HIV-infected patients with tuberculosis who initiate nonnucleoside reverse-transcriptase-based anti-retroviral treatment in combination with rifampicin-based antituberculosis treatment demonstrate increases in total cholesterol, low-density cholesterol, and high-density cholesterol levels but no change in blood glucose level after 1 year. Cholesterol increases were more frequent among patients receiving efavirenz.
Abstract
Background. Our aim was to study the incidence and pattern of dyslipidemia among human immunodeficiency virus (HIV)–infected patients with tuberculosis (TB) who received once-daily antiretroviral therapy (ART).
Methods. Antiretroviral-naive HIV-infected patients with TB were recruited to a trial of once-daily nonnucleoside reverse-transcriptase inhibitor (NNRTI)–based ART and treated with rifampicin-based thrice-weekly antituberculosis treatment (ATT); participants were randomized to receive didanosine (250/400 mg) and lamivudine (300 mg) with either efavirenz (600 mg) or nevirapine (400 mg) once-daily after an intensive phase of ATT. Fasting triglyceride (TG) level, total cholesterol (TC) level, low-density cholesterol (LDL-c) level and high-density cholesterol (HDL-c) level were measured at baseline and at 6 and 12 months. Lipid levels at 6 and 12 months were compared with baseline values with use of repeated measures analyses. McNemar test was used to compare the proportion of patients with lipid abnormality at baseline versus at 12 months, and χ2 test was used to compare between the 2 groups.
Results. Of 168 patients (79% men; mean age, 36 years; mean weight, 42 kg; median CD4+ cell count, 93 cells/mm3), 104 received efavirenz-based ART, and 64 received nevirapine-based ART. After 6 months, TC levels increased by 49 mg/dL, LDL-c levels by 30 mg/dL, and HDL-c levels increased by 18 mg/dL (P < .001 for all). At baseline and at 12 months, TC was >200 mg/dL for 1% and 26% of patients, respectively; LDL-c level was >130 mg/dL for 3% and 23%, respectively; HDL-c level was <40 mg/dL for 91% and 23%, respectively; and blood glucose level was >110 mg/dL for 14% and 13%, respectively. TC level >200 mg/dL was more common among patients who received efavirenz than among those who received nevirapine (32% vs 16%; P = .04).
Conclusions. HIV-infected patients with TB who initiate NNRTI-based ART undergo complex changes in lipid profile, highlighting the importance of screening and treating other cardiovascular disease risk factors in this population.
Dyslipidemia is a well-recognized complication of human immunodeficiency virus (HIV) infection and of highly active antiretroviral therapy (HAART), and it has been linked to an increased risk for cardiovascular morbidity in HIV-1–infected patients [1]. Previous studies have shown that some antiretroviral drugs, such as stavudine and protease inhibitors (PI), increase blood levels of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-c), and triglycerides (TG) and have variable effects on levels of high-density lipoprotein cholesterol (HDL-c) [2,3]. By contrast, antiretroviral therapy (ART) regimens containing nonnucleoside reverse-transcriptase inhibitors (NNRTIs) have been less well studied, although receipt of nevirapine is associated with less atherogenic lipid profiles [4].
Data on the effect of these regimens on lipid profiles in genetically different populations from countries such as India are scarce. With the dramatic scale-up of access to ART in many countries with differing baseline risk factors for cardiovascular disease (CVD), it is important to determine the prevalence of ART-associated complications, such as dyslipidemia, in different patient populations. Patients initiating ART in these settings may experience different rates and types of lipid abnormalities than patients in resource-sufficient countries because of differences in genetic background, dietary intake, and lifestyle factors. In addition, patients in resource-limited settings are more likely to have advanced HIV disease and poor nutritional status and to begin treatment with non PI-based regimens.
A small study from Pune, India, found that treatment with stavudine, lamivudine, and nevirapine was associated with substantial increases in TC and TG levels in HIV-infected patients treated for 18–20 months [5]. However, with respect to the widely used first-line ART regimens in India, which are nevirapine- or efavirenz-based, there are no comparative data on the differing effects of these regimens on plasma lipids in this population. We studied lipid profiles before initiation and after 6 and 12 months of a once-daily NNRTI-based antiretroviral regimen, given along with antituberculosis treatment (ATT), in adults with HIV and tuberculosis (TB) co-infection who participated in a once-daily ART clinical trial in Tamilnadu, India.
