Among otherwise healthy college women with a newly-diagnosed acute UTI, drinking 8 oz of 27% cranberry juice twice a day did not decrease the 6-month incidence of a second UTI compared to those drinking a placebo.
Abstract
Background. A number of observational studies and a few small or open randomized clinical trials suggest that the American cranberry may decrease incidence of recurring urinary tract infection (UTI).
Methods. We conducted a double-blind, placebo-controlled trial of the effects of cranberry on risk of recurring UTI among 319 college women presenting with an acute UTI. Participants were followed up until a second UTI or for 6 months, whichever came first. A UTI was defined on the basis of the combination of symptoms and a urine culture positive for a known uropathogen. The study was designed to detect a 2-fold difference between treated and placebo groups, as was detected in unblinded trials. We assumed 30% of participants would experience a UTI during the follow-up period.
Results. Overall, the recurrence rate was 16.9% (95% confidence interval, 12.8%–21.0%), and the distribution of the recurrences was similar between study groups, with the active cranberry group presenting a slightly higher recurrence rate (20.0% vs 14.0%). The presence of urinary symptoms at 3 days, 1–2 weeks, and at ≥1 month was similar between study groups, with overall no marked differences.
Conclusions. Among otherwise healthy college women with an acute UTI, those drinking 8 oz of 27% cranberry juice twice daily did not experience a decrease in the 6-month incidence of a second UTI, compared with those drinking a placebo.
Urinary tract infection (UTI) is one of the most commonly acquired bacterial infections in ambulatory and hospitalized populations. Approximately 11% of all women aged ≥18 years in the United States have a UTI each year. The incidence of UTI is highest among women aged 18–24 years, approaching 1 of 5 infections per year [ 1]. Although the risk of bladder UTI (cystitis) progression to pyelonephritis is negligible among otherwise healthy women, cystitis has a propensity to recur. Among otherwise healthy women aged 18–39, the 6-month risk of recurrence following a first UTI is 24% [ 2]. Approximately 5% of women with an initial UTI have multiple episodes within a year. Major risk factors for UTI among women aged 18–39 years are engaging in sexual intercourse and having a history of UTI.
Recurring UTI results in substantial direct and indirect costs to the individual, estimated at $1.6 billion annually in 1995 dollars [ 3]. One unmeasured and only recently appreciated cost to the individual is the impact of repeated courses of antibiotics on normal microbiota. A 5-day course of ciprofloxacin— often prescribed for UTI— affects ∼one-third of all taxa in the gut [ 4]. Some taxa do not rebound to pretreatment levels for as long as 6 months. Treating UTI with antibiotics selects for antibiotic resistance among uropathogens and other bacteria found in and on the human body.
Uropathogens are increasingly resistant to antibiotics in the United States and worldwide [ 5– 9]. Women with recurring UTI are prescribed repeated courses of antibiotics both as treatment and as a preventive strategy. Because antibiotic therapy is a major driver of resistance, and adversely affects the normal microbiota, preventive strategies that reduce the need for antibiotic therapy are particularly important.
The American cranberry (Vaccinium macrocarpon) is a well-known folk remedy used to prevent UTI. Laboratory analyses [ 10, 11], observational studies [ 12], and a few small or open randomized trials [ 13– 17] suggest that regularly drinking cranberry juice decreases the risk of UTI. In a randomized, blinded study involving 137 women aged ≥45 with ≥2 UTI in the past year, cranberry juice capsules were almost as effective as prophylactic trimethoprim sulfamathoxazole in preventing recurrences [ 18]. In vitro experiments have demonstrated that cranberry reduces adherence of Escherichia coli to uroepithelial cells. E. coli accounts for up to 80% of UTIs acquired in the community [ 19]. The effect of cranberry on E. coli adherence is dose-dependent; exposure to cranberry also can displace pre-attached E. coli [ 20].
