The results from two methodologically identical double-blind studies indicate that telavancin is noninferior to vancomycin based on clinical response in the treatment of hospital-acquired pneumonia due to Gram-positive pathogens.
Abstract
Background. Telavancin is a lipoglycopeptide bactericidal against gram-positive pathogens.
Methods. Two methodologically identical, double-blind studies (0015 and 0019) were conducted involving patients with hospital-acquired pneumonia (HAP) due to gram-positive pathogens, particularly methicillin-resistant Staphylococcus aureus (MRSA). Patients were randomized 1:1 to telavancin (10 mg/kg every 24 h) or vancomycin (1 g every 12 h) for 7–21 days. The primary end point was clinical response at follow-up/test-of-cure visit.
Results. A total of 1503 patients were randomized and received study medication (the all-treated population). In the pooled all-treated population, cure rates with telavancin versus vancomycin were 58.9% versus 59.5% (95% confidence interval [CI] for the difference, –5.6% to 4.3%). In the pooled clinically evaluable population (n = 654), cure rates were 82.4% with telavancin and 80.7% with vancomycin (95% CI for the difference, –4.3% to 7.7%). Treatment with telavancin achieved higher cure rates in patients with monomicrobial S. aureus infection and comparable cure rates in patients with MRSA infection; in patients with mixed gram-positive/gram-negative infections, cure rates were higher in the vancomycin group. Incidence and types of adverse events were comparable between the treatment groups. Mortality rates for telavancin-treated versus vancomycin-treated patients were 21.5% versus 16.6% (95% CI for the difference, –0.7% to 10.6%) for study 0015 and 18.5% versus 20.6% (95% CI for the difference, –7.8% to 3.5%) for study 0019. Increases in serum creatinine level were more common in the telavancin group (16% vs 10%).
Conclusions. The primary end point of the studies was met, indicating that telavancin is noninferior to vancomycin on the basis of clinical response in the treatment of HAP due to gram-positive pathogens.
Hospital-acquired pneumonia (HAP) is the second most common nosocomial infection and the leading cause of mortality attributable to these critical infections [ 1– 3]. Staphylococcus aureus, particularly methicillin-resistant S. aureus (MRSA), is now a major cause of HAP [ 4– 6]. Rates of clinical failure in patients with HAP due to MRSA are high [ 7, 8]. Currently, only vancomycin and linezolid are recommended for treatment of HAP due to MRSA [ 9]. Results from recent pneumonia trials with new antibiotics active against MRSA have not been encouraging [ 10– 12]. Therefore, additional antistaphylococcal agents for treatment of HAP are urgently needed.
Telavancin is a lipoglycopeptide antibacterial agent exhibiting potent, concentration-dependent bactericidal effects via a dual mechanism of action that combines inhibition of cell wall synthesis and disruption of membrane barrier function [ 13– 15]. In vitro, telavancin is rapidly bactericidal against clinically important gram-positive bacteria, including MRSA, vancomycin-intermediate S. aureus, and penicillin-resistant S. pneumoniae [ 13, 16, 17].
Telavancin penetrates well into the epithelial lining fluid and alveolar macrophages of healthy subjects, achieving concentrations up to 8-fold and 85-fold, respectively, above telavancin's minimum inhibitory concentration (MIC) for 90% (MIC90) of MRSA strains (.5 μg/mL) [ 16, 18]. Unlike daptomycin (a cyclic lipopeptide), telavancin remains active in vitro in the presence of pulmonary surfactant [ 16]. Telavancin is approved in the United States and Canada for the treatment of adult patients with complicated skin and skin-structure infections due to susceptible gram-positive pathogens.
The current studies were designed to assess the clinical efficacy and safety of telavancin compared with vancomycin in the treatment of HAP due to gram-positive organisms, with a focus on infections due to MRSA. Partial results of these studies have been previously reported.
METHODS
The Assessment of Telavancin for Treatment of Hospital-Acquired Pneumonia (ATTAIN) studies were 2 identical randomized, double-blind, comparator-controlled, parallel-group phase III trials, 0015 and 0019 (NCT00107952 and NCT00124020), with patients enrolled between January 2005 and June 2007. The institutional review board at each site approved the protocol, and all patients or their authorized representatives provided written informed consent.
Patient selection.
Male and nonpregnant female patients aged ≥18 years were eligible for enrollment if they had pneumonia acquired after 48 h in an inpatient acute or chronic care facility or that developed within 7 days after being discharged. Patients were required to have ≥2 of the following: cough, purulent sputum, auscultatory findings, dyspnea, tachypnea, or hypoxemia; or identification of an organism consistent with a respiratory pathogen isolated from respiratory tract or blood. In addition, patients were also required to have ≥2 of the following: fever (temperature >38°C) or hypothermia (rectal/core temperature <35°C); respiratory rate >30 breaths/min; pulse rate ≥120 beats/min; altered mental status; need for mechanical ventilation; and white blood cell count >10,000 cells/mm3, <4500 cells/mm3, or >15% immature neutrophils (band forms). All patients were required to have new or progressive infiltrates, consolidation, with or without pleural effusion on chest radiograph (or computed tomography), and an adequate respiratory specimen for Gram stain and culture.
Patients were excluded if they had any of the following: receipt of potentially effective systemic antibiotic therapy for gram-positive pneumonia for >24 h immediately prior to randomization (unless there was documented clinical failure after 3 days of therapy or if the pathogen was resistant in vitro to previous treatment); only gram-negative bacteria seen on Gram stain or culture; baseline QTc interval >500 msec, uncompensated heart failure; absolute neutrophil count <500 cells/mm3; or pulmonary disease that precludes evaluation of therapeutic response (eg, lung cancer, active tuberculosis, cystic fibrosis, or granulomatous disease).
Randomization and treatment regimens.
The ATTAIN trials were double-blinded studies in which patients were randomized (1:1) to receive either intravenous (IV) telavancin at a dosage of 10 mg/kg every 24 h or vancomycin at a dosage of 1 g IV every 12 h for 7–21 days. Randomization was conducted through an interactive voice response system using permuted block algorithm and stratifying by country group, presence of diabetes, and ventilatory status. The vancomycin regimen could be monitored and adjusted according to the institutional policy at each site but had to be performed such that blinding was not compromised. The dose of telavancin was adjusted in patients with creatinine clearance ≤50 mL/min. For patients with pneumonia due to suspected or proven methicillin-susceptible S. aureus (MSSA), a switch to antistaphylococcal penicillin from vancomycin was permitted. In patients with polymicrobial (mixed gram-positive/gram-negative) infection, concomitant therapy with aztreonam or piperacillin-tazobactam was allowed.