METHODS
This dyslipidemia study was nested within a prospective randomized controlled clinical trial called “Efficacy and Safety of Once Daily Nevirapine or Efavirenz Based Antiretroviral Therapy when Co-administered with Rifampicin Based Antitubercular Therapy (ClinicalTrials.gov Number: NCT00332306).” HIV-1–infected adults with active TB and with CD4+ cell counts <250 cells/mm3 seen at the Tuberculosis Research Centre, India, from May 2006 through June 2008 were eligible to participate. Patients with prior ART or ATT, HIV-2 infection, substance abuse, major complications of HIV disease, psychiatric illnesses, or serum transaminase levels >2.5 times the upper limit of normal were excluded.
This planned sub-study of the “Once-daily ART” trial collected additional clinical and laboratory data as part of the trial to monitor the safety and efficacy of administering different ART regimens in combination with rifampicin-based ATT. Therefore, it was possible to study the development and evolution of dyslipidemia prospectively in this study.
All patients enrolled in the “Once-daily ART” study initiated a standard anti-TB regimen with 4 drugs (isoniazid 600 mg; rifampicin 450 or 600 mg, determined on the basis of body weight ≤60 or >60 kg, respectively; ethambutol 1200 mg; and pyrazinamide 1500 mg) for the first 2 months and 2 drugs (isoniazid and rifampicin) for the subsequent 4 months. All drugs were administered thrice-weekly (2EHRZ3/4RH3) under direct observation in accordance with national guidelines. After the first 2 months of ATT, participants were randomized to a once-daily ART regimen with either nevirapine (400 mg per day, after a lead-in period of 200-mg doses administered once daily) or efavirenz (600 mg per day), along with didanosine (250 or 400 mg per day for body weight <60 or ≥60 kg, respectively) and lamivudine (300 mg per day).
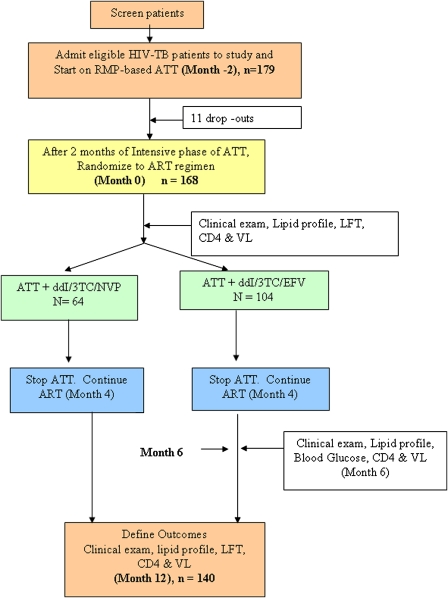
The time of ATT initiation was defined as Month -2 (ie, 2 months before the initiation of ART), and time of ART initiation was defined as month 0 or baseline. Subsequent months are chronological with reference to month 0. ART was administered under direct supervision 3 days per week and supplied to the patient for self administration for the remaining days. Although ATT was stopped at 6 months, which was month 4 of the ART study, ART was continued (Figure 1), and end points were determined.
Figure 1.
Schematic of once-daily ART study design. 3TC, lamivudine; ATT, antituberculosis treatment; ART, antiretroviral therapy; CD4, CD4+ cell count; ddI, didanosine; EFV, efavirenz; exam, examination; HIV, human immunodeficiency virus; LFT, liver function test; NVP, nevirapine; RMP, rifampin; TB, tuberculosis VL, viral load.
Study Population and Data Collection
During the 12-month follow-up period, participants were examined clinically every month, and height and weight were measured. Blood samples were collected after a 12-h overnight fast for glucose and lipid profile (TC, TG, LDL-c and HDL-c) at baseline, 6 and 12 months after ART in accordance with the randomized trial protocol. Glucose and lipid profiles, including TG, TC, HDL-c, and LDL-c levels, were measured using an automated analyzer (Olympus AU400, Japan), CD4+ cell counts were measured by FACS count, and viral load was measured by Roche COBAS Amplicor (kit version 1.5) every 6 months.
