Abstract
Background. Influenza A viruses cause occasional pandemics and frequent epidemics. Licensed influenza vaccines that induce high antibody titers to the highly polymorphic viral surface antigen hemagglutinin must be re-formulated and readministered annually. A vaccine providing protective immunity to the highly conserved internal antigens could provide longer-lasting protection against multiple influenza subtypes.
Methods. We prepared a Modified Vaccinia virus Ankara (MVA) vector encoding nucleoprotein and matrix protein 1 (MVA−NP+M1) and conducted a phase I clinical trial in healthy adults.
Results. The vaccine was generally safe and well tolerated, with significantly fewer local side effects after intramuscular rather than intradermal administration. Systemic side effects increased at the higher dose in both frequency and severity, with 5 out of 8 volunteers experiencing severe nausea/vomiting, malaise, or rigors. Ex vivo T-cell responses to NP and M1 measured by IFN-γ ELISPOT assay were significantly increased after vaccination (prevaccination median of 123 spot-forming units/million peripheral blood mononuclear cells, postvaccination peak response median 339, 443, and 1443 in low-dose intradermal, low-dose intramuscular, and high-dose intramuscular groups, respectively), and the majority of the antigen-specific T cells were CD8+.
Conclusions. We conclude that the vaccine was both safe and remarkably immunogenic, leading to frequencies of responding T cells that appear to be much higher than those induced by any other influenza vaccination approach. Further studies will be required to find the optimum dose and to assess whether the increased T-cell response to conserved influenza proteins results in protection from influenza disease.
Licensed influenza vaccines, whether inactivated or live attenuated, are designed to induce humoral immunity to hemagglutinin (HA). Seasonal influenza vaccines are a mixture of A/H1N1, A/H3N2, and B antigens. Vaccine effectiveness is 70%–90% when the circulating virus is well matched to the vaccine, but may fall below 50% when the circulating strain has drifted significantly from the vaccine strain [ 1], particularly in people over 60 years of age [ 2]. Annual revaccination is required to maintain immunity against seasonal influenza viruses.
Fears of an H5N1 pandemic resulted in the generation and testing of H5-specific vaccines, which may require the use of an adjuvant or multiple doses to achieve a protective level of immunity following vaccination [ 3]. H5N1 viruses have continued to mutate in avian populations, and in clinical trials of an unadjuvanted H5 vaccine, serological cross-reactivity to variant H5 viruses even within the same clade was only 20%–30% [ 4], although use of an adjuvant may improve this. Since swine origin H1N1 began to circulate in humans in April 2009, vaccine manufacturers have produced pandemic-specific vaccines, and the first doses became available in October 2009, 6 months after the virus was first identified.
Clearly, a vaccine that could provide heterosubtypic protection against all influenza A viruses would be of great benefit, and, if effective and widely used, could prevent another pandemic from occurring. The efficacy of influenza vaccines designed to induce subtype cross-reactive T cells to internal influenza antigens such as nucleoprotein (NP), which is highly conserved between all influenza A subtypes, has been demonstrated in many species of animal model [ 5– 8], and this approach has the potential to replace or supplement seasonal and pandemic-specific vaccination in humans. Influenza challenge studies in humans with low neutralizing antibody titers to the challenge virus (measured by the HA inhibition assay) have demonstrated a negative correlation between T-cell response to viral antigens and influenza disease and virus shedding [ 9]. Protection is thought to be mediated chiefly by CD8+ T cells, but protective immunity is short-lived [ 10], although reexposure to influenza virus within a few years of the first infection may result in a subclinical infection and boosting of the T-cell response. Lee et al [ 11] reported that memory T cells recognizing influenza antigens were detected in over 90% of those tested, and showed cross-recognition of at least one H5N1 internal protein. The magnitude of the responses varied considerably, and is presumably related to the time elapsed since the most recent exposure to influenza virus. However, low-level memory T-cell responses to influenza antigens have the potential to be boosted to protective levels, by further exposure to the virus or by vaccination. Live attenuated influenza vaccines have been shown to induce modest T-cell responses in children, but did not significantly boost T-cell responses to influenza in adults with T-cell responses induced by natural exposure [ 12].