Assessments.
Clinical assessments were performed at baseline and daily throughout study treatment, at the end of therapy (EOT), and at follow-up/test of cure visit (FU/TOC). Laboratory assessments were performed every 3 days up to the EOT. FU/TOC assessment was conducted 7–14 days after EOT.
Respiratory samples (invasive or noninvasive) and 2 blood culture specimens were obtained at baseline for Gram stain and culture [ 9]. Isolated pathogens were submitted to a central laboratory for confirmation of identity and susceptibility testing [ 19].
Telavancin plasma samples for pharmacokinetic analyses were obtained at some centers, as were vancomycin trough levels, in accordance with site-specific procedures.
Efficacy and safety variables.
Clinical responses at FU/TOC were defined as follows. Cure was defined as improvement or lack of progression of baseline radiographic findings at EOT and resolution of signs and symptoms of pneumonia at FU/TOC. Failure was defined as persistence or progression of signs and symptoms or progression of radiological signs of pneumonia at EOT; termination of study medications due to “lack of efficacy” and initiation within 2 calendar days of a different potentially effective antistaphylococcal medication; death on or after day 3 attributable to primary infection; or relapsed infection at TOC after termination of study medications. Indeterminate response was defined as the inability to determine outcome. Adverse events (AEs), vital signs, electrocardiograms, and laboratory parameters were also evaluated.
Analysis populations.
The all-treated (AT) population included all randomized patients who received ≥1 dose of study medication. The modified all-treated (MAT) population consisted of patients in the AT population who had a respiratory pathogen identified from baseline samples (or from blood cultures if no respiratory sample was positive). The clinically evaluable (CE) population consisted of patients in the AT population who were protocol-adherent or who died on or after study day 3, if death was attributable to the HAP episode under study. The microbiologically evaluable (ME) population consisted of CE patients who had a gram-positive respiratory pathogen recovered from baseline respiratory specimens or blood cultures. The safety population included patients who received ≥1 dose of study medication.
Statistical analyses.
The primary efficacy end point of each study was clinical response at FU/TOC in the AT and CE populations. Failure at EOT was carried forward to FU/TOC. Two-sided 95% confidence intervals (CIs) were calculated on the difference in response rate; pooled-study CIs were stratified on study. The primary efficacy end point was tested for the noninferiority of telavancin compared with vancomycin in both the AT and CE populations in each study, using a prespecified non-inferiority margin of 20% and a 1-sided significance level of .025. Assuming that 35% of enrolled patients would be in the CE group and that cure rates would be 60% in both treatment groups, 312 enrolled patients per treatment arm would provide 109 CE patients and statistical power of 86% to achieve noninferiority. A key prespecified secondary objective was to perform a 2-study pooled analysis of telavancin superiority compared with vancomycin treatment in patients with pneumonia due to MRSA. Post hoc analyses of cure rates at FU/TOC visit by baseline pathogen characteristics (methicillin resistance status, vancomycin MIC, and evidence for mixed gram-negative/gram-positive infection) were also performed; statistical inferential statements are not adjusted for multiple comparisons.
Deaths through FU/TOC were summarized. If there was no FU/TOC visit, any death reported to have occurred within 28 days after end of treatment was included in the analysis. Within this article, results are presented from analysis of the pooled datasets from the 2 studies. Primary efficacy results are also presented for each study separately.
RESULTS
Disposition of patients.
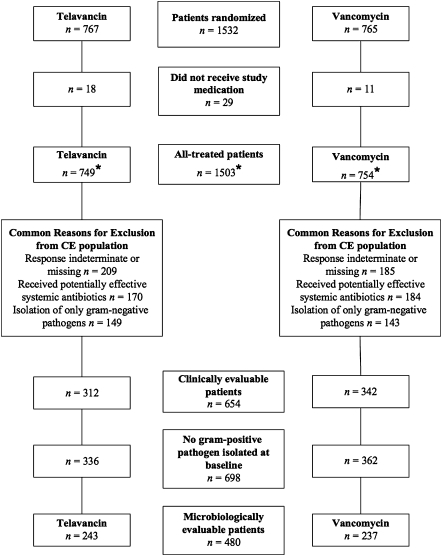
A total of 1532 patients from 274 study sites in 38 countries were randomized ( Figure 1). In all, 1503 patients received ≥1 dose of study medication (telavancin, n = 749; vancomycin, n = 754; AT population). The most common reasons for exclusion from the CE population were indeterminate or missing responses at FU/TOC, receipt of potentially effective nonstudy systemic antibiotics, and isolation of only gram-negative bacteria ( Figure 1). The most common reasons for missing or indeterminate responses at FU/TOC were patient death due to causes other than HAP and presence of gram-negative pathogen only, respectively.
Figure 1.
Patient disposition for studies 0015 and 0019. Patients could have >1 reason for exclusion from either the clinically evaluable (CE) or microbiologically evaluable (ME) populations. *Among those randomized to receive vancomycin, 20 patients had treatment switched to antistaphylococcal penicillins, and 2 patients in study 0019 who were randomized to receive vancomycin actually received telavancin. These 2 patients are included in the vancomycin group for the efficacy analysis (all-treated population) but were included in the telavancin group for the safety analysis. Because of this protocol deviation, neither patient was included in the CE population.
Baseline and demographic characteristics.
Baseline and demographic variables were comparable between treatment groups ( Table 1). Patients aged ≥65 years accounted for more than one-half of those enrolled and treated in both treatment groups. More than one-half of the patients were in intensive care units at baseline. Common co-morbidities included diabetes mellitus, chronic obstructive pulmonary disease, and acute and/or chronic renal failure. Almost two-thirds of patients had multilobar infiltrates, and nearly one-third had pleural effusions. Bacteremia was present in ∼6% of patients. More than one-half of the patients received antibiotic therapy for ≥24 h prior to enrollment. Among these patients, the most common reasons allowing enrollment were clinical failure despite prior antibiotic therapy and development of pneumonia while receiving antibacterials for other indications.