Dyslipidemia was defined according to the US National Cholesterol Education Program III guidelines [6]. These include TC level >200 mg/dL, LDL-c level >130 mg/dL, triglyceride level >150 mg/dL, and HDL-c level <40 mg/dL. Fasting hyperglycemia was defined as blood glucose level >110 mg/dL. For the purposes of our study, an elevation of any one of the lipid parameters to a level above these limits was considered as an abnormal lipid profile.
Statistical Analysis
The distribution of each variable was checked cross-sectionally at baseline and at 6 and 12 months. All unusual values were verified. Mean values and standard deviations were tabulated for normally distributed variables; median values and 25th and 75th percentiles were tabulated for skewed variables. We compared baseline lipid levels and various baseline demographic and clinical characteristics between treatment groups using the Student’s t test or the Wilcoxon rank-sum test, as appropriate. Lipid levels at 6 and 12 months were compared with baseline levels using repeated measures analyses, with skewed variables transformed to attain normality. We also compared changes in lipid levels between treatment groups using repeated measures analyses. We compared the proportion of patients with lipid abnormality at baseline versus at 12 months using the McNemar test for correlated proportions and the χ2 test to compare between the 2 treatment groups. All tests used alpha=.05 as the cutoff point for statistical significance. Analyses were performed using SAS software, version 9.1 (SAS Institute).
The study was approved by the ethics committee of the Tuberculosis Research Centre, and written informed consent was obtained from all patients prior to enrollment in the primary randomized controlled trial.
RESULTS
Of the 179 patients enrolled into the once-daily ART study, 168 patients (132 men and 36 women) were eligible for the current analysis (11 were not randomized). Follow-up data were available for 140 patients at 6 and 12 months (15 patients died, 11 were lost to follow-up, and 2 missed study visits). Data from patients who died or were lost to follow-up were not included in this analysis.
Patient Characteristics
At baseline, the 168 HIV-infected patients with TB (male sex, 79%) had a mean age of 36 years, mean body weight of 42 kg, a median CD4+ cell count of 93 cells/mm3, and a mean HIV load of 362,154 copies/mL. A total of 104 patients were randomized to the efavirenz arm, and 64 were randomized to the nevirapine arm (the Data Safety Monitoring Board stopped enrollment of patients into the nevirapine arm after the first interim analysis but allowed enrollment to continue in the efavirenz arm). At baseline, patients did not differ significantly between the 2 treatment arms with respect to body weight, CD4+ cell counts, viral load, lipid profile, or liver enzyme levels (Table 1). Forty-four percent of the patients consumed alcohol (often or habitually). Drug intake was directly observed 3 days a week, overall adherence was >90%, and the majority of subjects had undetectable HIV loads after 12 months of ART.
Table 1.
Demographic and Clinical Characteristics of Patients at Baseline
| Variable | Patients receiving ddI/3TC/EFV (n = 104) | Patients receiving ddI/3TC/NVP (n = 64) | P |
| Age, years | 35 (6.9) | 37 (7.7) | .06 |
| Weight, kg | 41.9 (7.9) | 42.0 (7.7) | .89 |
| Male sex, no. (%) of patients | 81 (78) | 51 (80) | .78 |
| Alcohol habituated, no. (%) of patients | 43 (41) | 31 (48) | .14 |
| CD4+ cell count, median cells/mm3 (IQR) | 90 (53–130) | 75 (34–130) | .14 |
| Viral load, median copies/mL (IQR) | 259000 (81200–531000) | 203000 (87900–370000) | .79 |
| Triglycerides level, median mg/dL (IQR) | 123 (96–164) | 113 (92–173) | .74 |
| HDL-cholesterol level, median mg/dL (IQR) | 26 (20–32) | 24 (20–32) | .66 |
| Total cholesterol level, mg/dL | 124 (34) | 126 (32.6) | .65 |
| LDL-c level, mg/dL | 82 (26) | 79 (25.9) | .49 |
| Fasting glucose level, mg/dL | 97 (26) | 91 (13) | .11 |
NOTE. Data are mean values (± standard deviation) unless otherwise indicated. For comparison between groups, the Student’s t test was used for mean values and the Wilcoxon rank-sum test was used for median values. 3TC, lamivudine; ddI, didanosine; EFV, efavirenz; HDL-c, high-density lipoprotein cholesterol; IQR, interquartile range; LDL-c, low-density lipoprotein cholesterol; NPV, nevirapine.
