Abstract
Anterior-posterior axis specification in the mouse requires signalling from a specialised extra-embryonic tissue called the anterior visceral endoderm (AVE). AVE precursors are induced at the distal tip of the embryo and move to the prospective anterior. Embryological and genetic analysis has demonstrated that the AVE is required for anterior patterning and for correctly positioning the site of primitive streak formation by inhibiting Nodal activity. We have carried out a genetic ablation of the Hex-expressing cells of the AVE (Hex-AVE) by knocking the Diphtheria toxin subunit A into the Hex locus in an inducible manner. Using this model we have identified that, in addition to its requirement in the anterior of the embryo, the Hex-AVE sub-population has a novel role between 5.5 and 6.5dpc in patterning the primitive streak. Embryos lacking the Hex-AVE display delayed initiation of primitive streak formation and miss-patterning of the anterior primitive streak. We demonstrate that in the absence of the Hex-AVE the restriction of Bmp2 expression to the proximal visceral endoderm is also defective and expression of Wnt3 and Nodal is not correctly restricted to the posterior epiblast. These results, coupled with the observation that reducing Nodal signalling in Hex-AVE ablated embryos increases the frequency of phenotypes observed, suggests that these primitive streak patterning defects are due to defective Nodal signalling. Together, our experiments demonstrate that the AVE is not only required for anterior patterning, but also that specific sub-populations of this tissue are required to pattern the posterior of the embryo.
Introduction
In mouse, the first definitive axis of the embryo to form is the anterior-posterior (A-P) axis. At approximately 5.25dpc, a group of visceral endoderm cells at the distal tip of the egg cylinder differentiate into a morphologically distinct tissue, known as the distal visceral endoderm (DVE). DVE cells adopt a tall, columnar epithelial morphology, distinguishing them from the surrounding squamous visceral endoderm [1], [2], [3], [4]. Shortly after its formation, the DVE tilts and begins to move unilaterally over the underlying epiblast [2], [4], [5]. The direction of this movement determines the prospective anterior of the embryo and the DVE, now referred to as the anterior visceral endoderm (AVE), is essential for correctly positioning the A-P axis (reviewed in [3], [6]).
Embryological and genetic analysis has demonstrated that the AVE is required for anterior patterning (reviewed in [7], [8], [9]). Microsurgical ablation of the AVE at the onset of gastrulation leads to forebrain truncations [10] and ablation at 5.5dpc abolishes the expression of anterior neuroectoderm markers [11]. Analysis of mouse mutants where gene function has been specifically lost in extra-embryonic tissues has further demonstrated the role of the AVE in forebrain specification [12], [13], [14].
The AVE has also been shown to inhibit primitive streak formation. Ectopic AVE transplantation experiments have indicated that the AVE represses posterior markers [1], [15] and analysis of mutants with defective AVE movements [1], [14], [16], [17], [18], or no AVE formation [19], [20], [21], have suggested that the AVE inhibits primitive streak formation in the anterior of the embryo. Analysis of Cerl−/−;Lefty1−/− compound mutants has shown that the AVE does this via the inhibition of Nodal signalling [22].
The AVE is thought to comprise multiple populations of cells expressing different molecular markers [4], [23]. The homeobox gene Hex is one of the earliest markers of the AVE [5] and the Hex-expressing cells of the AVE have been proposed to represent a different population of cells from those expressing Lefty1 or Cerl1 [23]. To date experiments carried out to address the role of the AVE have analysed the function of this tissue as a whole or the role of specific genes within the AVE but not the importance of specific sub-populations of AVE cells. To address what roles the Hex-AVE may have during A-P axis development we have knocked a Cre inducible diphtheria toxin A cassette (DTA) [24], [25] into the Hex locus. We demonstrate that in contrast to the reported roles of the AVE in repressing Nodal activity, Hex-expressing cells of the AVE are required at 6.5dpc to pattern the anterior primitive streak.
Methods
Mouse strains
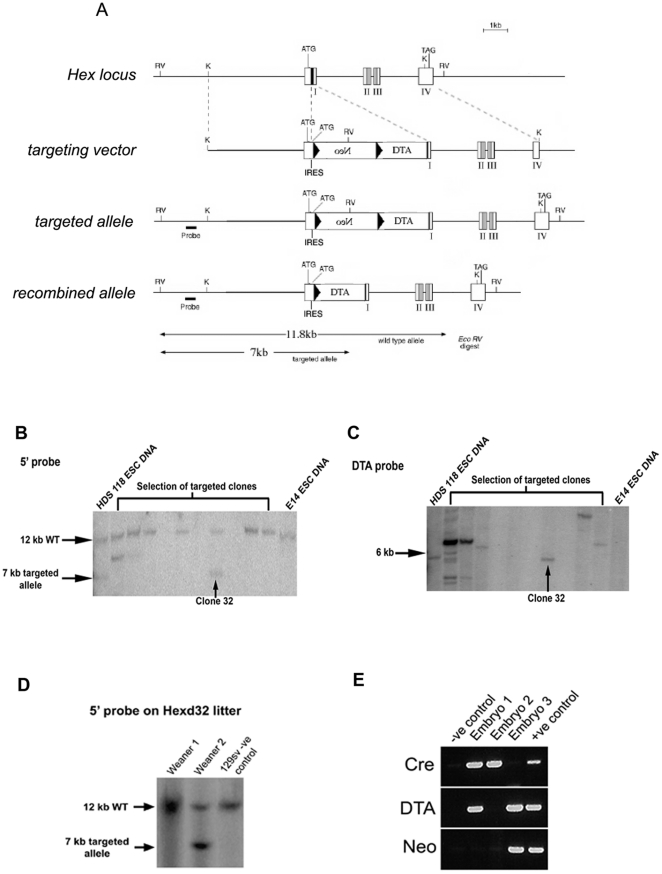
The LoxPneoSTOPRLoxP-DTA construct that has previously been used to ablate the roofplate [26], was introduced by gene targeting in ES cells into the Hex locus (Fig.1). Correctly targeted cells were used to generate Hexd mice and these were crossed to either β-actin-Cre [27] or Sox2Cre mice [28]. A small number of embryos resulting from the β-actin-Cre × Hexd cross escaped genetic ablation, were born, fertile and termed Hexdact/+. Genetic interaction experiments were performed using Nodal-LacZ mice [29]. Hex-GFP mice were used as a method of visualising Hex-expressing cells [30]. All mice were maintained and treated under the Home Office's animals (scientific procedures) Act 1986 under the Home Office approved Project Licence 70/5267.