If cranberry were effective in reducing recurring UTI among women at highest risk, benefits to the individual and to society would be substantial. The lack of adequately powered double-blind, randomized placebo-controlled trials of cranberry juice effectiveness in reducing risk of recurring UTI led us to conduct this study among college women presenting for treatment with acute urinary tract infection. Our overall goal was to determine the effect of regular drinking of cranberry juice cocktail (CJC) on reducing the rate of recurrent UTI and duration of symptoms, compared with placebo juice.
METHODS
Study Design
We conducted a prospective, randomized, double-blind comparison of the therapeutic efficacy of 8 oz of CJC drunk twice daily in preventing recurring UTI.
Participants
All consenting women presenting for urinalysis at the University of Michigan Health Service laboratory with symptoms of UTI between August 2005 and October 2007 were eligible for enrollment in the study if they were 18–40 years of age, had ≥3 urinary symptoms (painful and frequent urination, and report of either urgency, hematuria, or supra-pubic pressure), and would be in Ann Arbor for the next 6 months. Criteria for exclusion included antibiotic treatment over the last 48 h, hospitalization, or catheterization within the previous 2 weeks, kidney stones, diabetes, pregnancy or cranberry allergy, and a negative urine culture result. Culture results were obtained following enrollment and randomization. Participants were followed up for 6 months or until they experienced a confirmed UTI, whichever came first. Because we enrolled women from a college population, with a follow-up period of 6 months, we conducted recruitment primarily during the fall semester each year of the trial.
Therapeutic Regimen
Participants were randomly assigned to drink either 8 oz of 27% low-calorie cranberry juice cocktail twice per day or 8 oz of placebo juice twice per day for the test period of 6 months. Study juices were packaged and distributed by Fisher BioServices. Cranberry juice was provided by Ocean Spray Cranberries and was formulated under contract with NCCAM to fulfill research requirements of RFA.AT-03-004 grantees. A Drug Master File for this research grade—low-calorie juice cocktail (LCJC)—is on file with the United States Food and Drug Administration. Research juice was formulated to be similar to the commercially available Ocean Spray LCJC and was sweetened with Splenda (sucralose), exactly as is the retail juice. Commercially grown cranberries from Vaccinum macrocarpon Aiton were used for the juice production. Batches of LCJC were standardized for proanthocyanidin content. Proanthocyanidin is the cranberry juice component that is thought to produce the antiadhering activity against E. coli [ 20]. Each dose consisted of one 8-oz bottle (240 mL) containing a mean proanthocyanidin concentration of 112 mg per dose (range, 83–136 mg; standard deviation, ±14.1 mg), as measured by Fisher Bioservices by the DMAC(N,N-dimethyacetamicle) method. The placebo juice was formulated by Ocean Spray to imitate the flavor (sugar and acidity) and color of the cranberry beverage, without any cranberry content. In addition to other food and pharmaceutical-grade substances, both juices contained ascorbic acid in their formulations. Fisher Bioservices used identical bottles for the cranberry juice and the placebo beverage. All study juice (cranberry and placebo) was stored under refrigerated conditions (2–8oC) until delivery to study participants.
Patients were instructed to refrain from cranberry- or blueberry-containing foods during the study period. Study juice was delivered to participant's home every 1–2 weeks starting on the day of enrollment in order to promote compliance.
Clinical Assessments
At baseline, 3- and 6-month visits, and visits associated with a UTI symptomatic episode, participants donated a clean-catch, midstream urine specimen and self-collected vaginal and rectal specimens, which were cultured for the presence of uropathogens. Participants also completed questionnaires regarding UTI symptoms, risk and behavioral factors associated with their UTIs, diet, compliance (after baseline), gastrointestinal or any other symptoms, and medical history. Questionnaires were completed by pencil and paper on site (at enrollment and 3 and 6 months follow-up or at recurrence). We contacted participants for a telephone interview at 3 days and by email to complete a Web-based questionnaire at 1 week (which, if not completed, became a telephone interview at 2 weeks). We repeated these contacts at 1, 2, 4, and 5 months after enrollment. Web-based questionnaires were conducted using a password-protected system.