Baseline Lipids
The baseline lipid levels were comparable by treatment group, sex, and body weight. TG levels were significantly higher in those patients with higher viral loads (141 vs 122 mg/dL; P < .03). HDL-c levels were lower in persons <35 years of age (25 vs 28 mg/dL; P = .03) and those with CD4+ cell counts <90 cells/mm3 (24 vs 29 mg/dL; P = .01). These cutoff values were based on the median levels of the baseline demographic characteristics (data not shown). At baseline, TG level was >150 mg/dL in 44 patients (31%), TC level was >200 mg/dL in 2 patients, HDL-c was <40 mg/dL in 124 patients (91%), LDL-c was >130 mg/dL in 4 patients (3%), and plasma glucose level was >110 mg/dL in 19 patients.
Longitudinal Lipid Values
After 6 months of ART, significant increases were observed in TC level (125 vs 174 mg/dL; P < .001), LDL-c level (83 vs 112 mg/dL; P < .001), and HDL-c level (26 vs 44 mg/dL; P < .001) (Table 2). At 12 months of ART, patient mean weight was 50.5 kg, and median CD4+ cell count had increased from 90 to 363 cells/mm3. As shown in Table 2, the TC, LDL-c, fasting glucose, and HDL-c levels were significantly higher at 12 months than at baseline. The TG levels were comparable (P = .72). After 12 months of treatment, TC level increased by a mean of 54 mg/dL, and LDL-c level increased by 30 mg/dL, whereas HDL-c level increased by a mean of 23.6 mg/dL (median increase, 21 mg/dL) (Table 2).
Table 2.
Pattern of Change in Serum Lipid Levels for the Overall Group receiving Once-Daily Nonnucleoside Reverse-Transcriptase Inhibitor–Based Antiretroviral Therapy
| Lipid parameter | Baseline (n = 140) | At 6 months (n = 140) | Change at 6 months | Pa (6 months vs baseline) | At 12 months (n = 140) | Change at 12 months | Pa (12 months vs baseline) |
| Triglyceride levels, median mg/dL (IQR) | 119 (94, 164) | 125 (96, 172) | 6 (-35, 51) | 0.50 | 121 (88, 165) | 1 (-44, 47) | 0.72 |
| HDL-c level,median mg/dL (IQR) | 26 (20, 33) | 44 (38, 53) | 18 (13, 28) | <.0001 | 47(40, 55) | 21(13, 33) | < .0001 |
| Total cholesterol level, mean mg/dL (±SD) | 125±33.5 | 174±40.1 | 49±43.2 | <.0001 | 179±37.6 | 54±42.6 | < .0001 |
| LDL-c level, mean mg/dL (±SD) | 82.7±26 | 112±29.6 | 29.6±32 | <.0001 | 113±28.2 | 30.5±34 | < .0001 |
| Fasting glucose level, mean mg/dL (±SD) | 94±24 | 98±20 | 4±30.9 | 0.20 | 99±17.2 | 5±29.4 | 0.19 |
NOTE. Log triglyceride levels and square root HDL were used in the repeated measures analyses to attain normality for the skewed variables. HDL-c, high-density lipoprotein cholesterol; IQR, interquartile range; LDL-c, low-density lipoprotein cholesterol; SD, standard deviation.
P values were calculated with repeated measures analyses in Proc Mixed.
At 12 months, the proportion of patients with TC levels >200 mg/dL had increased significantly, from 1% to 26% (P < .001). However, the proportion of patients with HDL-c levels <40 mg/dL decreased significantly, from 91% to 23% (P < .001). The proportion of patients with TG levels >150 mg/dL did not change significantly (31% vs 32%; P = .89) nor did the proportion of patients with fasting glucose levels >110 mg/dL (14% vs 13%), as shown in Table 3.
Table 3.
Comparing Proportion of Patients with Abnormal Lipid and Glucose Levels at Baseline and after 12 Months of ART
| No. (%) of patients with abnormal lipid levels |
|||||
| After 12 monthsof ART (n = 140) |
|||||
| Lipid parameters | Atbaseline (n = 140) | Total | Newly developed | Persisted from baseline | Pa |
| Total cholesterol level >200 mg/dL | 2 (1.4) | 37 (26) | 36 (26) | 1 | < .001 |
| Triglyceride level >150 mg/dL | 44 (31) | 45 (32) | 28 (20) | 17 (12) | .89 |
| HDL cholesterol level <40 mg/dL | 124 (91) | 31 (23) | 0 | 31 (23) | < .001 |
| LDL cholesterol level >130 mg/dL | 4 (3) | 32 (23) | 31 (23) | 1 | < .001 |
| Fasting blood glucose level >110 mg/dL | 19 (14) | 17 (13) | 15 (11) | 2 | .13 |
NOTE. ART, antiretroviral therapy.