Table 1.
Baseline and Demographic Characteristics for the Pooled Studies All-Treated Population
| Characteristic | Telavancin (n= 749) | Vancomycin (n= 754) | P |
| Age, mean years ± SD | 62 ± 18.5 | 63 ± 17.7 | .40 |
| Age ≥65 years | 397 (53) | 408 (54) | .68 |
| Female sex | 262 (35) | 285 (38) | .26 |
| Race | .68 | ||
| White | 515 (69) | 526 (70) | |
| African American | 25 (3) | 20 (3) | |
| Asian | 172 (23) | 178 (24) | |
| Other | 37 (5) | 30 (4) | |
| Diabetes | 203 (27) | 191 (25) | .45 |
| Congestive heart failure | 130 (17) | 141 (19) | .50 |
| COPD | 173 (23) | 178 (24) | .86 |
| Chronic renal failure | 43 (6) | 52 (7) | .40 |
| Acute renal failure | 73 (10) | 64 (8) | .42 |
| CrCL ≤50 mL/min | 255 (34) | 250 (33) | .74 |
| Hemodialysis | 14 (2) | 14 (2) | 1.0 |
| Admission to ICU | 431 (58) | 440 (58) | .75 |
| Use of vasopressor/inotropic | 54 (7) | 89 (12) | .003 |
| Shock | 29 (4) | 41 (5) | .18 |
| ARDS | 33 (4) | 30 (4) | .70 |
| ALI | 51 (7) | 33 (4) | .04 |
| APACHE II score, mean ± SD a | 15 ± 6.1 | 16 ± 6.2 | .11 |
| APACHE II score ≥20 | 167 (22) | 191 (25) | .18 |
| Bacteremia at onset b | 48 (6) | 44 (6) | .67 |
| VAP | 216 (29) | 211 (28) | .73 |
| Signs of pneumonia | |||
| Fever (temperature >38°C) | 558 (74) | 552 (73) | .60 |
| WBC count >10,000 cells/mm3c | 412 (65) | 408 (65) | .95 |
| Purulent secretions | 677 (90) | 705 (94) | .03 |
| PaO2/FiO2, mean ± SD d | 254 ± 142 | 244 ± 125 | .69 |
| Heart rate >120 beats/min | 139 (19) | 144 (19) | .79 |
| Respiratory rate >30 breaths/min | 242 (32) | 246 (33) | .91 |
| SIRS e | 624 (83) | 632 (84) | .84 |
| Radiological characteristics | |||
| Multilobar infiltrates | 473 (63) | 460 (61) | .40 |
| Pleural effusion | 237 (32) | 244 (32) | .78 |
| Prior antibiotic use (>24 h) | 391 (52) | 427 (57) | .09 |
| Developed pneumonia while on antibiotic treatment for other indication | 189 (25) | 208 (28) | .94 |
| Clinical failure of prior antibiotics | 215 (29) | 211 (28) | .12 |
| Resistant organisms | 92 (12) | 102 (14) | .94 |
NOTE. Data are no. (%) of patients, unless otherwise indicated. ALI, acute lung injury [ 20]; APACHE, Acute Physiology and Chronic Health Evaluation; ARDS, adult respiratory distress syndrome [ 21]; COPD, chronic obstructive pulmonary disease; CrCL, creatinine clearance; ICU, intensive care unit; SD, standard deviation; SIRS, systemic inflammatory response syndrome; VAP, ventilator-associated pneumonia; WBC, white blood cells.
Complete APACHE II scores (21) were available for 396 and 406 patients in the telavancin and vancomycin arms, respectively. For the remaining patients, APACHE II scores were computed with the available data plus missing components imputed with a value of zero.
Any respiratory pathogen recovered from baseline blood cultures.
Denominator is based on patients with a baseline WBC count.
Relates to patients receiving mechanical ventilation.
Defined as the presence of 2 or more of the following 4 criteria: (1) temperature >38°C or <36°C; (2) heart rate >90 beats/min; (3) respiration >20 breaths/min or PaCO2 <32 mm Hg; (4) leukocyte count >12,000 cells/mm3 or <4000 cells/mm3, or >10% immature (band) cells.
Most of the respiratory sampling was via expectorated sputum or endotracheal aspirates (particularly in intubated patients), with <20% of patients undergoing more-invasive procedures ( Table 2). S. aureus was the most common gram-positive pathogen isolated from the respiratory tract; the majority (60%) of S. aureus isolates were MRSA ( Table 2). Mixed infections (ie, infections due to gram-positive and gram-negative pathogens) were present in 27% of patients. The most common gram-negative pathogens were Pseudomonas aeruginosa, Klebsiella pneumoniae, and Acinetobacter species.
Table 2.
Microbiological Characteristics at Study Entry for the Pooled Studies Microbiologically Evaluable Population
| No. (%) of patients |
||
| Characteristic | Telavancin(n = 243) | Vancomycin(n = 237) |
| Respiratory tract samples a | ||
| Sputum | 96 (40) | 115 (49) |
| Endotracheal aspiration | 100 (41) | 92 (39) |
| Invasive techniques b | 41 (17) | 31 (13) |
| Other c | 3 (1) | 4 (2) |
| Type of respiratory pathogen d | ||
| Gram-positive only | 175 (72) | 174 (73) |
| Mixed (gram-positive and gram-negative) | 68 (28) | 63 (27) |
| Respiratory samples | ||
| Staphylococcus aureuse | 215 (88) | 213 (90) |
| MRSA | 136 (56) | 154 (65) |
| MSSA | 83 (34) | 61 (26) |
| Streptococcus pneumonia | 20 (8) | 21 (9) |
| Pseudomonas aeruginosa | 34 (14) | 22 (9) |
| Acinetobacter species | 17 (7) | 13 (5) |
| Klebsiella pneumoniae | 11 (5) | 20 (8) |
| Other gram-negative organisms | 24 (10) | 19 (8) |
| Blood d | ||
| S. aureus | 14 (6) | 9 (4) |
| MRSA | 9 (4) | 6 (3) |
| MSSA | 5 (2) | 3 (1) |
| Gram-negative pathogens | 1 (<1) | 6 (3) |
NOTE. MRSA, methicillin-resistant S. aureus; MSSA, methicillin-susceptible S. aureus.