At enrollment, we instructed study participants to contact the study staff if they experienced any urinary symptoms. We also stressed to them the importance of following the study protocol. All participants were sent home with a sample collection bag and collection instructions should they experience urinary symptoms during off-clinic hours. All patients were treated for their index UTI with antibiotics deemed adequate by their treating physicians, without interference from the study. A medical record review was performed at the end of the study to confirm UTI diagnosis and to identify recurrences or adverse events in case we missed them during our follow-up. Our study protocol was approved by the University of Michigan Institutional Review Board and monitored by a data safety monitor from a different university.
Laboratory Assessments
Urinary specimens were streaked for isolation on MacConkey agar and blood agar plates for bacterial separation. A positive urine culture result was defined as ≥1000 cfu/mL urine of a known uropathogen [ 21]. Predominant colonies and colonies with unique morphologies were saved for further testing using standard methods. If multiple isolates were obtained from a single individual, they were tested for similarity using Enterobacterial Repetitive Intergenic Consensus (ERIC).
Definitions and Data Analysis
The primary end point was a confirmed UTI, defined using the same criteria as used to determine eligibility for enrollment, that occurred ≥15 days after trial enrollment or if at <15 days was with a different bacteria type or strain. ERIC PCR typing to compare enrollment and recurring UTI E. coli isolates was performed for those with a positive culture for E. coli within the first 15 days. If the recurrence isolates were identical to those found in the enrollment episode the recurrence was considered a treatment failure and the patient remained in the study. Compliance was based on self-report. A participant was considered to be compliant if she responded “no” to the question, “During the past 4 weeks, were there 2 or more days when you did not drink your cranberry juice?” and reported drinking 2 containers of juice on a typical day in the last month, and at least 1 container of juice on the previous day.
Demographic characteristics, behaviors, and medical history of the 2 treatment groups at time of enrollment were compared using the Student’s t test for continuous variables and χ2 for categorical variables. Risk of UTI was estimated per group in an intent to treat analysis. In the intent to treat analysis, we further adjusted for recent sexual behavior and a history of previous UTI in a time-dependent analysis, because these are strong determinants of recurrence. Survival until recurrence was plotted using Kaplan-Meier, and differences in survival between groups were tested using the log-rank test.
Randomization
Participants were randomly assigned to a lot number at time of enrollment. The Biometrics and Outcomes Research Core at the University of Michigan, which served as the Data Coordinating Center (DCC), provided a randomization schedule for the juice supplier by assigning labels that were placed on each box of juice.
Concealment of Allocation
At enrollment, study recruiters logged participants in a Web-based enrollment form that contained only the participant identification number and the number of the box given to the participant. When it was time to provide the participant with an additional supply of juice, the recruiter entered the participant's identification number into the same Web-based form and the database returned the number on a box that contained the same treatment regimen. In this manner the DCC was unblinded, but the investigators and their staff remained blinded to the assigned regimen until the trial was completed.
Sample Size
The study was powered to detect a 2-fold difference between treated and placebo groups, as was detected in unblinded trials, assuming that 30% of participants would experience a UTI during the follow-up period. Using a 2-sided test of significance, the study has 80% power to identify a change of this magnitude when there are 120 subjects per group. However, in an intent-to-treat analysis, if there is greater loss to follow up in the treatment group because of adverse effects, compared with the placebo group, the effect of loss to follow up is greater than simply correcting for the proportion lost. Therefore, to allow for an intent-to-treat analysis, we planned on recruiting 200 subjects per group.
RESULTS
Patient Characteristics
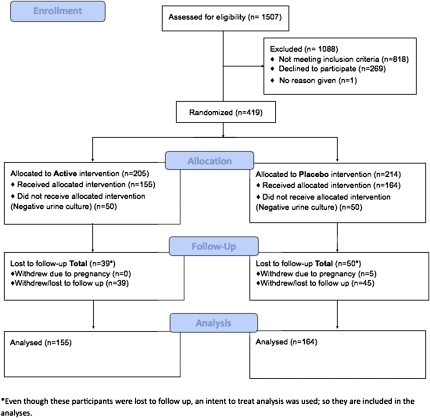
A total of 1507 women were screened for participation; 269 were unwilling to participate, and 818 were not eligible. Of the 419 enrolled and randomized women, 100 had a negative urine culture result and were therefore ineligible for participation. The remaining 319 eligible, participating women, were randomized: 155 to cranberry juice and 164 to the placebo beverage ( Figure 1).
