On the Thai-Myanmar border, Plasmodium vivax is the most common cause of parasitological failure following treatment for acute falciparum malaria. Slowly eliminated antimalarials significantly reduce the risk of early recurrence.
Abstract
(See editorial commentary by Baird, on pages 621–623.)
Background. Plasmodium vivax malaria commonly follows treatment of falciparum malaria in regions of co-endemicity. This is an important cause of preventable morbidity.
Methods. We examined the factors contributing to the risk of recurrence of P. vivax infection after treatment of acute falciparum malaria in a series of clinical trials conducted on the Thai-Myanmar border from 1991 through 2005.
Results. Overall, 10,549 patients (4960 children aged <15 years and 5589 adults) were treated for falciparum malaria; of these patients, 9385 (89.0%) had Plasmodium falciparum monoinfection and 1164 (11.0%) had mixed P. falciparum/P. vivax infections according to microscopic examinations performed at screening. The cumulative proportion of patients with P. falciparum infection recurrence by day 63 was 21.5% (95% confidence interval [CI], 20.3%–22.8%), and the cumulative proportion with P. vivax infection recurrence was 31.5% (95% CI, 30.1%–33.0%). Significant risk factors for P. vivax infection recurrence were mixed infection at enrollment, male sex, younger age, lower hematocrit, higher asexual P. falciparum parasite density (P < .001 for all factors), and P. falciparum gametocytemia at enrollment (P = .001). By day 63, the cumulative risk of vivax malaria after P. falciparum monoinfection was 51.1% (95% CI, 46.1%–56.2%) after treatment with rapidly eliminated drugs (t1/2 <1 day), 35.3% (95% CI, 31.8%–39.0%) after treatment with intermediate half-life drugs (t1/2 1–7 days), and 19.6% (95% CI, 18.1%–21.3%) after treatment with slowly eliminated drugs (t1/2 > 7 days) (P < .001, by test for trend). Artemisinin-based combinations containing mefloquine or piperaquine, compared with the artemether-lumefantrine and artesunate-atovaquone-proguanil combinations, were associated with a 3.6-fold to 4.2-fold lower adjusted hazard ratio for P. vivax infection recurrence within 63 days after pure or mixed P. falciparum infections (P < .001, for comparisons with artesunate-mefloquine).
Conclusions. On the Thai-Myanmar border, P. vivax is the most common cause of parasitological failure after treatment for falciparum malaria. Slowly eliminated antimalarials reduce the risk of early P. vivax infection recurrence.
In Southeast Asia, the incidence of Plasmodium vivax infection after treatment of falciparum malaria is substantially greater than would be expected on the basis of entomological inoculation rates [1–7]. The reasons for this are not clear. One postulate is that contemporaneous inoculation of P. vivax and Plasmodium falciparum occurs relatively frequently and that acute P. falciparum infection suppresses P. vivax parasitemia below levels detectable by light microscopy [1, 8]. According to this hypothesis, most recurrent P. vivax infections after treatment of falciparum malaria are relapses that are due to simultaneously acquired hypnozoites [1, 8]. An alternative theory is that either P. falciparum infection or its treatment somehow precipitate blood-stage relapse from dormant, previously acquired hypnozoites [8].
Whatever the underlying mechanism, P. vivax infection recurrence after falciparum malaria carries significant morbidity, impairs clinical and hematological recovery [3, 9], and worsens the socioeconomic burden of malaria [10]. Because asexual P. vivax parasitemia after blood-stage treatment is frequently associated with concurrent gametocytemia [3, 9, 11], it is also likely to have an important role in sustaining transmission of P. vivax [12]. The efficacy of antimalarial treatment for preventing P. vivax infection recurrence is therefore an important consideration for malaria control strategies.
We have used pooled data from a large series of clinical trials conducted at Shoklo Malaria Research Unit on the Thai-Myanmar border between 1991 and 2005 to establish the effect of demographic and clinical factors as well as antimalarial elimination kinetics on the risk of P. vivax infection recurrence after P. falciparum or mixed P. vivax/P. falciparum malaria.
METHODS
Study Sites
The studies included in this analysis were performed from 1991 through 2005 at camps for displaced persons of the Karen ethnic minority and border clinics that served mainly Karen and Burmese migrant workers along the northwestern border of Thailand. In the mid-1990s, the local annual incidence of malaria was approximately 1 episode per person-year, 53% of which were due to P. vivax, 37% of which were due to P. falciparum, and 10% of which were due to mixed infection (determined according to the results of examination with light microscopy) [13]. Virtually all P. falciparum infections and ∼90% of P. vivax infections were symptomatic [13]. Standard treatment of uncomplicated falciparum malaria was mefloquine monotherapy (25 mg base/kg total dose) from 1991 through 1994 and was mefloquine (25 mg base/kg) plus artesunate (12 mg/kg over 3 days) thereafter [14].
Design of the Studies
This analysis includes 24 studies that investigated 25 different antimalarial treatment regimens. None included routine administration of primaquine (Table 1). Sixteen of the studies were randomized controlled trials of different treatments for uncomplicated falciparum malaria with or without concomitant P. vivax infection; the remainder were single-arm clinical trials conducted to assess drug efficacy or safety. None included children who weighed <5 kg or pregnant women. Two studies restricted recruitment to children ≤15 years of age, and 1 study restricted recruitment to children <5 years of age (Table 1).
Table 1.
