Summary
Crosslinking of FcγRIIB and the BCR by immune complexes (ICs) can downregulate antigen-specific B cell responses. Accordingly, FcγRIIB deficiencies have been associated with B cell hyperactivity in patients with SLE and mouse models of lupus. However, we have previously shown that murine IgG2a-autoreactive AM14 B cells respond robustly to chromatin- associated ICs through a mechanism dependent on both the BCR and endosomal TLR9, despite FcγRIIB coexpression. To further evaluate the potential contribution of FcγRIIB to the regulation of autoreactive B cells, we have now compared the IC-triggered responses of FcγRIIB-deficient and FcγRIIB-sufficient AM14 B cells. We find that FcγRIIB-deficient cells respond significantly better than FcγRIIB-sufficient cells when stimulated with DNA ICs that incorporate low affinity TLR9 ligand (CG-poor dsDNA fragments). AM14 B cells also respond to RNA-associated ICs through BCR/TLR7 coengagement, but such BCR/TLR7 dependent responses are normally highly dependent on IFNα costimulation. However, we now show that AM14 FcγRIIB-/- B cells are very effectively activated by RNA ICs without supplemental IFNα priming. These results demonstrate that FcγRIIB can effectively modulate both BCR/TLR9 and BCR/TLR7 endosomal-dependent activation of autoreactive B cells.
Keywords: Autorective B cells, endogenous TLR ligands, inhibitory Fc receptor
Introduction
Fc gamma receptors (FcγRs) play a major role in the regulation of antibody-dependent effector mechanisms. Most FcγR+ cells express both activating and inhibitory receptors, and the magnitude and nature of the immune response depends upon the balance of signals transmitted by each cell-specific combination of signals. By contrast, B cells only express the inhibitory receptor FcγRIIB, and here it is thought to downregulate responses to antigens already bound by antibody [1].
In accordance with its suppressive function, mice with a deletion in the FcγRIIB gene develop enhanced humoral responses to both foreign [2] and self-antigens [3]. The level of FcγRIIB expression has been further correlated with systemic autoimmune disease in both animal models and patient populations. SLE-prone mice such as NZB, BXSB and MRL/lpr inherently express lower than normal levels of FcγRIIB in activated or germinal-center B cells due to polymorphisms in the FcγRIIB gene promoter [4]. Moreover, SLE patients fail to appropriately upregulate FcγRIIB expression on memory B cells and plasma cells [5-7]. Importantly, reconstitution of FcγRIIB-/- mice with FcγRIIB+ B cells confers protection from disease, as does increasing the level of FcγRIIB expression through retroviral transduction [8]. Together, these data suggest B cell expression of FcγRIIB is essential for the maintenance B cell peripheral tolerance.
Early studies demonstrated that ICs, composed of rabbit F(ab′)2 anti-IgM bound by mouse IgG, activated B cells significantly less well than F(ab′)2 anti-IgM alone [9]. However, chromatin/DNA-associated ICs, present in the sera of autoimmune mice, very effectively activate both IgG2a-reactive high affinity 20.8.3 and low affinity AM14 B cells [10, 11]. AM14 B cell activation required engagement of both the BCR and TLR9 [12]. TLR9 was originally described as a pattern recognition receptor specific for particular DNA sequences, designated CpG motifs, frequently found in bacterial but not mammalian DNA [13]. Nevertheless, the role of TLR9 in the detection of DNA-associated ICs, as described above, clearly demonstrated that TLR9 also detects mammalian DNA.
To better understand the nature of the endogenous TLR9 ligand, we have constructed dsDNA fragment ICs that incorporate biotinylated DNA fragments bound by an IgG2a anti-biotin mAb. Stimulation of AM14 B cells with ICs containing dsDNA fragments corresponding to the CG-rich sequences derived from endogenous CpG islands strongly activate AM14 B cell proliferation, while ICs containing dsDNA fragments representative of the overall mammalian genome do not [14]. The availability of DNA fragments that can engage TLR9 to varying degrees provides a useful tool for examining the regulation of autoreactive B cell activation. Like TLR9, TLR7 is also located in endosomal compartments, however this receptor recognizes single-stranded RNA [15-17]. In an analogous manner to the BCR/TLR9 paradigm, RNA ICs promote AM14 B cell responses through a mechanism that involves both the BCR and TLR7 [18]. However, AM14 B cell responses to RNA IC are generally more dependent on coactivation with type I IFNs.