Figure 1.
Disposition of participants in clinical trial of cranberry juice 2 times/day versus placebo. Number of study participants enrolled, allocated, followed, and analyzed shown using CONSORT 2010 Flow Diagram.
The demographic and behavioral characteristics and medical history of the 2 groups were similar at time of enrollment ( Table 1). The mean patient age in both groups was 21 years, Almost all (99%) participants had a history of ≥1 UTI; 200 (62.7%) participants reported ≥4 UTIs in their life, and 149 (46.7%) reported ≥3 UTIs in the previous 12 months. Almost all (98%) had ever engaged in sexual activity (defined as vaginal-penile intercourse, anal-penile intercourse, and/or oral-genital contact), and engaged in vaginal intercourse an average of 2–3 times per week. Most participants (92%) reported having only 1 sex partner during the previous 4 weeks. Birth control methods were similar between groups. The majority were students, and less than 10% of both groups were married or living as married. Race and ethnicity were distributed similarly by group. The most common organisms isolated from the index and recurring UTI were E. coli ( Table 2).
Table 1.
Participant Characteristics at Time of Enrollment in Clinical Trial of Cranberry Juice 2 Times/Day Versus Placebo.
| Characteristic | Cranberry (n = 155) | Placebo (n = 164) |
| Age, mean ±SD, years | 21.2 (3.4) | 21.2 (3.5) |
| No. previous UTI ever ±SD | 3.6 (4.0) | 3.8 (3.1) |
| No. UTI in past 12 months ±SD | 1.31(2.17) | 1.27 (1.48) |
| Frequency vaginal intercourse in previous 4 weeks, mean ±SD | 9.9 (7.2) | 10.2 (7.0) |
| No, of sex partners past 4 weeks, mean ±SD | 1.10 (.30) | 1.07 (.28) |
| Birth control method, n (%)* | ||
| None | 12 (8.0) | 15 (9.7) |
| Diaphragm | 1 (.7) | 2 (1.3) |
| Condoms with spermicide | 50 (33.8) | 44 (28.6) |
| Condoms without spermicide | 53 (35.8) | 61 (39.6) |
| Spermicide | 4 (2.7) | 2 (1.3) |
| Oral contraceptives | 112 (75.2) | 118 (75.2) |
| Completed college, n (%) | 38 (24.7) | 38 (23.4) |
| Married or living as married, n (%) | 11 (7.2) | 14 (8.6) |
| Race/ethnicity,* n (%) | ||
| Hispanic or Latino | 9 (5.9) | 10 (6.2) |
| White | 108 (72.5) | 126(81.3) |
| Black | 9 (6.0) | 13 (8.4) |
| Asian | 35 (23.5) | 16 (10.3) |
NOTE. Otherwise healthy young women (319) with a culture-confirmed urinary tract infection 2005–2007.
*Groups not mutually exclusive
Table 2.
Distribution of Bacterial Organisms Causing Index and Recurring Urinary Tract Infection by Treatment Group. Clinical trial of cranberry juice 2 times/day versus placebo.
