Abstract
Epithelial ovarian cancer is one of the most lethal gynecologic cancers and the fifth most frequent cause of female cancer deaths in the United States. Despite dramatic treatment successes in other cancers through the use of molecular agents targeted against genetically defined events driving cancer development and progression, very few insights into epithelial ovarian cancer have been translated from the laboratory to the clinic. If advances are to be made in the early diagnosis, prevention, and treatment of this disease, it will be critical to characterize the common and private (personalized) genetic defects underlying the development and spread of epithelial ovarian cancer. The tumor suppressor Kruppel-like factor 6 and its alternatively spliced, oncogenic isoform, Kruppel-like factor 6 splice variant 1, are members of the Kruppel-like zinc finger transcription factor family of proteins, which have diverse roles in cellular differentiation, development, proliferation, growth-related signal transduction, and apoptosis. Inactivation of Kruppel-like factor 6 and overexpression of Kruppel-like factor 6 splice variant 1 have been associated with the progression of a number of human cancers and even with patient survival. This article summarizes our recent findings demonstrating that a majority of epithelial ovarian cancer tumors have Kruppel-like factor 6 allelic loss and decreased expression coupled with increased expression of Kruppel-like factor 6 splice variant 1. The targeted reduction of Kruppel-like factor 6 in ovarian cancer cell lines results in marked increases in cell proliferation, invasion, tumor growth, angiogenesis, and intraperitoneal dissemination in vivo. In contrast, the inhibition of Kruppel-like factor 6 splice variant 1 decreases cellular proliferation, invasion, angiogenesis, and tumorigenicity; this provides the rationale for its potential therapeutic application. These results and our recent demonstration that the inhibition of Kruppel-like factor 6 splice variant 1 can dramatically prolong survival in a preclinical mouse model of ovarian cancer are reviewed and discussed.
Keywords: Kruppel-like factor 6, Kruppel-like factor 6 splice variant 1, molecular pathogenesis, ovarian cancer
Epithelial ovarian cancer (EOC) is one of the most lethal gynecologic cancers and the fifth most frequent cause of female cancer deaths in the United States.1 Despite dramatic treatment successes in other cancers through the use of molecular agents targeted against the genetically defined, specific alterations driving cancer development and progression, very few insights into EOC have been translated from the laboratory to the clinic, and overall mortality rates remain dismal. If advances are to be made in the early diagnosis, prevention, and treatment of this disease, it will be critical to characterize the genetic defects underlying the development and spread of ovarian cancer. Such an approach will lead to the identification of biomarkers allowing both earlier detection and the development of personalized therapeutics.
The tumor suppressor Kruppel-like factor 6 (KLF6) and its alternatively spliced, oncogenic isoform, Kruppel-like factor 6 splice variant 1 (KLF6-SV1), are members of the Kruppel-like zinc finger transcription factor family of proteins, which have diverse roles in the regulation of cellular differentiation, development, proliferation, growth-related signal transduction, and apoptosis.2,3 KLF6 inactivation and KLF6-SV1 overexpression have been associated not only with the progression of a number of human cancers but more importantly with patient survival.4– 19 This article summarizes our recent findings demonstrating that a majority of EOC tumors have KLF6 allelic loss and decreased expression coupled with increased expression of KLF6-SV1. The targeted reduction of KLF6 in ovarian cancer cell lines results in marked increases in cell proliferation, invasion, tumor growth, angiogenesis, and intraperitoneal dissemination in vivo. In contrast, KLF6-SV1 inhibition decreases cellular proliferation, invasion, angiogenesis, and tumorigenicity; this highlights its potential therapeutic application. These results are reviewed, and the possibility of therapeutically targeting KLF6-SV1 for the treatment of EOC is discussed.
FRAMEWORK OF GENE DISCOVERY IN EPITHELIAL OVARIAN CANCER
As with many other cancers, altered expression and mutations in proto-oncogenes, tumor suppressor genes, and DNA repair genes are believed to be critical to the initiation, progression, and spread of ovarian cancer. In general, ovarian cancers are known to undergo complex cytogenetic aberrations and are often aneuploid.20,21 Although numerous studies have attempted to correlate and systematize these long-known cytogenetic findings, the genes that are the targets of these relatively common alterations have not been identified or proven to be bona fide ovarian cancer genes.
One of the main theoretical arguments for this lack of success in gene identification has centered on the fact that past technologies lacked the necessary resolution to identify these genes. Therefore, a number of more recent studies have turned to high-density single nucleotide polymorphism arrays to identify areas of genomic copy number changes. For example, a recent study using a 500,000 element array, with an average intermarker distance of 2500 bp, identified not only large-scale genomic copy number changes but also several hundred small regions of alterations of less than 500 kb.22 These gains and losses would not have been detected by more traditional low-resolution techniques, including cytogenetics, bacterial artificial chromosome array comparative genomic hybridization, microsatellite mapping, and even the earlier versions of single nucleotide polymorphism–based arrays. Importantly, the regions harbored within the small gains and losses identified in this most recent study contain a number of known cancer genes, including fibroblast growth factor receptor 1 and retinoblastoma 1, and this immediately elevates them to candidate status.22 A parallel genome-wide approach has used gene expression profiling to capture the global view of transcriptional changes in ovarian cancer.23,24 These studies have primarily focused on defining gene signatures as predictors of clinical outcome25–29 or chemotherapy response.30– 33 Although this offers great promise for future individualized treatment options, validation of the identified signatures in additional sample sets will be required.