Details of Treatment Regimens and Characteristics of Patients
| Code | Total treatment dose (total regimen duration, total number of doses) | Year(s) studied | t½ | No. of patients | Male sex, no. (%) of patients | Age, median years (90% range) | Parasitemia, median parasites/μL (90% range) |
| AAP | Artesunate 12 mg/kg (3 days, 3 doses) + atovaquone 45 mg/kg (3 days, 3 doses) + proguanil 24 mg/kg (3 days, 3 doses) | 1998–2000 | Int | 526 | 353 (67) | 20 (7–41) | 4408 (176–86,219) |
| AM7 | Artemether 12 mg/kg (7 days, 7 doses) | 1993–1996 | Short | 206 | 114 (55) | 15 (2–33) | 4850 (273–73,853) |
| AP | Atovaquone 45 mg/kg (3 days, 3 doses) + proguanil 24 mg/kg (3 days, 3 doses) | 1998–2000 | Int | 528 | 354 (67) | 20 (7–43) | 3841 (142–66,870) |
| AS3 | Artesunate 12 mg/kg (3 days, 3 doses) | 1992–1994 | Short | 5 | 3 (60) | 14 (1–25) | 105,278 (4428–151,926) |
| AS5 | Artesunate 12 mg/kg (5 days, 5 doses) | 1992–1995 | Short | 153 | 86 (56) | 5 (1–25) | 13,842 (424–430,713) |
| AS7 | Artesunate 12 mg/kg (7 days, 7 doses) | 1992–1996 | Short | 452 | 245 (54) | 10 (2–29) | 6972 (331–149,142) |
| AS7T7 | Artesunate 12 mg/kg (7 days, 7 doses) + tetracycline 112 mg/kg (7 days, 7 doses) | 1993–1995 | Short | 20 | 12 (60) | 14 (9–39) | 9396 (1065–205,230) |
| COA4 | Artemether 6.8 mg/kg (3 days, 4 doses) + lumefantrine 48 mg/kg (3 days, 4 doses) | 1995–1997 | Int | 387 | 265 (68) | 21 (9–41) | 4529 (278–88,957) |
| COA6a | Artemether 10.2 mg/kg (60 h, 6 doses) + lumefantrine 72 mg/kg (96 h, 6 doses) | 1996–1998 2000–2002 | Int | 1115 | 757 (68) | 20 (7–45) | 6414 (489–88,297) |
| COA6b | Artemether 10.2 mg/kg (96 h, 6 doses) + lumefantrine 72 mg/kg (96 h, 6 doses) | 1996–1997 | Int | 87 | 62 (71) | 22 (11–41) | 5460 (1023–78,561) |
| DP+ | DHA 6.3 mg/kg (3 days, 4 doses) + piperaquine 51.3 mg/kg (3 days, 4 doses) + either artesunate 400 mg (3 days, 4 doses) or extra DHA to achieve total dose of 12 mg/kg (3 days, 4 doses) | 2002–2003 | Long | 174 | 125 (72) | 20 (6–45) | 16,830 (415–105,630) |
| DP3 | DHA 6.3 mg/kg (3 days, 3 doses) + piperaquine 51.3 mg/kg (3 days, 3 doses) | 2003–2004 | Long | 170 | 104 (61) | 21 (6–43) | 11,304 (496–75,360) |
| DP4 | DHA 6.3 mg/kg (3 days, 4 doses) + piperaquine 51.3 mg/kg (3 days, 4 doses) | 2002–2004 | Long | 340 | 216 (64) | 22 (7–44) | 13,816 (802–94,878) |
| M25 | Mefloquine 25 mg/kg (1–2 days, 1–2 doses) | 1991–1994 | Long | 949 | 543 (57) | 14 (4–38) | 3818 (213–36,754) |
| MA | Artesunate 10 mg/kg (1 day, 3 doses) + mefloquine 15 mg/kg (1 day, 1 dose) | 1991 | Long | 323 | 190 (59) | 15 (3–38) | 3486 (249–23,652) |
| MAM1 | Artemether 4–10 mg/kg (1 day, 2–3 doses) + mefloquine 25 mg/kg (1 day, 1 dose) | 1992 | Long | 19 | 10 (53) | 20 (11–50) | 6739 (253–228,592) |
| MAM3 | Artemether 12 mg/kg (3 days, 3 doses) + mefloquine 25 mg/kg (1 day>, 1 dose) | 1993–1994 | Long | 180 | 86 (48) | 16 (5–42) | 5299 (326–78,442) |
| MAS1 | Artesunate 4 mg/kg (1 day, 1 dose) + mefloquine 25 mg/kg (1 day, 1 dose) | 1992 | Long | 152 | 94 (62) | 16 (4–35) | 4847 (315–26,892) |
| MAS3 | Artesunate 12 mg/kg (3 days, 3 doses) + mefloquine 25 mg/kg (1–2 days in 1–2 doses) | 1992–2005 | Long | 4106 | 2,533 (62) | 14 (5–39) | 7300 (349–93,085) |
| MAS5 | Artesunate 12 mg/kg (5 days, 5 doses) + mefloquine 25 mg/kg (1 day, 1 dose) | 1992–1995 | Long | 57 | 29 (51) | 6 (2–23) | 326,874 (14,472–707,962) |
| MAS7 | Artesunate 12 mg/kg (7 days, 7 doses) + mefloquine 25 mg/kg (1 day, 1 dose) | 1993–1995 | Long | 139 | 82 (59) | 7 (3–12) | 270,957 (162,778–597,555) |
| MASF | Artesunate 12 mg/kg (3 days, 3 doses) + mefloquine 25 mg/kg (3 days, 3 doses) in fixed combination | 2004–2005 | Long | 247 | 170 (69) | 20 (6–45) | 14,469 (342–92,547) |
| MQIV | Quinine 40 mg/kg (1 day, 3 doses) + mefloquine 25 mg/kg (1 day, 1 dose) | 1993 | Long | 31 | 18 (58) | 9 (4–29) | 309,177 (150,850–562,186) |
| Q7 | Quinine 210 mg/kg (7 days, 7 doses) | 1992–1993 | Short | 28 | 16 (57) | 5 (2–8) | 3819 (130–26,158) |
| Q7T7 | Quinine 210 mg/kg (7 days, 7 doses) + tetracycline 112 mg/kg (7 days, 7 doses) | 1992–1994 | Short | 155 | 97 (63) | 15 (9–34) | 4284 (294–79,409) |
| Total | 1991–2005 | 10,549 | 6,564 (62) | 15 (5–40) | 6586 (328–101,284) |
NOTE. DHA, dihydroartemisinin; int, intermediate; t½, elimination half-life category.
