Abstract
Background
Open-label oral immunotherapy (OIT) protocols have been used to treat small numbers of patients with peanut allergy. Peanut OIT has not been evaluated in double-blind, placebo-controlled trials.
Objective
To investigate the safety and effectiveness of OIT for peanut allergy in a double blind, placebo-controlled study.
Methods
In this multicenter study, peanut-allergic children ages 1-16 years received OIT with peanut flour or placebo. Initial escalation, build-up, and maintenance phases were followed by an oral food challenge at approximately one year. Titrated skin prick tests (SPT) and laboratory studies were performed at regular intervals.
Results
Twenty-eight subjects were enrolled in the study. Three peanut OIT subjects withdrew early in the study due to allergic side effects. During the double-blind, placebo-controlled food challenge, all remaining peanut OIT subjects (N=16) ingested the maximum cumulative dose of 5000 mg (approximately 20 peanuts), while placebo subjects (N=9) ingested a median cumulative dose of 280 mg (range, 0-1900 mg) [p<0.001]. In contrast to the placebo group, the peanut OIT group showed reductions in SPT size (p<0.001), IL-5 (p=0.01), and IL-13 (p=0.02) and increases in peanut-specific IgG4 (p<0.001). Peanut OIT subjects had initial increases in peanut-specific IgE (p<0.01) but did not show significant change from baseline by the time of OFC. The ratio of FoxP3 hi: FoxP3 intermediate CD4+CD25+ T cells increased at the time of OFC (p=0.04) in peanut OIT subjects.
Conclusion
These results conclusively demonstrate that peanut OIT induces desensitization and concurrent immune modulation. The present study continues and is evaluating the hypothesis that peanut OIT causes long-term immune tolerance.
Keywords: peanut allergy, oral immunotherapy, desensitization, food allergy
Introduction
Food allergy is a major health concern in industrialized countries, affecting approximately 3.9% of children.1 Peanut allergy is one of the most common forms of food allergy, with approximately 3 million Americans reporting allergy to peanuts or tree nuts, and the prevalence appears to be increasing.2 Peanut and tree nut allergy account for the vast majority of life-threatening or fatal allergic reactions to foods.3, 4 In addition, peanut allergy is often lifelong.5 Current treatment options are limited to strict peanut avoidance and ready access to epinephrine. Challenges for patients and families with food allergy are considerable,6 and accidental ingestion is common.7 Anxiety impairs social functioning in food-allergic individuals, who report poorer health-related quality of life than those with diabetes mellitus.8
These findings underscore the need for active treatment strategies. Animal and human studies have examined potential therapies for peanut allergy, including allergen-nonspecific and allergen-specific modalities; however, recent meta-analyses highlight the shortage of controlled studies in the field.9, 10 TNX-901, a humanized monoclonal antibody, prevents binding of IgE to its high-affinity receptor on mast cells and basophils and was found to increase the threshold of peanut protein inducing symptoms in peanut-allergic individuals from less than one peanut to almost twelve peanuts.11 However, the prohibitive cost of monoclonal antibody treatment may limit this approach. A combination of traditional Chinese herbal medications, food allergy herbal formula 2 (FAHF-2), has shown promise in eliminating anaphylaxis to peanut in murine12 and phase I studies.13
Allergen immunotherapy, an allergen-specific treatment, refers to the administration of increasing amounts of an allergen to individuals with IgE-mediated allergy in order to diminish the allergic response to the substance on subsequent encounters. Traditional subcutaneous immunotherapy with aqueous peanut extract was attempted but had an unacceptably high rate of systemic reactions, despite favorable challenge outcomes.14
In pilot studies, our group has shown that open-label peanut oral immunotherapy (OIT) was relatively safe when performed in a supervised medical setting by trained personnel15 and was associated with clinical desensitization for the majority of subjects completing more than eight months of treatment.16 In order to establish the safety and efficacy of peanut OIT as an allergen-specific therapy for peanut allergy, we conducted the first randomized, double-blind, placebo-controlled study of OIT in children with peanut allergy. The primary endpoint was the amount of peanut protein ingested at food challenge by peanut OIT and placebo subjects after one year of treatment; additionally, we studied relevant immunologic mechanisms. We hypothesized that subjects receiving peanut OIT would be able to ingest more peanut protein than subjects receiving placebo.
Methods
Subject recruitment
Subjects age 1 to 16 years were recruited from the Allergy and Immunology clinics at Arkansas Children's Hospital and Duke University Medical Center or surrounding community physician offices.
Subject selection
Children with a clinical history of reaction to peanut within 60 minutes of ingestion, a peanut CAP-FEIA >15 kU/L (Phadia AB; Pharmacia, Inc, Uppsala, Sweden) or >7 kU/L if a significant reaction occurred within 6 months of enrollment, and a positive skin prick test ([SPT] ≥3 mm of negative control) were enrolled. Subjects were excluded if they had a history of severe peanut anaphylaxis (hypoxia, hypotension, or neurological compromise), moderate to severe persistent asthma, poorly-controlled atopic dermatitis, oat allergy (due to the oat-based placebo), or inability to discontinue antihistamines for skin testing and food challenges.
