Abstract
Tumors with a hypoxic component, including human Y79 retinoblastoma cells, express a specific gap junction protein, Connexin 46 (Cx46), which is usually only found in naturally hypoxic tissues such as the differentiated lens. The aim of this study was to investigate if Cx46 down-regulation would suppress Y79 tumor formation in vivo. Five-week old nude mice were subcutaneously implanted with human Y79 retinoblastoma cells and treated with intratumor siRNA injections of 30 µg Cx46 siRNA (n=6), 30 µg non-silencing siRNA (n=6), or no siRNA treatment (n=6) every 2 days for a maximum of 10 treatments. Tumor volume (TV) was calculated from the recorded caliper measurements of length and width. Excised tumors were measured and weighed. Western blot analyses were performed to evaluate Cx46 and Cx43 expression in tumors which received Cx46 siRNA, non-silencing siRNA, or no siRNA treatment. Tumor histopathology was used to assess tumor features. Cx46 siRNA treated Y79 tumors had a reduced TV (287 mm3 ± 77 mm3) when compared to the tumors of mice receiving the negative control siRNA (894 mm3 ± 218 mm3; P ≤ 0.03) or no siRNA (1068 mm3 ± 192 mm3; P ≤ 0.002). A 6-fold knockdown of Cx46 and a 3-fold rise in Cx43 protein expression was observed from western blots of tumors treated with Cx46 siRNA compared to mice treated with non-silencing siRNA. Knockdown of Cx46 with siRNA had an antitumor effect on human Y79 retinoblastoma tumors in the nude mouse model. The results suggest that anti-Cx46 therapy may be a potential target in the future treatment of retinoblastoma.
Keywords: Gap Junction, GJA3, Connexin 46, Retinoblastoma, Hypoxia, Y79 Retinoblastoma Cells, Xenograft
1. Introduction
Ocular retinoblastoma is a rare, pediatric ocular tumor, caused by mutations in the retinoblastoma tumor suppressor gene (RB1), which may occur in both eyes of affected children. Current treatment methods with chemotherapy using carboplatin, vincristine, and etoposide, cryotherapy, laser photocoagulation, and external beam radiation therapy have greatly improved patient outcome against this neoplasia without enucleation (Antoneli, et al., 2006; Wong, et al., 1997). However, even in successful cases, significant side effects have been reported, including secondary malignant tumor development and deformities at the irradiation site, ischemic necrosis of the optic nerve, ototoxicity, and bone marrow suppression with potentially subsequent systemic septicemia (Jehanne, et al., 2009; Abramson, et al., 2004; Benz, et al., 2000). Currently, there is a need for an initial therapy and drug development for therapies, which are less toxic and can be used for extended periods to prevent or suppress tumor growth and protect the remaining unaffected eye to prolong vision in these young children.
The retina is usually highly oxygenated due to extensive vascularization, however, retinoblastoma, like many solid tumors, has a hypoxic stage where oxygen levels are approximately 1.5%, allowing tumor cells to have a growth advantage over normal tissue (Vaupel, et al., 2001). Growth at 1% oxygen inside the early tumor is supported by the up-regulation of the regulatory transcription factor complex, hypoxia-inducible factor (HIF). Many genes, often controlled by HIF, contribute to the maintenance of hypoxia and are involved in early tumor formation and progression (Semenza, 2007). One of these genes is the gap junction protein, connexin 46 (Cx46), which is naturally expressed in the hypoxic lens but can also be found in ischemic tissues such as wounds, infarcts, and traumatized or avascularized tissues (Harris and Locke, 2009). Our lab has recently identified Cx46 expression in solid tumors with a hypoxic component.
Gap junctions are membrane channels that allow intercellular communication through the passage of small molecules, ions, and metabolites. Connexins, the proteins that make up the gap junctions, are expressed in a tissue specific manner (Ruch, 1994) and normal connexin expression is often altered in neoplasia. Connexins began to be investigated as tumor suppressors when it was observed that Cx32 knockout mice were more likely to develop liver tumor formation in comparison to their wild type littermates (Temme, et al., 1997). It is suspected that many tumors have a decrease in their normal connexin expression and may also have an impaired ability to form gap junctions with connexin proteins (Qin, et al., 2002). The loss of gap junction communication and gap junction proteins has been associated with the development of neoplastic and malignant progression (Lee, et al., 1991; Laird, et al., 1999; Kanczuga-Koda, et al., 2003, 2005). Several connexins have been investigated for their growth inhibitory effects but Cx43 has the best documented effects as a tumor suppressor (Habermann, et al., 2002; McLachlan, et al., 2006; Hirschi, et al., 1996; Qin, et al., 2002; Shao, et al., 2005; Kanczuga-Koda, et al., 2003, 2005). Several tissues, such as prostate and breast tissues, naturally express Cx43 and it is required for normal development and function. However, in both prostate and breast cancer, expression of Cx43 is significantly down regulated (Habermann, et al., 2002; McLachlan, et al., 2006). In the normal prostate samples, nearly all specimens were positive for Cx43, while nearly two thirds of prostate cancer tissues were negative for Cx43 and Cx43 was present in only 10% of poorly differentiated prostate tumors (Habermann, et al., 2002). While overexpression of Cx43 restored growth control in MDA-MB-435 human breast tumor cells in vitro (Hirschi, et al., 1996), Cx43 upregulation suppressed tumor growth in MDA-MB-231 human breast tumors in vivo (Qin, et al., 2002). Lastly, Cx43 knockdown with siRNA prompted development of an aggressive tumor phenotype (Shao, et al., 2005). Studies of Cx43 with retinoblastoma protein (Rb1) also suggest that Cx43 suppresses tumor formation (Sanchez-Alvarez, et al., 2006).
One very important feature of tumors is their adaptation to hypoxia which then favors tumor growth and survival beyond that of normal tissue. We previously reported that the hypoxia-specific gap junction protein, Cx46, is upregulated in MCF-7 breast cancer cells and human breast tumors but is not found in normal breast tissue. Downregulation of Cx46 suppressed tumor growth in xenograft MCF-7 cell tumors (Banerjee, et al., 2010). We hypothesized that the presence of Cx46 promotes tumor growth in hypoxia. Our lab has also shown that overexpression of Cx46 is sufficient to protect a gap junction deficient cell line, neuronal 2A cells (N2A), from hypoxia-induced cell death while overexpression of Cx43 does not offer any protection compared to wild-type cells. Furthermore, downregulation of Cx46 in lens epithelial cell lines, which naturally thrive in hypoxia in vitro, rendered these cells sensitive to the effects of hypoxia (Banerjee, et al., 2010).
Retinoblastoma, as well as many other tumors, has an early hypoxic growth stage, allowing tumor cells to have a growth advantage over normal tissues. In human lens epithelial cells, a reciprocal relationship exists between the expression of the tumor suppressor, Cx43, and the hypoxia-specific, Cx46. The reciprocal relationship may also be present in tumors and, therefore, the tumor suppressor effects of Cx43 may be absent, promoting tumor growth (Burr DB, et al. ARVO Abstract 1575, 2010). The purpose of this study was to determine if Cx46 is expressed in the human Y79 retinoblastoma cell line. In this study, we investigate Cx46 as a novel gap junction protein which confers resistance and protects cells from hypoxic death. We found that human Y79 retinoblastoma cells and tumors highly express Cx46 and have minimal expression of Cx43. Our hypothesis is that Cx46 down-regulation, using short interfering RNAs (siRNA), will prevent or suppress Y79 retinoblastoma tumor development in vivo.
2. Methods
2.1 Cell Culture
Y79 cells, a human retinoblastoma cell line, were purchased from the American Type Culture Collection (ATCC), suspended and grown in RPMI-1640 medium (Invitrogen), supplemented with 20% premium fetal bovine serum (FBS) (Atlanta Biologicals), 50 U/ml of penicillin, 50 µg/ml streptomycin, and 50 µg/ml gentamycin (Gibco). Neuro2A cells (N2A) were grown in Low Glucose DMEM (Invitrogen) supplemented with 10% premium FBS (Atlanta Biologicals) with 50 U/ml of penicillin, 50 µg/ml streptomycin, and 50 µg/ml gentamycin (all Invitrogen). All cultures were maintained in a humidified 37°C atmosphere of ambient air (21% O2) and 5% CO2.
2.2 Hypoxia and Cell Viability Assay
Hypoxia was defined as 1% O2, 5% CO2 and was maintained in a dual-controlled chamber (Bio-Spherix, ProOx Model C21, Redfield, NY) inserted into a temperature-controlled incubator, using N2 as a displacement gas, at 37°C and 100% relative humidity. Normoxic conditions were defined to be 21% O2, 5% CO2 at 37°C with 100% relative humidity. Y79 cells were suspended in RPMI-1640 media, grown in 100 mm dishes, seeded at 2.25×105/plate, and incubated for up to 7 days in normoxia (21% O2, 5% CO2) or hypoxia (1% O2, 5% CO2), using pre-equilibrated hypoxic media for the hypoxic studies. Cell count and viability were assessed every 24 hours by removing an aliquot of the cell suspension. Cell viability and number was measured by automated trypan blue staining and counting using an Auto T4 Cellometer and associated software (Nexcelom Bioscience) set up for Y79 counting.
