Abstract
Background
Leptospirosis is a zoonotic infectious disease that affects both humans and animals. The existing genetic tools for Leptospira spp. have improved our understanding of the biology of this spirochete as well as the interaction of pathogenic leptospires with the mammalian host. However, new tools are necessary to provide novel and useful information to the field.
Methodology and Principal Findings
A series of promoter-probe vectors carrying a reporter gene encoding green fluorescent protein (GFP) were constructed for use in L. biflexa. They were tested by constructing transcriptional fusions between the lipL41, Leptospiral Immunoglobulin-like A (ligA) and Sphingomielynase 2 (sph2) promoters from L. interrogans and the reporter gene. ligA and sph2 promoters were the most active, in comparison to the lipL41 promoter and the non-induced controls. The results obtained are in agreement with LigA expression from the L. interrogans Fiocruz L1-130 strain.
Conclusions
The novel vectors facilitated the in vitro evaluation of L. interrogans promoter activity under defined growth conditions which simulate the mammalian host environment. The fluorescence and rt-PCR data obtained closely reflected transcriptional regulation of the promoters, thus demonstrating the suitability of these vectors for assessing promoter activity in L. biflexa.
Introduction
Leptospira interrogans is the main causative agent of leptospirosis, a zoonotic infectious disease with worldwide distribution. Chronically infected reservoir hosts, such as rats, do not exhibit overt disease but are colonized by leptospires in their renal tubules and shed bacteria in the urine. Humans become infected by exposure to contaminated water, soil, or urine [1]. Severe manifestations of the disease, as observed in Weil's disease, are frequent and associated with significant mortality, up to 15% [1], [2]. In addition, leptospirosis may evolve to severe pulmonary haemorrhage syndrome (SPHS), for which case fatality is >50% [3], [4].
The Leptospira genus is composed of 20 recognized species and includes strains that belong to the saprophyte, intermediate or pathogen groups [5]. Currently, almost 300 serovars are recognized, of which more than 200 are considered to be pathogenic [2], [5], [6], [7]. The available genome sequences for pathogenic [8], [9], [10] and saprophytic [11] Leptospira spp. have been employed to search for new diagnostic reagents and vaccine candidates for leptospirosis.
Prompt diagnosis and early treatment of leptospirosis are essential to avoid severe outcomes [12]. The early phase leptospirosis is often misdiagnosed due to its presentation with nonspecific clinical signs [1], low sensitivity and frequent poor specificity of the results exhibited by the microscopic agglutination test (MAT) and the commercially available assays [7], [13], [14], [15], [16], [17], [18], [19].
The use of vaccines as prevention measures appears to be a cost-effective approach to prevent worldwide diseases. Commercially available whole-cell vaccines confer protection in a limited and incomplete manner, limiting their use among humans. E. g. whole-cell preparations produce only short-term immunity, requiring administration semi-annually; present low cross-protection and adverse reactions due to both residual media components and leptospiral lipopolysaccharide [2], [6], [7], [20], [21]. Efforts have been made for over a decade towards identifying immunoreactive [22], [23], [24], [25], [26], [27], [28] or protective [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41] antigens via recombinant DNA technology. Despite these advances, limitations still remain to be overcome. The rational identification of novel candidate antigens is therefore necessary and the development of genetic tools to Leptospira spp. may be helpful for this purpose.
The mechanisms of Leptospira pathogenesis remain unclear, despite the efforts to identify virulence factors and their role in the pathogen-host interaction. To this aim, new genetic tools have been developed in the last years [11], [42], [43]. Functional characterization of outer membrane and cytoplasmic proteins [44], [45], [46], [47], [48], [49], and more recently the generation and study of knock-out mutants [50], [51], [52], [53], [54], [55], [56] have provided important contribution.
Many efforts have been done to understand the influence of environmental signals on the leptospiral transcriptome and proteome, aiming to identify antigens involved in pathogenesis [44], [46], [47], [48], [57], [58]. Temperature, physiological osmolarity, iron availability and the growth phase, in addition to the multitude of factors existing in the host serum and during pathogen-host cell contact, are known to affect the expression of several leptospiral proteins [57], [58], [59], [60], [61], [62]. pH was also found to be responsible for protein regulation, such as LipL36 and P31LipL45 (Qlp42), in the kidney tubules of hamsters [61], [63]. However, many leptospiral coding sequences (CDSs) still remain to be characterized [64]. The L. interrogans genome carries a considerably large number of genes supposedly involved in response regulation [9]. L. borgpetersenii, which possesses a reduced ability to survive outside the host, contains a lower number of regulatory genes [10]. However, the saprophyte L. biflexa contains a larger number of putative transcription factors than the other sequenced species. This suggests that L. biflexa can be used as a model to study the gene regulation of pathogenic Leptospira spp. [11], [57]. Despite the sequence diversity between both species, these findings suggest that pathogens and saprophytes might share some similar mechanisms to respond to the environment.
The extent to which the manipulation of in vitro conditions can be used to reproduce the full spectrum of mammalian host signals, which trigger differential gene expression in pathogenic Leptospira spp. remains uncertain. In this study, we sought to develop a new genetic tool to help elucidating the biology of Leptospira spp. We found that our promoter-probe methodology is useful for assessing promoter activity under defined conditions. The comparative analysis of the fluorescence produced by a specific L. biflexa reporter strain, PAG (carrying a copy of the ligA promoter fused to the gfp gene), and LigA expression from L. interrogans Fiocruz L1-130 strain, grown in vitro under the same systematic conditions, validated the use of L. biflexa as a model to assess L. interrogans promoter activity. The reporter strain containing a copy of the sphingomyelinase 2 promoter, Psph2 (P2G) was also strongly induced by the conditions tested, above an established cut-off, whereas the non-virulence factor promoter control, from the lipL41 lipoprotein gene, was not. We believe this promoter-probe methodology may support the existing methodologies to the identification of novel virulence factors of pathogenic Leptospira spp.
Methods
Bacterial strains and growth conditions
Bacterial strains and constructs are listed in Table 1. Strains were obtained from the collection of the Faculdade de Medicina Veterinária e Zootecnia, Universidade de São Paulo, São Paulo, Brazil; Laboratoire de Biologie des Spirochetes, Institut Pasteur, Paris, France and the ATCC. The virulence of the low-passage L. interrogans serovar Copenhageni Fiocruz L1-130 strain was maintained by passage through Golden Syrian hamsters. Low-passage refers to strains that were sub-cultured in EMJH liquid medium up to 10 times. All strains were cultured at 30°C in liquid EMJH medium supplemented with 1% rabbit serum [65], [66]. Chemically competent E. coli TOP10F cells were used as host for genetic manipulation of plasmids. E. coli transformants were typically selected on LB agar plates containing spectinomycin (50 µg/ml) or ampicillin (100 µg/ml). Electrocompetent L. biflexa sorovar Patoc strain Patoc 1 was prepared as previously described [55], and transformed with replicating shuttle vectors containing the promoter-probe cassettes (Table 1). EMJH plates were prepared using 1% agar and supplemented with spectinomycin at 50 µg/ml.
Table 1. Primers, plasmids and strains employed in this study.
| Designation | Sequence (5′-3′)/genotype |
| PRIMERS | |
| PlipL41300F | CCGCTCGAGAGATAAGATCCAACCCAAAAGTTG |
| PlipL41300R | GGCGGATCCATGAAAAGTAACACCAATCCTGTTTGA |
| PligA300F | CCGCTCGAGTTGGTTTTATAGAAATCAGCAATGATCC |
| PligA300R | GGCGGATCCATAAACACTCACTCTAATTGTTTTATTTGAA |
| Psph2600F | CCGCTCGAGAAACAAAGAATACATACTATAACGTGAATTC |
| Psph2600R | GGCGGATCCATCGTTCTCTATCTCCATTCTGTATGTTTG |
| Gfp5 | GTCGACGAGCTCGAGGGATCCATGAGTAAAGGAGAAGAA |
| Gfp3 | TCAGATCTATTTGTGATGGTGATGGTGATGGTATAGTTCATCC |
| fD1 | AGAGTTTGATCYTGGYTYAG |
| rP2 | ACGGCTACCTTGTTACGACTT |
| NligA5 | GCGGATCCTCCGTTACCGCAGCGGAACTTACTGAGAT |
| NligA3 | CCCAAGCTTTTACCAGGCTCGATTACTTTT |
| PLASMIDS | |
| pGem-T easy | TA cloning vector; Ampr |
| pGem-T easy/gfp | pGem-T easy carrying a copy of the gfp gene |
| pGem-T easy/PlipL41/gfp | pGem-T easy/gfp carrying a copy of the PlipL41 promoter upstream gfp |
| pGem-T easy/PligA/gfp | pGem-T easy/gfp carrying a copy of the PligA promoter upstream gfp |
| pGem-T easy/Psph2/gfp | pGem-T easy/gfp carrying a copy of the Psph2 promoter upstream gfp |
| pSLe94 | E. coli/L. biflexa shuttle vector; Spcr |
| pSLe94/PlipL41/gfp | E. coli/L. biflexa shuttle vector carrying the cassete PlipL41 promoter-gfp |
| pSLe94/PligA/gfp | E. coli/L. biflexa shuttle vector carrying the cassete PligA promoter-gfp |
| pSLe94/Psph2/gfp | E. coli/L. biflexa shuttle vector carrying the cassete Psph2 promoter-gfp |
| STRAINS | |
| Escherichia coli Top 10 | F− mcrA Δ(mrr-hsdRMS-mcrBC) Φ80lacZΔM15 ΔlacX74 recA1 ara Δ139 Δ(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG galK rpsL (Strr) endA1 nupG |
| L. biflexa sv. Patoc str. Patoc1 | Wild-type saprophytic strain |
| L. interogans sv. Copenhageni str. Fiocruz L1-130 | Wild-type pathogenic strain |
| P41G | Str. Patoc1 carrying the pSLe94/PlipL41/gfp shuttle vector |
| PAG | Str. Patoc1 carrying the pSLe94/PligA/gfp shuttle vector |
| P2G | Str. Patoc1 carrying the pSLe94/Psph2/gfp shuttle vector |
Bacterial cultures of the indicated strains (wt and knock-in mutants) were grown in LB medium overnight at 37°C with vigorous shaking (E. coli) or in EMJH medium at 30°C with moderate agitation (Leptospira spp).
