Low levels of nevirapine-resistant HIV after failed prophylaxis compromise first-line nevirapine-based therapy. Initial therapy with lopinavir/ritonavir in nevirapine-exposed children raised the threshold level of pretreatment resistance (≥25% of the viral population) at which reuse of nevirapine-based therapy was affected.
Abstract
Background. Nevirapine resistance after failed prophylaxis to prevent mother-to-child human immunodeficiency virus (HIV) transmission can compromise subsequent nevirapine-based highly active antiretroviral therapy (HAART).
Methods. Nevirapine-exposed children who achieved virologic suppression with lopinavir/ritonavir-based induction HAART before switch to nevirapine-based HAART or who continued the lopinavir/ritonavir regimen were studied. Nevirapine-resistant HIV was quantified (≥1% frequency) in plasma before therapy and archived in peripheral blood mononuclear cells after induction HAART with ultradeep pyrosequencing. The primary endpoint was virologic failure (confirmed viremia ≥1000 copies/mL by 52 weeks) on nevirapine-based HAART, and Receiver operating characteristic analysis identified threshold levels of resistance associated with failure.
Results. Nevirapine resistance mutations were detected in plasma at a median frequency of 25.6% in 41 (33%) of 124 children starting HAART at median 9 months of age. After a median nine months of induction HAART, nevirapine-resistant HIV remained archived in cells in 59 (61%) of 96 children (median 13.6% of cells). The threshold frequency of nevirapine resistance in plasma most predictive of virologic failure on nevirapine-based HAART was 25%. Children maintaining resistance before therapy at or above this threshold frequency had a 3.5 fold higher risk of failure (95% confidence interval, 1.1–10.8) than children without detectable plasma resistance. In contrast, virologic failure was not independently associated with age, resistance in plasma below 25% frequencies, or archived in cells.
Conclusions. Virologic suppression with lopinavir/ritonavir-based HAART in nevirapine-exposed children raises the threshold level of resistance at which reuse of nevirapine-based therapy is compromised. Standard genotyping may allow identification of children likely to benefit from an induction-switch approach.
Exposure to a single dose of nevirapine to prevent mother-to-child transmission of human immunodeficiency virus (HIV) [1–5] results in the selection of resistant virus to high frequencies in plasma (>20% of virus population) in approximately 36% of women and 53% of infants [6]. The frequency of nevirapine-resistant HIV decays after prophylaxis but persists at low levels in plasma in infants for up to 1 year and in women for up to 3 years [7–10]. The frequency of HIV-infected cells containing nevirapine-resistant virus also decays during the first year [10] but remains detectable with replication-competent virus in about 8% of women followed for a median of 2 years [11].
Development of nevirapine resistance from the single prophylactic dose affects the effectiveness of first-line highly active antiretroviral treatment (HAART) with nevirapine in women and children [12–15]. A large clinical trial demonstrated that 83.2% of single-dose nevirapine exposed children who maintained resistance prior to HAART therapy at levels detected by standard genotyping failed nevirapine-based HAART, compared with 18.2% of nevirapine-exposed children without detectable resistance [13]. Even low levels of nevirapine resistance in plasma, whose detection requires sensitive genotyping assays, places women (hazard ratio [HR], 3.8 [95% confidence interval {CI}, 1.1–13.9]) [14] and infants (risk ratio, 2.4 [95% CI, 0.9–7.8]) at an increased risk of treatment failure with nevirapine-based regimens [15]. In women exposed to single-dose nevirapine, detection of resistant HIV in proviral DNA, at frequencies as low as 5% prior to nevirapine-based HAART, was also associated with treatment failure [16]; however, a similar effect was not seen in children [15].
In the NEVEREST trial, among children whose viremia was first controlled with lopinavir/ritonavir–based induction HAART before nevirapine was reused for treatment, approximately 20% experienced rebound viremia (≥1000 copies/mL), compared with 2% of children who continued to receive lopinavir/ritonavir–based HAART [17]. These findings suggest that archived nevirapine-resistant HIV from failed prophylaxis in children may diminish the effectiveness of treatment with nevirapine despite induction therapy. We used ultradeep pyrosequencing, a newer sequencing approach that quantifies drug-resistant HIV present at frequencies as low as 1% at all known sites [18], to assess the prevalence and frequency of nevirapine-resistant HIV in plasma and long-lived cells, and examined their effect on virologic response when nevirapine was reused for treatment.