Patients with severe disease according to World Health Organization criteria [15] were excluded, although the studies of intravenous quinine plus mefloquine and of the 5-day and 7-day courses of artesunate in combination with mefloquine included patients with uncomplicated hyperparasitemia (>4% parasitised red blood cells) (Table 1). Follow-up was standardized for all studies and lasted 28 days (6 studies; 1398 patients), 42 days (11 studies; 5354 patients), or 63 days (7 studies; 3797 patients). Patients were seen every day until they were afebrile and had experienced parasite clearance and were then seen weekly thereafter. In the event of illness that occurred between these visits, patients were asked to return to the clinic for treatment. Fully informed consent was obtained before enrollment in all of the studies. The studies were approved by the ethics committees of the Faculty of Tropical Medicine, Mahidol University, and Oxford University (OXTREC).
Study Data
Basic demographic and clinical details were recorded at enrollment, including age, sex, parasitemia, temperature, and in most cases, hematocrit and white blood cell (WBC) count. Symptoms, temperature, and parasite count were assessed at follow-up visits. Diagnosis of Plasmodium infection and subsequent species identification were established by examination of Giemsa-stained thick and thin blood films. Parasitemia was reported as the number of asexual parasites per 500 WBCs or per 1000 red blood cells and subsequently converted to a count per microliter using the patient's WBC count or hematocrit, if available. Population means or assumed values of 8300 WBCs/μL and 35%, respectively, were used when necessary. Asexual parasite densities in mixed infection were given as a summed total in the majority of studies and were given separately for both species in a minority. For this analysis, we used the summed total.
Patients were censored and deemed to have experienced treatment failure if there were signs of early treatment failure due to either malaria parasite species [16], if asexual P. falciparum or P. vivax parasitemia persisted beyond 7 days, or if either species reappeared in the circulation up to 63 days after initial clearance. Patients who did not experience failure were censored on the date of their last negative blood smear result.
Statistical Analysis
The primary outcome for this analysis was recurrence of P. vivax infection up to 63 days after treatment for P. falciparum or mixed P. falciparum/P. vivax infection. Potential risk factors examined were species of infection at enrollment (P. falciparum or mixed infection), age, sex, initial loge parasite density, baseline hematocrit, and P. falciparum gametocytemia at enrollment (yes or no). We compared nonparametric continuous data using the Kruskal-Wallis test, unpaired proportions using the χ2 test, and paired proportions using McNemar's test. The impact of antimalarial drugs was assessed in 2 separate comparisons. First, we examined outcomes for all antimalarial drugs or combinations grouped by their terminal elimination half-lives (t1/2) (Table 1; short was defined as t1/2 < 1 day, intermediate was defined as t1/2 > 1 day and < 1 week, and long was defined as t1/2 > 1 week). Second, we compared outcomes between individual artemisinin combination therapies. The Kaplan–Meier function and log-rank test were used for univariable analyses. Multivariable analyses were done using the Cox proportional hazards model with gamma frailty to account for heterogeneity of results between studies [17] (examined using the Wald test for significance of interaction terms in preliminary models). Fulfillment of the proportional hazards assumption was assessed using log-log plots for each of the model covariables. All analyses were done using Stata software, version 10.1 (Stata Corporation).
RESULTS
From 1991 through 2005, 10,549 patients (4960 children aged <15 years and 5589 adults) were treated for falciparum malaria, of whom 9385 (89.0%) had P. falciparum monoinfections and 1164 (11.0%) had mixed infections. Overall, 2925 patients (27.7%) had recurrence of parasitaemia, 1570 (53.7%) with monoinfection due to P. vivax alone, 1269 (43.4%) with monoinfection due to P. falciparum alone, and 86 (2.9%) with mixed infections. The median time to recurrence was 28 days for those with P. falciparum monoinfection, 35 days for those with P. vivax monoinfection, and 33 days for those with mixed infection (P < .001 for overall difference). The number and characteristics of individuals receiving each of the treatment regimens are shown in Table 1. According to Kaplan–Meier analyses, the cumulative proportion of patients experiencing treatment failure due to any species by day 63 was 45.6% (95% confidence interval [CI], 44.1%–47.0%), the proportion experiencing treatment failure due to P. falciparum infection (either monoinfection or mixed infection) was 21.5% (95% CI, 20.3%–22.8%) and due to P. vivax (either monoinfection or mixed infection) was 31.5% (95% CI, 30.1%–33.0%). Overall, 3.5% (36 of 1024) of recurrences with asexual P. falciparum infection were associated with patent P. falciparum gametocytemia. Gametocyte data for recurrences of P. vivax infection were not available.