Modified Vaccinia virus Ankara (MVA) is a highly attenuated virus that has been used to boost T-cell responses to recombinant antigens encoded by the virus in many clinical studies aimed at developing new vaccines for malaria, human immunodeficiency virus (HIV), and tuberculosis (TB). MVA has an excellent safety profile, and has been tested in children [ 13] as well as HIV-positive [ 14] and latently TB-infected individuals [ 15]. MVA has been used to boost both CD4+ and CD8+ responses primed by prior DNA, fowlpox [ 16], adenovirus [ 17], or Bacille Calmette-Guerin immunization [ 18], or HIV infection [ 14]. Since adults have been primed by prior exposure to influenza, MVA expressing conserved internal antigens of influenza such as NP and matrix protein 1 (M1) could be used to boost cross-reactive T-cell responses to protective levels, providing broad immunity to all subtypes of influenza A. An illustration of the conservation of the vaccine antigens is given in Table 1, showing the identity and divergence of the amino acid sequences of NP and M1 in the vaccine MVA−NP+M1 and human isolates of H3N2, H1N1, H5N1, and swine origin H1N1. The identity and divergence of HA are given for comparison. The high degree of identity with H3N2 is not surprising since the vaccine antigens are derived from the H3N2 virus A/Panama/2007/99, but both antigens are more than 90% identical with homologues from seasonal H1N1, swine origin H1N1, and H5N1 viruses, whereas identity drops as low as 43% between the HA proteins of the same 4 viruses.
Table 1.
Sequence Identity (top) and Divergence (Bottom) Between Antigens in MVA-NP+M1 and Other Influenza A Viruses
| A: Nucleoprotein | |||||
| Vaccine | H3N2 | H1N1 | H5N1 | SO H1N1 | |
| Vaccine | X | 98.0 | 91.8 | 91.4 | 90.2 |
| H3N2 | 2.0 | X | 91.4 | 90.8 | 89.8 |
| H1N1 | 8.7 | 9.2 | X | 92.0 | 90.0 |
| H5N1 | 9.2 | 9.9 | 8.5 | X | 93.6 |
| SO H1N1 | 10.5 | 11.0 | 10.8 | 6.7 | X |
| B: Matrix protein 1 | |||||
| Vaccine | H3N2 | H1N1 | H5N1 | SO H1N1 | |
| Vaccine | X | 99.2 | 94.9 | 92.9 | 92.1 |
| H3N2 | 0.8 | X | 95.7 | 92.9 | 92.1 |
| H1N1 | 5.3 | 4.5 | X | 93.3 | 93.7 |
| H5N1 | 7.5 | 7.5 | 7.1 | X | 96.0 |
| SO H1N1 | 8.4 | 8.4 | 6.6 | 4.1 | X |
| C: Hemagglutinin | |||||
| Vaccine | H3N2 | H1N1 | H5N1 | SO H1N1 | |
| Vaccine | X | N/A | N/A | N/A | N/A |
| H3N2 | N/A | X | 42.6 | 44.0 | 42.8 |
| H1N1 | N/A | 100.0 | X | 79.3 | 63.1 |
| H5N1 | N/A | 97.3 | 24.2 | X | 63.8 |
| SO H1N1 | N/A | 100.0 | 50.4 | 49.1 | X |
NOTE. Calculated using DNAStar MegAlign 8.0 after Jotun Hein alignment. Percent identity = (Matches x 100)/Length of aligned region (with gaps); divergence is calculated by comparing sequence pairs in relation to the reconstructed phylogeny. Viruses are H3N2: A/Pennsylvania/PIT08/2008 (NP: CY035057, M1: CY035055, HA: CY035054), H1N1: A/Washington/AF06/2007 (NP: CY037330, M1: CY037328, HA: CY037327), H5N1: A/Beijing/01/2003 (NP: EF587278, M1: EF587280, HA: EF587277), SO H1N1: A/Canada-NS/RV1535/2009 (NP: FJ998216, M1: FJ998210, HA: FJ998207).
We now report on the safety and immunogenicity of MVA−NP+M1, a vaccine designed to boost preexisting T-cell responses to conserved influenza antigens in a Phase I clinical study in healthy adult volunteers.
MATERIALS AND METHODS
Sequence Alignments
Sequences were obtained from the National Center for Biotechnology Information GenBank and aligned using Lasergene DNAStar 8.0 MegAlign, Jotun Hein method.
Vaccine Design and Manufacture
The vaccine antigen expressed from MVA consists of the complete NP and M1 from A/Panama/2007/99 joined by a 7 amino acid linker sequence, and is expressed from the Vaccinia P7.5 promoter inserted at the thymidine kinase locus of MVA. Generation of the recombinant virus and subsequent Good Manufacturing Practice (GMP) manufacture used primary chick embryo fibroblast (CEF) cells. GMP manufacture and release testing of the vaccine were carried out by Impfstoffwerk (Dessau-Tornau, Germany).