METHODS
Study Participants, Intervention, and Monitoring
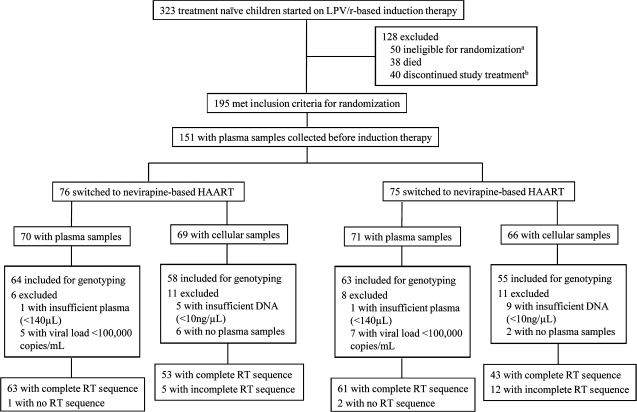
The study participants were a subset of children from the recently reported NEVEREST trial [17]. Subtype C HIV-infected South African children aged between 2 and 24 months who had received single-dose nevirapine were treated with induction HAART containing lopinavir/ritonavir, lamivudine, and stavudine before being randomized to continue the lopinavir/ritonavir regimen or switch to therapy with nevirapine, lamivudine, and stavudine [17]. Children who were eligible for randomization (decrease in plasma viremia to <400 copies/mL for ≥3 months within 12 months of induction HAART; n = 195) and who started HAART at entry into NEVEREST (n = 151) were eligible for our study (Figure 1) [17]. Forty-four children were not eligible because of referral into the trial after HAART was initiated. Stored pretreatment plasma samples and peripheral blood mononuclear cells (PBMCs) collected during induction HAART when plasma viral loads were <400 copies/mL were available from 141 and 135 children, respectively.
Figure 1.
Flow of participants through the study. HAART, highly active antiretroviral therapy; LPV/r, lopinavir/ritonavir; RT, reverse transcriptase. aIneligible for randomization because of nonsuppression, initiation of tuberculosis therapy, or increased levels of liver enzymes. bDiscontinued therapy because of study withdrawal, move from study area, or loss to follow-up.
HIV Genotyping of Pretreatment Plasma and PBMCs
At the time of our study, ultradeep pyrosequencing was validated for quantifying drug-resistant HIV at ≥1% frequency when plasma viral loads were ≥100,000 copies/mL [18]. Of the 141 children eligible for our study, genotyping was not possible for 14, because plasma volumes were insufficient or viral loads were <100,000 copies/mL (Figure 1). Analysis of resistance in cells was possible in 113 of 135 children whose viral loads were suppressed with induction HAART, had sufficient genomic DNA (>10 ng/μL), and paired pretreatment plasma samples (Figure 1).
Viral RNA from plasma (140 μL) and genomic DNA from PBMCs were extracted using column-based nucleic acid isolation (Qiagen). cDNA was synthesized from viral RNA with subtype C gene-specific primers. Four single-round polymerase chain reactions were performed from cDNA or standard input of genomic DNA (350 ng) for amplification of HIV reverse transcriptase regions encompassing 2 clusters of nevirapine resistance sites (amino acid positions 98–108 and 181–190) [18]. Reactions with positive results were sequenced by 454 Life Sciences. For children who failed nevirapine-based HAART, standard genotyping was performed with an in-house assay on plasma samples collected at the earliest time point when failure was confirmed [19].
Sequence Analyses
Sequences generated by ultradeep pyrosequencing were analyzed using GS Amplicon Variant Analyzer software [18]. Sequences derived by standard genotyping were analyzed as previously described [19–21]. Drug resistance mutations were characterized on the basis of recommendations from the International AIDS Society-USA and Stanford University drug resistance database [22, 23]. Nevirapine-resistant virus was quantified as a sum percentage of HIV variants in plasma or HIV-infected cells containing any of the following mutations: L100I, K101E/P, K103N/S, V106A/M, V108I, Y181C/I/V, Y188C/L/H, and G190A/S/E.
Statistical Analyses
Children with viral loads of ≥1000 copies/mL at 2 or more visits within 52 weeks after switch to nevirapine-based HAART, a safety end point in the parent trial [17], were defined as experiencing virologic failure. The time at confirmed viremia of ≥1000 copies/mL was used as the time of virologic failure in time-to-event analyses. The main covariates of interest were the prevalence and frequency of nevirapine-resistant HIV in pretreatment plasma and cells. Receiver operating characteristic (ROC) analysis was used to investigate whether a threshold frequency of nevirapine-resistant HIV in pretreatment plasma or cells predicted virologic failure. This threshold frequency was calculated on the basis of the maximum Youden index (sensitivity – [1 – specificity]) associated with failure. Kaplan-Meier analysis and Cox proportional hazard regression were used to assess the impact of nevirapine-resistant HIV on the probability and time to failure, after adjusting for potential confounders (pretreatment viral load and CD4+ T cell percentages, and age at HAART initiation). The correlation between frequencies of nevirapine-resistant HIV in pretreatment plasma and cells was assessed using Spearman rank correlation. Baseline characteristics of children randomized to the nevirapine and lopinavir/ritonavir arms were compared using Fisher exact test for categorical variables and Wilcoxon rank-sum test for continuous variables. Data analysis was performed using STATA software, version 10.0 (StataCorp).
