With the advent of prostate-specific antigen (PSA) screening in the mid- to late 1980s, the United States saw a rapid increase in incidence rates of prostate cancer (PCa). With only about half of the US male population over age 50 opting for regular PSA testing, the lifetime risk of PCa has approached 16% [1]. One could further extrapolate a man’s lifetime risk as approaching 25–30% with aggressive search for the disease using, for example, PSA cut-offs of 2.5 ng/ml or PSA velocity prompts, as currently recommended by some organizations, including the National Comprehensive Cancer Network [2]. This rate contrasts starkly with contemporary lifetime risk of PCa death estimates near 3% and illustrates the two fundamental problems with population wide screening for the disease: (1) The majority of cancers detected by screening will never cause symptoms or morbidity, and (2) the majority of cancer deaths from PCa will not be averted by screening. Although much attention has centered on differences in the outcomes of two large PCa screening studies, it should not be overlooked that both studies affirmed these two problems.
Some might suggest that over detection of PCa is not a problem because if the tumor detected is potentially insignificant, the patient can simply be observed. This contention is inaccurate for two reasons. First, almost all cancers are actively treated in the United States, with rates >90% in some series [3,4]. Second, this advice does not take into consideration the harsh reality of consequences of a cancer diagnosis, even if a strategy of surveillance is elected, including (1) the very significant risk that a “potentially significant” cancer was missed by biopsy; (2) a rate approaching 40% of likelihood of definitive treatment within a decade or so; (3) the lifelong anxiety associated with a cancer diagnosis; and, increasingly problematic, (4) the growing numbers of reported episodes of sepsis after prostate biopsy, perhaps related to the increasing rates of fluoroquinolone-resistant bacteria [5]. Although no national surveys of this complication have been reported to our knowledge, we are aware in discussions with multiple institutions that this complication is becoming serious and common. In rare instances, deaths have resulted from seemingly inconsequential outpatient prostate biopsies, but an episode of sepsis becomes a real and formidable problem for the patient on surveillance: Does he continue to have regular surveillance biopsies? Does he opt instead for treatment to avoid them? Does he simply continue to monitor his own PSA, knowing full well that it may not be a sufficiently sensitive measure for the development of consequential disease?
It is against these concerns that the authors present their data on PSA screening in men who have received dutasteride [6]. We have previously identified a similar phenomenon with the 5α-reductase inhibitor (5-ARI) finasteride, noting that the performance of PSA for screening (ie, sensitivity, specificity) is improved in men receiving finasteride [7]. In analysis of the Prostate Cancer Prevention Trial (PCPT), we found that the area under the receiver operating characteristic curve (AUC) of PSA for PCa detection was significantly improved from 0.681 in the placebo arm (n = 5112) to 0.757 in the finasteride arm (n = 4579). For high-grade disease (Gleason grade ≥7), the AUC increased from 0.781 to 0.838. For the practicing urologist, this finding means that if PSA is “elevated” in a man on finasteride, compared to a man not on finasteride, it is significantly more likely that PCa is the cause, and if PSA is not elevated, it is more likely that the patient does not have cancer. This phenomenon is probably best understood using a common practice as an example whereby a urologist typically refers men with PSA >4.0 ng/ml to biopsy. According to results from the PCPT, this urologist will detect only 24% of the cancer cases (sensitivity) and will miss 76% (false negatives) [8]. In contrast, this urologist will correctly diagnose 92.7% of the men without PCa (specificity) and will only subject the remaining 7% to an unnecessary biopsy (false positives). If we insist on a test with the same specificity for a man on finasteride and take into account that finasteride approximately halves PSA, then the results of the PCPT suggest that the PSA value that would maintain a specificity of 92.7% for the man on finasteride would be a level >1.6 ng/ml [7]; however, the test in this case would obtain a much greater sensitivity of 38%. In other words, the urologist would detect >50% more of the cancer cases. Importantly, the PCPT revealed this level of improvement not just for all PCa but also for high-grade PCa, suggesting that with fewer biopsies, it would be easier and more efficient to detect potentially lethal cancers in men who are receiving finasteride.
These data clearly illustrate that PSA screening is far more effective for a man who is receiving a 5-ARI such as finasteride. Since making this observation, we have wondered whether the prevention qualities of finasteride could be merged with its improvements in diagnosis of PCa with PSA. Imagine the patient who presents with a PSA of 2.9 ng/ml. With this somewhat elevated PSA value, he has a meaningful risk of PCa; if he is 65 yr of age and has no other risk factors, such as a positive family history, increased age, or African American race, his risk of cancer on biopsy is 29% and his risk of high-grade disease is 5.3%. Other risk factors would further increase this risk. With this information, the man might have two questions: Do I have cancer? If not, could I prevent its development since I will be at higher risk for the future? One approach to these logical questions might be to perform a prostate biopsy now and, if the biopsy is negative, consider the use of finasteride or dutasteride to reduce his subsequent cancer risk. Given that most men such as this will not have cancer and only a small fraction will have high-grade disease, an alternative approach could be the reverse order: Begin finasteride, repeat the PSA, and then consider performing a biopsy based on subsequent PSA values. This latter approach ought to do two things: prevent about a quarter of the cancers that would be expected to develop in this higher risk population and reduce the number of unnecessary biopsies.
