We tested whether impaired systemic immunoregulation and hyperactive immune responses are associated with an immune reconstitution inflammatory syndrome, CMV IRU. We found instead that T-regs in CMV IRU patients are functionally intact, while virus-specific immune responses and Th17 cells are compromised
Abstract
Background. The immune reconstitution inflammatory syndromes (IRIS) are a spectrum of inflammatory conditions associated with opportunistic infections and occurring in ∼16% of human immunodeficiency type 1 (HIV-1)–infected patients given antiretroviral therapy. It has been proposed that these conditions are linked by a dysregulated immune system that is prone to exaggerated responses. However, immunologic studies have been limited by the availability of longitudinal samples from patients with IRIS and appropriate matched control subjects. Cytomegalovirus (CMV) immune recovery uveitis (IRU) is an IRIS occurring in up to 38% of patients with CMV retinitis. Although the pathologic immune responses occur in the eye, immune dysregulation that allows for development of pathologic responses is presumably caused by faulty systemic immune cell reconstitution.
Methods. We examined CMV-specific T cell responses, regulatory T (Treg) cell function and polyclonal T cell responses, including IL-17 production, in 25 patients with CMV IRU and 49 immunorestored control subjects with CMV retinitis who did not develop IRU.
Results. Patients with CMV IRU had poor CMV-specific CD4+ T cell responses, as compared with control subjects, whereas CD8+ T cell responses were comparable. Patients with CMV IRU were characterized by smaller numbers of circulating Th17 cells. Deficiency in anti-CMV responses was not associated with differences in Treg cell function.
Conclusions. The Treg cell compartment is intact in patients with CMV IRU, and these patients do not develop exaggerated systemic CMV-specific or polyclonal immune responses. Cases are instead characterized by more profound depletion of Th17 cells and poor antiviral immune responses. CMV IRU may be most likely to develop in persons experiencing the greatest degree of immune dysfunction before initiating highly active antiretroviral therapy.
A subset of patients with AIDS who are immunorestored with highly active antiretroviral treatment (HAART) develop inflammatory conditions known collectively as immune reconstitution inflammatory syndromes (IRIS [1]). Patients most likely to be affected are those initiating HAART with low CD4 cell counts and pre-existing opportunistic infections [1, 2]. The symptoms of IRIS are often localized to sites of previous infection [3], and IRIS in patients with a history of cytomegalovirus retinitis (CMVR) can manifest as inflammation of the posterior uveal tract of the eye [4]. These facts have led to the suggestion that IRIS is caused by adaptive immune responses to persistent pathogen-derived antigens [2].
More recently, understanding of the importance of regulatory T (Treg) cells in controlling immune responses to self antigens has prompted the suggestion that failure to reconstitute Treg cells may predispose to IRIS [5]. We hypothesized that CMV-specific cytotoxic T lymphocytes are hyperactive in patients with CMV IRU because of deficiency in the number or function of Treg cells and that evidence of this immune perturbation, caused by the immune restoration process, would be observed in circulating T cells. To test this hypothesis, we studied Treg cell control over T cell responses in peripheral blood mononuclear cells (PBMCs) from patients with CMVR in a multicenter observational study who did or did not develop IRU.
MATERIAL AND METHODS
Participants
Participants gave written informed consent. Guidelines and standards of the US Department of Health and Human Services and of University of California, San Francisco, were followed. The institutional review board of the University of California, San Francisco, approved the study. Procedures followed were in accordance with the Helsinki Declaration as revised in 1983.
Patients were immunorestored persons with a documented history of CMVR who were enrolled in the Longitudinal Study of Ocular Complications of AIDS (LSOCA) and had a PMBC specimen available at a time point when active uveitis was diagnosed by a study ophthalmologist (based on slit-lamp and dilated funduscopic examination revealing evidence of intraocular inflammation). For individuals with newly diagnosed IRU, the index visit chosen for study was that when IRU was first diagnosed, if PBMCs were available, or the next subsequent visit when PMBCs were available. For patients with evidence of prior IRU (eg, macular edema), the index visit chosen was the first subsequent one at which there was active uveitis and PMBCs were available. For patients with a newly diagnosed case of CMV IRU, a pre-index visit was defined as the study visit occurring 3 months before the index visit. In all pre-index visits, concurrent ophthalmologic examination revealed no evidence of active uveitis or macular edema from prior uveitis.