RESULTS
Patient Characteristics
HIV was successfully genotyped in 124 of 127 children with sufficient pretreatment plasma at viral loads ≥100,000 copies/mL (63 children in the nevirapine arm and 61 children in the lopinavir/ritonavir arm; Figure 1). Similar to the parent trial [17], these 124 children started therapy at median 9 months of age (interquartile range [IQR], 5–14 months; Table 1) and received induction HAART with lopinavir/ritonavir for median 9 months (IQR, 7–12 months) before randomization. The presence of archived nevirapine-resistant HIV in cells while viremia was suppressed with induction HAART was assessed in 96 children (53 in the nevirapine arm and 43 in the lopinavir/ritonavir arm; Figure 1) who also had paired plasma genotypes. Pretreatment viral loads, CD4+ T cell percentages, World Health Organization (WHO) clinical staging, and months of receipt of induction HAART were similar for the 2 treatment groups (Table 1).
Table 1.
Patient Characteristics
| Characteristic | Children receiving nevirapine (n = 63) | Children receiving lopinavir/ritonavir (n = 61) | Pa |
| Plasma viral load at study entry | |||
| <750,000 copies/mL | 25 (40) | 21 (34) | |
| ≥750,000 copies/mL | 38 (60) | 40 (66) | .58 |
| CD4+ T cells at study entry, median % (IQR) | 20 (14–24) | 19 (14–26) | .96 |
| Age at study entry, median days (IQR) | 264 (195–407) | 303 (151–505) | .17 |
| Male sex | 27 (52) | 28 (51) | >.99 |
| WHO stage of HIV infection prior to starting antiretroviral therapy | 33 (52) | 31 (51) | .26 |
| 1 | 10 (16) | 13 (21) | |
| 2 | 4 (6) | 0 (0) | |
| 3 | 28 (49) | 29 (48) | |
| 4 | 18 (29) | 19 (31) | |
| Duration of induction therapy prior to randomization, median days (IQR) | 278 (199–371) | 281 (224–362) | .58 |
NOTE. Data are no. (%) of patients, unless otherwise specified. HIV, human immunodeficiency virus; IQR, interquartile range; WHO, World Health Organization.
Fisher exact test was used to compare categorical variables, and Wilcoxon rank sum test was used to compare continuous variables.
Nevirapine Resistance in Plasma Prior to HAART
For analysis of plasma genotypes, a median of 10,868 sequence reads per patient (IQR, 7715–14,551 sequence reads per patient) were obtained by means of ultradeep pyrosequencing. The prevalence of nevirapine resistance prior to HAART was 33% (41 of 124 children; Figure 2A). The median frequency of nevirapine-resistant virus in plasma was 25.6% (IQR, 14.4%–73.7%). Most children (32 [78%] of 41) had only 1 resistance mutation; however, 8 (20%) had 2 mutations and 1 child had 3 mutations.
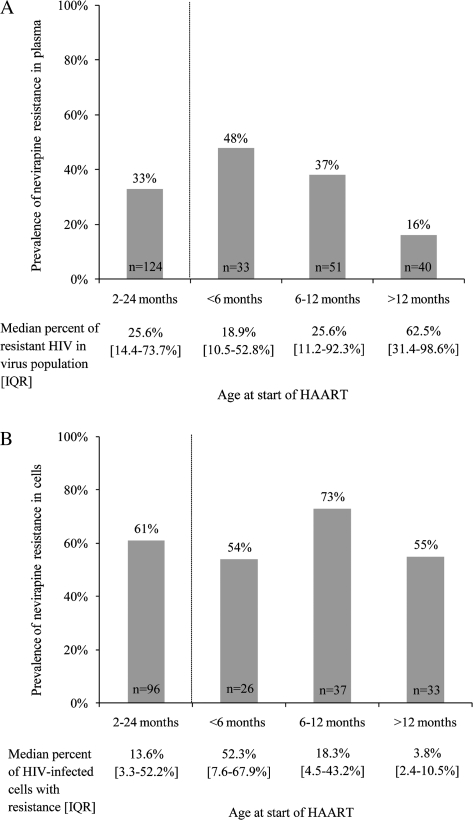
Figure 2A.
Overall prevalence and median frequency of nevirapine-resistant human immunodeficiency virus (HIV) in the pretreatment plasma virus population in children initiating highly active antiretroviral therapy (HAART) between 2 and 24 months of age. Also shown are the prevalence of resistance for children <6 months, 6–12 months, and >12 months of age at start of HAART (Fisher exact test P = .007) and their associated frequencies of resistant HIV in the plasma virus population (Wilcoxon rank sum test P = .23). IQR, interquartile range.Figure 2B. Overall prevalence and median frequency of human immunodeficiency virus (HIV)–infected cells with nevirapine resistance mutations in peripheral blood mononuclear cells collected from children whose viral load was suppressed with lopinavir/ritonavir–based highly active antiretroviral therapy (HAART) for a median of 9 months. Also shown are the prevalence of resistance in cells for children <6 months, 6–12 months, and >12 months of age at start of HAART (Fisher exact test P = .19) and their associated frequencies of HIV-infected cells with resistance (Wilcoxon rank sum test P = .03). IQR, interquartile range; PBMCs, peripheral blood mononuclear cells.