Liquid EMJH pH 6.7 was prepared by adding concentrated HCl until the pH of choice was reached. Spermine (Sigma) was dissolved according to the manufacturer's instructions and supplemented in cultures at 200 µM. Physiologic osmolarity was induced by supplementation with 120 mM NaCl [44]. Before induction, cultures were grown at 30°C in EMJH until the late-exponential phase was reached (culture density of 108 to 109/ml). Growth was monitored by measuring the OD420 using an Ultrospec 2100 pro spectrophotometer (GE Healthcare). Cells were harvested (OD420 0.5) and immediately stored at −20°C.
Bioinformatics analyses
The sequences used for this study were obtained from the complete genome sequence of L. interrogans serovar Copenhageni strain Fiocruz L1-130 by using the SpiroScope (http://www.genoscope.cns.fr/agc/mage) database [67] and Leptospira Genome Project (http://aeg.lbi.ic.unicamp.br) database [9]. The Neural Network Promoter Prediction v.2.2 [68], TRES – Transcription Regulatory Element Search, which performs searches within the TRANSFAC database [69] and the PromScan program, that generates an alignment of known sequences and matrix frequency [70], were used to scan DNA sequences for potential binding sites. Repeats were identified by the EMBOSS programs available at http://bioweb.pasteur.fr/nucleic/intro-en.html#repeat.
Construction of reporter vectors
The gfp gene [43] was amplified by PCR with Gfp5/Gfp3 primers pair, which introduced the SmaI, SacI, XhoI, BamHI, SmaI restriction sites. The resulting fragment was cloned into pGEM-T Easy (Promega) yielding the pGEM-T Easy/gfp construct. The PlipL41, PligA and Psph2 promoters from L. interrogans serovar Copenhageni strain Fiocruz L1-130 were amplified by PCR using primers PlipL41300F/PlipL41300R, PligA300F/PligA300R and Psph2600F/Psph2600R, which introduced the XhoI and BamHI restriction sites, and cloned into pGEM-T Easy/gfp via the same sites. Promoter-probe vectors containing the L. interrogans promoters-gfp cassettes were constructed in the E. coli-L. biflexa pSLe94 shuttle-vector [71]. The cassettes were removed from pGEM-T Easy (Promega) by SmaI digestion and cloned in via PvuII restriction site to give the new vectors, pSLe94/PlipL41/gfp, pSLe94/PligA/gfp and pSLe94/Psph2/gfp (Table 1). Electroporation of leptospires was performed as previously described [55], [71]. After 1 to 2 weeks of incubation, spectinomycin resistant transformants were used to inoculate liquid medium.
Reporter gene assays
Cells for spectrofluorometry measurements were resuspended in 300 µl of deionized water and distributed 100 µl per well in a black 96-well OptiPlate-96F microplate (PerkinElmer). GFP fluorescence was measured using a 650-10 spectrofluorometer (PerkinElmer) at an excitation wavelength of 485 nm and an emission wavelength of 538 nm. The fluorescence intensity from samples was expressed as arbitrary fluorescence units, obtained at a wavelength of maximum emission. The mean specific activity from at least three independent assays is indicated in the results.
Fluorescence microscopy
Qualitative expression of GFP was examined under UV light for detection of fluorescence. The cultures were induced and samples were collected at representative time-points, spun down and the resulting pellets were washed twice with 1× PBS to remove the culture medium. The pellets were then resuspended in 1× PBS, 20% Glycerol. Aliquots of 20 µl of the bacterial suspensions were applied to slides and sealed with a concentrated formaldehyde resin. Images of leptospires on cover slips were acquired using an IX81 inverted microscope (Olympus) equipped with 20× and 40× objectives and the CellR software (version 3.1). The UV filter sets used were DAPI (excitation: 350 nm – range is 330 to 380 nm, Emission: 460 nm) and DIC (transmitted light).
RNA isolation and rt-PCR
Cells were harvested (OD420 0.25) and RNA was isolated from bacteria with TRIzol reagent (Invitrogen), as described by the manufacturer. Then, isolated RNA (2 µg) was treated with DNaseI (Invitrogen), following manufacturer's instructions. cDNA was synthesized using SuperScript III First-strand (Invitrogen) according to the manufacturer's protocol. Samples were quantified and checked for purity using an Ultrospec 2100 pro spectrophotometer (GE healthcare) and agarose gel electrophoresis. The cDNA (representing 100 ng of RNA per reaction) was amplified with Taq DNA polymerase (Invitrogen) using primers pairs fD1/rP2 (control) [72], Gfp5/Gfp3 for reporter strains [43], or NligA5/NligA3, for L. interrogans serovar Copenhageni strain Fiocruz L1-130.
Whole-cell ELISA
Whole-cell ELISA experiments were performed using a modification of previously described methods [45], [73]. Flat-bottom polystyrene high-binding microtitre plates (Corning) were coated overnight at 4°C with 100 ml per well of 108 ml−1 whole L. interrogans serovar Copenhageni strain Fiocruz L1-130, which were previously centrifuged at 4,000×g and resuspended in 0.05 M sodium carbonate buffer (pH 9.6). Plates were blocked overnight at 4°C, and washed three times with 150 µl Leptospira Enrichment EMJH (Difco). Wells were incubated for 1 h at room temperature with 100 µl per well of a 1∶2,000 dilution of rabbit anti-LigA polyclonal serum (Kindly provided by Dr. Albert I. Ko and Dr. Paula Ristow, Gonçalo Moniz Research Centre, Oswaldo Cruz Foundation, Brazil) in Leptospira Enrichment EMJH, and washed three times with 200 µl PBS containing 0.05% Tween 20 (PBS-T). Wells were incubated with 100 µl per well of a 1∶5,000 dilution of horseradish-peroxidase-linked donkey whole-antibody anti-rabbit IgG (Sigma) for 1 h at room temperature, followed by two washes with 200 ml PBS-T, and three washes with PBS. The reactions were developed by adding 50 µl per well of ο-phenylenediamine (OPD) (1 mg/ml) in citrate phosphate buffer (pH 5.0) plus 1 µl/ml H2O2 was added (100 µl per well) for 10 min in the dark, at room temperature. The reaction was stopped by adding a 50 µl volume of 4 M H2SO4, and the absorbance was measured at 492 nm. Each ELISA experiment was repeated three times.
Statistical analysis
Differences between average values were tested for significance by performing an unpaired, two-sided Student's t-test [74]. Differences were considered statistically significant when the resulting p values were ≤0.05.
Results
In vitro induction of virulent Leptospira interrogans
Many efforts have been made to identify and quantify the leptospiral transcriptome and proteome during growth under different conditions. But, so far, little is known about the influence that mammalian host conditions exert over promoter activity. Therefore, we initiated our investigation by studying ligA gene transcription and protein expression profiles using L. interrogans serovar Copenhageni strain Fiocruz L1-130 grown, for up to 24 h, under a combination of conditions that include the mammalian physiological osmolarity (∼300 mosmol–120 mM), temperature (37°C), urine pH (6.7) and the supplementation with spermine, a component belonging to the intracellular environment. We focused our study on the first 24 h of induction, to observe the changes that L. interrogans promoters undergo during the early stages of host invasion.
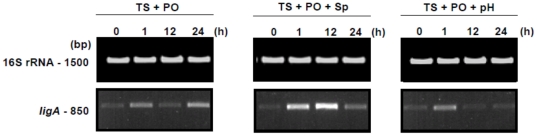
Initially, we evaluated the influence of the different conditions by rt-PCR analysis (Figure 1). It was performed on RNA extracted from both non-induced and induced cultures of the virulent, low-passage, L. interrogans serovar Copenhageni strain Fiocruz L1-130. Low level transcription (ligA-based) was detected before induction of the virulent strain, while a clear difference in the abundance of ligA transcript could be seen after induction, and between the time intervals. Three time-points were evaluated (1, 12 and 24 h post induction – p.i.) and, interestingly, ligA transcription was not constant after induction (Figure 1). Under most conditions, the level of ligA transcript was higher than the non-induced control immediately one hour p.i. However, only spermine was able to up-regulate ligA for an extended period, up to 12 h p.i., while the other treatments exhibited down-regulation (Figure 1). At 24 h p.i., only the temperature upshift and physiological osmolarity conditions stimulated up-regulation in comparison to the previous time-point (Figure 1).
Figure 1. Influence of the in vitro conditions on native LigA expression by L. interrogans serovar Copenhageni str. Fiocruz L1-130.
The effect of the various combinations of conditions (see Results section) on LigA expression was assessed by rt-PCR. Columns depict the systematic treatments: TS – Temperature upshift from 30°C to 37°C, PO – Physiological osmolarity, Sp – Spermine induction, pH – Urine pH induction. Within each gel the upper and lower bands correspond to the internal PCR control (16S rRNA) and ligA cDNA, respectively (domains 10–12). The lanes contain the amplified cDNA per sample time-point, both pre-treatment (0 h) and post induction (1, 12 and 24 h). Agarose gels were stained with GelRed (Invitrogen). No bands were observed in control samples run without template (data not shown). Samples were standardized according to an OD420 0.25. Data from a representative significant study are shown.
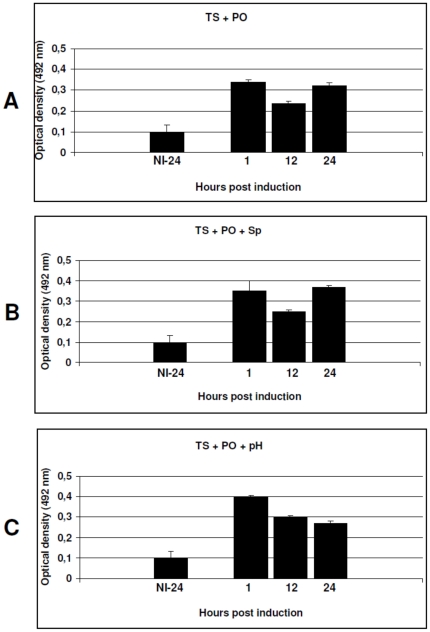
To examine the effect of the conditions over the expression patterns of native LigA, we cultivated L. interrogans serovar Copenhageni strain Fiocruz L1-130 until late-exponential phase before induction. The combination of both temperature upshift and physiological osmolarity promoted induction of protein synthesis (Figure 2A). An up-regulation 4.98-fold higher than the non-induced control was observed at one hour p.i. (p<0.01). The influence of spermine was also evaluated in the kinetics assay and stimulated LigA expression above the control levels immediately after induction, one hour p.i. (5.95-fold, p<0.01), (Figure 2B). The urine pH condition was simulated by reducing the pH from 7.2 to 6.7 and the highest up-regulation was obtained by this treatment, one hour p.i. (5.92-fold, p<0.01), (Figure 2C). But it was the only condition to be followed by decreasing expression levels. The analysis of native LigA expression complemented the results obtained by rt-PCR, although correlation was not always observed between RNA and protein profiles (Figure 1 versus 2).