Study Population
Twenty-eight subjects were recruited for immunization studies under a protocol approved by the United Kingdom’s Medicines and Healthcare products Regulatory Agency and Gene Therapy Advisory Committee and were enrolled only after obtaining written informed consent (www.clinicaltrials.gov, identifier: NCT00942071). Inclusion criteria required volunteers to be aged 18–50 years, resident in the Oxford area, and seronegative for HIV antibodies, hepatitis B surface antigen, and hepatitis C antibodies. Women who were pregnant or lactating, and volunteers who had previously received an MVA (but not vaccinia) vaccine, or who had a history of egg allergy or anaphylaxis following vaccination, were excluded. No information about prior seasonal influenza vaccination was recorded, but volunteers did not fall into the target population for vaccination within the UK and were unlikely to have received vaccination.
Vaccination and Follow-up Regime
Following receipt of information about the study, volunteers attended a screening visit to assess their suitability for the study. Each group was completed and vaccine safety assessed before enrolling the next group. All eligible volunteers were enrolled into the next available group. A single vaccination was administered at a subsequent visit, with the dose and route of vaccination varying between the study groups. Group 1 received 5 × 107 pfu intradermally (dose volume 385 microliters), group 2 received the same dose intramuscularly, and group 3 received 2.5 × 108 pfu intramuscularly (dose volume 1920 microliters). Blood was drawn to assess the T-cell response to NP and M1 on day of vaccination and 1, 3, 8, 12, 24, and 52 weeks after vaccination. Volunteers also attended a follow-up visit 2 days after vaccination; adverse events were elicited by open questions at that visit and all visits up to week 12 and were also recorded on a diary card by the volunteer for the first week after vaccination. Mild events were defined as awareness of a symptom that was easily tolerated, moderate as discomfort enough to cause interference with usual activity, and severe as incapacitating, inability to perform usual activities, requiring absenteeism or bed rest. Information about influenza-like illness was also recorded, with no volunteer reporting symptoms within the first 3 weeks following vaccination, and very few reports of coryzal illness at later time points. Vaccinations were carried out from August to November 2008 (group 1), February to March 2009 (group 2), and March to May 2009 (group 3). Circulating seasonal influenza A strains during this period were H3N2—A/Brisbane/10/2007 and H1N1—A/Brisbane/59/2007.
Ex Vivo IFN-γ ELISPOT
Ex vivo interferon-gamma enyzyme-linked immunosorbent spot (IFN-γ ELISPOT) assays were performed using cryopreserved peripheral blood mononuclear cells (PBMCs). PBMCs were cryopreserved in fetal calf serum (FCS) (Biosera Ltd) with 10% dimethyl sulfoxide (Sigma) at −80°C in a Mr Frosty container, then transferred and stored in liquid nitrogen. PBMCs were thawed quickly in warm R10 (R10: RPMI 1640 with 10% FCS, 100 IU/mL penicillin, .1 mg/mL streptomycin (all Sigma), and 2 mM L-glutamine (GIBCO/Invitrogen), washed and resuspended in R10 with 2 μl/mL of 25 U/mL Benzonase nuclease (Novagen) and left to rest overnight at 37°C. The following day the cells were washed and counted for use in the assays. The ex vivo IFN-γ ELISPOT was carried out as previously described [ 19]. Fifteen- to 20-mer peptides overlapping by 10 amino acid residues, spanning the whole of the NP+M1 insert, were used to stimulate PBMCs at a concentration of 10 μg/ml. The peptides were split into 8 pools of 10 peptides; pools 1–6 contained peptides from the NP sequence and pools 6–8 contained peptides from the M1 sequence. Fifty microliters of PBMCs (2 × 105 cells) and 50 μl of the peptides were added in triplicate. R10 was used as a negative control and phytohaemagglutinin (PHA) at a final concentration of 10 μg/mL was used as a positive control. Following a 18–20-hour incubation at 37°C, the ELISPOT plates were dried and read with an AID ELISPOT reader (AID Diagnostika). The results are expressed as spot-forming units (SFUs) per million PBMCs calculated by subtracting the mean R10 negative control response from the mean peptide pool response. To determine the ELISPOT response to the vaccine insert, the response to each peptide pool was summed following background subtraction. Plates were excluded if a response over 100 SFUs per million was seen in the R10 wells or under 1000 SFU in the PHA wells.
