Abstract
Background
EPHX1 is a key enzyme in metabolizing some exogenous carcinogens such as products of cigarette-smoking. Two functional polymorphisms in the EPHX1 gene, Tyr113His and His139Arg can alter the enzyme activity, suggesting their possible association with carcinogenesis risk, particularly of some tobacco-related cancers.
Methodology/Principal Findings
A comprehensive systematic review and meta-analysis was performed of available studies on these two polymorphisms and cancer risk published up to November 2010, consisting of 84 studies (31144 cases and 42439 controls) for Tyr113His and 77 studies (28496 cases and 38506 controls) for His139Arg primarily focused on lung cancer, upper aerodigestive tract (UADT) cancers (including oral, pharynx, larynx and esophagus cancers), colorectal cancer or adenoma, bladder cancer and breast cancer. Results showed that Y113H low activity allele (H) was significantly associated with decreased risk of lung cancer (OR = 0.88, 95%CI = 0.80–0.96) and UADT cancers (OR = 0.86, 95%CI = 0.77–0.97) and H139R high activity allele (R) with increased risk of lung cancer (OR = 1.18, 95%CI = 1.04–1.33) but not of UADT cancers (OR = 1.05, 95%CI = 0.93–1.17). Pooled analysis of lung and UADT cancers revealed that low EPHX1 enzyme activity, predicted by the combination of Y113H and H139R showed decreased risk of these cancers (OR = 0.83, 95%CI = 0.75–0.93) whereas high EPHX1 activity increased risk of the cancers (OR = 1.20, 95%CI = 0.98–1.46). Furthermore, modest difference for the risk of lung and UADT cancers was found between cigarette smokers and nonsmokers both in single SNP analyses (low activity allele H: OR = 0.77/0.85 for smokers/nonsmokers; high activity allele R: OR = 1.20/1.09 for smokers/nonsmokers) and in combined double SNP analyses (putative low activity: OR = 0.73/0.88 for smokers/nonsmokers; putative high activity: OR = 1.02/0.93 for smokers/ nonsmokers).
Conclusions/Significance
Putative low EPHX1 enzyme activity may have a potential protective effect on tobacco-related carcinogenesis of lung and UADT cancers, whereas putative high EPHX1 activity may have a harmful effect. Moreover, cigarette-smoking status may influence the association of EPHX1 enzyme activity and the related cancer risk.
Introduction
Human microsomal epoxide hydrolase (EPHX1 or mEH, EC 3.3.2.9) plays an important role during xenobiotic detoxification of exogenous chemicals such as polycyclic aromatic hydrocarbons (PAHs) which are produced during the use of coal tar, coke, bitumen, or during cigarette smoking [1]–[3]. On the other hand, it is also involved in the xenobiotic activation of some carcinogens [4]–[6]. EPHX1 also hydrolyzes arene, alkene, and aliphatic epoxides, which are metabolic products from PAHs and aromatic amines by cytochrome P450 and other phase I enzymes catalysis [1].
The human EPHX1 gene is 35.48 kb with nine exons and eight introns on chromosome 1q42.1. To date, more than 110 single nucleotide polymorphisms (SNPs) have been identified according to the NCBI's dbSNP database. Two SNPs among them, Tyr113His (rs1051740, in exon 3) and His139Arg (rs2234922, in exon 4), have been well characterized both in vitro studies and epidemiological investigation. Early in vitro studies showed that EPHX1 enzymatic activity was decreased by approximately 40% in subjects with the His113 allele (low EPHX1 activity allele) and increased by at least 25% with the Arg139 allele (high EPHX1 activity allele) [7], [8]. Given the known differential effect of EPHX1 alleles in the detoxification of procarcinogens, it has been proposed that these polymorphisms may affect cancer risk. Later population studies found that these two functional polymorphisms were strongly associated with susceptibility to a number of cancers, such as lung cancer [9]–[12], upper aerodigestive tract (UADT) cancers [13], [14], colorectal cancer or adenoma [15], [16], bladder cancer [17], breast cancer [18]. Based on the genotype combination of these two functional polymorphisms, Benhamou and colleagues [9] classified EPHX1 activity as putative low activity (113HH/139HH, 113HH/139HR and 113YH/139HH), intermediate activity (113HH/139RR, 113YY/139HH and 113YH/139HR) and high activity (113YH/139RR, 113YY/139HR and 113YY/139RR). They also found a significant association with lung cancer risk between cases exhibiting putative high and intermediate EPHX1 activity compared to low activity cases in Caucasian cigarette smokers [9]. A previous meta-analysis of the association of these SNPs with lung cancer revealed that the low-activity genotype (HH) of EPHX1 polymorphism Y113H was associated with decreased risk of lung cancer while the high-activity genotype (RR) of polymorphism H139R was associated with a modest increase risk of lung cancer among Caucasians. Moreover, the predicted low activity by genotype combination of two polymorphisms was associated with a modest decrease of lung cancer risk [19]. However, it has not been well clarified whether EPHX1 enzymatic activity is associated with cancer risk.
The present comprehensive meta-analysis of published epidemiological studies aims to systematically evaluate putative EPHX1 enzyme activity and risk of cancers predicted by single polymorphism of Y113H/H139R and by combined double polymorphisms, and to identify the association between these two functional polymorphisms and risk of some tobacco-related cancers.
Materials and Methods
Search strategy
All case-control studies of EPHX1 polymorphisms and cancer risk published up to November 1, 2010 were identified through comprehensive searches in PubMed, EMBASE, ISI Web of Science and Google Scholar. The search terms used were: EPHX1, microsomal epoxide hydrolase and mEH in combination with polymorphism, variation, genotype, genetic and mutation, and in combination with cancer, tumor, tumour, carcinoma, adenoma and adenocarcinoma. For each identified study, additional studies were sought from its references, citations and from the PubMed option ‘Related Articles’.
Selection
The following criteria were employed to determine inclusion of a study in this meta-analysis: 1) a case–control study evaluating at least one of these two polymorphisms (Y113H and H139R) and cancer risk; 2) no overlapping data. All data were independent of each other. For the same or overlapping data in the studies published by the same researchers, we selected the most recent study with a larger number of participants; 3) full-text articles; 4) published in English language journals.
Data Extraction
The collected data items included: first author, published year, cancer type, study design, original country, sample ethnicity, sample size, genotype counts and genotyping method. The data were independently extracted by two investigators (Li and Zhu) and rechecked by Hu. All item-specific ambiguities were clarified by investigators' consultation. Different case-control groups in one study were considered as independent studies. Cigarette smoking status was strategically classified as current smokers and nonsmokers.
Quantitative data synthesis
To evaluate the association of EPHX1 polymorphisms with carcinogenesis risk, we treated wild-type Y of Y113H and H of H139R as intermediate activity alleles and treated wild-type YY of Y113H and HH of H139R as intermediate activity genotypes. They are the comparison references for calculating odds ratios. Thus comparisons are Low activity vs. Intermediate activity (H vs. Y, YH vs. YY and HH vs. YY) and High activity vs. Intermediate activity (R vs. H, HR vs. HH and RR vs. HH) (Table S1).
EPHX1 enzymatic activity was also predicted by double polymorphisms based on the method of Benhamou 1998 [9] namely low activity (113HH/139HH, 113HH/139HR and 113YH/139HH), intermediate activity (113HH/139RR, 113YY/139HH and 113YH/139HR) and high activity (113YH/139RR, 113YY/139HR and 113YY/139RR) (Table S1).
Random-effects methods [20] were used to calculate pooled odds ratios (ORs) and the associated 95% confidence intervals (CIs).
The Cochran's Q statistic [21] and the inconsistency index I2 [22] were used to evaluate the between-study heterogeneity. Random effect meta-regression models with restricted maximum likelihood estimation were employed to evaluate the different variance among the individual ORs when heterogeneity was detected. The pre-specified possible sources of inter-study heterogeneity were: cancer type, ethnicity of population (Caucasian, East Asian, South Asian, African or Mixed population), study design (hospital-based case-control study, population-based case-control study or nested case-control study), sample size (≥500 or <500) and HWE violation (violated or not violated). Furthermore, the sensitive analysis method proposed by Patsopoulos et al. was implemented to identify studies which may be the main source of the measured heterogeneity [23].
To detect potential publication bias, funnel plots [24] were applied by plotting individual study log OR against the standard error of the log OR. Plots should resemble a symmetrical inverted funnel if ascertainment bias was absent. Publication bias was also assessed using Egger's test [25], by which asymmetry in a funnel plot could be tested.
Except for heterogeneity statistics (where significance was declared if P-value < 0.10), all results were considered “significant” if the corresponding P-value was < 0.05. All P-values were 2-sided. The statistical analyses were performed using STATA 11.0 (STATA Corp, College Station, Texas).
Results
Flow of included studies
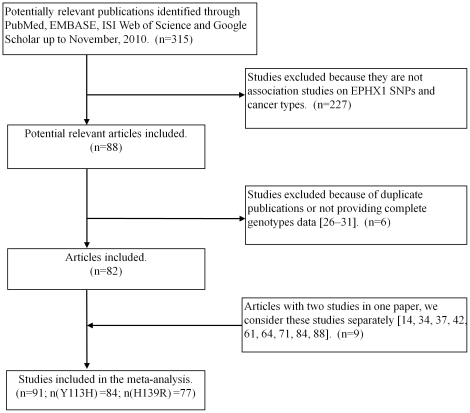
Initially a total of 315 potentially relevant publications up to November 1, 2010 were identified through PubMed, EMBASE, ISI Web of Science and Google Scholar. 227 studies were excluded because of insufficient information related to pre-specified inclusion criteria. Further six studies were excluded because of a duplicated publication or for not providing complete genotypes data [26]–[31]. The reasons for exclusion of each case-control study were detailed in Text S1. Finally, 82 articles [9]–[18], [32]– were selected in the meta-analysis, of which 9 articles [14], [34], [37], [42], [61], [64], [71], [84], [88] had two independent studies and were considered separately. Therefore, a total of 91 studies, of which 84 studies (31144 cases and 42439 controls) for Tyr113His and 77 studies (28496 cases and 38506 controls) for His139Arg dominated by lung cancer, UADT, colorectal cancer, colorectal adenoma, bladder cancer, breast cancer, liver cancer and blood cancers (leukemia, lymphoma and multiple myeloma) were included in the meta-analysis based on our search strategy and eligibility criteria (Table S2 and Figure 1).