Figure 2. Native protein expression stimulated by the in vitro conditions mimicking those of the mammalian host.
The expression levels of LigA were evaluated by ELISA, following exposure to the various conditions and combinations: TS – Temperature upshift from 30°C to 37°C, PO – Physiological osmolarity, Sp – Spermine induction, pH – Urine pH induction. (A) Temperature upshift and physiological osmolarity. (B) Temperature upshift, physiological osmolarity and spermine (intracellular level). (C) Temperature upshift, physiological osmolarity and pH reduction from 7.2 to 6.7. A non-induced L. interrogans serovar Copenhageni str. Fiocruz L1-130 (NI-24) was cultivated in vitro for 24 h under standard conditions as a control. Protein expression was measured at one, 12 and 24 h post-induction and the results are expressed as mean optical density ± standard error (bars). The assay was standardized according to an OD420 of 0.25. Data from two independent and significant experiments are shown.
Prediction of regulatory sequences by Bioinformatics
Previous studies demonstrated that upon induction by physiological osmolarity and temperature shift from 20°C to 37°C and 30°C to 37°C the lipL41, ligA and sph2 genes are differentially expressed in L. interrogans serovar Copenhageni strain Fiocruz L1-130, L. kirschneri serovar Grippotyphosa strain RM52 and L. interrogans serovar Lai strain 56601 [44], [46], [47], [48]. The rational to select the PlipL41, PligA and Psph2 promoters to compose and standardize our study was based on previous studies that characterized them extensively at the RNA and protein levels.
The PlipL41, PligA and Psph2 promoters were predicted by bioinformatics based on the analysis of the L. interrogans serovar Copenhageni strain Fiocruz L1-130 genome sequence (AE016823). Different regions were identified, which are composed of direct and inverted repeats (data not shown).
To examine whether the computationally determined sequences comprise leptospiral regulatory elements, we fused the DNA segments to a promoterless gfp reporter. Both elements were subsequently cloned into an E. coli-L. biflexa shuttle vector depicted in Table 1, yielding the plasmids pSLe94/PlipL41/gfp, pSLe94/PligA/gfp and pSLe94/Psph2/gfp (Table 1). To ensure the accurate inclusion of the DNA sequences containing the regulatory elements in the study, we designed primers to amplify 300 bp upstream of the first ATG of lipL41 and ligA, and 600 bp upstream of sph2. The larger length of the amplified fragment upstream of sph2 was adopted to include possible distal elements that may play a role in the activity of the Psph2 promoter, since the intergenic space to the next ORF, LIC12630 (hypothetical protein), is 650 bp. In the case of the lipL41 and ligA genes the intergenic spaces are 114 bp and 456 bp, respectively. The plasmid constructs were used to transform L. biflexa serovar Patoc strain Patoc I and generated the reporter strains P41G (lipL41 promoter), PAG (ligA promoter) and P2G (sph2 promoter), which were induced for production of fluorescence by the green fluorescent protein (GFP).
Application of promoter-probe vectors in L. biflexa
A series of replicating promoter-probe vectors utilizing gfp were constructed to examine changes in promoter activity in response to a set of defined conditions (Table 1). The constructs were based on the low-copy number shuttle-vector pSLe94 (Table 1), which contains the LE1 origin of replication that is capable of replication within saprophytic Leptospira spp. To validate the usefulness of these vectors as genetic tools to investigate promoter regulation, transcriptional fusions were constructed including the promoters from the well-characterized genes lipL41, ligA and sph2 of L. interrogans serovar Copenhageni strain L1-130. GFP reporter strains were established by electroporation of the promoter-specific reporter vectors into L. biflexa. The effectiveness of the reporter strains for assaying transcriptional activity was assessed quantitatively.
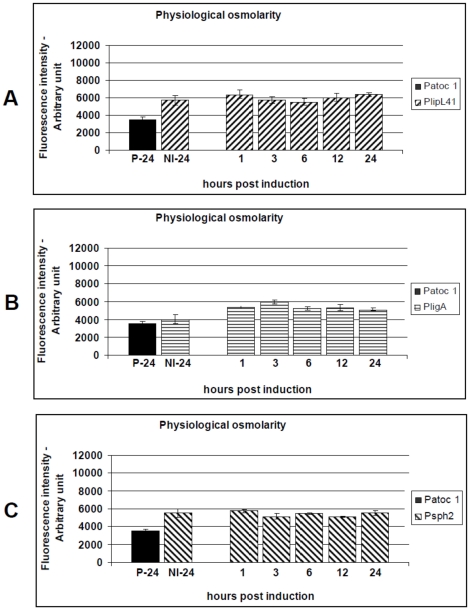
All three reporter strains of L. biflexa were initially cultivated on EMJH agar plates, at 30°C, and then transferred to liquid EMJH, both containing low-salt concentration (70 mosmol per liter NaCl – 28 mM). When cultures reached the late-exponential phase they were aliquoted and either adjusted to 120 mM NaCl or temperature-upshfited from 30°C to 37°C. Quantitative assessment of fluorescence showed that, under physiological osmolarity conditions, the highest promoter activity was detected in the PAG reporter strain, compared to the control, p<0.01 (Figure 3). The PligA promoter was induced in the presence of 120 mM NaCl and the fluorescence reached the highest level 3 hours p.i. The average intensity at this time-point was 1.48-fold higher than the non-induced control (Figure 3B). Neither the PlipL41 nor Psph2 promoters were significantly induced (Figure 3A and 3C). Of note, there were no obvious differences between the growth curves of the wild-type and the reporter strains of L. biflexa, suggesting that the GFP expression did not cause any disadvantage to the host strain (data not shown).
Figure 3. Kinetics of GFP production by the L. biflexa reporter strains (P41G, PAG and P2G) following induction by NaCl to a physiological level.
Cultures of the reporter strains containing a single-copy transcriptional reporter and either the wild-type lipL41 (A), ligA (B) or sph2 (C) promoter regions were induced with ∼300 mosmol NaCl (physiological level). An induced non-transformed L. biflexa serovar Patoc str. Patoc 1 (P-24) was induced for 24 h as a control. The uninduced reporter strains (NI-24) were included as additional controls. Transcriptional activity was presented as the mean ± standard error (bars). Fluorescence levels from triplicate samples of each culture were standardized according to an OD420 0.5 and are expressed as arbitrary fluorescence units. Data from a representative significant study are shown.
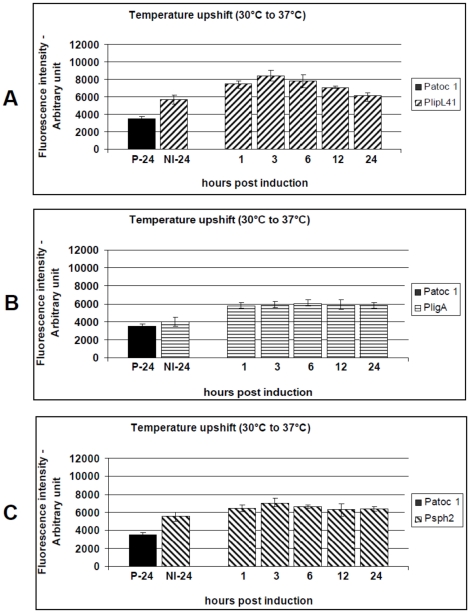
The influence of the temperature upshift, from 30°C to 37°C, on promoter activity was also assessed. The levels of fluorescence produced by the P41G, PAG and P2G reporter strains were significantly higher than the non-induced controls (p<0.01). The PlipL41 and Psph2 promoters reached their maximum activity 3 hours p.i. (Figure 4A and 4C). At this time point there was significant (p<0.01) up-regulation of 1.46-fold and 1.26-fold, respectively. The P41G reporter strain exhibited the highest promoter activity, under this condition, followed by P2G. PligA promoter activity was most influenced, with expression 1.50-fold higher than the non-induced control, p<0.01. Interestingly, PligA and Psph2 activities remained constant during the assay conditions (Figure 4B and 4C). The induction of PlipL41 and Psph2 by the temperature upshift led to production of fluorescence levels significantly higher than those induced under conditions of physiological osmolarity, p<0.05 (Figure 3A versus 4A and 3C versus 4C), while the PligA activity induced by the temperature upshift did not differ from that observed by salt supplementation, at most of the time-points investigated (Figure 3B versus 4B).
Figure 4. Kinetics of GFP production by the L. biflexa reporter strains (P41G, PAG and P2G) after a temperature upshift to 37°C.
Cultures of the reporter strains P41G (A), PAG (B) or P2G (C) were induced by temperature upshift from 30°C to 37°C (physiological temperature). L. biflexa serovar Patoc str. Patoc 1 (P-24) induced for 24 h and uninduced reporter strains grown (NI-24) were included as controls. Transcriptional activity was measured for 24 h and presented as the mean ± standard error (bars). Fluorescence from triplicate samples of each culture were standardized according to an OD420 0.5 and are expressed as arbitrary fluorescence units. Data from a representative significant study are shown.
Effect of the combination of environmental signals on L. interrogans promoter activity
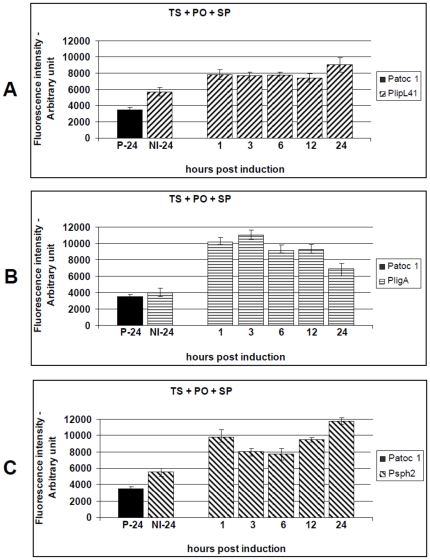
To identify promoters that are differentially induced in response to a combination of conditions, we performed systematic experiments with different, overlapping, stimuli. Subcultures were initially induced under conditions of physiological osmolarity and normal human body core temperature (37°C). The average fluorescence intensity produced by the reporter strains was highly significant when compared to the non-induced controls, p<0.01. For P41G, the highest promoter activity was observed 3 hours p.i. (1.54-fold), and remained constant throughout the assay (Figure 5A). Both PligA and Psph2 were induced to the highest levels 1 hour p.i., 2.59-fold and 1.62, respectively (p<0.01). However, they did not maintain a constant activity during the assay (Figure 5B and 5C). Additionally, PlipL41 was induced to a similar extent by the temperature upshift and the combination of physiological osmolarity and temperature (Figure 4A versus 5A). PligA and Psph2 were significantly induced, 1.74-fold and 1.57-fold, respectively (Figure 3B versus 5B and 3C versus 5C), and 1.73-fold and 1.28-fold, respectively (Figure 4B versus 5B and 4C versus 5C), compared to the single-condition treatments (physiological osmolarity or temperature upshift versus their combination).