| Enrollment |
Recurrence |
|||
| Organism | Cranberry (n = 155) n (%) | Placebo (n = 164) n (%) | Cranberry (n = 30) n (%) | Placebo (n = 24) n (%) |
| Escherichia coli | 121 (78.1) | 131 (79.9) | 28 (93.3) | 14 (58.3) |
| Staphylococcus saprophyticus | 9 (5.8) | 14 (8.5) | 0 (0) | 1 (4.2) |
| Enterococcus species | 9 (5.8) | 4 (2.4) | 1 (3.3) | 1 (4.2) |
| Klebsiella species | 9 (5.8) | 4 (2.4) | 0 (0) | 1 (4.2) |
| Proteus mirabilis | 2 (1.3) | 4 (2.4) | 1 (3.3) | 1 (4.2) |
| Streptococcus agalactiae | 9 (5.8) | 12 (7.3) | 1 (3.3) | 5 (20.8) |
| Other | 7 (4.5) | 12 (7.3) | 0 (0) | 1 (4.2) |
NOTE. Otherwise healthy young women (319) with a culture-confirmed urinary tract infection 2005–2007.
Some individuals had >103 cfu/mL of more than one bacteria, so numbers do not total the number of participants
Two hundred thirty (72%) of 319 participating women completed the entire protocol. Eighty-four were lost to follow-up or dropped out voluntarily, and 5 became pregnant and were excluded ( Figure 1). Participation rates for scheduled follow-up visits were similar between study groups. Overall, 116 (75%) of treatment and 114 (70%) of placebo participants completed the 6-month follow-up visit or had a prior recurrence.
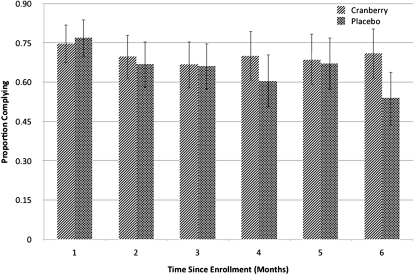
Because there is no validated test for a biological marker of cranberry juice consumption, and blinded assessments of potential tests did not distinguish between groups, compliance was assessed based on self-report. Monthly self report of compliance with the study protocol (see Methods) was very similar between the 2 groups ( Figure 2).
Figure 2.
Self-reported compliance with study protocol by treatment group and month. Clinical trial of cranberry juice 2 times/day versus placebo. Otherwise healthy young women (319) with a culture-confirmed urinary tract infection 2005–2007.
Outcome Associated with Treatment
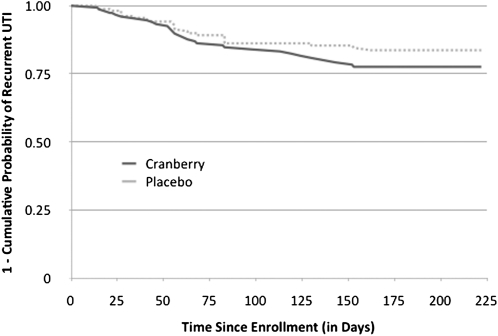
We observed 54 culture-confirmed recurrent UTI episodes (31 in cranberry and 23 in placebo). Overall, the recurrence rate was 16.9%, lower than the expected 30%. In an intent to treat analysis the distribution of the recurrences was similar between study groups, with the active cranberry group presenting a slightly higher recurrence rate (cumulative incidence rate, 19.3% vs 14.6%; log-rank P = .21). Kaplan-Meier curves of survival to recurrence are shown in Figure 3. The presence of urinary symptoms and of vaginal symptoms at 3 days, 1–2 weeks, and at ≥1 month was similar between study groups, with overall no marked differences. On monthly follow-up questionnaires, gastrointestinal symptoms were reported twice as frequently for those receiving placebo than cranberry, with the differences statistically significant in months 3 and 5. Serious adverse events occurred equally in both groups, and none were deemed to be attributable to treatment after review by the data safety monitor.
Figure 3.
Kaplan Meier curves of survival to urinary tract infection recurrence by juice assignment. clinical trial of cranberry juice 2 times/day versus placebo. Otherwise healthy young women (319) with a culture-confirmed urinary tract infection 2005–2007. The cranberry group had a higher failure rate than placebo (20% vs 14%) but the difference was not statistically significant.
