Viral decay rates during efavirenz-based therapy were compared between HIV-infected patients without (N=40) and with tuberculosis coinfection on concurrent antituberculous therapy (N=34). Phase 1 and II viral decay rates were similar in the two groups (P>0.05). Overall, concurrent antituberculous therapy did not reduce the efficacy of the HIV treatment.
Abstract
Viral decay rates during efavirenz-based therapy were compared between human immunodeficiency virus (HIV)–infected patients without tuberculosis (n = 40) and those with tuberculosis coinfection who were receiving concurrent antituberculous therapy (n = 34). Phase I and II viral decay rates were similar in the 2 groups (P > .05). Overall, concurrent antituberculous therapy did not reduce the efficacy of the HIV treatment.
Tuberculosis (TB) remains a major cause of mortality in human immunodeficiency virus (HIV)–infected persons [1]. Although highly active antiretroviral therapy (HAART) during antituberculous therapy is associated with a substantial reduction in mortality [2–5], it is often deferred because of concerns about pill burden, drug-drug interactions, immune reconstitution inflammatory syndrome (IRIS), and drug toxicities [6]. Tuberculosis enhances HIV replication, and co-infected patients experience significant increases in HIV plasma viral loads when effective antituberculous therapy alone is used [7, 8]. The initiation of HAART in HIV-infected patients is associated with a rapid decrease in HIV RNA within the first week of therapy (phase I decay), followed by a slower rate of decline (phase II decay) [9–14]. Because viral decay rates are used as a measure of antiretroviral regimen efficacy [10, 11, 14] and long-term effectiveness [12, 14], it is important to determine whether therapy for TB coinfection reduces viral decay rates. In this pilot study, we compared viral decay rates during efavirenz-based HAART between HIV-infected Ghanaian patients without TB coinfection and those with TB coinfection who were receiving antituberculous therapy. In secondary analysis, we investigated whether virus decay rates are predictive of virologic outcome at weeks 24 and 48.
METHODS
Study Patients
HIV-infected antiretroviral-naive patients with CD4+ lymphocyte count ≤ 250 cells/μL without TB and with TB coinfection were enrolled at the Korle-Bu Teaching Hospital (Accra, Ghana) from November 2006 through December 2007. The 2 groups were matched for baseline CD4+ lymphocyte count <100 cells/μL and ≥100 cells/μL. The study was approved by the Nogouchi Memorial Institute for Medical Research, Ghana. Informed written consent was obtained from all patients.
Treatment Regimens
All patients received generic didanosine-buffered tablets at a dosage of 400 mg (for those with body weight >60 kg) or 300 mg (for those with body weight <60 kg), 300 mg of lamivudine, and 600 mg of efavirenz once daily. In the co-infected patients, antituberculous therapy was started immediately upon TB diagnosis, and HAART was initiated between 4 and 90 days of the initiation of antituberculous therapy (median time to HAART initiation, 33 days). Antituberculous therapy consisted of isoniazid, rifampin, pyrazinamide, and ethambutol daily for 2 months followed by isoniazid and ethambutol daily for 6 months or isoniazid and rifampin daily for 4 months. Adherence to HAART, assessed monthly by pill count and patient self-report, was found to be good in all patients through week 24 of HAART.
Clinical and Laboratory Monitoring
Clinical evaluations were performed at study entry and at all follow-up visits. Plasma viral load were obtained on days 0, and 3 and at weeks 1, 2, 4, 12, 24, and 48 of HAART. CD4+ lymphocyte counts were performed at entry and at weeks 4, 12, 24, and 48. Mid-dose efavirenz concentrations were determined at weeks 4 and 8 of HAART. CD4+ lymphocyte count was measured by FAScount (Becton-Dickinson), and HIV-1 RNA quantification determined by polymerase chain reaction amplification (Roche Amplicor). Virologic failure was defined as failure to suppress HIV RNA level to <400 copies/mL by week 24 of HAART or a viral rebound to >400 copies/mL at week 48 after achieving suppression at week 24. Virologic rebound was confirmed with subsequent testing, but no HIV genotype testing was available. Efavirenz plasma concentrations were measured using a validated high-performance liquid chromatography method.[15]
Statistical Models and Analysis
A biexponential nonlinear mixed-effects (NLME) model of HIV viral dynamics was used to estimate viral decay rates [16]. The biologically meaningful parameters include P1 and P2, representing the amount of virus produced and cleared from productively infected cells and long-lived infected cells, respectively, and d1 and d2, representing the decay rates of 2 phases of plasma HIV RNA clearance.