Figure 1. Flow chart of study selection.
Study characteristics
Detailed characteristics of the aggregated data for 91 case-control studies are listed in Table S2. Minor allele frequency of Y113H and H139R of controls in different populations graphed as Figure S1. Among overall studies, 24 studies (6418 cases and 9516 controls) that further evaluated the putative EPHX1 enzyme activity and cancer risk by the method described by Benhamou et al. [9] are characterized in Table S3.
Quantitative data synthesis
EPHX1 polymorphisms Y113H and H139R and cancer risk
The associations of each of EPHX1 Y113H and H139R polymorphisms with cancer risk were analyzed. Summary ORs for cancer risk of EPHX1 Y113H and H139R polymorphisms in different cancer types were shown in Table 1. The overall OR by the random-effects model showed no significant association between Y113H or H139R and cancer risk except between the heterozygote versus wild-type Y113H allele (YH vs. YY), which exhibited a slightly decreased cancer risk (OR = 0.94, 95%CI = 0.90–0.99; P = 0.016). Results of analyzing these two polymorphisms in different cancer types revealed that the low activity allele (H) of Y113H was highly associated with decreased risk of lung cancer (OR = 0.88, 95%CI = 0.80–0.96; P = 0.005) and UADT (OR = 0.86, 95%CI = 0.77–0.97; P = 0.014); the high activity allele (R) of H139R was significantly associated with increased risk of lung cancer (OR = 1.18, 95%CI = 1.04–1.33; P = 0.010) but not of UADT (OR = 1.05, 95%CI = 0.93–1.17, P = 0.447). However, the homozygous variant (RR) of H139R showed increased risk of UADT cancers (OR = 1.34, 95%CI = 0.98–1.82, P = 0.065). Towards other assessed cancers, i.e., colorectal cancer, colorectal adenoma, breast cancer, bladder cancer, blood cancers (leukemia, lymphoma and multiple myeloma) or liver cancer, the study revealed only modest decreased or increased effects on cancer risk: Y113H for blood cancers (YH vs. YY: OR = 0.78, 95%CI = 0.60–1.00) and H139R for colorectal cancer (HR vs. HH: OR = 0.91, 95%CI = 0.84–1.00). No statistically significant association was observed for each polymorphism with other cancer cases. Interestingly, though not significant, the low activity of Y113H showed increased risk of bladder cancer (H vs. Y: OR = 1.17, 95%CI = 0.92–1.49; HH vs. YY: OR = 1.27, 95%CI = 0.84–1.92) whereas high activity of H139R showed decreased risk of bladder cancer (R vs. H: OR = 0.89, 95%CI = 0.76–1.05; RR vs. HH: OR = 0.73, 95%CI = 0.84–1.92) (Table 1).
Table 1. Summary ORs for association of EPHX1 polymorphisms Y113H and H139R with different cancers.
| Y113H | H139R | |||||||||
| Cancer group | Low vs. | Random effects | P-value | P-value for | I2 | High vs. | Random effects | P-value | P-value for | I2 |
| (Studies and cases/controls) | Intermediate | OR (95% CI) | heterogeneity | Intermediate | OR (95% CI) | heterogeneity | ||||
| Overall | (N = 84; 31144/42439) | (N = 77; 28496/38506) | ||||||||
| H vs. Y | 0.99 (0.95–1.03) | 0.574 | <0.001 | 61.4% | R vs. H | 1.02 (0.98–1.06) | 0.470 | 0.002 | 35.5% | |
| YH vs. YY | 0.94 (0.90–0.99) | 0.016 | <0.001 | 43.2% | HR vs. HH | 1.00 (0.95–1.04) | 0.868 | 0.011 | 29.1% | |
| HH vs. YY | 1.02 (0.94–1.12) | 0.592 | <0.001 | 59.2% | RR vs. HH | 1.08 (0.98–1.20) | 0.141 | 0.043 | 23.0% | |
| Lung cancer | (N = 18; 4819/9049) | (N = 18; 6742/9151) | ||||||||
| H vs. Y | 0.88 (0.80–0.96) | 0.005 | 0.033 | 41.7% | R vs. H | 1.18 (1.04–1.33) | 0.010 | < 0.001 | 68.3% | |
| YH vs. YY | 0.86 (0.78–0.95) | 0.003 | 0.272 | 15.2% | HR vs. HH | 1.19 (1.04–1.36) | 0.012 | 0.001 | 59.4% | |
| HH vs. YY | 0.81 (0.65–1.00) | 0.048 | 0.037 | 41.9% | RR vs. HH | 1.22 (0.92–1.63) | 0.162 | 0.018 | 45.7% | |
| UADT cancers | (N = 15; 3285/5324) | (N = 14; 2963/4867) | ||||||||
| H vs. Y | 0.86 (0.77–0.97) | 0.014 | 0.002 | 58.8% | R vs. H | 1.05 (0.93–1.17) | 0.447 | 0.110 | 33.1% | |
| YH vs. YY | 0.77 (0.66–0.90) | 0.001 | 0.027 | 45.9% | HR vs. HH | 1.00 (0.87–1.17) | 0.874 | 0.138 | 29.9% | |
| HH vs. YY | 0.82 (0.66–1.03) | 0.084 | 0.010 | 51.8% | RR vs. HH | 1.34 (0.98–1.82) | 0.065 | 0.252 | 18.5% | |
| Colorectal cancer | (N = 11; 5283/6903) | (N = 10; 4456/5669) | ||||||||
| H vs. Y | 1.04 (0.96–1.13) | 0.310 | 0.089 | 40.3% | R vs. H | 0.95 (0.88–1.02) | 0.144 | 0.857 | 0.0% | |
| YH vs. YY | 1.05 (0.97–1.14) | 0.199 | 0.820 | 0.0% | HR vs. HH | 0.91 (0.84–1.00) | 0.041 | 0.806 | 0.0% | |
| HH vs. YY | 1.11 (0.89–1.39) | 0.351 | 0.005 | 61.8% | RR vs. HH | 1.01 (0.82–1.26) | 0.897 | 0.545 | 0.0% | |
| Colorectal adenoma | (N = 8; 4012/4057) | (N = 9; 4857/4929) | ||||||||
| H vs. Y | 0.95 (0.88–1.03) | 0.224 | 0.259 | 21.4% | R vs. H | 1.05 (0.98–1.13) | 0.193 | 0.490 | 0.0% | |
| YH vs. YY | 0.94 (0.86–1.04) | 0.239 | 0.510 | 0.0% | HR vs. HH | 1.04 (0.95–1.13) | 0.418 | 0.696 | 0.0% | |
| HH vs. YY | 0.92 (0.78–1.09) | 0.319 | 0.212 | 27.1% | RR vs. HH | 1.13 (0.92–1.38) | 0.233 | 0.460 | 0.0% | |
| Breast cancer | (N = 6; 6090/7797) | (N = 4; 4543/6899) | ||||||||
| H vs. Y | 0.98 (0.90–1.07) | 0.696 | 0.123 | 42.3% | R vs. H | 0.96 (0.89–1.02) | 0.200 | 0.835 | 0.0% | |
| YH vs. YY | 0.97 (0.90–1.04) | 0.411 | 0.618 | 0.0% | HR vs. HH | 0.95 (0.87–1.03) | 0.202 | 0.749 | 0.0% | |
| HH vs. YY | 1.06 (0.81–1.39) | 0.680 | 0.002 | 72.8% | RR vs. HH | 0.94 (0.78–1.15) | 0.557 | 0.634 | 0.0% | |
| Bladder cancer | (N = 5; 1810/1869) | (N = 4; 1614/1656) | ||||||||
| H vs. Y | 1.17 (0.92–1.49) | 0.192 | 0.002 | 76.6% | R vs. H | 0.89 (0.76–1.05) | 0.168 | 0.262 | 25.0% | |
| YH vs. YY | 1.25 (0.90–1.75) | 0.183 | 0.006 | 72.1% | HR vs. HH | 0.91 (0.78–1.06) | 0.240 | 0.610 | 0.0% | |
| HH vs. YY | 1.27 (0.84–1.92) | 0.266 | 0.015 | 67.4% | RR vs. HH | 0.73 (0.40–1.32) | 0.300 | 0.155 | 42.8% | |
| Blood cancers | (N = 7; 1844/2028) | (N = 10; 2217/3067) | ||||||||
| H vs. Y | 0.98 (0.86–1.11) | 0.743 | 0.255 | 22.8% | R vs. H | 0.95 (0.86–1.05) | 0.318 | 0.426 | 1.3% | |
| YH vs. YY | 0.78 (0.60–1.00) | 0.046 | 0.059 | 50.6% | HR vs. HH | 0.96 (0.82–1.12) | 0.607 | 0.241 | 22.0% | |
| HH vs. YY | 1.08 (0.85–1.37) | 0.513 | 0.329 | 13.2% | RR vs. HH | 0.96 (0.62–1.48) | 0.838 | 0.136 | 35.2% | |
| Liver cancer | (N = 4; 368/859) | (N = 3; 212/556) | ||||||||
| H vs. Y | 1.05 (0.74–1.48) | 0.790 | 0.036 | 65.0% | R vs. H | 1.11 (0.80–1.54) | 0.537 | 0.222 | 33.6% | |
| YH vs. YY | 0.90 (0.59–1.39) | 0.639 | 0.087 | 54.4% | HR vs. HH | 1.18 (0.83–1.68) | 0.366 | 0.738 | 0.0% | |
| HH vs. YY | 1.16 (0.57–2.40) | 0.681 | 0.049 | 61.8% | RR vs. HH | 1.05 (0.34–3.29) | 0.927 | 0.116 | 53.6% | |
UADT, upper aerodigestive tract; N, number of studies.
