Abstract
Highly tumorigenic cancer cell (HTC) populations have been identified for a variety of solid tumors and assigned stem cell properties. Strategies for identifying HTCs in solid tumors have been primarily empirical rather than rational, particularly in epithelial tumors, which are responsible for 80% of cancer deaths. We report evidence for a spatially restricted bladder epithelial (urothelial) differentiation program in primary urothelial cancers (UCs) and in UC xenografts. We identified a highly tumorigenic UC cell compartment that resembles benign urothelial stem cells (basal cells), co-expresses the 67-kDa laminin receptor and the basal cell-specific cytokeratin CK17, and lacks the carcinoembryonic antigen family member CEACAM6 (CD66c). This multipotent compartment resides at the tumor-stroma interface, is easily identified on histologic sections, and possesses most, if not all, of the engraftable tumor-forming ability in the parental xenograft. We analyzed differential expression of genes and pathways in basal-like cells versus more differentiated cells. Among these, we found significant enrichment of pathways comprising “hallmarks” of cancer, and pharmacologically targetable signaling pathways, including Janus kinase-signal transducer and activator of transcription, Notch, focal adhesion, mammalian target of rapamycin, epidermal growth factor receptor (erythroblastic leukemia viral oncogene homolog [ErbB]), and wingless-type MMTV integration site family (Wnt). The basal/HTC gene expression signature was essentially invisible within the context of nontumorigenic cell gene expression and overlapped significantly with genes driving progression and death in primary human UC. The spatially restricted epithelial differentiation program described here represents a conceptual advance in understanding cellular heterogeneity of carcinomas and identifies basal-like HTCs as attractive targets for cancer therapy.
Keywords: Differentiation, Malignancy, Stem cell microenvironment interactions, Stromal cells
Introduction
Tumor growth is conceptually analogous to organ formation in the embryo and to repair in adults, yet the precise relationships between these events are poorly understood. In particular, there is mounting evidence suggesting that tumors, like developing organs, comprise hierarchies of cells with phenotypically distinct subpopulations. Thus, cellular heterogeneity in tumors may represent the operation of differentiation programs rather than solely random events. Such heterogeneity is an important part of the functional organization of somatic tissues, reflecting a cell's differentiation state and governing its phenotypic repertoire, including its gene expression and its ability to produce daughter cells. In many stratified polarized epithelia, such as the urothelial lining of the bladder, differentiation proceeds from the basement membrane at the epithelial-stromal interface toward the luminal surface and is inversely correlated to replicative activity [1, 2]. Accordingly, the basal layer is the proposed stem/progenitor compartment for urothelium responsible for generating enough cells to maintain homeostasis for the life of the animal, whereas the fully differentiated superficial cells have limited ability to replicate and function primarily to provide a distensible barrier that excludes excreted solutes in the urine, preventing them from re-entering the bloodstream [3]. Aptly named intermediate cells separate basal and superficial cells and are intermediate in their differentiation and replicative ability [2].
Carcinomas, cancers derived from epithelia, are responsible for the vast majority of cancer deaths [4]. Cell populations within carcinomas are heterogeneous in their contributions to tumor growth and spread, complicating the ability of scientists to understand and treat cancer [5]. Several investigators have used empirically derived surface markers to isolate phenotypically homogeneous and highly tumorigenic subpopulations of cells within solid cancers (reviewed in [5, 6]). Upon transfer to a new host environment, these phenotypically homogeneous cancer cell populations have enhanced potency in forming a new tumor and are able to recreate the phenotypic diversity of the original tumor [5, 7–9]. Through predominantly empirical approaches, highly tumorigenic cancer cells (HTCs) have now been isolated from a handful of carcinomas, including those arising in breast, pancreas, colon, ovary, skin, and the upper aerodigestive tract (reviewed in 6). These studies indicate that cells within tumors are hierarchically organized with respect to their tumor-forming potential, but yield limited insight into the basis of this hierarchy.
In normal epithelial tissues, cellular hierarchies are organized by differentiation programs [10], and differentiation has long been a recognized feature of cancer, including carcinomas [11]. As such, differentiation may represent a launching point from which to understand cancer cell heterogeneity. Although undifferentiated carcinomas are sometimes observed, most cases, even those with widespread metastases, express markers corresponding to the most differentiated cell types in the epithelia in which they arise. For example, two thirds of urothelial carcinomas express uroplakins, markers of differentiated superficial cells [12], and mRNA transcripts encoding these markers can be found in metastatic tumor cells isolated from blood or lymph nodes [13, 14]. From these results, one could suggest several possible relationships between cell differentiation and the ability to form new tumors at distant sites, including the following: (a) carcinoma cells aberrantly express differentiation markers that have no relationship to their metastatic potential; (b) fully differentiated urothelial carcinoma cells have significant long-term growth potential; and (c) relatively undifferentiated cells drive tumor formation and subsequently differentiate (see ref 15). The third possibility centers on differentiation as an organizing principle for the identification, isolation, and understanding of highly tumorigenic subpopulations of carcinoma cells. Here, we identify evidence for differentiation of urothelial carcinomas that is analogous to normal urothelial differentiation. In particular, we identify a basal-like urothelial cancer cell subpopulation that (a) resides at and depends on the tumor-stroma interface and (b) possesses most, if not all, of the tumor-forming ability of the parental tumor. We establish novel and easily tractable model systems for analyzing differential expression of genes and pathways in basal-like versus more differentiated cells. Using these systems, we demonstrate differential activity of pathways comprising the “hallmarks” of cancer [16] and supported by several specific inter- and intracellular signaling cascades for which mechanism-based therapies are available or in development. We further support the potential utility of such therapies with data-linking expression of the basal HTC gene signature with progression of human urothelial carcinoma in human patients.
Materials and Methods
Cells and Tissues
With Institutional Review Board approval, a tissue array with 55 cases of invasive urothelial carcinomas, summary stage I–IV, was constructed as described [17]. A genitourinary pathologist (DMB) scored the presence of cancer in the array and immunohistochemical staining for CK17 and CK18 (for staining methods, see below) as absent, present with peripheral pattern (strongest at the tumor-stroma interface), or present with equivalent pattern (intensity not increased at tumor-stromal interface). Cases were scored as positive if they fulfilled either of two criteria: intense staining in more than 5% of cells or moderate staining in more than 10% of cells. Two cases lacked cancer on the slide and were censored from the study. The SW780 (human bladder TCC) cell line was obtained from the American Type Culture Collection (Manassas, VA, http://www.atcc.org) and cultured in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, Carlsbad, CA, http://www.invitrogen.com) supplemented with 10% fetal bovine serum (FBS; Sigma, St. Louis, http://www.sigmaaldrich.com).