Percentages do not add to 100% because a small proportion of patients did not have a respiratory sample taken (telavancin group) or had >1 sampling method (vancomycin group).
Invasive techniques included bronchoalveolar lavage (BAL), mini-BAL, and blind bronchial suctioning.
Other methods were specified as quantitative tracheal lavage and protected specimen brush.
Includes 4 patients in the telavancin group and 1 patient in the vancomycin group with pathogens isolated exclusively from blood cultures.
Includes 4 patients in the telavancin group and 2 patients in the vancomycin group with both MRSA and MSSA.
The MIC90 for both MRSA and MSSA was .5 μg/mL for telavancin and 1 μg/mL for vancomycin. The mean (± standard deviation [SD]) predose plasma concentration (Ctrough) of telavancin for patients in the 2 studies were 10–12 μg/mL. Telavancin mean peak plasma concentration was ∼70 μg/mL. In those patients for whom >1 vancomycin serum trough level was obtained (n = 226), mean trough levels were ≥5 μg/mL in 94% (n = 212) and ≥10 μg/mL in 66% (n = 149) of patients.
Primary outcome measures.
Cure rates at FU/TOC in the 2 treatment groups were similar in each study ( Table 3). Results from studies 0015 and 0019 each met the criterion for noninferiority of telavancin compared with vancomycin. The 95% CI for the treatment difference between the 2 regimens from each study overlapped, supporting pooling of the data. Thus, cure rates in the pooled AT population were 58.9% for telavancin and 59.5% for vancomycin (95% CI for the difference, –5.6% to 4.3%), whereas in the pooled CE patients, cure rates were 82.4% for telavancin and 80.7% for vancomycin (95% CI for the difference, –4.3% to 7.7%). The most commonly listed reason for failure at FU/TOC in AT and CE patients in both treatment groups was treatment failure at EOT ( Table 4).
Table 3.
Cure Rates for Hospital-Acquired Pneumonia at Follow-up/Test-of-Cure Visit
| Study, analysis population | Telavancin group,% (proportion) of patients | Vancomycin group, a% (proportion) of patients | Treatment difference,% of patients (95% CI) |
| Study 0015 | |||
| AT | 57.5 (214/372) | 59.1 (221/374) | −1.6 (–8.6 to 5.5) |
| CE | 83.7 (118/141) | 80.2 (138/172) | 3.5 (–5.1 to 12.0) |
| Study 0019 | |||
| AT | 60.2 (227/377) | 60.0 (228/380) | 0.2 (–6.8 to 7.2) |
| CE | 81.3 (139/171) | 81.2 (138/170) | 0.1 (–8.2 to 8.4) |
| Pooled data | |||
| AT | 58.9 (441/749) | 59.5 (449/754) | −0.7 (–5.6 to 4.3) |
| CE | 82.4 (257/312) | 80.7 (276/342) | 1.7 (–4.3 to 7.7) |
| ME | 79.0 (192/243) | 76.8 (182/237) | 2.2 (–5.2 to 9.7) |
NOTE. AT, all-treated population; CE, clinically evaluable population; CI, confidence interval; ME, microbiologically evaluable population.
Includes 20 AT patients and 6 CE patients who received antistaphylococcal penicillins instead of vancomycin.
Table 4.
Reasons for Treatment Failure at Follow-up/Test-of-Cure Visit for Pooled Studies
| Telavancin,% (proportion) of patients |
Vancomycin,
a % (proportion) of patients |
|||
| Variable | AT group | CE group | AT group | CE group |
| Failure at EOT | 11.1 (83/749) | 15.7 (49/312) | 13.5 (102/754) | 17.3 (59/342) |
| Death on or after day 3 attributable to HAP | 3.1 (23/749) | 5.4 (17/312) | 2.1 (16/754) | 3.5 (12/342) |
| Death after EOT attributable to HAP | 0.7 (5/749) | 0.3 (1/312) | 0.1 (1/754) | 0.0 (0/342) |
| Relapsed pneumonia | 1.3 (10/749) | 1.3 (4/312) | 2.1 (16/754) | 2.0 (7/342) |
| Total | 13.2 (99/749) | 17.6 (55/312) | 15.9 (120/754) | 19.3 (66/342) |
NOTE. AT, all-treated population; CE, clinically evaluable population; EOT, end of treatment; HAP, hospital-acquired pneumonia.
Includes 6 patients who received antistaphylococcal penicillin instead of vancomycin.
Secondary outcome measures.
In patients with pneumonia due to MRSA with or without other pathogens, the clinical response at FU/TOC between the treatment groups was similar ( Table 5). Cure rates were higher in the telavancin group in patients with monomicrobial infection due to S. aureus, and this was consistent for both patients with MRSA and those with MSSA. Telavancin cure rates were also higher in patients infected with S. aureus that demonstrated a vancomycin MIC ≥1 μg/mL ( Table 5). Lower cure rates in patients with mixed infections were observed in the telavancin group. In patients with mixed infections who received adequate gram-negative coverage, cure rates were similar between the 2 groups ( Table 5).
Table 5.
Cure Rates at Follow-up/Test-of-Cure Visit by Baseline Pathogen for the Pooled Microbiologically Evaluable Population
| Infection type | Telavancin,% (proportion) of patients | Vancomycin, a % (proportion) of patients | Treatment difference,% of patients (95% CI) |
| All Staphylococcus aureusb | 78.1 (171/219) | 75.2 (161/214) | 3.0 (–5.0 to 11.0) |
| All MRSA c | 74.8 (104/139) | 74.7 (115/154) | 0.4 (–9.5 to 10.4) |
| Monomicrobial S. aureus | 84.2 (123/146) | 74.3 (113/152) | 9.9 (0.7 to 19.1) |
| Vancomycin MIC ≤0.5 μg/mL d | 89.2 (33/37) | 78.6 (22/28) | 10.1 (−9.0 to 28.8) |
| Vancomycin MIC ≥1 μg/mL e | 87.1 (74/85) | 74.3 (78/105) | 12.5 (0.5 to 23.0) f |
| MRSA | 81.8 (72/88) | 74.1 (86/116) | 7.9 (–3.5 to 19.3) |
| MSSA | 87.9 (51/58) | 75.0 (27/36) | 12.2 (–4.2 to 28.8) |
| Streptococcus pneumoniae | 90.0 (18/20) | 85.7 (18/21) | 5.9 (–19.1 to 29.7) |
| Mixed infections g | 66.2 (45/68) | 79.4 (50/63) | –12.6 (–26.9 to 3.2) |
| Mixed infections with adequate gram-negative therapy h | 63.2 (12/19) | 66.7 (14/21) | –0.8 (–28.9 to 25.7) |
NOTE. CI, confidence interval; MIC, vancomycin minimum inhibitory concentration; MRSA, methicillin-resistant S. aureus; MSSA, methicillin-susceptible S. aureus.