For the N2A hypoxia studies, N2A cells were stably transfected with pEGFP-Cx43GFP or pEGFP-Cx46GFP using Fugene 6 (Roche) followed by selection for 6–8 weeks in 1000 ug/ml G418 (Research Products International). Stable lines were maintained in the presence of 500 ug/ml G418. For the hypoxia cell viability studies, N2A lines were plated at 15,000 cells/well in an OptiLux clear bottom 96 well plate (BD Biosciences) and let attach for 6–12 hours in normoxic culture conditions. Media was then replaced with 100 ul antibiotic free growth media equilibrated to either 1% or 21% oxygen for hypoxic or normoxic assay and placed into the hypoxia or normoxia incubators, respectively. At the end of the indicated treatment period, 20 ul of CellTiter Blue Cell Viability Assay (Promega) fluorescent substrate was added per well, mixed, incubated respectively for 2 hours, and then read on a Carry Eclipse fluorescent plate reader using the excitation/emission wavelength(slit) settings of 560nm(10)/590nm(20) at a 400V PMT setting. Fluorescence is directly proportional to the number of live cells.
2.3 Y79 siRNA Knockdown and Viability Studies
Optimization of the siRNA transfection protocol of Y79 was performed in vitro prior to the in vivo pilot/proof-of-concept study. 2.0×105 Y79 cells were plated in a total of 0.4 mL of complete RPMI media 16–24 hours prior to siRNA transfection and placed in normoxic growth conditions. Each siRNA were mixed with various amounts of HiPerfect (Qiagen), according to the manufacturers suggestions in serum-free RPMI, at ratios of 1 ug siRNA : 3 uL HiPerfect, 1:6 and 1:12. Various combinations were tried and the remaining transfections were completed using 250 nM siRNA and 15 uL of HiPerfect (as to avoid cell death) in a total of 100 uL mixture per well of a 12 well plate with a 20 minute incubation at room temperature. 0.1 mL of transfection mixture was added to the 0.4 mL of pre-incubated Y79 cells, mixed and allowed to transfect in normoxic conditions for 6 hours prior to the addition of 0.5 mL complete RPMI equilibrated to either 21% or 1% oxygen. For the knockdown studies, siRNA transfected Y79 cells (250 nM) were kept in normoxic growth conditions for a further 18, 42 or 66 hour incubation period (to give 24, 48 or 72 hours post-transfection time points), then harvested and lysed according to the protocol below. For the viability studies, siRNA transfected cells (250 nM) were incubated in normoxic or hypoxic conditions for a further 18, 42 or 66 hours (to give 24, 48 and 72 hour post-transfection time points). At the end of the incubation period, the cell suspension was transferred to a microcentrifuge tube and mixed 1:1 with trypan blue solution. Cell viability and number was measured by automated trypan blue staining and counting using an Auto T4 Cellometer and associated software (Nexcelom Bioscience) set up for Y79 counting.
2.4 Transplantation of Human Y79 Retinoblastoma Cells in Nude Mice
The study protocol and procedures were approved by the Kansas State University Institutional Animal Care and Use Committee. All experiments were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The human Y79 retinoblastoma cell tumor nude mouse model has been previously characterized (Kimura, et al., 2008). The human Y79 retinoblastoma cell line was suspended in Iscove’s culture medium (Invitrogen) supplemented with 20% FBS. Five-week old, mixed sex, homozygous Nu/Nu nude mice (strain NuFoxn1) (n=18), purchased from Charles River Laboratories (Wilmington, MA), were subcutaneously transplanted with 1×107 human Y79 retinoblastoma cells in 0.5 ml total volume of a 1:1 mixture of basement membrane matrix (BD Biosciences) and Iscove’s medium supplemented with FBS, in the left dorsal region. Assessment of tumor development was performed daily. Once a tumor was palpable, typically 3 to 7 days after cell transplantation, treatment was initiated via intratumor siRNA injections. Mice were divided into 3 treatment groups with 6 mice in each group. Groups received intratumor injections of 30 µg Cx46 siRNA, 30 µg of non-silencing Allstars Negative Control siRNA, or they remained sham treated. Anti-Cx46 siRNA (Target sequence: CGC ATG GAA GAG AAG AAG AAA) and negative, non-silencing siRNA (catalog #1027281) were purchased from Qiagen (Valencia, CA). Intratumor siRNA treatment was given every other day for a maximum of 10 treatments.
Mice were examined every other day prior to siRNA treatment and the length (L) and width (W) of the tumors were measured with calipers to the nearest millimeter and recorded. Tumor volume was estimated using the following formula: TV = (LxW2)/2. The tumor size measured immediately prior to the first siRNA injection was considered the day 0 measurement. Once a tumor became larger than the 15mm in any dimension or 10 siRNA treatments had been administered, mice were euthanized by CO2 inhalation followed by thoracotomy, as a secondary method. Tumors were immediately excised, measured in 3 dimensions (mm), and weighed (mg). Tumors were sectioned in half, one section was snap frozen in a cryogenic vial to be homogenized for western blot analysis, while the remaining half was immersion-fixed in 10% formalin. Formalin-fixed tumor tissues were embedded in paraffin, serially sectioned and processed for standard hematoxylin and eosin (H&E) staining. Sections were pathologically evaluated for tumor features and cellular characteristics by a board-certified veterinary pathologist.
2.5 Western Blot
Cx46 and Cx43 expression were measured by Western blot analyses and were carried out as previously described (Akoyev and Takemoto, 2007). Y79 cells and tumors were homogenized in RIPA buffer containing a protease and phosphatase inhibitor cocktail (Calbiochem). Homogenates were sonicated for 10 seconds, three times and then centrifuged at 2000 × g for 15 minutes at 4°C. Equal amounts of protein, determined by the Bio-Rad Protein Assay, were used. Supernatant proteins were separated on an 8% SDS-PAGE gel and transferred to nitrocellulose membranes (OPTI-TRAN, MidWest Scientific). Membranes were blocked overnight at 4°C in 5% nonfat powdered milk in TDN buffer and then were incubated with the rabbit anti-Cx46 (1:1000 dilution) or rabbit anti-Cx43 (1:7500 dilution) antibodies overnight at 4°C, next incubated with a goat anti-rabbit-HRP secondary antibody (1:10000 dilution, Thermo Scientific Pierce, 31460). Rabbit anti-Cx46 antibody (US Biologicals, C7858-07A) and rabbit anti-C-terminal Cx43 antibody (Sigma-Aldrich, C6219) are commercially available. β-actin (Sigma-Aldrich, A5441) and α-tubulin (Sigma-Aldrich, T6074) were used as a loading controls. The blot was visualized using SuperSignal West Femto substrate (Pierce). Blots were digitized and analyzed using UN-SCAN-IT software (Silk Scientific).
2.6 Statistical Analyses
Commercial software (Origin; Microcal Software, Inc., Northampton, MA) was used for statistical analyses. Results were expressed as the mean ± SEM and differences of P ≤ 0.05, using a one-way analysis of variance, were considered statistically significant. The n are given with each figure.
3. Results
3.1 Cx46 Expression in Y79 Retinoblastoma Cells in vitro
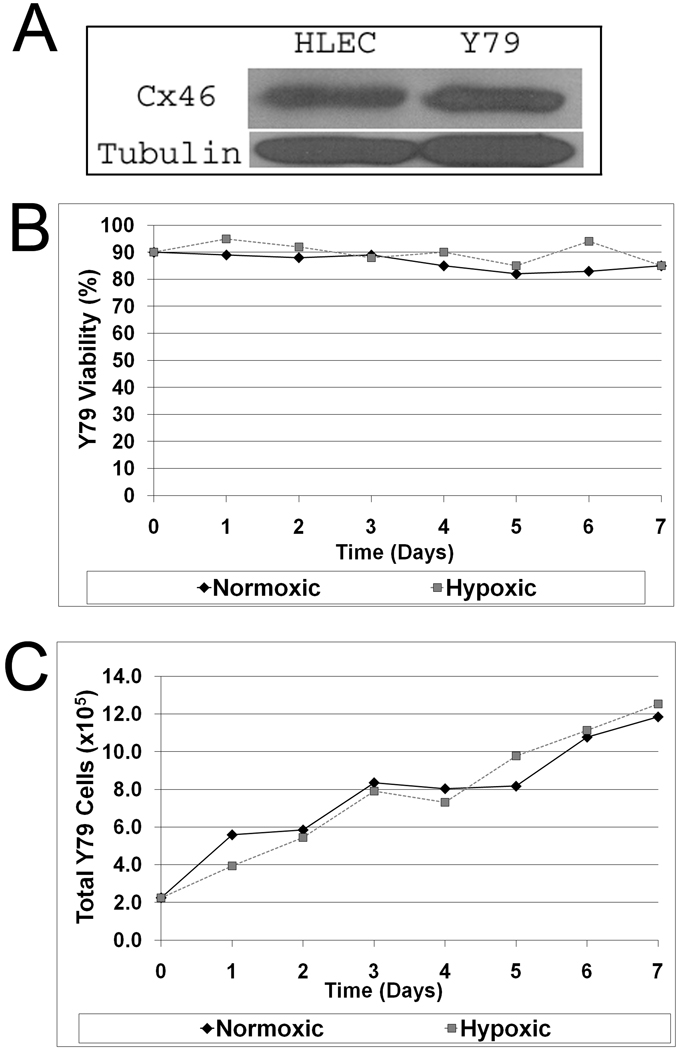
To investigate the effects of Cx46 in retinoblastoma growth, we had to establish that the human Y79 retinoblastoma cell line demonstrated Cx46 expression. Figure 1A shows that Cx46 protein is expressed endogenously in Y79 cells when compared to human lens epithelial cells (HLEC), which normally express Cx46 and naturally thrive in hypoxia. Y79 cells were then grown in both normoxic and hypoxic conditions as described in the Materials and Methods and assessed for cell viability and growth (Figs. 1B and 1C). Cells remained viable and grew in both hypoxic (1% O2) and normoxic (21% O2) growth conditions. After 7 days in normoxic growth conditions, there was a 5-fold increase in the number of Y79 cells with ≥80% viability. In hypoxic growth conditions, there also was a 5-fold increase in the number of Y79 cells and with ≥80% viability after 7 days. Similar results are presented in the Supplemental Data Figure S1. In contrast, when N2A cells are challenged with hypoxic growth conditions, these cells do not survive past 18 hours. However, only N2A cells expressing the carboxy-terminal GFP tagged wild type rat Cx46 survive in 1% oxygen. N2A cells expressing the related Cx43-GFP fusion protein do not survive hypoxia and behave similar to untransfected and hypoxia sensitive N2A cells (Supplemental Data Fig. S2). These data demonstrate that Cx46 confers resistance to hypoxia, and, that our hypoxia chamber is functional.