Given the technological, cost, and sample set limitations inherent in these global mechanistic studies, an alternative approach has focused on characterizing the genetic mutations and pathways altered in hereditary forms of ovarian cancer. It is estimated that approximately 10% of ovarian carcinomas occur in women with a familial predisposition to this cancer.34 Two major syndromes are recognized. The first, hereditary breast-ovarian cancer syndrome (and a possible variant, site-specific ovarian cancer), is associated with mutations in breast cancer gene 1 (BRCA1) and breast cancer gene 2 (BRCA2). Estimated lifetime risk ranges from 16% to 60% in women who are carriers for BRCA1 mutations, whereas BRCA2 mutation carriers have estimated risk ranges of 16% to 27%.35– 37 The second is hereditary nonpolyposis colorectal cancer (Lynch syndrome II), which is characterized by a predisposition to colorectal, endometrial, and ovarian cancers and is associated with mutations in the genes encoding mismatch repair enzymes.38 Unfortunately, the identification of these genes has not yielded great insight into the genetic basis of sporadic cases of EOC, which represent the overwhelming majority of women. BRCA1 and BRCA2 mutations account for approximately 10% of unselected cases. Mutations in the mismatch repair genes account for approximately 2% of cases. The importance, however, of defining the existence of a hereditary predisposition cannot be overstated because this may allow for gynecologic cancer surveillance for those at risk and lead to the optimal therapeutic strategy for those women in whom EOC develops. A number of investigators have provided suggestive evidence for the benefit of prophylactic oophorectomy in females with germline mutations of these genes.39– 42 A number of caveats do exist and include findings from a recent study that controlled for the type of chemotherapy used, lead-time biases, and mean age at diagnosis; it demonstrated that BRCA-positive patients have a more favorable clinical outcome than nonhereditary EOC patients.43
A third approach to identifying tumor suppressor genes and oncogenes is the candidate gene approach. The candidate gene approach, which selects individual genes for further analysis on the basis of either their function in relevant biological pathways or their known role in other cancers, has yielded an ever increasing number of genes implicated in sporadic ovarian cancer. The caveat in these studies is that the identified genes have risk-associated genetic effects that are relatively small. Apart from providing this increasing list of candidates for future study, these molecular studies have provided evidence that each ovarian tumor subtype has a distinct set of genetic alterations.44 For example, mutations in tumor protein 53, v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog, and v-raf murine sarcoma viral oncogene homolog B1 have been reported in serous tumors, whereas endometrioid cancers have been associated with mutations in cadherin-associated protein beta 1 (β-catenin) and protein tyrosine phosphatase.44 These findings indirectly emphasize the fact that substantial molecular heterogeneity exists between the different cancer subtypes.
Using the framework of candidate gene analysis, we recently investigated the role of KLF6 and the alternatively spliced isoform KLF6-SV1 in EOC. As detailed in this article, our results suggest that the altered expression of these 2 genes is both frequent and critical to EOC development and progression.
KRUPPEL-LIKE FACTOR 6 AND KRUPPEL-LIKE FACTOR 6 SPLICE VARIANT 1 IN HUMAN CANCERS
The tumor suppressor KLF6 is a member of the Kruppel-like zinc finger transcription factor family of proteins, which are involved in regulating differentiation, development, cellular proliferation, growth-related signal transduction, and apoptosis.2,3 KLF6 was originally cloned and independently isolated by 3 different groups in different tissues: hepatic mesenchymal stellate cells,45 placental cells,46 and peripheral blood lymphocytes from a B-cell chronic lymphocytic leukemia patient.47 Although its physiological role was unknown, the group at Mount Sinai led by Scott Friedman was the first to demonstrate KLF6’s role as a tumor suppressor.4 Since then, KLF6 inactivation has been implicated in a number of human cancers, including colorectal,6 non–small cell lung,7 gastric,8 astrocytic glioma,9,10 nasopharyngeal,11 and hepatocellular carcinomas.12– 14
Beyond its role as a tumor suppressor gene, KLF6 has also established a novel paradigm in cancer genetics. We recently demonstrated in one of the largest multi-institutional association studies of prostate cancer that the presence of a germline single nucleotide polymorphism in the KLF6 gene is associated with an increased lifetime prostate cancer risk by increasing the expression of an alternatively spliced, biologically active KLF6 cytoplasmic isoform, KLF6-SV1.16 KLF6-SV1 is generated by alternative 5′-splice site selection and, because of an out-of-frame splicing event, retains only the N-terminal activation domain of KLF6 but replaces the terminal DNA-binding zinc fingers with a novel 21 amino acid carboxy domain.16 Intriguingly, although KLF6-SV1 expression is present in both normal and cancerous tissues, the expression of this isoform is significantly up-regulated in multiple cancers, including prostate cancer, glioblastoma, and lung cancer.10,18,19 Moreover, its activity directly opposes KLF6’s tumor-suppressive effects on cell proliferation, colony formation, invasion, and in vivo tumor growth.48
Expression levels of KLF6-SV1 have now been shown to have a prognostic association with lung18 and prostate19 cancers. In prostate cancer, increased expression levels of KLF6-SV1 at the time of prostatectomy were associated with a >4-year survival difference in men. Patients with high levels of KLF6-SV1 expression had a median survival of approximately 30 months versus 80 months in patients with low KLF6-SV1 expression.19 In lung cancer, increased KLF6-SV1 expression levels were associated with a 6.5-year difference in median survival between patients in the lowest and highest tertiles.18 Taken together, these results suggest not only that KLF6 loss and KLF6-SV1 overexpression occur in human cancer but also that this dysregulation may be predictive of the stage, long-term outcome, and survival in different cancers.
KRUPPEL-LIKE FACTOR 6 AND KRUPPEL-LIKE FACTOR 6 SPLICE VARIANT 1 IN EPITHELIAL OVARIAN CANCER
Given these findings, we first began exploring the role of KLF6 and KLF6-SV1 in EOC by determining the frequency of gene loss and then the expression levels of KLF6 and KLF6-SV1 in human tumors.49 A total of 68 paired EOC tumor/normal samples were assayed, and allelic loss was present in 70% of serous carcinomas (32/46), the most common histology. In addition, as expected because of the different genetic backgrounds of the various histological subtypes discussed previously, the loss of heterozygosity pattern was subtype-specific (Table 1). KLF6 loss of heterozygosity was also significantly correlated with the tumor stage (P < 0.025) and grade (P < 0.05), which are determinants of patient survival. As shown in Figure 1, these allelic losses were associated with markedly decreased expression levels of KLF6. Interestingly, in contrast to a number of other cancer types, no KLF6 DNA sequence mutations were detected, and this suggests that changes in KLF6 expression levels are more important in EOC than somatic mutation.
Table 1.
Summary of Allelic Loss of KLF6 in Epithelial Ovarian Cancer
| Histological Type | Allelic Loss of KLF6 |
|---|---|
| Serous | 32/46 (70%)* |
| Endometrioid | 4/8 (50%) |
| Mucinous | 1/9 (11%) |
| Clear cell | 0/5 (0%) |
| Total | 37/68 (54%) |
Abbreviations: KLF6, Kruppel-like factor 6.