Control subjects were chosen among participants with a documented history of CMVR who never received a diagnosis of IRU, were immunerestored by HAART, and had PBMC specimens available in the LSOCA repository from the same visit as the patient (±1 visit). For each patient, 2 control subjects were selected, matched to patients by absolute CD4+ T cell count at the time of the index visit, the time interval since CMVR diagnosis, and duration of LSOCA follow-up.
Samples
Cryopreserved PBMCs were obtained from the LSOCA repository (Thermo Electron, Rockville, MD). Samples with <50% viability or <10% recovery were discarded.
Phenotypic Analysis
The frequency of Treg cells was determined by a previously described phenotypic flow assay [6]. The absolute number of Treg cells was calculated by multiplying the frequency among CD4+ T cells by the absolute CD4 cell count determined as part of the LSOCA protocol. FoxP3 is a transcription factor expressed by natural Treg cells and required for their development [7].
Cytokine Flow Cytometry (CFC)
The fraction of CD4+ and CD8+ T cells responsive to CMV pp65 or IE-1 was determined by CFC, as described elsewhere [8]. CD107a is a marker mobilized to the cell surface on degranulation of T cells [9].
Treg Functional Assays
Whenever available, PBMC specimens from the 2 subsequent LSOCA visits after the index visit for each patient and control subject were used in flow-sorting experiments to test Treg cell function in suppressing proliferation by other T cells. Specimens were stained with aqua amine-reactive dye and antibodies against CD3, CD4, CD25 (IL-2 receptor alpha chain, expressed by Treg cells [10]), and CD127 (IL-7 receptor alpha chain, not present on Treg cells [6]). CD3+CD4+CD25+CD127low cells were separated from other cells with use of a FACS ARIA sorter. Aliquots that were depleted by flow sorting and control aliquots not depleted (but run through the sorter, to control for physical effects) were divided into microtiter wells. In additional wells, sort-purified Treg cells were added back to Treg-depleted cells to determine whether Treg cells measured in our phenotypic assays suppressed proliferation and were functional. The cells were labeled with carboxyfluorescein diacetate succinimidyl ester and then stimulated with CMV pp65/IE peptides or surface-bound anti-CD3 antibodies for 5 days.
Statistical Analysis
Plotting and statistical analysis were performed using Stata/IC, version 10.1 (Stata). Conditional logistic regression was used to test for differences between the CMV IRU and control groups that required case-control matching.
RESULTS
Patient Characteristics
Twenty-five LSOCA participants met case criteria, 18 of whom had newly diagnosed CMV IRU (all had a PBMC specimen available from the visit when IRU was initially diagnosed). The index visit was an active recurrence of uveitis for the other 7 patients. Two matched control subjects were found for 24 of the patients, with 1 control subject for the remaining patient.
Summary statistics for our case and control groups demonstrate successful matching (Table 1). The median interval between CMVR diagnosis and CMV IRU diagnosis (ie, index visit) was 47.5 months (range, 3–128 months) for those with newly diagnosed CMV IRU. The median interval between CMVR diagnosis and the index visit for those with recurrent uveitis was 112 months (range, 44–180 months). The interval between CMVR diagnosis and the index visit for control subjects was within 20% of the matching case interval for 39 of the 49 control specimens. The historical nadir absolute CD4 cell count documented before enrollment in LSOCA was 47 cells/μL for control subjects and 18 cells/μL for patients (P = .33, by Wilcoxon rank-sum test).
Table 1.
