(See the article by Douglas et al, on Pages 612–620)
Douglas et al [1] describe patency with Plasmodium vivax in the 63 days after treatment of malaria caused by Plasmodium falciparum in >10,000 subjects in Thailand over a 14-year period who received 25 different therapies. Therapy for acute malaria aims at the asexual stages of the organism infecting blood. Among the many blood schizontocidal drugs that achieve this therapeutic effect, none eliminate dormant stages in the liver, which are known as hypnozoites. Regardless of the species being treated, if hypnozoites are present, relapse may occur in the absence of treatment with primaquine, which is the only registered hypnozoitocide. The patients evaluated by Douglas et al [1] did not receive hypnozoitocidal therapy for the simple reason that it is not indicated for falciparum malaria. Parasitemia with P. vivax occurred in 20%–51% of these patients, with that rate correlated to the rapidity of excretion of drugs administered against P. falciparum.
Figure 1.
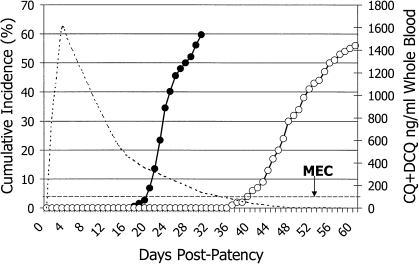
The graph illustrates cumulative incidence (left axis) of relapse among several hundred patients infected with Plasmodium vivax from Southeast Asia and the Western Pacific regions and treated with either rapidly excreted quinine (solid points) or slowly excreted chloroquine (hollow points). Blood levels of chloroquine and its major metabolite desethylchloroquine (right axis) slowly decrease to below the minimally effective concentration (MEC) at approximately day 35, coinciding with commencement of relapse. Reproduced with permission from Baird [7]. Antimicrob Agents Chemother 2004; 48:4075–83. Copyright American Society for Microbiology.
Malaria manifests as many infections of distinct biological character, with susceptibility to distinct classes of drugs, and distinct clinical or epidemiological consequences [2]. Anopheline mosquitoes transmit all of the 5 species of the genus Plasmodium known to naturally infect humans. Each passes through a series of liver and blood stages of asexual development that massively expand the numbers of individual parasites. In a biological sense, that expansion aims solely at positioning male and female sexual forms (called gametocytes) where they can access the gut of feeding anopheline mosquitoes—the only site where these parasites execute the sexual recombination essential to their propagation. Humans simply represent a means for the plasmodia to traffic among their mosquito definitive hosts.
Two species of plasmodia infecting humans hedge the probability that biting anophelines will be present to capitalize the relatively risky venture into blood. Among the infectious sporozoites of P. vivax and Plasmodium ovale introduced by biting anophelines, an unknown and variable fraction arrest development after invading human hepatocytes [3]. These clinically silent hypnozoites later commence development and emerge into the bloodstream to cause another round of malaria, which is termed a relapse.
The probability, interval, and frequency of relapse in the absence of primaquine treatment vary geographically in a manner suggesting linkage to a high probability of a relative abundance of anophelines [4]. Only ∼30% of individuals with infection due to P. vivax from the temperate Korean peninsula, for example, experience relapse after 8 months and only once; whereas almost all individuals with infections due to P. vivax from the perpetually warm and wet climate of New Guinea experience relapse within 4 weeks and experience relapse ≥5 times. These climate-specific relapse behaviors persist among strains transferred to another hemisphere, and they thus appear to be genetically programmed [3, 4]. In Thailand, ∼60% of patients treated for acute vivax malaria with rapidly excreted blood schizontocides experienced relapse within 28 days after patency [5]. When slowly excreted blood schizontocides (eg, chloroquine or mefloquine) were applied, no relapses appeared by day 28, because drug lingering in blood killed the asexual blood stages emanating from activated hypnozoites. When drug levels slip below minimally effective concentrations, relapses may occur [6]. Figure 1, reprinted from Baird [7], illustrates this principle. The natural relapse rate after quinine therapy for the primary attack was 60% at day 28. After chloroquine therapy for the primary attack, relapse did not commence until levels of drug slipped below the minimally effective concentration at approximately day 35, and the relapse rate reached 50% by day 63. It would be supposed, therefore, that delaying relapse would not impact the broader risks of morbidity and mortality in the community that are incurred by the failure to administer hypnozoitocidal therapy.