Establishing and Propagating Urothelial Cancer Xenografts
All animal work conformed to approved Institutional Animal Care and Use Committee protocols. The urothelial cancer (UC) xenograft XBL8 was established from a sample of a stage pT3N0Mx invasive urothelial carcinoma from a woman in her 70s and passaged once in athymic mice prior to use. Cancer tissue dissected from cystectomy specimens in the Surgical Pathology suite at The Johns Hopkins Hospital was transported in an ice-cold RPMI-1640 medium (Sigma-Aldrich) and minced in sterile phosphate buffered saline (PBS) (Invitrogen) with sterile surgical blades into approximately 1-mm fragments. Fragments were washed twice with PBS and resuspended in DMEM/10% FBS medium. Xenografts were further propagated by injecting 106 cultured cells (SW780) or approximately 100 mg of minced primary tumor fragments (XBL8 xenograft) suspended in 200 μl of a 1:1 mixture of DMEM/10% FBS and Matrigel (BD Biosciences, San Diego, http://www.bdbiosciences.com) subcutaneously into the flanks of 6-week-old female athymic nude mice (Harlan, Indianapolis, IN, http://www.harlan.com).
Xenograft Dissociation into Single-Cell Suspensions
Prior to reaching 1 cm in greatest dimension, tumor xenografts were harvested from euthanized animals, minced in sterile PBS, and passed through a CD1 cell dissociation sieve (Sigma). Cell aggregates were washed once with sterile PBS and dissociated with 0.5 mg/ml the bacillus-derived neutral metaloprotease (Invitrogen) in DMEM for 8–10 h at 37°C, followed by two rounds of filtration through 40-μm nylon mesh (BD Biosciences). The resulting single-cell suspensions were washed twice with PBS and resuspended in PBS. Cells obtained from this procedure showed higher than 75% viability evaluated by trypan blue exclusion assay (Invitrogen) (data not shown).
Cell Sorting
SW780/67-kDa Laminin Receptor
SW780 xenograft single cells were resuspended in PBS containing 2% heat-inactivated FBS (fluorescence-activated cell sorting [FACS] buffer) at 1 × 107 cells/ml. Nonspecific binding was blocked by incubation with 10% goat serum (Sigma) in FACS buffer for 30 minutes at 4°C. 67LR (67LR, 67-kDa laminin receptor) (Mlu5; Alexis Biochemicals, Plymouth Meeting, PA, http://www.alexis-biochemicals.com) monoclonal antibody was then added to 2.5 μg/ml and incubated for 40 minutes on ice. After three 5-minute washes with FACS buffer, cell suspensions were further incubated with phycoerythrin-conjugated goat anti-mouse IgM (#406507, 2.5 μg/ml; BioLegend, San Diego, CA, http://www.biolegend.com) and fluorescein isothiocyanate (FITC)-conjugated antibodies against mouse H-2Dk and H-2Kb/H-2Db major histocompatibility antigens (mMHC1, #110305 and #114605, 1:20 dilution; BioLegend) for 40 minutes on ice, protected from light. Cells were then washed three times with FACS buffer and resuspended in PBS containing 2% bovine serum albumin and 1 μg/ml of 7-aminoactinomycin D (Sigma-Aldrich). Isotype-matched negative controls and single-color controls were included with each experiment. Analysis and cell sorting were performed on a Vantage cell sorter (BD Biosciences) using Cellquest software. Side scatter and forward scatter profiles were used to eliminate cell doublets and debris. 7-Aminoactinomycin D positive cells were gated out to eliminate dead cells. Mouse MHC1-FITC staining was used to eliminate contamination from cells of mouse origin. SW780 xenograft tumor cells were sorted into 67LR bright (67LR+mMHC1-;) or dim (67LR−mMHC1-) populations on the basis of 67LR staining profile, or bulk- or control-sorted (mMHC1-) cells. The purity of every population was 97–99% evaluated by postsorting analysis (supporting information Fig. 1).
XBL8/CEACAM6
XBL8 xenograft single cells were prepared and stained as above except that a CEACAM6 antibody (catalog number MS-1731, 2.2 μg/ml; NeoMarkers, Fremont, CA, http://www.labvision.com) was used for the first incubation, followed by a cocktail of FITC-conjugated goat anti-mouse IgG (F2653, 1:100; Sigma) and phycoerythrin-conjugated anti-human CD44 (#312306, 1:40; BioLegend). XBL8 tumor cells were sorted into CEACAM6-positive and CEACAM6-negative/CD44 positive populations (supporting information Fig. 2).
Tumorigenicity Assays
Different doses of sorted cell populations (Fig. 4) were injected as above. Tumor formation was visually monitored weekly for 4 months.
Figure 4.
Basal-like cancer cells are enriched for tumor-propagating ability in xenograft assays. (A): CK17 and 67LR fail to colocalize in vitro. (B): In contrast, the two markers strictly colocalize in single cells cultured briefly after dissociation from xenografts grown subcutaneously in athymic mice. (C): Number of tumors identified at different starting doses of 67LR bright-, 67LR dim-, and control-sorted cells from SW780 xenografts. Note that all of the tumor-forming ability is associated with 67LR bright (basal) cells, which are approximately 10-fold more potent in initiating tumor xenografts than the parental tumor (control-sorted cells). (D): Number of tumors identified at different starting doses of CD66c (CEACAM6) dim (basal) or bright (central) cells isolated from passage 2 of the XBL8 human UC xenograft. Abbreviations: CK, cytokeratin; DAPI, 4′,6-diamidino-2-phenylindole.
RNA Preparation and Quality Assessment
Total RNA was purified using the RNeasy Mini-kit (Qiagen, Valencia, CA, http://www1.qiagen.com) and treated with RNase-free DNase I (Roche Diagnostics, Basel, Switzerland, http://www.roche-applied-science.com). RNA samples were quantified with NanoDrop ND-1000 (NanoDrop Products, Wilmington, DE, http://www.nanodrop.com) followed by quality assessment with 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, http://www.agilent.com). RNA concentrations for individual samples were greater than 250 ng/μl, with 28S/18S ratios greater than 1.4 and the RNA integrity number for each sample was greater than 9.7.
Probe Synthesis, Hybridization, and Labeling
RNA samples (1.5 μg total) were amplified, labeled, hybridized to 44,000 feature Whole Human Genome (hgug4112a) microarrays, and hybridization was detected according to the manufacturer's instructions (Agilent Technologies).