As expected, the persistence of nevirapine-resistant HIV in plasma differed significantly by age. Younger children starting HAART had a significantly higher prevalence of resistance: 16 (48%) of 33 infants aged <6 months, compared with 19 (37%) of 51 and 6 (15%) of 40 of children aged 6–12 and >12 months, respectively (P = 0.005; Figure 2A). However, when resistance was detectable in plasma, there was a trend toward higher frequencies in older children. The median frequencies of nevirapine-resistant virus were 18.9% (IQR, 10.5%–52.8%) for children aged <6 months, 25.6% (IQR, 11.2%–92.3%) for children aged 6–12 months, and 62.5% (IQR, 31.4%–98.6%) for children aged >12 months (P = .23; Figure 2A).
In plasma, Y181C was the most common mutation detected and was present in samples from 31 (76%) of 41 children with resistant virus (median frequency, 25.6% [IQR, 13.4%–71.9%]). The K103N mutation was detected in samples from 27% of children (median frequency, 14.6% [IQR, 1.3%–82.7%]). Three other nevirapine resistance mutations were also detected, although at relatively low levels: G190A/E (15% prevalence; median frequency, 4.4% [IQR, 2.1%–12.4%]), V106A/M (7% prevalence; median frequency, 1.5% [IQR, 1.2%–7.4%]), and K101E (in 1 child in 3.0% of plasma virus).
Archived Nevirapine Resistance during Suppressive Induction HAART
From a median of 12,696 sequence reads per patient (IQR, 10,630–17,728 sequence reads per patient), archived nevirapine resistance mutations were detectable in 59 (61%) of 96 children at a median of 18 months of age (IQR, 15–25 months; Figure 2B). The median frequency of cells with nevirapine-resistant HIV during suppressive induction HAART was 13.6% (IQR, 3.3%–52.2%). Moreover, the frequencies of infected cells with nevirapine resistance directly correlated with frequencies of resistant virus in plasma prior to therapy (r = 0.580; P < .001). As observed in plasma, most children (39 [66%] of 59) had 1 nevirapine resistance mutation; 13 (22%) had 2 mutations, 6 (10%) had 3 mutations, and 1 child had 4 mutations.
We did not detect a difference in prevalence of archived nevirapine resistance according to age. Fourteen (54%) of 26 children aged <6 months, 27 (73%) of 37 children aged 6–12 months, and 18 (55%) of 33 children aged >12 months at the start of HAART had archived nevirapine-resistant HIV (P = .19; Figure 2B). However, younger children had significantly higher frequencies of infected cells with resistant virus: a median of 52.3% (IQR, 7.6%–67.9%) in children initiating therapy at age <6 months, 18.3% (IQR, 4.5%–43.2%) in children initiating therapy at age 6–12 months, and 3.8% (IQR, 2.4%–10.5%) in children initiating therapy at age >12 months (P = .03; Figure 2B).
All 8 nevirapine resistance mutations [22, 23] were detected in cells. As observed in plasma, Y181C was the most frequent mutation detected and was present in specimens from 34 (58%) of 59 children with resistance. The median frequency of HIV-infected cells with Y181C was 22.4% (IQR, 6.5%–52.5%). G190A/E mutations were also commonly detected in cells (44%) and were present in a median of 2.6% of infected cells (IQR, 1.3%–3.5%). The K103N mutation was present in 14% of children in a median of 37.0% of infected cells (IQR, 4.0%–76.4%), and V106A/M mutations were present in 17% of children in a median of 4.8% of infected cells (IQR, 1.5%–28.5%). The mutations L100I, K101E, V108I, and Y188C/H were detected in 2%–7% of children at median frequencies ranging from 1.2% to 7.3% of cells.