Douglas et al [1] offer a different view by showing that risk of relapse diminished significantly when blood schizontocidal therapy for acute falciparum malaria included a slowly excreted drug. In other words, the longer-lasting drugs diminished the burden of blood infection in the community of treated patients by eliminating blood stages derived from hypnozoites. This may be reconciled to the predominantly experimental challenge data shown in Figure 1 by supposing that natural infection is associated with more-limited numbers of hypnozoites and, therefore, is associated with fewer relapses [8]. Hypnozoites were effectively killed off by the elimination of their blood stage progeny, which perhaps represented a significant proportion of the hypnozoite pool in such patients.
Although Douglas et al [1] cautiously put forth the broader advantage to malaria control gained by applying slowly excreted therapies, they close with perhaps a more important point: the application of primaquine against hypnozoites represents a more complete solution to the significant problem of relapse. As their data show, the blood schizontocidal approach to the relapse problem was only partially effective (∼60%; relapse rate, 20% vs 51% with slowly vs. rapidly excreted therapies, respectively). Moreover, relapses that occur after day 63 may deliver similar rates of relapse, regardless of the excretion rate of the blood schizontocide. Among American soldiers infected in the Pacific theater during World War II, the median number of relapses was 4.2, and many of these relapses occurred up to 3 years after exposure [9, 10]. The blood schizontocidal approach to diminishing relapse rates may be effectively summarized as “better than nothing” in the current context of therapeutic paradigms and practice in areas of endemicity.
Potentially severe hemolytic toxicity among patients with glucose-6-phosphate dehydrogenase deficiency (G6PDd) largely explains the gross underutilization of primaquine by most health care providers in areas of malaria endemicity. They hesitate to administer a potentially life-threatening therapy against an infection that is not supposed to be threatening to life. The vast majority of practitioners have no access to the relatively expensive and technically challenging screening tests for G6PDd. They cannot know who is at risk for primaquine toxicity and cannot rationally choose when not to prescribe it. This is the root of the problem with unchecked relapse and is the basis of the appeal of the partial solution provided by slowly excreted blood schizontocides for acute malaria.
A very important aspect of the far broader neglect of vivax malaria is the failure to develop a simple, inexpensive, and heat-stable point-of-care diagnostic tool for G6PDd. This failure impels us to accept the inability to bring primaquine to bear against endemic malaria and to consider partially effective solutions to the serious problem of relapse. Effective malaria control, much less successful elimination, will require a different approach.
Consider a therapeutic paradigm that includes a rapid diagnostic test (RDT) for G6PDd that reliably identifies the minority of people at risk for primaquine toxicity. Standard primaquine therapy is administered over a 14-day period as a means of mitigating risk to patients with undiagnosed G6PDd. Exposing patients to daily doses of 15–30 mg of primaquine provides opportunity to cease dosing before serious harm occurs [11]. The peculiar total dose concept with primaquine—in which delivery of a total dose of 210–420 mg over 7 days, 14 days, or even 8 weeks has little bearing upon efficacy [12]—opens the possibility of higher dosages over shorter duration [13]. A reliable RDT for G6PDd would enable safer and more-effective treatment against hypnozoites. Such a tool would also raise the possibility of treating all patients with malaria, regardless of the species diagnosed, with anti-relapse therapy.
The division of prescribed therapies across species of plasmodia may derive from practice in zones where malaria is not endemic. Most patients with malaria who are seen in that setting (eg, travelers) likely had a single encounter with an infected anopheline mosquito and will usually harbor a single species. In zones of endemicity, however, patients have cumulative exposures. If Thailand, where the disease is endemic, is typical, then more than half of patients are co-infected with at least 2 species. The people in any given community in which the disease is endemic who are demonstrated to be at risk with 1 species (by diagnosis) are also at risk for infection by the other species (whether infection due to that species is diagnosed or not) [14–16]. It stands to reason that patients with falciparum malaria in Thailand had a high risk of co-infection with hypnozoites of P. vivax, because these sympatric parasites share the same human and mosquito hosts. The data reported by Douglas et al [1] and the data reported by others and summarized by them should remove doubt on this important point. Providing therapy that is effective against P. falciparum but not against P. vivax is reasonable only when treatment is hamstrung by the toxicity of primaquine.
Confidence in the safety of primaquine therapy in most patients should prompt consideration of anti-relapse therapy after a diagnosis of P. falciparum malaria in areas in which this species occurs with P. vivax. This approach could provide a complete solution to the problem of relapse in zones of malaria endemicity. Fielding an RDT for G6PDd that provides certainty of primaquine safety could revolutionize chemotherapeutic strategy and efficacy in zones of malaria endemicity. There may be no more important task than this across the broad array of work required to control and eliminate malaria.