Statistical Analysis
Detailed description of methods used for array data annotation, processing and analysis, and public data sets used in the present study are described in the supporting information Methods section. Full MIAME-compliant information on gene expression experiments is reported as accompanying supporting information data. Raw expression data are available at http://astor.som.jhmi.edu/marchion/uroCSC.html. Data will be permanently hosted in the Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE16255). All analyses were performed with packages from R/Bioconductor (http://www.bioconductor.org) [18, 19]. For dual-color arrays, no background subtraction was performed, and within-array “loess” and between-arrays “scale” normalization methods were applied to log2 expression ratios [20]. For all probes, moderated t statistics (by empirical Bayes shrinkage of standard errors, log-odds ratios of differential expression) and adjusted p values (Benjamini and Hochberg method [21]) were obtained after fitting a linear model that accounted for correlation of biological replicates and labeling and group effects. Affymetrix data were normalized at the probe level using the robust multichip average algorithm [22]. Functional themes were obtained from Gene Ontology [23] and KEGG [24]. Enrichment analysis was performed by testing the hypothesis that each functional gene set is more highly ranked than a size-matched list consisting of randomly selected genes (10,000 simulations were performed), and by one-sided Wilcoxon test. Multiple testing correction was performed to adjust the p values using the Benjamini and Hochberg method [21].
Real-Time Reverse Transcription/Polymerase Chain Reaction Assays
First-strand cDNA synthesis, primed with random hexamers, was conducted with 1 μg of RNA isolated as above using Ready-To-Go You-Prime First-Strand polymerase chain reaction beads (GE Healthcare Life Scioences, Piscataway, NJ, http://www.gelife sciences.com) according to the manufacturer's instructions. The synthesized 33 μl of cDNA was diluted to 600 μl with diethylpyrocarbonate-H2O and 5 μl of diluted cDNA was incubated on an iCycler (1 cycle of 95°C for 5 minutes; 40 cycles of 95°C for 15 seconds, 60°C for 30 seconds, and 72°C for 30 seconds; followed by a melting-curve assay) with a 1-μl mixture of gene-specific forward and reverse primers (each 5 mM), 10 μl of 2× SYBR Green SuperMix (Bio-Rad, Hercules, CA, http://www.bio-rad.com), and 4 μl of diethylpyrocarbonate-H2O. Primer sequences are listed in supporting information Methods Table 1. Bio-Rad MyiQ software was used to calculate threshold cycle (CT) values for all target genes and for the reference gene hypoxanthine phosphoribosyltransferase (HPRT). The expression values for 67LR bright tumor cell population are presented as a fold expression in relation to 67LR dim tumor cell population; the actual values were calculated using the 2−ΔΔCT equation, where ΔΔCT = [CTTarget − CTHPRT](67LR bright) − [CTTarget − CTHPRT](67LR dim).
Immunodetection of Proteins
Immunodetection of proteins was performed as previously described [25]. The primary antibodies used are listed in supporting information Methods Table 2. For double-label immunohistochemistry (Fig. 2D), CK17-specific antibodies were detected with horseradish peroxidase-conjugated rabbit anti-mouse antibodies and visualized using Vector SG (Vector Laboratories, Burlingame, CA, http://www.vectorlabs.com) chromagen, followed by extensive washing with PBS and PBS/3% H2O2, and 10% goat serum block; then Ki67-specific antibodies were detected with a goat anti-rabbit–horseradish peroxidase secondary antibody and visualized with 3,3′-diaminobenzidine tetrahydrochloride chromagen.
Figure 2.

Urothelial differentiation of human SW780 urothelial carcinoma xenografts. (A): Basal-like urothelial cells at the tumor-stroma interface showing intense immunoperoxidase staining of CK17, similar to the CK17 staining pattern of benign urothelial basal cells (inset). Staining for CK18 (B) and CK20 (C) in the interior of tumor nodules corresponding to their superficial expression in benign urothelium (insets). (D): Double immunoperoxidase staining for the proliferation-associated protein Ki67 (brown) and the basal cell marker CK17 (blue/green) indicates approximately equal proliferation rates in basal-like and non-basal-like cells. Abbreviations: CK, cytokeratin; S, stroma; T, tumor; U, urothelium.
Results
Urothelial Differentiation in Urothelial Carcinomas
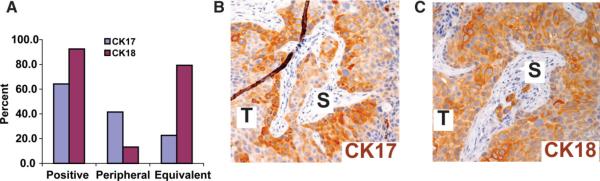
On the basis of the established biology of multilayered epithelia, we hypothesized that if highly tumorigenic urothelial cancer cells exist, they might share biological properties with stem/progenitor cells in the basal compartment of normal urothelium. By immunohistochemical analysis, almost two thirds (n = 34 of 53) of high-grade invasive primary human urothelial carcinomas analyzed expressed the urothelial basal cell marker CK17 (Fig. 1A). In two thirds of positive cases, intense staining for CK17 was preferentially detected at the tumor-stroma interface (peripheral pattern, Fig. 1B), analogous to the basal cell compartment in benign urothelium (Fig. 2A, inset). The remaining one third of cases showed equivalent (random or diffuse) CK17 staining (not shown). The intermediate cell marker CK18 was detected in 93% of cases, but lacked the stroma-oriented expression pattern seen for CK17 (Fig. 1C). Thus, in the majority of cases, the expression patterns of CK17 and CK18 in urothelial carcinomas mimic aspects of their physiological expression in normal urothelium, with increasing differentiation as a function of distance from the basement membrane.
Figure 1.
Immunohistochemical staining for CKs demonstrates aspects of urothelial differentiation in urothelial carcinomas. (A): Prevalence and patterns of CK17 and CK18 expression in primary urothelial carcinomas. (B): Representative photomicrograph showing peripheral staining for the basal cell marker cytokeratin 17 that is most intense at the tumor-stroma interface and (C) staining for the intermediate cell marker cytokeratin 18 that shows equivalent intensity at the peripheral and interior aspects of tumor nodules. Abbreviations: CK, cytokeratin; S, stroma; T, tumor.
We further investigated urothelial differentiation in normal human urothelium and in human urothelial carcinoma xenografts. Immunohistochemical analysis of urothelial differentiation markers demonstrated intense staining of CK17 in normal urothelial basal cells (inset, Fig. 2A) and an analogous layer of CK17-expressing cells in SW780 UC xenografts (Fig. 2A) at the tumor-stroma interface. CK18 expression was undetectable in basal cells and peripherally located CK17-expressing cancer cells (Fig. 2B), but was present in intermediate and superficial urothelial cells (inset, Fig. 2B) and in cancer cells in the center of tumor nodules. (Fig. 2B). CK20 staining was detected in superficial (umbrella) cells of normal urothelium (inset, Fig. 2C) and in a more limited subset of internally positioned cancer cells within UC xenograft nodules (Fig. 2C). Interestingly, although the basal compartment has the highest proliferation rate in normal urothelium [6], proliferation indices were approximately equal in CK17-expressing and nonexpressing cells (Fig. 2D and data not shown). With the exception of this abnormal proliferation pattern, these results indicate that UC nodules recapitulate normal basal-to-superficial urothelial differentiation, starting at the periphery and terminating in the interior. This differentiation can be supported, apparently, by either human bladder smooth muscle, as in the primary human tumors, or by mouse dermis, as in the xenografts.