Randomization
A randomization table was generated to assign subjects in a 2:1 ratio to receive peanut flour or placebo. Allocation was performed prior to enrollment and saved in a locked database accessible only by laboratory personnel to keep clinical staff and subjects unaware of upcoming assignments. Investigators, subjects, and families remained blinded to the assigned intervention as well as all laboratory studies until completion of the food challenge.
Peanut and placebo flour and dosing
Premeasured peanut flour (from Partially Defatted Peanut Flour 12% Fat Light Roast; Golden Peanut Company, Alpharetta, Ga; 2 g flour = 1 g peanut protein) or placebo (toasted oat flour; Arrowhead Mills, Hereford, Tx) doses were mixed in a food vehicle of the subject's choosing and taken in 2-3 bites. Approximately 240 mg peanut protein equals 1 whole peanut.17 Intact allergen content in the soluble extract of roasted peanut flour is ∼7% Ara h 2 and ∼8% Ara h 1.
OIT protocol
While receiving the intervention (peanut flour or placebo), subjects were instructed to continue a strict peanut-free diet and to keep a diary of any missed doses or adverse symptoms. An epinephrine auto-injector was provided to all subjects. A member of the study team was available by pager and phone throughout the study.
Initial day escalation phase
The initial day escalation phase was performed on the research unit at each institution with appropriate emergency medications available. Dosing began at 0.1 mg peanut protein or placebo; doses were approximately doubled every 30 minutes until 6 mg was reached or the subject had symptoms. The highest tolerated dose was the starting dose for the buildup phase and was given on the research unit the following day. Subjects not tolerating at least 1.5 mg were withdrawn from the study.
Home dosing
Subjects were instructed to ingest each dose mixed in a vehicle food daily. Based on patterns observed during our open-label study,18 we advised subjects to hold dosing if febrile or ill and to take all doses on a full stomach. Dosing was resumed at home if the subject missed less than three daily doses; subjects returned for an observed dose if 3-5 doses were missed.
Build-up visits
Subjects returned every two weeks for approximately 44 weeks for dose escalations. Doses were increased by 50-100% until the 75 mg dose and were then increased by 25-33% until the 4000 mg maintenance dose was reached.
Maintenance phase
After reaching the maintenance dose of peanut flour or placebo, subjects ingested the dose daily for one month and then returned for the first oral food challenge at week 48.
Oral food challenge (OFC)
A double-blind, placebo-controlled food challenge was performed after four weeks of maintenance therapy. Prior to the OFC, subjects were asked to restrict the use of antihistamines, beta agonists, theophylline, and montelukast.16 All subjects were challenged to both peanut and oat flour in a blinded manner. Challenges were administered by a nurse or physician who was also blinded to the testing materials. The challenge consisted of peanut or oat flour given in increasing doses every 10-20 minutes up to a cumulative dose of 5000 mg of protein.
Safety
The safety of peanut OIT when compared to placebo was studied during the four phases of the protocol. Certain mild allergic side effects (i.e. mild oral pruritus, contact urticaria) can be expected when administering oral immunotherapy and do not require treatment or result in a change in the treatment plan. We defined “clinically-relevant” side effects as those categorized as moderate or severe by study personnel (>1 on a symptom scale ranging from 0-3) and those requiring treatment with antihistamines or epinephrine. We calculated the rate of clinically-relevant side effects during the initial day escalation, dose escalation visits, and OFC and the rate of epinephrine use during any phase of the study.
Purified peanut protein reagent
Peanut proteins were extracted from defatted peanut flour (Golden Peanut Co) in PBS, clarified by centrifugation (30,000g for 30 minutes), and sterilized by filtration. The protein concentration was determined by using the bicinchoninic acid assay (BCA; Pierce, Rockford, Ill).
Titrated skin prick testing (SPT)
Titrated SPT (1:20, 1:200, 1:2000, 1:20,000) with peanut extract (Greer Laboratories, Lenoir, NC) and saline and histamine controls were performed at enrollment and at the time of OFC. Tests to peanut were measured and followed at the same dilution that resulted in a wheal > 5 mm at the baseline visit. Wheal size was calculated as the average of the largest diameter and the perpendicular midpoint diameter.
Assays for IgE, IgG, and IgG4
Peanut-specific IgE, IgG, and IgG4 levels were measured in serum using the ImmunoCAP 100 instrument (Phadia AB) according to manufacturer's instructions.
Secreted cytokine assays
Subjects with cultured peripheral blood mononuclear cells (PBMCs) at baseline, nine months, and the time of OFC underwent cytokine analysis. PBMCs were isolated from ∼30 mL heparinized blood using Ficoll-based density separation (LymphoH; Atlanta Biologicals, Lawrenceville, Ga). For cytokine assays, PBMCs were suspended in culture media (RPMI-1640; Mediatech) with 10% autologous plasma and were cultured at 37°C in 5% CO2 humidified atmosphere for 72 hours in the presence of 200 μg/ml crude peanut extract or media alone. Culture supernatants were analyzed for a panel of five relevant cytokines (IL-5, IL-13, IL-10, IFN-gamma, and TGF-beta) by ELISA according to manufacturer's instructions (R&D Systems, Minneapolis, MN). Reported values were calculated by results of crude peanut extract stimulation minus culture media alone.