We had previously shown that FcγRIIB deficiency did not affect the capacity of high affinity IgG2a-specific B cells to respond to chromatin ICs [11]. At the time, we surmised that the cell surface expression of FcγRIIB precluded its capacity to regulate signaling cascades emanating from TLR7 and TLR9, which were predominantly found in endosomal compartments. The capacity of FcγRIIB has now been re-examined in the context of low affinity IgG2a-reactive AM14 B cells activated by chromatin/DNA and RNA ICs. We find that FcγRIIB can regulate AM14 IC responses to DNA ICs only when the complexes contain CpG-poor DNA. FcγRIIB further modulates AM14 B cell responses to RNA ICs, both in the absence and presence of IFNα.
Results
FcγRIIB specifically downregulates the AM14 B cell response to intact goat anti-mouse IgM
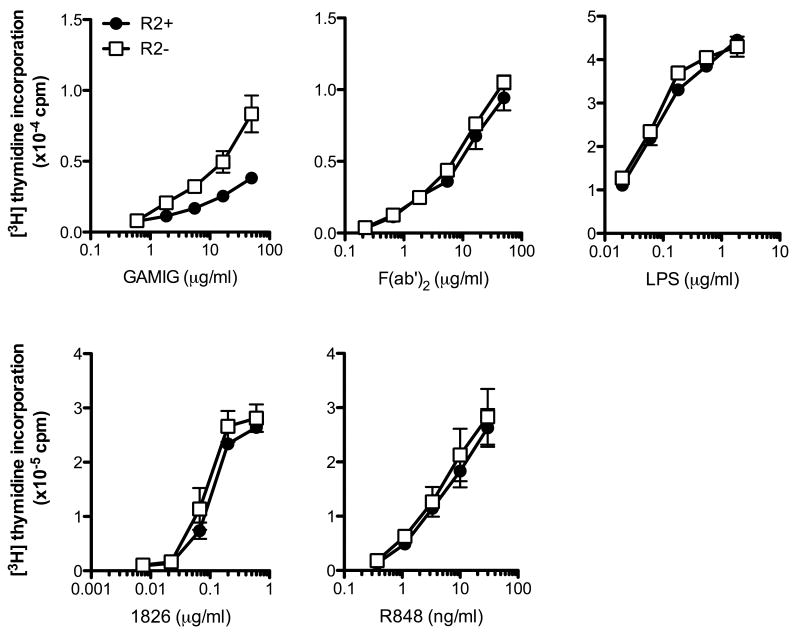
To determine whether BCR-mediated responses of AM14 B cells are appropriately modulated by FcγRIIB, FcγRIIB+ (R2+) and FcγRIIB-/- (R2-) AM14 B cells were stimulated with intact and F(ab′)2 fragments of goat anti-mouse IgM (GAMIG). As predicted from previous studies with non-Tg B cells [19], R2+AM14 B cells displayed an attenuated response to GAMIG when compared to R2- AM14 B cells although they responded comparably to increasing concentrations of F(ab′)2 fragments of GAMIG (Fig 1). Expression of FcγRIIB did not affect the responses to standard TLR ligands; R2+ and R2- AM14 and non-transgenic B cells responded comparably to ligands known to engage both the cell surface (LPS) and endosomal (CpG 1826 and R848) TLRs (Fig 1 and results not shown). Even though FcγRIIB-/- mice on the C57Bl/6 deficient background can develop spontaneous autoimmune disease [3], all the mice used for these studies were between 6-8 weeks of age and these data demonstrate that they maintained normal responses to BCR, TLR9 and TLR7 engagement.
Figure 1. R2- B cells display an enhanced response to GAMIG but not to F(ab′)2 anti-IgM or TLR ligands.
B cells from AM14 R2+ and R2- mice (top panels) and from non-transgenic R2+ and R2- mice (bottom panels) (R2+ (●); R2- (□)) were stimulated with increasing concentrations of F(ab′)2 fragment of goat anti-mouse IgM (F(ab′)2), intact goat anti-mouse IgM (GAMIG), LPS, 1826 and R848, and proliferation determined by [3H] thymidine incorporation. Shown are mean±SD of a representative experiment of four (top panels) and mean±SEM of five independent experiments (bottom panels).