Effect of Nevirapine Resistance on Virologic Control with Nevirapine-based HAART
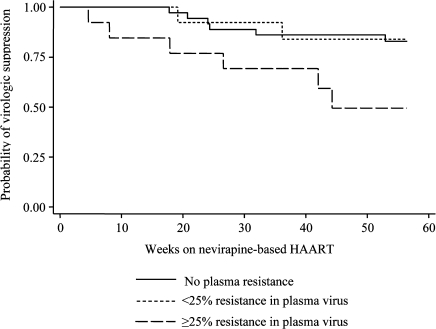
As in the parent trial [17], confirmed viremia of more than 1000 copies/mL by 52 weeks was more common in children who switched to nevirapine-based HAART than in children who continued to receive lopinavir/ritonavir–based HAART (14 [22%] of 63 vs 2 [3%] of 61; P = .002). We examined whether frequencies of nevirapine-resistant HIV in plasma prior to therapy would adversely affect virologic suppression after switch to nevirapine-based HAART. We did not detect a difference in the risk of virologic failure for children with nevirapine resistance at ≥1% frequency compared with those without detectable resistance (HR, 2.0 [95% CI, 0.7–5.9]; P = .19). ROC analysis was used to identify a threshold frequency of nevirapine-resistant virus in plasma of 25% to maximize the sensitivity and specificity of predicting failure in nevirapine-treated children despite initial treatment with the lopinavir/ritonavir regimen. Indeed, children who had pretreatment nevirapine resistance at or above this threshold frequency of 25% had a 3.5-fold higher risk of virologic failure than children without resistance (95% CI for HR, 1.1–10.8; P = .03). There was no suggestion of a difference in the risk of failure between children with resistance in plasma below 25% frequency and those without resistance (HR, 0.9 [95% CI, 0.2–4.5]; P = .91). Of the 13 children with nevirapine resistance at ≥25% frequency (median frequency, 83.9% [IQR, 38.9%–95.8%]), 6 (46%) experienced failure, whereas 2 (15%) of 13 children with <25% frequency of resistance (median frequency, 14.4% [IQR, 3.0%–16.8%]) and 6 (16%) of 37 children with no resistance experienced failure on nevirapine-based therapy. The observed differences in the time to virologic failure for these 3 groups are displayed in Figure 3.
Figure 3.
Kaplan-Meier estimates of virologic suppression (<1000 copies/mL at ≥2 visits) by 52 weeks after switch to nevirapine-based highly active antiretroviral therapy (HAART) for children in the study with resistance at ≥25% frequency (long-dashed line), <25% frequency (short-dashed line), and no detectable resistance in pretreatment plasma (<1%; solid line).
Archived nevirapine resistance detected in cells just prior to randomization was not predictive of virologic failure for patients receiving nevirapine-based HAART (HR, 1.2 [95% CI, 0.3–4.5]; P = .77). Unlike for resistance detected in plasma, we were unable to identify a threshold frequency of nevirapine-resistant cells associated with failure. The frequencies of nevirapine-resistant cells were, however, linked with the frequencies of resistant virus maintained in pretreatment plasma for children treated with nevirapine-based HAART. All 10 of the children who had a frequency of resistance in plasma of more than the threshold of 25% had detectable archived resistance in a median of 75.3% of infected cells (IQR, 49.6%–87.7%). This archived resistance was higher in prevalence and frequencies than in children who started HAART with <25% of plasma virus with nevirapine resistance (10 [77%] of 13 children; a median of 23.1% of infected cells [IQR, 7.6%–52.2%]) and without plasma resistance (17 [57%] of 30 children; a median of 4.1% of infected cells [IQR, 2.4%–7.6%]; P = .02).
We also examined whether other factors were predictive of virologic control with the switch to nevirapine-based HAART. Pretreatment viral loads and CD4+ T cell percentages were not significantly associated with virologic failure. Children who started induction HAART by 6 or 6–12 months of age were also not at an increased risk of virologic failure compared with children who started at age >12 months (<6 months: HR, 0.8 [95% CI, 0.3–2.5]; P = .73; 6–12 months: HR, 0.2 [95% CI, 0.02–1.6]; P = .12). Even when age at start of HAART was included in multivariate analysis, the association between plasma resistance at ≥25% threshold frequency and virologic failure persisted (HR, 6.4 [95% CI, 1.5–27.4]; P = .01).
Antiretroviral Drug Resistance Mutations among Children with Virologic Failure
HIV genotypes were amplified from specimens obtained from 12 of 14 children who experienced failure on nevirapine-based therapy (Table 2). Overall, adherence was considered suboptimal (<95% adherence) for only 2 of 12 patients who experienced failure (patients 2 and 11). Seven (58%) of the 12 children experienced failure with mutations that were either detected in pretreatment plasma or archived in cells before reexposure to nevirapine. Three children experienced failure with nonnucleoside reverse transcriptase inhibitor (NNRTI) mutations not detected in pretreatment plasma or cells (patients 1, 4, and 5), and 2 with wild-type virus (patients 7 and 12). Ten children (83%) experienced failure with Y181C, and 5 children with mutations not typically seen during failure involving nevirapine (K101E, V106A/M, and G190A; Table 2). The lamivudine resistance mutation, M184V, was detected in 8 children (75%), but none experienced failure with thymidine analog mutations (Table 2).
Table 2.