Intracellular Cytokine Staining
One to 2 million cryopreserved PBMCs were stimulated with a single pool of all NP+M1 peptides at a final concentration of 4 μg/mL and 1 μg/mL of co-stimulatory antibodies αCD28 and αCD49d (BD Pharmingen). Cells were incubated for 18 hours at 37°C. After the first 2 hours of incubation, 10 μg/mL brefeldin A and monensin (eBiosciences) was added. PBMCs were stained with: CD3 Alexa Fluor 700 (eBioscience-UCHT1), CD8-APC-AF780 (eBioscience-RPAT8), CD4-QD655 (Invitrogen-S3.5), IFN-γ FITC (eBioscience-4S.B3), CD14 Pacific Blue (Invitrogen-TuK4), CD19 Pacific Blue (Invitrogen-SJ25-C1), and VIVID Pacific Blue (Invitrogen). Over 300,000 gated lymphocyte events were acquired on a Beckton Dickinson LSRII flow cytometer using FACSDiva software (BD Biosciences) and analyzed using Flow Jo, Version 8.3 (Tree Star Inc). Unstained cells and single stained anti-human compensation beads (BD Biosciences) were used as controls to automatically calculate compensation. All antibodies were titrated for optimal staining.
Statistical Analysis
Fisher's exact test was used to detect significant differences in adverse events between the 3 vaccine groups. If such a difference existed, groups 1 and 2 were compared and the difference in proportions presented, and similarly for groups 2 and 3. Nonparametric tests were used to determine differences in the ELISPOT data; Wilcoxon signed rank test was performed to test for differences in the ELISPOT responses between time points within a vaccine group, and Mann-Whitney U test was performed to detect differences in ELISPOT responses between different vaccine groups.
RESULTS
MVA−NP+M1 Is Safe in Healthy Volunteers
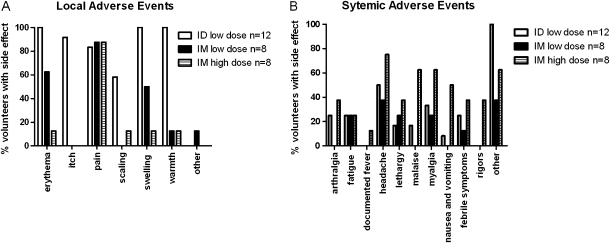
Volunteers were given a single dose 5 × 107 pfu intradermally (group 1, 12 subjects), 5 × 107 pfu intramuscularly (group 2, 8 subjects) or 2.5 × 108 pfu intramuscularly (group 3, 8 subjects). Adverse events are presented in Figure 1. Volunteers receiving the vaccine via the intramuscular route, at either dose, experienced significantly less erythema, itch, swelling, and warmth at the injection site than those vaccinated intradermally, regardless of the vaccine dose. All local adverse events were grade 1 severity apart from 1 volunteer in group 1 and 2 volunteers in group 2, who each experienced one grade 2 adverse event. No significant differences in systemic adverse events were reported by the volunteers receiving the low-dose vaccine by either route (no grade 3 adverse events in either group), but there was a significant increase in malaise, nausea/vomiting, and rigors in the group receiving the high-dose vaccination with 5 volunteers experiencing 1 or more severe adverse events ( Figure 1B).
Figure 1.
Local and systemic adverse events recorded after vaccination. Black: group 1 (n = 12). White: group 2 (n = 8). Striped: group 3 (n = 8). (A) Local adverse events. Significantly less (P < .05, Fisher's exact test) erythema, itch, swelling, and warmth at the injection site were detected in those receiving intramuscular vaccine than those vaccinated intradermally, regardless of the vaccine dose. Significantly less scaling was recorded in the low-dose compared with the high-dose intramuscular group. (B) Systemic adverse events. No significant differences in systemic adverse events were reported by the volunteers receiving the low-dose vaccine by either route, but there was a significant increase in malaise, nausea/vomiting, and rigors in the group receiving the high dose (P < .05, Fisher's exact test). Severe adverse events only occurred in the high-dose group, with 2 volunteers reporting severe pain at the injection site, 1 reporting malaise, 1 vomiting, 2 rigors, and 1 sweating. All severe adverse events resolved within 48 hours of vaccination, apart from 1 volunteer reporting severe pain at the injection site on the 3 days following vaccination. The majority of mild and moderate adverse events also took place within 48 hours of vaccination, although mild erythema at the injection site lasted for up to 49 days for those receiving intradermal vaccination.