FcγRIIB-deficient AM14 B cells can respond to chromatin ICs but not protein ICs
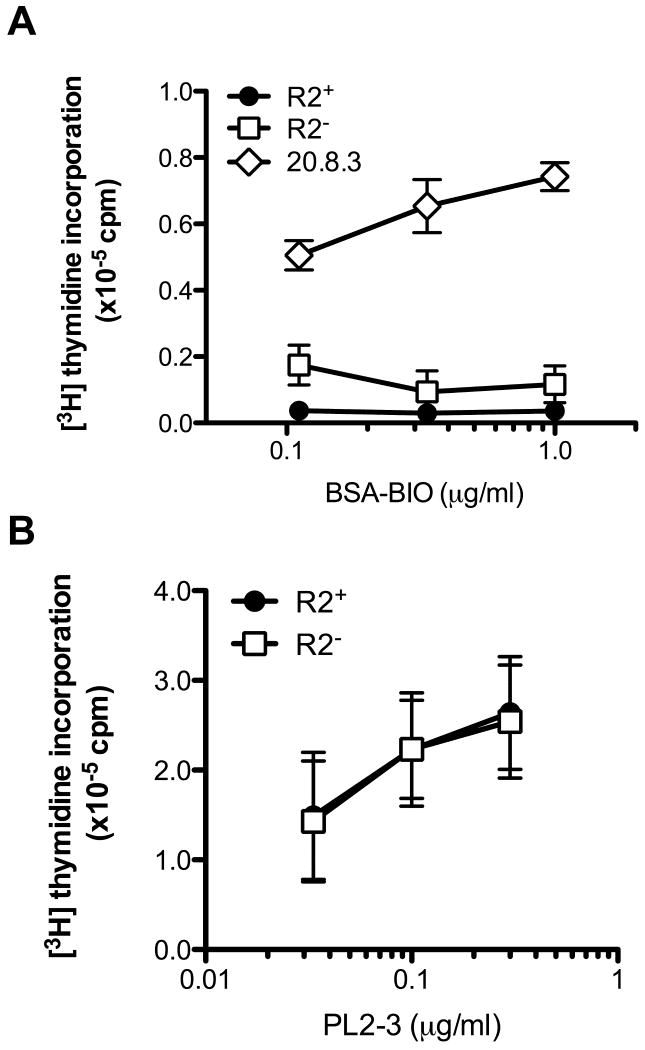
AM14 B cells express a receptor specificity commonly produced by spontaneously activated autoreactive B cells [20] that reacts weakly with IgG2a [21]. 20.8.3 BCR Tg B cells express a higher affinity receptor for IgG2a, initially elicited by an allotype-disparate immunization [22]. In contrast to 20.8.3 B cells, AM14 B cells do not proliferate when stimulated with ICs consisting of IgG2a bound to proteins [11]. Protein ICs do, however, induce upregulation of activation markers in AM14 B cells [23], although this signal is insufficient to stimulate cell cycle entry, possibly due to engagement of the inhibitory FcγRIIB. To determine whether the loss FcγRIIB would enable AM14 B cells to proliferate in response to protein ICs, R2+ and R2- AM14 B cells were stimulated with ICs consisting of biotinylated-BSA bound by the IgG2a anti-biotin mAb 1D4. Even in the absence of the inhibitory receptor, AM14 B cells failed to proliferate in response to these protein ICs. By comparison, 1D4/Bio-BSA ICs, but not 1D4 or Bio-BSA alone, did induce 20.8.3 B cell proliferation (Fig 2A and data not shown). These results demonstrate that the inability of AM14 B cells to proliferate in response to protein ICs is not simply due to engagement of FcγRIIB.
Figure 2. R2+ and R2- AM14 B cells fail to respond to protein ICs and respond comparably to chromatin ICs.
(A) R2+ AM14 (●), R2- AM14 (□) and 20.8.3 (◊) splenic B cells were stimulated with protein ICs formed by combining increasing concentrations of biotinylated BSA (BSA-Bio) and 1.5 μg/ml anti-biotin IgG2a. (B) R2+ (●) and R2- (□) AM14 B cells were stimulated with increasing concentrations of PL2-3. In all panels, proliferation was determined at 24 h by [3H] thymidine incorporation. Shown are mean±SEM of four independent experiments.
R2- and R2+ AM14 B cells respond comparably to PL2-3
The chromatin-reactive mAb PL2-3 binds uncharacterized DNAse-sensitive components of cell debris and strongly activates AM14 B cells through a mechanism dependent on both the BCR and TLR9. To evaluate the role of FcγRIIB in the regulation of AM14 B cell responses to these chromatin ICs, R2+ and R2- AM14 B cells were stimulated with increasing concentrations of PL2-3. However, in multiple experiments we found that the dose response curves for these two populations were essentially identical (Fig. 2B). These results were similar to those obtained previously with the PL2-3 activated 20.8.3 cells and appeared to further support the notion that FcγRIIB did not regulate optimal responses emanating from an endosomal TLR when ligated in conjunction with BCR engagement.
