Abstract
The teasel, Dipsacus fullonum is known to catch invertebrates in its water filled leaf bases, but experimental testing of reproductive benefits of this have been lacking. We report the effects of insect supplementation/removal and water removal during spring/summer on Dipsacus in two field populations. There were no significant treatment effects on biomass, but addition of dead dipteran larvae to leaf bases caused a 30% increase in seed set and the seed mass:biomass ratio. This study provides the first empirical evidence for reproductive benefit from carnivory in Dipsacus fullonum.
Introduction
The phenomenon of carnivory by plants has been recognised and studied since Charles Darwin [1], and is known to have evolved at least 6 times [2]. Its advantages to the plant are thought to involve the gain of significant amounts of nitrogen and phosphorus, explaining in part why the true carnivory is typically found in perennial plants of acid, nutrient-poor boggy soils [2], [3], [4]. However, intermediate states are known between normality and full carnivory, and most wild plants will increase their growth (hence, potentially, reproductive output) given the additional nitrogen and phosphorus from decaying animal remains. Here we report on evidence for reproductive benefits from carnivory in a plant showing none of the ecological or life history traits of standard carnivorous species.
The teasel, Dipsacus fullonum (Dipsacaceae) is a biennial herb, forming a rosette in its first year before growing quickly in the spring of its second year to a flowering height of between 0.5–2.5 m. [5]. Dipsacus has long been observed to catch insects and other invertebrates in their leaf basins which fill with rainwater, leading to speculation about it benefitting from carnivory [6], [7], [9], although textbooks on carnivorous plants have not considered Dipsacus [3], [8]. Dipsacus is unlike typical carnivorous plants in being associated with dry disturbed ground, often calcareous and nitrogen enriched - conditions lethal to true carnivorous plants [10]. It scores 7 on the Ellenberg scale for nitrogen [11], is biennial, and its size greatly exceeds most carnivorous plants. Here we report experimental tests of the evolutionary benefit of carnivory to Dipsacus, using total seedset as the best simple estimate of reproductive output of a biennial plant [12].
Methods
(1) Site description
Two teasel populations were used on separate spoil mounds, c. 10 m high, on Wimbledon Common, SW London, made from London clay mixed with some building waste, pH 8.0. The sites were labelled Site 1 (TQ2284073116 ) and Site 2 (TQ2284072823), c. 200 m apart. Field- grown Dipsacus were initially labelled, mapped and measured (number of leaves) in early 2009 while still at the rosette stage.
(2) Treatments
The design was a factorial combination of three different insect-supplement treatments (1: remove all dead insects, 2: leave all and add a maggot per clasping leaf base, 3: control) were crossed with two different water treatments (1: removal by puncturing leaf base, 2: control) equally at two sites, in a size-stratified design (based on rosette size overwinter, with treatments applied equally to the upper and lower halves of the size distribution), each with 3 replicates, giving a total sample size of 72 plants. Larvae of Calliphora vomitoria L. (maggots) were used as insect bodies; for the first 5 treatments the maggots were frozen (mean wet mass 0.024 g), followed by two additions of fresh maggots (mean wet mass 0.075 g), giving a season total of 0.27 g (fresh) insect bodies per leaf base or 1.08 g for a typical (2 stem-leaved) plant. Dry mass (105C) of maggots was found to be 0.307* fresh mass, hence a typical plant would receive 0.33 g dry mass supplementary insect food.
Treatments began 28th May 2009. Water removal and insect removal treatments took place once per week while maggot addition treatments took place every two weeks, reflecting the time for water/insects to build up and maggots to decompose respectively. Water was removed from the leaf basins by using a scalpel to make a small incision near the bottom of each basin and twisting the blade to allow water to drain out. One maggot was added into each leaf basin on the central stem, of which there were between 2–4 per plant depending on its size and age. Treatments continued until 20th August, by which time most of the plants' leaves were dry and could hold no water.
Plants were harvested in early September 2009 (above-ground only), taking care to conserve their seeds. Seeds were manually removed from dried heads, and their biomass recorded along with total above-ground plant biomass (dry: 105C).
(3) Statistical methods
Data were checked for normality, log-transformed where necessary, then analysed by ANOVA (for differences) and Pearson's correlation (for associations) using SPSS17. Variables that required log-transformation (biomass and seedmass) are presented after back-transformation of summary statistics.