Stroma-Dependent Co-localization of the 67-kDa Laminin Receptor and the Basal Cell Marker CK17
In looking for useful surface markers for purification of basal-like UC cells, we focused on proteins expected to be differentially expressed at the tumor-stroma interface, a region demarcated by the basement membrane. Like benign epithelial cells, tumor cells can secrete basement membrane components such as laminin [26]. Accordingly, we identified 67LR, previously noted to be upregulated in approximately 80% of high-grade invasive UC [27], as a surface marker that preferentially decorated basal cells and colocalized with CK17 in SW780 xenografts (Fig. 3A, 3B). In contrast, SW780 cells grown in culture showed no obvious relationship between CK17 and 67LR expression (Fig. 4A), suggesting that tumor-stroma interactions play an important role in UC differentiation. Together, these observations support the existence of a readily identifiable, spatially defined niche for carcinoma HTCs that is analogous to the normal urothelial stem cell niche.
Figure 3.
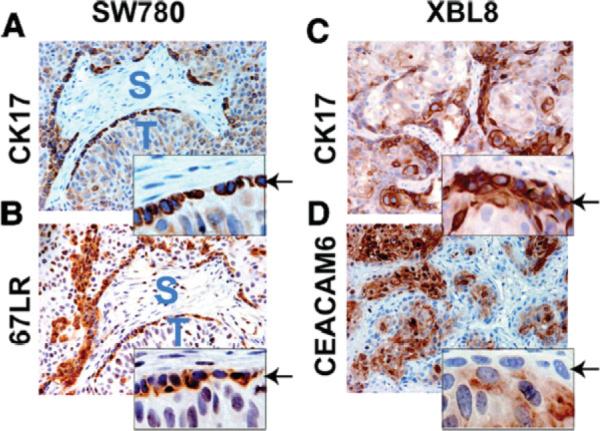
CK17-positive basal cancer cells and CK17-negative differentiated cancer cells express the surface markers 67LR and CEACAM6, respectively. (A): Photomicrograph of SW780 human UC xenograft showing basal localization of CK17 (top) and 67LR (bottom) on adjacent sections. Insets show a higher magnification view of the tumor-stromal interface. (B): Photomicrograph showing CK17 and CEACAM6 expression. Note mutually exclusive expression of the two proteins, with basal-like cells at the tumor-stroma interface, showing evidence of CK17 expression but not CEACAM6. CEACAM6-positive cells are in the internal aspect of tumor nodules. Abbreviations: CK, cytokeratin; S, stroma; T, tumor; UC, urothelial cancer.
We further confirmed this observation using a first-passage human UC xenograft, XBL8. In these experiments, basal-like cells were readily identifiable at the tumor-stroma interface (Fig. 3C, 3D) on the basis of low levels of CEACAM6 (identified by gene expression profiling, see below). Immunohistochemical staining of normal urothelium (supporting information Fig. 3) showed an analogous pattern; CEACAM6 protein was undetectable in urothelial basal cells, but readily identified in intermediate and superficial cells.
With tumor-stroma interactions in mind, we tested the relationship between 67LR levels and tumorigenicity of SW780 UC cells grown alone in culture or purified from stroma-containing xenografted tumors. As expected, fractionating cultured SW780 cells according to 67LR levels revealed no relationship between expression of the receptor and tumor-forming potency upon subcutaneous inoculation into athymic (nude) mice (data not shown).
Enhanced Xenograft Formation by Basal-like UC Cells
In contrast to SW780 cells grown in vitro, 67LR expression in tumor cells grown as xenografts in vivo correlated dramatically with basal differentiation as determined by CK17 expression (Fig. 4B). 67LR bright cells constituted approximately 15% of the human cells in these analyses (supporting information Fig. 1), a fraction similar to that seen for HTCs in other carcinomas [6]. We assayed the ability of these cells to initiate new tumors in immunodeficient mice by injecting 200–20,000 67LR bright and 67LR dim cells subcutaneously and waiting 4 months for discernable tumors to appear. Compared to bulk or the control-sorted SW780 cells, 67LR bright cells were approximately 5- to 10-fold more potent at initiating new tumors in vivo (Fig. 4C). More importantly, the ability to form new tumors was an exclusive or nearly exclusive property of the basal cell compartment in SW780 xenografts (Fig. 4C). This was particularly remarkable given the approximately equal proliferation rates of basal-like and nonbasal cells noted above (Fig. 2D). The enhanced tumor-forming potential of basal-like cells might therefore reflect their ability to undergo unlimited rounds of replication, their enhanced capacity to modify the host environment to favor tumor growth, or both.
We confirmed the enhanced tumorigenicity of basal-like UC cells using XBL8 xenografts and fractionating them by expression of CEACAM6 and the hyaluronic acid receptor CD44. Other groups have recently reported enhanced growth of tumor cells that express high levels of CD44 and preferentially localize to the tumor-stroma interface [28, 29], including in vitro growth of UC cells [29]. However, in our studies CD44 expression was not consistently limited to the tumor-stroma interface (supporting information Fig. 4), and CD44 expression was substantial in cancer cells with and without basal cell differentiation (supporting information Fig. 4). CEACAM6 dim/CD44 bright basal-like XBL8 UC cells (supporting information Fig. 2) constituted approximately 3% of the parental tumor and were dramatically more potent than CEACAM6 bright nonbasal cells in forming tumors (Fig. 4D). These results, validated in two separate tumor models and with use of independent sorting strategies, further support the enhanced tumor-forming potential of basal-like UC cells.
Histologic and immunohistochemical analysis of basal cell-derived tumors demonstrated the presence of basal-like cells and more differentiated cells—recapitulating their arrangement in the parental tumor (supporting information Figs. 5, 6). These results indicate that basal HTCs share functional properties normal to urothelial basal stem cells, including enhanced growth potential and multipotency.
Comprehensive Gene Expression Analysis of Basal HTCs
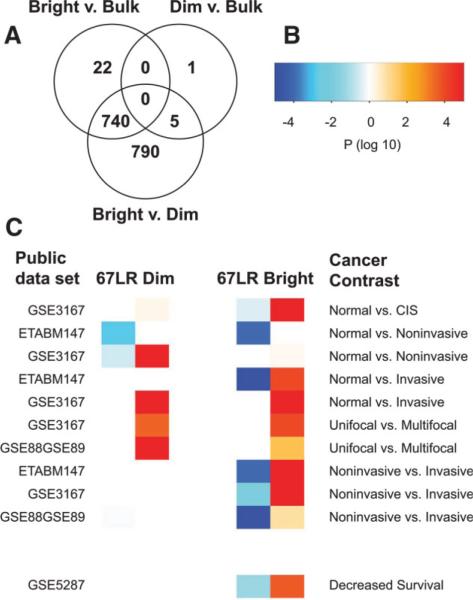
We investigated the cellular pathways underlying cancer progression and multipotency by mRNA expression analysis of sorted 67LR bright and 67LR dim fractions of human SW780 UC xenografts. Our preliminary analysis of 44,000 feature whole genome Agilent array hybridizations identified robust differential expression of more than 1,000 genes at the adjusted p < .05 level (corrected for multiple comparisons). Complete lists of differentially expressed genes are included in supporting information Tables A, B, E. We showed that gene expression in 67LR dim- and sham-sorted SW780 cells were essentially indistinguishable from each other and markedly different from 67LR bright basal-like cells (Fig. 5A). This result indicates that the basal-like cells are distinct from the tumor population as a whole, and that the UC basal HTC gene expression profile is completely hidden within the gene expression profile of the unfractionated tumor.