Subject Participant Characteristics (n = 74)
| Control subjects (n = 49) | Patients with CMV IRU (n = 25) | P values | |||
| Characteristic | Mean | Mean | |||
| Number | Percent | Number | Percent | ||
| Age at index visita | Mean: 45.9 | Mean: 41.5 | 0.15 | ||
| <40 years | 10 | 20.4 | 9 | 36.0 | |
| 40–43.99 years | 10 | 20.4 | 8 | 32.0 | |
| 44–48.99 years | 14 | 28.6 | 5 | 20.0 | |
| ≥49 years | 15 | 30.6 | 3 | 12.0 | |
| Race/ethnicity | 0.49 | ||||
| White | 25 | 51.0 | 10 | 40.0 | |
| Black | 13 | 26.5 | 9 | 36.0 | |
| Hispanic | 10 | 20.4 | 5 | 20.0 | |
| American Indian | 0 | 0 | 1 | 4.0 | |
| Other | 1 | 2.0 | 0 | 0 | |
| Sex | 0.19 | ||||
| Male | 43 | 87.8 | 19 | 76.0 | |
| Female | 6 | 12.2 | 6 | 24.0 | |
| Prior Cidofovir prescription | |||||
| Yes | 0 | 0 | 5 | 20.0 | |
| No | 0 | 0 | 12 | 48.0 | |
| Unknown | 49 | 100 | 8 | 32.0 | |
| CD4 cell count,acells/μL | Mean: 412 | Mean: 410 | 0.86 | ||
| <250 | 12 | 24.5 | 7 | 28.0 | |
| 250–355 | 11 | 22.4 | 7 | 28.0 | |
| 356–502 | 14 | 28.6 | 5 | 20.0 | |
| >502 | 12 | 24.5 | 6 | 24.0 | |
| CD4 cell count, nadir cells/μL | Mean: 47 | Mean: 18 | 0.33 | ||
| Viral load,a logcopies/mL | Mean: 2.6 | Mean: 2.8 | 0.52 | ||
| <1.7 | 19 | 38.8 | 6 | 24.0 | |
| 1.7–2.699 | 14 | 28.6 | 10 | 40.0 | |
| 2.7–3.3 | 3 | 6.1 | 1 | 4.0 | |
| >3.3 | 10 | 20.4 | 7 | 28.0 | |
a Measured at the index visit.
Patients Developing CMV IRU Have Weak Antiviral CD4+ T Cell Responses at Diagnosis
We initially assessed T cell responses in patients and control subjects at the index visit, when patients had active uveitis. We hypothesized that differences observed at this time point could suggest whether systemic T cell responses were related to the underlying disease immunopathology. Sixteen case-control sets had evaluable samples from a patient and at least 1 control subject at this time point.
T cells were evaluated for expression of IFN-γ, TNF-α, or IL-2 and for mobilization of CD107a after stimulation with CMV peptide pools. Using a Boolean gating strategy, we obtained data for 15 possible combinations of cytokine expression and CD107a mobilization for both CD4+ and CD8+ T cells. Cells expressing 6 of these combinations were extraordinarily rare in the entire dataset and were not analyzed further (typically <.01%; IL-2 only, IFN-γ+TNF-α–IL-2+CD107a–, IFN-γ–TNF-α–IL-2+CD107a+, IFN-γ+TNF-α–IL-2–CD107a+, IFN-γ+TNF-α–IL-2+CD107a+, and IFN-γ–TNF-α+IL-2+CD107a+.)
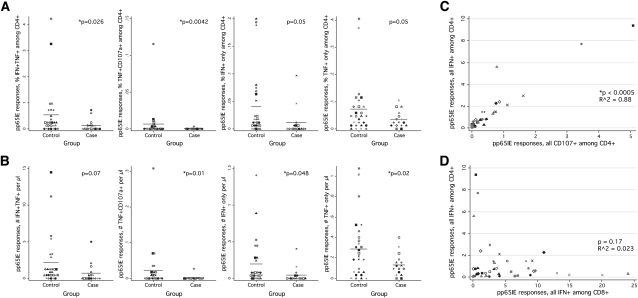
Analysis of the 9 remaining CD4+ T cell populations demonstrated consistently lower responses in the CMV IRU group. The mean sum of these responses, for example, was 1.62% among control subjects and .74% among patients (P = .11 by conditional logistic regression; data not shown); the mean sum of mono- and bifunctional responses was .74% among control subjects and .28% among patients (P = .03; data not shown). As assessed by IFN-γ and TNF-α production in bifunctional cells, pp65/IE-specific CD4+ T cell responses were significantly lower in patients with CMV IRU than in control subjects (P = .026) (Figure 1A, leftmost panel) this was true whether responses were assessed as a fraction of CD4+ T cells (Figure 1A) or as an absolute number of circulating cells (Figure 1B). The difference observed was not restricted to IFN-γ and TNF-α production; statistically significant differences were also seen in the fraction and/or number of cells producing TNF-α and CD107a, IFN-γ alone, or TNF-α alone (Figures 1A and 1B, second to fourth panels).
Figure 1.