The allelic loss of KLF6 was significantly more frequently found in serous carcinoma (P < 0.02).
Fig 1.
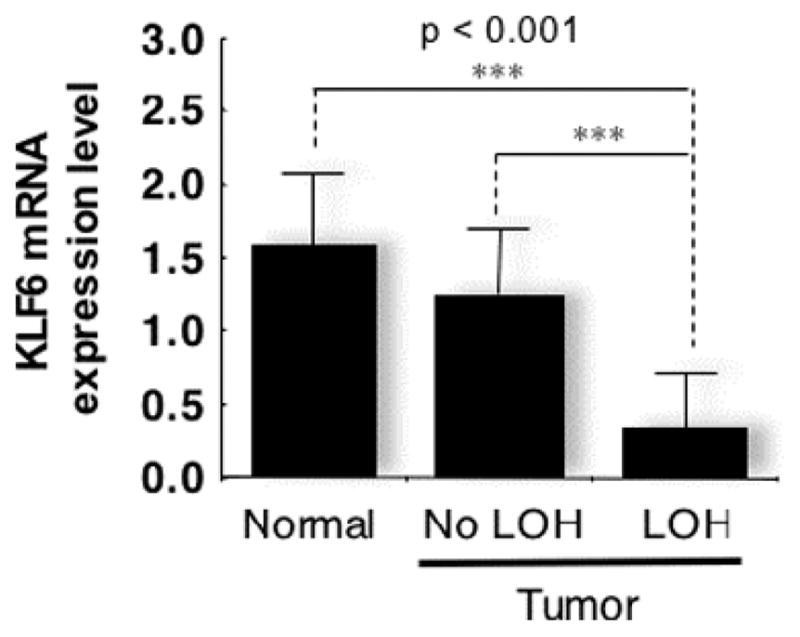
Loss of KLF6 expression in epithelial ovarian cancer tumors with LOH. Abbreviations: KLF6, Kruppel-like factor 6; LOH, loss of heterozygosity; mRNA, messenger RNA.
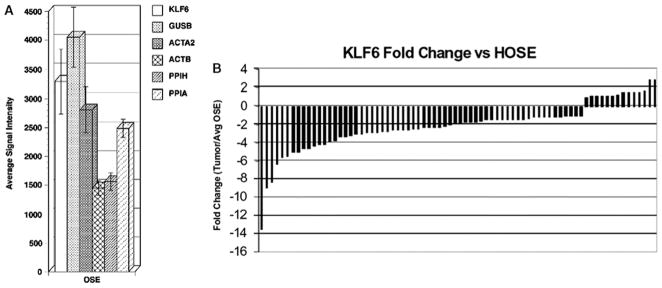
Given that EOC is believed to arise from ovarian surface epithelial cells, we also compared the level of KLF6 expression in these cells to EOC. KLF6 expression was decreased in most high-grade ovarian cancers. Specifically, KLF6 expression in a set of 10 ovarian surface epithelial cells was compared with its expression across 76 stage III EOC samples by Affymetrix GeneChip analysis. As shown in Figure 2, KLF6 expression was decreased in 62 (82%) tumor samples in comparison with ovarian surface epithelial cells, and the overall decrease was approximately 2-fold (P < 0.05). Taken together, these findings are consistent with KLF6’s role as an EOC tumor suppressor.
Fig 2.
KLF6 expression in OSE brushings versus select housekeeping genes. (A) KLF6 microarray signal intensity values were averaged for 10 OSE brushings and compared to the average expression levels of 5 housekeeping genes: GUSB, ACTA2, ACTB, PPIH, and PPIA. The KLF6 expression level was comparable to the housekeeping gene levels, and this indicates that KLF6 is reliably expressed in ovarian epithelial cells. (B) KLF6 expression in 76 stage III EOC samples versus KLF6 expression in OSE brushings. The KLF6 expression in each microdissected EOC sample was compared to the average expression in all 10 OSE samples. The fold changes were normalized to zero. Abbreviations: ACTA2, actin alpha 2 smooth muscle aorta; ACTB, actin beta; EOC, epithelial ovarian cancer; GUSB, glucuronidase beta; HOSE, human ovarian surface epithelial; KLF6, Kruppel-like factor 6; OSE, ovarian surface epithelium; PPIA, peptidylprolyl isomerase A (cyclophilin A); PPIH, peptidylprolyl isomerase H (cyclophilin H).
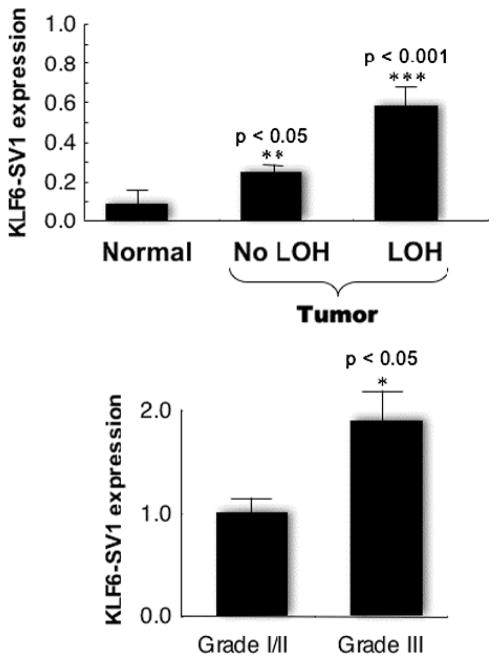
We next explored whether KLF6-SV1 was also expressed in ovarian tissue and whether KLF6 alternative splicing was dysregulated in EOC. RNA from 33 tumors and a panel of 5 normal ovarian tissue samples was analyzed by quantitative reverse-transcript polymerase chain reaction using isoform-specific primers. All tumors except 1 (32/33, 97%) expressed KLF6-SV1. Strikingly, KLF6-SV1 expression was increased nearly 5-fold on average (P < 0.001) in the EOC samples (Figure 3, top panel) with respect to normal tissue. In addition, KLF6-SV1 was up-regulated approximately 2-fold in poorly differentiated grade III tumors in comparison with well to moderately differentiated grade I or II tumors (P < 0.01; Figure 3, lower panel). Increased KLF6-SV1 expression was particularly notable in view of the corresponding overall decrease in wild-type KLF6 expression.