MVA−NP+M1 Vaccination Boosts IFN-γ Secreting Antigen-Specific T Cells
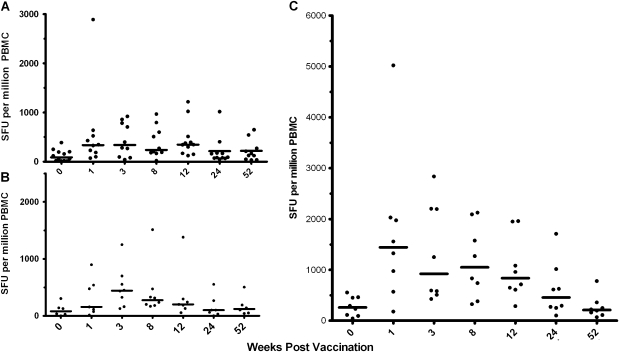
Ex vivo IFN-γ ELISPOT responses to the whole NP and M1 vaccine insert were measured in cryopreserved PBMCs at baseline (week 0) and at 1, 3, 8, 12, 26, and 52 weeks after immunization ( Figure 2A–C). All volunteers had measurable responses to NP and M1 prior to vaccination (median 123 SFU/million PBMC). A significant increase in the number of SFUs detected following vaccination was seen in all 3 groups as measured by Wilcoxon signed rank test at weeks 1 and 3. Responses were also significantly above the prevaccination level at weeks 8 and 12 for group 1, and weeks 8, 12, and 24 for group 3. The route of immunization did not appear to affect the magnitude of the immune response at low dose (no significant difference between groups 1 and 2) whereas the increase in dose from 5 × 107 pfu (group 2) to 2.5 × 108 pfu (group 3) resulted in significant increase in immune response at weeks 1, 3, 8, 12, and 24.
Figure 2.
Ex vivo IFN-γ ELISPOT responses to the vaccine insert. Median with individual ex vivo IFN-γ ELISPOT responses from vaccinated volunteers at baseline (week 0), and weeks 1, 3, 8, 12, 24, and 52 weeks after immunization. (A) group 1; (B) group 2; (C) group 3. Wilcoxon signed rank test was used to determine significant differences in the post- and prevaccination time points. (A) week 1, P = .0059; week 3, P = .0098; week 8, P = .0078; week 12, P = .0049. (B) week 1, P = .0313; week 3, P = .0313. (C) week 1, P = .0078; week 3, P = .0078; week 8, P = .0078; week 12, P = .0078; week 24, P = .023. Significant differences were detected between groups 2 (B) and 3 (C) at all postvaccination time points apart from week 52 (Mann-Whitney U test: week 1, P = .006; week 3, P = .04; week 8, P = .01; week 12, P = .02; week 24, P = .012).
Vaccination Boosts Both CD4+ and CD8+ Antigen-Specific T Cells
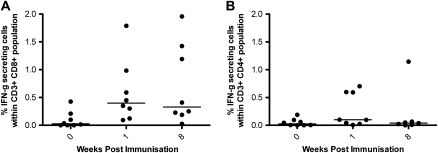
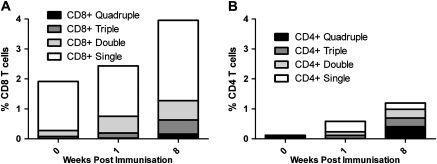
Intracellular cytokine staining (ICS) analysis was carried out to determine whether the IFN-γ detected in the ex vivo ELISPOT was produced by CD3+CD4+ or CD3+CD8+ T cells. ICS was carried out at week 0, week 1, and week 8 on cryopreserved PBMCs from all volunteers in group 3. The CD4+ and CD8+ T-cell responses following background subtraction are shown in Figure 3. A significant increase in IFN-γ production from CD8+ T cells was detected following vaccination at week 1 and week 8. The percentage of antigen-specific CD8+ cells producing IFN–γ was higher than the corresponding population of CD4+ T cells both before and after vaccination. Interleukin-2 and tumour necrosis factor alpha (IL-2 and TNF-α) production and CD107a expression were also analyzed in the CD4+ and CD8+ populations, revealing quadruple, triple, double, and single functional cells in both populations ( Figure 4). CD107a, a marker of degranulation and cytotoxicity, was present both with and without IFN-γ.
Figure 3.