Hematocrit data were available for 90.7% of patients (9565 of 10,549) at enrollment and 58.9% of patients (1724 of 2925) at the time of treatment failure. In total, 14.5% of patients (1382 of 9565) were anemic (hematocrit <30%) at enrollment to the studies. Of those who did not have parasitological failure, 13.5% of patients (925 of 6869) were anemic at baseline, compared with 4.0% of patients (192 of 4755) at the last follow-up visit (P < .001). The corresponding figures at baseline and at the time of recurrence were 14.2% of patients (169 of 1189) versus 11.3% of patients (78 of 692) for those who experienced treatment failure due to P. falciparum (P = .1) and 18.7% of patients (296 of 1586) versus 7.2% of patients (78 of 1091) for those who experienced treatment failure due to P. vivax (P < .001). Patients who had recurrent P. falciparum monoinfection, P. vivax monoinfection, or mixed infection were anemic at the time of failure in 11.9% (75 of 633), 7.3% (75 of 1032), and 5.1% (3 of 59) of cases, respectively (P = .004 for overall difference).
Symptomatology data were available at the time of parasitological failure for 68.3% of study participants (1997 of 2925). Recurrences with P. falciparum monoinfection, P. vivax monoinfection, and mixed infections were associated with symptoms in 65.5% (537 of 820), 44.3% (495 of 1118), and 71.2% (42 of 59) of cases, respectively (P < .001 for overall difference). At the time of recurrence, the proportion of patients who were febrile (temperature >37.5°C) or had a history of fever within the last 24 h was 51.7% (455 of 880) for those with P. falciparum monoinfections, 33.6% (386 of 1,148) for those with P. vivax monoinfections, and 61.4% (35 of 57) for those with mixed infections (P < .001 for overall difference).
Of patients who had recurrent P. falciparum monoinfection, P. vivax monoinfection, or mixed infection, 41.2% (523 of 1269), 30.5% (479 of 1570) and 58.1% (50 of 86), respectively, presented outside of routine weekly follow-up and therefore presumably of their own volition (P < .001 for overall difference). P. vivax infection recurrences after treatment with short, intermediate, and long half-life combinations were symptomatic in 58.3% (158 of 271), 42.7% (230 of 539), and 40.6% (149 of 367) of cases, respectively (P < .001 for overall difference).
Risk Factors for Recurrence of P. vivax Infection
The cumulative risk of P. vivax infection recurrence by day 63 after P. falciparum monoinfection was 29.4% (95% CI, 27.9%–30.9%), and the risk after mixed infection was 49.3% (95% CI, 44.3%–54.5%); adjusted hazard ratio (AHR), 2.47; 95% CI, 2.15–2.85; P < .001 (Tables 2 and 3). Univariable analyses showed a statistically significant increase in the risk of P. vivax infection recurrence after pure P. falciparum infection with decreasing age, low hematocrit (<30%), increasing loge asexual parasite density, and presence of P. falciparum gametocytemia (Table 2). Male patients were significantly more likely to have recurrent P. vivax infection after both monoinfection due to P. falciparum and mixed infections (Tables 2 and 3; AHR, 1.27; 95% CI, 1.14–1.41; P < .001).
Table 2.
Baseline Risk Factors for Plasmodium vivax Recurrence, by Initial Species Isolated
|
Plasmodium falciparum infection |
Mixed P. falciparum/P. vivax infection |
|||||||
| Variable | No. of cases | Treatment failurea | 95% CI | P | No. of cases | Treatment failure,%a | 95% CI | P |
| Age group | ||||||||
| <5 years | 802 | 39.4 | 33.7–45.6 | <.001b | 204 | 56.2 | 43.1–70.1 | .6b |
| 5–15 years | 3347 | 35.4 | 32.7–38.3 | 607 | 52.9 | 45.9–60.1 | ||
| >15 years | 5236 | 24.6 | 22.8–26.5 | 353 | 41.4 | 33.7–50.1 | ||
| Sex | ||||||||
| Male | 5925 | 30.3 | 28.4–32.3 | .03 | 639 | 53.7 | 47.0–60.7 | .02 |
| Female | 3460 | 27.8 | 25.5–30.3 | 525 | 43.7 | 36.7–51.5 | ||
| Hematocrit, % | ||||||||
| <30 | 1252 | 38.6 | 34.4–43.1 | <.001 | 130 | 47.2 | 35.1–61.2 | .4 |
| ≥30 | 7318 | 27.9 | 26.3–29.6 | 865 | 50.4 | 44.9–56.2 | ||
| Loge parasitemia | ||||||||
| <25th centile (∼1400 parasites/μL) | 2454 | 23.3 | 20.7–26.2 | <.001b | 158 | 39.3 | 28.2–53.0 | .005b |
| 25th–50th centile (1400–6600 parasites/μL) | 2223 | 26.8 | 23.7–30.1 | 391 | 50.0 | 41.1–59.6 | ||
| 50th – 75th centile (6600–35,900 parasites/μL) | 2184 | 29.4 | 26.4–32.5 | 429 | 40.1 | 32.0–49.3 | ||
| >75th centile (>35,900 parasites/μL) | 2524 | 36.9 | 33.9–40.0 | 186 | 69.4 | 59.5–78.8 | ||
| P. falciparum gametocytemia at enrollment | ||||||||
| No | 8847 | 28.7 | 27.2–30.3 | <.001 | 1116 | 48.3 | 43.2–53.6 | .008 |
| Yes | 437 | 41.4 | 34.4–49.2 | 43 | 69.1 | 48.5–87.4 | ||
| Total | 9385 | 29.4 | 27.9–30.9 | 1164 | 49.3 | 44.3–54.5 | ||
NOTE. CI, confidence interval.