Comparing the proportion of patients with abnormal lipid levels at baseline and at 12 months with use of McNemar test.
Changes in Lipid Levels by Type of ART Regimen
We found no statistically significant differences between the nevirapine and efavirenz groups in mean lipid and glucose level changes. However, the proportion of patients at 12 months with total cholesterol >200 mg/dL was significantly higher in the efavirenz arm than in the nevirapine arm (32% vs 16%; P = .04). The proportion of patients with other abnormal lipid parameters was not statistically different between the groups (Table 4). No significant changes were observed in plasma glucose levels in either group. At 12 months, of the 36 patients who had an elevated total cholesterol level (>200 mg/dL), only 3 patients had a detectable viral load (viral load >400 copies/mL), whereas the rest had viral loads <400 copies/mL.
Table 4.
Proportion of Patients with Abnormal Lipid Levels after 12 Months of ART Categorized by Treatment Groups
| No. (%) of patients |
|||||
| Lipid level after 12 months of ART | Efavirenz-based regimen (n = 90) | Nevirapine-based regimen(n = 50) | Odds Ratio | 95% confidence limits | Pa |
| Triglyceride level>150 mg/dL | 30 (33) | 15 (30) | 0.86 | 0.41, 1.81 | .69 |
| Total cholesterol level>200 mg/dL | 29 (32) | 8 (16) | 0.40 | 0.17, .96 | .04 |
| HDL level <40 mg/dL | 26 (29) | 11 (22) | 1.42 | 0.63, 3.21 | .39 |
| LDL level >130 mg/dL | 23 (26) | 9 (18) | 0.65 | 0.27, 1.53 | .32 |
| FBS level >110mg/dL | 14 (16) | 3 (6) | 0.34 | 0.09, 1.24 | .09 |
NOTE. ART, antiretroviral therapy; FBS, fasting blood sugar; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
By χ2 test.
DISCUSSION
In this cohort of patients in South India with TB and advanced HIV disease, who were treated with both anti-TB and antiretroviral drugs, we found that, at baseline, total cholesterol, LDL-c and HDL-c levels were low, whereas triglyceride levels were in the normal range. Fasting glucose level was in the normal range at baseline and remained so throughout the study. After 12 months of successful NNRTI-based ART, HDL levels, as well as total and LDL cholesterol levels, increased significantly. The major changes in lipid profiles occurred during the first 6 months of ART and were maintained without significant change thereafter. At 1 year, there was no difference in the overall lipid profiles between patients who received nevirapine and those who received efavirenz-based regimens. Although HDL levels increased in the majority of patients who received treatment, a significant proportion (23%) continued to have levels below the lower limit of normal. There was an increase in the number of patients with abnormal TC levels (26%) at the end of the study, regardless of the ART regimen.
Studies in resource sufficient countries have documented that patients with advanced HIV infection have decreased levels of TC, LDL-c and HDL-c, with the extent of lipid abnormalities correlating with disease severity. TC and LDL-c levels routinely show an increase with the use of effective ART [7–9]. Recent findings suggest that the use of NNRTI-based therapy results in an elevation in HDL-cholesterol levels and therefore may be less atherogenic than protease inhibitor–based treatments [10]. Nevirapine-containing regimens have been associated with more-favorable changes in lipid profiles (lesser increase in TC, TG, LDL-c levels and greater increase in HDL-c levels) than efavirenz-containing regimens [11, 12]. Although we did not find major differences in lipid profiles between the 2 NNRTI treatment regimens, a greater proportion of patients who received efavirenz developed TC levels >200 mg/dL after 12 months of treatment. Because of multiple testing, this finding should be interpreted with caution.