Figure 1.
Y79 cells express Cx46 and thrive under hypoxic conditions. (A) Y79 whole cell lysates probed with rabbit anti-Cx46 antibody and mouse anti-tubulin antibody, demonstrating Cx46 expression in human Y79 retinoblastoma cells. (B) Viability of Y79 cells under normoxia (37°C humidified ambient air, 5% CO2) and hypoxia (37°C humidified 1% oxygen displaced by N2, 5% CO2) was monitored for 7 days and recorded. (C) Total Y79 cell numbers were counted for 7 days under normoxic and hypoxic growth conditions. Data trends from several experiments indicate that Y79 cells express Cx46 and can survive and thrive in hypoxic conditions (error not calculated). Representative experimental data shown, additional data in supplement.
3.2 Transfection with anti-Cx46 siRNA negatively affects Y79 hypoxic cell growth in vitro
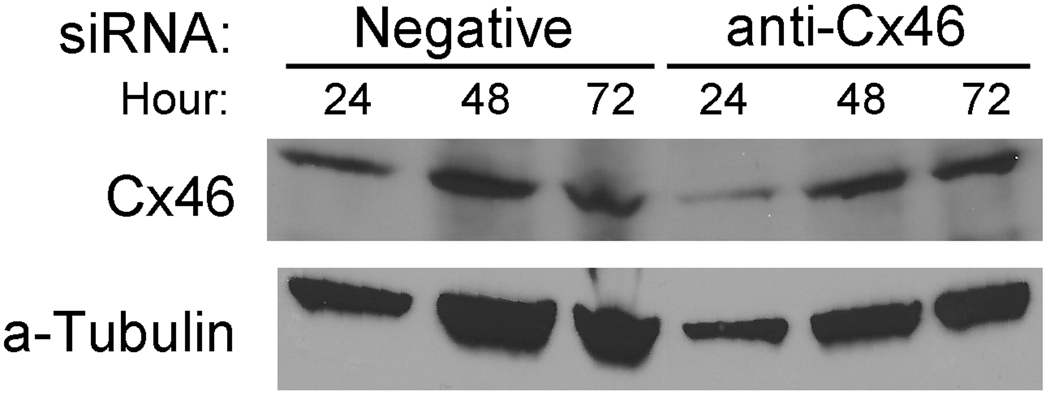
Since Y79 cells thrive in hypoxic growth conditions, we next tested if knocking down Cx46 expression levels affected the growth characteristics of Y79 cells. Once knockdown conditions were optimized (Supplemental Data Fig. S3), it was clear that a high amount of siRNA was required to have an effect on Cx46 protein expression levels (also observed in the in vivo tumor studies). Y79 cells are very resistant to many transfection methods, and this high amount of siRNA may be necessary because of low transfection efficiency (Farber et al, 2000; Mitra et al, 2010; White et al, 2001). Next, the effects of anti-Cx46 siRNA were assessed by western blot (Fig. 2) and by measuring cell count and viability (Fig. 3).
Figure 2.
Western blot of siRNA knockdown in Y79 cells in vitro. Y79 cells treated with 250 nM and 15 uL HiPerfect for each siRNA show were grown in normoxic conditions, lysed at the timepoints shown and western blotted for Cx46 expression. Optimal knockdown of Cx46 was shown at 24 hours.
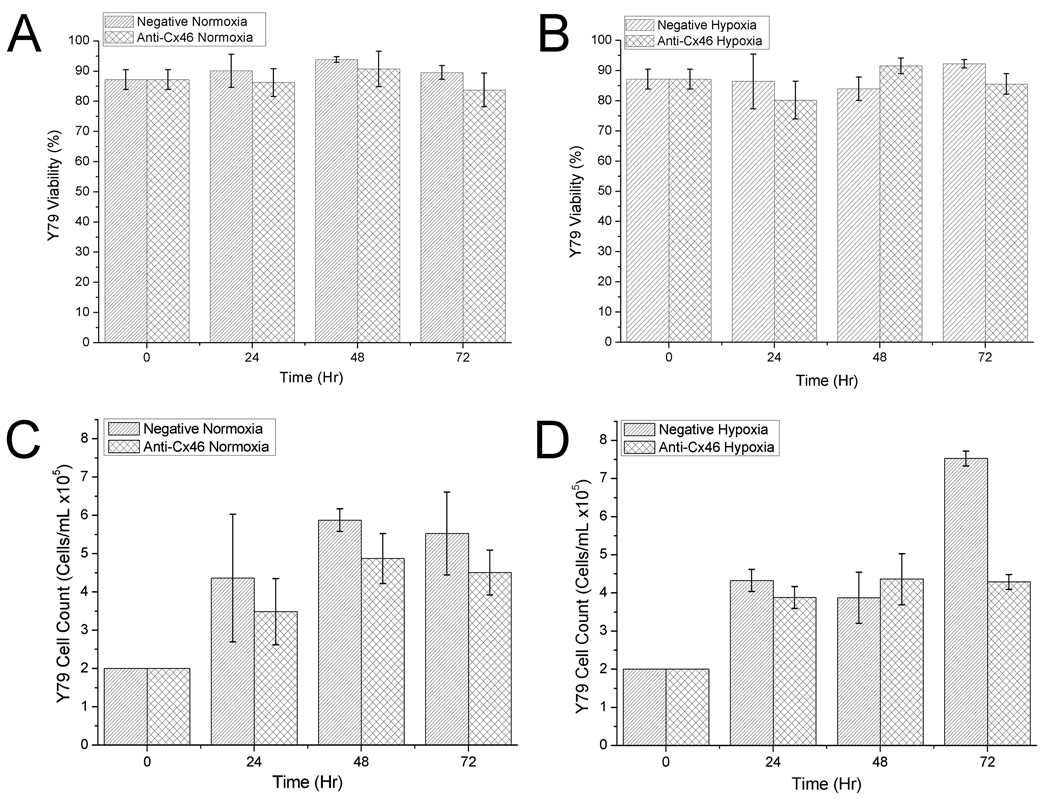
Figure 3.
Viability assay of siRNA knockdown in Y79 cells in vitro. Y79 cells treated with 250 nM and 15 uL HiPerfect for each siRNA were subjected to normoxic and hypoxic growth conditions as shown. Viability (A, B) was measured and cell counts (C, D) taken to assess the knockdown effect of Cx46 on Y79 cell growth characteristics. Each data point shown with standard error (n = 4).
Western blotting of Y79 cells transiently transfected with anti-Cx46 siRNA (Fig. 2) revealed that maximal Cx46 knockdown occurred at 24 hours post-transfection and rebounded at 48 and 72 hours. It is difficult to draw solid conclusions from the western blots based on the fact that the Y79 cell line is hard to transfect with any amount of siRNA. Increasing the HiPerfect transfection reagent increases cell death and using other lipid-based compounds did not yield better results (data not shown). Other researchers have used viral transfection to achieve a high level of transfection in Y79 cells (White, et al 2001).
Y79 cells transfected with the negative control siRNA showed no discernable negative effects from the transfection in both normoxic (Figs. 3A and 3C) and hypoxic (Figs. 3B and 3D) growth conditions over 72 hours; viability remained high and cell counts increased. Cells transfected with anti-Cx46 siRNA showed no discernable effects in normoxia compared to the negative control in both viability and cell count (Figs. 3A, 3C) however, when subjected to hypoxia, viability (Fig. 3B) dropped slightly at 24 hours, recovered at 48 hours, but remained lower than the control at 72 hours. Y79 cell number in cells treated with anti-Cx46 siRNA did not increase significantly at any of the time points tested, while cell number greatly increased at 72 hr in the negative siRNA treated cells. Taken together with the western blot data, the knockdown of Cx46 levels did cause a decrease in Y79 cell count at 72 hr after siRNA transfection. However, this was much less than that observed in the normal N2A cells. This may suggest that knock down of Cx46 has additional effects for its anti-tumor activity. This could be through an increase in Cx43 once the Cx46 is decreased (see below).