Figure 1. Genetic ablation of the AVE.
(A) Strategy for knock-in of DTA into the Hex locus. (B) Identification of Hexd+/− ES clones using an external 5′ probe. (C) Identification of Hexd+/− ES clones using an internal DTA probe. (D) Identification of Hexd+/− mice using an external 5′ probe. (E) Identification of Hexdact/+ embryos by PCR.
Genotyping of mice and embryos
DNA for genotyping was prepared according to standard procedures [31], [32]. Mice and embryos from the β-actin-Cre × Hexd cross were genotyped using the following primers DTA: CGACAATAAATACGACGCTGCGGG and CATCGCATCTTGGCCACGTTTTCC; Cre: CCAGCTAAACATGCTTCATC and CGCTCGACCAGTTTAGTTAC; neomycin: CAAGATGGATTGCACGCAGG and CGGCAGGAGCAGGTGAGAT; from the Hexdact/+ x Hexdact/+ cross using the neomycin primers and primers for the wild-type allele CGGAGGCGAATCTGAAGCCAG and GCATACAGCGGGACTCCCACG; HexGFP mice using TGCAGTGCTTCAGCCGCTAC and CCAGCAGGACCATGTGATCG; and Nodal.LacZ mice using CGCCAGCTGGCGTAATAGCGAAG and GATGGGCGCATCGTAACCGTGCA.
Embryo culture
Embryos were cultured in 50 ng/ml BMP2 (R&D Systems) in pre-equilibrated drops. Each 80 µL drop comprised inhibitor diluted in DMEM:rat serum (1:1) covered with mineral oil. Embryos were incubated at 37°C, 5% CO2 overnight (typically 16 hours), then fixed in 4% paraformalde'hyde overnight at 4°C or stained for β-galactosidase activity.
Whole-mount in situ hybridization (WISH) β-galactosidase staining and confocal acquisition
WISH was carried out as previously described [5]. Staining for LacZ was carried out according to standard procedures [31]. For confocal analysis, embryos were stained with DAPI-Vectashield and TRITC-phalloidin mounting medium for 20 mins, mounted in 1∶1 Glycerol:PBS and analysed with a Leica DM IRB upright confocal microscope.
Results
Genetic ablation of the Hex-AVE between 5.5dpc and 6.5dpc
To genetically ablate the Hex-AVE we expressed diphtheria toxin subunit A (DTA) in the Hex-expressing cells of the AVE. This approach has been successfully used to ablate other cell populations within the embryo [26], [33]. We targeted the LoxPneoSTOPRLoxP-DTA construct, containing a Cre-inducible DTA, into the Hex locus to generate Hexd mice (Fig.1). Hexd mice were crossed to β-actin Cre mice to generate embryos with a DTA-expressing Hex allele, termed Hexdact/+. Analysis of this cross revealed that at weaning there were 52% fewer than expected Hexdact/+ mice. Given that close to mendelian numbers were observed at 9.5dpc, this data suggests that about half of the Hexdact/+ embryos died between this stage and weaning (Table S1). Those mutant animals that survived till weaning developed into apparently normal adults suggesting that the Hex locus carrying the DTA allele may be silenced during development in a proportion of embryos. When Hexdact/+ adult mice were crossed to wildtype mice we observed 53% fewer than expected mutant mice at weaning (Table S2), indicating the ablation system could be reactivated in the gametes of Hexdact/+ mice.
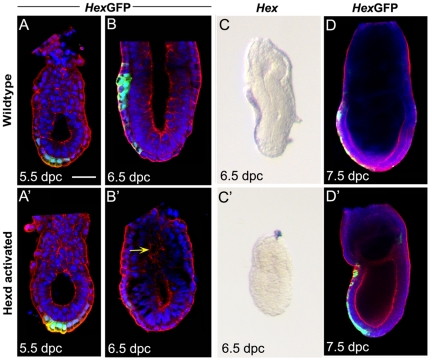
The Hex-GFP reporter line [30] provides a marker of Hex expressing cells that is independent of the Hex locus. We therefore analysed Hexdact/+ embryos carrying this reporter to address whether the Hex expressing cells were being ablated. Analysis of Hex-GFP expression at 5.5dpc revealed that the vast majority of Hexdact/+ embryos showed expression at this stage (n = 14/15; Fig.2A′). In contrast to this, at 6.5dpc 33% (n = 4/12) of Hexdact/+ embryos had lost Hex-GFP expression and presented a disrupted AVE (Fig.2B). These embryos also failed to show Hex transcript expression at this stage (Fig.2C). This data indicates that the Hex expressing cells of the AVE (Hex-AVE) had been ablated by 6.5dpc in one third of Hexdact/+ embryos. At 7.5dpc the vast majority of Hexdact/+ embryos were morphologically normal and all those analysed showed Hex-GFP expression in the definitive endoderm (Fig.2D) suggesting that those embryos with an ablated Hex-AVE were able to initiate gastrulation.
Figure 2. Ablation of the Hex-AVE in Hexdact/+ embryos.
(A–D) Hex-GFP and Hex expression in wild-type and Hexdact/+ embryos. (A–A′) At 5.5dpc n = 1/7 Hexdact/+ embryos have lost expression. Green arrow points to Hex-GFP domain of AVE; (B–B′) at 6.5dpc n = 4/12 have severely reduced or lost expression, yellow arrow indicating disorganised pro-amniotic cavity; (C–C′) Hex transcript is reduced or lost in n = 6/6 Hexdact/+ embryos at 6.5dpc, but (D) not affected at 7.5dpc in these embryos, n = 0/4. Scale bar 40 µm in A–A″; 60 µm in B; 90 µm in C and 100 µm in D. GFP (green) Nuclear stain (blue); F-actin (red).
A very small proportion of Hexdact/+ embryos (4.4%; n = 11/250) at 6.5dpc and 7.5dpc were retarded and displayed a small, disorganised egg cylinder. Marker analysis on these embryos is presented in Fig.S1 but for the sake of clarity these embryos are not considered any further in the text.