Fig 3.
KLF6-SV1 is overexpressed in nearly all epithelial ovarian cancer, regardless of the LOH status, and its expression increases with a higher tumor grade. Abbreviations: KLF6-SV1, Kruppel-like factor 6 splice variant 1; LOH, loss of heterozygosity.
EFFECTS OF KRUPPEL-LIKE FACTOR 6 AND KRUPPEL-LIKE FACTOR 6 SPLICE VARIANT 1 INHIBITION
Clinically Relevant Cancer Cell Phenotypes
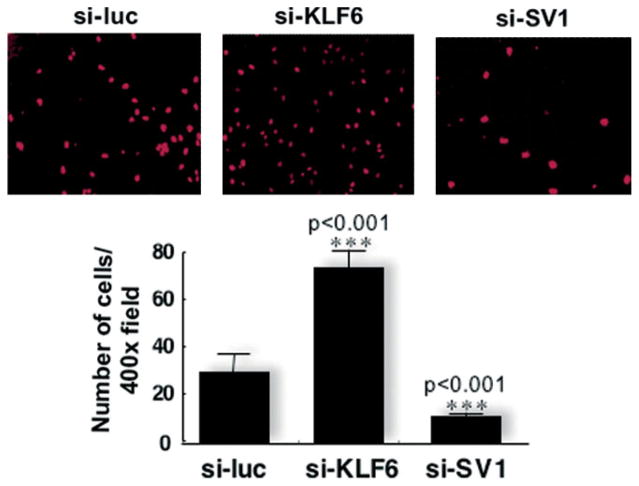
Because our data suggested that increased KLF6-SV1 expression was present in virtually all serous EOC, we then decided to directly explore the biological role of KLF6-SV1 as it relates to proliferation and invasion/metastasis.49 We used small interfering RNA (siRNA), which we previously demonstrated to be highly specific,48 to down-regulate KLF6-SV1 expression. Stable ovarian cancer (SKOV-3) cell lines were generated with pSUPER small interfering luciferase (si-luc; a control), pSUPER small interfering Kruppel-like factor 6 (si-KLF6), and pSUPER small interfering splice variant 1 (si-SV1). In these polyclonal pools, KLF6 was reduced approximately 60% and KLF6-SV1 was reduced approximately 75% on protein and RNA levels. Cellular proliferation differences between these siRNA stable cell lines were then measured. In the si-KLF6–expressing cell line, proliferation was increased approximately 2-fold (P < 0.001). Conversely, the targeted reduction of KLF6-SV1 resulted in a 25% reduction in proliferation (P < 0.01). We next tested the effects of KLF6-SV1 inhibition on invasive capacity. Although targeted KLF6 reduction resulted in a 2.5-fold increase (P < 0.001) in cellular invasion, KLF6-SV1 inhibition decreased invasion by approximately 60% (P < 0.001; Figure 4).
Fig 4.
Invasion capacity in small interfering RNA stable cells. The inhibition of KLF6-SV1 decreases tumor invasion and metastasis. The upper panels are representative photomicrographs of the underside of a Matrigel insert stained with 4′,6-diamidino-2-phenylindole. The lower panel shows the number of cells per visual field (400× magnification). The cell number was determined from an analysis of 4 representative fields and 3 independent experiments. The statistical analysis was performed with the Student t test with a 2-tailed distribution and 2 samples of equal variance. Abbreviations: si-KLF6, pSUPER small interfering Kruppel-like factor 6; si-luc, pSUPER small interfering luciferase; si-SV1, pSUPER small interfering splice variant 1.
We next directly examined the role of KLF6 and KLF6-SV1 in tumor growth in vivo. Stable SKOV-3 cell lines expressing si-luc, si-KLF6, or si-SV1 were subcutaneously injected into nude mice, which were monitored weekly for tumor growth and then sacrificed at the end of 6 weeks. Most conspicuously, all mice transplanted with si-SV1 cells (n = 5 mice) failed to form persistent tumors. The small tumors that initially developed regressed after 3 weeks. In contrast, reduction of the tumor suppressor KLF6 doubled the tumor growth rate and mass (Figure 4).
The extension of these experiments to define more long-term effects also demonstrated that decreased KLF6, which may occur through a dominant-negative interaction with KLF6-SV1, results in increased intraperitoneal dissemination in vivo. Specifically, the role of KLF6 in EOC progression was examined in an in vivo ovarian cancer mouse model that recapitulates advanced-stage disease with intraperitoneal carcinomatosis and ascites production. The SKOV-3 si-luc or si-KLF6 cell lines were injected into the peritoneal cavity of BALB/c nu/nu mice, and tumor growth was followed by weekly measurements of abdominal circumferences.
All si-KLF6 mice developed pronounced abdominal swelling from ascites accumulation and intraperi-toneal carcinomatosis 6 weeks after tumor cell inoculation, at which point animals from both groups were euthanized, and the tumor burdens were assessed. In the si-KLF6 mice, tumors were present on the peritoneal, intestinal, and diaphragmatic surfaces (Figure 5; arrows). The average ascites volume in the si-KLF6 group was 600 uL, with an average vascular endothelial growth factor (VEGF) concentration of 870 pg/mL. In marked contrast, no overt metastatic tumors or ascitic fluid collections were observed in the matched si-luc control mice at the same experimental time point.
Fig 5.
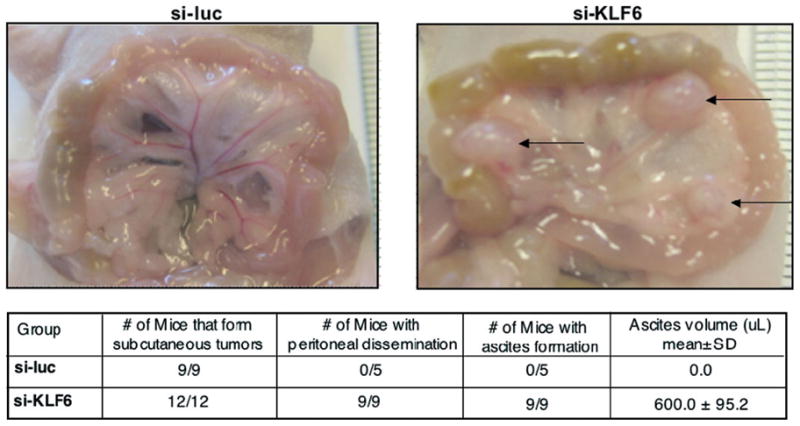
The inhibition of Kruppel-like factor 6 increases intraperitoneal tumor dissemination and vascular endothelial growth factor expression. Abbreviations: SD, standard deviation; si-KLF6, pSUPER small interfering Kruppel-like factor 6; si-luc, pSUPER small interfering luciferase.