Includes 5 microbiologically evaluable patients who received antistaphylococcal penicillins instead of vancomycin.
S. aureus with and without concomitant pathogens; includes 4 patients in the telavancin group and 1 patient in the vancomycin group with pathogens isolated exclusively from blood cultures.
MRSA with and without concomitant pathogens.
All vancomycin MICs = 0.5 μg/mL, except for 1 patient in the telavancin group with MIC ≤0.25 μg/mL.
All vancomycin MICs = 1.0 μg/mL, except for 2 patients in the telavancin group with MIC = 2.0 μg/mL.
P = .03.
Mixed gram-positive and gram-negative infections.
Inadequate gram-negative coverage was defined as not having received an antibiotic to which the recovered gram-negative pathogen was susceptible until study day 3 or later or not receiving such an antibiotic at all during study treatment.
Safety analysis.
The overall incidence of AE was comparable in the 2 groups ( Table 6). For study 0015, 80 (21.5%) of 372 telavancin-treated patients died, and 62 (16.6%) of 374 vancomycin-treated patients died (95% CI for the difference, –0.7% to 10.6%). For study 0019, 70 (18.5%) of 379 telavancin-treated patients died, and 78 (20.6%) of 378 vancomycin-treated patients died (95% CI for the difference, –7.8% to 3.5%).
Table 6.
Safety parameters for the Pooled Studies Safety Population
| No. (%) of patients |
||
| Safety parameter | Telavancin group (n= 751) | Vancomycin group a (n= 752) |
| Death b | 150 (20.0) | 140 (18.6) |
| Any TEAE | 616 (82) | 613 (82) |
| Any serious AE | 234 (31) | 197 (26) |
| Discontinued medication due to TEAE | 60 (8) | 40 (5) |
| TEAE ≥5% in any treatment arm | ||
| Diarrhea | 85 (11) | 92 (12) |
| Renal impairment c | 74 (10) | 57 (8) |
| Anemia | 64 (9) | 85 (11) |
| Constipation | 70 (9) | 71 (9) |
| Hypokalemia | 61 (8) | 80 (11) |
| Hypotension | 48 (6) | 52 (7) |
| Nausea | 40 (5) | 31 (4) |
| Decubitus ulcer | 39 (5) | 44 (6) |
| Insomnia | 34 (5) | 47 (6) |
| Peripheral edema | 34 (5) | 38 (5) |
NOTE. AE, adverse event; TEAE, treatment-emergent adverse event.
Includes 20 patients who received antistaphylococcal penicillin instead of vancomycin and 2 patients randomized to vancomycin who actually received telavancin.
Point estimate (95% confidence interval) on the treatment difference (telavancin minus vancomycin) in death rate, –1.4% (–2.6% to 5.3%).
Includes renal impairment, renal insufficiency, acute renal failure, chronic renal failure, and creatinine level increase.
Most common treatment-emergent AEs (TEAEs) in both treatment groups were diarrhea, anemia, hypokalemia, constipation, and renal impairment ( Table 6). The incidences of serious AEs (SAEs) and TEAEs leading to discontinuation of study medication were higher in the telavancin group (31% vs 26% and 8% vs 5%, respectively). The most common SAEs in patients receiving telavancin and those receiving vancomycin were septic shock (4% vs 4%), respiratory failure (3% vs 3%), and multiorgan failure (3% vs 2%). The most frequently reported AE leading to study medication discontinuation was acute renal failure (1.2%) in telavancin-treated patients and septic shock (0.7%) in vancomycin-treated patients.
Potentially clinically significant increases in serum creatinine levels (>50% increase from baseline and a maximum value >1.5 mg/dL) were more common in the telavancin group than in the vancomycin group (16% vs 10%). Other than creatinine level increases, the most common abnormalities in both treatment groups were anemia, abnormal serum potassium levels, and hepatic enzyme abnormalities ( Table 7). All of these abnormalities occurred with similar frequencies in the 2 treatment groups.
Table 7.
Laboratory Abnormalities in Patients with Normal Values at Baseline for the Pooled Studies Safety Population
| Proportion (%) of patients | ||
| Variable | Telavancin group | Vancomycin group |
| Hematocrit | ||
| Male sex, ≤30% | 13/99 (13) | 17/106 (16) |
| Female sex, ≤28% | 15/97 (15) | 16/93 (17) |
| WBC count <2800 cells/μL | 1/251 (<1) | 6/243 (2) |
| Platelet count ≤75,000 platelets/μL | 6/370 (2) | 10/403 (2) |
| AST level ≥3 ULN | 23/359 (6) | 17/358 (5) |
| ALT level ≥3 ULN | 22/398 (6) | 33/411 (8) |
| Alkaline phosphatase level ≥2 ULN | 23/469 (5) | 40/505 (8) |
| Potassium level <3 meq/L | 50/587 (9) | 37/579 (6) |
| Potassium level >5.5 meq/L | 33/587 (6) | 32/579 (6) |
| Creatinine level increase a | 111/716 (16) | 69/723 (10) |
NOTE. ALT, alanine aminotransferase; AST, aspartate aminotransferase; ULN, upper limit of normal; WBC, white blood cell.
Serum creatinine level increased >50% from baseline and with a maximum value >1.5 mg/dL regardless of the initial value; includes patients with abnormal serum creatinine levels at baseline.
Prolongation of Fridericia-corrected QT interval (QTcF) by >60 msec occurred in 8% and 7% of the telavancin-treated and vancomycin-treated patients, respectively. A maximum QTcF interval value >500 msec occurred in a similar proportion of patients (2%) in the 2 groups. None of the patients experienced arrhythmias attributable to a prolonged QTcF interval.