In vivo proof-of-concept and dosage pilot study
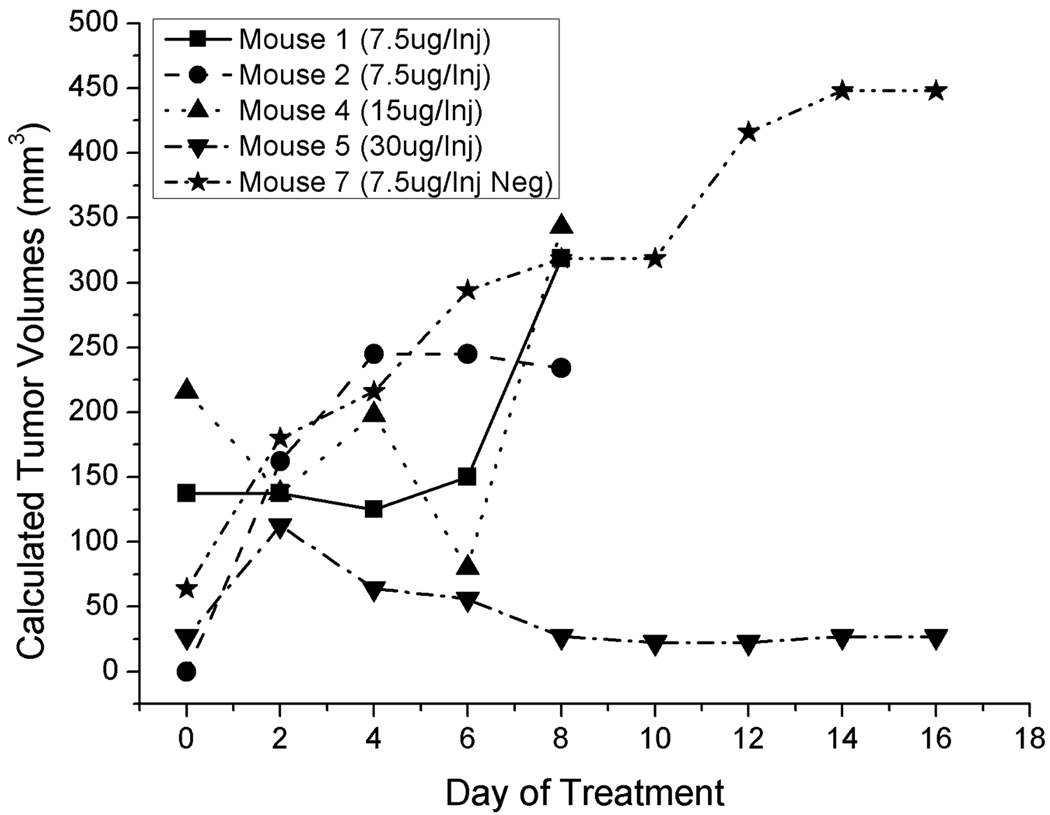
An initial proof-of-concept and dosage study was performed prior to performing a statistically correct in vivo study. Knowing that a high amount of siRNA was required for Cx46 knockdown in Y79 cells in vitro, and using previous knowledge from a related study (Banerjee et al, 2010), 4 treatment groups of two mice each were used to determine the best in vivo dosage amount. Three dosages of 7.5 ug, 15 ug and 30 ug/injection of the anti-Cx46 siRNA and a single dosage of 7.5 ug/injection of negative control siRNA were tested. Figure 4 shows the effects of each dosage on the Y79 tumors in mice confirmed to have a tumor and Supplemental Table S1 shows the individual tumor data compiled from the study. 7.5 ug and 15 ug/injection of anti-Cx46 siRNA had no effect on the growth of the Y79 xenografts, while the highest dose of 30 ug/injection showed a reduction in tumor growth and burden (Fig. 4). Statistical analysis of this initial study was not performed as it was a pilot study. For the primary in vivo study, 30 ug/injection of anti-Cx46 siRNA was chosen for the dosage amount.
Figure 4.
Results of anti-Cx46 siRNA dosing and proof-of-concept in vivo study. 4 groups of mice (n = 2) were used in the study to assess the most effective dosing regimen for the primary study. Tumors receiving anti-Cx46 siRNA were allowed to grow until they became a health concern for the mice harboring the tumors, then the mice were euthanized to prevent any further suffering. A dosage of 30 ug/Injection every other day was used for the primary study. Tabulated data for each mouse can be found Supplemental Data Table 1.
3.4 Cx46 siRNA suppresses Y79 Tumor growth in vivo
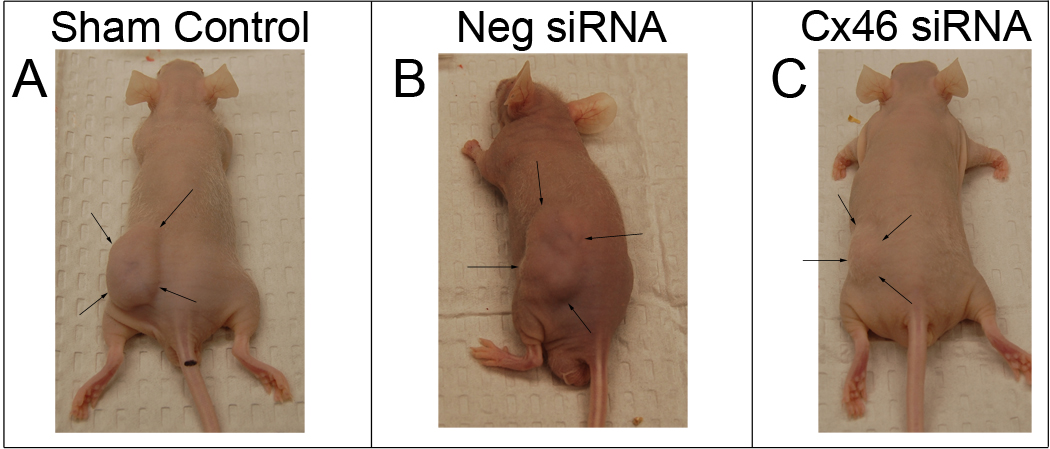
The effects of Cx46 gene silencing was investigated on xenograft Y79 cell tumors in vivo. All experimental and control mice developed tumors at the site of injection. The gross appearance of mice 14 days post-Y79 cell implantation in untreated, control tumors or following 5 treatments with non-silencing siRNA or Cx46 siRNA, are shown, outlined with arrows, in Figure 5. In control mice without siRNA treatment (Fig. 5A) and in mice in the non-silencing siRNA treatment group (Fig. 5B), large, subcutaneous and vascularized tumors, were observed where Y79 cells had been injected in their dorsal area. Mice who received intratumor Cx46 siRNA therapy (Fig. 5C) had an extensive reduction in tumor size and vascularization. Images of representative excised tumors are shown in the Supplementary Data Figure S4.
Figure 5.
Intratumor Cx46 siRNA suppresses the growth of Y79 tumors in vivo. Macroscopic appearance of Y79 tumors in nude mice 14 days post-transplantation of human Y79 retinoblastoma cells in (A) untreated control tumors, or following 5 siRNA treatments, with (B) 30 µg non-silencing siRNA or (C) 30 µg Cx46 siRNA. Arrows outline the representative tumors in the left dorsal region of the mice. Tabulated data for each mouse can be found Supplemental Data Table 2.