Figure 5.
Gene expression in SW780 human UC xenograft cell subpopulations: 67LR bright cancer HTC genes drive aspects of human UC progression. (A): Venn diagram of differentially expressed genes (Log Odds >3.0) indicates that 67LR bright cells are very distinct from 67LR dim- and control (bulk)-sorted cells, whereas bulk and 67LR dim cells have no significant differences in expression (even although 67LR bright cells are a component of the bulk population). (B): Color values represent log 10 transformed p values for association of gene expression in panel (C). Positive values (in the white-red scale) are used for concordant upregulation, whereas negative values (in the white-blue scale) are used for concordant downregulation (C). Concordant variation of gene expression between different stages of UC progression (obtained from publically available data sets) (cancer contrasts) and either 67LR bright basal HTCs (heat map on the right) or 67LR dim nontumorigenic cells (heat map on the left). Orange/red boxes show concordant upregulation in more advanced clinical stages and in cancer cells. Blue boxes show concordant downregulation in UC progression and in cancer cells. The gene expression program of 67LR bright HTCs proved to be concordantly enriched in many more contrasts than the gene expression program of 67LR nontumorigenic dim cells. Abbreviations: HTC, highly tumorigenic cancer cell; UC, urothelial cancer.
Hallmarks of Cancer Are Enriched among Differentially Expressed Genes
We confirmed differential expression of 17 of 17 genes by real-time reverse transcription/polymerase chain reaction (supporting information Fig. 7). Focusing on contrasts between 67LR bright and 67LR dim populations, we performed analysis of functional annotation [30] to reveal significant (adjusted p < .05) enrichment of genes associated with hallmark characteristic activities of cancer [16] including apoptosis, cell growth, and proliferation (Table 1; supporting information Tables D, E). There was also significant enrichment of genes comprising a variety of pharmacologically tractable signaling pathways, including Janus kinase-signal transducer and activator of transcription, Notch, focal adhesion, mammalian target of rapamycin, ErbB, and Wnt [31–36], which may form the basis for novel mechanism-based therapies for UC. Additional analysis of functional annotation results are available as supporting information Tables D, E and can be browsed online at http://astor.som.jhmi.edu/~marchion/uroCSC.html.
Table 1.
Gene expression was comprehensively profiled in 67LR bright highly tumorigenic cancer cells and 67LR dim cells
| Source | Identifier | Pathway | P | Genes up in 67LR bright | Genes down in 67LR bright |
|---|---|---|---|---|---|
| KEGG | 4510 | Focal adhesion | <10−5 | ITGA6, ITGAV, CAV1, LAMB3, LAMC2, PGF, SPP1, CAV3, IGFIR, LAMA3 | BIRC3 |
| KEGG | 4110 | Cell cycle | <10−5 | SFN, CDKNIC | CDC2 |
| KEGG | 4012 | ErbB signaling pathway | .0009 | NRG2, HBEGF, MYC | AREG |
| KEGG | 4330 | Notch signaling pathway | .0004 | DLL1, MFNG | |
| KEGG | 4150 | mTOR signaling pathway | .005 | HIF1A, PGF, DDIT4 | |
| KEGG | 4310 | Wnt signaling pathway | .0045 | MYC, NFATC1, WNT10A | PRKACB |
| GO | 6979 | Response to oxidative stress | .0013 | APOE, SOD2, HMOX1, PRNP, KRT1 | IDH1 |
| GO | 6916 | Anti-apoptosis | <10−5 | APOE, NOL3, NRG2, BNIP3, SOCS3, HMOX1, PRNP, CRYAB, IGF1R | BIRC3 |
| GO | 6281 | DNA repair | <10−5 | SOD2, FANCE | |
| GO | 1558 | Cell growth | .002 | IGFBP2, DLC | PRSS2, PLCE1, ING3 |
The table lists selected functional pathways that were significantly enriched (adjusted p < .05) among differentially expressed genes between 67LR bright and dim cells, along with selected genes in each pathway showing statistically significant (adjusted p < 0.001) differential expression. Genes are listed according to official symbols in descending order of statistical significance. We added Kremen2 to the Wnt pathway category, but this addition was not considered in statistical analysis.
Components of the Wnt Signaling Pathway
The Wnt signaling pathway regulates stem cell maintenance and differentiation in a variety of tissues [37] and previous work has demonstrated Wnt pathway activation in UC, deriving most frequently from epigenetic events [38–40]. By gene expression analysis, WNT signaling was highly significant (p < .03). 67LR bright HTCs showed highly significant upregulation (adjusted p < 10−6 in comparison to levels in 67LR dim cells) of several activating pathway components, including WNT4, −6, and −10A ligands, their FZD6 receptor, and the Wnt effector/transcriptional co-activator β-catenin. These cells also showed significant downregulation (adjusted p < 10−6) of the Wnt ligand antagonist DKK1. KREMEN2 levels were markedly elevated in 67LR bright HTCs, which, in the setting of low DKK levels, can convert KREMEN proteins from Wnt pathway antagonists to pathway agonists by stabilizing the Wnt ligand coreceptors LRP5 and LRP6 [41, 42]. Wnt ligand binding leads to stabilization of β-catenin and allows it to activate Wnt pathway target genes. Although such targets vary across tissue types, several common Wnt targets, including MYC and MMP7 [43, 44], were significantly (adjusted p < 10−5) upregulated in 67LR bright cells. These mRNA expression profiling results demonstrate selective expression of WNT activating components and target genes in 67LR bright basal/HTCs. In addition, immunofluorescent staining of SW780 human UC xenografts with β-catenin-specific antibodies demonstrated basal-specific cytoplasmic accumulation of the Wnt pathway effector β-catenin (supporting information Fig. 8), consistent with moderate levels of pathway activity (see [45]). Little staining was observed in the interior nontumorigenic regions of tumor nodules. Taken together, these results show regulation of WNT pathway components in a pattern expected to stimulate WNT signaling activity and expression of target genes in basal/HTCs. Further studies will be needed to elucidate the function of WNT in HTCs. The SW780 cell line xenograft system should be well-suited for such studies.