The estimated mean viral decay rates were compared between the 2 groups using the nonparametric O'Brian rank sum test for simultaneous test of viral decay rates in both phases or Wilcoxon rank-sum test for individual test of viral decay rates in a single phase [17]. Wilcoxon signed-rank test was used to examine the difference in viral decay rates between the group with virologic failure and the group without failure at weeks 24 and 48. Survival analysis was performed to evaluate the effect of TB coinfection and viral decay rates on time-to-virological failure. Significance was determined at the alpha = .05 level.
RESULTS
Study Population
Of the 74 patients, 34 (46%) had TB coinfection. Of the co-infected patients, 26 (76.5%) had pulmonary TB, of whom 8 (30.8%) had sputum smear results that were positive for acid-fast bacilli. Eight patients (23.5%) had extrapulmonary TB (3 had disseminated TB, 3 had tuberculous meningitis, and 1 each had pericardial and abdominal TB). Patients with HIV and TB coinfection were more likely than the patients with HIV monoinfection to be male (73.5% vs 27.5%; P < .001) and to have a lower body mass index (defined as the weight in kilograms divided by the square of height in meters; median body mass index, 17.3 vs 19.7; P = .044). The co-infected and HIV-infected patients had comparable baseline CD4+ lymphocyte counts (median, 76 vs 88 cells/μL; P = .733) and plasma viral loads (median, 320,000 vs 199,000 copies/mL; P = .222). Median efavirenz mid-dose concentration was similar between the 2 groups.
Viral Decay Rates and Relationship with Treatment Outcome
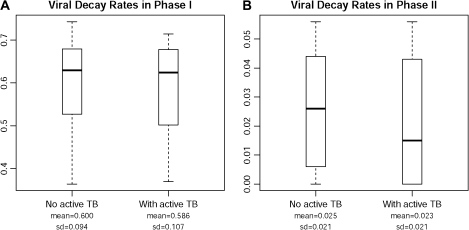
The distribution of viral decay rates by TB coinfection status is shown in Figure 1. The mean (± standard deviation) phase I viral decay rate was .586 (.107) per day in the co-infected patients and .600 (.094) per day in the patients without active TB (P = .726). The mean phase II decay rates were .023 (.021) and .025 (.021) per day in patients with and those without active TB (P = .415), respectively. Log-rank test revealed that TB coinfection and concurrent antituberculous therapy had no significant effect on time-to-virological failure (P = .125), but phase I decay rate (P = .04) and phase II decay rate (P = .01) were significantly related to time-to-virological failure. These results were confirmed by a Cox proportional hazard model. The estimated hazard ratio (95% confidence interval) for virological failure in patients with TB coinfection, compared with that for those without TB, was 2.043 (0.499–8.371; P = .321). The estimated risk of virological failure decreases by >99% if a patient's phase I or II viral decay rate increases by 1 unit (P < .05).
Figure 1.
Phase I (A) and phase II (B) viral decay rates in human immunodeficiency virus–infected patients with and without active tuberculosis (TB). Shown are median values and range (box, 25th–75th percentiles). SD, standard deviation.
Extended Follow-up Outcome
Of the 40 HIV-infected patients, 6 (15%) discontinued the study (4 with TB IRIS and 1 each with pregnancy and poor adherence), 3 (8%) died, and 3 (8%) were lost to follow-up before week 48. The characteristics of the 4 HIV-infected patients who developed TB IRIS during HAART are shown in Table 1. Of the 34 patients with HIV and TB coinfection, 4 (12%) discontinued the study (2 with pregnancy and 1 each with poor adherence and withdrawal of consent), 4 (12%) died, and 5 (15%) were lost to follow-up. There were no treatment discontinuations caused by drug adverse effects in either group.