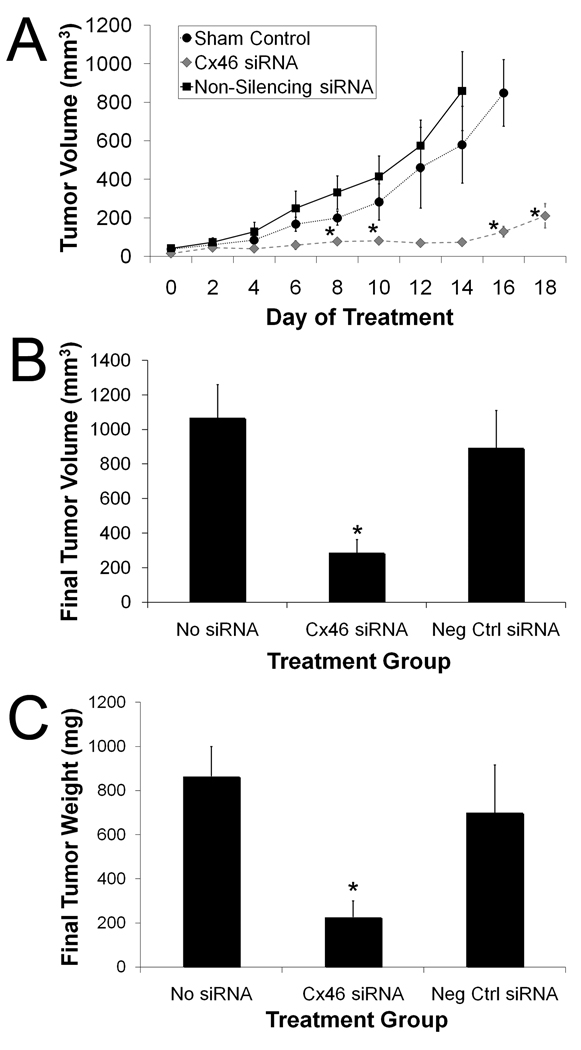
During the 21-day treatment period, the tumor growth rate and the final tumor volumes were greater in the untreated control and non-silencing siRNA treatment groups than in the mice treated with Cx46 siRNA (Fig. 6). Y79 cell tumors grew rapidly in the untreated control and the non-silencing siRNA treatment groups while the rate of tumor growth was significantly reduced in the Cx46 siRNA treatment group (Fig. 6A). The reduction in tumor volumes of Cx46 siRNA treated mice were statistically significant (P ≤ 0.05), when compared to non-silencing siRNA or no siRNA treated groups. The Cx46 siRNA group began to be statistically different from the non-silencing siRNA treatment group beginning 8 days after treatment was initiated and continued to be significant throughout the study (P ≤ 0.02). The Cx46 siRNA group began to show significance on day 6 (P ≤ 0.04) and was also significant on day 8 (P ≤ 0.02), day 14 (P ≤ 0.04), and day 16 (P ≤ 0.003) from the untreated, control group, however, days 10 and 12 were not statistically significant (P ≥ 0.05). Mice treated with intratumor Cx46 siRNA injections had significantly smaller final tumor volumes (287 mm3 ± 77 mm3) when compared to untreated control mice (1068 mm3 ± 192 mm3; P ≤ 0.002) or to mice who received intratumor injections of non-silencing siRNA (894 mm3 ± 218 mm3; P ≤ 0.03) (Fig. 6B). Although the non-silencing siRNA group tumor volumes were lower than the untreated sham group, this was not statistically significant. The tumor weights of the treatment groups paralleled the final tumor volumes closely (Fig. 6C). Mice in the Cx46 siRNA treatment group had significantly reduced mean final tumor weight (226 mg ± 75 mg) in comparison to mice treated with non-silencing siRNA (700 mg ± 218mg; P ≤ 0.05) or the untreated control mice (864 mg ± 136 mg; P ≤ 0.01). Data for all mice used in this study can be found in the supplementary data in Table S2.
Figure 6.
Tumor volume in Cx46 siRNA, non-silencing siRNA, and no siRNA treatment groups of mice transplanted with Y79 cells. Tumor volume of the transplanted mice was measured for a maximum of 21 days, with the administration of 30 µg Cx46 siRNA (n = 6), 30 µg non-silencing siRNA (n = 6), or no treatment (n = 6). (A) The calculated tumor volumes were averaged by treatment group and day over the course of the study (mean ± SE). The tumor volumes of Cx46 siRNA treated mice (n = 6) were statistically significant (* = P ≤ 0.05), when compared to non-silencing siRNA treatment group (n = 6) beginning on 8 days after treatment was initiated and continued to be significant throughout the study. The Cx46 siRNA treatment group was statistically different (P ≤ 0.05) from the untreated, no siRNA group (n = 6) beginning on day 6 and was also significant on days 8, 14, and 16. (B) Final measurements were taken on the excised tumors and the volumes were averaged by group. There was a significant reduction in final tumor volume after treatment with Cx46 siRNA (287 mm3 ± 77 mm3, n = 5) when compared to the non-silencing siRNA (894 mm3 ± 218 mm3, n = 5) or untreated (1068 mm3 ± 192 mm3, n = 6) groups (P ≤ 0.03 and P ≤ 0.002, respectively). (C) Cx46 siRNA treated mice had a decreased tumor weight. Final tumor weights of groups treated with Cx46 siRNA, non-silencing siRNA (negative control siRNA) ,or no siRNA, shown as mean tumor weight (mg) ± SE (n = 6 for each group). The weight of tumors treated with Cx46 siRNA (226 mg ± 75 mg) were significantly less than the tumors of mice treated with non-silencing siRNA (700 mg ± 218 mg, * = P ≤ 0.05) or no siRNA (864 mg ± 136 mg, P ≤ 0.01).
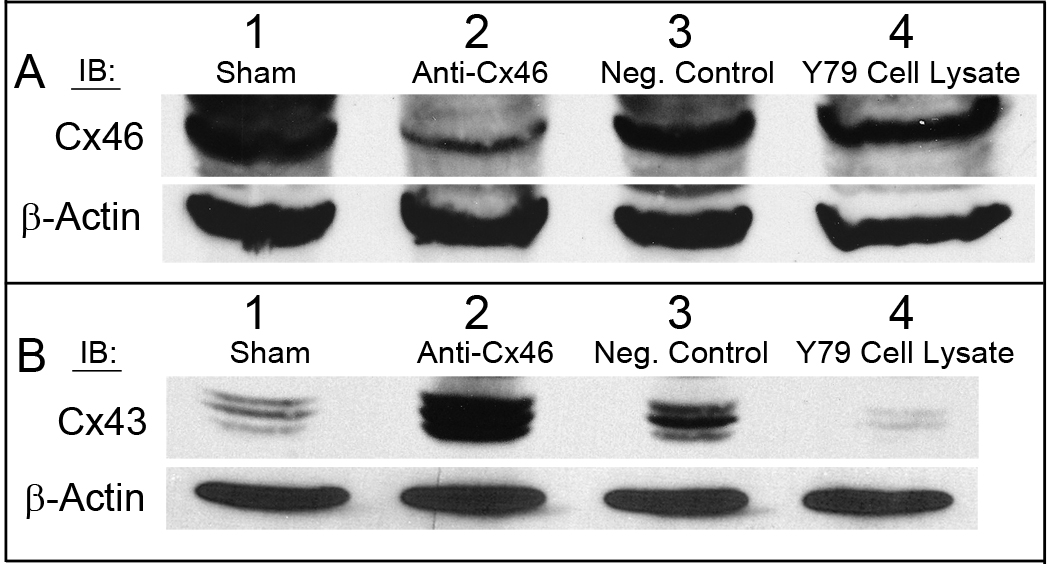
Y79 tumor homogenates were analyzed by western blot and probed with anti-Cx46 and anti-Cx43 antibodies using β-actin as a loading control. Blots were digitized and analyzed as described in the Materials and Methods (Supplementary Fig. S5). Not surprisingly, Cx46 expression was markedly decreased in tumors treated with Cx46 siRNA (Fig. 7A), however, upregulation of Cx43 protein was observed in a reciprocal manner (Fig. 7B). Both the sham untreated control and non-silencing siRNA treatment groups demonstrated high expression of Cx46 protein (Fig. 7A) and had low Cx43 protein expression (Fig. 7B). A 5-fold reduction in Cx46 and 6-fold rise in Cx43 expression were observed in mice treated with Cx46 siRNA in comparison to mice who received no siRNA treatment (Supplementary Fig. S5). Similarly, mice treated with Cx46 siRNA had a 6-fold decrease in Cx46 and 3-fold increase in Cx43 expression compared to mice treated with non-silencing siRNA. The increase in the expression of the Cx43 tumor suppressor protein may contribute significantly to the observed tumor reduction.
Figure 7.
Decreased expression of Cx46 with a reciprocal increase in Cx43 protein expression in Y79 tumors of mice treated with Cx46 siRNA. Conversely, tumors treated with a non-silencing siRNA or were untreated showed Cx46 upregulation and Cx43 downregulation. Equal amounts of Y79 tumor homogenate were analyzed for (A) Cx46 and (B) Cx43 where β-actin was used as the loading control. Lane 1: Tumor #14, sham treated tumor; Lane 2: Tumor #5, anti-Cx46 siRNA treated tumor; Lane 3: Tumor #11, AllStars negative control siRNA treated tumor; Lane 4: Y79 cell lysate positive control for Cx46 expression. Representative tumor western blots shown.