Nevirapine Resistance in Pretreatment Plasma, Long-lived Cells, and at Rebound Viremia for Children Experiencing Virologic Failure after Switch to Nevirapine-Based Highly Active Antiretroviral Therapy
| Patient | NNRTI resistance in pretreatment plasma (% mutation frequency) | NNRTI resistance in long-lived cells (% mutation frequency) | Resistance at rebound viremia with nevirapine maintenance HAART |
|
| NNRTI mutations | NRTI mutations | |||
| 1 | WT | WT | K101E, G190A | M184V |
| 2 | WT | WT | Y181C | M184V |
| 3 | WT | K103N (1.8); Y181C (26.1); G190E (4.5) | Y181C | M184V |
| 4 | WT | G190E (1.6) | V106A | M184V |
| 5 | WT | G190E (4.2) | V106A | M184V |
| 6 | WT | V106M (1.5); Y181C (14.0); G190E (2.9) | Y181C, V106M | M184V |
| 7 | Y181C (38.9) | Y181C (2.9) | K103N, Y181C | L74V, M184V |
| 8 | Y181C (25.4) | Y181C (46.8); G190E (2.8) | Y181C | None |
| 9 | Y181C (25.6) | Y181C (63.3) | K101E, Y181C | M184V |
| 10 | Y181C (92.4) | Y181C (93.3) | Y181C | None |
| 11 | Y181C (1.8); G190E (1.2) | WT | WT | None |
| 12 | Y181C (16.8) | Y181C (7.6) | WT | None |
NOTE. NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; WT, wild-type HIV.
Discussion
Persistence of nevirapine resistance after exposure to the single prophylactic dose, even at very low frequencies (0.2%), increases the risk of virologic failure with nevirapine-based therapy in both women [14, 24, 25] and infants [15]. In our study, however, we found that when children were first effectively treated with a lopinavir/ritonavir–based regimen, the presence of nevirapine resistance at a frequency of ≥1% of plasma virus did not increase the risk of virologic failure with use of nevirapine-based therapy. Rather, the persistence of nevirapine-resistant HIV in plasma at frequencies of 25% or higher was what placed children at higher risk (3.5-fold) of failure. Indeed, children in the NEVEREST trial were more likely to experience failure with nevirapine-based HAART than with lopinavir/ritonavir–based HAART when resistance was identified by means of standard genotyping prior to therapy [17]. The small number of failures in our study may have limited our ability to detect a statistical difference in the risk of failure for children with <25% frequency of nevirapine-resistant virus, although our data were not suggestive of a higher risk. Nevertheless, this observation warrants additional study, because it differs from findings when nevirapine was administered as part of first-line therapy [15].
The detection of nevirapine resistance in cells of children while viremia was effectively suppressed with induction therapy with protease inhibitors suggests that archiving of resistant HIV occurs in a substantial proportion of children (61%) after nevirapine prophylaxis. Archived resistance was not predictive of virologic failure on reexposure to nevirapine, supporting the notion that not all archived, drug-resistant HIV present after prophylaxis are replication competent and clinically relevant [26]. Analysis of the replication competence of the archived pool was not feasible in this study, nor was genetic linkage of archived variants to rebounding virus, because ultradeep pyrosequencing only generated short sequence reads. However, children with resistant HIV at frequencies of ≥25% in plasma also had higher frequencies of cells with resistant virus just prior to randomization. This may reflect a fitness advantage of these variants to replicate to high levels in plasma in the absence of nevirapine and to establish replication-competent reservoirs likely to compromise therapy within 1 year after reexposure to nevirapine. Given that preexisting low-frequency, NNRTI resistance can compromise first-line therapy with NNRTIs [12, 14, 15, 18, 24], it is possible that induction therapy also cleared short-lived cells infected with nevirapine-resistant HIV that would otherwise contribute to a poorer treatment response. Whether a longer duration of induction HAART with lopinavir/ritonavir would allow clearance of cells contributing high frequencies of nevirapine-resistant virus in plasma is unclear but important to study.
In our study, unlike in studies of women and infants who had received single-dose nevirapine and were receiving first-line nevirapine-based HAART, we did not find the time to rechallenge with nevirapine after prophylaxis affected treatment response. Initiation of nevirapine-based HAART within 6 months in women and 7–12 months in children after receipt of the single prophylactic dose was associated with lower rates of virologic suppression than in those who received delayed treatment or no treatment with nevirapine [12, 13, 15, 16, 28–32]. Since the prevalence of nevirapine resistance in plasma and the frequencies of infected cells with resistance were higher in younger children in our study, it is possible that induction therapy modified this effect by clearing short-lived infected cells with actively replicating nevirapine-resistant virus. Whether this was confounded by predominance of resistance at frequencies of ≥25% in plasma in older children starting HAART (5/6 with resistance) remains unclear.
This study reports a high threshold level of nevirapine resistance in plasma (25%) to predict poor virologic response with reuse of nevirapine after prophylaxis. These findings suggest that standard genotyping assays may be sufficient to identify nevirapine-exposed children likely to respond to an induction-switch approach. Because nevirapine is a key component of strategies to prevent mother-to-child HIV transmission [33] and of HAART for infants in resource-poor countries [27], insights into circumstances that would allow reuse of NNRTIs remain critical.