Cytomegalovirus (CMV)–specific T cell responses among patients and control subjects at the index visit. A, The fraction of pp65/IE-specific CD4+ T cells among patients with CMV immune recovery uveitis (IRU) or control subjects as assessed by cytokine flow cytometry (CFC) of cell aliquots from the index visit. The populations shown did not express other cytokines; for example, IFN+TNF+ cells plotted in the figure did not express IL-2 or mobilize CD107a. Only case-control sets having at least 1 patient and 1 control subject are plotted, and only these sets were analyzed statistically. The various symbols shown in each dotplot are indicative of matched sets; for example, values for Patient 1 and the associated control subject(s) are shown with a black circle. The P values shown were calculated using Stata for a conditional logistic regression of CMV IRU status against the CFC response variable shown on the Y axis, using the case-control set number as the grouping variable. B, The absolute number of cells producing the indicated cytokines in response to pp65/IE stimulation, in cells per microliter. C, Relationship between 2 different measures of the CD4+ T cell response to virus, IFN-γ production and CD107a mobilization. The symbols used for plotting are identical to those used in A and B (ie, all samples from a specific case-control set are plotted with a shared symbol). D, Relationship between a measure of the CD4+ T cell response to virus and the CD8+ T cell response.
We tested 2-variable conditional logistic models to determine if production of different cytokine combinations by CD4+ T cells might have independent associations with CMV IRU disease. We found that these models were not superior to single-variable models, most likely because of the small number of evaluable case-control sets (n = 16) and the near colinearity of CD4+ T cell responses determined using different cytokine combinations (Figure 1C).
In contrast to CD4+ T cells, no statistically significant difference was observed between patients and control subjects for any CD8+ T cell population. We found this result to be surprising, both in view of data collected by others using a vitreal sample [11] and because of our expectation that CD4+ and CD8+ T cell responses to CMV would be correlated with each other. In fact, CD4+ and CD8+ T cell responses to CMV antigen had no statistically significant association in our dataset (Figure 1D). Furthermore, combinations of CMV-specific CD4+ and CD8+ T cell cytokine expression patterns did not discriminate between patients and control subjects (using cutoff values; data not shown).
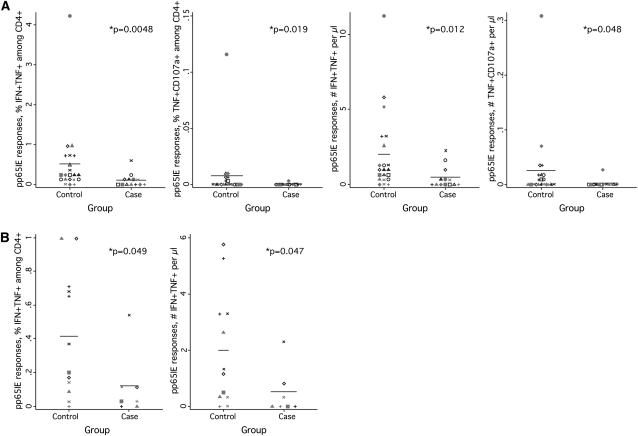
Finally, because prior exposure to cidofovir, a drug used to treat active CMVR, can cause chronic uveitis as an adverse drug effect, we analyzed our dataset when restricted in either of 2 ways (Figure 2): (1) by eliminating 3 patients known to have received cidofovir in the past, leaving 13 case-control sets, or (2) by limiting the data to 7 patients known for certain to have never received the drug. With use of either restriction, differences between case and control groups remained significant (Figures 2A and 2B).
Figure 2.
Cytomegalovirus (CMV)–specific T cell responses in patients and control subjects not exposed to cidofovir. A, Dotplots for patients who either did not receive cidofovir or were not known to have received it (n = 13) and their respective control subjects. From left to right, the dotplots show the fraction of cells making IFN-γ and TNF-α, the fraction making TNF-α and mobilizing CD107a, the number making IFN-γ and TNF-α, and the number making TNF-α and mobilizing CD107a. B, Dotplots for patients known not to have received cidofovir (n = 7). The left plot shows the fraction of cells making IFN-γ and TNF-α; the right plot shows the absolute number of such cells.