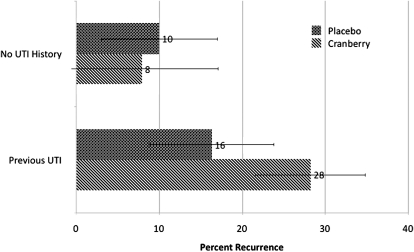
Because recent sexual activity and history of previous UTI are strong predictors of recurrence, we further analyzed the data adjusting for these factors using a time-dependent analysis. Adjustment for frequency of sexual activity in the previous month and history of UTI at time of enrollment made no difference in the risk of recurrence by treatment (unadjusted hazard ratio of cranberry versus placebo (1.4; 95% CI, 0.8–2.4); adjusted for sexual frequency and UTI history (1.6; 95% CI, .9–3.0)). There was no difference in frequency of sexual activity in the month before recurrence and frequency of sexual activity in the last month among those whose UTI did not recur. Risk of recurrence was significantly higher among those with a history of 2 or more UTIs (22% vs 10%; P = .0007). However, there was no significant effect of cranberry juice in risk of recurrence in those with or without UTI history ( Figure 4).
Figure 4.
Risk of a recurring urinary tract infection (UTI) by history of UTI and juice assignment. Participants (92/155) taking cranberry and taking placebo (87/164) reported a history of one or more UTI. Error bars show 95% confidence intervals. Clinical Trial of Cranberry Juice 2 times/day versus placebo. Otherwise healthy young women (319) with a culture-confirmed urinary tract infection 2005–2007.
DISCUSSION
We report results of a double-blind, randomized, placebo controlled trial of the effects of drinking cranberry juice on risk of recurring UTI among college-aged women. The trial was developed to detect a 2-fold difference in incidence of recurring UTI with alpha of .05 and power of 99%. The power was estimated assuming we would observe a 30% recurrence rate among controls, consistent with that reported in observational studies [ 2, 22]. Contrary to expectation, we found that drinking an 8-oz dosage of cranberry juice twice per day gave no protection against the risk of recurring UTI among college-aged women. Although previous studies in similar study populations (young, otherwise healthy sexually active women) have shown a significant reduction in UTI recurrences, these studies were not blinded [ 13] or were underpowered [ 14]. Our trial study groups were similar with respect to socio-demographic variables and known UTI risk factors, and we observed no differences in the duration or severity of UTI symptoms.
We observed a recurrence rate of 16.9% overall—almost half that expected based on the literature [ 2, 22]. It is possible that the placebo inadvertently contained the active ingredient(s) in cranberry juice. While the active ingredient was previously believed to be proanthocyanidin, and the placebo was tested accordingly, the actual active ingredient is uncertain [ 20]. Both placebo and cranberry juice contained ascorbic acid, which has also been suggested to prevent UTI [ 23], but has not been demonstrated to reduce risk in controlled trials. Another possibility to explain our results is that our study protocol kept all participants better hydrated and led them to urinate more frequently, decreasing bacterial growth and/or reducing mild urinary symptoms.
Whereas there are no other adequately powered placebo-controlled trials of cranberry juice among otherwise healthy college women [ 24], some trials have been performed in special populations. A randomized controlled trial of 305 individuals with neuropathic bladder following spinal cord injury found that ingestion of cranberry tablets did not reduce rates of UTI relative to the standard regimen [ 25]. However, a randomized controlled trial among 150 women aged 21–72 years who were randomized to receive placebo juice and placebo tablets versus placebo juice (filtered water, pineapple juice, and food coloring) and cranberry tablets, compared with cranberry juice and placebo tablets found cranberry juice and cranberry tablets reduced the number of symptomatic UTI per year (to 20% and 18% respectively) compared with placebo (to 32%) (P < .05). Of note, the rate among those taking cranberry in this trial is consistent with what we observed among both placebo and treatment groups [ 15]. Of interest, in the only other large trial of cranberry juice compared with placebo juice, conducted among 376 British in-patients ≥60 years, the incidence of symptomatic UTI was also lower than anticipated in the placebo group, but there was no significant difference between groups [ 26]. Similar to the juices used in our trial, both active and placebo juice contained ascorbic acid.