E-Cadherin Expression
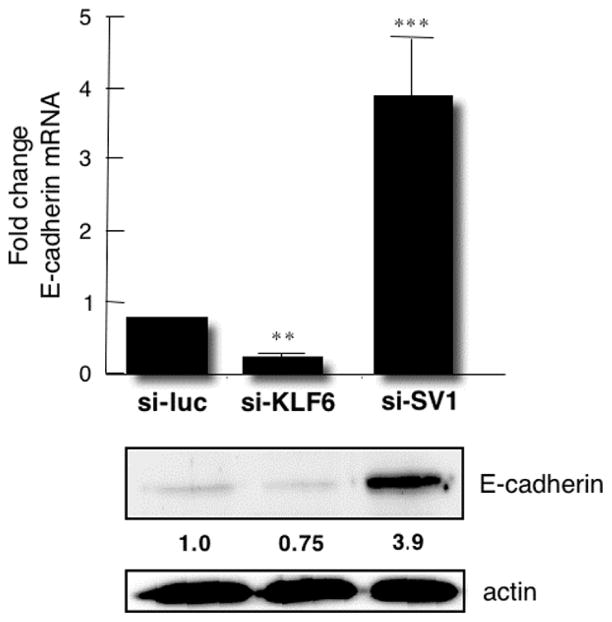
In contrast to well-differentiated ovarian tumors, E-cadherin expression has been shown to be reduced during ovarian tumor progression and metastasis.50,51 As shown in Figure 6, the inhibition of KLF6 and the inhibition of KLF6-SV1 have opposite and markedly powerful effects on E-cadherin expression. Although the inhibition of KLF6 decreases E-cadherin by more than half, KLF6-SV1 inhibition increases levels approximately 5-fold. To determine whether E-cadherin is a direct transcriptional target of KLF6, we generated a series of E-cadherin promoter deletion constructs. The sequential deletion of all 4 binding elements within a site having a GGGCGG nucleotide sequence (GC-box) revealed that the −150 and −117 motifs were necessary and sufficient for full KLF6-mediated activation.52 To establish a direct interaction between KLF6 and the native E-cadherin promoter in vivo, chromatin immunoprecipitation analysis was also performed. The KLF6 protein occupies the GC-box elements identified in vitro. Therefore, the differences in cellular invasion and proliferation observed in the stable SKOV3 cell lines discussed previously may be due to these KLF6-mediated alterations in E-cadherin.
Fig 6.
The inhibition of Kruppel-like factor 6 and Kruppel-like factor 6 splice variant 1 has markedly antagonistic effects on E-cadherin expression. Abbreviations: mRNA, messenger RNA; si-KLF6, pSUPER small interfering Kruppel-like factor 6; si-luc, pSUPER small interfering luciferase; si-SV1, pSUPER small interfering splice variant 1.
Vascular Endothelial Growth Factor Expression
The second candidate target that we analyzed was VEGF. Given the striking effects of targeted KLF6 and KLF6-SV1 inhibition on in vivo tumor growth, we examined the effects of their inhibition on the expression of VEGF, a critical regulator of angiogenesis and EOC tumor development.49 Differences in all 4 transcripts encoding monomeric VEGF, excluding isoform 121, were examined by quantitative reverse-transcript polymerase chain reaction. Inhibition of KLF6-SV1 decreased the secretion of VEGF by approximately 50% (as detected in messenger RNA and protein levels by both Western blotting and enzyme-linked immunosorbent assay) in stable SKOV-3 cell lines in culture. In contrast, KLF6 inhibition led to increased VEGF expression and secretion.
These effects in cultured cells were also sustained in vivo and were associated with tumor growth differences in the mouse xenograft model. There was a significant increase in both VEGF messenger RNA (P < 0.001) and protein levels in the si-KLF6–derived tumors. On average, these tumors expressed approximately 15-fold higher VEGF concentrations as measured by enzyme-linked immunosorbent assay.
FUTURE DIRECTIONS: KRUPPEL-LIKE FACTOR 6 SPLICE VARIANT 1 IS A NOVEL THERAPEUTIC TARGET
Ovarian cancer is one of the leading causes of death from gynecologic malignancy, with more than 190,000 new cases each year worldwide. Most patients present with advanced-stage disease, in which the tumor has disseminated/metastasized within the peritoneum, and the cornerstone of treatment is surgical debulking and platinum-based chemotherapy. Although initially responsive to cisplatin, the majority of women will eventually succumb to recurrence and chemoresistance, including 50% of women who have no evidence of disease after primary therapy. Because ovarian cancer recurrences are primarily confined within the peritoneum, from a treatment perspective, the disease can be considered a compartmentalized disease. In fact, the intraperitoneal administration of chemotherapy was first proposed 30 years ago,53 and currently used agents, when given via the intraperitoneal route, have been shown to have distinct pharmacokinetic advantages, including higher concentrations and longer half-lives.54 Most importantly, numerous studies have suggested that intraperitoneal dosing results in increased patient survival.54
Our studies demonstrating overexpression of KLF6-SV1 in EOC and the effect of KLF6-SV1 inhibition on a number of critical tumor phenotypes have led us to propose that KLF6-SV1 represents a novel therapeutic target in ovarian cancer. Of particular note with respect to potential therapeutic targeting are our studies demonstrating not only that overexpression of KLF6-SV1 is associated with decreased survival and metastatic spread of certain cancers but also that siRNA-mediated inhibition of KLF6-SV1 has such dramatic effects on tumor behavior in vitro and in vivo.19,48,49 Thus, the intraperitoneal delivery of an agent, possibly siRNA-based, to inhibit KLF6-SV1 activity would represent a novel paradigm in targeted ovarian cancer treatment. Because platinum-based chemotherapeutics are a first-line treatment for ovarian cancer, it is particularly notable that KLF6 down-regulation has also been associated with cisplatin resistance in an ovarian cancer cell line.55
On the basis of these findings and this rationale, we examined the therapeutic efficacy of an intraperitoneally delivered, next-generation chemically modified siRNA molecule targeting KLF6-SV1.56 SKOV3 ovarian cancer cells, which were stably transfected with a luciferase expression vector, were injected into the peritoneum of mice, and the growth and spread of the cells were followed in real time. Survival, the endpoint of the study, was increased in a dose-dependent manner. Treatment of the mice with the highest dose of si-KLF6-SV1 (20 mg/kg) resulted in a tripling of the median survival from 114 to 311 days, whereas overall survival more than doubled, surpassing 450 days. Mechanistically, KLF6-SV1 was demonstrated to be a prosurvival/antiapoptotic protein that binds to phorbol-12-myristate-13-acetate-induced protein 1, the proapoptotic BH3-only protein, and it targets them both for degradation.56