Ablation of Hex-expressing cells is accompanied by a reduction in other AVE markers
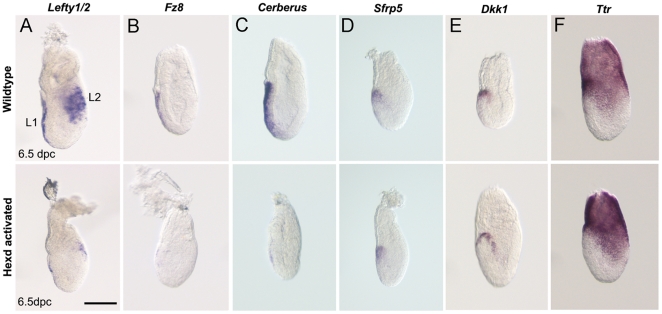
To further characterise the extent to which the AVE is affected in Hexdact/+ mutants, the expression of five AVE markers was analysed at 6.5dpc. WISH analysis revealed AVE patterning defects in 30% of Hexdact/+ embryos (n = 26/86), a similar proportion to the 33% of Hexdact/+ embryos that had lost Hex-GFP expression at 6.5dpc. These AVE patterning defects consisted of a vast reduction or total absence of Lefty1/2, Fz8 and Cerl1 transcript (Fig.3A–C) but little or no change in the expression of Sfrp5 and Dkk1-two markers of the anterior portion of the AVE (Fig.3D–E). Despite the AVE patterning defects caused by ablation of the Hex expressing cells, the rest of the visceral endoderm remained intact and showed no evidence of a loss of integrity, as confirmed by the normal expression of Ttr at 6.5dpc (Fig.3F). These results suggest that between 5.5dpc and 6.5dpc the Hex domain of expression in the AVE (Hex-AVE) significantly overlaps with that of Lefty1, Fz8 and Cerl and therefore is likely to mark a common population of cells. However, we cannot exclude that the loss of expression of Lefty1, Fz8 and Cerl in Hexdact/+ embryos is due to a non-cell autonomous effect caused by the loss of the Hex-AVE. The lack of overlap with the Sfrp5 and Dkk1 domains suggests that these genes mark a different subpopulation of AVE cells from the Hex expressing sub-population at these stages. Furthermore, the fact that expression of these genes is correctly positioned at the anterior of Hexdact/+ embryos indicates that correct placement of cells expressing these genes is independent of the Hex-AVE after 5.5dpc.
Figure 3. AVE defects in Hexdact/+ embryos.
(A) Lefty1/2, (B) Fz8, (C) Cerl, (D) Sfrp5, (E) Dkk1 and (F) Ttr expression in control and Hexdact/+ embryos at 6.5dpc. Hexdact/+ embryos with reduced or lost expression in the AVE = Lefty1/2 n = 13/28; Fz8 n = 4/13; Cerl, n = 7/16; Sfrp5, n = 2/14; Dkk1, n = 0/15; Ttr normal in n = 16/16; Lefty1/2 (L1 and L2) lost from the posterior epiblast in n = 7/28. Scale bar 90 µm.
The patterning of the primitive streak is impaired after Hex-AVE ablation
To explore the consequences of Hex-AVE ablation, the expression of primitive streak markers were analysed in Hexdact/+ embryos. At 6.5dpc Lefty2 and Eomes are normally expressed in the posterior epiblast, but the expression of both these genes were significantly reduced in Hexdact/+ embryos (Fig.3A and Fig.4A). In contrast, the expression of T and Cripto in the posterior epiblast was normal in Hexdact/+ embryos at this stage (Fig.4B–C). Together these data suggest that the onset of expression of some primitive streak markers is delayed when the AVE is ablated between 5.5-6.5dpc.
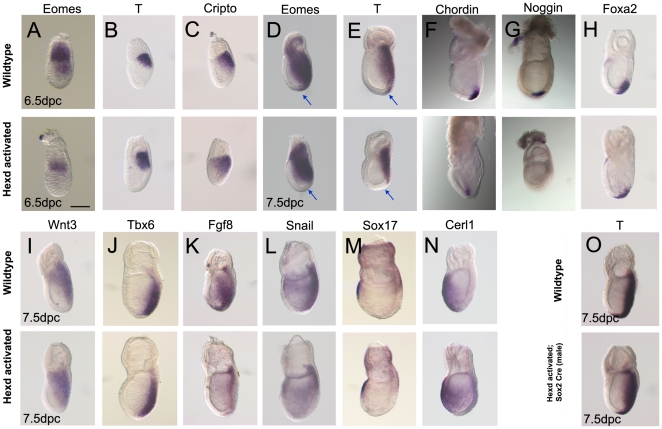
Figure 4. Defective primitive streak formation in Hexdact/+ mutants.
(A–C) Eomes, T and Cripto expression in wildtype and Hexdact/+ mutant embryos at 6.5dpc. (D–N) Expression of Eomes, T, Chordin, Noggin, Foxa2, Wnt3, Tbx6, Fgf8, Snail, Sox17 and Cerl in control and Hexdact/+ mutant embryos at 7.5dpc. Hexdact/+ embryos with normal expression: T (6.5dpc) n = 7/7; Cripto n = 9/9 Wnt3 n = 11/11; Tbx6 n = 13/13; Foxa2 n = 8/8; Cerl n = 14/14; Sox17 n = 14/14. Hexdact/+ embryos with reduced or lost expression Eomes (6.5dpc) n = 2/11; Eomes (7.5dpc) n = 3/18; T (7.5dpc) n = 3/14; Chordin n = 2/11; Noggin n = 6/15, Fgf8 n = 5/17; Snail n = 3/14. (O) T expression in control and (O′) Hexd+/−;Sox2Cre+/− embryos at 7.5dpc (n = 16/16). Blue arrow indicates the anterior primitive streak in D and E. Scale bar 90 µm.
During gastrulation epiblast cells traverse through the primitive streak and adopt a mesodermal or endodermal fate (reviewed in [6]). WISH analysis revealed that whilst Hexdact/+ embryos displayed robust expression of T and Eomes in the extraembryonic mesoderm and intermediate primitive streak, these mutants displayed a lack of expression in the mesodermal derivatives of the anterior primitive streak (Fig.4D–E). In addition to these patterning defects, Hexdact/+ mutant embryos also exhibited reduced expression of both Fgf8 and Snail in the anterior portion of the primitive streak (Fig.4K–L). We also observed a decrease or loss of expression of the anterior primitive streak markers Chordin and Noggin at 7.5dpc (Fig.4F–G), further suggesting that this region is affected in Hexdact/+ embryos. However, Foxa2 was expressed normally in these embryos, indicating that not all anterior primitive streak markers/derivatives are affected (Fig.4H). Normal expression of Wnt3 and Tbx6 suggested that posterior and intermediate primitive streak formed normally (Fig.4I–J). These results indicated that some mesoderm cells were unable to extend anteriorly or were incorrectly patterned in AVE ablated embryos. In accordance with these subtle defects a third of Hexdact embryos showed mild forebrain patterning defects at 9.5dpc (Fig.S2 and Table S3).