In conclusion, among otherwise healthy college women with a history of ≥1 UTIs, drinking 8 oz of 27% cranberry juice twice per day did not decrease the 6-month incidence of another UTI, compared with drinking a placebo juice.
Funding
This study was supported by a grant from the National Center for Alternative Medicine at the National Institutes of Health, R01AT002086
Acknowledgments
We thank Charlotte Williams and other laboratory personnel at the University Health Service Clinical Laboratory; Dr. Robert Winfield, Dr. Robert Ernst, and the other clinicians and nurses at the University Health Service at the University of Michigan, who made this study possible; Dr. Jack D. Sobel, , who graciously served as Data Safety Monitor; our study recruiters Patricia Arballo-Spong, Surair Bashir, Marisol LaFontaine, Mimi Lee, Kachel St Aimie Joanna Tatomir, Harolyn Tarr, Chelsey Timmer, and Julia Weinert; our juice delivery crew Ahmad Hatahet Akrofi Koram, Caitlin Murphy, Walter Olds, Joseph Rancour, Ian Spicknall, and Jung Shin; the staff at the Biometrics and Outcomes Research Center, particularly Sarr Blumson and Mariann Christy; and our study participants, for their time and effort.
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Foxman B, Brown P. Epidemiology of urinary tract infections: transmission and risk factors, incidence, and costs. Infect Dis Clin North Am. 2003;17:227–241. doi: 10.1016/s0891-5520(03)00005-9. [DOI] [PubMed] [Google Scholar]
- 2.Foxman B, Gillespie B, Koopman J, et al. Risk factors for second urinary tract infection among college women. Am J Epidemiol. 2000;151:1194–1205. doi: 10.1093/oxfordjournals.aje.a010170. [DOI] [PubMed] [Google Scholar]
- 3.Foxman B, Barlow R, D'Arcy H, et al. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000;10:509–515. doi: 10.1016/s1047-2797(00)00072-7. [DOI] [PubMed] [Google Scholar]
- 4.Dethlefsen L, Huse S, Sogin ML, et al. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schito GC, Naber KG, Botto H, et al. The ARESC study: an international survey on the antimicrobial resistance of pathogens involved in uncomplicated urinary tract infections. Int J Antimicrob Agents. 2009;34:407–413. doi: 10.1016/j.ijantimicag.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Moreira JRED, Siqueira ICD, Alcantara AP, et al. Antimicrobial resistance of Escherichia coli strains causing community-acquired urinary tract infections among insured and uninsured populations in a large urban center. J Chemother. 2006;18:255–260. doi: 10.1179/joc.2006.18.3.255. [DOI] [PubMed] [Google Scholar]
- 7.Zhanel GG, Hisanaga TL, Laing NM, et al. Antibiotic resistance in outpatient urinary isolates: final results from the North American urinary tract infection Collaborative Alliance (NAUTICA) Int J Antimicrob Agents. 2005;26:380–388. doi: 10.1016/j.ijantimicag.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Alos JI, Serrano MG, Gomez-Garces JL, et al. Antibiotic resistance of Escherichia coli from community-acquired urinary tract infections in relation to demographic and clinical data. Clin Microbiol Infect. 2005;11:199–203. doi: 10.1111/j.1469-0691.2004.01057.x. [DOI] [PubMed] [Google Scholar]
- 9.Karlowsky JA, Kelly LJ, Thornsberry C, et al. Trends in antimicrobial resistance among urinary tract infection isolates of Escherichia coli from female outpatients in the United States. Antimicrob Agents Chemother. 2002;46:2540–2545. doi: 10.1128/AAC.46.8.2540-2545.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt DR, Sobota AE. An examination of the anti-adherence activity of cranberry juice on urinary nonurinary bacterial isolates. Microbios. 1988;55:173–181. [PubMed] [Google Scholar]