How can these research findings now be translated to the clinic? Given our own work with siRNA, this would seem to be offer a first logical step. Indeed, the horizon for the use of siRNA/RNA interference seems to beckon with the potential for a pharmacopeia of tomorrow for many human diseases.57 Already, a number of recent studies have begun to highlight the potential efficacy and specificity of siRNA/RNA interference–based therapies and have moved well beyond the earliest demonstrations of gene silencing in mammalian cell culture.58 For example, a number of investigative groups have demonstrated the in vivo effectiveness of gene silencing in mouse and/or nonhuman primate models of a number of human disorders ranging from respiratory syncytial virus infection59 to hypercholesteremia.60 The prospects for the treatment of human cancers have also been long recognized, and delivery issues remain some of the major obstacles.61 Theoretically, targeting these disorders by the direct delivery of siRNA into the diseased tissue would represent the most attractive option. The direct delivery route would ensure a high concentration of siRNA and would be predicted to have the least likelihood of unanticipated systemic effects.
Beyond siRNA-mediated treatment, another approach to inhibiting KLF6-SV1 would involve the identification of small molecules that could target it for degradation. The high-throughput screening of large-scale chemical libraries could be used to identify those compounds that selectively lead to KLF6-SV1 inhibition or degradation. On the basis of an ever expanding appreciation of the relevance of KLF6 and KLF6-SV1 to human cancer, the immediate translation of these strategies first to animal models and then to the clinic is a high priority. We believe that developing methods to therapeutically regulate KLF6-SV1 ultimately will play an important role in treating a number of localized and metastatic cancers.
Footnotes
DISCLOSURES
Potential conflict of interest: Drs. Narla and Martignetti are named inventors on patent applications filed by MSSM related to the use of KLF6 and KLF6-SV1 in cancer diagnostics and treatment.
References
- 1.Jemal A, Siegel R, Ward E, et al. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Bieker JJ. Kruppel-like factors: three fingers in many pies. J Biol Chem. 2001;276:34355–34358. doi: 10.1074/jbc.R100043200. [DOI] [PubMed] [Google Scholar]
- 3.Black AR, Black JD, Azizkhan-Clifford J. Sp1 and Kruppel-like factor family of transcription factors in cell growth regulation and cancer. J Cell Physiol. 2001;188:143–160. doi: 10.1002/jcp.1111. [DOI] [PubMed] [Google Scholar]
- 4.Narla G, Heath KE, Reeves HL, et al. KLF6, a candidate tumor suppressor gene mutated in prostate cancer. Science. 2001;294:2563–2566. doi: 10.1126/science.1066326. [DOI] [PubMed] [Google Scholar]
- 5.Chen C, Hytinen ER, Sun X, et al. Deletion, mutation, and loss of expression of KLF6 in human prostate cancer. Am J Pathol. 2003;162:1349–1354. doi: 10.1016/S0002-9440(10)63930-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reeves HL, Narla G, Ogunbiyi O, et al. Kruppel-like factor 6 (KLF6) is a tumor suppressor gene frequently inactivated in colorectal cancer. Gastroenterology. 2004;126:1090–1103. doi: 10.1053/j.gastro.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Ito G, Uchiyama M, Kondo M, et al. Kruppel-like factor 6 is frequently down-regulated and induces apoptosis in non-small cell lung cancer cells. Cancer Res. 2004;64:3838–3843. doi: 10.1158/0008-5472.CAN-04-0185. [DOI] [PubMed] [Google Scholar]
- 8.Cho YG, Kim CJ, Park CH, et al. Genetic alterations of the KLF6 gene in gastric cancer. Oncogene. 2005;24:4588–4590. doi: 10.1038/sj.onc.1208670. [DOI] [PubMed] [Google Scholar]
- 9.Jeng YM, Hsu HC. KLF6, a putative tumor suppressor gene, is mutated in astrocytic gliomas. Int J Cancer. 2003;105:625–629. doi: 10.1002/ijc.11123. [DOI] [PubMed] [Google Scholar]
- 10.Camacho-Vanegas O, Narla G, Teixeira MS, et al. Functional inactivation of the KLF6 tumor suppressor gene by loss of heterozygosity and increased alternative splicing in glioblastoma. Int J Cancer. 2007;121:1390–1395. doi: 10.1002/ijc.22809. [DOI] [PubMed] [Google Scholar]
- 11.Chen HK, Liu XQ, Lin J, et al. Mutation analysis of KLF6 gene in human nasopharyngeal carcinomas [in Chinese] Ai Zheng. 2002;21:1047–1050. [PubMed] [Google Scholar]
- 12.Tal-Kremer S, Reeves HL, Narla G, et al. Frequent inactivation of the tumor suppressor Kruppel-like factor 6 (KLF6) in hepatocellular carcinoma. Hepatology. 2004;40:1047–1052. doi: 10.1002/hep.20460. [DOI] [PubMed] [Google Scholar]
- 13.Wang S, Chen X, Zhang W, Qiu F. KLF6 mRNA expression in primary hepatocellular carcinoma. J Huazhong Univ Sci Technol Med Sci. 2004;24:585–587. doi: 10.1007/BF02911362. [DOI] [PubMed] [Google Scholar]