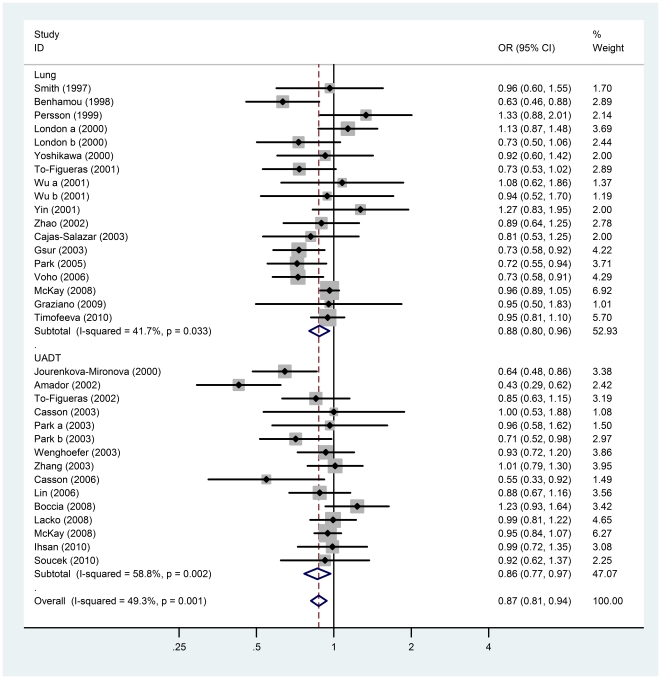
As lung and UADT cancers share a similar etiology and association with tobacco usage [104], we pooled lung and UADT cancers together to further explore the cancer risk of polymorphisms Y113H and H139R. We found that the low activity allele (H) of Y113H presented a significant association with decreased cancer risk (OR = 0.87, 95%CI = 0.81–0.94; P = 0.0002) (Table 2 and Figure 2), whereas the high activity allele (R) of H139R presented a modest association with increased cancer risk (OR = 1.12, 95%CI = 1.03–1.22; P = 0.011) (Table 2).
Table 2. Summary ORs for association of EPHX1 polymorphisms Y113H and H139R with pooled lung and upper aerodigestive tract (UADT) cancers.
| Y113H | H139R | |||||||||
| Study group | N | Random effects OR (95% CI) | P-value | P-value for | I2 | N | Random effects OR (95% CI) | P-value | P-value for | I2 |
| Low vs. Intermediate (H vs. Y) | heterogeneity | High vs. Intermediate (R vs. H) | heterogeneity | |||||||
| Overall | ||||||||||
| Lung + UADT | 33 | 0.87 (0.81–0.94) | 0.0002 | 0.001 | 49.3% | 32 | 1.12 (1.03–1.22) | 0.011 | <0.001 | 58.0% |
| Ethnicity | ||||||||||
| Caucasian | 21 | 0.87 (0.81–0.94) | 0.0005 | 0.015 | 44.5% | 24 | 1.07 (0.97–1.15) | 0.228 | 0.001 | 58.7% |
| Asian | 6 | 1.02 (0.89–1.16) | 0.806 | 0.553 | 0.0% | 3 | 1.52 (1.13–2.05) | 0.006 | 0.438 | 0.0% |
| African | 3 | 0.83 (0.63–1.09) | 0.175 | 0.619 | 0.0% | 3 | 1.26 (1.01–1.57) | 0.040 | 0.741 | 0.0% |
| Study design | ||||||||||
| Population-based | 21 | 0.88 (0.80–0.96) | 0.006 | 0.026 | 41.3% | 18 | 1.15 (1.01–1.31) | 0.033 | <0.001 | 64.2% |
| Hospital-based | 9 | 0.82 (0.68–0.98) | 0.031 | 0.004 | 64.3% | 10 | 1.09 (0.96–1.25) | 0.187 | 0.310 | 14.5% |
| Sample size | ||||||||||
| ≥500 | 10 | 0.91 (0.84–0.98) | 0.022 | 0.061 | 44.7% | 10 | 1.02 (0.92–1.13) | 0.695 | 0.001 | 67.6% |
| <500 | 23 | 0.85 (0.76–0.95) | 0.004 | 0.005 | 48.5% | 22 | 1.21 (1.07–1.37) | 0.002 | 0.038 | 38.0% |
| Smoke status * | ||||||||||
| Nonsmokers | 7 | 0.85 (0.69–1.06) | 0.152 | 0.942 | 0.0% | 4 | 1.09 (0.70–1.68) | 0.709 | 0.130 | 46.9% |
| Smokers | 7 | 0.77 (0.65–0.91) | 0.002 | 0.664 | 0.0% | 7 | 1.20 (0.93–1.55) | 0.152 | 0.119 | 40.8% |
N, number of studies; * YH+HH vs. YY for Y113H; HR+RR vs. HH for H139R.
Figure 2. Forest plots describing the association of EPHX1 polymorphism Y113H with lung and upper aerodigestive tract (UADT) cancers.
ORs were calculated by comparing the low activity allele H vs. the intermediate activity allele Y in lung and UADT cancers. P-values of the ORs are calculated with the DerSimonian-Laird method using a random effects model and measurements of heterogeneity are based on Cochran's Q-test and the inconsistency index I 2.
The ethnicity, study design, sample size and smoke status of pooled lung and UADT cancer risk carrying the low enzymatic activity allele (H) of Y113H or the high enzymatic activity allele (R) of H139R was calculated and a modest difference between cigarette smokers and nonsmokers was observed (Table 2). The odds ratio for the low activity allele (H) of polymorphism Y113H in smokers was 0.77 (95%CI = 0.65–0.91, P = 0.002) and 0.85 (95%CI = 0.69–1.06, P = 0.152) in nonsmokers. The odds ratio for high activity allele (R) of polymorphism H139R was 1.20 (95%CI = 0.93–1.55, P = 0.152) in smokers and 1.09 (95%CI = 0.70–1.68, P = 0.709) in nonsmokers.
Putative EPHX1 enzyme activity and risk of lung and UADT cancers
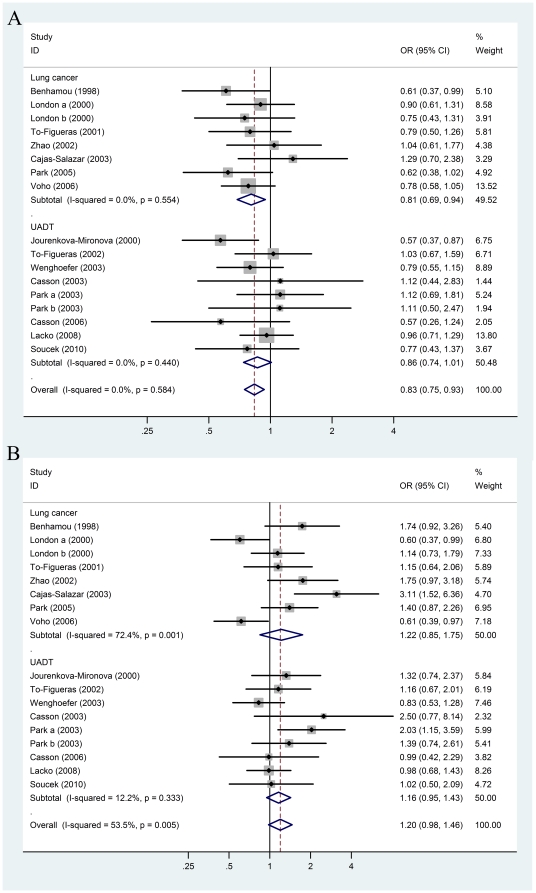
In order to evaluate the association of these two functional polymorphisms and their enzyme activity with carcinogenesis risk, we analyzed the association of EPHX1 enzyme activity predicted by genotype combination of polymorphism Y113H and H139R with risk of lung and UADT cancers. In an overall comparison to the putative intermediate EPHX1 activity, low EPHX1 activity decreased risk of lung and UADT cancers significantly (OR = 0.83; 95%CI = 0.75–0.93, P = 0.001) and high EPHX1 activity increased the cancer risk (OR = 1.20; 95%CI = 0.98–1.46; P = 0.081) (Table 3 and Figure 3).