Kaplan-Meier cumulative failure estimates (%) at day 63.
Log-rank test for trend.
Table 3.
Multivariable Cox Proportional Hazards Models Showing the Effect of Baseline Factors and Antimalarial Drugs on Risk of Plasmodium vivax Recurrence
| Recurrence with P. vivax |
|||
| AHR | 95% CI | P | |
| All drugs | |||
| Drug half-life | |||
| Short (t1/2 < 1 day) | 1 | … | … |
| Intermediate (t1/2 1–7 days) | .43 | .29–.63 | <.001 |
| Long (t1/2 > 7 days) | .12 | .08–.18 | <.001 |
| Species at enrollment | |||
| Pure P. falciparum | 1 | … | … |
| Mixed P. falciparum/P. vivax | 2.47 | 2.15–2.85 | <.001 |
| Age, per year increase | .98 | .97–.98 | <.001 |
| Sex | |||
| Female | 1 | … | … |
| Male | 1.27 | 1.14–1.41 | <.001 |
| Hct, per percentage point increase | .98 | .97–.99 | <.001 |
| Loge parasite density, per loge order | 1.09 | 1.07–1.12 | <.001 |
| P. falciparum gametocytemia | |||
| No | 1 | … | … |
| Yes | 1.38 | 1.14–1.69 | .001 |
| Artemisinin combination therapiesa | |||
| Artesunate + mefloquine combinations | 1 | … | … |
| DHA + piperaquine combinations | 1.12 | .79–1.58 | .5 |
| Artemether + mefloquine combinations | .80 | .42–1.51 | .5 |
| Artemether + lumefantrine | 3.57 | 2.91–4.37 | <.001 |
| Artesunate + atovaquone + proguanil | 4.20 | 2.79–6.31 | <.001 |
NOTE. CI, confidence interval; DHA, dihydroartemisinin; Hct, hematocrit; AHR, adjusted hazard ratio.
Model also includes species at enrollment, age, sex, hematocrit, loge parasite density, and P. falciparum gametocytemia at enrollment.
Effect of Antimalarial Drugs on Risk of Recurrence of P. vivax Infection
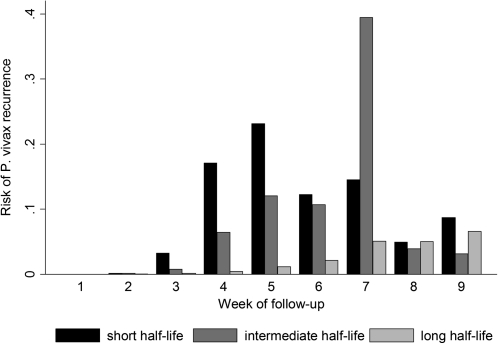
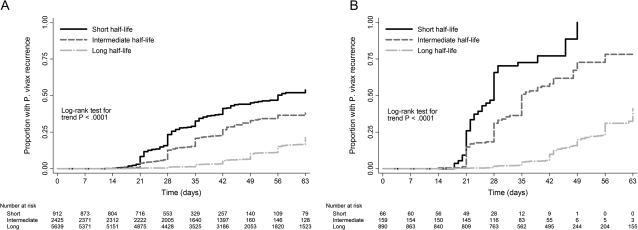
The median times to P. vivax infection recurrence after treatment with short, intermediate, and long half-life regimens were 28, 29, and 49 days, respectively (P < .001 for overall difference; Figure 1). Treatment with slowly eliminated antimalarials was associated with a significant trend to decreasing risk of P. vivax infection recurrence up to 63 days after both malaria due to P. falciparum monoinfection and malaria due to mixed infection (P < .001 for trend in both cases; Figure 2). The cumulative proportion of patients treated with a rapidly eliminated antimalarial who had a recurrence of P. vivax infection after pure falciparum malaria was 53.8% (95% CI, 48.5%–59.3%), compared with 21.1% (95% CI, 19.5%–22.9%) among those treated with slowly eliminated regimens (P < .001). All patients with mixed-species infections who were treated with a rapidly eliminated antimalarial had a recurrent infection within 49 days of follow-up. The adjusted hazard ratios for P. vivax infection recurrence after either P. falciparum infection or mixed infection for patients receiving long or intermediate half-life regimens were 0.43 (95% CI, 0.29–0.63; P < .001) and 0.12 (95% CI, 0.08–0.18; P < .001), respectively, when compared with those receiving rapidly eliminated antimalarials (Table 3).
Figure 1.
Risk of Plasmodium vivax recurrence after Plasmodium falciparum monoinfection or mixed P. vivax/P. falciparum malaria by week of follow-up and antimalarial half-life.
Figure 2.
Kaplan–Meier failure estimates for the cumulative risk of Plasmodium vivax recurrence after Plasmodium falciparum infection (A) and following mixed P. falciparum/P. vivax infection (B) by antimalarial half-life.