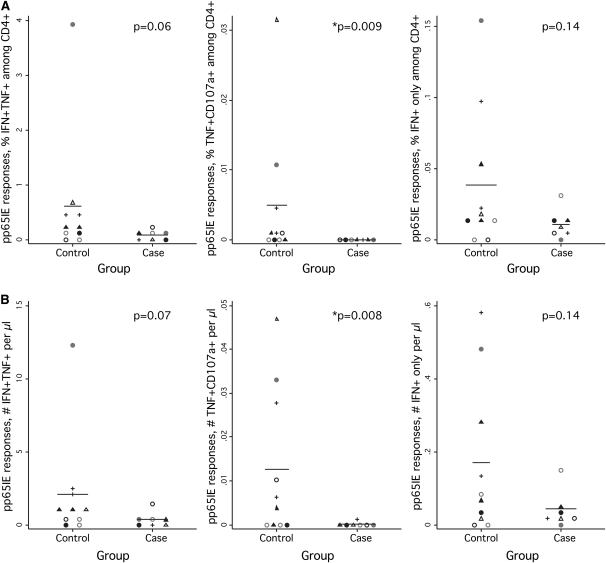
Immune Response Patterns Predictive of CMV IRU before Diagnosis
Our study was also designed to assess immune responses before IRU diagnosis, with the goal of identifying immune response patterns that might be predictive of disease development. Matched samples from only 7 case-control sets were evaluable in this analysis. Nevertheless, we again observed a pattern of lower CD4+ T cell responses in patients with CMV IRU, compared with control subjects. For example, patients with CMV IRU had a significantly lower fraction and absolute number of cells synthesizing TNF-α and mobilizing CD107a, compared with control subjects who did not develop the condition (P < .01 in both cases) (Figures 3A and 3B, middle panels). IFN-γ responses were also lower in patients with CMV IRU, although these differences did not reach statistical significance (Figures 3A and 3B, left and right panels).
Figure 3.
Cytomegalovirus (CMV)–specific T cell responses in patients and control subjects at the pre-index visit. A, The dotplots show the fraction of pp65/IE-specific CD4+ T cells among patients with CMV immune recovery uveitis (IRU) or control subjects, as assessed by cytokine flow cytometry (CFC) of cell aliquots from the pre-index visit. Symbols and statistics are as described for Figure 1. B, Dotplots showing the absolute number of cells producing the indicated cytokines in response to pp65/IE stimulation, in cells per microliter.
As was true of samples collected at the index visit, no differences in CD8+ T cell responses were observed between patients and control subjects (not shown). Elimination of case-control sets with patients exposed to cidofovir did not change the conclusions drawn: P values for the CMV-specific cell populations shown in Figure 3A were .06, .02, and .04, respectively.
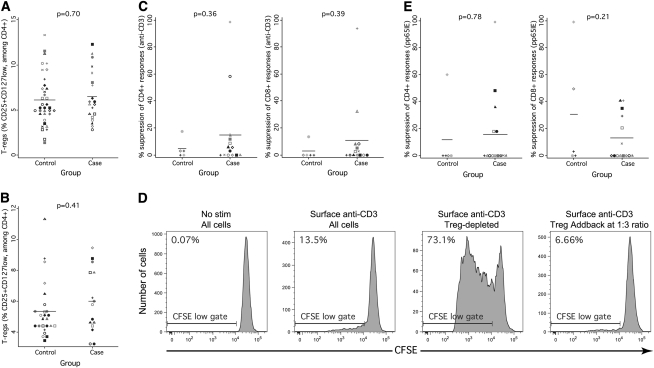
Treg Control Over CMV-Specific and –Nonspecific T Cells in Patients with CMV IRU
Although we had initially speculated that patients with CMV IRU might have fewer or less-functional Treg cells than control subjects, the data collected using CFC assays (Figures 1– 3) suggested the alternative possibility—that patients with CMV IRU might have excessive numbers or functional activity of Treg cells .
We evaluated numbers of Treg cells at the index and pre-index visits either as a fraction of CD4+ T cells or as an absolute number. Three Treg cell phenotypes were assessed (CD25+CD127low, CD25+FOXP3+, and CD25+CD127lowFoxP3+). Data for the first phenotype are shown in Figure 4A (at the index visit) and Figure 4B (at the pre-index visit) and show no difference between patients with CMV IRU and control subjects. Data collected using the 3 different phenotypes were highly intercorrelated and yielded qualitatively identical results (ie, no difference between patients and control subjects). Assessment of the data in terms of absolute circulating cell numbers also showed no differences.
Figure 4.