- 14.Kremer-Tal S, Narla G, Chen Y, et al. Downregulation of KLF6 is an early event in hepatocarcinogenesis, and stimulates proliferation while reducing differentiation. J Hepatol. 2007;46:645–654. doi: 10.1016/j.jhep.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng D, Sheta EA, Powell SM, et al. Alterations in Barrett’s-related adenocarcinomas: a proteomic approach. Int J Cancer. 2008;1226:1303–1310. doi: 10.1002/ijc.23258. [DOI] [PubMed] [Google Scholar]
- 16.Narla G, DiFeo A, Reeves HL, et al. A germline DNA polymorphism associated with increased prostate cancer risk enhances alternative splicing of the KLF6 tumor suppressor gene. Cancer Res. 2005;65:1213–1222. doi: 10.1158/0008-5472.CAN-04-4249. [DOI] [PubMed] [Google Scholar]
- 17.Teixeira MS, Camacho-Vanegas O, Fernandez Y, et al. KLF6 allelic loss is associated with tumor recurrence and markedly decreased survival in head and neck squamous cell carcinoma. Int J Cancer. 2007;121:1976–1983. doi: 10.1002/ijc.22926. [DOI] [PubMed] [Google Scholar]
- 18.DiFeo A, Feld L, Rodriguez E, et al. A functional role for KLF6-SV1 in lung adenocarcinoma prognosis and chemotherapy response. Cancer Res. 2008;68:965–970. doi: 10.1158/0008-5472.CAN-07-2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narla G, DiFeo A, Fernandez Y, et al. KLF6-SV1 overexpression accelerates human and mouse prostate cancer progression and metastasis. J Clin Invest. 2008;118:2711–2712. doi: 10.1172/JCI34780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taetle R, Aickin M, Yang JM, et al. Chromosome abnormalities in ovarian adenocarcinoma: I. Nonrandom chromosome abnormalities from 244 cases. Genes Chromosomes Cancer. 1999;25:290–300. [PubMed] [Google Scholar]
- 21.Höglund M, Gisselsson D, Hansen GB, et al. Ovarian carcinoma develops through multiple modes of chromosomal evolution. Cancer Res. 2003;63:3378–3385. [PubMed] [Google Scholar]
- 22.Gorringe KL, Jacobs S, Thompson ER, et al. High-resolution single nucleotide polymorphism array analysis of epithelial ovarian cancer reveals numerous microdeletions and amplifications. Clin Cancer Res. 2007;13:4731–4739. doi: 10.1158/1078-0432.CCR-07-0502. [DOI] [PubMed] [Google Scholar]
- 23.Konstantinopoulos PA, Spentzos D, Cannistra SA. Gene-expression profiling in epithelial ovarian cancer. Nat Clin Pract Oncol. 2008;5:577–587. doi: 10.1038/ncponc1178. [DOI] [PubMed] [Google Scholar]
- 24.Farley J, Ozbun LL, Birrer MJ. Genomic analysis of epithelial ovarian cancer. Cell Res. 2008;18:538–548. doi: 10.1038/cr.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berchuck A, Iversen ES, Lancaster JM, et al. Prediction of optimal versus suboptimal cytoreduction of advanced-stage serous ovarian cancer with the use of microarrays. Am J Obstet Gynecol. 2004;190:910–925. doi: 10.1016/j.ajog.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Berchuck A, Iversen ES, Lancaster JM, et al. Patterns of gene expression that characterize long-term survival in advanced stage serous ovarian cancers. Clin Cancer Res. 2005;11:3686–3696. doi: 10.1158/1078-0432.CCR-04-2398. [DOI] [PubMed] [Google Scholar]
- 27.Hartmann LC, Lu KH, Linette GP, et al. Gene expression profiles predict early relapse in ovarian cancer after platinum-paclitaxel chemotherapy. Clin Cancer Res. 2005;11:2149–2155. doi: 10.1158/1078-0432.CCR-04-1673. [DOI] [PubMed] [Google Scholar]
- 28.Lancaster JM, Dressman HK, Whitaker RS, et al. Gene expression patterns that characterize advanced stage serous ovarian cancers. J Soc Gynecol Investig. 2004;11:51–59. doi: 10.1016/j.jsgi.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Spentzos D, Levine DA, Ramoni MF, et al. Gene expression signature with independent prognostic significance in epithelial ovarian cancer. J Clin Oncol. 2004;22:4700–4710. doi: 10.1200/JCO.2004.04.070. [DOI] [PubMed] [Google Scholar]
- 30.Dressman HK, Berchuck A, Chan G, et al. An integrated genomic-based approach to individualized treatment of patients with advanced-stage ovarian cancer. J Clin Oncol. 2007;25:517–525. doi: 10.1200/JCO.2006.06.3743. [DOI] [PubMed] [Google Scholar]
- 31.Helleman J, Jansen MP, Span PN, et al. Molecular profiling of platinum resistant ovarian cancer. Int J Cancer. 2006;118:1963–1971. doi: 10.1002/ijc.21599. [DOI] [PubMed] [Google Scholar]
- 32.Selvanayagam ZE, Cheung TH, Wei N, et al. Prediction of chemotherapeutic response in ovarian cancer with DNA microarray expression profiling. Cancer Genet Cytogenet. 2004;154:63–66. doi: 10.1016/j.cancergencyto.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 33.Spentzos D, Levine DA, Kolia S, et al. Unique gene expression profile based on pathologic response in epithelial ovarian cancer. J Clin Oncol. 2005;23:7911–7918. doi: 10.1200/JCO.2005.02.9363. [DOI] [PubMed] [Google Scholar]
- 34.Brown GJ, St John DJ, Macrae FA, Aittomäki K. Cancer risk in young women at risk of hereditary nonpolyposis colorectal cancer: implications for gynecologic surveillance. Gynecol Oncol. 2001;80:346–349. doi: 10.1006/gyno.2000.6065. [DOI] [PubMed] [Google Scholar]
- 35.Brose MS, Rebbeck TR, Calzone KA, et al. Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J Natl Cancer Inst. 2002;94:1365–1372. doi: 10.1093/jnci/94.18.1365. [DOI] [PubMed] [Google Scholar]
- 36.Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62:676–689. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bosze P, Bast RC, Berchuck A, et al. Consensus statements on prognostic factors in epithelial ovarian carcinoma. Report of the consensus meeting organized by the European Society of Gynaecological Oncology. Eur J Gynaecol Oncol. 2000;21:513–526. [PubMed] [Google Scholar]