The patients in our study had both advanced HIV and TB, which will continue to be a frequent occurrence in individuals starting ART in resource-limited areas. The baseline lipid profiles may somewhat reflect the severity of nutritional compromise seen with these co-morbidities. The changes that we observed in lipid profiles in this study (namely, increased TC, HDL-c, and LDL-c levels) may therefore, at least in part, represent the return to normal lipid values when both TB and HIV infection are treated, inflammation decreases, nutritional status and immune function improve, and HIV viremia is controlled. Patients who showed an increase in lipid levels in our study were all virologically suppressed after 12 months of ART and all gained weight. The changes that we saw in TC and LDL-c were disadvantageous, with a significantly increased proportion of patients who developed abnormally high levels. But the changes in HDL-c level were advantageous, with a significant decrease in the proportion of patients who had abnormally low HDL levels. A sizeable proportion of patients in our study (23%), however, continued to demonstrate a low level of HDL cholesterol.
A study from Pune, India, looked at the lipid profile of patients receiving ART (stavudine, lamivudine, and nevirapine) for a mean of 20 months [5]. Their study population was roughly comparable by age and immunologic status to our study population but did not include patients with active TB [5]. In that study, 41% of patients, compared with 26% of the patients in our study, developed TC levels >200 mg/dL, whereas 45% of their patients, in contrast to 32% of our patients, developed TG levels >150 mg/dL. This increase in the proportion of patients who showed abnormal levels in the Pune study may be attributable to differences in TB co-infection, socioeconomic status, longer duration of treatment, and diet, all of which can affect lipid changes in patients receiving ART. A study from Uganda involving patients who received stavudine, lamivudine, and either nevirapine or efavirenz and were followed-up for 24 months, recorded a 10% increase in the number of patients with TC levels >200 mg/dL, a 20% increase in patients with TGL levels >200 mg/dL, and 6% increase in the number of patients with LDL-c level >150 mg/dL at the end of 24 months [13]. The increases in HDL-c and TC level in our study are similar to those in the 2NN study (which was an open-label, randomized, comparative trial of first-line ART in treatment-naive patients, with regimens based on stavudine plus lamivudine plus either efavirenz or nevirapine dosed either at 400 mg once or 200 mg twice daily or both NNRTIs administered simultaneously), where an increase in HDL-c level of 49% was seen in the nevirapine arm [8]. There are no studies from India that compare the lipid profile between these 2 different NNRTIs.
We did not find marked differences by sex in pre-treatment or post-treatment lipid levels, which have been documented in studies of HIV-infected patients elsewhere [8]. However, all of the patients in our study had low pretreatment lipid levels, likely resulting from advanced HIV infection or AIDS, infection with TB, serious undernutrition, or poor dietary intake. These factors may have diminished potential differences, if any, in lipid profiles by sex.
Our findings should be interpreted within the context of the study and its limitations. We did not collect individual-level dietary history and cannot comment on the role of dietary intake. We do not have data on regional body composition or blood pressure, so we were unable to assess the risk of metabolic syndrome or coronary heart disease or develop predictors for the same in this population. Furthermore, our follow-up end point at 12 months may not have been enough to assess long-term changes in lipids.
The Indian population in general has a high risk of cardiovascular disease (because of genetic and other factors), and there is concern that HIV infection and treatment with ART may increase that risk [14]. Our observations that ∼25% of patients who receive an NNRTI-based regimen have an abnormal lipid profile at 1 year should alert physicians to this outcome and encourage testing. Although the current World Health Organization guidelines do not recommend routine monitoring of lipid levels for patients receiving first-line antiretroviral treatment [15], patients would benefit from an assessment of lipid profiles and other cardiovascular risk factors followed by counseling on risk-reduction strategies. As patients continue to enjoy longer lives as a result of effective treatment, it is important to consider and minimize long-term adverse effects of the disease and its treatment.
Acknowledgments
We thank Dr V. Kumaraswami and Dr Paul Kumaran of the Tuberculosis Research Centre, India, for their valuable support; Dr Alice Tang and Mrs Sally Skinner of Tufts University School of Medicine, for assistance with data analysis and interpretation; the staff of the Departments of Clinical Research, Biochemistry, and Bacteriology of Tuberculosis Research Centre, for their contributions to the conduct of the study; Ms D. Kalaivani, for secretarial assistance; the faculty of the Master’s Program in Clinical Research at the Sackler School of Graduate Biomedical Education; and the participants in this study.