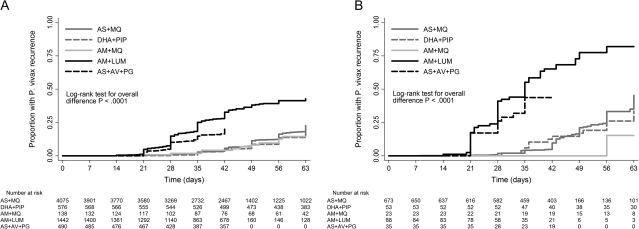
The median times to P. vivax infection recurrence after artesunate-atovaquone-proguanil, artemether-lumefantrine, artesunate-mefloquine, dihydroartemisinin-piperaquine, and artemether-mefloquine treatment were 28, 29, 49, 49, and 56 days, respectively (P < .001 for overall difference). Of the artemisinin combination therapies, those regimens containing mefloquine or piperaquine appeared to be equally effective at preventing P. vivax infection recurrence in both univariable and multivariable analyses (Figure 3 and Table 3). The shorter-acting combinations, artemether-lumefantrine and artesunate-atovaquone-proguanil, were associated with 3.6-fold and 4.2-fold increases in risk of P. vivax infection recurrence, respectively, when compared with artesunate-mefloquine treatment (P <.001 in both cases) (Table 3).
Figure 3.
Kaplan–Meier failure estimates for the cumulative risk of Plasmodium vivax recurrence after Plasmodium falciparum infection (A) and following mixed P. falciparum/P. vivax infection (B) for artemisinin combination therapies. AS+MQ, artesunate plus mefloquine; DHA + PIP, dihydroartemisinin plus piperaquine; AM+MQ, artemether plus mefloquine; AM+LUM, artemether plus lumefantrine; AS+AV+PG, artesunate plus atovaquone plus proguanil.
DISCUSSION
In a large series of clinical trials conducted on the Thai-Myanmar border, P. vivax infection accounted for substantially more malaria recurrences within 63 days of treatment for falciparum or mixed malaria than did P. falciparum infection. Because P. vivax is more frequently associated with gametocytemia [3, 9, 11] and is more transmissible at low parasite densities [18], the most commonly transmitted parasite after treatment for falciparum malaria, paradoxically, was not P. falciparum, but P. vivax.
Statistically significant baseline risk factors for P. vivax infection recurrence after acute falciparum malaria included initial mixed-species infection, male sex, younger age, higher total asexual parasitemia, lower hematocrit, and the presence of P. falciparum gametocytemia. Slowly eliminated antimalarial regimens, such as those containing mefloquine or piperaquine, were associated with a markedly lower risk of P. vivax infection recurrence than were rapidly eliminated drugs.
High asexual P. falciparum parasitemia is a well-recognized risk factor for subsequent P. falciparum recrudescence [19–23]. In the present analysis, we have shown that it also increases the risk of P. vivax infection recurrence. One potential explanation for this phenomenon is that higher P. falciparum density, lower hematocrit, and younger age are proxy markers of malaria naivety and hence poor immunity to both P. falciparum and P. vivax infections. If this is true, relapses due to P. vivax hypnozoites acquired at or around the same time as the index P. falciparum infection would have a greater chance of reaching patency. Simultaneous or near simultaneous infection due to P. falciparum and P. vivax is probably relatively common. Mason et al [24] showed that 10.5% of patients treated for P. vivax malaria in Bangkok subsequently had a recurrence of P. falciparum infection within 28 days. Because P. falciparum does not have a dormant form, and because there is no local malaria transmission in Bangkok, these parasites are most likely to have been acquired at the same time as the P. vivax infections.
An alternative, but potentially complimentary, hypothesis is that high parasitemia and low hematocrit are indicators of greater disease severity and hence of pathophysiological and immunological derangement, a consequence of which may be stimulation of P. vivax infection relapse and/or failure to suppress growth of recurrent blood stage infection. This mechanism would be equally plausible regardless of whether the relapsing P. vivax hypnozoites had been acquired at the same time or prior to the index P. falciparum infection. Because the excess risk of P. vivax infection recurrence is seen even after slowly eliminated therapies, these putative factors would either have to be long-lasting or induce a prolonged stream rather than a single pulse of relapsing merozoites from the liver.
Highly sensitive polymerase chain reaction–based assays typically reveal a much higher prevalence of concurrent mixed-species infection than does examination with light microscopy [5, 25–28]. This suggests that a sizeable proportion of patients with microscopically confirmed P. falciparum monoinfection in regions of co-endemicity actually have subpatent P. vivax parasitemia. In our study, patients presenting with falciparum gametocytemia were at 1.38 times the risk of early recurrence with P. vivax infection, compared with the risk among patients without gametocytemia. The presence of gametocytes is more likely in patients with chronic, asymptomatic infections and may therefore be suggestive of multiple previous exposures to both Plasmodium species and thus a greater risk of subpatent vivax infection at enrollment.
Our pooled meta-analysis included a large number of individuals who were treated with multiple different antimalarial regimens. The individual trials were conducted in similar physical environments, which helped to ensure the comparability of their results. Nevertheless, several sources of inter-study heterogeneity remain. Some of these could be partially addressed in multivariable models by controlling for differences in the age structure and median parasite density of study participants. Other known and unknown sources of heterogeneity, such as differences in dosing schedules for individual regimens and temporal differences in local malaria incidence, could not be controlled for. By using Cox models with gamma frailty, we have presented an averaged effect of specific regimens across the different studies [17].
The long-term benefits of prolonged post-exposure prophylaxis against recurrent parasitemia have yet to be determined. With the exception of the antifolate drugs, antimalarial compounds active against P. falciparum have excellent efficacy against the blood stages of P. vivax, and thus, the drug regimens included in this analysis should have cleared initial subpatent P. vivax infections [29]. The risk of P. vivax reinfection in this region is low (<5% during a 42-day period) [13, 30]. One can therefore assume that most of the observed P. vivax infection recurrences were relapses. Hypnozoites have the potential to seed multiple relapses, and it is not known whether prevention of just one of these by use of a slowly eliminated antimalarial will reduce the total number of relapses or simply delay the occurrence of the next relapse. If the former is true, the total morbidity from a given vivax infection could be reduced, and total gametocyte carriage and, hence, transmissibility would also be expected to decrease. A greater period of post-exposure prophylaxis against recurrence of infection due to any Plasmodium species should also facilitate fuller hematological and clinical recovery [3, 9].