DISCUSSION
The results of the ATTAIN trials reported herein demonstrate that telavancin has clinical response outcomes that are noninferior to those of vancomycin in the treatment of patients with HAP due to the gram-positive bacteria, such as S. aureus (MRSA and MSSA) and S. pneumoniae. Importantly, these findings, which incorporate data for more than 1500 patients from >250 sites around the world, are robust and consistent across all efficacy populations.
The ATTAIN trials, which enrolled almost 300 ME patients with monomicrobial S. aureus pneumonia, demonstrated that telavancin therapy achieved higher cure rates than did vancomycin therapy in the group of patients with pneumonia due to S. aureus. Higher cure rates were also observed in the telavancin group among patients infected with S. aureus that had a vancomycin MIC ≥1 mg/L.
Importantly, telavancin was effective in the treatment of patients with pneumonia due to MRSA, as well as in the treatment of those patients with pneumonia due to MSSA. The high cure rates obtained in the telavancin group support the use of telavancin as empirical therapy for suspected S. aureus pneumonia as well as its use as targeted therapy for both MRSA and MSSA infections.
A lower cure rate was associated with telavancin therapy in the subgroup of patients with mixed infections, although the difference was not statistically significant. Because telavancin has no activity against gram-negative pathogens, these findings could be a result of inadequate gram-negative therapy. This is supported by the finding that, in the subset of patients with mixed infections who received adequate gram-negative coverage, cure rates were similar in the 2 treatment groups.
Although more patients in the telavancin group experienced SAEs or had treatment discontinued due to an AE, compared with patients in the vancomycin group, the incidences of most common AEs were similar in the 2 treatment groups. Clinically significant increases in serum creatinine level were more frequent among telavancin-treated patients. In the majority of patients in both groups with significant creatinine increases, the impairment in renal function had resolved or was resolving at the last follow-up visit. The numbers of patients with QTcF interval prolongation >60 msec or those with absolute QTcF interval >500 msec were comparable between the treatment groups. Death rates were higher for telavancin-treated patients than they were for vancomycin-treated patients in study 0015, whereas the opposite trend was seen in study 0019. Although the ATTAIN trials were not optimally designed for a mortality end point, the differences observed in these studies were not statistically significant.
The strengths of the ATTAIN trials should be underscored. First, when the ATTAIN trials were conducted, the combined populations of these identically designed trials provided, to our knowledge, the largest cohort of patients studied to date for HAP. Similarly, to date, the S. aureus and MRSA subgroups are the largest such subgroups available of patients with HAP. Third, the breadth and diversity of the patient population make the results generalizable to many settings. Lastly, a significant proportion of patients enrolled in these studies were critically ill.
These studies also have limitations. First, the standard of care in much of the world for diagnosis of HAP does not include semi-invasive diagnostic procedures (eg, bronchoalveolar lavage), and the limited number of patients who underwent these procedures makes determination of the exact etiology of HAP potentially less reliable than would otherwise be the case. However, the noninvasive diagnostic techniques used in these studies followed the guidelines of the American Thoracic Society and the Infectious Diseases Society of America [ 9] and are supported by a large ventilator-associated pneumonia study that demonstrated similar outcomes for patients undergoing invasive or noninvasive diagnostic approaches [ 22]. Second, our comparator antibiotic (vancomycin) has been cited as potentially inferior to linezolid for patients with MRSA pneumonia. However, the results of the post hoc analysis of 2 previous studies of linezolid versus vancomycin are controversial [ 23]. As a result, vancomycin continues to be commonly used to treat patients with HAP in whom MRSA infection is suspected or identified [ 24]. Third, the vancomycin dosage was adjusted in accordance with institutional policies. Although such policies differed between sites, they reflect the real use of vancomycin during the time period in which these studies were conducted. Despite the unresolved controversy regarding the clinical value of determining serum levels of vancomycin, the large majority of patients for whom vancomycin levels were obtained had mean trough levels that were considered “adequate” (ie, 5–15 μg/mL) at the time the studies were conducted. Baseline renal status, as well as co-morbidities known to predispose patients to renal dysfunction, should be taken into consideration before treatment is initiated. Renal function should be monitored in all patients receiving telavancin.