Acknowledgments
We thank the children and their families who participated in this trial, and the NEVEREST study team for their assistance with the conduct of the study, data collection, and verification.
Potential conflicts of interest. All authors: no conflicts.
Financial support. This study was supported by the National Institutes of Child Health and Human Development, National Institutes of Health (NIH) (grant number RO1HD057784-04 to D.P.). The NEVEREST clinical trial was supported by the National Institutes of Child Health and Human Development, NIH (grant number HD47177 to L.K.) and the Secure the Future Foundation.
References
- 1.Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 2.Jackson JB, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet. 2003;362:859–68. doi: 10.1016/S0140-6736(03)14341-3. [DOI] [PubMed] [Google Scholar]
- 3.Bedri A, Gudetta B, Isehak A, et al. Extended-dose nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: an analysis of three randomised controlled trials. Lancet. 2008;372:300–13. doi: 10.1016/S0140-6736(08)61114-9. [DOI] [PubMed] [Google Scholar]
- 4.Kumwenda NI, Hoover DR, Mofenson LM, et al. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. N Engl J Med. 2008;359:119–29. doi: 10.1056/NEJMoa0801941. [DOI] [PubMed] [Google Scholar]
- 5.Chasela C, Hudgens M, Jamieson D, et al. Paper presented at: 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention. Capetown, South Africa: Both maternal HAART and daily infant nevirapine (NVP) are effective in reducing HIV-1 transmission during breastfeeding in a randomized trial in Malawi: 28 week results of the Breastfeeding, Antiretroviral and Nutrition (BAN) Study. 19–22 July. 2009. [Google Scholar]
- 6.Arrive E, Newell ML, Ekouevi DK, et al. Prevalence of resistance to nevirapine in mothers and children after single-dose exposure to prevent vertical transmission of HIV-1: a meta-analysis. Int J Epidemiol. 2007;36:1009–21. doi: 10.1093/ije/dym104. [DOI] [PubMed] [Google Scholar]
- 7.Flys T, Nissley DV, Claasen CW, et al. Sensitive drug-resistance assays reveal long-term persistence of HIV-1 variants with the K103N nevirapine (NVP) resistance mutation in some women and infants after the administration of single-dose NVP: HIVNET 012. J Infect Dis. 2005;192:24–9. doi: 10.1086/430742. [DOI] [PubMed] [Google Scholar]
- 8.Moorthy A, Gupta A, Bhosale R, et al. Nevirapine resistance and breast-milk HIV transmission: effects of single and extended-dose nevirapine prophylaxis in subtype C HIV-infected infants. PLoS One. 2009;4:e4096. doi: 10.1371/journal.pone.0004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmer S, Boltz V, Martinson N, et al. Persistence of nevirapine-resistant HIV-1 in women after single-dose nevirapine therapy for prevention of maternal-to-fetal HIV-1 transmission. Proc Natl Acad Sci U S A. 2006;103:7094–9. doi: 10.1073/pnas.0602033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loubser S, Balfe P, Sherman G, Hammer S, Kuhn L, Morris L. Decay of K103N mutants in cellular DNA and plasma RNA after single-dose nevirapine to reduce mother-to-child HIV transmission. AIDS. 2006;20:995–1002. doi: 10.1097/01.aids.0000222071.60620.1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wind-Rotolo M, Durand C, Cranmer L, et al. Identification of nevirapine-resistant HIV-1 in the latent reservoir after single-dose nevirapine to prevent mother-to-child transmission of HIV-1. J Infect Dis. 2009;199:1301–9. doi: 10.1086/597759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lockman S A5208/OCTANE study team. Programs and abstracts of the 16th Conference on Retroviruses and Opportunisitic Infection. (Montreal, Canada): Lopinavir/ritonavir+tenofovir/emtricitabine is superior to nevirapine+tenofovir/emtricitabine for women with prior exposure to single-dose nevirapine: a5208 (“OCTANE”) 2009. Paper#955. [Google Scholar]
- 13.Palumbo P, Violari A, Lindsey J, et al. 5th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention. Capetown, South Africa. Alexandria, VA: Foundation for Retrovirology and Human Health, 2009.: Nevirapine (NVP) vs lopinavir-ritonavir (LPV/r)-based antiretroviral therapy (ART) in single dose nevirapine (sdNVP)-exposed HIV-infected infants: preliminary results from the IMPAACT P1060 trial. 19–22 July. 2009 [abstract LBPEB12] [Google Scholar]
- 14.Boltz V, Zheng Y, Lockman S, et al. Program and abstracts of the 17th Conference on Retroviruses and Opportunistic Infections. (Boston, MA): NNRTI-resistant variants detected by allele-specific PCR predict outcome of NVP-containing ART in women with prior exposure to sdNVP: results from the OCTANE/A5208 study. Alexandria, VA: Foundation for Retrovirology and Human Health, 2010. Paper#154. [Google Scholar]