CD3+CD4+ and CD3+CD8+ IFN-γ responses to vaccine insert as measured by intracellular cytokine staining. Intracellular IFN-γ responses after background subtraction in (A) CD3+CD8+ and (B) CD3+CD4+ cell populations stimulated with 1 pool of peptides spanning the complete NP+M1 vaccine insert. Volunteers in group 3 were tested at weeks 0, 1, and 8. Median % IFN-γ+ within CD3+CD8+ cells at week 1 = .4% and week 8 = .33%; median % IFN-γ+ cells within CD3+CD4+ population at week 1 = .098% and week 8 = .039%.
Figure 4.
IFN-γ, IL-2, TNF-α, and CD107a multifunctional cells detected by ICS in CD3+CD8+ and CD3+CD4+ populations. Mean percentage of quadruple (black), triple (dark gray), double (light gray), and single (white) functional cells detected within the CD8+ (A) and CD4+ (B) populations. Within the CD8+ population, the most frequently detected triple positive cells were CD107a+IFN-γ+TNF-α+; the most frequently detected double positive cells were CD107a+TNF-α+; and CD107a+ cells were the most frequently detected single positive cells. Within the CD4+ cells, the most frequently detected triple positive cells were CD107a+IFN-γ+IL-2+; the most frequently detected double positive cells were IFN-γ+IL-2+; and TNF-α+ cells were the most frequently detected single positive cells. At all time points the frequency of antigen-specific cytokine positive cells was greater in the CD8+ population (week 0, CD8+ = 1.92% and CD4+ = .12%; week 1, CD8+ = 2.43% and CD4+ = .58%; week 8, CD8+ = 3.96% and CD4+ = 1.19%).
DISCUSSION
We report here the first clinical study of a novel influenza vaccine designed to boost cross-reactive immune responses to all influenza A subtypes. Many studies have reported intradermal vaccination with MVA, and the side effect profile seen with MVA−NP+M1 is comparable [ 20]. The same dose administered by the intramuscular route resulted in significantly fewer local, but not systemic, adverse events. At the higher dose of 2.5 × 108 pfu administered as an intramuscular injection, there was an increase in both the frequency and severity of systemic adverse events compared with the lower dose of 5 × 107 pfu. For future studies with this vaccine the dose will be reduced to 1.5 × 108 pfu. The magnitude of the immune response to vaccination determined by ex vivo IFN-γ ELISPOT did not differ with the route of administration, but increased at the higher dose.
ICS analysis for IFN-γ production by CD3+CD4+ and CD3+CD8+ cells was also carried out in the high-dose intramuscular group and showed that more antigen-specific CD3+CD8+ T cells than CD3+CD4+ T cells were present after vaccination. IL-2, TNF-α, and CD107a were also produced by antigen-specific cells. Further studies are required to determine which T-cell phenotypes, whether lytic or cytokine-producing, are capable of prevention of disease following exposure to influenza virus.
A vaccine that boosts cross-reactive T-cell responses to conserved internal antigens of influenza has the potential to modify or prevent disease and virus shedding in vaccinees, thus reducing morbidity and transmission whether the virus causing the infection is one that continually circulates in humans or is a different subtype with the potential to cause a pandemic. Vaccines based on HA protein must be produced not only for each virus subtype, but for the continually evolving sequences within each subtype. MVA-vectored vaccines can be produced at large scale for human vaccination, and are safe for use. MVA−NP+M1 could be used alone, or in combination with an anti-HA antibody inducing component, to provide broad protection against all influenza A viruses, thereby improving vaccine efficacy over that currently achieved, particularly in influenza seasons when the circulating virus has drifted from the vaccine strain, and to provide protection when a global pandemic occurs, regardless of the virus subtype.
Currently the magnitude of T-cell response to NP and M1 required to prevent influenza disease in humans is not known. However, the magnitude of the induced T-cell responses measured here are noteworthy. A median response of 1443 SFU/million PBMCs at the peak time point substantially exceeds the T-cell responses induced in any of large numbers of phase I and phase II trials of potent vectored vaccines in HIV, malaria, and cancer [ 21, 22]. In the STEP trial of an adenovirus vectored vaccine against HIV-1, the geometric mean T-cell response at peak was around 300 SFU/million PBMCs to the vaccine antigens. The much higher immunogenicity identified here likely results from the level of T-cell response prior to vaccination attributable to natural influenza virus exposure, combined with the remarkable boosting ability of MVA-vectored vaccines. A similar potent boosting of preexisting T-cell responses and induction of polyfunctional T-cell responses is observed with an MVA vector encoding a TB antigen [ 23]. In contrast, a trial of the cold-adapted influenza virus vaccine, FluMist, found that although it could induce modest T-cell responses in children, it did not significantly boost T-cell responses to influenza in adults with T-cell responses induced by natural exposure [ 12].