ICs containing suboptimal TLR9 ligands can activate R2- AM14 B cells
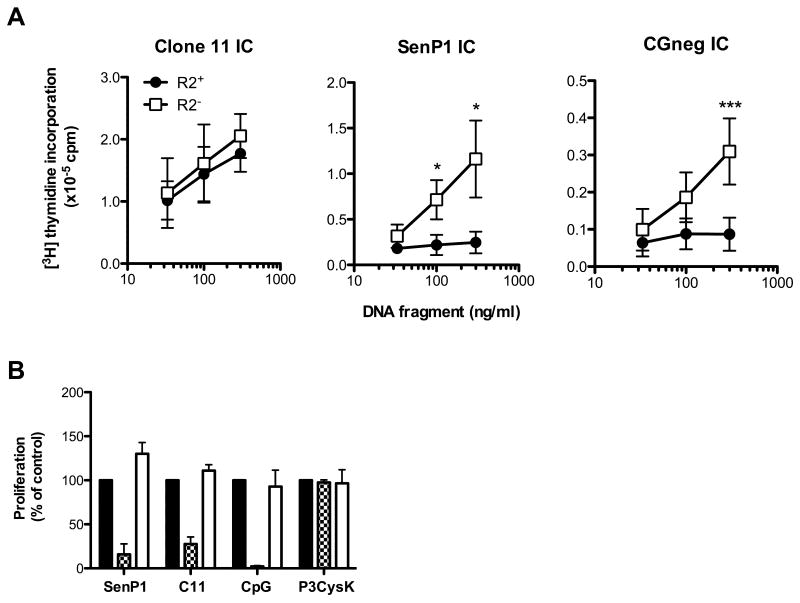
Since FcγRIIB has been reported to bind IgG2a antibodies with relatively low affinity [24], we reasoned that our ICs elicited a relatively weak FcγRIIB inhibitory signal. Therefore it was possible that PL2-3 ICs elicited a strong TLR9 signal not easily regulated by FcγRIIB. Even though TLR9-expressing AM14 cells respond more robustly to DNA fragments enriched for CG dinucleotides than to CG-poor DNA fragments [14, 25], CG-poor DNA fragments can still be bound by TLR9 [26]. To extend our analysis to weak TLR9 ligands, normally incapable of promoting AM14 cell cycle entry, we decided to use ICs that contained defined dsDNA fragments derived from CG-poor portions of the genome. CG-poor dsDNA is the prevalent class of DNA found in the mammalian genome, and representative sequences such as SenP1, a 557 fragment containing only 4 CG dinucleotides routinely induce minimal activation of AM14 B cells [14]. CGneg, a sequence completely devoid of CG dinucleotides, was constructed to examine TLR9 specificity, and also fails to promote AM14 B cell proliferation [11]. By contrast, Clone 11 is a 573 bp long dsDNA fragment corresponding to a CG-rich unmethylated sequence found in the promoter region of the murine pre-ribosomal RNA gene complex. Such CG-rich regions, denoted CpG islands, comprise about 2% of the mammalian genome [27]. IgG2a ICs incorporating Clone 11 are potent activators of AM14 B cells [14].
To determine whether ICs containing CG-poor dsDNA fragments could activate R2- AM14 B cells, we used ICs consisting of 1D4 bound to Bio-SenP1 or Bio-CGneg. As a control for CG-rich DNA, we used 1D4 bound to Bio-Clone 11 ICs. Similar to the results obtained with PL2-3, Clone 11 IC-activated R2+ and R2- AM14 B cells had almost identical dose-response curves. However, the R2- AM14 B cells proliferated significantly better than the R2+ AM14 B cells when stimulated with SenP1 or CGneg ICs (Fig. 3A). These results indicate that FcγRIIB does indeed regulate B cell responses to endogenous TLR9 ligands, however its regulatory capacity is only revealed with weak TLR9 ligands.
Figure 3. R2- AM14 B cells respond to dsDNA fragment ICs containing non-optimal TLR9 ligands through a TLR9-dependent mechanism.