In summary, the 2 large identically designed, double-blinded, randomized ATTAIN trials demonstrate that telavancin is effective in the treatment of patients with HAP caused by gram-positive pathogens. In the overall population, telavancin has an acceptable risk profile for the treatment of patients with HAP.
Members of the ATTAIN Study Group
C-S. Abboud, N. Abdullah, A. Allworth, J. Altclas, T. Amgott, A. D. Aquila, K. Ashutosh, J.W. Baddley, C.X. Bai, A. Bajpai, I. Balik, M.I.C. Barker, M.G. Bayasi, C. Beltrán, A.K. Bhattacharya, P. K. Bhattacharya, D. L. Bowton, J. Brodnan, U. Bucksteg, J. Cardoso, Y. Castaing, C. Chahin, K. Chang, C. Charters, Y.H. Chen, J. Cheníček, R. Chetambath, H.J. Choi, J.Y. Choi, N. Chowta, Y.C. Chuang, M. Clavel, M. Confalonieri, G. Criner, D. Curcio, F. De Rozario, F. Della Corte, G. Deng, S. Dhalla, A. Dhar, F. M. Díaz, R. H. Dretler, O. Dziublyk, J. Edington, A. El Sohl, W. Flynn Jr., P. Fogarty, F. Fôret, J. Fratzia, C. Freuler, C. Galletti, J.B. Garcia-Diaz, V. Gasparovic, M. J. Gehman, A. Germar, A. Gerogianni, M. Gerson, D. Ghelani, M. Giannokou-Peftoulidou, M. Giladi, V. Golin, R. Grinbaum, I. Gudelj, I. Gugila, J.B. Gupta, O. Hadjiiski, G.L. Hara, M. Heiner, K. Holn, M. Hojman, C. H. Huang, S.C. Hwang, K. H. In, A. Itzhaki, T. Janasková, M. Kaaki, T. Kavardzhiklieva, S. Keenan, G. Kekstas, A. Khoja, W.J. Kim, Y.K. Kim, U. Kivistik, S.K. Kochar, J. Koirala, I. Koksal, A. Komnos, F. Koura, J. Kraatz, L. Kucharski, I. Kuzman, J. LaForge, D. Lakey, Z. Lazic, A. Leditschke, J-Y. Lefrant, F. Lewis, J. Lipman, S. Liu, H. Lopes, T. Louie, C. Lovesio, J. Mador, M. Magaña, A.A. Mahayiddin, M. Makhviladze, I. Maia, J. Malone-Lee, E. Martinez, H. Metev, H. Minkowitz, M. Mitic-Miliki, N. Monogarova, B.C. Montaldo, M. C. Montalban, P. Montravers, E. Moore, P. Mootsikapun, R.A. Mori, G. Nackaerts, V. Nayyar, R. Norviliene, P. O'Neill, I. Oren, W. O'Riordan, A. Ortiz, A.H.D. Pacheco, H.K. Park, Y.S. Park, M.J. Park, S. Peake, T. Pejcic, Y. Pesant, G. Philteos, G. Pittoni, V. Platikanov, J. Plutinsky, I. Potasman, J.P. Pretorius, D. Pryluka, J. Pullman, R. Raz, N.M. Razali, M. Riachy, C. Rodriguez, Y. Roldan, E. Romero, C. Rondina, R. Salazar, J. Santiagual, M.K. Sarna, F.A. Sarubbi, P. Sepulveda, T. Sheftel, Y. Shehabi, P. Shitrit, E. Shmelev, J. Showalter, R. Siebert, V. Simanenkov, G. Simmons, J. Sirotiakova, V. Skerk, S. Song, H. Standiford, C. Stefanov, R. Stienecker, K. Stock, M. Street, R. Tabukashvili, D. Talwar, A. Tamariz, C. Tanaseanu, A. Timerman, T. Todisco, S. Towfigh, V. Tsvetkov, N. Tudoric, A. Valavicius, R. Valentini, C. Van Dyk, H. Van Rensburg, G. Villamizar, J-L. Vincent, C. Walker, G.F. Wang, J.H. Wang, L.S. Wang, K. Weiss, J. Welker, Z. Wen, L.A. Witty, C. Wu, P. Wu, C.T. Yang, K-Y. Yang, L. Yashyna, S. Yi, S.J. Yong, V. Yovtchev, M. M. Yusoff, T. Zhang, X. Zhou, R. Zimlichman, E. Zonova, and F. Zveibil.
Acknowledgments
Financial support. The research and publication process was supported jointly by Theravance, Inc. and Astellas Pharma Global Development, Inc.
Manuscript preparation. Theravance, Inc. (South San Francisco) provided assistance with statistical analyses. Medical writing and editorial support was provided by Ivo Stoilov and Zeena Nackerdien, Envision Scientific Solutions, funded by Astellas Pharma Global Development, Inc.
Potential conflicts of interest. E.R. has served as a consultant for Theravance, Inc., Astellas, Pfizer, Bayer, Wyeth, Merck, Atox, Ortho-McNeill, and sanofi-aventis. G.R.C. has served as a consultant for Theravance, Inc.; has received support from Cubist Pharmaceuticals and Theravance, Inc.; and serves as a consultant for Cerexa, Merck, Pfizer, Cempra, and Astellas. E.C.N. received honoraria from Theravance, Inc. and research support from Astellas and Johnson & Johnson. M.G.R. has received research support from Theravance, Inc., Hospira, Novartis, and Chiron for conducting clinical trials. G.R. has received a research grant from Pfizer. M.S.N. has served as a consultant and received honoraria from Pfizer, Merck, Astra-Zeneca, Johnson & Johnson, Theravance, Inc., Schering-Plough, and Nektar and had received research support from Nektar and Pfizer. M.H.K. has received research support from Merck, Pfizer, and Astellas. A.F.S. has either served as a consultant or investigator or has delivered promotional lectures for Astellas, Theravance, Inc., Pfizer, Merck, Johnson & Johnson, Boehringer Ingelheim, GSK, sanofi-aventis, Canyon and Medicine Comp. P.L. has served as a consultant for Smiths Medical and received honoraria from Wyeth, King Pharmaceutical, and Adolor Corporation. A.L.L. has received research support from Ortho-McNeill, Cerexa, Targanta, Optimer, and Theravance, Inc. and has received honoraria from Cubist Pharmaceuticals. C.M.L. has served as a consultant and received honoraria from Pfizer, Merck, Astra-Zeneca, and Bayer and has received independent research support from Pfizer. A.T. has served as a speaker for Astellas, Novartis, and Bayer and has research support from Pfizer. M.M.K. is a former employee of Theravance, Inc. F.C.G. is an employee of Theravance, Inc. S.L.B. is an employee of Theravance, Inc. H.D.F. is a former employee of Theravance, Inc. M.E.S. has served as a consultant for Theravance, Inc. and Trius Therapeutics, has received honoraria from Astellas, and received research support from Theravance, Inc.