Results
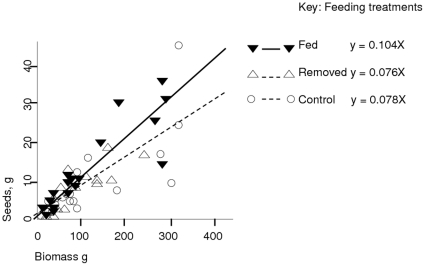
Raw data are supplied in the supplementary excel file Table S1. Throughout the growing season, Dipsacus were observed to collect dead insects in leaf bases (mainly coleoptera, hemiptera, lepidoptera and diptera). Experimentally applied maggots took 2–4 weeks to disappear, with decayed remains of the previous maggot usually visible when a new one was added. Data on above-ground biomass and seed production are summarised in Table 1, with anova results in Table 2 (suppressing the higher order interaction terms). Post-harvest, the biggest source of variation in both the seed mass and biomass was between sites, with plants from site 2 having over five times the seed and biomass as plants from site 1 (p<0.001; Table 1). This had been evident from their smaller over-wintering rosettes with fewer leaves (15.9 sd 3.2 against 27.9 sd 9.7; p<0.01). Number of overwintering leaves correlated well with eventual biomass (r = 0.84, p<0.01) and seed production (r = 0.66 p<0.01). The proportion of final biomass that was seeds did not alter with plant size or site. There were no significant treatment effects on biomass, nor any of cutting drainage holes in each leaf basin. By contrast the effect of the supplementary insect feeding treatment on seed production, and the seed mass:biomass ratio, were both significant (both p<0.05), with highest values in maggot-supplemented plants (Table 1, Figure 1).
Table 1. Effects of feeding treatments on biomass and seed production in teasels Dipsacus fullonum (mean+-sd ); data have been pooled over two populations and two water removal treatments, and (for biomass and seed mass) were log-transformed, averaged then back-transformed.
| Feeding treatment: | Site | ||||
| Insect fed | control | Insect removal | Site 1 | Site 2 | |
| Biomass (total), g | 57.9+−3.0 A | 46.8+−2.7 A | 55.8+−3.3 A | 22.4+-1.9a | 127.2+-2.0b |
| Seedmass, g | 5.6+−3.2B | 3.0+−3.8 A | 3.5+−3.8 AB | 1.8+-2.3a | 9.0+-3.33b |
| Seeds as % biomass | 10.2+−2.9 B | 7.8+−4.0 A | 7.3+−3.7 A | 8.7+-3.9a | 8.1+-3.6a |
Values followed by the same letter do not differ at p = 0.05 by Duncan's post-hoc test.
Table 2. F values from Anova tests on biomass and seed production in teasel Dipsacus fullonum: significant results are in bold.
| Factor | Df(all with 57 df for error) | Biomass (total) Log-transformed | SeedmassLog-transformed | Seed as % biomass |
| Site (S) | 1 | 274 ** | 55.2 ** | 0.8 |
| Sizeclass (C) | 1 | 74.6 ** | 16.4 ** | 0.4 |
| Insect addition/removal treatment (I) | 2 | 1.5 | 3.2 * | 4.0 * |
| Water removal (W) | 1 | 0.7 | 0.01 | 0.4 |
| S*C | 1 | 0.6 | 0.1 | 0.1 |
| S*I | 2 | 1.2 | 2.4 | 0.8 |
| S*W | 1 | 0.6 | 0.02 | 0.0 |
| C*I | 2 | 0.9 | 0.8 | 0.5 |
| C*W | 1 | 6.9 * | 4.3 * | 0.1 |
| I*W | 1 | 2.1 | 0.2 | 0.0 |
Abbreviations: * - p<0.05, ** - p<0.01.
Figure 1. A graph of seed mass against vegetative biomass for Dipsacus plants, showing feeding treatment.
Discussion
For a plant to be considered carnivorous, a key criterion is that experimental manipulations of its insect food supply can be shown to produce growth or developmental responses. Increases in size and biomass have been shown for Pinguicula, Drosera and Utricularia supplemented with appropriate animal bodies [10], [13]–[15], a result that was not duplicated here.
The dominant pattern in Dipsacus biomass was attributable to the natural variation between sites, initially manifest as differences in the sizes of over-wintering rosettes and probably explicable by unquantified differences in soil chemistry. The correlation between number of rosette leaves and final biomass was highly significant (r = 0.84, p<0.01), agreeing with the model that the plant's final size is largely determined by energy capture in the previous growing season [16]. Surprisingly, cutting each leaf base to prevent water buildup had no effect on any growth parameter. Insect nutrition had no detectable effect on biomass, either in absolute terms or as deviation from size predicted from its over-wintering rosette. However, the seed production and the seed mass:biomass ratio differed between insect-feeding treatments increasing as expected if insects were supplying mineral nutrition (Table 1). Similarly, Thum [15] listed increased seedset along with other indices of overall size when Drosera were fed supplemental flies (although based on a pseudo-replicated design). Wakefield [17] found that supplemental feeding of Sarracenia with flies did not increase size or biomass but did increase their nutrient content: in this case seed set was not quantified. Meyers [18] reported Utricularia's growth is reduced by half in the absence of prey, but again seedset was not quantified.