(A) Clone 11 ICs, SenP1 ICs and CGneg ICs were preformed by combining 1D4 (5 μg/ml) with increasing concentrations of Bio-dsDNA fragments and then added to R2+ or R2- AM14 B cells. Results shown are the mean±SEM of three (SenP1 and Clone 11 IC) or five (CGneg IC) independent experiments. * = p<0.05, and *** = p<0.001 indicate significant differences between R2+ and R2- B cells as calculated using the unpaired Student t test. (B) R2- AM14 B cells were stimulated with SenP1 ICs, Clone 11 ICs (C11), ODN 1826 (CpG), or Pam3CysK4 (P3CysK) in the absence (black bars) or presence of TLR9 inhibitor INH-18 (checkered bars) or control INH-48 (open bars). Results shown are the mean±SEM of three independent experiments. In all panels, proliferation was measured at 24 h by [3H]-thymidine incorporation.
To verify that the enhanced R2- AM14 B cell response to SenP1 ICs was still TLR9-dependent, we tested the effect of the TLR9 inhibitor, oligodeoxynucleotide (ODN) INH-18, and the control (non-inhibitory) ODN, INH-48 [28]. The R2- AM14 B cell responses to SenP1 ICs were blocked by INH-18 but not by INH-48 (Fig. 3B). These results demonstrate that in the absence of FcγRIIB-mediated inhibition, AM14 B cells respond to otherwise non-stimulatory DNA through a TLR9-dependent mechanism.
FcγRIIB also regulates autoreactive B cell responses to RNA ICs
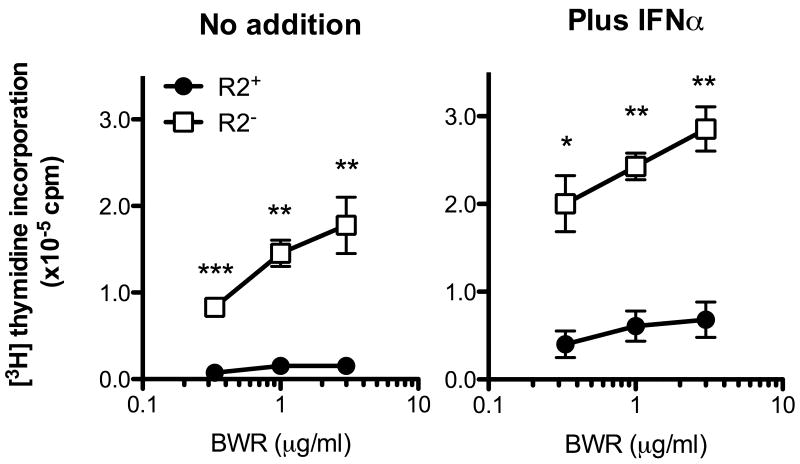
AM14 B cells respond to RNA-containing ICs through coengagement of the BCR and TLR7. TLR7-dependent AM14 B cell responses to RNA ICs are modest when compared to TLR9-dependent responses to CG-rich DNA ICs, but can be significantly enhanced by addition of IFNα [18]. To determine whether the absence of FcγRIIB promoted AM14 B cell responses to RNA ICs, we stimulated R2+ and R2- AM14 cells with increasing concentrations of the RNA-specific IgG2a mAb BWR4 [29]. In the absence of IFNα, R2- AM14 cells proliferated significantly better than R2+ AM14 cells, which were only weakly activated by BWR4 (Fig 4). This response was further enhanced by the addition of IFNα, as both the R2+ and the R2- AM14 B cells proliferated even more robustly. These results show that FcγRIIB normally downregulates the response to RNA-associated ICs both in the absence and presence of IFNα, and in its absence, B cells can now respond to these common autoantigens.
Figure 4. FcγRIIB downregulates AM14 B cell responses to RNA ICs in the absence and presence of IFNα.
R2+ (●) and R2- (□) AM14 B cells were stimulated with increasing concentrations of IgG2a anti-RNA BWR4 in the absence (left panel) or the presence (right panel) of 300 U/ml of IFNα. Proliferation was measured by [3H] thymidine incorporation. Results shown are mean±SEM of three independent experiments. * p<0.05; ** p<0.01 and *** p<0.001 indicate significant differences between R2+ and R2- B cells as calculated using the unpaired Student t test.
Discussion
In the current study, we have used both spontaneous and defined ICs to examine the role of FcγRIIB in the activation of autoreactive B cells. PL2-3 (anti-histone) and BWR4 (anti-RNA) are both IgG2a mAbs isolated from autoimmune-prone mice, and when added to primary B cells in culture, they bind to undefined DNA-/RNA-associated components of cell debris to form ICs. These PL2-3 and BWR4 ICs activate AM14 B cells through mechanisms that are TLR9 and TLR7-dependent, respectively. However, our previous studies have shown that the AM14 response to BWR4 and other RNA-associated ICs is markedly enhanced by the addition of type I IFN [18]. These effects presumably reflect the capacity of type I IFN to dramatically increase the level of TLR7 expression in B cells [30] and lower the BCR signaling threshold [14]. We also found that type I IFN enhanced the response to defined CG-poor dsDNA ICs, even though it only appeared to induce a minimal increase in the level of TLR9 expression [14].