Figure 5. Kinetics of GFP production by the L. biflexa reporter strains (P41G, PAG and P2G) after exposure to a combination of physiological salt levels and temperature upshift to 37°C.
Cultures of the reporter strains P41G (A), PAG (B) or P2G (C) were stimulated by a combination of physiological osmolarity (PO) and temperature upshift (TS). The induced wild-type strain (P-24) and the uninduced reporter strains (NI-24) were included as controls. Transcriptional activity was measured for 24 h and presented as the mean ± standard error (bars). Fluorescence from triplicate samples of each culture were standardized according to an OD420 0.5 and are expressed as arbitrary fluorescent units. Data from a representative significant study are shown.
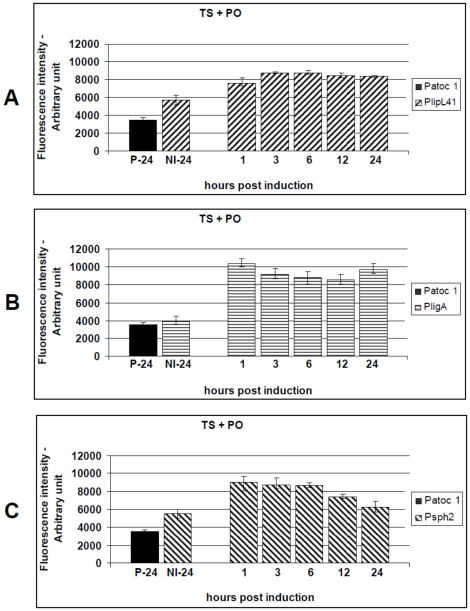
The results indicate these promoters were preferentially active, in vitro, when L. biflexa was cultured under conditions emulating the mammalian host environment. Thus, we decided to evaluate another combination of conditions, the effect of the urine pH plus the physiological osmolarity and the temperature upshift. The reporter strains were sub-cultured into EMJH medium supplemented with 120 mM NaCl, pH 6.7, and shifted from 30°C to 37°C. This resulted in the activation of all promoters as seen by increasing levels of fluorescence. The results from three independent experiments are shown in Figure 6. The PligA promoter was the most affected, reaching an activity 2.08-fold higher than the non-induced control (3 hours p.i.), followed by Psph2 (1.96-fold) and PLipL41 (1.58-fold), both at 24 hours p.i, p<0.01 (Figure 6). Curiously, we observed that both PligA and PlipL41 were slightly less stimulated by the combination that included pH 6.7 than that including salt and temperature only (p<0.05) (Figure 5A versus 6A and 5B versus 6B).
Figure 6. Kinetics of GFP production by the L. biflexa reporter strains (P41G, PAG and P2G), after exposure to a combination of physiological salt levels, temperature upshift to 37°C and urine pH 6.7.
Cultures of the reporter strains P41G (A), PAG (B) or P2G (C) were induced by a combination of physiological osmolarity (PO), temperature upshift (TS) and pH reduction from 7.2 to 6.7 (PH). The induced wild-type control (P-24) and the uninduced reporter strains (NI-24) were included as controls. Transcriptional activity was measured for 24 h and presented as the mean ± standard error (bars). Fluorescence from triplicate samples of each culture were standardized according to an OD420 0.5 and are expressed as arbitrary fluorescent units. Data from a representative significant study are shown.
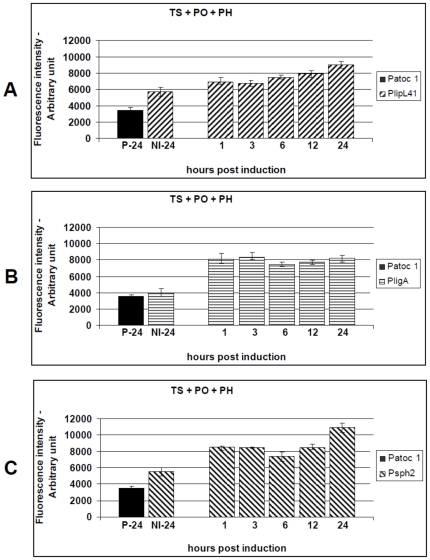
Previous studies have demonstrated that several bacteria undergo translational regulation in response to spermine [75]. This polyamine is only produced by eukaryotic cells, it can reach millimolar levels within them [76], and it has significant effects on various cellular processes in liver, kidney and brain cells and in lymphocytes [76], [77], [78], [79], [80], [81], [82]. Here, we investigated whether spermine contributed to L. interrogans promoter regulation. The L. biflexa reporter strains were grown at 30°C until late-exponential phase and then supplemented with 200 µM spermine, 120 mM NaCl and upshifted to 37°C. The ligA promoter was the most affected, the PAG reporter strain produced a fluorescence level 2.74-fold higher than the non-induced control (3 hours p.i.), followed by P2G (2.12-fold) and P41G (1.58-fold), both 24 hours p.i, p<0.01 (Figure 7). However, ligA promoter activity was not maintained, the average fluorescence decreased to 1.72-fold (24 hours p.i.), demonstrating that PligA is transiently induced by spermine (Figure 7B). The spermine-induced activity of both PligA and Psph2 promoters was the highest observed in this study (Figure 7B and 7C).
Figure 7. Kinetics of GFP production by the L. biflexa reporter strains (P41G, PAG and P2G), after exposure to a combination of physiological salt levels, temperature upshift to 37°C and supplementation with spermine.
Cultures of the reporter strains P41G (A), PAG (B) or P2G (C) were induced by a combination of physiological osmolarity (PO), temperature upshift (TS) and 200 µM spermine (intracellular level) (SP). The induced wild-type control (P-24) and the uninduced reporter strains (NI-24) were included as controls. Transcriptional activity was measured for 24 h and presented as the mean ± standard error (bars). Fluorescence from triplicate samples of each culture were standardized according to an OD420 0.5 and are expressed as arbitrary fluorescent units. Data from a representative significant study are shown.
In agreement with the native LigA expression pattern, the fluorescence produced by the PAG reporter strain reached the highest (4.98-fold versus 2.59-fold) and lowest (2.19-fold versus 2.13-fold) levels at one and 12 hours p.i., respectively, when induced by the combination of physiological osmolarity and temperature upshift (Figure 2A versus 5B and Table 2), while the LigA expression pattern induced by spermine was different from the fluorescence intensity produced by the PAG reporter strain (Figure 2B versus 7B). Finally, induction by pH 6.7 led to LigA expression that was consistent with the fluorescence levels produced by the PAG reporter strain (Figure 2C versus 6B and Table 2). Protein and fluorescence levels were highest one hour p.i., but the amount of native LigA decreased significantly (from 5.95-fold to 4.04-fold), while the fluorescence level produced by the PAG reporter strain remained constant (from 2.07-fold to 2.03-fold). The results obtained in this study, by the use of the promoter-probe methodology described, allowed us to establish a cut-off based on the determined fluorescence levels (Table 2). This information will be useful to support the future prediction of virulence factors as we are currently developing a follow-up study employing several other promoter sequences to construct a library of knock-in mutants.
Table 2. Comparison among top fluorescence intensities and protein expression levels reached by the strains in study.
| Treatments | Reporter strain | ||
| P41G | PAG | P2G | |
| Physiological osmolarity | 1.11 | 1.48 | 1.03 |
| Temperature upshift to 37°C | 1.46 | 1.50 | 1.26 |
| Physiological osmolarity/Temperature upshift | 1.54 | 2.59* | 1.62 |
| Physiological osmolarity/Temperature upshift/pH 6.7 | 1.58 | 2.08* | 1.96† |
| Physiological osmolarity/Temperature upshift/Spermine | 1.58 | 2.74* | 2.12* |
*Corresponds to signal intensity at least 2-fold higher than that observed for the non-induced control.
Corresponds to the border line of 2-fold higher signal intensity, compared to the non-induced control.
NA - Not applicable.
Relative to the parental virulent strain, L. interrogans serovar Copenhageni str. Fiocruz L1-130 strain.
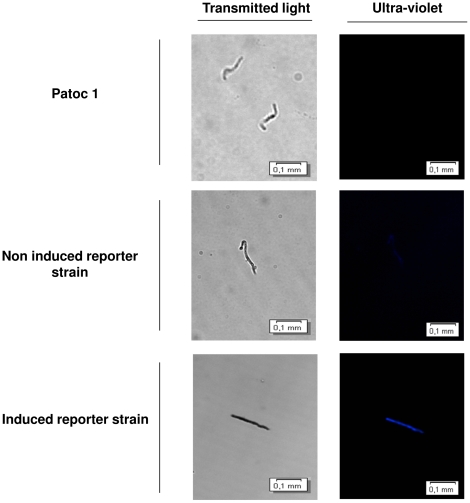
The ability of the promoter-probe system to allow for the differentiation between both non-induced and induced reporter strains suggests that the gfp regulation can be employed to accurately mimic the behaviour of the native genes. This is corroborated by similarities observed between the expression patterns of native LigA and the fluorescence levels produced by the PAG reporter strain, in comparison to the non-induced controls (Table 2). Qualitative assessment of these reporter strains clearly demonstrated the presence of fluorescent leptospires upon induction in vitro (Figure 8).
Figure 8. Inducible expression of GFP in the L. biflexa promoter-probe constructs.
Microscope images of the in vitro induced and uninduced P2G reporter strain transformed with pSLe94-sph2 promoter-gfp vector. Uninduced and physiological osmolarity/temperature upshift/spermine exposed leptospires were fixed and visualized by phase contrast and by GFP fluorescence. Wild-type L. biflexa str. Patoc 1 was used as the negative control.