Enriched Antioxidant and Detoxification Gene Expression in Highly Tumorigenic Cells
Expression analysis indicated that 67LR bright HTCs were significantly enriched for expression of potential chemoresistance pathways, including genes with oxidoreductase activity (p < 10−6), and greater than twofold upregulation of several individual genes with established protective roles in stem cells and/or cancer. These included antioxidant and detoxification enzymes such as aldehyde dehydrogenase, which mediates resistance to cyclophosphamide [46], heme oxygenase, which mediates resistance to gemcitabine and radiation therapy [47], and superoxide dismutase, which mediates resistance to both daunorubicin [48] and radiotherapy [49]. These results indicate the potential utility of studying chemo- and radio-responsiveness in SW780 xenografts, particularly since this model is easily monitored by histology and immunohistochemistry for effects on numbers of basal-like versus differentiated cells.
67LR Bright HTC Gene Expression Signature Is Associated with UC Progression and Death
As further indication of a role for basal-like cells in human UC biology, we found highly significant (in many cases well below p = .01) identity in genes that were concordantly (up and/or down) regulated in both 67LR bright cells and in increasingly aggressive forms of UC (Fig. 5). This result was somewhat surprising given the heterogeneous cell types, both benign and malignant, that were likely present in the human tissue samples. In publically available gene expression data from freshly resected UC cases and control tissues [50–53], basal-like HTC gene expression overlapped particularly strongly with genes distinguishing normal urothelium from UC, solitary (unifocal) from multifocal cancers, and noninvasive from invasive cancers (Fig. 5B, 5C). Even more remarkable was the significant enrichment (p < .001) of genes predicting poor survival in UC [53]. Since the basal HTC gene signature should be masked by more differentiated cells, as indicated above (Fig. 5A), these results suggest a particularly strong relationship between HTCs and aggressive behavior in UCs. Indeed, compared to gene expression in nontumorigenic cancer cells, basal HTC gene expression was far more significantly related to gene expression in UC progression (Fig. 5B, 5C). These results support a recent report relating HTC gene expression and poor prognosis in breast cancer [54] and further validate the biological and clinical relevance of our experimental xenograft system. Thus, basal-like cells, a minor fraction of UC tumors, may play a disproportionately important role in cancer progression.
Discussion
We have identified a differentiation program in the majority of urothelial carcinomas that mirrors normal urothelial differentiation. Our data support the notion that carcinomas have a basal population at the tumor-stroma interface that resembles benign urothelial stem cells, differentiates as it moves away from the basal compartment, and loses tumor-forming potential upon differentiation. Our finding that urothelial HTCs localize to the basal compartment and likely interact with adjacent stroma provides empirical support for proposed links between stem cell differentiation and the “invasive front” of carcinomas [15]. Accordingly, there may be a niche at the tumor-stroma interface that coordinates the balance between epithelial differentiation, carcinogenesis, and metastasis.
Further supporting the idea of a stromal niche, we found that mouse subcutaneous stroma was able to direct adjacent UC cells to differentiate in a way that is very much analogous to the differentiation of normal basal urothelial cells by bladder stroma. This differentiation was absent in vitro, but apparent in xenografts, whether inoculated from either low-passage xenografts or from long established cultured cells. In practical terms, these results demonstrate a potentially convenient system for assaying HTC and stem cell-related properties using cells readily available from public repositories and easily manipulated for engineered expression of genes of interest. In conceptual terms, these observations point to the potential utility of studying epithelial-stromal interactions and their malignant (carcinoma-stroma) counterparts in stem cell and HTC biology.
The enhanced tumor-forming potential of the basal compartment is accompanied by distinctive gene expression programs associated with fundamental (hallmark) properties of cancer [16] and pharmacologically tractable signaling pathways (Table 1 and supporting information Tables D, E). Basal HTC gene expression is masked by other cells in the tumor, but is clinically relevant as demonstrated by its strong overlap with genes driving neoplastic transformation, invasion, and death in UC (Fig. 5).
Other investigators have questioned whether high xenografting efficiency in animals can be equated with enhanced tumorigenicity in human patients [6]. Our data do not definitively exclude the possibility that the basal cell-like phenotype we observed is highly tumorigenic only in animals, but not in humans. However, in addition to xenografting efficiency, several other observations support the hypothesis that HTCs are disproportionately important in promoting human UC progression: (a) Similarity of basal-like HTCs to normal multipotent, self-renewing urothelial stem cells, (b) mulitpotency of HTCs in xenografting assays (supporting information Figs. 5, 6), and (c) concordant expression by HTCs of genes driving UC progression in humans (Fig. 5B, 5C). The latter observation will form the basis for correlative and clinical studies to more definitively address the roles of these cells.
Multipotency and high rates of tumorigenicity are the essential defining features of “cancer stem cells” [5–7, 9], which may or may not derive from or bear phenotypic similarity to benign tissue stem cells [7]. Although we have demonstrated these essential features in basal-like HTCs, we note that some investigators impose additional criteria such as superior orthotopic growth capabilities [9]. Since the cancer stem cell concept continues to evolve (see [7, 55]), we have chosen the more philosophically neutral nomenclature “HTC.”
We identified evidence for basal-like cells in approximately two thirds of human UC cases. The remaining third may have basal-like cells that do not express CK17 or they may lack basal-like cells altogether. Since basal-like differentiation in other carcinomas predicts poor prognosis (see [56, 57]), and we did not observe significantly different clinical features associated with CK17 expression, we favor the notion that CK17 expression alone is not sufficiently sensitive to identify all cases with basal-like differentiation. Future studies will aim to develop a panel of markers that reliably identify basal-like HTCs in patient material. Approaches based on CEACAM6 and 67LR may suffice, or be augmented with additional candidates from lists of differentially expressed genes (Table 1, supporting information Tables A–C). Once HTC panels are validated, additional gene-profiling studies should reveal common pathways for UC pathogenesis and may identify drug targets that are particularly important for long-term tumor growth. Alternatively, it may be possible to identify relevant pathways on a patient-by-patient basis through immunohistochemical colocalization of basal cell markers and pathway-specific activation markers as part of an individualized therapy approach. Similar immunohistochemical colocalization protocols are in widespread diagnostic use in surgical pathology laboratories (see [58]) and would be more practical for identification of HTCs in routine patient material than flow cytometry-based assays reported previously (reviewed in [6]).'
Supplementary Material
Acknowledgments
We thank A. Tam and L. Blosser for assistance with FACS, members of the Berman laboratory for comments on the manuscript, and D. Trusty, K. Lecksell, N. Abdallah, and H. Fedor for help with specimen acquisition and analysis. This work was supported by the following grants: NIH R01DK072000, P01CA077664, and K08DK059375, The Bladder Cancer Research Center at Johns Hopkins University, and Stemline Therapeutics (to D.M.B.); NSF DMS0342111 and NIH/NCRR 1U54RR023561-01A1 (to L.M.) and P50CA088843 (to G.P.).