We now show that FcγRIIB-deficiency eliminates the need for exogenously supplied type I IFN in both the response to BWR4 and CG-poor dsDNA. Therefore, quite remarkably either the addition of type I IFN or the loss of FcγRIIB can convert non-stimulatory or weak stimulatory autoantigen to a potent activator of autoreactive B cells. It follows that the activation of B cells with low affinity receptors for self-antigen reflects the integration of signals of variable strength emanating from both activating (BCR, TLR7/TLR9, IFN receptor) and inhibitory (FcγRIIB) receptors. A certain final signal strength must be achieved in order for the B cells to cross a proliferation “threshold”, and this threshold can be attained by either increasing the affinity of the TLR-derived signal or recalibrating the BCR signaling cascade. A relatively weak (IgG2a) FcγRIIB ligand is sufficient to limit the response to weak TLR signals (CG-poor dsDNA fragment ICs or BWR4).
The mechanisms responsible for crosstalk between surface receptors (BCR, FcγRIIB, IFNAR) and endosomal receptors (TLR7, TLR9) remain to be fully elucidated. It has been well established that FcγRIIB blocks ITAM-dependent BCR signaling through recruitment of the phosphatase SHIP and dephosphorylation of key molecules involved in the BCR signaling cascade [31]. In addition, common molecules activated by both the BCR and TLR signaling pathways could be targets for FcγRIIB inhibition. One such example is phosphoinositide 3 kinase (PI3K), a molecule required for BCR signaling [32] which was recently reported to participate in TLR9 responses [33]. Interestingly, PI3K is also involved in IFNα-dependent activation pathways [34]. The further elucidation of the mechanisms responsible for crosstalk between surface receptors (BCR, FcγRIIB, IFNAR) and endosomal receptors (TLR7, TLR9) will aid in our understanding of how FcγRIIB deficiencies promote autoreactive B cell activation in the context of autoimmune disease.
Materials and methods
Mice
AM14 H/L chain transgenic mice [12] were intercrossed with FcγRIIB-/- mice (Jackson Laboratory) to obtain experimental mice. All FcγRIIB-/- mice used in these studies were 6-8 weeks of age. High affinity IgG2a-reactive 20.8.3 mice [22] were kindly provided by Dr. M. Shlomchik (Yale University School of Medicine). Mice were maintained at the BUSM Laboratory Animal Sciences Center under pathogen-free conditions. All procedures were performed under the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care, and approved by Boston University School of Medicine Institutional Animal Care and Use Committee.
Reagents
ODN 1826 (CpG class B) was obtained from Coley Pharmaceuticals (Wellesley, MA) and the inhibitory oligonucleotide INH-18 and its control INH- 48 [28] were obtained from Integrated DNA Technologies, Inc. (Coralville, Iowa). The TLR2 ligand Pam3CysK4 was obtained from EMC Microcollections (Tuebingen, Germany), TLR7 ligand R848 was from Invitrogen (Carslbad, CA), intact and F (ab′) 2 fragment of goat anti-mouse IgM were from Jackson Immunoresearch (West Grove, PA), and IFNα was from PBL (Piscataway, NJ). CGneg, Clone 11, and SenP1 dsDNA fragments were prepared and biotinylated as previously described [11, 14]. The histone-reactive mAb PL2-3 [35] was kindly provided by Dr. M. Monestier (Temple University School of Medicine). The RNA-reactive IgG2a BWR4 [29] was kindly provided by Dr. D. Eilat (Hadassah University Hospital, Jerusalem, Israel).
B cell proliferation studies
Primary B cells were purified from spleens using anti-CD45RB magnetic beads (BD biosciences, San Jose, CA) and stimulated with TLR ligands, and ICs as described previously [14, 18]. ICs containing biotinylated Clone 11, CGneg and SenP1, or biotinylated BSA, were combined with the IgG2a anti-biotin mAb 1D4 [14] in RPMI and incubated at room temperature for 15-30 min prior to addition to B cells.
Acknowledgments
This work was supported by National Institutes of Health Grants AR050256 and AR35230 to A.M.R.