Regulatory T-cell analyses
Changes in the T regulatory cell (Treg) subset were analyzed in the nine subjects enrolled at Duke University Medical Center who reached OFC. PBMCs were suspended in culture media as described above and incubated for 7 days with crude peanut extract (200 μg/ml), tetanus toxoid (5 μg/ml; EMD Biosciences, Darmstadt, Germany), and medium alone (RPMI). Flow cytometry was performed, and CD4+CD25+ lymphocytes were gated for FoxP3intermediate and FoxP3hi signals using FlowJo software (TreeStar, Ashland, OR). In each FoxP3 gate, the percentage obtained after RPMI incubation was subtracted from CPE and tetanus toxoid values. The FoxP3hi:FoxP3int ratio was calculated as recently described19 and plotted at baseline and OFC.
Ethics
Approval was obtained through each institution's Institutional Review Board; procedures were in accordance with ethical standards of the responsible committee on human experimentation and the Helsinki Declaration of 1975, as revised in 1983. Written informed consent was obtained in accordance with each institution's ethics guidelines for research in children.
Statistical analysis
Fisher's exact test was used to compare baseline characteristics of active and placebo groups. Differences in the values over time compared to baseline were analyzed using the Wilcoxon Rank-Sum test (Stata 10, StataCorp LP, College Station, TX) on matched data, which was also used to test the primary hypothesis. In all analyses, p values < 0.05 were considered significant. The sample size target was 60 individuals, with random assignment to peanut and placebo of 2:1 and was designed to have 95% power to detect with a two-sided 5% level test a difference between a 20% desensitization rate for placebo-treated versus an 80% rate for peanut OIT-treated subjects. A predetermined analysis by the data safety monitoring board was to be done after their review of the initial OFC. Because of the significant difference in OFC outcomes, enrollment was stopped at the number of enrolled subjects.
Results
Study population
Twenty-eight subjects were enrolled between March 2007 and December 2008 (see Table 1). No screened subjects were excluded due to severe systemic reactions to peanut. The median age at enrollment was 69 months (range, 28-126). The active treatment group (9 males, 10 females) had a median baseline IgE level of 104 kU/L (range, 31-685 kU/L); the placebo group (9 males) had a median baseline IgE level of 57 kU/L (range, 20-188 kU/L).
Table 1. Baseline characteristics of subjects randomized to active or placebo intervention.
| Active | Placebo | |
|---|---|---|
| Number | 19 | 9 |
| Median age, months | 84 (38-126) | 69 (28-114) |
| Sex | 9 male, 10 female | 9 male |
| Race | 18 white, 1 biracial | 9 white |
| Subjects with asthma | 13 (68%) | 7 (78%) |
| Subjects with atopic dermatitis | 14 (74%) | 4 (44%) |
| Subjects with allergic rhinitis | 15 (79%) | 8 (89%) |
| Subjects with other food allergy | 14 (74%) | 8 (89%) |
| Median baseline peanut IgE, kU/L* | 106 (31-685) | 57 (20-188) |
| Median baseline titrated SPT, mm | 7 (5.5-15) | 7 (5.5-13) |
p=0·02
IgE = immunoglobulin E, SPT = skin prick test
Initial day escalation
During the initial day escalation, 26 (93%) of 28 subjects reached the maximum cumulative dose of 12 mg study protein (2 subjects ingested 15 mg study protein due to a repeated dose). Nine (47%) of 19 peanut OIT subjects experienced clinically-relevant side effects requiring antihistamine treatment. Of these, two also required treatment with epinephrine (see Table 2). Two peanut OIT subjects did not reach the 1.5 mg dose and were deemed initial day escalation failures. No placebo subjects had clinically-relevant symptoms or required treatment.
Table 2. Safety profile – Number of subjects requiring treatment with epinephrine.
| Active | Placebo | |
|---|---|---|
| Initial escalation day | 2/19 | 0/9 |
| Build-up visits | 0 | 0 |
| Home dosing | 0 | 1 |
| OFC | 0/16 | 3/9 |
OFC = oral food challenge
Build-up doses
Peanut OIT subjects had clinically-relevant symptoms after 1.2% of 407 buildup doses on the research unit. None required treatment with epinephrine or hospitalization. One peanut OIT subject withdrew from the study after the first dose escalation due to mild gastrointestinal symptoms precluding further dosing. No placebo subjects had clinically-relevant symptoms with dose escalation visits.
Home doses
No peanut OIT subjects needed epinephrine with home doses; one placebo subject was given epinephrine at home for symptoms with a placebo dose (see Table 2). Sixteen of 19 subjects (84%) reached the goal maintenance dose of 4000 mg.