Financial support. National AIDS Control Organization (NACO), India, National Institutes of Health (NIH) (Fogarty grant 2D43TW000237-17 to C.P.), NIH/National Institute of Allergy and Infectious Diseases (CFAR grant 1P30AI42353-12 to C.P.), and Center for Research Resources (grant UL1 RR025752).
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Grover SA, Coupal L, Gilmore N, et al. Impact of dyslipidemia associated with highly active antiretroviral therapy on cardiovascular risk and life expectancy. Am J Cardiol. 2005;95:586–91. doi: 10.1016/j.amjcard.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Jones R, Sawleshwarkar S, Michailidis C, et al. Impact of antiretroviral choice on hypercholesterolemia events: the role of the nucleoside reverse transcriptase inhibitor backbone. HIV Med. 2005;6:396–402. doi: 10.1111/j.1468-1293.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 3.Anastos K, Lu D, Shi Q, et al. Association of serum lipid levels with HIV serostatus, specific antiretroviral agents and treatment regimens. J Acquir Immune Defic Syndr. 2007;45:34–42. doi: 10.1097/QAI.0b013e318042d5fe. [DOI] [PubMed] [Google Scholar]
- 4.van der Valk M, Kastelein JJ, Murphy RL, et al. Nevirapine-containing antiretroviral therapy in HIV-1 infected patients results in an anti-atherogenic lipid profile. AIDS. 2001;15:2407–14. doi: 10.1097/00002030-200112070-00008. [DOI] [PubMed] [Google Scholar]
- 5.Pujari SN, Dravid A, Naik E, et al. Lipodystrophy and dyslipidemia among patients taking first-line, World Health Organization recommended highly active antiretroviral therapy regimens in Western India. J Acquir Immune Defic Syndr. 2005;39:199–202. [PubMed] [Google Scholar]
- 6.National Cholesterol Education Program (NCEP) Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 7.El Sadr WM, Mullin CM, Carr A, et al. Effects of HIV disease on lipid, glucose and insulin levels: results form a large antiretroviral naive cohort. HIV Med. 2005;6:114–21. doi: 10.1111/j.1468-1293.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 8.van der Valk M, Kastelein J, Murphy R, et al. Nevirapine-containing antiretroviral therapy in HIV-1 infected patients results in anti atherogenic profile. AIDS. 2001;15:2407–14. doi: 10.1097/00002030-200112070-00008. [DOI] [PubMed] [Google Scholar]
- 9.van der Valk M, Reiss P. Lipid profiles associated with antiretroviral drug choices. Curr Opin Infect Dis. 2003;16:19–23. doi: 10.1097/00001432-200302000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Wanke C, Gerrior J, Hendricks k, McNamara J, Schaefer E. Alterations in lipid profiles in HIV infected patients treated with protease inhibitor therapy are not influenced by diet. Nutr Clin Pract. 2005;20:668–73. doi: 10.1177/0115426505020006668. [DOI] [PubMed] [Google Scholar]
- 11.Fontas E, van Leth F, Sabin A, et al. Lipid profiles in HIV-infected patients receiving combination antiretroviral therapy: are different antiretroviral drugs associated with different lipid profiles? J Infect Dis. 2004;189:1056–74. doi: 10.1086/381783. [DOI] [PubMed] [Google Scholar]
- 12.van Leth F, Phanuphak P, Stroes E, et al. Nevirapine and efavirenz elicit different changes in lipid profiles in antiretroviral therapy-naive patients infected with HIV -1. PLos Med. 2004;1:e19. doi: 10.1371/journal.pmed.0010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchacz K, Weidle PJ, Moore D, et al. Changes in lipid profile over 24 months among adults on first-line highly active antiretroviral therapy in the home-based AIDS care program in rural Uganda. J Acquir Immune Defic Syndr. 2008;47:304–11. doi: 10.1097/qai.0b013e31815e7453. [DOI] [PubMed] [Google Scholar]
- 14.Das M, Pal S, Gosh A. Rural urban differences of cardiovascular disease risk factors in adult Asian Indians. Am J Hum Biol. 2008;20:440–5. doi: 10.1002/ajhb.20757. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents in resource-limited settings: towards universal access. 2006 revision. http://www.who.int/hiv/pub/guidelines/adult/en/ [Google Scholar]