List of abbreviations
- FcγRIIB
Fc gamma receptor II B
- IC
immune complex
- SLE
Systemic Lupus Erythematosus
- ODN
oligonucleotide
- SenP1
Sentrin-specific Peptidase 1
- GAMIG
goat anti-mouse IgM
Footnotes
Conflict of interests: The authors declare no financial or commercial conflict of interest.
References
- 1.Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. 1996;379:346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 3.Bolland S, Ravetch JV. Spontaneous autoimmune disease in Fc(gamma)RIIB-deficient mice results from strain-specific epistasis. Immunity. 2000;13:277–285. doi: 10.1016/s1074-7613(00)00027-3. [DOI] [PubMed] [Google Scholar]
- 4.Jiang Y, Hirose S, Abe M, Sanokawa-Akakura R, Ohtsuji M, Mi X, Li N, Xiu Y, Zhang D, Shirai J, Hamano Y, Fujii H, Shirai T. Polymorphisms in IgG Fc receptor IIB regulatory regions associated with autoimmune susceptibility. Immunogenetics. 2000;51:429–435. doi: 10.1007/s002510050641. [DOI] [PubMed] [Google Scholar]
- 5.Isaak A, Gergely P, Szekeres Z, Prechl J, Poor G, Erdei A, Gergely J. Physiological up-regulation of inhibitory receptors Fc gamma RII and CR1 on memory B cells is lacking in SLE patients. Int Immunol. 2008;20:185–192. doi: 10.1093/intimm/dxm132. [DOI] [PubMed] [Google Scholar]
- 6.Mackay M, Stanevsky A, Wang T, Aranow C, Li M, Koenig S, Ravetch JV, Diamond B. Selective dysregulation of the FcgammaIIB receptor on memory B cells in SLE. J Exp Med. 2006;203:2157–2164. doi: 10.1084/jem.20051503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su K, Yang H, Li X, Gibson AW, Cafardi JM, Zhou T, Edberg JC, Kimberly RP. Expression profile of FcgammaRIIb on leukocytes and its dysregulation in systemic lupus erythematosus. J Immunol. 2007;178:3272–3280. doi: 10.4049/jimmunol.178.5.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGaha TL, Sorrentino B, Ravetch JV. Restoration of tolerance in lupus by targeted inhibitory receptor expression. Science. 2005;307:590–593. doi: 10.1126/science.1105160. [DOI] [PubMed] [Google Scholar]
- 9.Phillips NE, Parker DC. Subclass specificity of Fc gamma receptor-mediated inhibition of mouse B cell activation. J Immunol. 1985;134:2835–2838. [PubMed] [Google Scholar]
- 10.Rifkin IR, Leadbetter EA, Beaudette BC, Kiani C, Monestier M, Shlomchik MJ, Marshak-Rothstein A. Immune complexes present in the sera of autoimmune mice activate rheumatoid factor B cells. J Immunol. 2000;165:1626–1633. doi: 10.4049/jimmunol.165.3.1626. [DOI] [PubMed] [Google Scholar]
- 11.Viglianti GA, Lau CM, Hanley TM, Miko BA, Shlomchik MJ, Marshak-Rothstein A. Activation of autoreactive B cells by CpG dsDNA. Immunity. 2003;19:837–847. doi: 10.1016/s1074-7613(03)00323-6. [DOI] [PubMed] [Google Scholar]
- 12.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 13.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 14.Uccellini MB, Busconi L, Green NM, Busto P, Christensen SR, Shlomchik MJ, Marshak-Rothstein A, Viglianti GA. Autoreactive B cells discriminate CpG-rich and CpG-poor DNA and this response is modulated by IFN-alpha. J Immunol. 2008;181:5875–5884. doi: 10.4049/jimmunol.181.9.5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 16.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 17.Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, Iwasaki A, Flavell RA. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci U S A. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ, Christensen SR, Shlomchik MJ, Viglianti GA, Rifkin IR, Marshak-Rothstein A. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diegel ML, Rankin BM, Bolen JB, Dubois PM, Kiener PA. Cross-linking of Fc gamma receptor to surface immunoglobulin on B cells provides an inhibitory signal that closes the plasma membrane calcium channel. J Biol Chem. 1994;269:11409–11416. [PubMed] [Google Scholar]