Table 3. Summary ORs for association of putative EPHX1 enzyme activity by Y113H/H139R genotype combination with lung and upper aerodigestive tract (UADT) cancers.
| Low vs. Intermediate | High vs. Intermediate | ||||||||
| Study group | N (cases/controls) | Random effects | P value | P-value for | I2 | Random effects | P value | P-value for | I2 |
| OR (95%CI) | heterogeneity | OR (95%CI) | heterogeneity | ||||||
| Overall | |||||||||
| Lung + UADT | 17 (2928/5436) | 0.83 (0.75–0.93) | 0.001 | 0.584 | 0.0% | 1.20 (0.98–1.46) | 0.081 | 0.005 | 53.5% |
| Ethnicity | |||||||||
| Caucasian | 15 (2692/5072) | 0.83 (0.74–0.94) | 0.002 | 0.483 | 0.0% | 1.20 (0.95–1.51) | 0.125 | 0.002 | 58.9% |
| African | 2 (236/364) | 0.85 (0.54–1.35) | 0.497 | 0.427 | 0.0% | 1.22 (0.86–1.76) | 0.287 | 0.625 | 0.0% |
| Study design | |||||||||
| Hospital-based | 6 (959/1063) | 0.75 (0.59–0.95) | 0.016 | 0.273 | 21.3% | 1.30 (0.96–1.76) | 0.087 | 0.177 | 34.6% |
| Population-based | 11 (1969/4373) | 0.88 (0.77–1.00) | 0.050 | 0.799 | 0.0% | 1.15 (0.88–1.50) | 0.303 | 0.004 | 60.9% |
| Sample size | |||||||||
| ≥500 | 5 (1296/3606) | 0.83 (0.71–0.97) | 0.020 | 0.633 | 0.0% | 0.85 (0.63–1.13) | 0.263 | 0.074 | 53.1% |
| <500 | 12(1632/1830) | 0.84 (0.70–0.99) | 0.036 | 0.393 | 5.3% | 1.44 (1.20–1.73) | 0.0001 | 0.411 | 3.5% |
| Smoke status | |||||||||
| Nonsmokers | 2 (191/1719) | 0.88 (0.61–1.28) | 0.517 | 0.535 | 0.0% | 0.93 (0.58–1.51) | 0.777 | 0.866 | 0.0% |
| Smokers | 4 (620/1132) | 0.73 (0.58–0.93) | 0.009 | 0.825 | 0.0% | 1.02 (0.68–1.53) | 0.928 | 0.136 | 46.0% |
Figure 3. Forest plots describing the association of putative EPHX1 enzyme activities with lung and upper aerodigestive tract (UADT) cancers.
ORs were calculated as (A) putative low activity vs. putative intermediate activity, and (B) putative high activity vs. putative intermediate activity predicted by genotype combination of polymorphisms Y113H/H139R in lung and UADT cancers. P-values of the ORs are calculated with the DerSimonian-Laird method using a random effects model and measurements of heterogeneity are based on Cochran's Q-test and the inconsistency index I 2.
The association of EPHX1 enzyme activity predicted by the combination of these two polymorphisms with the risk of cigarette smoker or nonsmoker in pooled lung and UADT cancers was further assessed. Similar results with the single SNP analysis were obtained: modest, non-significant differences of ORs of putative EPHX1 enzyme activity between cigarette smokers and nonsmokers. The odds ratio of putative low enzyme activity was 0.73 (95%CI = 0.58–0.93; P = 0.009) in smokers and 0.88 (95%CI = 0.61–1.28) in nonsmokers while the odds ratio of putative high enzyme activity was 1.02 (95%CI = 0.68–1.53) in smokers and the 0.93 (95%CI = 0.58–1.51) in nonsmokers (Table 3).
Between-study heterogeneity
Obvious Between-study heterogeneity was detected among pooled lung and UADT cancer studies on I2 measures of 49.3% (P = 0.001) and 58.0% (P<0.001) for H vs. Y of Y113H and R vs. H of H139R, respectively. After subgroup analysis by ethnicity, we found heterogeneity was only obvious in Caucasian (H vs. Y of Y113H: I2 = 44.5%, P = 0.015; R vs. H of H139R: I2 = 58.7%, P = 0.001) but not in Asian and African descents (Table 2).
By further univariate meta-regression analysis, we identified that it is population ethnicity (β coefficient = 0.12 (0.03–0.21), P = 0.021) that was a significant source of heterogeneity for R vs. H of H139R but not cancer type, study design, sample size, genotyping method or HWE-violation (Table S4). Actually, it has shown that the high activity allele (R) of H139R significantly increased the cancer risk in Asians (OR = 1.52, 95%CI = 1.13–2.05) and Africans (OR = 1.26, 95%CI = 1.01–1.57) but not in Caucasians (OR = 1.07, 95%CI = 0.97–1.15) in pooled analysis. These results emphasized that the population heterogeneity of polymorphism H139R was associated with cancer risk. None of cancer type, ethnicity, study design, sample size, genotyping method or HWE-violation was found to be the source of heterogeneity for H vs. Y of Y113H (Table S4).
In the analysis of putative EPHX1 enzyme activity and risk of lung and UADT cancers, obvious between-study heterogeneity was identified for high vs. intermediate (I2 = 53.5%, P = 0.005) (Table 3). Sample size was found the main source of heterogeneity (β coefficient = 0.53 (0.19–0.88), P = 0.005). When the studied samples were classified into large size subgroup (≥500, OR = 0.85, 95%CI = 0.63–1.13) and small size one (<500, OR = 1.44, 95%CI = 1.20–1.73), unexpectedly, it brought about the results that heterogeneity was still obvious in large sample subgroup (I2 = 53.1%, P = 0.074) but not in small sample subgroup (I2 = 3.5%, P = 0.411) (Table 3).
Sensitive analysis
Applying sensitive analysis method of Patsopoulos et al [23], we found studies of Jourenkova-Mironova [13], Benhamou [9] and Voho [41] contributed mostly to the heterogeneity in comparison of H vs. Y of Y113H in Caucasians. After excluded these three studies, the index I2 decreased significantly from 44.5% (P = 0.015) to 18.5% (P = 0.232) and odds ratio became 0.92 (95%CI = 0.87–0.98). In comparison of R vs. H of H139R, studies of Zienolddiny [12] and Graziano [44] contributed the most to heterogeneity in Caucasians. After excluded them, the index I2 decreased from 58.7% (P = 0.001) to 20.0% (P = 0.202) and odds ratio became 1.00 (95%CI = 0.94–1.06). These results indicated that the high activity allele (R) of H139R may be not associated with lung and UADT cancer risk in Caucasians when considering omitting heterogeneity-caused studies.
Three studies of Cajas-Salazar [40], Voho [41] and London a [34] were identified to contribute to the heterogeneity for high vs. intermediate of putative EPHX1 enzyme activity. After excluded them, the index I2 decreased from 53.5% (P = 0.005) to 0.0% (P = 0.42) and odds ratio became 1.23 (95% = 1.06–1.42). Interestingly, the both studies of Cajas-Salazar [40] and Voho [41] were belong to the large sample subgroup (≥500). Therefore, though in large sample subgroup putative high activity showed decreased risk of lung and UADT caners, the studies were quite heterogeneous and the authentic role of EPHX1 high activity might increase risk of lung and UADT caners as data showed above.
Publication bias
By Begg's funnel plot and Egger's test, the results revealed a significant publication bias for H vs. Y of Y113H (P = 0.003) in the pooled analysis of lung and UADT cancers but not for R vs. H of H139R (P = 0.141) (Figure S2). Among all studies, four studies of Zienolddiny [12], Ihsan [56], Graziano [44] and Wu a [37] were detected to deviate remarkably from other symmetrically distributed studies in the funnel plot (Figure S2). These four studies were exactly the source of heterogeneity from Patsopoulos et al's sensitive analysis in pooled dataset of lung and UADT cancers. When omitted these four studies, Egger's test P-value turned into 0.124, and I2 decreased from 57.6% (P for heterogeneity <0.001) to 12.6% (P for heterogeneity <0.276).
Discussion
The present meta-analysis provides the most comprehensive and up-to-date evidence on putative EPHX1 enzyme activity predicted from two genetic polymorphisms, Y113H and H139R, and risk of developing cancers. Though a number of early studies showed the two polymorphisms functionally affect the EPHX1 enzymatic activity [7], [8] and are associated with certain cancers [9]–[18], our systematic analyses revealed that both Y113H low enzymatic activity allele (H) and putative low EPHX1 enzyme activity, predicted by the combination of Y113H and H139R, were significantly associated with decreased risk of lung and UADT cancers, while the putative high EPHX1 enzyme activity was associated with increased risk of these cancers. Certainly, the actual EPHX1 enzyme activity should be measured in cancer case-control population to confirm cancer susceptibility of EPHX1 activity. Moreover, it showed modest difference of the risk of lung and UADT cancers between cigarette smokers and nonsmokers both in single SNP analyses and in combined double SNP analyses. Thus, cigarette-smoking status may influence the association of EPHX1 enzyme activity and the related cancer risk.
These findings are consistent with the known roles for EPHX1 enzyme in the detoxification and activation of exogenous carcinogens such as PAHs during tobacco smoking [3]. Lung and UADT cancers have been well characterized as causally related to cigarette smoking [104]. Smoking products such as tobacco-specific nitrosamines (4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, N’-nitrosonornicotine, etc.), polycyclic aromatic hydrocarbons (e.g. benzo[a]pyrene) and aromatic amines (e.g. 4-aminobiphenyl) are strongly toxic to epithelial cells and are potential carcinogens [105]. The EPHX1 enzyme has been proposed to transform epoxide intermediates from PAHs into more reactive carcinogens, such as benzo[a]pyrene-7,8-diol-9,10 epoxide (from benzo[a]pyrene), which is the most mutagenic and carcinogenic metabolite [4]–[6]. Thus, high EPHX1 enzymatic activity could increase the concentrations of carcinogens in the tissue. Hence, the EPHX1 variants, individually or collectively with other metabolic enzymes, may lead to cancer susceptibility.
EPHX1 enzyme activity is affected by single or combination of polymorphisms Y113H and H139R [7], [8]. The present cancer meta-analysis was motivated by the idea that performing both single-SNP analysis and two-SNP analysis may provide insights into the relationship between EPHX1 enzyme activity and cancer risk. In single SNP analysis, we performed per-allele comparisons (H vs. Y; R vs. H) and pairwise comparisons (YH vs. YY, HH vs. YY; HR vs. HH, RR vs. HH) regardless of the inheritance model (dominant, co-dominant, recessive). Our combination analysis of two SNPs using the method of Benhamou et al. [9] assumed that the inheritance model was co-dominant, as the classification of low, intermediate and high activity was based on the counts of the high activity allele (H both for Y113H and H139R) of combination genotypes. We have additionally tested the inheritance model of these two polymorphisms by the Bayesian model-free approach [106], [107]. For lung cancer the results suggested a co-dominant inheritance model for the polymorphisms Y113H (λ = 0.64, 0.22–0.99) and H139R (λ = 0.62, 0.27–0.99). But in UADT this method suggested near dominant for Y113H (λ = 0.77, 0.34–1.00) and near recessive model for H139R (λ = 0.12, 0.08–0.90).