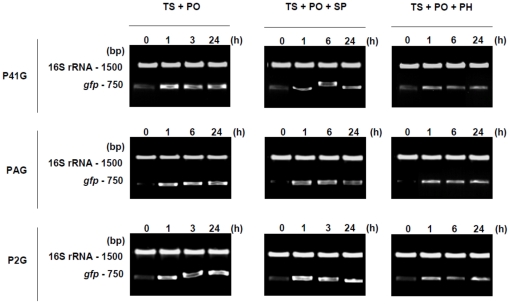
To further substantiate the data obtained by the use of the promoter-probe vectors, rt-PCR analysis was performed on RNA extracted from non-induced and induced cultures of the L. biflexa reporter strains (Figure 9). As expected, low level of transcripts (gfp-based) were detected before induction of the reporter strains. Three time-points were evaluated, 1, 3 or 6 and 24 h p.i., but only a slight difference was observed between the time-points for any given in vitro treatment, per reporter strain (Figure 9). Despite this, the amount of gfp mRNA varied considerably per treatment. Correlation between the fluorescence and transcription levels was not always evident, similarly to the lack of correlation observed during the analysis of the native strain (see Results). In both cases (native and reporter strains) the discrepancy observed between the mRNA and protein levels probably resulted from intrinsic regulatory mechanisms. Although we have no information on the signalling pathways involved, the L. interrogans promoters evaluated appear to behave similarly in both species, suggesting conservation of the regulatory mechanisms higher than expected. The transcription data complement those obtained by fluorescence measurement from the reporter strains, reinforcing the utility of the promoter-probe vectors as a genetic tool to test promoter activity in L. biflexa.
Figure 9. Influence of the in vitro conditions on gfp expression of the reporter strains.
The effect of each of the combinations of conditions on promoter activity was assessed by the level of gfp mRNA produced. Rows depict the reporter strains in this study, while columns show the various conditions used: TS – Temperature upshift from 30°C to 37°C; PO – Physiological osmolarity; SP – Spermine induction; PH – Urine pH induction. Within each lane, the upper band represents the internal PCR control (16S rRNA) and the lower band corresponds to the gfp cDNA amplified by rt-PCR. The lanes in each gel contain the amplified cDNA per time-point, both pretreatment (0 h) and post induction (1, 3 or 6 and 24 h). Agarose gels were stained with GelRed (Invitrogen). No bands were observed in control samples run without template (data not shown). Samples were standardized according to an OD420 0.25. Data from a representative significant study are shown.
Validation of the L. biflexa promoter-probe constructs was performed by comparison against the in vitro-induced pathogenic strain. In addition to the protein content, we compared the mRNA profiles produced by the wild-type virulent L. interrogans and the PAG reporter strain. Side-by-side analysis of two time-points (1 and 24 h p.i.) showed that all treatments produced similar patterns of both LigA and GFP expression, the latter being controlled by the native L. interrogans PligA promoter (Figure 1 versus 9). However, a noticeable difference was observed with respect to the transcript levels produced by the native ligA and the PligA-controlled gfp (Figure 1 versus 9). Of note, up-regulation of the PligA was noted despite the treatment (Table 2), therefore validating the use of this promoter-probe methodology as a genetic tool to assess the ability of pathogens promoters to respond host stimuli.
Discussion
In this study we developed and characterized in detail a L. biflexa promoter-probe methodology for the analysis of L. interrogans promoters. The three promoters regions used in this study resemble regulatory regions of other bacterial species, based on bioinformatics characterization only (data not shown). Although we did not support in silico findings with in vitro data we observed that PlipL41 promoter sequence is similar to the E. coli σ70 promoter, while PligA and Psph2 organization is more distant to it. The identification of different repeats into the promoter regions studied may be suggestive of specific response regulator binding sites. It is possible that the elements through which each promoter senses the changes on environmental cues may be different.
The lipL41, ligA and sph2 genes are known to be regulated in vitro by physiological conditions [44], [46], [47], [48]. In addition, the L. interrogans transcriptome was evaluated in response to multifactorial conditions, upon exposure to serum [58]. To further substantiate the knowledge of Leptospira genetics, P41G, PAG and P2G reporter strains were constructed to contain gfp under the conditional control of the aforementioned L. interrogans promoters. Analysis of cell extracts harvested from exponentially growing cultures revealed low GFP activity under routine growth conditions, while variable activity was observed post-induction. In agreement to previous findings obtained by transcriptomics, our data demonstrate the sensitivity of leptospiral promoters under the various conditions evaluated.
ligA is a paralog of ligB, and originated by duplication events of the first ten immunoglobulin-like domains [83], consequently both are expected to respond to host stimuli through similar mechanisms. Both proteins seem to contribute to the pathogenesis of Leptospira spp. as important adhesins [84], [85], [86]. In addition, spectral counts of both proteins by a mass-spectrometry-based strategy revealed that both LigA and LigB are present in 553 and 914 copies per cell, respectively [87]. Recently, Lo and colleagues were not able to detect LigB among L. interrogans serovar Lai samples shifted from environmental to human body temperature (37°C), demonstrating that temperature upshift alone may be insufficient for induction of expression of Lig proteins [88]. Likewise, in our study, the PligA promoter activity was only weakly detected during non-induced and single-condition induced PAG cells. However, fluorescence was more than doubled after induction by combinations of stimuli (Figure 5B– 7B). The same was observed for the expression of native LigA by L. interrogans serovar Copenhageni strain Fiocruz L1-130 (Figure 2).
Sph2 is expressed under routine in vitro conditions and temperature upshift to 37°C [87], [88], [89]. At the mRNA level, it is up-regulated by physiological osmolarity [47] and temperature [46]. In our study, we corroborated these findings with the observation of low levels of fluorescence in the non-induced P2G reporter strain and elevated levels of Psph2 promoter activity after induction by the combination of different mammalian host conditions (Figures 5C, 6, 7C). These findings suggest the mammalian host conditions may have contributed to this effect and reinforces the hypothesis that the sph2 gene is likely to be regulated at post-transcriptional level.
Interestingly, we observed LigA down-regulation when L. interrogans serovar Copenhageni was cultivated at pH 6.7 (Figure 2C). This is in agreement with previous findings that leptospiral antigens are down-regulated when leptospires are excreted in the urine [90]. Of note, this was not observed during fluorescence analysis (Figure 6). This suggests that specific response regulators may be triggered in the parental pathogenic strain, during renal colonization and/or urine shedding. In general, the activity of the PlipL41 promoter was not, or only weakly, influenced under the various conditions studied (Figure 3A– 7A), and this is consistent with previous observations [59], [88], [91], [92]. In contrast to the study of Nally and colleagues [93] that observed LipL41 downregulation upon experimental infection, PlipL41 promoter activity was not reduced when the P41G reporter strain was cultivated under the majority of the conditions analysed (Figure 3A– 7A). This suggests that the reduction of LipL41 expression in guinea pig-recovered L. interrogans may be due to post translational regulation, and may involve complex regulation systems such as host cell contact, protein degradation, and energy metabolism.
Of note, the PlipL41 promoter was initially selected as a constitutive promoter. However, we noted that this promoter was also up-regulated at some of the selected time-points. An ideal constitutive gene should exhibit constant activity independently to the cell state or environmental conditions. However, constitutive expression may not be related to constant promoter activity. Moreover, expression levels of constitutive genes such as the flagellin or the ribosomal RNA synthesis genes, have been shown to be altered by temperature-shift or ciprofloxacin supplementation [87], [88]. PlipL41 promoter activity was not always induced under the in vitro conditions. In addition, its activity was below the cut-off level (2-fold) established in this work for virulence factor promoters (Table 2). This lead us to believe that the P41G reporter strain may serve as a negative control in the identification of novel virulence factors.
Although L. interrogans is a pathogen known to infect the mammalian host through active penetration, there is uncertainty over the existence of an intracellular phase during this process. In this study, we demonstrated that spermine, a polyamine found in abundance in eukaryotic cells [76] alters L. interrogans promoters activity. Previous studies showed leptospiral survival and replication within human macrophages, a spermine-rich eukaryotic cell [94]. We observed that supplementation of reporter strain cultures with spermine was able to stimulate promoter activity. In addition, we present evidence that similar transcriptional and translational changes occurred during induction of the native proteins, supporting the hypothesis that pathogenic Leptospira spp. can recognize polyamines as a signal of the intracellular environment. Polyamine recognition is likely a component of a sophisticated system that integrates multiple environmental signals and regulates gene expression in intracellular bacteria, i.e. Francisella [95], [96], [97], and biofilm formation in Vibrio spp. and Yersinia spp. [77], [78]. As polyamines in the intracellular environment are likely to reach elevated levels, our findings on promoter regulation by spermine may be relevant bacterial pathogenesis in general.
It is not known what controls ligA or sph2 transcription initiation. However, previous studies have shown that both genes are up-regulated by physiological osmolarity [44], [47], [48] and temperature [46]. Also, recent work presented evidence of a functional redundancy between LigA and LigB [52]. The finding that the PligA promoter is active under different in vitro conditions, which simulate the mammalian host environment, raises the hypothesis that ligA absence from several pathogenic serovars is likely to be due to spontaneous deleterious events that may have occurred inside or outside the mammalian host environment [83], [98], without any influence on virulence or pathogenicity. Since most of the conditions studied induced constant activity of PligA in vitro (Figure 3B– 6B), it is possible that LigA may contribute to the early stages in the course of leptospirosis. The kinetics of native LigA expression also supports this hypothesis, although protein levels did vary (Figure 2).
We found that native LigA was up-regulated upon induction by the combined conditions of physiological osmolarity, temperature upshift to 37°C and spermine, with a 5.95-fold increase. The corresponding reporter strain, PAG, was up-regulated by the same treatment (2.74-fold), demonstrating a good correlation between the promoter-probe methodology and native protein expression in L. interrogans, suggesting that LigA is post-transcriptionally regulated (Table 2). This also suggests that L. interrogans protein expression may involve particular mechanisms or pathways to respond to specific host stimuli. The overall up-regulation of native LigA observed in comparison to the non-induced control was in agreement with the induction of the L. biflexa PAG reporter strain. Our results are in concordance with previous studies whereby the lig genes were expressed in vitro at both transcript and proteins levels [99], although a previous study using an L. interrogans serovar Pomona type Kennewicki strain was only able to detect the lig genes at the transcript level, [100]. We believe that these differences may be due to the different strains used. Moreover, ligB a paralogous gene to ligA, was up-regulated by an overnight temperature upshift from 30°C to 37°C (1.7-fold). Yet, physiological osmolarity stimulated ligA and ligB up-regulation (4.41 to 5.27-fold – based on the transcriptional signals employing oligonucleotides to both the ligA/ligB identical region and the ligB unique region, respectively) [46], [48]. Additionally, a recent study evaluated the influence of serum on ligB up-regulation (1.89-fold) [58]. Although we did not quantify the transcription of the ligA gene, a clear variation was observed in comparison to the non-induced controls, which is in agreement with previous findings (Figure 1). This reinforces the applicability of the promoter-probe vectors to assess promoter activity in L. biflexa. Based on these results, we conclude that (i) the combination of the in vitro conditions reliably simulated the host environment, (ii) L. biflexa can be used as a model to characterize L. interrogans promoters and (iii) this promoter-probe methodology may be helpful in the prediction of potential virulence factors of pathogenic Leptospira spp. Furthermore, previous studies showed ligA gene up-regulation shortly after temperature upshift [46], [88], thus reinforcing the hypothesis that LigA may contribute to the early stages of infection and host adaptation. In addition, the Psph2 promoter was up-regulated in the presence of spermine (2.12-fold).