These data allow an estimate of nitrogen fluxes. The total animal biomass added over the season was 0.33 g dry mass per plant; assuming diptera have a mean nitrogen content at 10% [19] this equates to approximately 33 mg nitrogen as animal tissue added to a plant over the season. It has not been possible to find a published value for the nitrogen content of Dipsacus seeds, but Mattson [20] gives a range of 1–6% for seeds in general with lower values for non-legumes. Even assuming a low value of 15 mg/g nitrogen in seeds, the supplemental feeding supplied enough nitrogen for less than 2.5 g of seeds, while the regression lines predicted a difference between fed and control plants' seedset of approximately 7 g for a 300 g plant (Figure 1), implying that the apparent seed gain involved more nitrogen than was added in food. This may be from some other nutrient being limiting, or a statistical artefact; either way the result needs duplicating.
These results provide the first empirical evidence for Dipsacus displaying one of the principal criteria for carnivory given by Juniper et al (3); the use of products absorbed from prey to increase fitness. The result needs to be duplicated, and there remain other criteria of carnivory still to be demonstrated in Dipsacus; does it actively attract insects to its basins, how are insects digested / broken down, and are there any specialist structures such as waxy scales which cause insects to slip?
Supporting Information
Raw data on biomass and seed mass described in the text, with metadata defining each plant's location, feeding treatment and watering treatment.
(XLS)
Acknowledgments
Thanks are due to the Wimbledon Common rangers for permission to work on the common, Amanda Morgan for technical help, and anonymous referees for constructive feedback.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The authors have no support or funding to report.
References
- 1.Darwin C. London: John Murray; 1875. The carnivorous plants.462 [Google Scholar]
- 2.Juniper BE, Robins RJ, Joel DM. London: Academic Press Ltd; 1989. The Carnivorous Plants (1st edition).353 [Google Scholar]
- 3.Ellison AM, Gotelli EJ. Evolutionary ecology of carnivorous plants. Trends Ecol Evol. 2001;16:623–629. [Google Scholar]
- 4.Werner PA. The biology of Canadian weeds 12. Dipsacus sylvestris Huds. Can J Plant Sci. 1975;55:783–794. [Google Scholar]
- 5.Lloyd FE. NY: Chronica bot. 9. Ronald press; 1942. The carnivorous plants. [Google Scholar]
- 6.Darwin F. On the protrusion of protoplasmic filaments from the glandular hairs on the leaves of the common teasel (Dipsacus sylvestris). . Proc Roy Soc Lond. 1877;26:245–271. [Google Scholar]
- 7.Christy M. The common teasel as a carnivorous plant. J Bot. 1923;61:33–45. [Google Scholar]
- 8.Slack AAP. London: The Carnivorous Plants, Ebury Press; 1979. 240 [Google Scholar]
- 9.Simons P. How exclusive are carnivorous plants? Carnivorous Plants Newsletter. 1981;10:65–68. [Google Scholar]
- 10.Adamec L. Mineral nutrition of carnivorous plants: a review. Bot Rev. 1997;63:273–299. [Google Scholar]
- 11.ECOFACT. ITE: Monks Wood; 1999. Research report volume 2: Technical annex – Ellenberg's indicator values for British plants. [Google Scholar]
- 12.Primack RB, Kang H. Measuring fitness and natural selection in wild plant populations. Ann rev ecol system. 1989;20:367–396. [Google Scholar]
- 13.Hanslin HM, Karlsson PS. Nitrogen uptake from prey and substrate as affected by prey capture level and plant reproductive status in four carnivorous species. Oecol. 1996;106:370–375. doi: 10.1007/BF00334564. [DOI] [PubMed] [Google Scholar]
- 14.Chandler GE, Anderson JW. Studies on the mutualism and growth of Drosera species with reference to the carnivorous habit, New Phytol. 1976;76:129–141. [Google Scholar]
- 15.Thum M. The significance of carnivory for Drosera in its natural habitat. I. The reactions of Drosera intermedia and Drosera rotundifolia to supplementary feeding. Oecol. 1989;81:401–411. doi: 10.1007/BF00376954. [DOI] [PubMed] [Google Scholar]
- 16.Werner PA. Predictions of fate from rosette size in teasel (Dipsacus fullonum). Oecol. 1975;20:197–201. doi: 10.1007/BF00347472. [DOI] [PubMed] [Google Scholar]
- 17.Wakefield AE, Gotelli NJ, Wittman NE, Ellison AM. Prey addition alters nutrient stoichiometry of the carnivorous plant Sarracenia purpurea. Ecol. 2005;86:1737–1743. [Google Scholar]
- 18.Meyers DG. Darwin's investigations of aquatic carnivorous plants of the genus Utricularia. Misconception, contribution and authority. Proc acad Nat Sci Philadel. 1982;134:1–11. [Google Scholar]
- 19.Fagan WF, Siemann E, Mitter C, Denno RF, Huberty AF, et al. Nitrogen in Insects: Implications for Trophic Complexity and Species Diversification. Am Nat. 2002;160:784–802. doi: 10.1086/343879. [DOI] [PubMed] [Google Scholar]
- 20.Mattson WJ. Herbivory in relation to nitrogen content. Ann Rev Ecol Syst. 1980;11:119–161. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Raw data on biomass and seed mass described in the text, with metadata defining each plant's location, feeding treatment and watering treatment.
(XLS)