The xenobiotic metabolism of smoking products is carried out by both Phase I (e.g. cytochrome P450 family, EPHX1) and Phase II (e.g. glutathione-S-transferases) enzymes since Phase I enzymes induce the formation of active carcinogens from procarcinogens, whereas Phase II enzymes conjugate these compounds and make them suitable for excretion [108]. It is reasonable to think that the overall carcinogenic effect of tobacco compounds should be measured as the final result of the combined action of the two categories of enzymes. Further study is necessary to confirm the qualitative gene-gene interaction of these xenobiotic metabolism enzymes as well as their interaction with tobacco smoking dose in relation to susceptibility of tobacco-related cancers.
The dispersion extent of effect sizes or between-study heterogeneity in a meta-analysis determines the difficulty in drawing overall conclusions to a great extent [109]. Because the dispersion in observed effects is partly spurious (it includes both real difference in effects and also random error), before trying to interpret the variation in effects we need to determine what part of the observed variation is real. A critical meta-analysis should appropriately quantify the heterogeneity and thoroughly ascertain the caused reasons [110] such as using subgroup analysis, sensitive analysis and meta-regression. In the present study, both meta-regression and subgroup analysis by ethnicity revealed that ethnicity is a source of heterogeneity and have a major influence on the cancer risk of these two EPHX1 polymorphisms. For instance, the Y113H low enzymatic activity allele (H) showed significant association with decreased risk of lung and UADT cancers in Caucasian (OR = 0.87, 95%CI = 0.81–0.94) but not significant in Asian (OR = 1.02, 95%CI = 0.89–1.16) (Table 2). The H139R high enzymatic activity allele (R) showed a more significant association with increased risk of cancer in both Asian (OR = 1.52, 95%CI = 1.13–2.05) and African (OR = 1.26, 95%CI = 1.01–1.57) than that in Caucasian (OR = 1.06, 95%CI = 0.97–1.15) (Table 2). The minor allele frequency of these polymorphisms in controls showed significant differences among different populations (Figure S1), which may have an impact on the statistical association analysis.
Study sample size was found to be the main source of heterogeneity for putative high vs. intermediate activity (Table S4) and the results are inconsistency when the studies were divided into subgroups of large sample size and small sample size (Table 3). Intuitively, larger samples studies should reach more convincing results. However, the large studies still exhibited as quite heterogeneous. Moreover, two large studies [40], [41] contributed mainly the between-study heterogeneity by applying method of Patsopoulos et al's sensitive analysis [23]. After omitted the heterogeneity-caused studies, putative high activity was significantly associated with increased risk of lung and UADT cancers (OR = 1.23, 95%CI = 1.06–1.42). Thus, putative high EPHX1 enzyme activity was supposed to increase risk lung and UADT cancers rather than decrease the risk as results from overall large sample subgroup analysis suggested.
Publication bias is another main limitation of meta-analysis which may arise from selective publication or selective inclusion of literatures. Obvious publication bias was detected from the analysis for R vs. H of H139R in pooled lung and UADT cancers. Studies of Zienolddiny [12], Ihsan [56], Graziano[44] and Wu a [37] were found remarkably deviated from other symmetrically distributed studies in the Begg's funnel plot (Figure S2). These four studies were exactly the source of heterogeneity by using Patsopoulos et al's sensitive analysis, which suggests that omitting heterogeneity-caused studies could reach relatively pertinent conclusions.
Supporting Information
Definition of EPHX1 activity predicted by single polymorphism Y113H/H139R and by combination of double polymorphisms.
(0.05 MB RTF)
Characteristics of published studies included in the meta-analysis.
(0.73 MB RTF)
Characteristics of the studies evaluated putative EPHX1 enzyme activity predicted by genotype combination of Y113H/H139R and cancer risk.
(0.17 MB RTF)
Results of random-effect meta-regression for search of the source of heterogeneity.
(0.10 MB RTF)
Minor allele frequency of polymorphisms Y113H and H139R among ethnicity of African, Caucasian, East Asian and South Asian in controls.
(0.03 MB TIF)
Begg's funnel plot with pseudo 95% confidence limits for publication bias detection. Each point represents a separate study and is plotted by individual study log OR again the standard error of the log OR. A, H vs. Y of Y113H; B, R vs. H of H139R.
(0.06 MB TIF)
Six case-control studies of EPHX1 polymorphisms and cancer risk were excluded for the following reasons.
(0.03 MB DOC)
Footnotes
Competing Interests: Dr. Ring is employed by Yigene Inc., but the company has no financial interest in this study. He has declared that he has had no involvements that might raise the question of bias in the work reported or in the conclusions, implications, or opinions stated. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Funding: This study was funded in part by a grant 2009DFA31940 and 2009ZX10001-017-06 from The Ministry of Science and Technology, China. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Dr. Ring is employed by Yigene Inc. and helped to prepare the manuscript. Yigene Inc. did have a role in the preparation of the manuscript.
References
- 1.Oesch F. Mammalian epoxide hydrases: inducible enzymes catalysing the inactivation of carcinogenic and cytotoxic metabolites derived from aromatic and olefinic compounds. Xenobiotica. 1973;3:305–340. doi: 10.3109/00498257309151525. [DOI] [PubMed] [Google Scholar]
- 2.Morisseau C, Hammock BD. Epoxide hydrolases: mechanisms, inhibitor designs, and biological roles. Annu Rev Pharmacol Toxicol. 2005;45:311–333. doi: 10.1146/annurev.pharmtox.45.120403.095920. [DOI] [PubMed] [Google Scholar]
- 3.Decker M, Arand M, Cronin A. Mammalian epoxide hydrolases in xenobiotic metabolism and signalling. Arch Toxicol. 2009;83:297–318. doi: 10.1007/s00204-009-0416-0. [DOI] [PubMed] [Google Scholar]
- 4.Sims P, Grover PL, Swaisland A, Pal K, Hewer A. Metabolic activation of benzo-(a)pyrene proceeds by a diol-epoxide. Nature. 1974;252:326–328. doi: 10.1038/252326a0. [DOI] [PubMed] [Google Scholar]
- 5.Seidegard J, DePierre JW. Microsomal epoxide hydrolase. Properties, regulation and function. Biochim Biophys Acta. 1983;695:251–270. doi: 10.1016/0304-419x(83)90014-8. [DOI] [PubMed] [Google Scholar]
- 6.Fretland AJ, Omiecinski CJ. Epoxide hydrolases: biochemistry and molecular biology. Chem Biol Interact. 2000;129:41–59. doi: 10.1016/s0009-2797(00)00197-6. [DOI] [PubMed] [Google Scholar]
- 7.Hassett C, Aicher L, Sidhu JS, Omiecinski CJ. Human microsomal epoxide hydrolase: genetic polymorphism and functional expression in vitro of amino acid variants. Hum Mol Genet. 1994;3:421–428. doi: 10.1093/hmg/3.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassett C, Lin J, Carty CL, Laurenzana EM, Omiecinski CJ. Human hepatic microsomal epoxide hydrolase: comparative analysis of polymorphic expression. Arch Biochem Biophys. 1997;337:275–283. doi: 10.1006/abbi.1996.9794. [DOI] [PubMed] [Google Scholar]
- 9.Benhamou S, Reinikainen M, Bouchardy C, Dayer P, Hirvonen A. Association between lung cancer and microsomal epoxide hydrolase genotypes. Cancer Res. 1998;58:5291–5293. [PubMed] [Google Scholar]
- 10.Gsur A, Zidek T, Schnattinger K, Feik E, Haidinger G, et al. Association of microsomal epoxide hydrolase polymorphisms and lung cancer risk. Br J Cancer. 2003;89:702–706. doi: 10.1038/sj.bjc.6601142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JY, Chen L, Elahi A, Lazarus P, Tockman MS. Genetic analysis of microsomal epoxide hydrolase gene and its association with lung cancer risk. Eur J Cancer Prev. 2005;14:223–230. doi: 10.1097/00008469-200506000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zienolddiny S, Campa D, Lind H, Ryberg D, Skaug V, et al. A comprehensive analysis of phase I and phase II metabolism gene polymorphisms and risk of non-small cell lung cancer in smokers. Carcinogenesis. 2008;29:1164–1169. doi: 10.1093/carcin/bgn020. [DOI] [PubMed] [Google Scholar]
- 13.Jourenkova-Mironova N, Mitrunen K, Bouchardy C, Dayer P, Benhamou S, et al. High-activity microsomal epoxide hydrolase genotypes and the risk of oral, pharynx, and larynx cancers. Cancer Res. 2000;60:534–536. [PubMed] [Google Scholar]