References
- 1.Kollef MH. Prevention of hospital-associated pneumonia and ventilator-associated pneumonia. Crit Care Med. 2004;32:1396–1405. doi: 10.1097/01.ccm.0000128569.09113.fb. [DOI] [PubMed] [Google Scholar]
- 2.Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in medical intensive care units in the United States. National nosocomial infections surveillance system. Crit Care Med. 1999;27:887–892. doi: 10.1097/00003246-199905000-00020. [DOI] [PubMed] [Google Scholar]
- 3.Craven DE, Steger KA. Epidemiology of nosocomial pneumonia: new perspectives on an old disease. Chest. 1995;108:1S–16S. doi: 10.1378/chest.108.2_supplement.1s. [DOI] [PubMed] [Google Scholar]
- 4.Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol. 2000;21:510–515. doi: 10.1086/501795. [DOI] [PubMed] [Google Scholar]
- 5.Vincent JL, Bihari DJ, Suter PM, et al. The prevalence of nosocomial infection in intensive care units in Europe: results of the European prevalence of infection in intensive care (EPIC) study. EPIC International Advisory Committee. JAMA. 1995;274:639–644. [PubMed] [Google Scholar]
- 6.Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest. 2005;128:3854–3862. doi: 10.1378/chest.128.6.3854. [DOI] [PubMed] [Google Scholar]
- 7.Rubinstein E, Cammarata S, Oliphant T, Wunderink R. Linezolid (PNU-100766) versus vancomycin in the treatment of hospitalized patients with nosocomial pneumonia: a randomized, double-blind, multicenter study. Clin Infect Dis. 2001;32:402–412. doi: 10.1086/318486. [DOI] [PubMed] [Google Scholar]
- 8.Wunderink RG, Rello J, Cammarata SK, Croos-Dabrera RV, Kollef MH. Linezolid vs vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest. 2003;124:1789–1797. [PubMed] [Google Scholar]
- 9.American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 10.Pertel PE, Bernardo P, Fogarty C, et al. Effects of prior effective therapy on the efficacy of daptomycin and ceftriaxone for the treatment of community-acquired pneumonia. Clin Infect Dis. 2008;46:1142–1151. doi: 10.1086/533441. [DOI] [PubMed] [Google Scholar]
- 11.Maroko R, Cooper A, Dukart G, Dartois N, Gandjini H. Program and abstracts of the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy. Chicago, IL: American Society for Microbiology; Results of phase 3 study comparing a tigecycline (TGC) regimen with an imipenem/cilastatin (IMI) regimen in treatment of patients (pts) with hospital-acquired pneumonia (HAP) [abstract L-730] pp. 17–20. September 2007. [Google Scholar]
- 12.Noel GJ, Strauss RS, Shah A, Bagchi P, the BAP00248 Study Group . Program and abstracts of the 48th Annual ICAAC/IDSA 46th Annual Meeting. Washington, DC: American Society for Microbiology/Infectious Diseases Society of America; Ceftobiprole vs. ceftazidime combined with linezolid for the treatment of patients with nosocomial pneumonia [abstract K-486] pp. 25–28. October 2008. [Google Scholar]
- 13.Leuthner KD, Cheung CM, Rybak MJ. Comparative activity of the new lipoglycopeptide telavancin in the presence and absence of serum against 50 glycopeptide non-susceptible staphylococci and three vancomycin-resistant Staphylococcus aureus. J Antimicrob Chemother. 2006;58:338–343. doi: 10.1093/jac/dkl235. [DOI] [PubMed] [Google Scholar]
- 14.Higgins DL, Chang R, Debabov DV, et al. Telavancin, a multifunctional lipoglycopeptide, disrupts both cell wall synthesis and cell membrane integrity in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2005;49:1127–1134. doi: 10.1128/AAC.49.3.1127-1134.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lunde CS, Hartouni SR, Janc JW, Mammen M, Humphrey PP, Benton BM. Telavancin disrupts the functional integrity of the bacterial membrane through targeted interaction with the cell wall precursor Lipid II. Antimicrob Agents Chemother. 2009;53:3375–3383. doi: 10.1128/AAC.01710-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gotfried MH, Shaw JP, Benton BM, et al. Intrapulmonary distribution of intravenous telavancin in healthy subjects and effect of pulmonary surfactant on in vitro activities of telavancin and other antibiotics. Antimicrob Agents Chemother. 2008;52:92–97. doi: 10.1128/AAC.00875-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pace JL, Krause K, Johnston D, et al. In vitro activity of TD-6424 against Staphylococcus aureus. Antimicrob Agents Chemother. 2003;47:3602–3604. doi: 10.1128/AAC.47.11.3602-3604.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krause KM, Renelli M, Difuntorum S, Wu TX, Debabov DV, Benton BM. In vitro activity of telavancin against resistant gram-positive bacteria. Antimicrob Agents Chemother. 2008;52:2647–2652. doi: 10.1128/AAC.01398-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corey GR, Rubinstein E, Lalani T, et al. Program and abstracts of the 48th Annual ICAAC/IDSA 46th Annual Meeting. Washington, DC: American Society for Microbiology/Infectious Diseases Society of America; Telavancin for hospital-acquired pneumonia caused by S. aureus: efficacy analysis according to the in vitro susceptibility to vancomycin [abstract K-528] pp. 25–28. October 2008. [Google Scholar]
- 20.Krause KM, Friedland HD, Barriere SL, Kitt MM, Benton BM. Program and abstracts of the 48th Annual ICAAC/IDSA 46th Annual Meeting. Washington, DC: American Society for Microbiology/Infectious Diseases Society of America; Activity of telavancin against gram-positive isolates from phase 3 studies of hospital-acquired pneumonia (ATTAIN) [abstract C1–149] pp. 25–28. October 2008. [Google Scholar]
- 21.Rubinstein E, Corey GR, Stryjewski ME, et al. Program and abstracts of the 18th European Congress of Clinical Microbiology and Infectious Diseases. Barcelona, Spain: Wiley-Blackwell; Telavancin for hospital-acquired pneumonia, including ventilator-associated pneumonia: the ATTAIN studies [abstract O75] pp. 19–22. April 2008. [Google Scholar]
- 22.Rubinstein E, Corey GR, Stryjewski ME, et al. Program and abstracts of the 48th Annual ICAAC/IDSA 46th Annual Meeting. Washington, DC: American Society for Microbiology/Infectious Diseases Society of America; Telavancin for treatment of hospital-acquired pneumonia (HAP) caused by MRSA and MSSA: the ATTAIN studies [abstract K-530] pp. 25–28. October 2008. [Google Scholar]
- 23.Shorr AF, Niederman M, Kollef MH, et al. Telavancin: a novel agent for ventilator-associated pneumonia due to Staphylococcus aureus. Chest. 2008;134:s11002. [Google Scholar]
- 24.Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard M07-A7. Wayne, PA: CSLI; 2009. [Google Scholar]
- 25.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 26.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 27.The Canadian Critical Care Trials Group. A randomized trial of diagnostic techniques for ventilator-associated pneumonia. N Engl J Med. 2006;355:2619–2630. doi: 10.1056/NEJMoa052904. [DOI] [PubMed] [Google Scholar]
- 28.Powers JH, Ross DB, Lin D, Soreth J. Linezolid and vancomycin for methicillin-resistant Staphylococcus aureus nosocomial pneumonia: the subtleties of subgroup analyses. Chest. 2004;126:314–315. doi: 10.1378/chest.126.1.314. author reply 315–316. [DOI] [PubMed] [Google Scholar]
- 29.Rubinstein E, Kollef MH, Nathwani D. Pneumonia caused by methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2008;46(Suppl 5):S378–S385. doi: 10.1086/533594. [DOI] [PubMed] [Google Scholar]