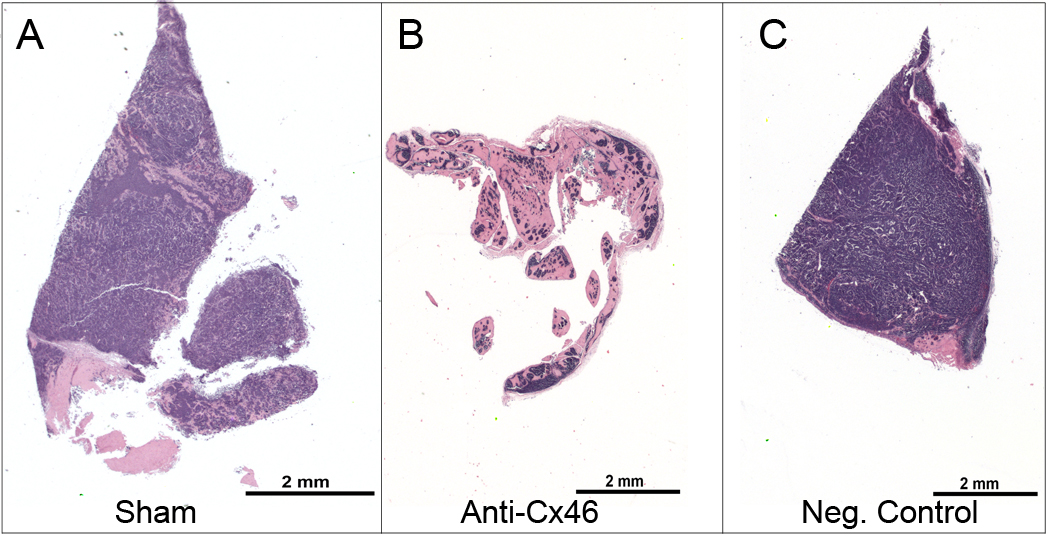
Histopathology of the tumor sections, shown in Figure 8, determined that mice treated with Cx46 siRNA had the least number and, therefore, the least density of Y79 cells when compared to mice treated with non-silencing siRNA or no siRNA. There were no differences in the Y79 cell density between the non-silencing siRNA treated mice and mice which received no siRNA treatment by observation of the stains, hematoxylin (purple, nuclei) and eosin (pink, cytoplasm and connective tissue) in Figure 8.
Figure 8.
Decreased Y79 tumor cell burden in mice treated with Cx46 siRNA. Excised tissues were fixed, embedded, sectioned and then stained with hematoxylin (purple, nuclei) and eosin (pink, cytoplasm and connective tissue). (A) Untreated sham Y79 tumor (cage 5, mouse 2) (B) Cx46 siRNA treated Y79 tumor (cage 2, mouse 2) (C) Non-silencing siRNA treated Y79 tumor (cage 4, mouse 2). The representative images were taken by a board-certified veterinary pathologist and show the microscopic appearance of the Y79 tumors isolated from the xenograft mice, treated with Cx46 siRNA, a non-silencing siRNA, or sham treated for a maximum of 21 days.
4. Discussion
A connection between GJIC and control of cell growth was established over 40 years ago (Loewenstein, 1979), which resulted in a multitude of research reporting a loss or diminished ability to adjoin amongst neoplastic cells or between cancer cells and nearby normal cells (reviewed in Mesnil, et al., 2005). The tissue specific expression of connexins was discovered along with the finding that reexpression of specific connexins was able to reestablish control of cell growth. Additionally, recovery of GJIC alone was not always capable of normalizing cell growth (Mesnil, et al, 1995). In other cases, reexpression of connexins was enough to restore cell growth control without restoring GJIC (Huang, et al., 1998). It has been suggested that connexins, independent of GJIC, may directly control growth, possibly by altering gene expression of neoplastic cells (Cronier, et al, 2009), and a change from the normal expression of connexin proteins may allow tumor progression. Gap junction proteins are, therefore, often termed tumor suppressors and are downregulated in tumor development and progression.
However, our current work shows that upregulation of Cx46 is observed in cancerous cells and may help solid tumor tissues in surviving hypoxic conditions or could cause tumor growth through the loss of Cx43. Human Y79 retinoblastoma cells express Cx46 and in contrast to connexin-deficient, wild-type neural N2A cells (Figure S2). Y79 cells are able to thrive in hypoxic conditions over the course of several days while maintaining viability. Similarly, human lens epithelial cells express both Cx46 and Cx43, a related and ubiquitously expressed gap junction protein, but can only survive prolonged hypoxic conditions while actively expressing Cx46. Gene knockdown by siRNA of Cx46 but not Cx43 makes HLECs susceptible to hypoxia induced cell death (Banerjee et al., 2010). Although the in vitro siRNA data presented here in Y79 cells is not completely conclusive, there is evidence that knockdown of Cx46 protein levels contributes to stagnated growth of Y79s in hypoxic conditions at 72 hr. It could be inferred that prolonged dosing of Y79 cells with anti-Cx46 could prolong the stagnation of Y79 cells and indeed this is what we show in vivo. Further evidence of Cx46-related hypoxic growth control is taken from N2A cells which lack gap junctions and can be engineered to express either Cx43 or Cx46. In these cell lines, only the Cx46 expressing cells show delayed cellular death in 1% oxygen (Figure S2). Cx46 does indeed appear to play a role in cellular resistance to hypoxia. Human Y79 retinoblastoma (this manuscript), human lens epithelial, rabbit lens epithelial, MCF-7 human breast cancer (Banerjee, et al., 2010), and the N2A-Cx46 cell line all express Cx46 and all are able to survive hypoxia (Figure 1; Figure S2). Knockdown of Cx46 in human and rabbit lens epithelial (Banerjee et al, 2010) and Y79 cell lines grown in hypoxic conditions clearly increases the negative effects of reduced oxygen.
Although retinoblastoma is well-known as a vascularized tumor, the early tumor is hypoxic and hypoxic regions exist in the advanced tumor stage (Maschek, et al., 2004; Boutrid, et al., 2008). The areas of the hypoxic tumor are increasingly refractory to conventional treatments, such as chemotherapy and radiation therapy, due to the slowly proliferating cells (Maschek, et al., 2004) since these therapies target rapidly dividing cells (Burnier, et al., 1990). With the resistant nature of hypoxic tumors and the local and systemic complications associated with current therapy protocols, the search for drugs that are non-cytotoxic to normal cells and can effectively target cancer cells is continuous. One approach would be to target a component of the cancerous cells which confers growth and viability of the tumor specifically during hypoxic events.
We chose to evaluate in vivo treatments using a subcutaneous model as opposed to the eye for several reasons. Y79 cells were used for the in vitro studies and subsequently using the same cell line allowed us to make direct correlations between the studies. In a preliminary Fisher rat eye retinoblastoma animal model (data not presented) we intraocularly treated with intratumor injections and it was very difficult to ocularly dose on an every other day basis without an increase in eye damage due to repeated injections as well as the increased risk of death due to repeated general anesthesia. The subcutaneous xenograft tumors presented in this paper were much easier to assess and reliably measured throughout the study which was especially helpful when testing an in vivo treatment protocol for the first time. Subsequent studies are needed to further develop and optimize a delivery system for the siRNA drug. Alternatively, other means to specifically lower Cx46 protein levels would need to be developed, such as use of a specific modified antibody similar to current antibody treatments for macular degeneration, or a long-lasting siRNA derivative.
Previously, siRNAs have been used successfully to provide a strong and specific knockdown of gene expression (Devroe and Silver, 2004), and are, therefore, ideal for the initial testing of targets. Nude mice injected with Cx46 siRNA exhibited a significant reduction in Y79 cell tumor growth, having a greater than 3-fold decrease in mean tumor volume, 21 days after initiation of treatment. Tumors treated with Cx46 siRNA had a projected knockdown of Cx46 but an unexpected increase in the expression of Cx43. While untreated tumors or tumors treated with non-silencing siRNA had an upregulation of Cx46 and subsequent downregulation of Cx43. This is the first time the reciprocal relationship between Cx46, a connexin upregulated in hypoxia, and Cx43, a known tumor suppressor, has been reported in vivo. Future studies would need to elucidate how loss of Cx46 results in up-regulation of the Cx43 tumor suppressor. These results demonstrate that Cx46 gene silencing using siRNA has an antitumor property in xenograft human Y79 retinoblastoma cell tumors. We hypothesize that the protumor effects of Cx46 may be partially due to its ability to confer resistance from hypoxic death to tumor cells expressing Cx46. Additionally, subsequent reappearance of Cx43 may then aid cells to acquire more “mortal” properties and could render the tumor cells more susceptible to current antitumor therapies. However, additional studies are needed to determine the details of how Cx43 and Cx46 are reciprocally controlled. Current work in our lab indicates that degradation of Cx43 is induced when Cx46 is expressed, and this degradation takes place in the proteasome (manuscript submitted). Additional studies on the mechanism of Cx43 reappearance upon anti-Cx46 siRNA treatment are outside the scope of this paper.
In conclusion, this study was the first to demonstrate the presence of Cx46 protein in human Y79 retinoblastoma cells. This is the second neoplastic cell line and related tumor tissue reported to express high levels of Cx46 and reciprocally low levels of Cx43 protein. Our findings reveal that local administration of anti-Cx46 siRNA can mediate effective knockdown of this target protein and suppress tumor growth in vivo. We observed no associated side effects with siRNA treatment in our subcutaneous Y79 retinoblastoma tumor model in nude mice. Since Cx46 is only naturally found in the hypoxic and differentiated lens, ill effects of gene silencing of this protein would likely be negligible; however, a potential yet treatable side effect with intraocular use may be cataract development. While siRNAs are not an optimal treatment due to their short half life and transient effects (Devroe and Silver, 2004; Behike, 2008), other anti-Cx46 treatment modalities more suitable for therapy, such as modified RNAi or an antibody, can be developed in the future. The findings of this study provide an exciting new direction in drug development for the treatment of retinoblastoma. In addition, Cx46 could provide an early marker for hypoxia and these findings could have broader significance for other hypoxia-related diseases.