- 14.Park JY, Schantz SP, Lazarus P. Epoxide hydrolase genotype and orolaryngeal cancer risk: interaction with GSTM1 genotype. Oral Oncol. 2003;39:483–490. doi: 10.1016/s1368-8375(03)00008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sachse C, Smith G, Wilkie MJ, Barrett JH, Waxman R, et al. A pharmacogenetic study to investigate the role of dietary carcinogens in the etiology of colorectal cancer. Carcinogenesis. 2002;23:1839–1849. doi: 10.1093/carcin/23.11.1839. [DOI] [PubMed] [Google Scholar]
- 16.Huang WY, Chatterjee N, Chanock S, Dean M, Yeager M, et al. Microsomal epoxide hydrolase polymorphisms and risk for advanced colorectal adenoma. Cancer Epidemiol Biomarkers Prev. 2005;14:152–157. [PubMed] [Google Scholar]
- 17.Srivastava DS, Mandhani A, Mittal RD. Genetic polymorphisms of cytochrome P450 CYP1A1 (*2A) and microsomal epoxide hydrolase gene, interactions with tobacco-users, and susceptibility to bladder cancer: a study from North India. Arch Toxicol. 2008;82:633–639. doi: 10.1007/s00204-007-0276-4. [DOI] [PubMed] [Google Scholar]
- 18.Spurdle AB, Chang JH, Byrnes GB, Chen X, Dite GS, et al. A systematic approach to analysing gene-gene interactions: polymorphisms at the microsomal epoxide hydrolase EPHX and glutathione S-transferase GSTM1, GSTT1, and GSTP1 loci and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2007;16:769–774. doi: 10.1158/1055-9965.EPI-06-0776. [DOI] [PubMed] [Google Scholar]
- 19.Kiyohara C, Yoshimasu K, Takayama K, Nakanishi Y. EPHX1 polymorphisms and the risk of lung cancer: a HuGE review. Epidemiology. 2006;17:89–99. doi: 10.1097/01.ede.0000187627.70026.23. [DOI] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Petiti DB. Oxford University Press; 1994. Meta-analysis Decision Analysis and Cost-Effectiveness Analysis, Vol.24. [Google Scholar]
- 22.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37:1148–1157. doi: 10.1093/ije/dyn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou W, Thurston SW, Liu G, Xu LL, Miller DP, et al. The interaction between microsomal epoxide hydrolase polymorphisms and cumulative cigarette smoking in different histological subtypes of lung cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:461–466. [PubMed] [Google Scholar]
- 27.de Assis S, Ambrosone CB, Wustrack S, Krishnan S, Freudenheim JL, et al. Microsomal epoxide hydrolase variants are not associated with risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2002;11:1697–1698. [PubMed] [Google Scholar]
- 28.Tsai YY, McGlynn KA, Hu Y, Cassidy AB, Arnold J, et al. Genetic susceptibility and dietary patterns in lung cancer. Lung Cancer. 2003;41:269–281. doi: 10.1016/s0169-5002(03)00238-1. [DOI] [PubMed] [Google Scholar]
- 29.Gemignani F, Landi S, Szeszenia-Dabrowska N, Zaridze D, Lissowska J, et al. Development of lung cancer before the age of 50: the role of xenobiotic metabolizing genes. Carcinogenesis. 2007;28:1287–1293. doi: 10.1093/carcin/bgm021. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberger A, Illig T, Korb K, Klopp N, Zietemann V, et al. Do genetic factors protect for early onset lung cancer ? A case control study before the age of 50 years. BMC Cancer. 2008;8:60. doi: 10.1186/1471-2407-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiran M, Chawla YK, Jain M, Kaur J. Haplotypes of microsomal epoxide hydrolase and x-ray cross-complementing group 1 genes in Indian hepatocellular carcinoma patients. DNA Cell Biol. 2009;28:573–577. doi: 10.1089/dna.2009.0921. [DOI] [PubMed] [Google Scholar]
- 32.Smith CA, Harrison DJ. Association between polymorphism in gene for microsomal epoxide hydrolase and susceptibility to emphysema. Lancet. 1997;350:630–633. doi: 10.1016/S0140-6736(96)08061-0. [DOI] [PubMed] [Google Scholar]
- 33.Persson I, Johansson I, Lou YC, Yue QY, Duan LS, et al. Genetic polymorphism of xenobiotic metabolizing enzymes among Chinese lung cancer patients. Int J Cancer. 1999;81:325–329. doi: 10.1002/(sici)1097-0215(19990505)81:3<325::aid-ijc2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 34.London SJ, Smart J, Daly AK. Lung cancer risk in relation to genetic polymorphisms of microsomal epoxide hydrolase among African-Americans and Caucasians in Los Angeles County. Lung Cancer. 2000;28:147–155. doi: 10.1016/s0169-5002(99)00130-0. [DOI] [PubMed] [Google Scholar]
- 35.Yoshikawa M, Hiyama K, Ishioka S, Maeda H, Maeda A, et al. Microsomal epoxide hydrolase genotypes and chronic obstructive pulmonary disease in Japanese. Int J Mol Med. 2000;5:49–53. doi: 10.3892/ijmm.5.1.49. [DOI] [PubMed] [Google Scholar]
- 36.To-Figueras J, Gene M, Gomez-Catalan J, Pique E, Borrego N, et al. Lung cancer susceptibility in relation to combined polymorphisms of microsomal epoxide hydrolase and glutathione S-transferase P1. Cancer Lett. 2001;173:155–162. doi: 10.1016/s0304-3835(01)00626-7. [DOI] [PubMed] [Google Scholar]
- 37.Wu X, Gwyn K, Amos CI, Makan N, Hong WK, et al. The association of microsomal epoxide hydrolase polymorphisms and lung cancer risk in African-Americans and Mexican-Americans. Carcinogenesis. 2001;22:923–928. doi: 10.1093/carcin/22.6.923. [DOI] [PubMed] [Google Scholar]
- 38.Yin L, Pu Y, Liu TY, Tung YH, Chen KW, et al. Genetic polymorphisms of NAD(P)H quinone oxidoreductase, CYP1A1 and microsomal epoxide hydrolase and lung cancer risk in Nanjing, China. Lung Cancer. 2001;33:133–141. doi: 10.1016/s0169-5002(01)00182-9. [DOI] [PubMed] [Google Scholar]
- 39.Zhao H, Spitz MR, Gwyn KM, Wu X. Microsomal epoxide hydrolase polymorphisms and lung cancer risk in non-Hispanic whites. Mol Carcinog. 2002;33:99–104. doi: 10.1002/mc.10023. [DOI] [PubMed] [Google Scholar]
- 40.Cajas-Salazar N, Au WW, Zwischenberger JB, Sierra-Torres CH, Salama SA, et al. Effect of epoxide hydrolase polymorphisms on chromosome aberrations and risk for lung cancer. Cancer Genet Cytogenet. 2003;145:97–102. doi: 10.1016/s0165-4608(03)00058-x. [DOI] [PubMed] [Google Scholar]
- 41.Voho A, Metsola K, Anttila S, Impivaara O, Jarvisalo J, et al. EPHX1 gene polymorphisms and individual susceptibility to lung cancer. Cancer Lett. 2006;237(1):102–108. doi: 10.1016/j.canlet.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 42.McKay JD, Hashibe M, Hung RJ, Wakefield J, Gaborieau V, et al. Sequence variants of NAT1 and NAT2 and other xenometabolic genes and risk of lung and aerodigestive tract cancers in Central Europe. Cancer Epidemiol Biomarkers Prev. 2008;17:141–147. doi: 10.1158/1055-9965.EPI-07-0553. [DOI] [PubMed] [Google Scholar]
- 43.Rotunno M, Yu K, Lubin JH, Consonni D, Pesatori AC, et al. Phase I metabolic genes and risk of lung cancer: multiple polymorphisms and mRNA expression. PLoS One. 2009;4:e5652. doi: 10.1371/journal.pone.0005652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Graziano C, Comin CE, Crisci C, Novelli L, Politi L, et al. Functional polymorphisms of the microsomal epoxide hydrolase gene: a reappraisal on an early-onset lung cancer patients series. Lung Cancer. 2009;63:187–193. doi: 10.1016/j.lungcan.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 45.Timofeeva M, Kropp S, Sauter W, Beckmann L, Rosenberger A, et al. Genetic polymorphisms of MPO, GSTT1, GSTM1, GSTP1, EPHX1 and NQO1 as risk factors of early-onset lung cancer. Int J Cancer. 2010;127:1547–61. doi: 10.1002/ijc.25175. [DOI] [PubMed] [Google Scholar]
- 46.Amador AG, Righi PD, Radpour S, Everett ET, Weisberger E, et al. Polymorphisms of xenobiotic metabolizing genes in oropharyngeal carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:440–445. doi: 10.1067/moe.2002.122586. [DOI] [PubMed] [Google Scholar]
- 47.To-Figueras J, Gene M, Gomez-Catalan J, Pique E, Borrego N, et al. Microsomal epoxide hydrolase and glutathione S-transferase polymorphisms in relation to laryngeal carcinoma risk. Cancer Lett. 2002;187:95–101. doi: 10.1016/s0304-3835(02)00406-8. [DOI] [PubMed] [Google Scholar]
- 48.Casson AG, Zheng Z, Chiasson D, MacDonald K, Riddell DC, et al. Associations between genetic polymorphisms of Phase I and II metabolizing enzymes, p53 and susceptibility to esophageal adenocarcinoma. Cancer Detect Prev. 2003;27:139–146. doi: 10.1016/s0361-090x(03)00033-3. [DOI] [PubMed] [Google Scholar]
- 49.Wenghoefer M, Pesch B, Harth V, Broede P, Fronhoffs S, et al. Association between head and neck cancer and microsomal epoxide hydrolase genotypes. Arch Toxicol. 2003;77:37–41. doi: 10.1007/s00204-002-0414-y. [DOI] [PubMed] [Google Scholar]