These speculative benefits must be weighed against potential disadvantages. Drugs with long terminal elimination half-lives will be present in the bloodstream at subtherapeutic concentrations for longer than rapidly eliminated drugs and will therefore provide a more powerful force for the spread of drug-resistant parasites [12, 31, 32]. The combination of mefloquine and artesunate has been used for the treatment of P. falciparum malaria along the northwestern border of Thailand both in trials and in routine practice since 1994. Recent studies have revealed an increase in the prevalence of PvMDR1 gene amplification in local P. vivax isolates, a polymorphism associated with reduced susceptibility to mefloquine [33]. Although post-hoc exploratory analyses (not presented) show that the risk of P. vivax infection recurrence after mefloquine-artesunate therapy has increased slightly with time, it is unclear whether this is due to emerging mefloquine tolerance or variation in background endemicity.
In this series of clinical trials, P. vivax was the most common cause of parasitological failure and was almost certainly the most frequently transmitted parasite after P. falciparum infection and mixed infection. The risk of P. vivax infection recurrence in the 9 weeks after initial falciparum malaria or mixed malaria is inversely correlated with antimalarial half-life. Slowly eliminated regimens should facilitate full clinical recovery and, if used on a large scale, may reduce transmission of both P. falciparum and P. vivax. Although additional work is required to establish the risk and deleterious effects of P. vivax infection recurrence in other regions, our study suggests that there is a coherent argument for the safe provision of a sterilizing course of antirelapse therapy (currently, 14 days of primaquine) for all patients with malaria in regions of co-endemicity.
Acknowledgments
We thank the staff of the Shoklo Malaria Research Unit for their work and all of the patients who participated in the studies.
Financial support. The Rhodes Trust (Scholarship to N.M.D) and The Wellcome Trust (Program grant to F.N. and N.J.W and Senior Research Fellowship in Clinical Science to R.N.P).
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Looareesuwan S, White NJ, Chittamas S, Bunnag D, Harinasuta T. High rate of Plasmodium vivax relapse following treatment of falciparum malaria in Thailand. Lancet. 1987;2:1052–5. doi: 10.1016/s0140-6736(87)91479-6. [DOI] [PubMed] [Google Scholar]
- 2.Ashley EA, Krudsood S, Phaiphun L, et al. Randomized, controlled dose-optimization studies of dihydroartemisinin-piperaquine for the treatment of uncomplicated multidrug-resistant falciparum malaria in Thailand. J Infect Dis. 2004;190:1773–82. doi: 10.1086/425015. [DOI] [PubMed] [Google Scholar]
- 3.Ratcliff A, Siswantoro H, Kenangalem E, et al. Two fixed-dose artemisinin combinations for drug-resistant falciparum and vivax malaria in Papua, Indonesia: an open-label randomised comparison. Lancet. 2007;369:757–65. doi: 10.1016/S0140-6736(07)60160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karunajeewa HA, Mueller I, Senn M, et al. A trial of combination antimalarial therapies in children from Papua New Guinea. N Engl J Med. 2008;359:2545–7. doi: 10.1056/NEJMoa0804915. [DOI] [PubMed] [Google Scholar]
- 5.Mayxay M, Pukrittayakamee S, Newton PN, White NJ. Mixed-species malaria infections in humans. Trends Parasitol. 2004;20:233–40. doi: 10.1016/j.pt.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Karbwang J, Bangchang KN, Thanavibul A, Bunnag D, Chongsuphajaisiddhi T, Harinasuta T. Comparison of oral artemether and mefloquine in acute uncomplicated falciparum malaria. Lancet. 1992;340:1245–8. doi: 10.1016/0140-6736(92)92947-e. [DOI] [PubMed] [Google Scholar]
- 7.Smithuis F, Kyaw MK, Phe O, et al. Effectiveness of five artemisinin combination regimens with or without primaquine in uncomplicated falciparum malaria: an open-label randomised trial. Lancet Infect Dis. 2010;10:673–81. doi: 10.1016/S1473-3099(10)70187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snounou G, White NJ. The co-existence of Plasmodium: sidelights from falciparum and vivax malaria in Thailand. Trends Parasitol. 2004;20:333–9. doi: 10.1016/j.pt.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Hasugian AR, Purba HLE, Kenangalem E, et al. Dihydroartemisinin-piperaquine versus artesunate-amodiaquine: superior efficacy and posttreatment prophylaxis against multidrug-resistant Plasmodium falciparum and Plasmodium vivax malaria. Clin Infect Dis. 2007;44:1067–74. doi: 10.1086/512677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, Anstey NM. Vivax malaria: neglected and not benign. Am J Trop Med Hyg. 2007;77:79–87. [PMC free article] [PubMed] [Google Scholar]