Supplementary Material
Figure S1. Y79 cells exhibit normal growth characteristics in 1% oxygen. 3×105 Y79 cells were placed in pre-equilibrated normoxic or hypoxic media (100mm dish) and incubated for up to 5 days in normoxia (37°C humidified 21% O2, 5% CO2) or hypoxia (37°C humidified 1% O2, 5% CO2, displaced with N2). Cells grew after a 2 day lag period while maintaining viability under hypoxic growth conditions, indicating that Y79 cells can thrive in a hypoxic environment.
Figure S2. Connexin 46 provides growth protection in 1% oxygen. Mouse neuronal2A cells (N2A) were stably transfected with plasmids encoding either Cx43-EGFP or Cx46-EGFP and then subjected to viability analysis using the CellTiter Blue fluorescence cell viability assay (n = 4, error shown inside shape labels). N2A cells lack endogenous Cx43 and Cx46. N2A cells expressing Cx46 were more viable than control N2A or N2A cells expressing Cx43-GFP after 12, 18, 24 and 36 hours in 1% oxygen.
Figure S3. Optimization of anti-Cx46 siRNA transfection in Y79 cells. (A) Initial studies of 10, 100 or 250 nM siRNA with ug:uL HiPerfect ratios given. Negative control (Neg) at 100 nM, ratio of 1ug siRNA:6 uL HiPerfect. (B) Secondary optimization of siRNA transfection using nM siRNA:uL HiPerfect ratios, as given. A ratio of 250 nM siRNA : 15 uL of HiPerfect used for the remaining in vitro siRNA assays. (C) 30 ug/lane of ARPE-19 and Y79 whole cell lysates used as Cx46 immunoblotting positive controls.
Figure S4. Gross appearance of freshly excised Y79 xenograft tumors. Mice were euthanized and the tumors were immediately excised, measured in three dimensions (mm), imaged and then formalin fixed. Representative tumors shown are from cage 2, mouse 1 and mouse 2 (anti-Cx46 siRNA treated); cage 4, mouse 2 (negative control); cage 5, mouse 2 (sham). Note the reduced amount of visible vascularization present in the anti-Cx46 siRNA treated tumors compared to either the sham or negative control tumor.
Figure S5. Quantitative protein expression densitometry of Cx46 and Cx43 protein levels in representative tumors. Blots were digitized then normalized to β-actin levels and the detected protein expression of Cx46 and Cx43 in different treatment groups was compared, using the no siRNA treatment as a control. A 5-fold reduction in Cx46 and 6-fold rise in Cx43 expression were observed in mice treated with Cx46 siRNA in comparison to mice who received no siRNA treatment. Mice treated with Cx46 siRNA had a 6-fold decrease in Cx46 and 3-fold increase in Cx43 expression compared to mice treated with non-silencing siRNA.
Table S1. Calculated volumes (mm3) of individual xenograft tumors used the proof-of-concept and dosage pilot study. Tumor volumes for individual tumors were measured and recorded on each injection day, then calculated using TV = [L*(W2)]/2. Column listed as “Tumors” shows which mice had confirmed tumors and which data subsequently comprised Figure 4. NT = Measurement not taken for that day; E = Euthanized.
Table S2. Measured volumes (mm3) of individual xenograft tumors used in the primary study. Tumor volumes for individual tumors were measured and recorded on each injection day, then calculated using TV = [L*(W2)]/2. NT = Measurement not taken for that day; E = Euthanized; indicates which tumors were not included in the volume calculations and statistics due to being an outlier; (*) indicates which tumors were used in western blotting experiments.
Acknowledgements
This work is supported by grant number NIH R01EY13421 to DJT from the National Eye Institute and partially funded by the Graduate Student Summer Stipend for DB and the Undergraduate Cancer Research Award to DML from the Johnson Center for Basic Cancer Research at Kansas State University. The authors would like to thank Kyathanahalli Janardhan at the Kansas State University College of Veterinary Medicine for the histopathological assistance.
Abbreviations
- HIF
hypoxia inducible factor
- Cx
connexin
- N2A
neuronal2A cells
- siRNA
short interfering RNA
- GJIC
gap junction intercellular communication
- HLEC
human lens epithelial cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Diana B. Burr, Email: dburr@vet.k-state.edu.
Samuel A. Molina, Email: smolina@ksu.edu.
Debarshi Banerjee, Email: debarshi@ksu.edu.
Derek M. Low, Email: dlow@kumc.edu.
Dolores J. Takemoto, Email: dtak@ksu.edu.
REFERENCES
- Abramson DH, Beaverson KL, Chang ST, Dunkel IJ, McCormick B. Outcome following initial external beam radiotherapy in patients with Reese-Ellsworth group Vb retinoblastoma. Arch Ophthalmol. 2004;122:1316–1323. doi: 10.1001/archopht.122.9.1316. [DOI] [PubMed] [Google Scholar]
- Akoyev V, Takemoto DJ. ZO-1 is required for protein kinase C gamma-driven disassembly of connexin 43. Cell Signal. 2007;19:958–967. doi: 10.1016/j.cellsig.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoneli CB, Ribeiro KC, Steinhorst F, Novaes PE, Chojniak MM, Malogolowkin M. Treatment of retinoblastoma patients with chemoreduction plus local therapy: experience of the AC Camargo Hospital, Brazil. J. Pediatr. Hematol. Oncol. 2006;28:342–345. doi: 10.1097/00043426-200606000-00004. [DOI] [PubMed] [Google Scholar]
- Banerjee D, Gakhar G, Madgwick D, Hurt A, Takemoto D, Nguyen TA. A novel role of gap junction connexin46 protein to protect breast tumors from hypoxia. Int J Cancer. 2010;127:839–848. doi: 10.1002/ijc.25107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behike MA. Chemical modification of siRNAs for in vivo use. Oligonucleotides. 2008;18:305–319. doi: 10.1089/oli.2008.0164. [DOI] [PubMed] [Google Scholar]
- Benz MS, Scott IU, Murray TG, Kramer D, Toledano S. Complications for systemic chemotherapy as treatment of retinoblastoma. Arch. Ophthalmol. 2000;118:577–578. [PubMed] [Google Scholar]
- Boutrid H, Jockovich ME, Murray TG, Pina Y, Feuer WJ, Lampidis TJ, Cebulla CM. Targeting hypoxia, a novel treatment for advanced retinoblastoma. Invest Ophthalmol Vis Sci. 2008;49:2799–2805. doi: 10.1167/iovs.08-1751. [DOI] [PubMed] [Google Scholar]
- Burnier MN, McLean IW, Zimmerman LE, Rosenberg SH. Retinoblastoma. The relationship of proliferating cells to blood vessels. Invest Ophthalmol Vis Sci. 1990;31:2037–2040. [PubMed] [Google Scholar]
- Cronier L, Crespin S, Strale PO, Defamie N, Mesnil M. Gap junctions and cancer: new functions for an old story. Antioxid. Redox. Signal. 2009;11:323–338. doi: 10.1089/ars.2008.2153. [DOI] [PubMed] [Google Scholar]
- Devroe E, Silver PA. Therapeutic potential of retroviral RNAi vectors. Expert Opin. Biol. Ther. 2004;4:319–327. doi: 10.1517/14712598.4.3.319. [DOI] [PubMed] [Google Scholar]
- Farber DB, Learner LE, Viczian AS. Methods in Molecular Medicine. New York: Springer; 2001. Vision Research Protocols. [Google Scholar]
- Habermann H, Ray V, Habermann W, Prins GS. Alterations of gap junction protein expression in human benign prostatic hyperplasia and prostate cancer. J. Urol. 2002;167:655–660. doi: 10.1016/S0022-5347(01)69118-3. [DOI] [PubMed] [Google Scholar]
- Harris AL, Locke D. Connexins: A Guide. New York: Springer; 2009. [Google Scholar]
- Hirschi KK, Xu CE, Tsukamoto T, Sager R. Gap junction genes Cx26 and Cx43 individually suppress the cancer phenotype of human mammary carcinoma cells and restore differentiation potential. Cell. Growth Differ. 1996;7:861–870. [PubMed] [Google Scholar]
- Huang RP, Fan Y, Hossain MZ, Peng A, Zeng ZL, Boynton AL. Reversion of the neoplastic phenotype of human glioblastoma cells by connexin 43 (cx43) Cancer Res. 1998;58:5089–5096. [PubMed] [Google Scholar]
- Jehanne M, Lumbroso-Le Rouic L, Savignoni A, Aerts I, Mercier G, Bours D, Desjardins L, Doz F. Analysis of ototoxicity in young children receiving carboplatin in the context of conservative management of unilateral or bilateral retinoblastoma. Pediatr. Blood Cancer. 2009;52:637–643. doi: 10.1002/pbc.21898. [DOI] [PubMed] [Google Scholar]
- Kanczuga-Koda L, Sulkowska M, Tomaszewski J, Koda M, Sulkowska M, Przystupa W, Golaszewska J, Baltaziak M. Connexins 26 and 43 correlate with Bak, but not with Bcl-2 protein in breast cancer. Oncol. Rep. 2005;14:325–329. [PubMed] [Google Scholar]