The observation that mesoderm markers are appropriately restricted to the posterior of the embryo indicates that the Hex-AVE is establishing the A–P axis in these embryos prior to its ablation. In total 24% (n = 24/100) of Hexdact/+ embryos showed defects in T, Eomes, Chrodin, Noggin, Fgf8 or Snail expression between 6.5–7.5dpc, a similar proportion to the 30% of Hexdact/+ embryos with a miss-patterned AVE at 6.5dpc. Therefore it is likely that the anterior primitive streak patterning defects were arising due to ablation of the Hex-AVE.
To rule out the possibility that patterning defects in the anterior primitive streak were not simply due to ablation of Hex-expressing cells of the anterior definitive endoderm (ADE), we analysed the expression of the definitive endoderm markers Sox17 and Cerl. The expression of neither of these markers was affected at 7.5dpc in Hexdact/+ embryos (Fig.4M–N). This, together with the normal Hex-GFP expression domain found in these embryos at this stage (Fig.2D), suggests that the ADE is not affected in mutant embryos. To confirm this observation, Hexd mice were crossed to male Sox2Cre mice, which activate DTA only in epiblast derivatives from 4.5dpc [28]. Unlike the observations in Hexdact/+ embryos, Hexd+/−;Sox2Cre+/− embryos showed no change in the expression of T at 7.5dpc (Fig.4O) indicating that the primitive streak defects of ablated mutants were a consequence of Hex-AVE ablation.
Ablation of the Hex-AVE leads to aberrant Nodal expression
The AVE and DVE secrete antagonists of Nodal signalling which restrict its activity to the posterior epiblast where it can induce the primitive streak (reviewed in [6]). However, Nodal signalling has also been implicated in the regulation of Eomes expression [34], which is defective in Hexdact/+ embryos. To test the effects of Hex-AVE ablation on Nodal signalling we analysed Nodal expression in Hexdact/+ embryos. At 6.5dpc, Nodal transcript is restricted to the posterior epiblast, but in 29% of the mutants Nodal expression was downregulated and found proximally with no posterior restriction (Fig.5A). This suggested that when the AVE is ablated between 5.5–6.5dpc, Nodal expression is not restricted to the posterior epiblast.
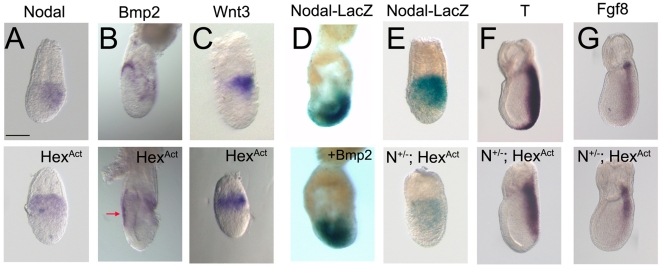
Figure 5. Nodal signalling is affected in Hexdact/+ embryos.
(A) Nodal expression is not restricted to the posterior in Hexdact/+ embryos at 6.5dpc (n = 8/28). (B) Bmp2 expression is expanded in the anterior visceral endoderm (red arrow) of Hexdact/+ embryos; n = 7/15. (C) Proximal expression of Wnt3 in Hexdact/+ embryos; n = 3/6. (D) Culture with BMP2 leads to ectopic NodalLacZ/+ expression (controls that are posterior n = 10/10; BMP2 treated posterior n = 1/8). (E) β-galactosidase expression in NodalLacZ/+and Hexdact/+;NodalLacZ/+ embryos at 6.5dpc (weak and proximal expression n = 6/8). (F–G) T and Fgf8 expression in control and Hexdact/+;NodalLacZ/+ embryos at 7.5dpc. Hexdact/+ embryos with reduced expression T n = 7/17 and Fgf8 n = 6/11. Scale bar 90 µm.
BMP4 has been shown to act at 6.5dpc in a positive regulation loop that amplifies Nodal signalling in the posterior epiblast via the activation of Wnt3 [35]. We have also shown that BMP signalling is required to sustain Nodal expression throughout the epiblast prior to gastrulation [36]. Bmp2 is expressed in the embryonic visceral endoderm at 5.0dpc [37] and in the AVE and posterior VE at 5.75dpc (Fig.S3), but at 6.5dpc is only observed in AVE cells that are adjacent to the embryonic-extraembryonic boundary (Fig.5B). Therefore Bmp2 expression is extinguished from the AVE as Nodal expression is restricted to the posterior, suggesting it may be cooperating with BMP4 in maintaining Nodal expression prior to 6.5dpc [36]. Analysis of Bmp2 expression in Hexdact/+ embryos shows that at 6.5dpc Bmp2 is expressed in all the anterior visceral endoderm and not just restricted to the anterior-most AVE as is seen in controls (Fig.5B and Fig.S3). Concomitant to Bmp2 miss-expression, we observe that Wnt3 expression, which is normally restricted to the posterior at this stage, is expressed throughout the proximal epiblast of Hexdact/+ embryos (Fig.5C). This indicates that the expression of a key element of the Nodal amplification loop is miss-localised at 6.5dpc.
To test whether BMP2 can sustain Nodal expression in a similar way to BMP4, we cultured 6.5dpc NodalLacZ/+ embryos overnight in the presence of BMP2 recombinant protein. Nodal-LacZ expression in these embryos was found throughout the epiblast, and not restricted to the posterior as occurs in controls (Fig.5D). This suggests that BMP2 can maintain Nodal expression in the anterior epiblast. Therefore the expression of Bmp2 throughout the anterior visceral endoderm at 6.5dpc in the Hex-AVE ablated embryos is one possible explanation for the miss-localised Nodal expression we observe in these embryos.