Disclosure of Potential Conflicts of Interest Partial funding for the study described in this article was provided by Stemline Therapeutics. Under a licensing agreement between Stemline Therapeutics and the Johns Hopkins University (JHU), D.M.B., W.M., and JHU are entitled to financial consideration for commercial use of inventions described in this article. Dr. Berman is a paid consultant to Stemline and owns Stemline stock options. The terms of this arrangement are managed by the JHU in accordance with its conflict of interest policies.
Footnotes
See www.StemCells.com for supporting information material available online.
References
- 1.Kurzrock EA, Lieu DK, Degraffenried LA, et al. Label-retaining cells of the bladder: Candidate urothelial stem cells. Am J Physiol Renal Physiol. 2008;294:F1415–F1421. doi: 10.1152/ajprenal.00533.2007. [DOI] [PubMed] [Google Scholar]
- 2.Farsund T. Cell kinetics of mouse urinary bladder epithelium. II. Changes in proliferation and nuclear DNA content during necrosis regeneration, and hyperplasia caused by a single dose of cyclophosphamide. Virchows Arch B Cell Pathol. 1976;21:279–298. [PubMed] [Google Scholar]
- 3.Lewis SA. Everything you wanted to know about the bladder epithelium but were afraid to ask. Am J Physiol Renal Physiol. 2000;278:F867–F874. doi: 10.1152/ajprenal.2000.278.6.F867. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Thun MJ, Ries LA, et al. Annual report to the nation on the status of cancer, 1975–2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst. 2008;100:1672–1694. doi: 10.1093/jnci/djn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lobo NA, Shimono Y, Qian D, et al. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–699. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- 6.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 7.Jordan CT. Cancer stem cells: Controversial or just misunderstood? Cell Stem Cell. 2009;4:203–205. doi: 10.1016/j.stem.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 9.Wang JC, Dick JE. Cancer stem cells: Lessons from leukemia. Trends Cell Biol. 2005;15:494–501. doi: 10.1016/j.tcb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Sun TT, Eichner R, Nelson WG, et al. Keratin classes: Molecular markers for different types of epithelial differentiation. J Invest Dermatol. 1983;81:109s–115s. doi: 10.1111/1523-1747.ep12540831. [DOI] [PubMed] [Google Scholar]
- 11.Grubb C, Janota I. Squamous differentiation in carcinoma in situ of the cervix uteri. A cyto-histological correlation of malignant intraepithelial lesions with invasive carcinoma. J Clin Pathol. 1967;20:7–14. doi: 10.1136/jcp.20.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang HY, Shariat SF, Sun TT, et al. Persistent uroplakin expression in advanced urothelial carcinomas: Implications in urothelial tumor progression and clinical outcome. Hum Pathol. 2007;38:1703–1713. doi: 10.1016/j.humpath.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li SM, Zhang ZT, Chan S, et al. Detection of circulating uroplakin-positive cells in patients with transitional cell carcinoma of the bladder. J Urol. 1999;162:931–935. doi: 10.1097/00005392-199909010-00093. [DOI] [PubMed] [Google Scholar]
- 14.Seraj MJ, Thomas AR, Chin JL, et al. Molecular determination of perivesical and lymph node metastasis after radical cystectomy for urothelial carcinoma of the bladder. Clin Cancer Res. 2001;7:1516–1522. [PubMed] [Google Scholar]
- 15.Brabletz T, Jung A, Spaderna S, et al. Opinion: Migrating cancer stem cells - an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5:744–749. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 16.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 17.Fedor HL, De Marzo AM. Practical methods for tissue microarray construction. Methods Mol Med. 2005;103:89–101. doi: 10.1385/1-59259-780-7:089. [DOI] [PubMed] [Google Scholar]
- 18.Ihaka R, Gentleman R. R: A language for data analysis and graphics. J Comput Graph Stat. 1996;5:299–314. [Google Scholar]
- 19.Gentleman RC, Carey VJ, Bates DM, et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smyth GK, Speed T. Normalization of cDNA microarray data. Methods. 2003;31:265–273. doi: 10.1016/s1046-2023(03)00155-5. [DOI] [PubMed] [Google Scholar]
- 21.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- 22.Irizarry RA, Bolstad BM, Collin F, et al. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashburner M, Ball CA, Blake JA, et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanehisa M. The KEGG database. Novartis Found Symp. 2002;247:91–101. discussion101–113,119–128,244–252. [PubMed] [Google Scholar]
- 25.Kleeberger W, Bova GS, Nielsen ME, et al. Roles for the stem cell associated intermediate filament Nestin in prostate cancer migration and metastasis. Cancer Res. 2007;67:9199–9206. doi: 10.1158/0008-5472.CAN-07-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiyoshima K, Oda Y, Kinukawa N, et al. Overexpression of laminin-5 gamma2 chain and its prognostic significance in urothelial carcinoma of urinary bladder: Association with expression of cyclooxygenase 2, epidermal growth factor receptor [corrected] and human epidermal growth factor receptor [corrected] 2. Hum Pathol. 2005;36:522–530. doi: 10.1016/j.humpath.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Diggle CP, Cruickshank S, Olsburgh JD, et al. Identification of genes up-regulated in urothelial tumors: The 67-kd laminin receptor and tumor-associated trypsin inhibitor. Am J Pathol. 2003;163:493–504. doi: 10.1016/S0002-9440(10)63678-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prince ME, Sivanandan R, Kaczorowski A, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang YM, Chang JW. Bladder cancer initiating cells (BCICs) are among EMA-CD44v6+ subset: Novel methods for isolating undetermined cancer stem (initiating) cells. Cancer Invest. 2008;26:725–733. doi: 10.1080/07357900801941845. [DOI] [PubMed] [Google Scholar]