Table 1.
Demographic and Clinical Characteristics of HIV-Infected Patients Who Developed Tuberculosis IRIS
| ID | Age, years | Sex | Weight, kg | CD4+ lymphocyte count, cells/μL | Baseline HIV RNA level, copies/mL | Phase I decay rate, per day | Phase II decay rate, per day | Time to IRIS diagnosis, days | Site of TB |
| AC19 | 31 | F | 36 | 13 | 147,000 | 0.640 | 0.0 | 17 | Pulmonarya,b |
| AC149 | 33 | F | 54 | 14 | 456,000 | 0.549 | 0.004 | 19 | Pulmonarya |
| AC160 | 37 | F | 34 | 42 | 389,000 | 0.452 | 0.009 | 77 | Abdominal |
| AC165 | 35 | M | 51 | 3 | 39,000 | 0.710 | 0.0 | 30 | Meningitis |
NOTE. HIV, human immunodeficiency virus; IRIS, immune reconstitution inflammatory syndrome; TB, tuberculosis;
Sputum smear positive for acid-fast bacilli.
Died at week 4 of antiretroviral therapy.
Of the patients who continued to receive HAART, 31 (94%) of 33 and 27 (96%) of 28 patients without TB achieved viral loads <400 copies/mL at weeks 24 and 48 of HAART, respectively. Of the co-infected patients, 21 (91%) of 23 and 16 (80%) of 20 also achieved viral loads <400 copies/mL at weeks 24 and 48 of therapy. The median (interquartile range [IQR]) increase in CD4+ lymphocyte count at weeks 24 and 48 in patients without and those with TB were 112 cells/μL (IQR, 43–189 cells/μL) versus 172 cells/μL (IQR, 120–243 cells/μL) and 206 cells/μL (IQR, 52–260 cells/μL) versus 234 cells/μL (IQR, 167–345 cells/μL).
DISCUSSION
In this study, initiation of efavirenz-based HAART within ∼1 month of starting antituberculous therapy in patients with HIV and TB coinfection did not appear to reduce the efficacy of the HIV treatment. The comparable phase I and II decay rates in the patients without and those with TB coinfection who were receiving antituberculous therapy indicate that the efficacy of the antiretroviral regimen was similar, because several studies have shown that viral decay rates reflect antiretroviral regimen potency and/or efficacy [11, 12,17–19].
Overall, clinical, immunological, and virological outcomes through week 48 of follow-up were similar in the 2 cohorts. This finding concurs with previously published studies from resource-rich settings that found that TB coinfection and antituberculous therapy did not compromise HIV treatment responses through 6 months of follow-up [20, 21]. Consistent with findings of other studies involving African populations [22, 23], we found efavirenz plasma mid-dose concentrations to be similar irrespective of concurrent antituberculous therapy. This may be due to the relative high proportion of patients who were considered to be “slow metabolizers” of efavirenz in our cohort [24].
All of the patients who developed TB IRIS were severely immunocompromised and did not have TB symptoms at HAART initiation. However, the majority manifested with TB disease within 30 days of HAART, which is consistent with reports of unmasked TB presenting as IRIS soon after initiation of HAART in areas of TB endemicity [25, 26]. Severely immunosuppressed HIV-infected patients without TB symptoms at HAART initiation should be monitored closely for the possibility of unmasked disease in these areas.
Despite the small size of our study population and our inability to adjust for multiple comparisons in the hypothesis testing, the findings of this pilot study suggest that TB coinfection and concurrent antituberculous therapy did not compromise the efficacy of an efavirenz-based regimen in co-infected patients, compared with HIV-infected patients matched for CD4+ lymphocyte count level.
Acknowledgments
We thank the study participants; the study coordinators, Adjoa Obo-Akwa and Esther Manche; and the study nurse, Janet May Ayi, of Korle Bu Teaching Hospital. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding organizations.