- 38.Chung DC, Rustgi AK. The hereditary nonpolyposis colorectal cancer syndrome: genetics and clinical implications. Ann Intern Med. 2003;138:560–570. doi: 10.7326/0003-4819-138-7-200304010-00012. [DOI] [PubMed] [Google Scholar]
- 39.Rebbeck TR, Lynch HT, Neuhausen SL, et al. for the Prevention and Observation of Surgical End Points Study Group. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346:1616–1622. doi: 10.1056/NEJMoa012158. [DOI] [PubMed] [Google Scholar]
- 40.Kauff ND, Satagopan JM, Robson ME, et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346:1609–1615. doi: 10.1056/NEJMoa020119. [DOI] [PubMed] [Google Scholar]
- 41.Schmeler KM, Lynch HT, Chen LM, et al. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N Engl J Med. 2006;354:261–269. doi: 10.1056/NEJMoa052627. [DOI] [PubMed] [Google Scholar]
- 42.Finch A, Beiner M, Lubinski J, et al. Salpingo-oophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with a BRCA1 or BRCA2 mutation. JAMA. 2006;296:185–192. doi: 10.1001/jama.296.2.185. [DOI] [PubMed] [Google Scholar]
- 43.Tan DS, Rothermundt C, Thomas K, et al. “BRCAness” syndrome in ovarian cancer: a case-control study describing the clinical features and outcome of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutations. J Clin Oncol. 2008;26:5530–5536. doi: 10.1200/JCO.2008.16.1703. [DOI] [PubMed] [Google Scholar]
- 44.Rabban JT, Bell DA. Current issues in the pathology of ovarian cancer. J Reprod Med. 2005;50:467–467. [PubMed] [Google Scholar]
- 45.Kim Y, Ratziu V, Choi SG, et al. Transcriptional activation of transforming growth factor beta1 and its receptors by the Kruppel-like factor Zf9/core promoter-binding protein and Sp1. Potential mechanisms for autocrine fibrogenesis in response to injury. J Biol Chem. 1998;273:33750–33758. doi: 10.1074/jbc.273.50.33750. [DOI] [PubMed] [Google Scholar]
- 46.Koritschoner NP, Bocco JL, Panzetta-Dutari GM, et al. A novel human zinc finger protein that interacts with the core promoter element of a TATA box-less gene. J Biol Chem. 1997;272:9573–9580. doi: 10.1074/jbc.272.14.9573. [DOI] [PubMed] [Google Scholar]
- 47.El Rouby S, Rao PH, Newcomb EW. Assignment of the human B-cell-derived (BCD1) proto-oncogene to 10p14-p15. Genomics. 1997;43:395–397. doi: 10.1006/geno.1997.4824. [DOI] [PubMed] [Google Scholar]
- 48.Narla G, DiFeo A, Yao S, et al. Targeted inhibition of the KLF6 splice variant, KLF6 SV1, suppresses prostate cancer cell growth and spread. Cancer Res. 2005;65:5761–5768. doi: 10.1158/0008-5472.CAN-05-0217. [DOI] [PubMed] [Google Scholar]
- 49.DiFeo A, Narla G, Hirshfeld J, et al. Roles of KLF6 and KLF6-SV1 in ovarian cancer progression and intraperitoneal dissemination. Clin Cancer Res. 2006;12:3730–3739. doi: 10.1158/1078-0432.CCR-06-0054. [DOI] [PubMed] [Google Scholar]
- 50.Darai E, Scoazec JY, Walker-Combrouze F, et al. Expression of cadherins in benign, borderline, and malignant ovarian epithelial tumors: a clinicopathologic study of 60 cases. Hum Pathol. 1997;28:922–928. doi: 10.1016/s0046-8177(97)90007-1. [DOI] [PubMed] [Google Scholar]
- 51.Hudson LG, Zeineldin R, Stack MS. Phenotypic plasticity of neoplastic ovarian epithelium: unique cadherin profiles in tumor progression. Clin Exp Metastasis. 2008;25:643–655. doi: 10.1007/s10585-008-9171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DiFeo A, Narla G, Camacho-Vanegas O, et al. E-cadherin is a novel transcriptional target of the KLF6 tumor suppressor. Oncogene. 2006;25:6026–6031. doi: 10.1038/sj.onc.1209611. [DOI] [PubMed] [Google Scholar]
- 53.Dedrick R, Myers C, Bungay PM, et al. Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat Rep. 1978;62:1–11. [PubMed] [Google Scholar]
- 54.Trimble EL, Thompson S, Christian MC, Minasian L. Intraperitoneal chemotherapy for women with epithelial ovarian cancer. Oncologist. 2008;13:403–409. doi: 10.1634/theoncologist.2007-0058. [DOI] [PubMed] [Google Scholar]
- 55.Macleod K, Mullen P, Sewell J, et al. Altered ErbB receptor signaling and gene expression in cisplatin-resistant ovarian cancer. Cancer Res. 2005;65:6789–6800. doi: 10.1158/0008-5472.CAN-04-2684. [DOI] [PubMed] [Google Scholar]
- 56.Difeo A, Huang F, Sangodkar J, et al. KLF6-SV1 is a novel antiapoptotic protein that targets the BH3-only protein NOXA for degradation and whose inhibition extends survival in an ovarian cancer model. Cancer Res. 2009;69:4733–4741. doi: 10.1158/0008-5472.CAN-08-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gewirtz AM. On future’s doorstep: RNA interference and the pharmacopeia of tomorrow. J Clin Invest. 2007;117:3612–3614. doi: 10.1172/JCI34274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elbashir SM, Harborth J, Lendeckel W, et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 59.Bitko V, Musiyenko A, Shulyayeva O, Barik S. Inhibition of respiratory viruses by nasally administered siRNA. Nat Med. 2005;11:50–55. doi: 10.1038/nm1164. [DOI] [PubMed] [Google Scholar]
- 60.Frank-Kamenetsky M, Grefhorst A, Anderson NN, et al. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc Natl Acad Sci U S A. 2008;105:11915–11920. doi: 10.1073/pnas.0805434105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pai SI, Lin YY, Macaes B, et al. Prospects of RNA interference therapy for cancer. Gene Ther. 2006;13:464–477. doi: 10.1038/sj.gt.3302694. [DOI] [PubMed] [Google Scholar]