Regulatory T cell number and function in patients with cytomegalovirus (CMV) immune recovery uveitis (IRU) and control subjects. A, The fraction of CD3+CD4+CD25+CD127low regulatory T (Treg) cells present in patients and control subjects at the index visit. Symbols and statistics are as described for Figure 1. B, The fraction of CD3+CD4+CD25+CD127low Treg cells present in patients and control subjects at the pre-index visit. C, Percentage suppression of CD4+ (left panel) and CD8+ (right panel) proliferative responses to stimulation with surface-bound anti-CD3 antibody. Greater numbers mean more suppression and greater Treg function. These suppression assays were performed using pooled peripheral blood mononuclear cell (PBMC) samples collected after the index visit. Cells aliquots were assayed either intact or after Treg depletion, as described in Methods, and the percentage suppression was calculated using the formula: (percent CFSElow in depleted sample—percent CFSElow in undepleted sample)/(percent CFSElow in depleted sample). D, Example plots demonstrating robust suppression in a patient with CMV IRU. From left to right, the flow cytograms demonstrate little proliferation in the unstimulated sample, detectable proliferation in the intact sample treated with surface-bound anti-CD3, more robust proliferation in a Treg-depleted sample, and suppressed proliferation in a sample to which a supraphysiologic number of Treg cells was added. E, Dotplots showing percentage suppression of CD4+ (left panel) and CD8+ (right panel) proliferative responses to stimulation with pp65/IE peptides.
We next evaluated Treg cell function with use of proliferation assays to test the impact of Treg cell depletion on T cell proliferation in patients and control subjects. Suppression of polyclonal responses was assessed using surface-bound anti-CD3 antibody as the stimulus in wells containing either intact cell populations or CD3+CD4+CD25+CD127low-depleted populations. Depletion was performed using fluorescence-activated cell sorting, to eliminate the possibility that any suppression observed was attributable to non-Treg cells that were nonetheless CD25+. In this assay, patients and control subjects exhibited equivalent Treg cell function (Figure 4C). The degree of suppression observed was similar to that of suppression that we previously observed in chronically HIV-infected individuals, with many individuals, including patients with CMV IRU, having little or no suppression attributable to CD3+CD4+CD25+CD127low Treg cells (data not shown) (Figure 4C). Only 2 patients of the 14 with CMV IRU who were tested exhibited unusually robust suppression, which was confirmed by add-back assays to be caused by CD3+CD4+CD25+CD127low cells (data not shown) (Figure 4D).
Suppression of CMV-specific T cell responses was tested using similar methods, except that pp65/IE peptides were used as the proliferation stimulus. In these assays, patients and control subjects also exhibited a similar degree of suppression, with many patients with CMV IRU demonstrating no detectable suppression (Figure 4E).
Patients with CMV IRU are Characterized by More Profound Th17 Cell Depletion than are Control Subjects
Several studies have recently demonstrated Th17 cell depletion from simian immunodeficiency virus–infected nonhuman primates and HIV-infected persons, the degree of which is predictive of disease progression [12, 13]. On the basis of the aforementioned results, we speculated that patients with CMV IRU who have poor CMV-specific T cell responses might have smaller numbers of Th17 cells and associated poor antiviral T cell responses.
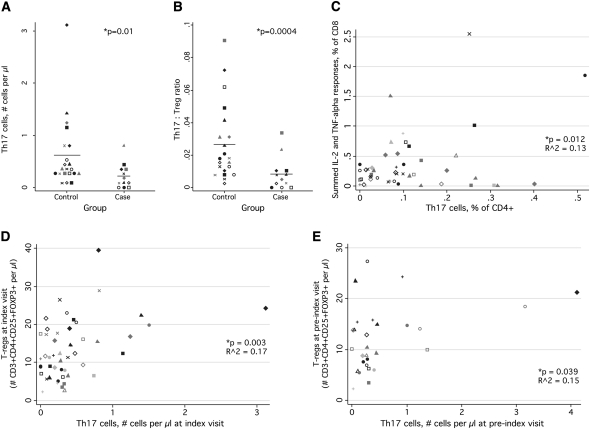
We found that patients with CMV IRU at the index visit were indeed characterized by a smaller number of Th17 cells than were control subjects (Figure 5A). Patients with CMV IRU also had a lower Th17:Treg cell ratio, a measure that has been used by some investigators to track Th17 cell depletion (Figure 5B) [12]. At the pre-index visit, in a smaller number of evaluable samples, no difference in either Th17 cells or the Th17:Treg cell ratio was observed (data not shown). The Th17 cell population was not associated with CMV-specific CD4+ T cell responses, contrary to our speculation, but was linearly related to CD8+ T cell responses (Figure 5C). Furthermore, Th17 cells were positively correlated to Treg cells at both the index (Figure 5D) and pre-index (Figure 5E) visits.
Figure 5.