Oral food challenge
Peanut OIT subjects reached OFC after a median of 12.4 months on treatment (range, 11.3-16.3 months) versus 11.7 months for placebo-treated subjects (range, 11-13.8 months) [p=0.07]. As shown in Figure 1, all peanut OIT subjects reaching OFC (N=16) ingested the maximum cumulative dose of 5000 mg (approximately 20 peanuts), while placebo subjects (N=9) ingested a median cumulative dose of 280 mg (range, 0-1900 mg) [p<0.001]. One peanut OIT subject had clinically-relevant symptoms (mild upper respiratory symptoms and moderate urticaria) after completing the challenge and received antihistamine treatment. No peanut OIT subject required epinephrine or hospitalization (see Table 2). Eight placebo subjects had clinically-relevant symptoms (one had grade 1 objective gastrointestinal symptoms and oral pruritus), three requiring treatment with epinephrine (see Table 2). No placebo subjects required hospitalization.
Figure 1. Cumulative amount of peanut protein ingested at OFC by peanut OIT and placebo subjects (*p<0.001) following 12 months of therapy.
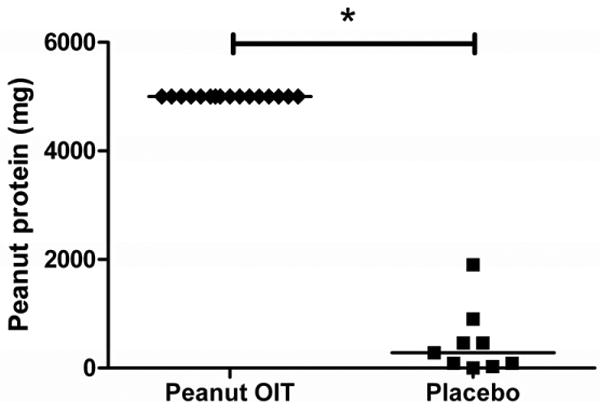
Individual subjects are shown as diamonds (peanut OIT) or squares (placebo); lines designate median values.
Titrated SPTs
As shown in Figure 2, in the peanut OIT group, titrated SPT size decreased from a median of 7 mm (range, 5.5-15 mm) at baseline to 1.75 mm (range, 0-10 mm) at the time of OFC (p<0.001). There was no significant change in SPT size in the placebo group; titrated SPT was 7 mm (range, 5.5-13 mm) at baseline and 4 mm (range, 0-12.5 mm) at OFC.
Figure 2. Titrated skin prick testing.
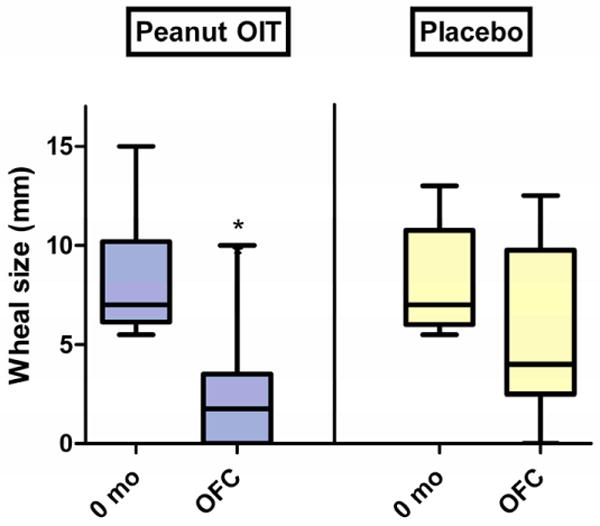
Change in median wheal size from baseline to time of OFC in peanut OIT and placebo subjects (*p<0·001). Boxes represent 25-75% quartiles; whiskers represent range. Lines designate median values.
Peanut-specific serum IgE, IgG, IgG4
The median baseline peanut IgE levels in the peanut OIT and placebo groups were 104 kU/L (range, 31-685 kU/L) and 57 kU/L (range, 20-188 kU/L), respectively (p=0.02). In peanut OIT subjects, median peanut-specific IgE increased nearly 3-fold by 2 months (to 308 kU/L, p<0.01) and was not significantly different from baseline at OFC (Figure 3a); placebo subjects showed no changes. Peanut OIT subjects had significant increases in peanut-specific IgG at all time points (Figure 3b). Peanut-specific IgG4 showed a significant increase from baseline at all time points with peanut OIT and did not change with placebo (Figure 3c).
Figure 3. Changes in serum immunoglobulin levels during treatment with peanut OIT and placebo.