We observed some conflicting evidence in the correlation between native ligA mRNA and LigA abundance levels (Figure 1 versus 2), similar to previous studies [88], [101], [102], [103], [104]. As previously suggested by Lo and colleagues [88], it is possible that the lack of correlation might be due to the longer half-life, greater stability or post transcriptional regulation of the mRNA transcript, i.e. as a result of the activity of small non-coding RNAs. Native LigA was detected at very low levels in the non-induced L. interrogans cells, while a significant up-regulation, at similar rates to recent studies [48], was observed when leptospires were grown under mammalian host conditions. In light of this information, we may conclude that the expression of both LigA and Sph2 is likely to be regulated at the post-transcriptional level.
Despite advances in the development of genetic tools for Leptospira spp. some basic questions remain unanswered. It is still unclear what regulators and pathways are associated with the expression of virulence factors. The level at which members of a given regulatory cascade exert induction/repression of transcription of LigA, Sph2 and other virulence factors is unknown. In this sudy we demonstrate that L. biflexa can serve as a model to study the genetics of L. interrogans. The pathogen-derived promoters exhibited activity in the L. biflexa, suggesting the existence of shared regulatory mechanisms among the saprophytes and pathogenic Leptospira spp. In addition, the establishment of a cut-off based on the promoter activity of virulence and non-virulence factors (Table 2), and the different fluorescence levels expressed by the reporter strains upon induction eliminated the possibility that the effects were simply due to stress responses. Furthermore, the suite of promoter-probe vectors developed in this study can be modified to make diverse translational fusions to investigate protein expression patterns and complex regulatory networks involved in leptospiral protein regulation.
In conclusion, this study demonstrates the potential of a novel genetic tool for the identification and characterization of virulence factors of Leptospira spp. Our transcription and expression findings suggest that a combination of in vitro signals may be important to accurately simulate the host environment. We expect to provide further information towards understanding Leptospira spp. genetics and that will eventually serve as a basis for further studies. The understanding of the extent to which leptospiral promoters are regulated by mammalian host conditions, as well as the expression kinetics, may reveal useful information about the biology of Leptospira spp. A knock-in mutant library of different promoters is currently under construction with this in mind.
Acknowledgments
We thank Dr. Marilene Demasi, Dr. Hugo A. Armelin and Dr. Toshie Kawano for their support with fluorescence and microscopy facilities, and Alexsander Seixas de Souza, Ivan N. Avino and Bogar O. A. Montoya for technical assistance with the experiments. We are also grateful to Dr. Mathieu Picardeau, Dr. Justin D. Radolf and Dr. Alan J. A. McBride for helpful comments and critical reviews of the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Gustavo M. Cerqueira holds a postdoctoral fellowship from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP). Ana L.T.O. Nascimento would like to acknowledge the support from FAPESP, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação Butantan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.McBride AJ, Athanazio DA, Reis MG, Ko AI. Leptospirosis. Curr Opin Infect Dis. 2005;18:376–386. doi: 10.1097/01.qco.0000178824.05715.2c. [DOI] [PubMed] [Google Scholar]
- 2.Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3:757–771. doi: 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 3.Segura ER, Ganoza CA, Campos K, Ricaldi JN, Torres S, et al. Clinical spectrum of pulmonary involvement in leptospirosis in a region of endemicity, with quantification of leptospiral burden. Clin Infect Dis. 2005;40:343–351. doi: 10.1086/427110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gouveia EL, Metcalfe J, de Carvalho AL, Aires TS, Villasboas-Bisneto JC, et al. Leptospirosis-associated severe pulmonary hemorrhagic syndrome, Salvador, Brazil. Emerg Infect Dis. 2008;14:505–508. doi: 10.3201/eid1403.071064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerqueira GM, Picardeau M. A century of Leptospira strain typing. Infect Genet Evol. 2009;9:760–768. doi: 10.1016/j.meegid.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Faine S, Adler B, Bolin C, Perolat P. Leptospira and Leptospirosis. Melbourne: MedScience; 1999. [Google Scholar]
- 7.Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14:296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren SX, Fu G, Jiang XG, Zeng R, Miao YG, et al. Unique physiological and pathogenic features of Leptospira interrogans revealed by whole-genome sequencing. Nature. 2003;422:888–893. doi: 10.1038/nature01597. [DOI] [PubMed] [Google Scholar]
- 9.Nascimento AL, Ko AI, Martins EA, Monteiro-Vitorello CB, Ho PL, et al. Comparative genomics of two Leptospira interrogans serovars reveals novel insights into physiology and pathogenesis. J Bacteriol. 2004;186:2164–2172. doi: 10.1128/JB.186.7.2164-2172.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bulach DM, Zuerner RL, Wilson P, Seemann T, McGrath A, et al. Genome reduction in Leptospira borgpetersenii reflects limited transmission potential. Proc Natl Acad Sci U S A. 2006;103:14560–14565. doi: 10.1073/pnas.0603979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Picardeau M, Bulach DM, Bouchier C, Zuerner RL, Zidane N, et al. Genome sequence of the saprophyte Leptospira biflexa provides insights into the evolution of Leptospira and the pathogenesis of leptospirosis. PLoS One. 2008;3:e1607. doi: 10.1371/journal.pone.0001607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. 2003. Human Leptospirosis: Guidance for Diagnosis, Surveillance and Control. Malta.
- 13.Levett PN. Usefulness of serologic analysis as a predictor of the infecting serovar in patients with severe leptospirosis. Clin Infect Dis. 2003;36:447–452. doi: 10.1086/346208. [DOI] [PubMed] [Google Scholar]
- 14.Bajani MD, Ashford DA, Bragg SL, Woods CW, Aye T, et al. Evaluation of four commercially available rapid serologic tests for diagnosis of leptospirosis. J Clin Microbiol. 2003;41:803–809. doi: 10.1128/JCM.41.2.803-809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Effler PV, Bogard AK, Domen HY, Katz AR, Higa HY, et al. Evaluation of eight rapid screening tests for acute leptospirosis in Hawaii. J Clin Microbiol. 2002;40:1464–1469. doi: 10.1128/JCM.40.4.1464-1469.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smits HL, Ananyina YV, Chereshsky A, Dancel L, Lai AFRF, et al. International multicenter evaluation of the clinical utility of a dipstick assay for detection of Leptospira-specific immunoglobulin M antibodies in human serum specimens. J Clin Microbiol. 1999;37:2904–2909. doi: 10.1128/jcm.37.9.2904-2909.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smits HL, Hartskeerl RA, Terpstra WJ. International multi-centre evaluation of a dipstick assay for human leptospirosis. Trop Med Int Health. 2000;5:124–128. doi: 10.1046/j.1365-3156.2000.00525.x. [DOI] [PubMed] [Google Scholar]
- 18.Smits HL, Chee HD, Eapen CK, Kuriakose M, Sugathan S, et al. Latex based, rapid and easy assay for human leptospirosis in a single test format. Trop Med Int Health. 2001;6:114–118. doi: 10.1046/j.1365-3156.2001.00675.x. [DOI] [PubMed] [Google Scholar]
- 19.Smits HL, Eapen CK, Sugathan S, Kuriakose M, Gasem MH, et al. Lateral-flow assay for rapid serodiagnosis of human leptospirosis. Clin Diagn Lab Immunol. 2001;8:166–169. doi: 10.1128/CDLI.8.1.166-169.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonrier C, Branger C, Michel V, Ruvoen-Clouet N, Ganiere JP, et al. Evidence of cross-protection within Leptospira interrogans in an experimental model. Vaccine. 2000;19:86–94. doi: 10.1016/s0264-410x(00)00129-8. [DOI] [PubMed] [Google Scholar]
- 21.Bolin CA, Thiermann AB, Handsaker AL, Foley JW. Effect of vaccination with a pentavalent leptospiral vaccine on Leptospira interrogans serovar hardjo type hardjo-bovis infection of pregnant cattle. Am J Vet Res. 1989;50:161–165. [PubMed] [Google Scholar]
- 22.Croda J, Ramos JG, Matsunaga J, Queiroz A, Homma A, et al. Leptospira immunoglobulin-like proteins as a serodiagnostic marker for acute leptospirosis. J Clin Microbiol. 2007;45:1528–1534. doi: 10.1128/JCM.02344-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oliveira TR, Longhi MT, de Morais ZM, Romero EC, Blanco RM, et al. Evaluation of leptospiral recombinant antigens MPL17 and MPL21 for serological diagnosis of leptospirosis by enzyme-linked immunosorbent assays. Clin Vaccine Immunol. 2008;15:1715–1722. doi: 10.1128/CVI.00214-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong H, Hu Y, Xue F, Sun D, Ojcius DM, et al. Characterization of the ompL1 gene of pathogenic Leptospira species in China and cross-immunogenicity of the OmpL1 protein. BMC Microbiol. 2008;8:223. doi: 10.1186/1471-2180-8-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin X, Chen Y, Yan J. Recombinant multiepitope protein for diagnosis of leptospirosis. Clin Vaccine Immunol. 2008;15:1711–1714. doi: 10.1128/CVI.00189-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srimanote P, Wongdeethai N, Jieanampunkul P, Samonkiert S, Leepiyasakulchai C, et al. Recombinant ligA for leptospirosis diagnosis and ligA among the Leptospira spp. clinical isolates. J Microbiol Methods. 2008;72:73–81. doi: 10.1016/j.mimet.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Bomfim MR, Ko A, Koury MC. Evaluation of the recombinant LipL32 in enzyme-linked immunosorbent assay for the serodiagnosis of bovine leptospirosis. Vet Microbiol. 2005;109:89–94. doi: 10.1016/j.vetmic.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Dey S, Mohan CM, Kumar TM, Ramadass P, Nainar AM, et al. Recombinant LipL32 antigen-based single serum dilution ELISA for detection of canine leptospirosis. Vet Microbiol. 2004;103:99–106. doi: 10.1016/j.vetmic.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 29.Haake DA, Mazel MK, McCoy AM, Milward F, Chao G, et al. Leptospiral outer membrane proteins OmpL1 and LipL41 exhibit synergistic immunoprotection. Infect Immun. 1999;67:6572–6582. doi: 10.1128/iai.67.12.6572-6582.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koizumi N, Watanabe H. Leptospiral immunoglobulin-like proteins elicit protective immunity. Vaccine. 2004;22:1545–1552. doi: 10.1016/j.vaccine.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Branger C, Chatrenet B, Gauvrit A, Aviat F, Aubert A, et al. Protection against Leptospira interrogans sensu lato challenge by DNA immunization with the gene encoding hemolysin-associated protein 1. Infect Immun. 2005;73:4062–4069. doi: 10.1128/IAI.73.7.4062-4069.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palaniappan RU, McDonough SP, Divers TJ, Chen CS, Pan MJ, et al. Immunoprotection of recombinant leptospiral immunoglobulin-like protein A against Leptospira interrogans serovar Pomona infection. Infect Immun. 2006;74:1745–1750. doi: 10.1128/IAI.74.3.1745-1750.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silva EF, Medeiros MA, McBride AJ, Matsunaga J, Esteves GS, et al. The terminal portion of leptospiral immunoglobulin-like protein LigA confers protective immunity against lethal infection in the hamster model of leptospirosis. Vaccine. 2007;25:6277–6286. doi: 10.1016/j.vaccine.2007.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faisal SM, Yan W, McDonough SP, Pan MJ, Chang CF, et al. Leptosome-associated leptospiral antigens conferred significant higher levels of protection than those associated with PC-liposomes in a hamster model. Vaccine. 2009 doi: 10.1016/j.vaccine.2009.08.051. [DOI] [PubMed] [Google Scholar]