A further notable finding is the greater CD8+ than CD4+ T-cell response after vaccination. In the only previous example of vaccine-induced T-cell response exceeding 1000 SFU/million PBMCs to an antigenic insert, the response was predominantly of CD4+ T cells [ 24]. This reflects the proportions of CD4+ and CD8+ T cells detected prior to vaccination in each case and adds to the growing evidence that MVA vectors can boost both CD4+ and CD8+ T cells effectively in humans.
Further planned studies will address the ability of this MVA vaccine to boost preexisting T-cell responses to the conserved influenza antigens NP and M1 in an extended age range, as well as the efficacy of the vaccine in preventing influenza disease and virus shedding via influenza virus challenge studies.
Acknowledgments
We gratefully acknowledge Saroj Saurya and Matt Cottingham for assistance with generating the recombinant MVA; Nicola Alder, Centre for Statistics in Medicine, University of Oxford, in presenting the analysis of the adverse events data; and Alison Lawrie, Katherine Gantlett, and Ian Poulton for assistance with the clinical trial. SCG and AVSH are Jenner Investigators, and are inventors on a patent covering use of MVA−NP+M1. No potential conflicts of interest exist for the other authors. This work was funded by the Wellcome Trust and by the NIHR Oxford Biomedical Research Centre.
Potential conflict of interest. All authors: no conflicts.
References
- 1.Centers for Disease Control and Prevention (CDC) Interim within-season estimate of the effectiveness of trivalent inactivated influenza vaccine–Marshfield, Wisconsin, 2007-08 influenza season. MMWR Morb Mortal Wkly Rep. 2008;57:393–398. [PubMed] [Google Scholar]
- 2.de Jong JC, Beyer WE, Palache AM, Rimmelzwaan GF, Osterhaus AD. Mismatch between the 1997/1998 influenza vaccine and the major epidemic A(H3N2) virus strain as the cause of an inadequate vaccine-induced antibody response to this strain in the elderly. J Med Virol. 2000;61:94–99. [PubMed] [Google Scholar]
- 3.Galli G, Medini D, Borgogni E, et al. Adjuvanted H5N1 vaccine induces early CD4+ T cell response that predicts long-term persistence of protective antibody levels. Proc Natl Acad Sci U S A. 2009;106:3877–3882. doi: 10.1073/pnas.0813390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nolan TM, Richmond PC, Skeljo MV, et al. Phase I and II randomised trials of the safety and immunogenicity of a prototype adjuvanted inactivated split-virus influenza A (H5N1) vaccine in healthy adults. Vaccine. 2008;26:4160–4167. doi: 10.1016/j.vaccine.2008.05.077. [DOI] [PubMed] [Google Scholar]
- 5.Breathnach CC, Clark HJ, Clark RC, Olsen CW, Townsend HG, Lunn DP. Immunization with recombinant modified vaccinia Ankara (rMVA) constructs encoding the HA or NP gene protects ponies from equine influenza virus challenge. Vaccine. 2006;24:1180–1190. doi: 10.1016/j.vaccine.2005.08.091. [DOI] [PubMed] [Google Scholar]
- 6.Donnelly JJ, Friedman A, Ulmer JB, Liu MA. Further protection against antigenic drift of influenza virus in a ferret model by DNA vaccination. Vaccine. 1997;15:865–868. doi: 10.1016/s0264-410x(96)00268-x. [DOI] [PubMed] [Google Scholar]
- 7.Epstein SL, Kong WP, Misplon JA, et al. Protection against multiple influenza A subtypes by vaccination with highly conserved nucleoprotein. Vaccine. 2005;23:5404–5410. doi: 10.1016/j.vaccine.2005.04.047. [DOI] [PubMed] [Google Scholar]
- 8.Laddy DJ, Yan J, Khan AS, et al. Electroporation of synthetic DNA antigens offers protection in nonhuman primates challenged with highly pathogenic avian influenza virus. J Virol. 2009;83:4624–4630. doi: 10.1128/JVI.02335-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMichael AJ, Gotch FM, Noble GR, Beare PA. Cytotoxic T-cell immunity to influenza. N Engl J Med. 1983;309:13–17. doi: 10.1056/NEJM198307073090103. [DOI] [PubMed] [Google Scholar]
- 10.McMichael AJ, Gotch FM, Dongworth DW, Clark A, Potter CW. Declining T-cell immunity to influenza, 1977-82. Lancet. 1983;2:762–764. doi: 10.1016/s0140-6736(83)92297-3. [DOI] [PubMed] [Google Scholar]