- 11.Awab GR, Pukrittayakamee S, Imwong M, et al. Dihydroartemisinin-piperaquine versus chloroquine to treat vivax malaria in Afghanistan: an open randomized, non-inferiority, trial. Malar J. 2010;9:105. doi: 10.1186/1475-2875-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douglas NM, Anstey NM, Angus BJ, Nosten F, Price RN. Artemisinin combination therapy for vivax malaria. Lancet Infect Dis. 2010;10:405–16. doi: 10.1016/S1473-3099(10)70079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luxemburger C, Thwai KL, White NJ, et al. The epidemiology of malaria in a Karen population on the western border of Thailand. Trans R Soc Trop Med Hyg. 1996;90:105–11. doi: 10.1016/s0035-9203(96)90102-9. [DOI] [PubMed] [Google Scholar]
- 14.Price R, van Vugt M, Nosten F, et al. Artesunate versus artemether for the treatment of recrudescent multidrug-resistant falciparum malaria. Am J Trop Med Hyg. 1998;59:883–8. doi: 10.4269/ajtmh.1998.59.883. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Severe and complicated malaria. Trans R Soc Trop Med Hyg. 1990;84:S1–S65. [PubMed] [Google Scholar]
- 16.World Health Organization. Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparum malaria. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- 17.Glidden DV, Vittinghoff E. Modelling clustered survival data from multicentre clinical trials. Statist Med. 2004;23:369–88. doi: 10.1002/sim.1599. [DOI] [PubMed] [Google Scholar]
- 18.Boyd MF, Kitchen SF. On the infectiousness of patients infected with Plasmodium vivax and Plasmodium falciparum. Am J Trop Med. 1937;s1–17:253–62. [Google Scholar]
- 19.Price RN, Nosten F, Luxemburger C, et al. Artesunate/mefloquine treatment of multi-drug resistant falciparum malaria. Trans R Soc Trop Med Hyg. 1997;91:574–7. doi: 10.1016/s0035-9203(97)90032-8. [DOI] [PubMed] [Google Scholar]
- 20.Ittarat W, Pickard AL, Rattanasinganchan P, et al. Recrudescence in artesunate-treated patients with falciparum malaria is dependent on parasite burden not on parasite factors. Am J Trop Med Hyg. 2003;68:147–52. [PubMed] [Google Scholar]
- 21.ter Kuile FO, Luxemburger C, Nosten F, Thwai KL, Chongsuphajaisiddhi T, White NJ. Predictors of mefloquine treatment failure: a prospective study of 1590 patients with uncomplicated falciparum malaria. Trans R Soc Trop Med Hyg. 1995;89:660–4. doi: 10.1016/0035-9203(95)90435-2. [DOI] [PubMed] [Google Scholar]
- 22.Fontanet AL, Walker AM. Predictors of treatment failure in multiple drug-resistant falciparum malaria: results from a 42-day follow-up of 224 patients in eastern Thailand. Am J Trop Med Hyg. 1993;49:465–72. doi: 10.4269/ajtmh.1993.49.465. [DOI] [PubMed] [Google Scholar]
- 23.White NJ. The assessment of antimalarial drug efficacy. Trends Parasitol. 2002;18:458–64. doi: 10.1016/s1471-4922(02)02373-5. [DOI] [PubMed] [Google Scholar]
- 24.Mason DP, Krudsood S, Wilairatana P, et al. Can treatment of P. vivax lead to a unexpected appearance of falciparum malaria? Southeast Asian J Trop Med Public Health. 2001;32:57–63. [PMC free article] [PubMed] [Google Scholar]
- 25.McKenzie FE, Sirichaisinthop J, Miller RS, Gasser RAJ, Wongsrichanalai C. Dependence of malaria detection and species diagnosis by microscopy on parasite density. Am J Trop Med Hyg. 2003;69:372–6. [PMC free article] [PubMed] [Google Scholar]
- 26.Siripoon N, Snounou G, Yamogkul P, Na-Bangchang K, Thaithong S. Cryptic Plasmodium falciparum parasites in clinical P. vivax blood samples from Thailand. Trans R Soc Trop Med Hyg. 2002;96:70–1. doi: 10.1016/s0035-9203(02)90246-4. [DOI] [PubMed] [Google Scholar]
- 27.Brown AE, Kain KC, Pipithkul J, Webster HK. Demonstration by the polymerase chain reaction of mixed Plasmodium falciparum and P. vivax infections undetected by conventional microscopy. Trans R Soc Trop Med Hyg. 1992;86:609–12. doi: 10.1016/0035-9203(92)90147-5. [DOI] [PubMed] [Google Scholar]
- 28.Gupta B, Gupta P, Sharma A, Singh V, Dash AP, Das A. High proportion of mixed-species Plasmodium infections in India revealed by PCR diagnostic assay. Trop Med Int Health. 2010;15:819–24. doi: 10.1111/j.1365-3156.2010.02549.x. [DOI] [PubMed] [Google Scholar]
- 29.Pukrittayakamee S, Chantra A, Simpson JA, et al. Therapeutic responses to different antimalarial drugs in vivax malaria. Antimicrob Agents Chemother. 2000;44:1680–5. doi: 10.1128/aac.44.6.1680-1685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Price RN, Nosten F, Luxemburger C, et al. Artesunate versus artemether in combination with mefloquine for the treatment of multidrug-resistant falciparum malaria. Trans R Soc Trop Med Hyg. 1995;89:523–7. doi: 10.1016/0035-9203(95)90094-2. [DOI] [PubMed] [Google Scholar]
- 31.White NJ. Antimalarial drug resistance. J Clin Invest. 2004;113:1084–92. doi: 10.1172/JCI21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Price RN, Douglas NM. Artemisinin combination therapy for malaria: beyond good efficacy. Clin Infect Dis. 2009;49:1638–40. doi: 10.1086/647947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suwanarusk R, Chavchich M, Russell B, et al. Amplification of pvmdr1 associated with multidrug-resistant Plasmodium vivax. J Infect Dis. 2008;198:1558–64. doi: 10.1086/592451. [DOI] [PMC free article] [PubMed] [Google Scholar]