Financial support. ACRiA grant from the Doris Duke Foundation (to M.L.), National Institutes of Health (NIH) K23 developmental award (NIH K23 AI071760 to A.K.), CDAAR (P30DA013868 to T.P.F.), Developmental Center for AIDS Research (D-CFAR), University of Rochester Medical Center (NIH/National Institute of Allergy and Infectious Diseases P30AI078498 to H.W. and H.Y.), The University of North Carolina at Chapel Hill, Center for AIDS research (#9P30 AI50410), and the Clinical Pharmacology and Analytical Chemistry Laboratory (CPACL), which performed the efavirenz concentrations.
Potential conflicts of interest. A.K. has previously received grant support and payment for lecture/speaker's bureau from BMS. T.F. holds stock/stock options in BMS and Gilead. All other authors: no conflicts.
References
- 1.World Health Organization. Global tuberculosis control: a short update to the 2009 report. WHO/HTM/TB/2009426 2010. http://whqlibdoc.who.int/publications/2009/9789241598866_eng.pdf. Accessed 26 August 2010. [Google Scholar]
- 2.Abdool Karim SS, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dheda K, Lampe FC, Johnson MA, Lipman MC. Outcome of HIV-associated tuberculosis in the era of highly active antiretroviral therapy. J Infect Dis. 2004;190:1670–6. doi: 10.1086/424676. [DOI] [PubMed] [Google Scholar]
- 4.Manosuthi W, Chottanapand S, Thongyen S, Chaovavanich A, Sungkanuparph S. Survival rate and risk factors of mortality among HIV/tuberculosis-coinfected patients with and without antiretroviral therapy. J Acquir Immune Defic Syndr. 2006;43:42–6. doi: 10.1097/01.qai.0000230521.86964.86. [DOI] [PubMed] [Google Scholar]
- 5.Velasco M, Castilla V, Sanz J, et al. Effect of simultaneous use of highly active antiretroviral therapy on survival of HIV patients with tuberculosis. J Acquir Immune Defic Syndr. 2009;50:148–52. doi: 10.1097/QAI.0b013e31819367e7. [DOI] [PubMed] [Google Scholar]
- 6.Burman WJ. Issues in the management of HIV-related tuberculosis. Clin Chest Med. 2005;26:283–94. doi: 10.1016/j.ccm.2005.02.002. vi-vii. [DOI] [PubMed] [Google Scholar]
- 7.Kalou M, Sassan-Morokro M, Abouya L, et al. Changes in HIV RNA viral load, CD4+ T-cell counts, and levels of immune activation markers associated with anti-tuberculosis therapy and cotrimoxazole prophylaxis among HIV-infected tuberculosis patients in Abidjan, Cote d'Ivoire. J Med Virol. 2005;75:202–8. doi: 10.1002/jmv.20257. [DOI] [PubMed] [Google Scholar]
- 8.Morris L, Martin DJ, Bredell H, et al. Human immunodeficiency virus-1 RNA levels and CD4 lymphocyte counts, during treatment for active tuberculosis, in South African patients. J Infect Dis. 2003;187:1967–71. doi: 10.1086/375346. [DOI] [PubMed] [Google Scholar]
- 9.Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–6. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 10.Hoetelmans RM, Reijers MH, Weverling GJ, et al. The effect of plasma drug concentrations on HIV-1 clearance rate during quadruple drug therapy. AIDS. 1998;12:F111–5. doi: 10.1097/00002030-199811000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Kuritzkes DR, Ribaudo HJ, Squires KE, et al. Plasma HIV-1 RNA dynamics in antiretroviral-naive subjects receiving either triple-nucleoside or efavirenz-containing regimens: ACTG A5166 s. J Infect Dis. 2007;195:1169–76. doi: 10.1086/512619. [DOI] [PubMed] [Google Scholar]
- 12.Polis MA, Sidorov IA, Yoder C, et al. Correlation between reduction in plasma HIV-1 RNA concentration 1 week after start of antiretroviral treatment and longer-term efficacy. Lancet. 2001;358:1760–5. doi: 10.1016/s0140-6736(01)06802-7. [DOI] [PubMed] [Google Scholar]