- 30.Schaeffer EM, Marchionni L, Huang Z, et al. Androgen-induced programs for prostate epithelial growth and invasion arise in embryogenesis and are reactivated in cancer. Oncogene. 2008;27:7180–7191. doi: 10.1038/onc.2008.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Golubovskaya VM, Nyberg C, Zheng M, et al. A small molecule inhibitor, 1,2,4,5-benzenetetraamine tetrahydrochloride, targeting the Y397 site of focal adhesion kinase decreases tumor growth. J Med Chem. 2008;51:7405–7416. doi: 10.1021/jm800483v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim BH, Yin CH, Guo Q, et al. A small-molecule compound identified through a cell-based screening inhibits JAK/STAT pathway signaling in human cancer cells. Mol Cancer Ther. 2008;7:2672–2680. doi: 10.1158/1535-7163.MCT-08-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rizzo P, Osipo C, Foreman K, et al. Rational targeting of Notch signaling in cancer. Oncogene. 2008;27:5124–5131. doi: 10.1038/onc.2008.226. [DOI] [PubMed] [Google Scholar]
- 34.Xue Q, Hopkins B, Perruzzi C, et al. Palomid 529, a novel small-molecule drug, is a TORC1/TORC2 inhibitor that reduces tumor growth, tumor angiogenesis, and vascular permeability. Cancer Res. 2008;68:9551–9557. doi: 10.1158/0008-5472.CAN-08-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wakeling AE. Inhibitors of growth factor signalling. Endocr Relat Cancer. 2005;12(Suppl 1):S183–S187. doi: 10.1677/erc.1.01014. [DOI] [PubMed] [Google Scholar]
- 36.Chen B, Dodge ME, Tang W, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nusse R. Wnt signaling and stem cell control. Cell Res. 2008;18:523–527. doi: 10.1038/cr.2008.47. [DOI] [PubMed] [Google Scholar]
- 38.Urakami S, Shiina H, Enokida H, et al. Combination analysis of hypermethylated Wnt-antagonist family genes as a novel epigenetic bio-marker panel for bladder cancer detection. Clin Cancer Res. 2006;12:2109–2116. doi: 10.1158/1078-0432.CCR-05-2468. [DOI] [PubMed] [Google Scholar]
- 39.Urakami S, Shiina H, Enokida H, et al. Epigenetic inactivation of Wnt inhibitory factor-1 plays an important role in bladder cancer through aberrant canonical Wnt/beta-catenin signaling pathway. Clin Cancer Res. 2006;12:383–391. doi: 10.1158/1078-0432.CCR-05-1344. [DOI] [PubMed] [Google Scholar]
- 40.Stoehr R, Krieg RC, Knuechel R, et al. No evidence for involvement of beta-catenin and APC in urothelial carcinomas. Int J Oncol. 2002;20:905–911. [PubMed] [Google Scholar]
- 41.Cselenyi CS, Lee E. Context-dependent activation or inhibition of Wnt-beta-catenin signaling by Kremen. Sci Signal. 2008;1:e10. doi: 10.1126/stke.18pe10. [DOI] [PubMed] [Google Scholar]
- 42.Hassler C, Cruciat CM, Huang YL, et al. Kremen is required for neural crest induction in Xenopus and promotes LRP6-mediated Wnt signaling. Development. 2007;134:4255–4263. doi: 10.1242/dev.005942. [DOI] [PubMed] [Google Scholar]
- 43.Crawford HC, Fingleton BM, Rudolph-Owen LA, et al. The metalloproteinase matrilysin is a target of beta-catenin transactivation in intestinal tumors. Oncogene. 1999;18:2883–2891. doi: 10.1038/sj.onc.1202627. [DOI] [PubMed] [Google Scholar]
- 44.He TC, Sparks AB, Rago C, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 45.Gao S, Eiberg H, Krogdahl A, et al. Cytoplasmic expression of E-cadherin and beta-Catenin correlated with LOH and hypermethylation of the APC gene in oral squamous cell carcinomas. J Oral Pathol Med. 2005;34:116–119. doi: 10.1111/j.1600-0714.2004.00275.x. [DOI] [PubMed] [Google Scholar]
- 46.Kastan MB, Schlaffer E, Russo JE, et al. Direct demonstration of elevated aldehyde dehydrogenase in human hematopoietic progenitor cells. Blood. 1990;75:1947–1950. [PubMed] [Google Scholar]
- 47.Berberat PO, Dambrauskas Z, Gulbinas A, et al. Inhibition of heme oxygenase-1 increases responsiveness of pancreatic cancer cells to anticancer treatment. Clin Cancer Res. 2005;11:3790–3798. doi: 10.1158/1078-0432.CCR-04-2159. [DOI] [PubMed] [Google Scholar]
- 48.Eisele L, Klein-Hitpass L, Chatzimanolis N, et al. Differential expression of drug-resistance-related genes between sensitive and resistant blasts in acute myeloid leukemia. Acta Haematol. 2007;117:8–15. doi: 10.1159/000096854. [DOI] [PubMed] [Google Scholar]
- 49.Josson S, Xu Y, Fang F, et al. RelB regulates manganese superoxide dismutase gene and resistance to ionizing radiation of prostate cancer cells. Oncogene. 2006;25:1554–1559. doi: 10.1038/sj.onc.1209186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dyrskjot L, Kruhoffer M, Thykjaer T, et al. Gene expression in the urinary bladder: A common carcinoma in situ gene expression signature exists disregarding histopathological classification. Cancer Res. 2004;64:4040–4048. doi: 10.1158/0008-5472.CAN-03-3620. [DOI] [PubMed] [Google Scholar]
- 51.Dyrskjot L, Thykjaer T, Kruhoffer M, et al. Identifying distinct classes of bladder carcinoma using microarrays. Nat Genet. 2003;33:90–96. doi: 10.1038/ng1061. [DOI] [PubMed] [Google Scholar]
- 52.Stransky N, Vallot C, Reyal F, et al. Regional copy number-independent deregulation of transcription in cancer. Nat Genet. 2006;38:1386–1396. doi: 10.1038/ng1923. [DOI] [PubMed] [Google Scholar]
- 53.Als AB, Dyrskjot L, von der Maase H, et al. Emmprin and survivin predict response and survival following cisplatin-containing chemo-therapy in patients with advanced bladder cancer. Clin Cancer Res. 2007;13:4407–4414. doi: 10.1158/1078-0432.CCR-07-0109. [DOI] [PubMed] [Google Scholar]
- 54.Liu R, Wang X, Chen GY, et al. The prognostic role of a gene signature from tumorigenic breast-cancer cells. New Engl J Med. 2007;356:217–226. doi: 10.1056/NEJMoa063994. [DOI] [PubMed] [Google Scholar]
- 55.Kern SE, Shibata D. The fuzzy math of solid tumor stem cells: A perspective. Cancer Res. 2007;67:8985–8988. doi: 10.1158/0008-5472.CAN-07-1971. [DOI] [PubMed] [Google Scholar]
- 56.Luna MA, el Naggar A, Parichatikanond P, et al. Basaloid squamous carcinoma of the upper aerodigestive tract. Clinicopathologic and DNA flow cytometric analysis. Cancer. 1990;66:537–542. doi: 10.1002/1097-0142(19900801)66:3<537::aid-cncr2820660322>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 57.Schneider BP, Winer EP, Foulkes WD, et al. Triple-negative breast cancer: Risk factors to potential targets. Clin Cancer Res. 2008;14:8010–8018. doi: 10.1158/1078-0432.CCR-08-1208. [DOI] [PubMed] [Google Scholar]
- 58.Adley BP, Yang XJ. Alpha-methylacyl coenzyme A racemase immunoreactivity in partial atrophy of the prostate. Am J Clin Pathol. 2006;126:849–855. doi: 10.1309/F3R88U2574Q8G1J3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.