Relationship of Th17 cells to cytomegalovirus (CMV) immune recovery uveitis (IRU) disease, CMV-specific T cells, and regulatory T (Treg) cells . A, The number of Th17 cells detected in patients and control subjects at the index visit. Symbols and statistics are as described for Figure 1. B, Dotplot showing the Th17:Treg ratio in patients and control subjects at the index visit. The ratio was calculated by dividing the fraction of Th17 cells among CD4+ T cells by the fraction of Treg cells (CD3+CD4+CD25+CD127low) among CD4+ T cells. C, Relationship between Th17 cells, shown on the X axis, and CMV-specific responses among CD8+ T cells, shown on the Y axis. The measure shown is a sum of the fraction of cells found to produce IL-2 alone, TNF-α alone, both cytokines combined, or both cytokines in combination with IFN-γ. D, Relationship between Th17 cells and Treg cells at the index visit. E, Relationship between Th17 cells and Treg cells at the pre-index visit.
Th17 cells were identified by measuring IL-17 production from CD4+ T cells after stimulation with anti-CD3 and anti-CD28, which are both polyclonal stimuli. To determine whether lower IL-17 production in patients with CMV IRU was attributable to a generalized loss of cytokine producing cells, we measured the ability of CD4+ T cells to make other cytokines. In contrast to IL-17 expression, there was no statistically significant difference in the ability of CD4+ T cells from CMV IRU and control groups to make IFN-γ, IL-2, or TNF-α after stimulation with anti-CD3 and anti-CD28 (data not shown).
DISCUSSION
We performed a case-control study to test the hypothesis that development of CMV IRU in immunorestored patients with AIDS is associated with systemic failure of Treg control over exaggerated immune responses, reflecting poor reconstitution of Treg cells. Surprisingly, we found that the number and function of Treg cells is comparable in patients with CMV IRU and matched control subjects, both before and after the development of disease. Furthermore, patients with CMV IRU displayed weaker anti-CMV CD4+ T cell responses than did control subjects and equivalent virus-specific CD8+ T cell responses, as well as equivalent ability to produce IFN-γ, IL-2, and TNF-α after polyclonal stimulation. This is in contrast to the only other IRIS for which T cell function before and at the time of IRIS diagnosis has been investigated. In HIV-infected patients with active tuberculosis prospectively evaluated when initiating antimycobacterial therapy and sequentially during HAART, a sharp systemic increase in mycobacterial antigen-specific Th1 IFN-γ–producing cells (measured by ELISPOT) has been observed in individuals developing tuberculosis IRIS [14]. We observed no similar explosion in systemic CMV-specific Th1 responses and no diminution of Treg cell numbers or function in patients with CMV IRU. One possibility is that CMV IRU results from a heightened sensitivity of lymphopenic hosts to CD4+ T cell responses, as was observed in an animal model of Mycobacterium avium immune resonstitution disease [15]. Different immune mechanisms may underlie the pathogenesis of different IRIS syndromes, depending on the opportunistic pathogen and, perhaps, the location of disease.
We also found that patients with CMV IRU were differentiated from control subjects by having a smaller number of CD4+ Th17 cells, which are not specific for CMV antigens. It has recently been recognized that Th17 cells are depleted in simian immunodeficiency virus and HIV disease and that the degree of depletion is predictive of disease progression [12, 13]. We speculate that lower numbers of Th17 cells among patients with CMV IRU may reflect greater losses throughout the course of HIV disease and a correspondingly greater level of immune dysfunction. This contention is supported by the fact that patients with CMV IRU had lower absolute CD4 counts at nadir than did control subjects (Table 1), although CD4 cell counts at nadir were not significantly predictive of either CMV IRU development or the size of the Th17 compartment at the index visit (data not shown). Furthermore, a restricted analysis of case-control sets in which control nadirs did not exceed case nadirs significantly (<5 cells/μL) yielded qualitatively identical conclusions to analysis of the whole dataset. These findings indicate that low CD4 nadirs themselves do not drive development of CMV IRU.
Although peripheral CMV-specific CD8+ T cell responses were neither predictive of nor associated with CMV IRU, other researchers have shown that at least some such cells are present in the eye [11]. Perhaps PBMC immune responses to CMV antigens other than pp65 and IE correlate with risk for CMV IRU. Other factors that we did not examine, such as the interval between initiating anti-CMV therapy and HAART [16] or the persistence of CMV antigen in the eye [17], might also be important in CMV IRU pathogenesis.