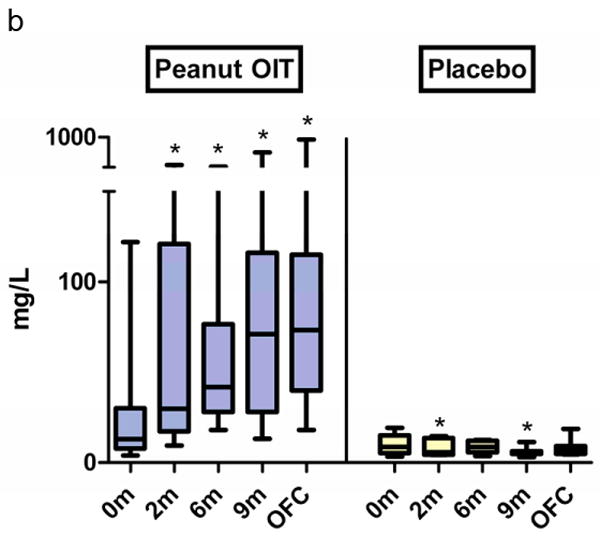
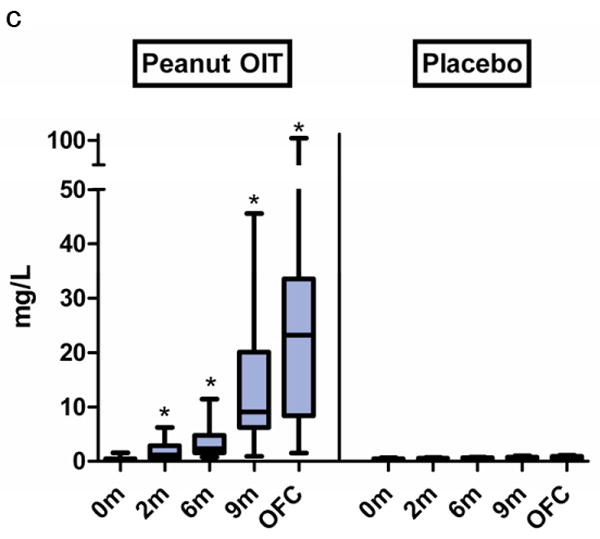
(a) Peanut-specific IgE. (*p≤0.01). (b) Peanut-specific IgG. (*p<0.05). (c) Peanut-specific IgG4. (*p≤0.001). Boxes represent 25-75% quartiles; whiskers represent range. Lines designate median values.
Secreted cytokines
A panel of 5 cytokines was measured at baseline, 9 months, and at the time of OFC in 8 peanut subjects and 9 placebo subjects who had cultured peripheral blood mononuclear cells (PBMCs) at these timepoints. IL-5 and IL-13 significantly decreased from baseline in peanut OIT subjects at 9 months and OFC (Figure 4a and 4b, p<0.03). There was a transient increase in TGF-β levels in peanut OIT subjects at 9 months (p=0.03, data not shown); levels returned to baseline at OFC. There was no change in IL-5, IL-13, or TGF-β in placebo-treated subjects. There was no significant change in IL-10 or IFN-γ in either peanut OIT or placebo subjects.
Figure 4. Changes in secreted cytokine responses for subjects receiving peanut OIT and placebo.
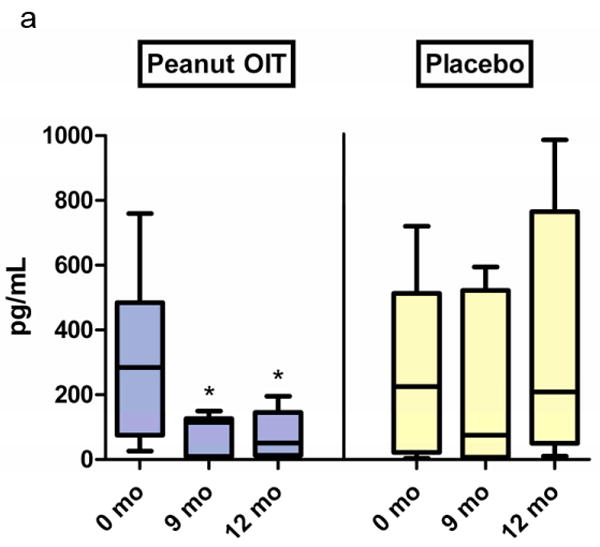
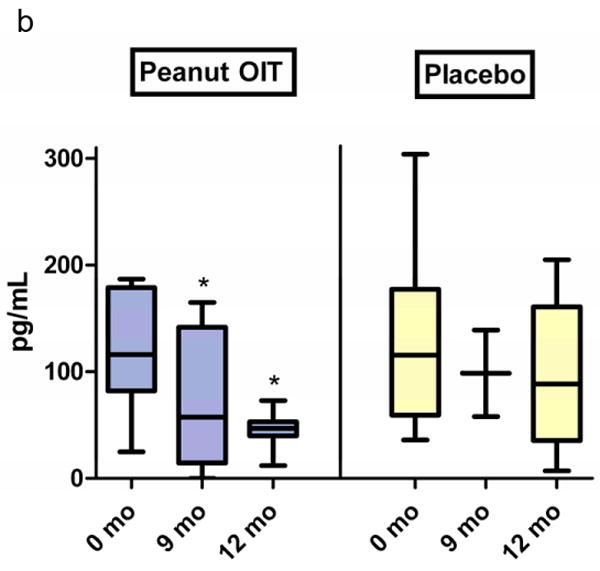
PBMCs were cultured with peanut protein for 72 hours; cytokines were measured via ELISA. (a) IL-5. (*p≤0·02). (b) IL-13. (*p≤0·03).
Regulatory T cells
Peanut OIT subjects had an increase from baseline in the ratio of FoxP3hi: FoxP3intermediate CD4+CD25+ Treg cells at the time of OFC (Figure 5a, p=0.04). Importantly, this change was not observed for the control antigen, tetanus toxoid. Placebo subjects had no change in Treg cells (Figure 5b).