The problem with simply accepting the data from the PCPT to make broad clinical recommendations is two-fold. First, the patient population is quite a bit different from that seen in a clinical urology practice. Second, the exposure time to finasteride was quite long, several years. Practically, most men who perceive their risk to be potentially elevated due to a PSA measure will not be willing to put off a prostate biopsy for this length of time. As a result of these issues, our decision was to design a clinical trial to answer the question whether PSA change with administration of finasteride improves detection of PCa. We have initiated a clinical trial, funded by the National Cancer Institute, that will soon begin enrolling men with a 20–60% risk of PCa, as determined by the PCPT Risk Calculator, and randomly assigning them to either finasteride or placebo. PSA will be measured monthly for a period of 3 mo, after which a prostate biopsy will be performed. We are planning on examining the performance of several additional biomarkers to determine how these markers may enhance the detection of both cancer and high-grade disease. Figure 1 displays the study design.
Fig. 1. Study design.
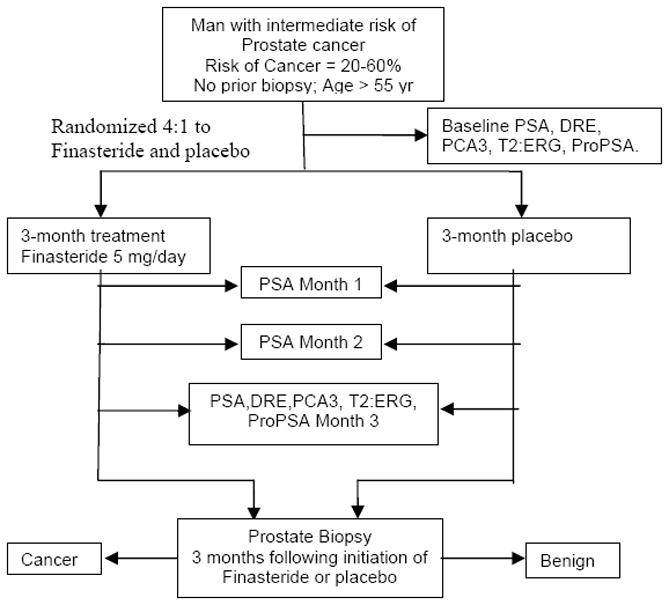
DRE = digital rectal examination; PCA3 = prostate cancer antigen 3; ProPSA = proenzyme prostate-specific antigen; PSA = prostate-specific antigen.
Mortality from PCa has fallen by almost 40% between 1990 and 2006 during the period of PSA testing [1]. Whether this fall is due to behavioral or pharmacologic preventive measures, improved treatment, or screening is not known. Although a small reduction in PCa mortality with screening was observed in the European screening trial but was not seen in the US trial, those of us who manage patients affected by the disease are aware of anecdotal cases in which we are convinced that early detection and treatment most likely averted the lethal consequences of this disease. Nonetheless, we cannot be so naïve to think that we can prevent all deaths or even the majority of deaths from this disease by simply screening more intensely. Our screening process must be based on the following tests:
For the man who does not have the disease: The screening test is negative.
For the man who has inconsequential disease: The screening test is negative.
For the man who has potentially lethal disease: The screening test is positive.
Now that we have evidence that 5-ARIs reduce a man’s risk of subsequent cancer diagnosis, we must develop tests that augment our screening tests:
For the man who will never develop PCa: The predictive test is negative.
For the man who will develop inconsequential disease: The predictive test is negative.
For the man who will develop potentially lethal disease: The predictive test is positive.
If these predictive tests are sufficiently sensitive, for the first two results, screening becomes unnecessary. After these predictive tests are developed, we may then revisit our prevention strategies in such a manner to merge prevention interventions with early detection interventions to ultimately develop a public health approach to PCa (1) that avoids PCa in as many men as possible as well as when it develops despite our preventive interventions and (2) that detects PCa sufficiently soon so as to allow treatment and cure. This approach is what our children and our children’s children deserve and expect of us.
Acknowledgments
Financial support
Financial assistance was provided by the Cancer Therapy and Research Center at the University of Texas Health Science Center San Antonio through the NCI Cancer Center Support Grant No. 2 P30 CA054174-17 and National Cancer Institute Grants U01 CA86402 and PO1 CA108964.
Footnotes
Conflicts of interest
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.NCCN Clinical Practice Guidelines in Oncology. Prostate cancer early detection. V.2.2010. National Comprehensive Cancer Network; Web site. http://www.nccn.org/professionals/physician_gls/PDF/prostate_detection.pdf. [DOI] [PubMed] [Google Scholar]
- 3.Shao YH, Albertsen PC, Roberts CB, et al. Risk profiles and treatment patterns among men diagnosed as having prostate cancer and prostate-specific antigen level below 4.0 ng/mL. Arch Int Med. 2010;170:1256–61. doi: 10.1001/archinternmed.2010.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncl. 2010;28:1117–23. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28:126–31. doi: 10.1200/JCO.2009.24.2180. [DOI] [PubMed] [Google Scholar]
- 6.van Leeuwen PJ, Kölble K, Huland H, Hambrock T, Barentsz J, Schröder FH. Prostate cancer detection and dutasteride: utility and limitations of prostate-specific antigen in men with previous negative biopsies. Eur Urol. doi: 10.1016/j.eururo.2010.09.035. In press. [DOI] [PubMed] [Google Scholar]
- 7.Thompson IM, Chi C, Ankerst DP, et al. Effect of finasteride on the sensitivity of PSA for detecting prostate cancer. J Natl Cancer Inst. 2006;98:1128–33. doi: 10.1093/jnci/djj307. [DOI] [PubMed] [Google Scholar]
- 8.Thompson IM, Ankerst DP, Chi C, et al. Operating characteristics of prostate-specific antigen in men with an initial PSA level of 3.0 ng/mL or lower. JAMA. 2005;294:66–70. doi: 10.1001/jama.294.1.66. [DOI] [PubMed] [Google Scholar]