- 50.Zhang JH, Jin X, Li Y, Wang R, Guo W, et al. Epoxide hydrolase Tyr113His polymorphism is not associated with susceptibility to esophageal squamous cell carcinoma in population of North China. World J Gastroenterol. 2003;9:2654–2657. doi: 10.3748/wjg.v9.i12.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Casson AG, Zheng Z, Porter GA, Guernsey DL. Genetic polymorphisms of microsomal epoxide hydroxylase and glutathione S-transferases M1, T1 and P1, interactions with smoking, and risk for esophageal (Barrett) adenocarcinoma. Cancer Detect Prev. 2006;30:423–431. doi: 10.1016/j.cdp.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 52.Lin YC, Wu DC, Lee JM, Hsu HK, Kao EL, et al. The association between microsomal epoxide hydrolase genotypes and esophageal squamous-cell-carcinoma in Taiwan: interaction between areca chewing and smoking. Cancer Lett. 2006;237:281–288. doi: 10.1016/j.canlet.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 53.Boccia S, Cadoni G, Sayed-Tabatabaei FA, Volante M, Arzani D, et al. CYP1A1, CYP2E1, GSTM1, GSTT1, EPHX1 exons 3 and 4, and NAT2 polymorphisms, smoking, consumption of alcohol and fruit and vegetables and risk of head and neck cancer. J Cancer Res Clin Oncol. 2008;134:93–100. doi: 10.1007/s00432-007-0254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lacko M, Roelofs HM, Te Morsche RH, Voogd AC, Oude Ophuis MB, et al. Microsomal epoxide hydrolase genotypes and the risk for head and neck cancer. Head Neck. 2008;30:836–844. doi: 10.1002/hed.20781. [DOI] [PubMed] [Google Scholar]
- 55.Varela-Lema L, Ruano-Ravina A, Juiz Crespo MA, Kelsey KT, Loidi L, et al. CYP1A1, mEH, and GSTM1 Polymophisms and Risk of Oral and Pharyngeal Cancer: A Spanish Case-Control Study. J Oncol. 2008;2008:741310. doi: 10.1155/2008/741310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ihsan R, Chattopadhyay I, Phukan R, Mishra AK, Purkayastha J, et al. Role of epoxide hydrolase 1 gene polymorphisms in esophageal cancer in a high-risk area in India. J Gastroenterol Hepatol. 2010;25:1456–1462. doi: 10.1111/j.1440-1746.2010.06354.x. [DOI] [PubMed] [Google Scholar]
- 57.Soucek P, Susova S, Mohelnikova-Duchonova B, Gromadzinska J, Moraviec-Sztandera A, et al. Neoplasma. 2010;57:415–421. doi: 10.4149/neo_2010_05_415. [DOI] [PubMed] [Google Scholar]
- 58.Harrison DJ, Hubbard AL, MacMillan J, Wyllie AH, Smith CA. Microsomal epoxide hydrolase gene polymorphism and susceptibility to colon cancer. Br J Cancer. 1999;79:168–171. doi: 10.1038/sj.bjc.6690028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Landi S, Gemignani F, Moreno V, Gioia-Patricola L, Chabrier A, et al. A comprehensive analysis of phase I and phase II metabolism gene polymorphisms and risk of colorectal cancer. Pharmacogenet Genomics. 2005;15:535–546. doi: 10.1097/01.fpc.0000165904.48994.3d. [DOI] [PubMed] [Google Scholar]
- 60.Robien K, Curtin K, Ulrich CM, Bigler J, Samowitz W, et al. Microsomal epoxide hydrolase polymorphisms are not associated with colon cancer risk. Cancer Epidemiol Biomarkers Prev. 2005;14:1350–1352. doi: 10.1158/1055-9965.EPI-04-0877. [DOI] [PubMed] [Google Scholar]
- 61.Tranah GJ, Chan AT, Giovannucci E, Ma J, Fuchs C, et al. Epoxide hydrolase and CYP2C9 polymorphisms, cigarette smoking, and risk of colorectal carcinoma in the Nurses' Health Study and the Physicians' Health Study. Mol Carcinog. 2005;44:21–30. doi: 10.1002/mc.20112. [DOI] [PubMed] [Google Scholar]
- 62.van der Logt EM, Bergevoet SM, Roelofs HM, Te Morsche RH, Dijk Y, et al. Role of epoxide hydrolase, NAD(P)H:quinone oxidoreductase, cytochrome P450 2E1 or alcohol dehydrogenase genotypes in susceptibility to colorectal cancer. Mutat Res. 2006;593:39–49. doi: 10.1016/j.mrfmmm.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 63.Kiss I, Orsos Z, Gombos K, Bogner B, Csejtei A, et al. Association between allelic polymorphisms of metabolizing enzymes (CYP 1A1, CYP 1A2, CYP 2E1, mEH) and occurrence of colorectal cancer in Hungary. Anticancer Res. 2007;27:2931–2937. [PubMed] [Google Scholar]
- 64.Skjelbred CF, Saebo M, Hjartaker A, Grotmol T, Hansteen IL, et al. Meat, vegetables and genetic polymorphisms and the risk of colorectal carcinomas and adenomas. BMC Cancer. 2007;7:228. doi: 10.1186/1471-2407-7-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cotterchio M, Boucher BA, Manno M, Gallinger S, Okey AB, et al. Red meat intake, doneness, polymorphisms in genes that encode carcinogen-metabolizing enzymes, and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2008;17:3098–3107. doi: 10.1158/1055-9965.EPI-08-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cleary SP, Cotterchio M, Shi E, Gallinger S, Harper P. Cigarette smoking, genetic variants in carcinogen-metabolizing enzymes, and colorectal cancer risk. Am J Epidemiol. 2010;172:1000–1014. doi: 10.1093/aje/kwq245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hlavata I, Vrana D, Smerhovsky Z, Pardini B, Naccarati A, et al. Association between exposure-relevant polymorphisms in CYP1B1, EPHX1, NQO1, GSTM1, GSTP1 and GSTT1 and risk of colorectal cancer in a Czech population. Oncol Rep. 2010;24:1347–1353. doi: 10.3892/or_00000992. [DOI] [PubMed] [Google Scholar]
- 68.Cortessis V, Siegmund K, Chen Q, Zhou N, Diep A, et al. A case-control study of microsomal epoxide hydrolase, smoking, meat consumption, glutathione S-transferase M3, and risk of colorectal adenomas. Cancer Res. 2001;61:2381–2385. [PubMed] [Google Scholar]
- 69.Ulrich CM, Bigler J, Whitton JA, Bostick R, Fosdick L, et al. Epoxide hydrolase Tyr113His polymorphism is associated with elevated risk of colorectal polyps in the presence of smoking and high meat intake. Cancer Epidemiol Biomarkers Prev. 2001;10:875–882. [PubMed] [Google Scholar]
- 70.Tiemersma EW, Bunschoten A, Kok FJ, Glatt H, de Boer SY, et al. Effect of SULT1A1 and NAT2 genetic polymorphism on the association between cigarette smoking and colorectal adenomas. Int J Cancer. 2004;108:97–103. doi: 10.1002/ijc.11533. [DOI] [PubMed] [Google Scholar]
- 71.Tranah GJ, Giovannucci E, Ma J, Fuchs C, Hankinson SE, et al. Epoxide hydrolase polymorphisms, cigarette smoking and risk of colorectal adenoma in the Nurses' Health Study and the Health Professionals Follow-up Study. Carcinogenesis. 2004;25:1211–1218. doi: 10.1093/carcin/bgh126. [DOI] [PubMed] [Google Scholar]
- 72.Mitrou PN, Watson MA, Loktionov AS, Cardwell C, Gunter MJ, et al. Role of NQO1C609T and EPHX1 gene polymorphisms in the association of smoking and alcohol with sporadic distal colorectal adenomas: results from the UKFSS Study. Carcinogenesis. 2007;28:875–882. doi: 10.1093/carcin/bgl194. [DOI] [PubMed] [Google Scholar]
- 73.Northwood EL, Elliott F, Forman D, Barrett JH, Wilkie MJ, et al. Polymorphisms in xenobiotic metabolizing enzymes and diet influence colorectal adenoma risk. Pharmacogenet Genomics. 2010;20:315–326. doi: 10.1097/FPC.0b013e3283395c6a. [DOI] [PubMed] [Google Scholar]
- 74.Sarmanova J, Susova S, Gut I, Mrhalova M, Kodet R, Adamek J, et al. Breast cancer: role of polymorphisms in biotransformation enzymes. Eur J Hum Genet. 2004;12:848–854. doi: 10.1038/sj.ejhg.5201249. [DOI] [PubMed] [Google Scholar]
- 75.Justenhoven C, Hamann U, Schubert F, Zapatka M, Pierl CB, et al. Breast cancer: a candidate gene approach across the estrogen metabolic pathway. Breast Cancer Res Treat. 2008;108:137–149. doi: 10.1007/s10549-007-9586-8. [DOI] [PubMed] [Google Scholar]
- 76.Khedhaier A, Hassen E, Bouaouina N, Gabbouj S, Ahmed SB, et al. Implication of Xenobiotic Metabolizing Enzyme gene (CYP2E1, CYP2C19, CYP2D6, mEH and NAT2) polymorphisms in breast carcinoma. BMC Cancer. 2008;8:109. doi: 10.1186/1471-2407-8-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sangrajrang S, Sato Y, Sakamoto H, Ohnami S, Laird NM, et al. Genetic polymorphisms of estrogen metabolizing enzyme and breast cancer risk in Thai women. Int J Cancer. 2009;125:837–843. doi: 10.1002/ijc.24434. [DOI] [PubMed] [Google Scholar]
- 78.The MARIE-GENICA Consortium. Postmenopausal estrogen monotherapy-associated breast cancer risk is modified by CYP17A1_-34_T>C polymorphism. Breast Cancer Res Treat. 2010;120:737–744. doi: 10.1007/s10549-009-0490-2. [DOI] [PubMed] [Google Scholar]
- 79.Brockmoller J, Kaiser R, Kerb R, Cascorbi I, Jaeger V, et al. Polymorphic enzymes of xenobiotic metabolism as modulators of acquired P53 mutations in bladder cancer. Pharmacogenetics. 1996;6:535–545. doi: 10.1097/00008571-199612000-00007. [DOI] [PubMed] [Google Scholar]
- 80.Broberg K, Bjork J, Paulsson K, Hoglund M, Albin M. Constitutional short telomeres are strong genetic susceptibility markers for bladder cancer. Carcinogenesis. 2005;26:1263–1271. doi: 10.1093/carcin/bgi063. [DOI] [PubMed] [Google Scholar]