Figure 5. Change in FoxP3 hi: FoxP3 intermediate CD4+CD25+ T cells from baseline to time of OFC.

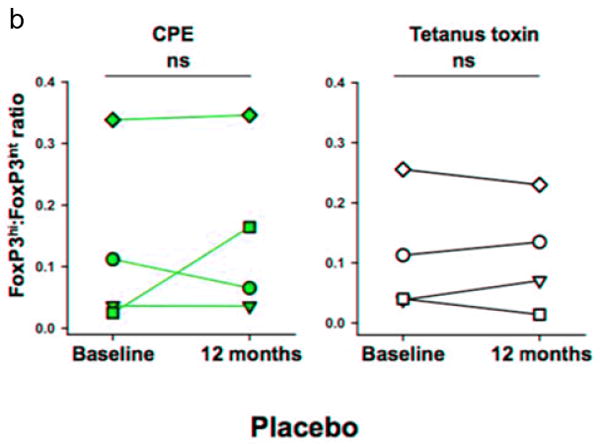
PBMCs were stimulated with crude peanut extract and tetanus toxin for 7 days, and then stained for Treg markers. Individual subjects are shown for (a) peanut OIT and (b) placebo groups. *p=0.04.
Discussion
This is the first double-blind, placebo-controlled study of peanut OIT as a treatment for peanut allergy. By establishing the safety and efficacy of allergen-specific desensitization, these data support the feasibility of using peanut OIT as an interventional therapy. Sixteen of 19 subjects (84%) completed one year of peanut OIT treatment; three (16%) were unable to complete the protocol. All 16 reaching OFC completed the 5000 mg peanut protein challenge, ingesting approximately 20 peanuts; only one subject required antihistamine therapy. Subjects receiving placebo ingested a median cumulative dose of 280 mg, or approximately 1 peanut, before stopping the OFC due to allergic symptoms. Desensitization represents an important advance in the treatment of food allergy by increasing the threshold of food antigen causing allergic symptoms. This degree of protection would likely prevent accidental peanut anaphylaxis, offering great benefit to affected patients and their families. Studies addressing the impact of peanut OIT on health-related quality of life are currently underway.20, 21
The immunologic changes during peanut OIT in antigen-specific immunoglobulins, mast cells, and T cells mirror those seen with traditional allergen immunotherapy and are similar to the natural development of tolerance to food allergens.22 In active subjects but not controls, peanut-specific IgE increased during the first year of OIT treatment but was not significantly different from baseline levels at OFC, whereas peanut-specific IgG and IgG4 increased as early as 2 months into treatment and continued to rise throughout the first year. Titrated skin prick test size decreased by the time of OFC. Peanut OIT induced a shift in allergen-specific cytokine production away from a Th2-type profile, with decreased IL-5 and IL-13 production. Peanut OIT subjects had an increased ratio of FoxP3 hi: FoxP3 intermediate CD4+CD25+ T cells at OFC, which may represent the induction of Treg cells important in suppressing the allergic response. Allergen-specific Treg cells may play a role in the natural resolution of milk allergy;23 it is possible that the same immunosuppressive functions play a role in OIT. The exact mechanism of Treg-induced immunosuppression remains unknown. It is interesting that there was only a transient increase in blood TGF-beta levels and no change in IL-10 levels; it is possible that blood cytokine levels do not reflect mucosal production or that mucosal Treg function differs from that seen in the periphery.
This study utilizes a rigorous study design to confirm and extend the findings seen in our open-label study16 and adds to the small body of literature of controlled interventional food allergy trials.10, 24 It is unlikely that all subjects in the intervention group naturally outgrew their allergy given their history and baseline immunologic parameters. Furthermore, all placebo subjects reacted at low doses of peanut protein after approximately one year in the study; none demonstrated natural tolerance acquisition. The only other placebo-controlled study of OIT for food allergy found an increased amount of milk protein ingested at challenge as well as increased milk-specific IgG4 levels in subjects receiving active treatment but not placebo.25 Although a number of subjects receiving active treatment had changes in the end-point titration SPT threshold, the median change did not reach statistical significance, and there was no change in milk-specific IgE, possibly due to methodologic differences (sample size, shorter study duration) or differing immune responses to different allergens.