- 35.Yan W, Faisal SM, McDonough SP, Divers TJ, Barr SC, et al. Immunogenicity and protective efficacy of recombinant Leptospira immunoglobulin-like protein B (rLigB) in a hamster challenge model. Microbes Infect. 2009;11:230–237. doi: 10.1016/j.micinf.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Faisal SM, Yan W, McDonough SP, Chang YF. Leptospira immunoglobulin-like protein A variable region (LigAvar) incorporated in liposomes and PLGA microspheres produces a robust immune response correlating to protective immunity. Vaccine. 2009;27:378–387. doi: 10.1016/j.vaccine.2008.10.089. [DOI] [PubMed] [Google Scholar]
- 37.Faisal SM, Yan W, Chen CS, Palaniappan RU, McDonough SP, et al. Evaluation of protective immunity of Leptospira immunoglobulin like protein A (LigA) DNA vaccine against challenge in hamsters. Vaccine. 2008;26:277–287. doi: 10.1016/j.vaccine.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 38.Chang YF, Chen CS, Palaniappan RU, He H, McDonough SP, et al. Immunogenicity of the recombinant leptospiral putative outer membrane proteins as vaccine candidates. Vaccine. 2007;25:8190–8197. doi: 10.1016/j.vaccine.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 39.Yan W, Faisal SM, McDonough SP, Chang CF, Pan MJ, et al. Identification and characterization of OmpA-like proteins as novel vaccine candidates for Leptospirosis. Vaccine. 28:2277–2283. doi: 10.1016/j.vaccine.2009.12.071. [DOI] [PubMed] [Google Scholar]
- 40.Faisal SM, Yan W, McDonough SP, Chang CF, Pan MJ, et al. Leptosome-entrapped leptospiral antigens conferred significant higher levels of protection than those entrapped with PC-liposomes in a hamster model. Vaccine. 2009;27:6537–6545. doi: 10.1016/j.vaccine.2009.08.051. [DOI] [PubMed] [Google Scholar]
- 41.Faisal SM, Yan W, McDonough SP, Mohammed HO, Divers TJ, et al. Immune response and prophylactic efficacy of smegmosomes in a hamster model of leptospirosis. Vaccine. 2009;27:6129–6136. doi: 10.1016/j.vaccine.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 42.Bourhy P, Louvel H, Saint GI, Picardeau M. Random insertional mutagenesis of Leptospira interrogans, the agent of leptospirosis, using a mariner transposon. Journal of Bacteriology. 2005;187:3255–3258. doi: 10.1128/JB.187.9.3255-3258.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aviat F, Slamti L, Cerqueira GM, Lourdault K, Picardeau M. Expanding the Genetic Toolbox for Leptospira Species by Generation of Fluorescent Bacteria. Appl Environ Microbiol. 2010;76:8135–8142. doi: 10.1128/AEM.02199-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsunaga J, Sanchez Y, Xu X, Haake DA. Osmolarity, a key environmental signal controlling expression of leptospiral proteins LigA and LigB and the extracellular release of LigA. Infect Immun. 2005;73:70–78. doi: 10.1128/IAI.73.1.70-78.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barbosa AS, Abreu PA, Neves FO, Atzingen MV, Watanabe MM, et al. A newly identified leptospiral adhesin mediates attachment to laminin. Infect Immun. 2006;74:6356–6364. doi: 10.1128/IAI.00460-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lo M, Bulach DM, Powell DR, Haake DA, Matsunaga J, et al. Effects of temperature on gene expression patterns in Leptospira interrogans serovar Lai as assessed by whole-genome microarrays. Infect Immun. 2006;74:5848–5859. doi: 10.1128/IAI.00755-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsunaga J, Medeiros MA, Sanchez Y, Werneid KF, Ko AI. Osmotic regulation of expression of two extracellular matrix-binding proteins and a haemolysin of Leptospira interrogans: differential effects on LigA and Sph2 extracellular release. Microbiology. 2007;153:3390–3398. doi: 10.1099/mic.0.2007/007948-0. [DOI] [PubMed] [Google Scholar]
- 48.Matsunaga J, Lo M, Bulach DM, Zuerner RL, Adler B, et al. Response of Leptospira interrogans to physiologic osmolarity: relevance in signaling the environment-to-host transition. Infect Immun. 2007;75:2864–2874. doi: 10.1128/IAI.01619-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Atzingen MV, Barbosa AS, De Brito T, Vasconcellos SA, de Morais ZM, et al. Lsa21, a novel leptospiral protein binding adhesive matrix molecules and present during human infection. BMC Microbiol. 2008;8:70. doi: 10.1186/1471-2180-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ristow P, Bourhy P, da Cruz McBride FW, Figueira CP, Huerre M, et al. The OmpA-like protein Loa22 is essential for leptospiral virulence. PLoS Pathog. 2007;3:e97. doi: 10.1371/journal.ppat.0030097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stevenson B, Choy HA, Pinne M, Rotondi ML, Miller MC, et al. Leptospira interrogans endostatin-like outer membrane proteins bind host fibronectin, laminin and regulators of complement. PLoS One. 2007;2:e1188. doi: 10.1371/journal.pone.0001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Croda J, Figueira CP, Wunder EA, Jr, Santos CS, Reis MG, et al. Targeted mutagenesis in pathogenic Leptospira species: disruption of the LigB gene does not affect virulence in animal models of leptospirosis. Infect Immun. 2008;76:5826–5833. doi: 10.1128/IAI.00989-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murray GL, Srikram A, Henry R, Puapairoj A, Sermswan RW, et al. Leptospira interrogans requires heme oxygenase for disease pathogenesis. Microbes Infect. 2009;11:311–314. doi: 10.1016/j.micinf.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 54.Murray GL, Srikram A, Hoke DE, Wunder EA, Jr, Henry R, et al. Major surface protein LipL32 is not required for either acute or chronic infection with Leptospira interrogans. Infect Immun. 2009;77:952–958. doi: 10.1128/IAI.01370-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murray GL, Morel V, Cerqueira GM, Croda J, Srikram A, et al. Genome-wide transposon mutagenesis in pathogenic Leptospira species. Infect Immun. 2009;77:810–816. doi: 10.1128/IAI.01293-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murray GL, Ellis KM, Lo M, Adler B. Leptospira interrogans requires a functional heme oxygenase to scavenge iron from hemoglobin. Microbes Infect. 2008;10:791–797. doi: 10.1016/j.micinf.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 57.Xue F, Dong H, Wu J, Wu Z, Hu W, et al. Transcriptional responses of Leptospira interrogans to host innate immunity: significant changes in metabolism, oxygen tolerance, and outer membrane. PLoS Negl Trop Dis. 2010;4:e857. doi: 10.1371/journal.pntd.0000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patarakul K, Lo M, Adler B. Global transcriptomic response of Leptospira interrogans serovar Copenhageni upon exposure to serum. BMC Microbiol. 2010;10 doi: 10.1186/1471-2180-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cullen PA, Cordwell SJ, Bulach DM, Haake DA, Adler B. Global analysis of outer membrane proteins from Leptospira interrogans serovar Lai. Infect Immun. 2002;70:2311–2318. doi: 10.1128/IAI.70.5.2311-2318.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haake DA, Martinich C, Summers TA, Shang ES, Pruetz JD, et al. Characterization of leptospiral outer membrane lipoprotein LipL36: downregulation associated with late-log-phase growth and mammalian infection. Infect Immun. 1998;66:1579–1587. doi: 10.1128/iai.66.4.1579-1587.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matsunaga J, Young TA, Barnett JK, Barnett D, Bolin CA, et al. Novel 45-kilodalton leptospiral protein that is processed to a 31-kilodalton growth-phase-regulated peripheral membrane protein. Infect Immun. 2002;70:323–334. doi: 10.1128/IAI.70.1.323-334.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nally JE, Artiushin S, Timoney JF. Molecular characterization of thermoinduced immunogenic proteins Q1p42 and Hsp15 of Leptospira interrogans. Infect Immun. 2001;69:7616–7624. doi: 10.1128/IAI.69.12.7616-7624.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barnett JK, Barnett D, Bolin CA, Summers TA, Wagar EA, et al. Expression and distribution of leptospiral outer membrane components during renal infection of hamsters. Infect Immun. 1999;67:853–861. doi: 10.1128/iai.67.2.853-861.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ko AI, Goarant C, Picardeau M. Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat Rev Microbiol. 2009;7:736–747. doi: 10.1038/nrmicro2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ellinghausen HC, Jr, McCullough WG. Nutrition of Leptospira Pomona and Growth of 13 Other Serotypes: Fractionation of Oleic Albumin Complex and a Medium of Bovine Albumin and Polysorbate 80. Am J Vet Res. 1965;26:45–51. [PubMed] [Google Scholar]
- 66.Johnson RC, Harris VG. Differentiation of pathogenic and saprophytic letospires. I. Growth at low temperatures. J Bacteriol. 1967;94:27–31. doi: 10.1128/jb.94.1.27-31.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vallenet D, Labarre L, Rouy Z, Barbe V, Bocs S, et al. MaGe—a microbial genome annotation system supported by synteny result. Nucleic Acids Research. 2006;34:13. doi: 10.1093/nar/gkj406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reese MG. Application of a time-delay neural network to promoter annotation in the Drosophila melanogaster genome. Computational Chemistry. 2001;26:6. doi: 10.1016/s0097-8485(01)00099-7. [DOI] [PubMed] [Google Scholar]
- 69.Matys V, Fricke E, Geffers R, Gossling E, Haubrock M, et al. TRANSFAC: transcriptional regulation, from patterns to profiles. Nucl Acids Res. 2003;31:5. doi: 10.1093/nar/gkg108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Studholme DJ, Dixon R. Domain architectures of sigma 54-dependent transcriptional activators. J Bacteriol. 2003;185:11. doi: 10.1128/JB.185.6.1757-1767.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Girons IS, Bourhy P, Ottone C, Picardeau M, Yelton D, et al. The LE1 bacteriophage replicates as a plasmid within Leptospira biflexa: construction of an L. biflexa-Escherichia coli shuttle vector. J Bacteriol. 2000;182:5700–5705. doi: 10.1128/jb.182.20.5700-5705.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:7. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matsunaga J, Werneid K, Zuerner RL, Frank A, Haake DA. LipL46 is a novel surface-exposed lipoprotein expressed during leptospiral dissemination in the mammalian host. Microbiology. 2006;152:3777–3786. doi: 10.1099/mic.0.29162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. GraphPad Software. Available: http://www.graphpad.com.