Hexdact/+ embryos display miss-patterning of the anterior primitive streak and these phenotypes are characteristic of decreased Nodal signalling [34], [38]. This, and the observation that we see miss-localisation of Nodal and Wnt3, led us to further investigate how Nodal signalling is affected in Hexdact/+/+ embryos. We reduced the levels of Nodal signalling by crossing Hexdact/+ and NodalLacZ/+ mice [29]. In NodalLacZ/+ embryos at 6.5dpc, β-galactosidase staining is posteriorly restricted and the A–P axis is correctly established (Fig.5E)[39]. In contrast to this, the majority of Hexdact/+; NodalLacZ/+ mutants examined at 6.5dpc displayed a clear reduction in β-galactosidase staining and its expression was observed throughout the proximal epiblast (75%; n = 6/8 Fig.5E). This indicates that in these embryos there is a clear reduction in Nodal signalling activity as well as an increase in the proportion of embryos showing no posterior restriction in Nodal expression when compared to Hexdact/+ mutants (75% of Hexdact/+; NodalLacZ/+embryos showed ectopic LacZ expression compared to 29% Hexdact/+ embryos that showed ectopic Nodal mRNA expression).
To confirm these observations we analysed T and Fgf8 expression in Hexdact/+; NodalLacZ/+ embryos at 7.5dpc. Hexdact/+ embryos on this genetic background displayed an absence of the anterior primitive streak domain of expression of T in 12.5% of cases analysed (n = 2/16) and of Fgf8 in 14% of cases (n = 1/7). In contrast to this 41% of Hexdact/+; NodalLacZ/+ mutants showed a lack of T expression in the anterior primitive streak (n = 7/17; Fig.5F) and 54% showed a decrease in Fgf8 expression in this domain (n = 6/11; Fig.5G). This indicates that reducing the levels of Nodal signalling increases the frequency of phenotypes observed in Hex-AVE-ablated embryos. It also suggests that in addition to the role of the AVE in restricting Nodal expression, Hex-AVE cells also assist in augmenting Nodal activity prior to primitive streak formation.
Discussion
The AVE is a signalling centre required for A–P axis specification (reviewed [3], [6]). To date the AVE has been shown to have roles in inhibiting Nodal signalling, promoting forebrain identity and inhibiting primitive streak formation. The results presented here indicate that the AVE has additional roles to these ones. We find that the Hex-AVE is required for the correct patterning of the anterior primitive streak. Therefore the AVE, in addition to patterning the anterior of the embryo, also patterns its posterior.
The AVE has been suggested to comprise multiple cell subpopulations [4], [23]. To address the role of the Hex-AVE we knocked the diphtheria toxin subunit A (DTA) into the Hex locus in a Cre inducible manner. DTA catalyses the inactivation of elongation factor 2, resulting in termination of protein synthesis and cell death at very low concentrations [25], [40], [41], [42], [43]. To monitor the time of loss of the Hex-AVE we used the Hex-GFP reporter line [30], which is independent of the Hex locus. We found that at 5.5dpc the vast majority of embryos showed Hex-GFP expression but that by 6.5dpc one third of embryos had lost this expression. This indicated that in these embryos the Hex-AVE was ablated between 5.5dpc and 6.5dpc. When we analysed how other AVE markers were being affected by ablation of the Hex-AVE we observed that the expression of Lefty1, Fz8 and Cerl were severely affected in 30% of Hexdact/+ embryos, suggesting that between 5.5dpc and 6.5dpc these genes are expressed in the Hex-AVE or that their expression is maintained by the Hex-AVE. In contrast to this we found no change in the expression of Sfrp5 and Dkk1 in Hexdact/+ embryos indicating that correct placement of cells expressing these genes is independent of the Hex-AVE after 5.5dpc. Together these observations provide strong support for the notion that the AVE is indeed composed of multiple cell subpopulations.
Our work has also shed light on what roles the AVE has on patterning the posterior of the embryo. Analysis of how ablation of the Hex-AVE impacted on gastrulation revealed that these cells were required to pattern the anterior primitive streak. This is indicated by the fact that 24% of Hexdact/+ embryos showed defects in T, Eomes, Chordin, Noggin, Fgf8 or Snail expression in the anterior primitive streak between 6.5–7.5dpc, a similar proportion to the 30% of Hexdact/+ embryos with a miss-patterned AVE at 6.5dpc. In contrast to this, forebrain specification occurred apparently normally in all Hexdact/+ embryos as suggested by the normal Otx2 expression at 7.5dpc (Fig.S3), indicating either that by 6.5dpc the Hex-AVE has already initiated anterior patterning in the epiblast or that other AVE subpopulations are able to perform these roles. At 9.5dpc a third of Hexdact/+ embryos showed mild forebrain patterning defects. These patterning defects are not as pronounced as those shown by zygotic mutations that affect genes required for AVE specification [44]. The mild forebrain defects observed in Hex-AVE ablated embryos could be due to either a role of the Hex-AVE in patterning the forebrain between 5.5 and 6.5dpc or a consequence of the subtle anterior primitive streak defects present in these embryos.
How do the primitive streak patterning defects we see in Hexdact/+ embryos arise? It has been shown that Wnt3 and Nodal act in an autoregulatory loop to amplify Nodal signalling in the posterior epiblast [35] and this is essential for patterning of the anterior primitive streak (reviewed [3], [6]). In this manuscript we show that in Hexdact/+ embryos Nodal and Wnt3 expression are miss-localised, suggesting that Nodal signalling is disrupted in these embryos. We also demonstrate that reducing Nodal levels in Hexdact/+ embryos increases the frequency of affected embryos indicating that Hex-AVE ablation is causing decreased Nodal signalling. A decrease in Nodal signalling has been shown to cause anterior primitive streak defects (reviewed in [6]). Also, mutation of Ectodermin, an intracellular inhibitor of Nodal signalling, causes an expansion of the anterior primitive streak [45]. Together, this suggests that a decrease in Nodal signalling is the most likely cause of the primitive streak defects we observe after ablation of the Hex-AVE.