This regimen was well-tolerated, with peanut OIT subjects experiencing clinically-relevant symptoms after only 1.2% of build-up doses. Importantly, no peanut OIT subjects required epinephrine treatment with dose escalation visits or home doses. During the open-label study, we observed certain patterns of allergic reactions with home dosing and were able to implement changes in this blinded study.18
The strengths of this study include its placebo-controlled design, favorable safety profile, and convincing clinical desensitization, which occurs in parallel with biologically-relevant immune modulation. However, there are also several important limitations to note. We use the term desensitization to signify a change in the amount of food antigen needed to cause allergic symptoms; this state is dependent on regular antigen exposure. In contrast, tolerance refers to long-term immunologic changes associated with the ability to ingest a food without symptoms and without ongoing therapy. Desensitization is a worthwhile therapeutic goal in that it offers individuals freedom from the risk of accidental ingestion in everyday settings; achieving long-term clinical tolerance would allow safe food ingestion without ongoing therapy by inducing lasting immunologic changes. This report does not address the induction of tolerance, which requires longer-term follow-up; the subjects in this study continue to receive peanut protein, and these longer-term outcomes will be followed. Additionally, baseline entry challenges are necessary to definitively evaluate the change in threshold dose with OIT treatment; future protocols will utilize challenges at study entry to define baseline allergen thresholds. The study's sample size is small, limiting the ability to generalize the results. Moreover, this protocol included only pediatric subjects, and further study is needed to evaluate the efficacy of OIT in adults with long-standing peanut allergy.
Peanut OIT is relatively safe under strict supervision; however, with current forms of OIT, as with other forms of immunotherapy, certain individuals are unable to endure associated side effects.15, 26 In this cohort, three subjects were unable to complete the initial day escalation or build-up dosing due to side effects. Further study may help identify the clinical and/or immunologic profiles of patients who are the best candidates for this therapy. Although many of our subjects had experienced systemic reactions to peanut prior to enrollment, we excluded patients with a history of severe anaphylaxis. This is an important limitation, as these severely affected patients may be more likely to seek treatment. Moreover, there remain numerous unanswered questions that must be addressed before OIT can be applied in widespread clinical use, including risks of OIT compared to avoidance, dosing regimen issues, patient selection, post-desensitization strategy, allocation of clinical resources, and reimbursement.27
When performed by experienced investigators in an appropriate setting, peanut OIT is a safe, allergen-specific therapy effective in inducing desensitization and providing protection against accidental exposure with ongoing therapy. Immunologic changes suggest downregulation of the allergic response. Further investigation of this promising intervention will address outstanding issues and continue to refine therapeutic protocols in hopes of offering an allergen-specific treatment option for food allergy.
Acknowledgments
Database design and support from Henry Beresford, Duke University School of Nursing. Clinical support from Duke Clinical Research Unit.
Funding sources: Funding support from the Food Allergy & Anaphylaxis Network; Food Allergy Initiative; Gerber Foundation; NIH Grant 1 R01-AI06874-01A1, NIH T32 Training Grant, and NIH Grant 1 UL1 RR024128-01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research (contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH); Dorothy & Frank Robins Family; and the National Peanut Board.
Abbreviations
- OIT
oral immunotherapy
- OFC
oral food challenge
- SPT
skin prick test
- Ig
immunoglobulin
- mAb
monoclonal antibody
- PBMC
peripheral blood mononuclear cell
- Treg
regulatory T cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Branum AM, Lukacs SL. Food allergy among U.S. children: trends in prevalence and hospitalizations. NCHS Data Brief. 2008 Oct;(10):1–8. [PubMed] [Google Scholar]
- 2.Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: a 5-year follow-up study. J Allergy Clin Immunol. 2003 Dec;112(6):1203–7. doi: 10.1016/s0091-6749(03)02026-8. [DOI] [PubMed] [Google Scholar]
- 3.Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001 Jan;107(1):191–3. doi: 10.1067/mai.2001.112031. [DOI] [PubMed] [Google Scholar]
- 4.Bock SA, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001-2006. J Allergy Clin Immunol. 2007 Apr;119(4):1016–8. doi: 10.1016/j.jaci.2006.12.622. [DOI] [PubMed] [Google Scholar]
- 5.Fleischer DM. The natural history of peanut and tree nut allergy. Curr Allergy Asthma Rep. 2007 Jun;7(3):175–81. doi: 10.1007/s11882-007-0018-y. [DOI] [PubMed] [Google Scholar]
- 6.Pieretti MM, Chung D, Pacenza R, Slotkin T, Sicherer SH. Audit of manufactured products: use of allergen advisory labels and identification of labeling ambiguities. J Allergy Clin Immunol. 2009 Aug;124(2):337–41. doi: 10.1016/j.jaci.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 7.Sicherer SH, Burks AW, Sampson HA. Clinical Features of Acute Allergic Reactions to Peanut and Tree Nuts in Children. Pediatrics. 1998 July 1;102(1):e6. doi: 10.1542/peds.102.1.e6. [DOI] [PubMed] [Google Scholar]