- 11.Ofek I, Goldhar J, Zafriri D, et al. Anti-Escherichia coli adhesin activity of cranberry and blueberry juices. N Engl J Med. 1991;324:1599. doi: 10.1056/NEJM199105303242214. [DOI] [PubMed] [Google Scholar]
- 12.Foxman B, Zhang L, Tallman P, et al. Virulence characteristics of Escherichia coli causing first urinary tract infection predict risk of second infection. J Infect Dis. 1995;172:1536–1541. doi: 10.1093/infdis/172.6.1536. [DOI] [PubMed] [Google Scholar]
- 13.Kontiokari T, Sundqvist K, Nuutinen M, et al. Randomised trial of cranberry-lingonberry juice and Lactobacillus GG drink for the prevention of urinary tract infections in women. BMJ. 2001;322:1571. doi: 10.1136/bmj.322.7302.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker EB, Barney DP, Mickelsen JN, et al. Cranberry concentrate: UTI prophylaxis. J Fam Pract. 1997;45:167–168. [PubMed] [Google Scholar]
- 15.Stothers L. A randomized trial to evaluate effectiveness and cost effectiveness of naturopathic cranberry products as prophylaxis against urinary tract infection in women. Can J Urol. 2002;9:1558–1562. [PubMed] [Google Scholar]
- 16.Wing DA, Rumney PJ, Preslicka CW, et al. Daily cranberry juice for the prevention of asymptomatic bacteriuria in pregnancy: a randomized, controlled pilot study. J Urol. 2008;180:1367–1372. doi: 10.1016/j.juro.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrara P, Romaniello L, Vitelli O, et al. Cranberry juice for the prevention of recurrent urinary tract infections: a randomized controlled trial in children. Scand J Urol Nephrol. 2009;43:369–372. doi: 10.3109/00365590902936698. [DOI] [PubMed] [Google Scholar]
- 18.McMurdo ME, Argo I, Phillips G, et al. Cranberry or trimethoprim for the prevention of recurrent urinary tract infections? A randomized controlled trial in older women. J Antimicrob Chemother. 2009;63:389–395. doi: 10.1093/jac/dkn489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stamm WE. Scientific and clinical challenges in the management of urinary tract infections. Am J Med. 2002;113:1S–4S. doi: 10.1016/s0002-9343(02)01053-7. [DOI] [PubMed] [Google Scholar]
- 20.Guay DR. Cranberry and urinary tract infections. Drugs. 2009;69:775–807. doi: 10.2165/00003495-200969070-00002. [DOI] [PubMed] [Google Scholar]
- 21.Warren JW, Abrutyn E, Hebel JR, et al. Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA) Clin Infect Dis. 1999;29:745–758. doi: 10.1086/520427. [DOI] [PubMed] [Google Scholar]
- 22.Hooton TM, Scholes D, Hughes JP, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. N Engl J Med. 1996;335:468–474. doi: 10.1056/NEJM199608153350703. [DOI] [PubMed] [Google Scholar]
- 23.Carlsson S, Wiklund NP, Engstrand L, et al. Effects of pH, nitrite, and ascorbic acid on nonenzymatic nitric oxide generation and bacterial growth in urine. Nitric Oxide. 2001;5:580–586. doi: 10.1006/niox.2001.0371. [DOI] [PubMed] [Google Scholar]
- 24.Jepson RG, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2008:CD001321. doi: 10.1002/14651858.CD001321.pub4. [DOI] [PubMed] [Google Scholar]
- 25.Lee BB, Haran MJ, Hunt LM, et al. Spinal-injured neuropathic bladder antisepsis (SINBA) trial. Spinal Cord. 2007;45:542–550. doi: 10.1038/sj.sc.3101974. [DOI] [PubMed] [Google Scholar]
- 26.McMurdo ME, Bissett LY, Price RJ, et al. Does ingestion of cranberry juice reduce symptomatic urinary tract infections in older people in hospital? A double-blind, placebo-controlled trial. Age Ageing. 2005;34:256–261. doi: 10.1093/ageing/afi101. [DOI] [PubMed] [Google Scholar]