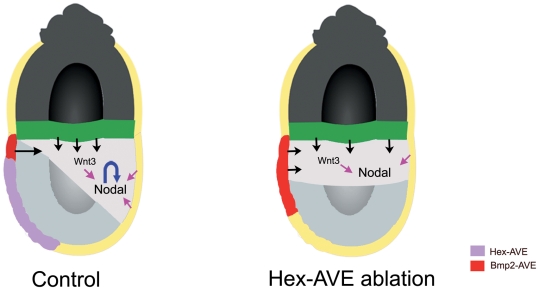
What could be causing the miss-localisation of Nodal and Wnt3 expression? We find that in Hexdact/+ embryos Bmp2 expression persists in the AVE at 6.5dpc and is not restricted to the proximal boundary as occurs in control embryos. There are two alternative explanations for this observation. Either the Hex-AVE is required to displace those cells expressing Bmp2 or the Hex-AVE is required for the downregulation of Bmp2 expression in the AVE. Previously we, and others, have shown that BMP signalling is required to maintain Nodal expression in the post-implantation epiblast [35], [36]. BMP4 secreted by the extra-embryonic ectoderm has been shown to be involved in this maintainance [35]. Here we show that culturing embryos in BMP2 leads to ectopic Nodal expression, suggesting that BMP2 can also maintain Nodal expression in the epiblast. Given that the ectopic domains of Nodal and Wnt3 expression in the epiblast of Hexdact/+ embryos are directly adjacent to the domain of the AVE where we observe ectopic Bmp2 expression, it is tempting to speculate that the ectopic Bmp2 expression is causing the ectopic Nodal expression in Hexdact/+ embryos (Figure 6). The failure to restrict Nodal expression to the posterior of the embryo is likely to lead to a lower threshold of Nodal signalling in this region of the embryo as the Nodal/Wnt3 amplification loop is disrupted, and this lower threshold of signalling will affect the specification of the anterior primitive streak derivatives that require the highest levels of signalling. In this way Nodal signalling is both upstream of the AVE, as it induces AVE gene expression [20], and downstream of this tissue, as some AVE genes, such as Bmp2, are required to maintain its expression. Ablation of Bmp2 specifically in the AVE would be required to confirm this hypothesis.
Figure 6. Model for how ablation of the Hex-AVE leads to proximal Wnt3 and Nodal expression.
Lack of the Hex-AVE leads to a failure to restrict Bmp2 expression to the proximal visceral endoderm and this leads to ectopic Wnt3 and Nodal expression in the anterior epiblast. This miss-localisation of Wnt3 and Nodal leads to the inability to correctly amplify Nodal in the posterior epiblast at 6.5dpc, causing defective specification of the anterior primitive streak.
In conclusion our experiments identify that the AVE is not only required for anterior patterning, but also that specific sub-populations of this tissue are required for the correct patterning of the posterior of the embryo.
Supporting Information
Marker analysis in severely affected Hexdact/+ embryos. (A-B) Hex-GFP and Cerl expression is lost and (C) Ttr expression expanded in the visceral endoderm of severely affected Hexdact/+ mutant embryos. (D) At 6.5dpc Eomes expression is lost from the epiblast and (E) Nodal expression is inappropriately expressed in the anterior epiblast in severely affected Hexdact/+ mutant embryos. (F) Wnt3 expression is down-regulated and restricted to the proximal epiblast and (G) T and (H) Foxa2 expression is lost in severely affected Hexdact/+ mutant embryos at 7.5dpc. (I) At 7.5dpc Oct4 is expressed in the epiblast of Hexdact/+ mutant embryos. Cerl, n = 1, Ttr, n = 2, Eomes n = 1, Nodal n = 1 Oct4 n = 1, Wnt3 n = 2, T n = 2, Foxa2 n = 2. Scale bar 60 µm in A-C; 70 µm in D-G.
(TIF)
Forebrain defects in Hexdact/+ embryos. (A) No change in Otx2 expression at 7.5dpc and (B-B′) reduced Six3 at 8.5dpc and (C) Foxg1 at 9.5dpc in Hexdact embryos. Arrow indicates the site of forebrain patterning defects.
(TIF)
Bmp2 expression in wild-type and Hexdact/+ embryos. (A) At 5.75dpc Bmp2 is expressed in the AVE and posterior VE. (B-C) Different views of two Hexdact/+ embryos showing ectopic Bmp2 expression at 6.5dpc.
(TIF)
Genotyping results of Hexd × β-actin Cre crosses at various embryonic stages and weaning age.
(PDF)
Genotyping results of Hexdact × +/+ crosses at various embryonic stages and weaning age.
(PDF)
Proportion of Hexdact embryos showing forebrain defects at 8.5dpc and 9.5dpc.
(PDF)
Acknowledgments
We thank Liz Robertson for making the NodalLacz mouse line available to us, and Jonathan Godwin for blastocyst injections. We also thank all members of the Molecular Embryology lab for critical discussion and technical help.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the Medical Research Council (http://www.mrc.ac.uk/index.htm) and the Lister Institute of Preventive Medicine (http://www.lister-institute.org.uk/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kimura C, Yoshinaga K, Tian E, Suzuki M, Aizawa S, et al. Visceral endoderm mediates forebrain development by suppressing posteriorizing signals. Dev Biol. 2000;225:304–321. doi: 10.1006/dbio.2000.9835. [DOI] [PubMed] [Google Scholar]
- 2.Rivera-Perez JA, Mager J, Magnuson T. Dynamic morphogenetic events characterize the mouse visceral endoderm. Dev Biol. 2003;261:470–487. doi: 10.1016/s0012-1606(03)00302-6. [DOI] [PubMed] [Google Scholar]
- 3.Srinivas S. The anterior visceral endoderm-turning heads. Genesis. 2006;44:565–572. doi: 10.1002/dvg.20249. [DOI] [PubMed] [Google Scholar]
- 4.Srinivas S, Rodriguez T, Clements M, Smith JC, Beddington RS. Active cell migration drives the unilateral movements of the anterior visceral endoderm. Development. 2004;131:1157–1164. doi: 10.1242/dev.01005. [DOI] [PubMed] [Google Scholar]
- 5.Thomas PQ, Brown A, Beddington RS. Hex: a homeobox gene revealing peri-implantation asymmetry in the mouse embryo and an early transient marker of endothelial cell precursors. Development. 1998;125:85–94. doi: 10.1242/dev.125.1.85. [DOI] [PubMed] [Google Scholar]