- 81.Figueroa JD, Malats N, Garcia-Closas M, Real FX, Silverman D, et al. Bladder cancer risk and genetic variation in AKR1C3 and other metabolizing genes. Carcinogenesis. 2008;29:1955–1962. doi: 10.1093/carcin/bgn163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hsu LI, Chiu AW, Huan SK, Chen CL, Wang YH, et al. SNPs of GSTM1, T1, P1, epoxide hydrolase and DNA repair enzyme XRCC1 and risk of urinary transitional cell carcinoma in southwestern Taiwan. Toxicol Appl Pharmacol. 2008;228:144–155. doi: 10.1016/j.taap.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 83.Sarmanova J, Benesova K, Gut I, Nedelcheva-Kristensen V, Tynkova L, et al. Genetic polymorphisms of biotransformation enzymes in patients with Hodgkin's and non-Hodgkin's lymphomas. Hum Mol Genet. 2001;10:1265–1273. doi: 10.1093/hmg/10.12.1265. [DOI] [PubMed] [Google Scholar]
- 84.Lebailly P, Willett EV, Moorman AV, Roman E, Cartwright R, et al. Genetic polymorphisms in microsomal epoxide hydrolase and susceptibility to adult acute myeloid leukaemia with defined cytogenetic abnormalities. Br J Haematol. 2002;116:587–594. doi: 10.1046/j.0007-1048.2001.03320.x. [DOI] [PubMed] [Google Scholar]
- 85.Clavel J, Bellec S, Rebouissou S, Menegaux F, Feunteun J, et al. Childhood leukaemia, polymorphisms of metabolism enzyme genes, and interactions with maternal tobacco, coffee and alcohol consumption during pregnancy. Eur J Cancer Prev. 2005;14:531–540. doi: 10.1097/00008469-200512000-00007. [DOI] [PubMed] [Google Scholar]
- 86.De Roos AJ, Gold LS, Wang S, Hartge P, Cerhan JR, et al. Metabolic gene variants and risk of non-Hodgkin's lymphoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1647–1653. doi: 10.1158/1055-9965.EPI-06-0193. [DOI] [PubMed] [Google Scholar]
- 87.Lincz LF, Scorgie FE, Robertson R, Enno A. Genetic variations in benzene metabolism and susceptibility to multiple myeloma. Leuk Res. 2007;31:759–763. doi: 10.1016/j.leukres.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 88.Gold LS, De Roos AJ, Brown EE, Lan Q, Milliken K, et al. Associations of common variants in genes involved in metabolism and response to exogenous chemicals with risk of multiple myeloma. Cancer Epidemiol. 2009;33:276–280. doi: 10.1016/j.canep.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Silveira Vda S, Canalle R, Scrideli CA, Queiroz RG, Tone LG. Role of the CYP2D6, EPHX1, MPO, and NQO1 genes in the susceptibility to acute lymphoblastic leukemia in Brazilian children. Environ Mol Mutagen. 2010;51:48–56. doi: 10.1002/em.20510. [DOI] [PubMed] [Google Scholar]
- 90.Chauhan PS, Ihsan R, Yadav DS, Mishra AK, Bhushan B, et al. DNA Cell Biol. 2010 Aug 23. [Epub ahead of print]; 2010. Association of Glutathione S-Transferase, EPHX, and p53 codon 72 Gene Polymorphisms with Adult Acute Myeloid Leukemia. [DOI] [PubMed] [Google Scholar]
- 91.Wong NA, Rae F, Bathgate A, Smith CA, Harrison DJ. Polymorphisms of the gene for microsomal epoxide hydrolase and susceptibility to alcoholic liver disease and hepatocellular carcinoma in a Caucasian population. Toxicol Lett. 2000;115:17–22. doi: 10.1016/s0378-4274(00)00166-1. [DOI] [PubMed] [Google Scholar]
- 92.Tiemersma EW, Omer RE, Bunschoten A, van't Veer P, Kok FJ, et al. Role of genetic polymorphism of glutathione-S-transferase T1 and microsomal epoxide hydrolase in aflatoxin-associated hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev. 2001;10:785–791. [PubMed] [Google Scholar]
- 93.Kirk GD, Turner PC, Gong Y, Lesi OA, Mendy M, et al. Hepatocellular carcinoma and polymorphisms in carcinogen-metabolizing and DNA repair enzymes in a population with aflatoxin exposure and hepatitis B virus endemicity. Cancer Epidemiol Biomarkers Prev. 2005;14:373–379. doi: 10.1158/1055-9965.EPI-04-0161. [DOI] [PubMed] [Google Scholar]
- 94.Kiran M, Chawla YK, Kaur J. Glutathione-S-transferase and microsomal epoxide hydrolase polymorphism and viral-related hepatocellular carcinoma risk in India. DNA Cell Biol. 2008;27:687–694. doi: 10.1089/dna.2008.0805. [DOI] [PubMed] [Google Scholar]
- 95.De Roos AJ, Rothman N, Brown M, Bell DA, Pittman GS, et al. Variation in genes relevant to aromatic hydrocarbon metabolism and the risk of adult brain tumors. Neuro Oncol. 2006;8:145–155. doi: 10.1215/15228517-2005-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sierra-Torres CH, Au WW, Arrastia CD, Cajas-Salazar N, Robazetti SC, et al. Polymorphisms for chemical metabolizing genes and risk for cervical neoplasia. Environ Mol Mutagen. 2003;41:69–76. doi: 10.1002/em.10132. [DOI] [PubMed] [Google Scholar]
- 97.Nishino K, Sekine M, Kodama S, Sudo N, Aoki Y, et al. Cigarette smoking and glutathione S-transferase M1 polymorphism associated with risk for uterine cervical cancer. J Obstet Gynaecol Res. 2008;34:994–1001. doi: 10.1111/j.1447-0756.2008.00798.x. [DOI] [PubMed] [Google Scholar]
- 98.Agudo A, Sala N, Pera G, Capella G, Berenguer A, et al. Polymorphisms in metabolic genes related to tobacco smoke and the risk of gastric cancer in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2006;15:2427–2434. doi: 10.1158/1055-9965.EPI-06-0072. [DOI] [PubMed] [Google Scholar]
- 99.Boccia S, Sayed-Tabatabaei FA, Persiani R, Gianfagna F, Rausei S, et al. Polymorphisms in metabolic genes, their combination and interaction with tobacco smoke and alcohol consumption and risk of gastric cancer: a case-control study in an Italian population. BMC Cancer. 2007;7:206. doi: 10.1186/1471-2407-7-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Spurdle AB, Purdie DM, Webb PM, Chen X, Green A, et al. The microsomal epoxide hydrolase Tyr113His polymorphism: association with risk of ovarian cancer. Mol Carcinog. 2001;30:71–78. doi: 10.1002/1098-2744(200101)30:1<71::aid-mc1015>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 101.Baxter SW, Choong DY, Campbell IG. Microsomal epoxide hydrolase polymorphism and susceptibility to ovarian cancer. Cancer Lett. 2002;177:75–81. doi: 10.1016/s0304-3835(01)00782-0. [DOI] [PubMed] [Google Scholar]
- 102.Ockenga J, Strunck S, Post C, Schulz HU, Halangk J, et al. The role of epoxide hydrolase Y113H gene variant in pancreatic diseases. Pancreas. 2009;38:e97–e101. doi: 10.1097/MPA.0b013e31819feeed. [DOI] [PubMed] [Google Scholar]
- 103.Mittal RD, Srivastava DL. Cytochrome P4501A1 and microsomal epoxide hydrolase gene polymorphisms: gene-environment interaction and risk of prostate cancer. DNA Cell Biol. 2007;26:791–798. doi: 10.1089/dna.2007.0630. [DOI] [PubMed] [Google Scholar]
- 104.IARC. Lyon: International Agency for Research on Cancer; 2004. Tobacco smoking and involuntary smoking. [Google Scholar]
- 105.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3:733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 106.Minelli C, Thompson JR, Abrams KR, Thakkinstian A, Attia J. The choice of a genetic model in the meta-analysis of molecular association studies. Int J Epidemiol. 2005;34:1319–1328. doi: 10.1093/ije/dyi169. [DOI] [PubMed] [Google Scholar]
- 107.Minelli C, Thompson JR, Abrams KR, Lambert PC. Bayesian implementation of a genetic model-free approach to the meta-analysis of genetic association studies. Stat Med. 2005;24:3845–3861. doi: 10.1002/sim.2393. [DOI] [PubMed] [Google Scholar]
- 108.Taioli E. Gene-environment interaction in tobacco-related cancers. Carcinogenesis. 2008;29:1467–1474. doi: 10.1093/carcin/bgn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1958. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 110.Higgins JP. Commentary: Heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol. 2008;37:1158–1160. doi: 10.1093/ije/dyn204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Definition of EPHX1 activity predicted by single polymorphism Y113H/H139R and by combination of double polymorphisms.
(0.05 MB RTF)
Characteristics of published studies included in the meta-analysis.
(0.73 MB RTF)
Characteristics of the studies evaluated putative EPHX1 enzyme activity predicted by genotype combination of Y113H/H139R and cancer risk.
(0.17 MB RTF)
Results of random-effect meta-regression for search of the source of heterogeneity.
(0.10 MB RTF)
Minor allele frequency of polymorphisms Y113H and H139R among ethnicity of African, Caucasian, East Asian and South Asian in controls.
(0.03 MB TIF)
Begg's funnel plot with pseudo 95% confidence limits for publication bias detection. Each point represents a separate study and is plotted by individual study log OR again the standard error of the log OR. A, H vs. Y of Y113H; B, R vs. H of H139R.
(0.06 MB TIF)
Six case-control studies of EPHX1 polymorphisms and cancer risk were excluded for the following reasons.
(0.03 MB DOC)