- 13.Wu H, Kuritzkes DR, McClernon DR, et al. Characterization of viral dynamics in human immunodeficiency virus type 1-infected patients treated with combination antiretroviral therapy: relationships to host factors, cellular restoration, and virologic end points. J Infect Dis. 1999;179:799–807. doi: 10.1086/314670. [DOI] [PubMed] [Google Scholar]
- 14.Wu H, Lathey J, Ruan P, et al. Relationship of plasma HIV-1 RNA dynamics to baseline factors and virological responses to highly active antiretroviral therapy in adolescents (aged 12–22 years) infected through high-risk behavior. J Infect Dis. 2004;189:593–601. doi: 10.1086/381500. [DOI] [PubMed] [Google Scholar]
- 15.Rezk NL, Crutchley RD, Yeh RF, Kashuba AD. Full validation of an analytical method for the HIV-protease inhibitor atazanavir in combination with 8 other antiretroviral agents and its applicability to therapeutic drug monitoring. Ther Drug Monit. 2006;28:517–25. doi: 10.1097/00007691-200608000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Wu H, Ding AA. Population HIV-1 dynamics in vivo: applicable models and inferential tools for virological data from AIDS clinical trials. Biometrics. 1999;55:410–8. doi: 10.1111/j.0006-341x.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- 17.Ding AA, Wu H. Assessing antiviral potency of anti-HIV therapies in vivo by comparing viral decay rates in viral dynamic models. Biostatistics. 2001;2:13–29. doi: 10.1093/biostatistics/2.1.13. [DOI] [PubMed] [Google Scholar]
- 18.Wu H, Ding AA, De Gruttola V. Why are the decay rates in plasma HIV-1 different for different treatments and in different patient populations? AIDS. 1999;13:429–30. doi: 10.1097/00002030-199902250-00022. [DOI] [PubMed] [Google Scholar]
- 19.Louie M, Hogan C, Di Mascio M, et al. Determining the relative efficacy of highly active antiretroviral therapy. J Infect Dis. 2003;187:896–900. doi: 10.1086/368164. [DOI] [PubMed] [Google Scholar]
- 20.Breen RA, Miller RF, Gorsuch T, et al. Virological response to highly active antiretroviral therapy is unaffected by antituberculosis therapy. J Infect Dis. 2006;193:1437–40. doi: 10.1086/503437. [DOI] [PubMed] [Google Scholar]
- 21.Hung CC, Chen MY, Hsiao CF, Hsieh SM, Sheng WH, Chang SC. Improved outcomes of HIV-1-infected adults with tuberculosis in the era of highly active antiretroviral therapy. AIDS. 2003;17:2615–22. doi: 10.1097/00002030-200312050-00008. [DOI] [PubMed] [Google Scholar]
- 22.Friedland G, Khoo S, Jack C, Lalloo U. Administration of efavirenz (600 mg/day) with rifampicin results in highly variable levels but excellent clinical outcomes in patients treated for tuberculosis and HIV. J Antimicrob Chemother. 2006;58:1299–302. doi: 10.1093/jac/dkl399. [DOI] [PubMed] [Google Scholar]
- 23.Ren Y, Nuttall JJ, Eley BS, et al. Effect of rifampicin on efavirenz pharmacokinetics in HIV-infected children with tuberculosis. J Acquir Immune Defic Syndr. 2009;50:439–43. doi: 10.1097/QAI.0b013e31819c33a3. [DOI] [PubMed] [Google Scholar]
- 24.Kwara A, Lartey M, Sagoe KW, Rzek NL, Court MH. CYP2B6 (c.516G–>T) and CYP2A6 (*9B and/or *17) polymorphisms are independent predictors of efavirenz plasma concentrations in HIV-infected patients. Br J Clin Pharmacol. 2009;67:427–36. doi: 10.1111/j.1365-2125.2009.03368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20:1605–12. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- 26.Lawn SD, Myer L, Edwards D, Bekker LG, Wood R. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS. 2009;23:1717–25. doi: 10.1097/QAD.0b013e32832d3b6d. [DOI] [PMC free article] [PubMed] [Google Scholar]