- 75.Carlson JPE, Horzempa J, O'Dee DM, Robinson CM, Neophytou P, et al. Global transcriptional response to spermine, a component of the intramacrophage environment, reveals regulation of Francisella gene expression through insertion sequence elements. J Bacteriol. 2009;191:10. doi: 10.1128/JB.00995-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Igarashi K, Kashiwagi K. Polyamines: mysterious modulators of cellular functions. Biochem Biophys Res Commun. 2000;271:6. doi: 10.1006/bbrc.2000.2601. [DOI] [PubMed] [Google Scholar]
- 77.Karatan E, Duncan TR, Watnick PI. NspS, a predicted polyamine sensor, mediates activation of Vibrio cholerae biofilm formation by nor spermidine. J Bacteriol. 2005;187:10. doi: 10.1128/JB.187.21.7434-7443.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Patel CN, Wortham BW, Lines JL, Fetherston JD, Perry RD, et al. Polyamines are essential for the formation of plague biofilm. J Bacteriol. 2006;188:9. doi: 10.1128/JB.188.7.2355-2363.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ware D, Jiang Y, Lin W, Swiatlo E. Involvement of potD in Streptococcus pneumoniae polyamine transport and pathogenesis. Infect Immun. 2006;74:10. doi: 10.1128/IAI.74.1.352-361.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yoshida M, Kashiwagi K, Shigemasa A, Taniguchi S, Yamamoto K, et al. A unifying model for the role of polyamines in bacterial cell growth, the polyamine modulon. J Biol Chem. 2004;279:6. doi: 10.1074/jbc.M404393200. [DOI] [PubMed] [Google Scholar]
- 81.Segal GM, Stueve T, Adamson JW. Spermine and spermidine are non-specific inhibitors of in vitro Hematopoiesis. Kidney Int. 1987;31:5. doi: 10.1038/ki.1987.11. [DOI] [PubMed] [Google Scholar]
- 82.Zahedi K, Bissler JJ, Wang Z, Josyula A, Lu L, et al. Spermidine/spermine N1-acetyltransferase overexpression in kidney epithelial cells disrupts polyamine homeostasis, leads to DNA damage, and causes G2 arrest. Am J Physiol Cell Physiol. 2007;292:12. doi: 10.1152/ajpcell.00451.2006. [DOI] [PubMed] [Google Scholar]
- 83.McBride AJ, Cerqueira GM, Suchard MA, Moreira AN, Zuerner RL, et al. Genetic diversity of the Leptospiral immunoglobulin-like (lig) genes in pathogenic Leptospira spp. Infect Genet Evol. 2009;9:196–205. doi: 10.1016/j.meegid.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matsunaga J, Barocchi MA, Croda J, Young TA, Sanchez Y, et al. Pathogenic Leptospira species express surface-exposed proteins belonging to the bacterial immunoglobulin superfamily. Mol Microbiol. 2003;49:929–945. doi: 10.1046/j.1365-2958.2003.03619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barocchi MA, Ko AI, Reis MG, McDonald KL, Riley LW. Rapid translocation of polarized MDCK cell monolayers by Leptospira interrogans, an invasive but nonintracellular pathogen. Infect Immun. 2002;70:6926–6932. doi: 10.1128/IAI.70.12.6926-6932.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Choy HA, Kelley MM, Chen TL, Moller AK, Matsunaga J, et al. Physiological osmotic induction of Leptospira interrogans adhesion: LigA and LigB bind extracellular matrix proteins and fibrinogen. Infect Immun. 2007;75:2441–2450. doi: 10.1128/IAI.01635-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Malmstrom J, Beck M, Schimidt A, Lange V, Deutch EW, et al. Proteome-wide cellular protein concentrations of the human pathogen Leptospira interrogans. Nature. 2009;460:4. doi: 10.1038/nature08184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lo M, Cordwell SJ, Bulach DM, Adler B. Comparative transcriptional and translational analysis of leptospiral outer membrane protein expression in response to temperature. PLoS Negl Trop Dis. 2009;3:e560. doi: 10.1371/journal.pntd.0000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Artiushin S, Timoney JF, Nally J, Verma A. Host-inducible immunogenic sphingomyelinase-like protein, Lk73.5, of Leptospira interrogans. Infect Immun. 2004;72:742–749. doi: 10.1128/IAI.72.2.742-749.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Monahan AM, Callanan JJ, Nally JE. Proteomic analysis of Leptospira interrogans shed in urine of chronically infected hosts. Infect Immun. 2008;76:4952–4958. doi: 10.1128/IAI.00511-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nally JE, Timoney JF, Stevenson B. Temperature-regulated protein synthesis by Leptospira interrogans. Infect Immun. 2001;69:400–404. doi: 10.1128/IAI.69.1.400-404.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Haake DA, Chao G, Zuerner RL, Barnett JK, Barnett D, et al. The leptospiral major outer membrane protein LipL32 is a lipoprotein expressed during mammalian infection. Infect Immun. 2000;68:2276–2285. doi: 10.1128/iai.68.4.2276-2285.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nally JE, Whitelegge JP, Bassilian S, Blanco DR, Lovett MA. Characterization of the outer membrane proteome of Leptospira interrogans expressed during acute lethal infection. Infect Immun. 2007;75:766–773. doi: 10.1128/IAI.00741-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li S, Ojcius DM, Liao S, Li L, Xue F, et al. Replication or death: distinct fates of pathogenic Leptospira strain Lai within macrophages of human or mouse origin. Innate Immun. 2009 doi: 10.1177/1753425909105580. [DOI] [PubMed] [Google Scholar]
- 95.Deng K, Blick RJ, Liu W, Hansen EJ. Identification of Francisella tularensis genes affected by iron limitation. Infect Immun. 2006;74:13. doi: 10.1128/IAI.01975-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Horzempa J, Carlson JPE, O'Dee DM, Shanks RM, Nau GJ. Global transcriptional response to mammalian temperature provides new insight into Francisella tularensis pathogenesis. BMC Microbiol. 2008;8 doi: 10.1186/1471-2180-8-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Horzempa J, Tarwacki DM, Carlson JPE, Robinson CM, Nau GJ. Characterization and application of a glucose-repressible promoter in Francisella tularensis. Appl Environ Microbiol. 2008;74:10. doi: 10.1128/AEM.02360-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cerqueira GM, McBride AJ, Picardeau M, Ribeiro SG, Moreira AN, et al. Distribution of the leptospiral immunoglobulin-like (lig) genes in pathogenic Leptospira species and application of ligB to typing leptospiral isolates. J Med Microbiol. 2009;58:1173–1181. doi: 10.1099/jmm.0.009175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Matsunaga J, Barocchi MA, Croda J, Young TA, Sanchez Y, et al. Pathogenic Leptospira species express surface-exposed proteins belonging to the bacterial immunoglobulin superfamily. Molecular Microbiology. 2003;49:929–945. doi: 10.1046/j.1365-2958.2003.03619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Palaniappan RU, Chang YF, Hassan F, McDonough SP, Pough M, et al. Expression of leptospiral immunoglobulin-like protein by Leptospira interrogans and evaluation of its diagnostic potential in a kinetic ELISA. J Med Microbiol. 2004;53:975–984. doi: 10.1099/jmm.0.45568-0. [DOI] [PubMed] [Google Scholar]
- 101.Ross PL, Huang YN, Marchese JN, Williamson B, Parker K, et al. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics. 2004;3:16. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- 102.Nie L, Wu G, Zgang W. Correlation of mRNA expression and protein abundance affected by multiple sequence features related to translational efficiency in Desulfovibrio vulgaris: a quantitative analysis. Genetics. 2006;174:15. doi: 10.1534/genetics.106.065862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Suzuki I, Simon WJ, Slabas AR. The heat shock response of Synechocystis sp. PCC 6803 analysed by transcriptomics and proteomics. J Exp Bot. 2006;57:6. doi: 10.1093/jxb/erj148. [DOI] [PubMed] [Google Scholar]
- 104.Chong PK, Burja AM, Radianingtyas H, Fazeli A, Wright PC. Translational and transcriptional analysis of Sulfolobus solfataricus P2 to provide insights into alcohol and ketone utilisation. Proteomics. 2007;7:12. doi: 10.1002/pmic.200600746. [DOI] [PubMed] [Google Scholar]