- 20.Wolfowicz CB, Sakorafas P, Rothstein TL, Marshak-Rothstein A. Oligoclonality of rheumatoid factors arising spontaneously in lpr/lpr mice. Clin Immunol Immunopathol. 1988;46:382–395. doi: 10.1016/0090-1229(88)90057-8. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson BA, Sharon J, Shan H, Shlomchik M, Weigert MG, Marshak-Rothstein A. An isotype switched and somatically mutated rheumatoid factor clone isolated from a MRL-lpr/lpr mouse exhibits limited intraclonal affinity maturation. J Immunol. 1994;152:4489–4499. [PubMed] [Google Scholar]
- 22.Wang H, Shlomchik MJ. High affinity rheumatoid factor transgenic B cells are eliminated in normal mice. J Immunol. 1997;159:1125–1134. [PubMed] [Google Scholar]
- 23.Avalos AM, Latz E, Mousseau B, Christensen SR, Shlomchik MJ, Lund F, Marshak-Rothstein A. Differential cytokine production and bystander activation of autoreactive B cells in response to CpG-A and CpG-B oligonucleotides. J Immunol. 2009;183:6262–6268. doi: 10.4049/jimmunol.0901941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nimmerjahn F, Bruhns P, Horiuchi K, Ravetch JV. FcgammaRIV: a novel FcR with distinct IgG subclass specificity. Immunity. 2005;23:41–51. doi: 10.1016/j.immuni.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Yasuda K, Richez C, Uccellini MB, Richards RJ, Bonegio RG, Akira S, Monestier M, Corley RB, Viglianti GA, Marshak-Rothstein A, Rifkin IR. Requirement for DNA CpG content in TLR9-dependent dendritic cell activation induced by DNA-containing immune complexes. J Immunol. 2009;183:3109–3117. doi: 10.4049/jimmunol.0900399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haas T, Metzger J, Schmitz F, Heit A, Muller T, Latz E, Wagner H. The DNA sugar backbone 2′ deoxyribose determines toll-like receptor 9 activation. Immunity. 2008;28:315–323. doi: 10.1016/j.immuni.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Cross SH, Bird AP. CpG islands and genes. Curr Opin Genet Dev. 1995;5:309–314. doi: 10.1016/0959-437x(95)80044-1. [DOI] [PubMed] [Google Scholar]
- 28.Lenert P, Yasuda K, Busconi L, Nelson P, Fleenor C, Ratnabalasuriar RS, Nagy PL, Ashman RF, Rifkin IR, Marshak-Rothstein A. DNA-like class R inhibitory oligonucleotides (INH-ODNs) preferentially block autoantigen-induced B-cell and dendritic cell activation in vitro and autoantibody production in lupus-prone MRL-Fas lpr/lpr mice in vivo. Arthritis Res Ther. 2009;11:R79. doi: 10.1186/ar2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eilat D, Fischel R. Recurrent utilization of genetic elements in V regions of antinucleic acid antibodies from autoimmune mice. J Immunol. 1991;147:361–368. [PubMed] [Google Scholar]
- 30.Green NM, Laws A, Kiefer K, Busconi L, Kim YM, Brinkmann MM, Trail EH, Yasuda K, Christensen SR, Shlomchik MJ, Vogel S, Connor JH, Ploegh H, Eilat D, Rifkin IR, van Seventer JM, Marshak-Rothstein A. Murine B cell response to TLR7 ligands depends on an IFN-beta feedback loop. J Immunol. 2009;183:1569–1576. doi: 10.4049/jimmunol.0803899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 32.Okkenhaug K, Bilancio A, Farjot G, Priddle H, Sancho S, Peskett E, Pearce W, Meek SE, Salpekar A, Waterfield MD, Smith AJ, Vanhaesebroeck B. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297:1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 33.Dil N, Marshall AJ. Role of phosphoinositide 3-kinase p110 delta in TLR4- and TLR9-mediated B cell cytokine production and differentiation. Mol Immunol. 2009;46:1970–1978. doi: 10.1016/j.molimm.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Uddin S, Yenush L, Sun XJ, Sweet ME, White MF, Platanias LC. Interferon-alpha engages the insulin receptor substrate-1 to associate with the phosphatidylinositol 3‖-kinase. J Biol Chem. 1995;270:15938–15941. doi: 10.1074/jbc.270.27.15938. [DOI] [PubMed] [Google Scholar]
- 35.Losman MJ, Fasy TM, Novick KE, Monestier M. Monoclonal autoantibodies to subnucleosomes from a MRL/Mp(-)+/+ mouse. Oligoclonality of the antibody response and recognition of a determinant composed of histones H2A, H2B, and DNA. J Immunol. 1992;148:1561–1569. [PubMed] [Google Scholar]