- 8.F-d Blok BMJ, Dubois AEJ, Vlieg-Boerstra BJ, Elberink JNGO, Raat H, DunnGalvin A, et al. Health-related quality of life of food allergic patients: comparison with the general population and other diseases. Allergy. 2009;65(2):238–44. doi: 10.1111/j.1398-9995.2009.02121.x. [DOI] [PubMed] [Google Scholar]
- 9.Chafen JJ, Newberry SJ, Riedl MA, Bravata DM, Maglione M, Suttorp MJ, et al. Diagnosing and managing common food allergies: a systematic review. JAMA. 2010 May 12;303(18):1848–56. doi: 10.1001/jama.2010.582. in press. [DOI] [PubMed] [Google Scholar]
- 10.Fisher HR, Toit GD, Lack G. Specific oral tolerance induction in food allergic children: is oral desensitisation more effective than allergen avoidance?: A meta-analysis of published RCTs. Arch Dis Child. 2010 Jun 3; doi: 10.1136/adc.2009.172460. in press. [DOI] [PubMed] [Google Scholar]
- 11.Leung DY, Sampson HA, Yunginger JW, Burks AW, Jr, Schneider LC, Wortel CH, et al. Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med. 2003 Mar 13;348(11):986–93. doi: 10.1056/NEJMoa022613. [DOI] [PubMed] [Google Scholar]
- 12.Srivastava KD, Kattan JD, Zou ZM, Li JH, Zhang L, Wallenstein S, et al. The Chinese herbal medicine formula FAHF-2 completely blocks anaphylactic reactions in a murine model of peanut allergy. J Allergy Clin Immunol. 2005 Jan;115(1):171–8. doi: 10.1016/j.jaci.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Patil SP, Yang N, Ko J, Lee J, Noone S, et al. Safety, tolerability, and immunologic effects of a food allergy herbal formula in food allergic individuals: a randomized, double-blinded, placebo-controlled, dose escalation, phase 1 study. Ann Allergy Asthma Immunol. 2010 Jul;105(1):75–84. doi: 10.1016/j.anai.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson HS, Lahr J, Rule R, Bock A, Leung D. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J Allergy Clin Immunol. 1997 Jun;99(6 Pt 1):744–51. doi: 10.1016/s0091-6749(97)80006-1. [DOI] [PubMed] [Google Scholar]
- 15.Hofmann AM, Scurlock AM, Jones SM, Palmer KP, Lokhnygina Y, Steele PH, et al. Safety of a peanut oral immunotherapy protocol in children with peanut allergy. J Allergy Clin Immunol. 2009 Aug;124(2):286–91. 91 e1–6. doi: 10.1016/j.jaci.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009 Aug;124(2):292–300. e1–97. doi: 10.1016/j.jaci.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.USDo Agriculture. USDA National Nutrient Database for Standard Reference, release 21. 2002 [cited December 21, 2009]; Available from: http://ars.usda.gov/Services/docs.htm?docid=17477.
- 18.Varshney P, Steele PH, Vickery BP, Bird JA, Thyagarajan A, Scurlock AM, et al. Adverse reactions during peanut oral immunotherapy home dosing. J Allergy Clin Immunol. 2009 Nov 11;124(6):1351–2. doi: 10.1016/j.jaci.2009.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mittag D, Scholzen A, Varese N, Baxter L, Paukovics G, Harrison LC, et al. The Effector T Cell Response to Ryegrass Pollen Is Counterregulated by Simultaneous Induction of Regulatory T Cells. J Immunol. 2010 May 1;184(9):4708–16. doi: 10.4049/jimmunol.0901036. [DOI] [PubMed] [Google Scholar]
- 20.Bird A, Daly D, Burks W, Hourihane JOB, DunnGalvin A. Impact of Oral Food-Specific Immunotheraphy (OIT) on Health Related Quality of Life (HRQL) of Children and Parents During Build Up of Tolerance. The Journal of allergy and clinical immunology. 2010 Feb;125(2):AB22. [Google Scholar]
- 21.Edie AH, Steele P, Kamilaris J, Stowe R, Dunn A, Chin W, et al. Improvement of Quality of Life in Children with Peanut Allergy upon Development of Tolerance to Peanut. The Journal of allergy and clinical immunology. 2010 Feb;125(2):AB52. [Google Scholar]
- 22.Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. Journal of Allergy and Clinical Immunology. 2007;119(4):780–9. doi: 10.1016/j.jaci.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 23.Shreffler WG, Wanich N, Moloney M, Nowak-Wegrzyn A, Sampson HA. Association of allergen-specific regulatory T cells with the onset of clinical tolerance to milk protein. Journal of Allergy and Clinical Immunology. 2009;123(1):43–52.e7. doi: 10.1016/j.jaci.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 24.Blumchen K, Ulbricht H, Staden U, Dobberstein K, Beschorner J, de Oliveira LC, et al. Oral peanut immunotherapy in children with peanut anaphylaxis. J Allergy Clin Immunol. 2010 Jul;126(1):83–91.e1. doi: 10.1016/j.jaci.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 25.Skripak JM, Nash SD, Rowley H, Brereton NH, Oh S, Hamilton RG, et al. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow's milk allergy. J Allergy Clin Immunol. 2008 Dec;122(6):1154–60. doi: 10.1016/j.jaci.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Longo G, Barbi E, Berti I, Meneghetti R, Pittalis A, Ronfani L, et al. Specific oral tolerance induction in children with very severe cow's milk-induced reactions. J Allergy Clin Immunol. 2008 Feb;121(2):343–7. doi: 10.1016/j.jaci.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 27.Thyagarajan A, Varshney P, Jones SM, Sicherer S, Wood R, Vickery BP, et al. Peanut oral immunotherapy is not ready for clinical use. J Allergy Clin Immunol. 2010 Jul;126(1):31–2. doi: 10.1016/j.jaci.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