- 6.Arnold SJ, Robertson EJ. Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nat Rev Mol Cell Biol. 2009;10:91–103. doi: 10.1038/nrm2618. [DOI] [PubMed] [Google Scholar]
- 7.Beddington RS, Robertson EJ. Anterior patterning in mouse. Trends Genet. 1998;14:277–284. doi: 10.1016/s0168-9525(98)01499-1. [DOI] [PubMed] [Google Scholar]
- 8.Beddington RS, Robertson EJ. Axis development and early asymmetry in mammals. Cell. 1999;96:195–209. doi: 10.1016/s0092-8674(00)80560-7. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Barbera JP, Beddington RS. Getting your head around Hex and Hesx1: forebrain formation in mouse. Int J Dev Biol. 2001;45:327–336. [PubMed] [Google Scholar]
- 10.Thomas P, Beddington R. Anterior primitive endoderm may be responsible for patterning the anterior neural plate in the mouse embryo. Curr Biol. 1996;6:1487–1496. doi: 10.1016/s0960-9822(96)00753-1. [DOI] [PubMed] [Google Scholar]
- 11.Miura S, Mishina Y. The DVE changes distal epiblast fate from definitive endoderm to neurectoderm by antagonizing nodal signaling. Dev Dyn. 2007;236:1602–1610. doi: 10.1002/dvdy.21166. [DOI] [PubMed] [Google Scholar]
- 12.Varlet I, Collignon J, Robertson EJ. nodal expression in the primitive endoderm is required for specification of the anterior axis during mouse gastrulation. Development. 1997;124:1033–1044. doi: 10.1242/dev.124.5.1033. [DOI] [PubMed] [Google Scholar]
- 13.Shawlot W, Wakamiya M, Kwan KM, Kania A, Jessell TM, et al. Lim1 is required in both primitive streak-derived tissues and visceral endoderm for head formation in the mouse. Development. 1999;126:4925–4932. doi: 10.1242/dev.126.22.4925. [DOI] [PubMed] [Google Scholar]
- 14.Rhinn M, Dierich A, Shawlot W, Behringer RR, Le Meur M, et al. Sequential roles for Otx2 in visceral endoderm and neuroectoderm for forebrain and midbrain induction and specification. Development. 1998;125:845–856. doi: 10.1242/dev.125.5.845. [DOI] [PubMed] [Google Scholar]
- 15.Tam PP, Steiner KA. Anterior patterning by synergistic activity of the early gastrula organizer and the anterior germ layer tissues of the mouse embryo. Development. 1999;126:5171–5179. doi: 10.1242/dev.126.22.5171. [DOI] [PubMed] [Google Scholar]
- 16.Ding J, Yang L, Yan YT, Chen A, Desai N, et al. Cripto is required for correct orientation of the anterior-posterior axis in the mouse embryo. Nature. 1998;395:702–707. doi: 10.1038/27215. [DOI] [PubMed] [Google Scholar]
- 17.Acampora D, Avantaggiato V, Tuorto F, Briata P, Corte G, et al. Visceral endoderm-restricted translation of Otx1 mediates recovery of Otx2 requirements for specification of anterior neural plate and normal gastrulation. Development. 1998;125:5091–5104. doi: 10.1242/dev.125.24.5091. [DOI] [PubMed] [Google Scholar]
- 18.Perea-Gomez A, Lawson KA, Rhinn M, Zakin L, Brulet P, et al. Otx2 is required for visceral endoderm movement and for the restriction of posterior signals in the epiblast of the mouse embryo. Development. 2001;128:753–765. doi: 10.1242/dev.128.5.753. [DOI] [PubMed] [Google Scholar]
- 19.Perea-Gomez A, Shawlot W, Sasaki H, Behringer RR, Ang S. HNF3beta and Lim1 interact in the visceral endoderm to regulate primitive streak formation and anterior-posterior polarity in the mouse embryo. Development. 1999;126:4499–4511. doi: 10.1242/dev.126.20.4499. [DOI] [PubMed] [Google Scholar]
- 20.Brennan J, Lu CC, Norris DP, Rodriguez TA, Beddington RS, et al. Nodal signalling in the epiblast patterns the early mouse embryo. Nature. 2001;411:965–969. doi: 10.1038/35082103. [DOI] [PubMed] [Google Scholar]
- 21.Kimura C, Shen MM, Takeda N, Aizawa S, Matsuo I. Complementary functions of Otx2 and Cripto in initial patterning of mouse epiblast. Dev Biol. 2001;235:12–32. doi: 10.1006/dbio.2001.0289. [DOI] [PubMed] [Google Scholar]
- 22.Perea-Gomez A, Vella FD, Shawlot W, Oulad-Abdelghani M, Chazaud C, et al. Nodal antagonists in the anterior visceral endoderm prevent the formation of multiple primitive streaks. Dev Cell. 2002;3:745–756. doi: 10.1016/s1534-5807(02)00321-0. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto M, Saijoh Y, Perea-Gomez A, Shawlot W, Behringer RR, et al. Nodal antagonists regulate formation of the anteroposterior axis of the mouse embryo. Nature. 2004;428:387–392. doi: 10.1038/nature02418. [DOI] [PubMed] [Google Scholar]
- 24.Collier RJ. Diphtheria toxin: mode of action and structure. Bacteriol Rev. 1975;39:54–85. doi: 10.1128/br.39.1.54-85.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collier RJ. Understanding the mode of action of diphtheria toxin: a perspective on progress during the 20th century. Toxicon. 2001;39:1793–1803. doi: 10.1016/s0041-0101(01)00165-9. [DOI] [PubMed] [Google Scholar]
- 26.Lee KJ, Dietrich P, Jessell TM. Genetic ablation reveals that the roof plate is essential for dorsal interneuron specification. Nature. 2000;403:734–740. doi: 10.1038/35001507. [DOI] [PubMed] [Google Scholar]
- 27.Lewandoski M, Meyers EN, Martin GR. Analysis of Fgf8 gene function in vertebrate development. Cold Spring Harb Symp Quant Biol. 1997;62:159–168. [PubMed] [Google Scholar]
- 28.Hayashi S, Lewis P, Pevny L, McMahon AP. Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Mech Dev. 2002;119(Suppl 1):S97–S101. doi: 10.1016/s0925-4773(03)00099-6. [DOI] [PubMed] [Google Scholar]