- 15.Macleod IJ, Rowley CF, Thior I, et al. Minor resistant variants in nevirapine-exposed infants may predict virologic failure on nevirapine-containing ART. J Clin Virol. 2010;48:162–7. doi: 10.1016/j.jcv.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jourdain G, Wagner TA, Ngo-Giang-Huong N, et al. Association between detection of HIV-1 DNA resistance mutations by a sensitive assay at initiation of antiretroviral therapy and virologic failure. Clin Infect Dis. 2010;50:1397–404. doi: 10.1086/652148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coovadia A, Abrams EJ, Stehlau R, et al. Reuse of nevirapine in exposed HIV-infected children after protease inhibitor-based viral suppression: a randomized controlled trial. JAMA. 2010;304:1082–90. doi: 10.1001/jama.2010.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simen BB, Simons JF, Hullsiek KH, et al. Low-abundance drug-resistant viral variants in chronically HIV-infected, antiretroviral treatment-naive patients significantly impact treatment outcomes. J Infect Dis. 2009;199:693–701. doi: 10.1086/596736. [DOI] [PubMed] [Google Scholar]
- 19.Ziemniak C, George-Agwu A, Moss WJ, Ray SC, Persaud D. A sensitive genotyping assay for detection of drug resistance mutations in reverse transcriptase of HIV-1 subtypes B and C in samples stored as dried blood spots or frozen RNA extracts. J Virol Methods. 2006;136:238–47. doi: 10.1016/j.jviromet.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 20.Kumar S, Tamura K, Nei M. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform. 2004;5:150–63. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
- 21.Los Alamos National Laboratory. HIV databases. 2008. http://www.hiv.lanl.gov/content/index. Accessed 25 February 2010. [Google Scholar]
- 22.Stanford University HIV Drug Resistance Database. 2008. http://hivdb.stanford.edu/. Accessed 25 February 2010. [Google Scholar]
- 23.Johnson VA, Brun-Vezinet F, Clotet B, et al. Update of the drug resistance mutations in HIV-1: spring 2008. Top HIV Med. 2008;16:62–8. doi: 10.1007/s11750-007-0034-z. [DOI] [PubMed] [Google Scholar]
- 24.Coovadia A, Hunt G, Abrams EJ, et al. Persistent minority K103N mutations among women exposed to single-dose nevirapine and virologic response to nonnucleoside reverse-transcriptase inhibitor-based therapy. Clin Infect Dis. 2009;48:462–72. doi: 10.1086/596486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rowley CF, Boutwell CL, Lee EJ, et al. Ultrasensitive detection of minor drug-resistant variants for HIV after nevirapine exposure using allele-specific PCR: clinical significance. AIDS Res Hum Retroviruses. 2010;26:293–300. doi: 10.1089/aid.2009.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chun TW, Carruth L, Finzi D, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–8. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. Report of the WHO technical reference group, paediatric HIV/ART care guideline group meet. 2008. http://www.who.int/hiv/pub/paediatric/WHO_Paediatric_ART_guideline_rev_mreport_2008.pdf. Accessed 1 January 2010. [Google Scholar]
- 28.Kuhn L, Semrau K, Ramachandran S, et al. Mortality and virologic outcomes after access to antiretroviral therapy among a cohort of HIV-infected women who received single-dose nevirapine in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2009;52:132–6. doi: 10.1097/QAI.0b013e3181ab6d5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jourdain G, Ngo-Giang-Huong N, Le Coeur S, et al. Program and abstracts of the 16th Conference on Retroviruses and Opportunistic Infections. (Montreal, Canada): 4-Year clinical and therapeutic consequences of intra-partum single-dose nevirapine for the prevention of perinatal HIV in women who subsequently initiated a nevirapine-based ART. Alexandria, VA: Foundation for Retrovirology and Human Health, 2009. Paper#954. [Google Scholar]
- 30.Chi BH, Sinkala M, Stringer EM, et al. Early clinical and immune response to NNRTI-based antiretroviral therapy among women with prior exposure to single-dose nevirapine. AIDS. 2007;21:957–64. doi: 10.1097/QAD.0b013e32810996b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jourdain G, Ngo-Giang-Huong N, Le CS, et al. Intrapartum exposure to nevirapine and subsequent maternal responses to nevirapine-based antiretroviral therapy. N Engl J Med. 2004;351:229–40. doi: 10.1056/NEJMoa041305. [DOI] [PubMed] [Google Scholar]
- 32.Lockman S, Shapiro RL, Smeaton LM, et al. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N Engl J Med. 2007;356:135–47. doi: 10.1056/NEJMoa062876. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization. Use of antiretroviral drugs for treating pregnant women and preventing HIV nfection in infants. 2009. http://www.who.int/hiv/pub/mtct/rapid_advice_mtct.pdf. Accessed 1 January 2010. [PubMed] [Google Scholar]