Nevertheless, our data are congruent with other reports showing that IRIS is associated with low CD4 cell counts at the start of antiretroviral therapy [2]. Th17 cell depletion may be either a reflection or a cause of more general CD4+ T cell loss, whereas severe Th17 cell depletion is associated with disease progression. Thus, CD4 cell count and Th17 cell number may both be measures of the severity of HIV disease before initiation of HAART. Association of these measures with development of IRIS suggests that the IRIS syndromes are rooted in immune conditions established at CD4 nadir, rather than failure of the immune system to re-establish immunoregulatory mechanisms after immunorestoration.
Supplementary Material
Supplementary materials are available at Clinical Infectious Diseases online (http://www.oxfordjournals.org/our_journals/cid/).
Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Acknowledgments
We thank Joseph M. McCune, MD, PhD, for many helpful discussions and for his support of this work.
Financial support. This work was supported by National Eye Institute (R21 EY018559 to M. J.), National Institute of Allergy and Infectious Diseases (K23 AI081540 to D. H.-O.’C.; P30AI027763 to the UCSF-GIVI Center for AIDS Research, which supports the Core Immunology Laboratory), and the National Center for Research Resources (UL1 RR024131 to UCSF CTSI).
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Price P, Murdoch DM, Agarwal U, Lewin SR, Elliott JH, French MA. Immune restoration diseases reflect diverse immunopathological mechanisms. Clin Microbiol Rev. 2009;22:651–63. doi: 10.1128/CMR.00015-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:251–61. doi: 10.1016/S1473-3099(10)70026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawn SD, Myer L, Bekker LG, Wood R. Tuberculosis-associated immune reconstitution disease: incidence, risk factors and impact in an antiretroviral treatment service in South Africa. AIDS. 2007;21:335–41. doi: 10.1097/QAD.0b013e328011efac. [DOI] [PubMed] [Google Scholar]
- 4.Nussenblatt RB, Lane HC. Human immunodeficiency virus disease: changing patterns of intraocular inflammation. Am J Ophthalmol. 1998;125:374–82. doi: 10.1016/s0002-9394(99)80149-4. [DOI] [PubMed] [Google Scholar]
- 5.Seddiki N, Sasson SC, Santner-Nanan B, et al. Proliferation of weakly suppressive regulatory CD4+ T cells is associated with over-active CD4+ T-cell responses in HIV-positive patients with mycobacterial immune restoration disease. Eur J Immunol. 2009;39:391–403. doi: 10.1002/eji.200838630. [DOI] [PubMed] [Google Scholar]
- 6.Hartigan-O'Connor DJ, Poon C, Sinclair E, McCune JM. Human CD4+ regulatory T cells express lower levels of the IL-7 receptor alpha chain (CD127), allowing consistent identification and sorting of live cells. J Immunol Methods. 2007;319:41–52. doi: 10.1016/j.jim.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 8.Jacobson MA, Tan QX, Girling V, et al. Poor predictive value of cytomegalovirus (CMV)-specific T cell assays for the development of CMV retinitis in patients with AIDS. Clin Infect Dis. 2008;46:458–66. doi: 10.1086/525853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Betts MR, Brenchley JM, Price DA, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 10.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 11.Mutimer HP, Akatsuka Y, Manley T, et al. Association between immune recovery uveitis and a diverse intraocular cytomegalovirus-specific cytotoxic T cell response. J Infect Dis. 2002;186:701–5. doi: 10.1086/342044. [DOI] [PubMed] [Google Scholar]
- 12.Favre D, Lederer S, Kanwar B, et al. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009;5:e1000295. doi: 10.1371/journal.ppat.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brenchley JM, Paiardini M, Knox KS, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–35. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bourgarit A, Carcelain G, Martinez V, et al. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS. 2006;20:F1–7. doi: 10.1097/01.aids.0000202648.18526.bf. [DOI] [PubMed] [Google Scholar]
- 15.Barber DL, Mayer-Barber KD, Antonelli LR, et al. Th1. driven immune reconstitution disease in Mycobacterium avium infected mice. Blood. 2010;116:3485–93. doi: 10.1182/blood-2010-05-286336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ortega-Larrocea G, Espinosa E, Reyes-Teran G. Lower incidence and severity of cytomegalovirus-associated immune recovery uveitis in HIV-infected patients with delayed highly active antiretroviral therapy. AIDS. 2005;19:735–8. doi: 10.1097/01.aids.0000166100.36638.97. [DOI] [PubMed] [Google Scholar]
- 17.Kosobucki BR, Goldberg DE, Bessho K, et al. Valganciclovir therapy for immune recovery uveitis complicated by macular edema. Am J Ophthalmol. 2004;137:636–8. doi: 10.1016/j.ajo.2003.11.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.