Eur Radiol. 2007;17:1226–34. doi: 10.1007/s00330-006-0522-9. [DOI] [PubMed] [Google Scholar]
- 25.Veiseh O, Sun C, Gunn J, et al. Optical and MRI multifunctional nanoprobe for targeting gliomas. Nano Lett. 2005;5:1003–8. doi: 10.1021/nl0502569. [DOI] [PubMed] [Google Scholar]
- 26.Daldrup-Link HE, Rudelius M, Metz S, et al. Cell tracking with gadophrin-2: a bifunctional contrast agent for MR imaging, optical imaging, and fluorescence microscopy. Eur J Nucl Med Mol Imaging. 2004;31:1312–21. doi: 10.1007/s00259-004-1484-2. [DOI] [PubMed] [Google Scholar]
- 27.Sumner JP, Conroy R, Shapiro EM, et al. Delivery of fluorescent probes using iron oxide particles as carriers enables in-vivo labeling of migrating neural precursors for magnetic resonance imaging and optical imaging. J Biomed Opt. 2007;12:051504. doi: 10.1117/1.2800294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oweida AJ, Dunn EA, Karlik SJ, et al. Iron-oxide labeling of hematogenous macrophages in a model of experimental auto-immune encephalomyelitis and the contribution to signal loss in fast imaging employing steady state acquisition (FIESTA) images. J Magn Reson Imaging. 2007;26:144–51. doi: 10.1002/jmri.21005. [DOI] [PubMed] [Google Scholar]
- 29.Sutton EJ, Henning TD, Pichler BJ, et al. Cell tracking with optical imaging. Eur Radiol. 2008;18:2021–32. doi: 10.1007/s00330-008-0984-z. [DOI] [PubMed] [Google Scholar]
- 30.Brekke C, Morgan SC, Lowe AS, et al. The in vitro effects of a bimodal contrast agent on cellular functions and relaxometry. NMR Biomed. 2007;20:77–89. doi: 10.1002/nbm.1077. [DOI] [PubMed] [Google Scholar]
- 31.Lu CW, Hung Y, Hsiao JK, et al. Bifunctional magnetic silica nanoparticles for highly efficient human stem cell labeling. Nano Lett. 2007;7:149–54. doi: 10.1021/nl0624263. [DOI] [PubMed] [Google Scholar]
- 32.Modo M, Cash D, Mellodew K, et al. Tracking transplanted stem cell migration using bifunctional, contrast agent-enhanced, magnetic resonance imaging. Neuroimage. 2002;17:803–11. [PubMed] [Google Scholar]
- 33.Bulte JW, Douglas T, Witwer B, et al. Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat Biotechnol. 2001;19:1141–7. doi: 10.1038/nbt1201-1141. [DOI] [PubMed] [Google Scholar]
- 34.Daldrup-Link HE, Rudelius M, Oostendorp RA, et al. Targeting of hematopoietic progenitor cells with MR contrast agents. Radiology. 2003;228:760–7. doi: 10.1148/radiol.2283020322. [DOI] [PubMed] [Google Scholar]
- 35.Chudakov DM, Lukyanov S, Lukyanov KA. Fluorescent proteins as a toolkit for in vivo imaging. Trends Biotechnol. 2005;23:605–13. doi: 10.1016/j.tibtech.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Perlitz C, Licha K, Scholle FD, et al. Comparison of two tricarbocyanine-based dyes for fluorescence optical imaging. J Fluoresc. 2005;15:443–54. doi: 10.1007/s10895-005-2636-x. [DOI] [PubMed] [Google Scholar]
- 37.Arbab AS, Yocum GT, Rad AM, et al. Labeling of cells with ferumoxides-protamine sulfate complexes does not inhibit function or differentiation capacity of hematopoietic or mesenchymal stem cells. NMR Biomed. 2005;18:553–9. doi: 10.1002/nbm.991. [DOI] [PubMed] [Google Scholar]
- 38.Bulte JW, Kraitchman DL. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004;17:484–99. doi: 10.1002/nbm.924. [DOI] [PubMed] [Google Scholar]
- 39.Bulte JW, Kraitchman DL, Mackay AM, Pittenger MF. Chondrogenic differentiation of mesenchymal stem cells is inhibited after magnetic labeling with ferumoxides. Blood. 2004;104:3410–2. doi: 10.1182/blood-2004-06-2117. author reply 3412–3. [DOI] [PubMed] [Google Scholar]
- 40.Hinds KA, Hill JM, Shapiro EM, et al. Highly efficient endosomal labeling of progenitor and stem cells with large magnetic particles allows magnetic resonance imaging of single cells. Blood. 2003;102:867–72. doi: 10.1182/blood-2002-12-3669. [DOI] [PubMed] [Google Scholar]
- 41.Hoehn M, Wiedermann D, Justicia C, et al. Cell tracking using magnetic resonance imaging. J Physiol. 2007;584:25–30. doi: 10.1113/jphysiol.2007.139451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henning TD, Sutton EJ, Kim A, et al. The influence of ferucarbotran on the chondrogenesis of human mesenchymal stem cells. Contrast Media Mol Imaging. 2009;4:165–73. doi: 10.1002/cmmi.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kassab K. Photophysical and photosensitizing properties of selected cyanines. J Photochem Photobiol B. 2002;68:15–22. doi: 10.1016/s1011-1344(02)00325-1. [DOI] [PubMed] [Google Scholar]
- 44.Becker W, Emmrich F, Horneff G, et al. Imaging rheumatoid arthritis specifically with technetium 99m CD4-specific (T-helper lymphocytes) antibodies. Eur J Nucl Med. 1990;17:156–9. doi: 10.1007/BF00811445. [DOI] [PubMed] [Google Scholar]
- 45.Biswal S, Resnick DL, Hoffman JM, Gambhir SS. Molecular imaging: integration of molecular imaging into the musculoskeletal imaging practice. Radiology. 2007;244:651–71. doi: 10.1148/radiol.2443060295. [DOI] [PubMed] [Google Scholar]
- 46.Gaal J, Mezes A, Siro B, et al. 99m Tc-HMPAO labelled leukocyte scintigraphy in patients with rheumatoid arthritis: a comparison with disease activity. Nucl Med Commun. 2002;23:39–46. doi: 10.1097/00006231-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 47.Weissleder R. A clearer vision for in vivo imaging. Nat Biotechnol. 2001;19:316–7. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- 48.Boddington SE, Sutton EJ, Henning TD, et al. Labeling human mesenchymal stem cells with fluorescent contrast agents: the biological impact. Mol Imaging Biol. 2010 Apr 9; doi: 10.1007/s11307-010-0322-0. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