Acknowledgments
Financial support. The Wellcome Trust.
Potential conflicts of interest. Author certifies no potential conflicts of interest.
References
- 1.Douglas, et al. [Google Scholar]
- 2.Baird JK. Eliminating malaria—all of them. Lancet. 2010;376:1883–5. doi: 10.1016/S0140-6736(10)61494-8. [DOI] [PubMed] [Google Scholar]
- 3.Shute PG, Lupascu G, Branzei P, et al. A strain of Plasmodium vivax characterized by prolonged incubation: the effect of numbers of sporozoites on the length of the prepatent period. Trans R Soc Trop Med Hyg. 1976;70:474–81. doi: 10.1016/0035-9203(76)90132-2. [DOI] [PubMed] [Google Scholar]
- 4.Contactos PG, Collins WE, Jeffery GM, Krotoski WA, Howard WA. Studies on the characterization of Plasmodium vivax strains from South America. Am J Trop Med Hyg. 1972;21:707–12. doi: 10.4269/ajtmh.1972.21.707. [DOI] [PubMed] [Google Scholar]
- 5.Pukrittayakamee S, Chantra A, Simpson JA, et al. Therapeutic responses to different antimalarial drugs in vivax malaria. Antimicrob Agents Chemother. 2000;44:1680–5. doi: 10.1128/aac.44.6.1680-1685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baird JK, Leksana B, Masbar S, et al. Diagnosis of resistance to chloroquine by Plasmodium vivax: timing of recurrence and whole blood chloroquine levels. Am J Trop Med Hyg. 1997;56:621–6. doi: 10.4269/ajtmh.1997.56.621. [DOI] [PubMed] [Google Scholar]
- 7.Baird JK. Chloroquine resistance in Plasmodium vivax. Antimicrob Agents Chemother. 2004;48:4075–83. doi: 10.1128/AAC.48.11.4075-4083.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coatney GR, Collins WE, Warren M, Contacos PG. The primate malarias. Washington, DC: US Government Printing Office; 1971. p. 37. [Google Scholar]
- 9.Eyles DE, Young MD. Studies on imported malarias; the parasitological pattern of relapsing Plasmodium vivax in military patients. J Natl Malar Soc. 1948;7:23–37. [PubMed] [Google Scholar]
- 10.Hill E, Amatuzio DS. Southwest Pacific vivax malaria; clinical features and observations concerning duration of clinical activity. Am J Trop Med. 1949;29:203–14. [PubMed] [Google Scholar]
- 11.Alving AS, Johnson CF, Tarlov AR, et al. Mitigation of the hemolytic effect of primaquine and enhancement of its action against exoerythrocytic forms of the Chesson strain of Plasmodium vivax by intermittent regimens of drug administration. Bull World Health Organ. 1960;22:621–31. [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt LH, Fradkin R, Vaugh D, Rasco J. Radical cure of infections with Plasmodium cynomolgi: a function of total 8-aminoquinonline dose. Am J Trop Med Hyg. 1977;26:1116–28. doi: 10.4269/ajtmh.1977.26.1116. [DOI] [PubMed] [Google Scholar]
- 13.Baird JK, Rieckmann K. Can primaquine therapy for vivax malaria be improved? Trends Parasitol. 2003;19:115–20. doi: 10.1016/s1471-4922(03)00005-9. [DOI] [PubMed] [Google Scholar]
- 14.Mayxay M, Pukrittayakamee S, Newton PN, White NJ. Mixed species malaria infections in humans. Trends Parasitol. 2004;20:233–40. doi: 10.1016/j.pt.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Brown AE, Kain KC, Pipithkul J, Webster HK. Demonstration by the polymerase chain reaction of mixed Plasmodium falciparum and P. vivax infections undetected by conventional microscopy. Trans R Soc Trop Med Hyg. 1992;86:609–12. doi: 10.1016/0035-9203(92)90147-5. [DOI] [PubMed] [Google Scholar]
- 16.Siripoon N, Snounou G, Yamogkul P, Na-Bangchang K, Thaitthong S. Cryptic Plasmodium falciparum parasites in clinical P. vivax blood samples from Thailand. Trans R Soc Trop Med Hyg. 2002;96:70–1. doi: 10.1016/s0035-9203(02)90246-4. [DOI] [PubMed] [Google Scholar]