- 29.Collignon J, Varlet I, Robertson EJ. Relationship between asymmetric nodal expression and the direction of embryonic turning. Nature. 1996;381:155–158. doi: 10.1038/381155a0. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez TA, Casey ES, Harland RM, Smith JC, Beddington RS. Distinct enhancer elements control Hex expression during gastrulation and early organogenesis. Dev Biol. 2001;234:304–316. doi: 10.1006/dbio.2001.0265. [DOI] [PubMed] [Google Scholar]
- 31.Nagy A, Gertsenstein M, Vintersten K, Behringer R. Cold Spring Harbor, New York: Cold Spring Harbor laboratory Press; 2003. Manipulating the Mouse Embryo.764 [Google Scholar]
- 32.Martinez-Barbera JP, Rodriguez TA, Beddington RS. The homeobox gene Hesx1 is required in the anterior neural ectoderm for normal forebrain formation. Dev Biol. 2000;223:422–430. doi: 10.1006/dbio.2000.9757. [DOI] [PubMed] [Google Scholar]
- 33.Ivanova A, Signore M, Caro N, Greene ND, Copp AJ, et al. In vivo genetic ablation by Cre-mediated expression of diphtheria toxin fragment A. Genesis. 2005;43:129–135. doi: 10.1002/gene.20162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnold SJ, Hofmann UK, Bikoff EK, Robertson EJ. Pivotal roles for eomesodermin during axis formation, epithelium-to-mesenchyme transition and endoderm specification in the mouse. Development. 2008;135:501–511. doi: 10.1242/dev.014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ben-Haim N, Lu C, Guzman-Ayala M, Pescatore L, Mesnard D, et al. The nodal precursor acting via activin receptors induces mesoderm by maintaining a source of its convertases and BMP4. Dev Cell. 2006;11:313–323. doi: 10.1016/j.devcel.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Di-Gregorio A, Sancho M, Stuckey DW, Crompton LA, Godwin J, et al. BMP signalling inhibits premature neural differentiation in the mouse embryo. Development. 2007;134:3359–3369. doi: 10.1242/dev.005967. [DOI] [PubMed] [Google Scholar]
- 37.Mesnard D, Guzman-Ayala M, Constam DB. Nodal specifies embryonic visceral endoderm and sustains pluripotent cells in the epiblast before overt axial patterning. Development. 2006;133:2497–2505. doi: 10.1242/dev.02413. [DOI] [PubMed] [Google Scholar]
- 38.Vincent SD, Dunn NR, Hayashi S, Norris DP, Robertson EJ. Cell fate decisions within the mouse organizer are governed by graded Nodal signals. Genes Dev. 2003;17:1646–1662. doi: 10.1101/gad.1100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norris DP, Brennan J, Bikoff EK, Robertson EJ. The Foxh1-dependent autoregulatory enhancer controls the level of Nodal signals in the mouse embryo. Development. 2002;129:3455–3468. doi: 10.1242/dev.129.14.3455. [DOI] [PubMed] [Google Scholar]
- 40.Breitman ML, Clapoff S, Rossant J, Tsui L-C, Glode M, et al. Genetic ablation: targeted expression of a toxin genes causes microphthalmia in transgenic mice. Science. 1987;238:1563–1565. doi: 10.1126/science.3685993. [DOI] [PubMed] [Google Scholar]
- 41.Maxwell IH, Maxwell F, Glode LM. Regulated expression of a diphtheria toxin A-chain gene transfected into human cells: possible strategy for inducing cancer cell suicide. Cancer Res. 1986;46:4660–4664. [PubMed] [Google Scholar]
- 42.Palmiter RD, Behringer RR, Quaife CJ, Maxwell F, Maxwell IH, et al. Cell lineage ablation in transgenic mice by cell-specific expression of a toxin gene. Cell. 1987;50:435–443. doi: 10.1016/0092-8674(87)90497-1. [DOI] [PubMed] [Google Scholar]
- 43.Harrison GS, Maxwell F, Long CJ, Rosen CA, Glode LM, et al. Activation of a diphtheria toxin A gene by expression of human immunodeficiency virus-1 Tat and Rev proteins in transfected cells. Hum Gene Ther. 1991;2:53–60. doi: 10.1089/hum.1991.2.1-53. [DOI] [PubMed] [Google Scholar]
- 44.Martinez Barbera JP, Clements M, Thomas P, Rodriguez T, Meloy D, et al. The homeobox gene Hex is required in definitive endodermal tissues for normal forebrain, liver and thyroid formation. Development. 2000;127:2433–2445. doi: 10.1242/dev.127.11.2433. [DOI] [PubMed] [Google Scholar]
- 45.Morsut L, Yan KP, Enzo E, Aragona M, Soligo SM, et al. Negative control of Smad activity by ectodermin/Tif1{gamma} patterns the mammalian embryo. Development. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Marker analysis in severely affected Hexdact/+ embryos. (A-B) Hex-GFP and Cerl expression is lost and (C) Ttr expression expanded in the visceral endoderm of severely affected Hexdact/+ mutant embryos. (D) At 6.5dpc Eomes expression is lost from the epiblast and (E) Nodal expression is inappropriately expressed in the anterior epiblast in severely affected Hexdact/+ mutant embryos. (F) Wnt3 expression is down-regulated and restricted to the proximal epiblast and (G) T and (H) Foxa2 expression is lost in severely affected Hexdact/+ mutant embryos at 7.5dpc. (I) At 7.5dpc Oct4 is expressed in the epiblast of Hexdact/+ mutant embryos. Cerl, n = 1, Ttr, n = 2, Eomes n = 1, Nodal n = 1 Oct4 n = 1, Wnt3 n = 2, T n = 2, Foxa2 n = 2. Scale bar 60 µm in A-C; 70 µm in D-G.
(TIF)
Forebrain defects in Hexdact/+ embryos. (A) No change in Otx2 expression at 7.5dpc and (B-B′) reduced Six3 at 8.5dpc and (C) Foxg1 at 9.5dpc in Hexdact embryos. Arrow indicates the site of forebrain patterning defects.
(TIF)
Bmp2 expression in wild-type and Hexdact/+ embryos. (A) At 5.75dpc Bmp2 is expressed in the AVE and posterior VE. (B-C) Different views of two Hexdact/+ embryos showing ectopic Bmp2 expression at 6.5dpc.
(TIF)
Genotyping results of Hexd × β-actin Cre crosses at various embryonic stages and weaning age.
(PDF)
Genotyping results of Hexdact × +/+ crosses at various embryonic stages and weaning age.
(PDF)
Proportion of Hexdact embryos showing forebrain defects at 8.5dpc and 9.5dpc.
(PDF)