- Kanczuga-Koda L, Sulkowska M, Koda M, Reszec J, Famulski W, Baltaziak M, Sulkowski S. Expression of connexin 43 in breast cancer in comparison with mammary dysplasia and normal mammary gland. Folia Morphol. 2003;62:439–442. [PubMed] [Google Scholar]
- Kimura K, Usui Y, Hattori T, Yamakawa N, Goto H, Usui M, Okada S, Shirato K, Tomoda A. Phenoxazine derivative, 2-amino-4,4α-dihydro-4α,7-dimethyl-3H-phenoxazine-3-one suppresses growth of human retinoblastoma cell line Y79 in vitro and in vivo. Oncol. Rep. 2008;19:3–10. [PubMed] [Google Scholar]
- Laird DW, Fistouris P, Batist G, Alpert L, Huynh HT, Carystinos GD, Alaoui-Jamali MA. Deficiency of connexin43 gap junctions is an independent marker for breast tumors. Cancer Res. 1999;59:4104–4110. [PubMed] [Google Scholar]
- Lee SW, Tomasetto C, Sager R. Positive selection of candidate tumor-suppressor genes by subtractive hybridization. Proc. Natl. Acad. Sci. U.S.A. 1991;88:2825–2829. doi: 10.1073/pnas.88.7.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein WR. Junctional intercellular communication and the control of growth. Biochim. Biophys. Acta. 1979;560:1–65. doi: 10.1016/0304-419x(79)90002-7. [DOI] [PubMed] [Google Scholar]
- Maschek G, Savaraj N, Priebe W, Braunschweiger P, Hamilton K, Tidmarsh GF, De Young LR, Lampidis TJ. 2-deoxy-D-glucose increases the efficacy of adriamycin and paclitaxel in human osteosarcoma and non-small cell lung cancers in vivo. Cancer Res. 2004;64:31–34. doi: 10.1158/0008-5472.can-03-3294. [DOI] [PubMed] [Google Scholar]
- McLachlan E, Shao Q, Wang HL, Langlois S, Laird DW. Connexins act as tumor suppressors in three-dimensional mammary cell organoids by regulating differentiation and angiogenesis. Cancer Res. 2006;66:9886–9894. doi: 10.1158/0008-5472.CAN-05-4302. [DOI] [PubMed] [Google Scholar]
- Mesnil M, Crespin S, Avanzo JL, Zaidan-Dagli ML. Defective gap junctional intercellular communication in the carcinogenic process. Biochim. Biophys. Acta. 2005;1719:125–145. doi: 10.1016/j.bbamem.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Mesnil M, Krutovskikh V, Piccoli C, Elfgang C, Traub O, Willecke K, Yamasaki H. Negative growth control of HeLa cells by connexin genes: connexin species specificity. Cancer Res. 1995;55:629–639. [PubMed] [Google Scholar]
- Mitra M, Kandalam M, Verma RS, UmaMaheswari K, Krishnakumar S. Genome-wide changes accompanying the knockdown of Ep-CAM in retinoblastoma. Mol. Vis. 2010;16:828–842. [PMC free article] [PubMed] [Google Scholar]
- Qin H, Shao Q, Curtis H, Galipeau J, Belliveau DJ, Wang T, Alaoui-Jamali MA, Laird DW. Retroviral delivery of connexin genes to human breast tumor cells inhibits in vivo tumor growth by a mechanism that is independent of significant gap junctional intercellular communication. J. Biol. Chem. 2002;277:29132–29138. doi: 10.1074/jbc.M200797200. [DOI] [PubMed] [Google Scholar]
- Ruch RJ. The role of gap junctional intercellular communication in neoplasia. Ann. Clin. Lab. Sci. 1994;24:216–231. [PubMed] [Google Scholar]
- Sanchez-Alvarez R, Paino T, Herrero-Gonzalez S, Medina JM, Tabernero A. Tolbutamide reduces glioma cell proliferation by increasing connexin43, which promotes the up-regulation of p21 and p27 and subsequent changes in retinoblastoma phosphorylation. Glia. 2006;54:125–134. doi: 10.1002/glia.20363. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Regulation of tissue perfusion in mammals by hypoxia-inducible factor. Exp. Physiol. 2007;92:988–991. doi: 10.1113/expphysiol.2006.036343. [DOI] [PubMed] [Google Scholar]
- Shao Q, Wang H, McLachlan E, Veitch GI, Laird DW. Down-regulation of Cx43 by retroviral delivery of small interfering RNA promotes an aggressive breast cancer cell phenotype. Cancer Res. 2005;65:2705–2711. doi: 10.1158/0008-5472.CAN-04-2367. [DOI] [PubMed] [Google Scholar]
- Temme A, Buchmann A, Gabriel HD, Nelles E, Schwarz M, Willecke K. High incidence of spontaneous and chemically induced liver tumors in mice deficient for connexin32. Curr. Biol. 1997;7:713–716. doi: 10.1016/s0960-9822(06)00302-2. [DOI] [PubMed] [Google Scholar]
- Vaupel P, Kelleher DK, Hockel M. Oxygen status of malignant tumors: pathogenesis of hypoxia and significance for tumor therapy. Semin. Oncol. 2001;28:29–35. doi: 10.1016/s0093-7754(01)90210-6. [DOI] [PubMed] [Google Scholar]
- White JB, Taylor RE, Pittler SJ. Reproducible high efficiency gene transfer into Y79 retinoblastoma cells using adenofection. J. Neurosci. Methods. 2001;106:1–7. doi: 10.1016/s0165-0270(00)00368-x. [DOI] [PubMed] [Google Scholar]
- Wong FL, Boice JD, Abramson DH, Tarone RE, Kleinerman RA, Stovall M, Goldman MB, Seddon JM, Tarbell N, Fraumeni JF, Jr, Li FP. Cancer incidence after retinoblastoma. Radiation dose and sarcoma risk. J. Am. Med. Assoc. 1997;278:1262–1267. doi: 10.1001/jama.278.15.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Y79 cells exhibit normal growth characteristics in 1% oxygen. 3×105 Y79 cells were placed in pre-equilibrated normoxic or hypoxic media (100mm dish) and incubated for up to 5 days in normoxia (37°C humidified 21% O2, 5% CO2) or hypoxia (37°C humidified 1% O2, 5% CO2, displaced with N2). Cells grew after a 2 day lag period while maintaining viability under hypoxic growth conditions, indicating that Y79 cells can thrive in a hypoxic environment.
Figure S2. Connexin 46 provides growth protection in 1% oxygen. Mouse neuronal2A cells (N2A) were stably transfected with plasmids encoding either Cx43-EGFP or Cx46-EGFP and then subjected to viability analysis using the CellTiter Blue fluorescence cell viability assay (n = 4, error shown inside shape labels). N2A cells lack endogenous Cx43 and Cx46. N2A cells expressing Cx46 were more viable than control N2A or N2A cells expressing Cx43-GFP after 12, 18, 24 and 36 hours in 1% oxygen.
Figure S3. Optimization of anti-Cx46 siRNA transfection in Y79 cells. (A) Initial studies of 10, 100 or 250 nM siRNA with ug:uL HiPerfect ratios given. Negative control (Neg) at 100 nM, ratio of 1ug siRNA:6 uL HiPerfect. (B) Secondary optimization of siRNA transfection using nM siRNA:uL HiPerfect ratios, as given. A ratio of 250 nM siRNA : 15 uL of HiPerfect used for the remaining in vitro siRNA assays. (C) 30 ug/lane of ARPE-19 and Y79 whole cell lysates used as Cx46 immunoblotting positive controls.
Figure S4. Gross appearance of freshly excised Y79 xenograft tumors. Mice were euthanized and the tumors were immediately excised, measured in three dimensions (mm), imaged and then formalin fixed. Representative tumors shown are from cage 2, mouse 1 and mouse 2 (anti-Cx46 siRNA treated); cage 4, mouse 2 (negative control); cage 5, mouse 2 (sham). Note the reduced amount of visible vascularization present in the anti-Cx46 siRNA treated tumors compared to either the sham or negative control tumor.
Figure S5. Quantitative protein expression densitometry of Cx46 and Cx43 protein levels in representative tumors. Blots were digitized then normalized to β-actin levels and the detected protein expression of Cx46 and Cx43 in different treatment groups was compared, using the no siRNA treatment as a control. A 5-fold reduction in Cx46 and 6-fold rise in Cx43 expression were observed in mice treated with Cx46 siRNA in comparison to mice who received no siRNA treatment. Mice treated with Cx46 siRNA had a 6-fold decrease in Cx46 and 3-fold increase in Cx43 expression compared to mice treated with non-silencing siRNA.
Table S1. Calculated volumes (mm3) of individual xenograft tumors used the proof-of-concept and dosage pilot study. Tumor volumes for individual tumors were measured and recorded on each injection day, then calculated using TV = [L*(W2)]/2. Column listed as “Tumors” shows which mice had confirmed tumors and which data subsequently comprised Figure 4. NT = Measurement not taken for that day; E = Euthanized.
Table S2. Measured volumes (mm3) of individual xenograft tumors used in the primary study. Tumor volumes for individual tumors were measured and recorded on each injection day, then calculated using TV = [L*(W2)]/2. NT = Measurement not taken for that day; E = Euthanized; indicates which tumors were not included in the volume calculations and statistics due to being an outlier; (*) indicates which tumors were used in western blotting experiments.