- 11.Lee LY, Ha DLA, Simmons C, et al. Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals. J Clin Invest. 2008;118:3478–3490. doi: 10.1172/JCI32460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He XS, Holmes TH, Zhang C, et al. Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. J Virol. 2006;80:11756–11766. doi: 10.1128/JVI.01460-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bejon P, Peshu N, Gilbert SC, et al. Safety profile of the viral vectors of attenuated fowlpox strain FP9 and modified vaccinia virus Ankara recombinant for either of 2 preerythrocytic malaria antigens, ME-TRAP or the circumsporozoite protein, in children and adults in Kenya. Clin Infect Dis. 2006;42:1102–1110. doi: 10.1086/501459. [DOI] [PubMed] [Google Scholar]
- 14.Dorrell L, Yang H, Iversen AK, et al. Therapeutic immunization of highly active antiretroviral therapy-treated HIV-1-infected patients: safety and immunogenicity of an HIV-1 gag/poly-epitope DNA vaccine. AIDS. 2005;19:1321–1323. doi: 10.1097/01.aids.0000180104.65640.16. [DOI] [PubMed] [Google Scholar]
- 15.Sander CR, Pathan AA, Beveridge NE, et al. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in Mycobacterium tuberculosis-infected individuals. Am J Respir Crit Care Med. 2009;179:724–733. doi: 10.1164/rccm.200809-1486OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vuola JM, Keating S, Webster DP, et al. Differential immunogenicity of various heterologous prime-boost vaccine regimens using DNA and viral vectors in healthy volunteers. J Immunol. 2005;174:449–455. doi: 10.4049/jimmunol.174.1.449. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert SC, Schneider J, Hannan CM, et al. Enhanced CD8 T cell immunogenicity and protective efficacy in a mouse malaria model using a recombinant adenoviral vaccine in heterologous prime-boost immunisation regimes. Vaccine. 2002;20:1039–1045. doi: 10.1016/s0264-410x(01)00450-9. [DOI] [PubMed] [Google Scholar]
- 18.McShane H, Pathan AA, Sander CR, et al. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med. 2004;10:1240–1244. doi: 10.1038/nm1128. [DOI] [PubMed] [Google Scholar]
- 19.Berthoud TK, Fletcher H, Porter D, Thompson F, Hill AV, Todryk SM. Comparing human T cell and NK cell responses in viral-based malaria vaccine trials. Vaccine. 2009;28:21–27. doi: 10.1016/j.vaccine.2009.09.132. [DOI] [PubMed] [Google Scholar]
- 20.Webster DP, Dunachie S, McConkey S, et al. Safety of recombinant fowlpox strain FP9 and modified vaccinia virus Ankara vaccines against liver-stage P. falciparum malaria in non-immune volunteers. Vaccine. 2006;24:3026–3034. doi: 10.1016/j.vaccine.2005.10.058. [DOI] [PubMed] [Google Scholar]
- 21.Dunachie SJ, Walther M, Epstein JE, et al. A DNA prime-modified vaccinia virus ankara boost vaccine encoding thrombospondin-related adhesion protein but not circumsporozoite protein partially protects healthy malaria-naive adults against Plasmodium falciparum sporozoite challenge. Infect Immun. 2006;74:5933–5942. doi: 10.1128/IAI.00590-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Priddy FH, Brown D, Kublin J, et al. Safety and immunogenicity of a replication-incompetent adenovirus type 5 HIV-1 clade B gag/pol/nef vaccine in healthy adults. Clin Infect Dis. 2008;46:1769–1781. doi: 10.1086/587993. [DOI] [PubMed] [Google Scholar]
- 23.Beveridge N, Price D, Casazza J, et al. Immunisation with BCG and recombinant MVA85A induces long-lasting, polyfunctional Mycobacterium tuberculosis-specific CD4+ memory T lymphocyte populations. Eur J Immunol. 2007;37:3089–3100. doi: 10.1002/eji.200737504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whelan KT, Pathan AA, Sander CR, et al. Safety and immunogenicity of boosting BCG vaccinated subjects with BCG: comparison with boosting with a new TB vaccine, MVA85A. PLoS ONE. 2009;4:e5934. doi: 10.1371/journal.pone.0005934. [DOI] [PMC free article] [PubMed] [Google Scholar]