Abstract
The skin is the largest organ of the body that produces a flexible and self-repairing barrier and protects the body from most common potentially harmful physical, environmental, and biological insults. Solar ultraviolet (UV) radiation is one of the major environmental insults to the skin and causes multi-tiered cellular and molecular events eventually leading to skin cancer. The past decade has seen a surge in the incidence of skin cancer due to changes in life style patterns that have led to a significant increase in the amount of UV radiation that people receive. Reducing excessive exposure to UV radiation is desirable; nevertheless this approach is not easy to implement. Therefore, there is an urgent need to develop novel strategies to reduce the adverse biological effects of UV radiation on the skin. A wide variety of natural agents have been reported to possess substantial skin photoprotective effects. Numerous preclinical and clinical studies have elucidated that natural agents act by several cellular and molecular mechanisms to delay or prevent skin cancer. In this review article, we have summarized and discussed some of the selected natural agents for skin photoprotection.
Keywords: Ultraviolet Radiation, Skin Cancer, Natural Agents, Photoprotection, Recative Oxygen Species, Oxidative Stress
1. Introduction
Skin is the organ most accessible to sunlight, directly suffers from the deleterious effects of ultraviolet (UV) radiation [1–3]. Solar UV radiation is the main cause for the vast majority of cutaneous malignancies in the Caucasian populations. According to an estimate by the American Cancer Society, the incidence of newly diagnosed skin cancer in the United States alone is estimated to exceed 1 million per year [4]. The non-melanoma skin cancers (NMSCs), comprising of the basal cell carcinomas (BCCs) and squamous cell carcinomas (SCCs), are the most frequently diagnosed cutaneous malignancies and account for approximately 80% and 16% respectively, of all skin cancers. However, melanoma the malignant form of skin cancer accounts approximately 4% of all new cases of skin cancers diagnosed in the USA [1,4]. In fact, NMSCs develop almost exclusively on sun-exposed areas of the skin in individuals who burn easily and tan rarely. It is important to mention that among all skin cancers; unquestionably NMSCs are readily treatable and most preventable. Nonetheless, melanoma, while substantially less common, is often fatal.
Earth is continuously irradiated by light photons coming from the sun such as infrared light (780–5000 nm), visible light (400–780 nm), and UV light (290–400 nm). Approximately 5% of the radiant energy from the sun is emitted in the UV range and is divided into three categories dependent on wavelength, UVC (200–280 nm), UVB (280–320 nm) and UVA (320–400 nm). Solar UV radiation is a potent mutagen and both epidemiological and molecular evidence have established it as the main cause of human skin cancers [5,6]. UVC is extremely damaging to the skin because these wavelengths have enormous energy and induces genotoxic stress. Fortunately, UVC is prevented from reaching the earth, as it is largely absorbed by atmospheric ozone layer. On the other hand, UV radiation, especially UVA and UVB, which reaches the earth and penetrates the skin, causes variety of adverse effects [7,8]. UVB constitutes only about 4–5% of UV radiation is thought to be the most active constituent of solar radiation. UVB is more genotoxic and capable of causing much more cell damage than UVA. However, it has less penetrating power than UVA and acts mainly on the epidermal basal layer of the skin. Solar UVB radiation induces sunburn, inflammation, DNA damage, oxidative stress, free radical production, immunosuppression, photoaging and skin cancer [2,9–12].
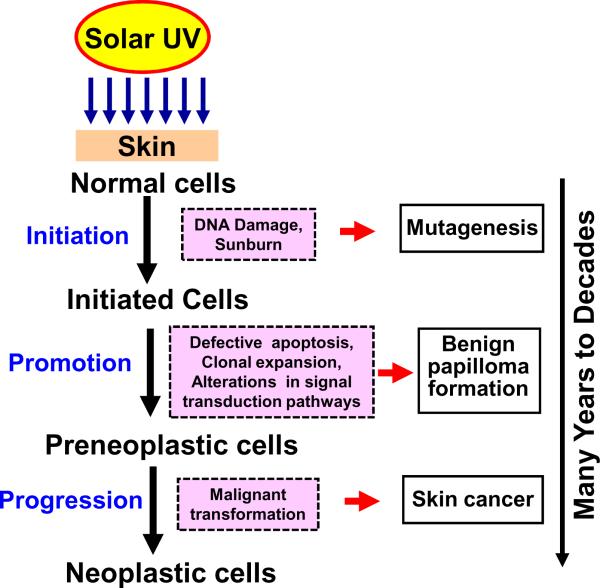
Solar UVB radiation-induced skin cancer development is a multistage process involving three distinct stages exemplified by initiation, promotion and progression (Figure 1). Each of these stages is mediated by means of alterations in various cellular, biochemical and molecular changes. Tumor initiation, the first step in the photocarcinogenesis process involves genetic alterations that ultimately leads to DNA mutation in normal cells, is essentially an irreversible process. Tumor promotion involves clonal expansion of initiated cells by alterations in signal transduction pathways and is considered to be reversible. Tumor progression involves malignant transformation of papillomas to carcinomas [13,14]. As initiation process occurs rapidly and therefore strategies to prevent initiation process by intervention are difficult to envision. Solar UV radiation-induced skin cancer takes many years or even decades to develop, the best opportunity to intervene might be in the tumor promotion or progression phases of carcinogenesis as these steps are the slow and rate limiting stages. This has raised the credibility of photoprotection as a serious and practical approach to control skin cancers.
Figure 1.
Stages of solar UV radiation-induced skin cancer
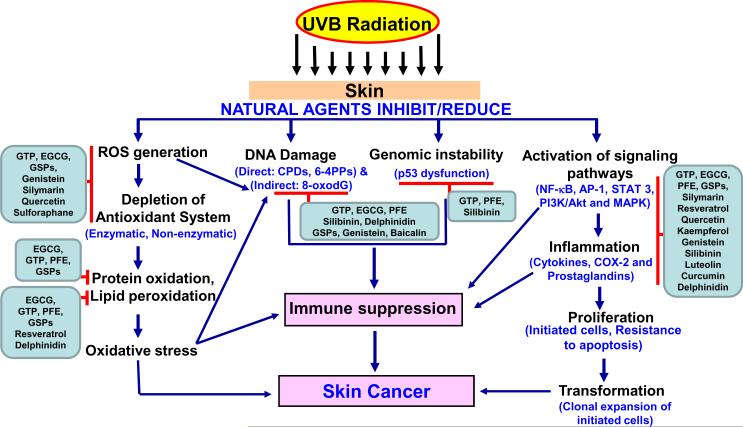
Natural agents, with potential antioxidant, anti-inflammatory, anti-mutagenic, anti-carcinogenic and immunomodulatory properties, and that have the ability to exert striking inhibitory effects on diverse cellular and molecular events are gaining considerable attention for the prevention of UV-induced skin damage [1,2,15]. Because of these properties natural agents are gaining popularity as more and more skin care products containing botanical ingredients are introduced in the market. Individuals can modify their dietary habits and lifestyles in combination with a careful use of skin care products, because exposure to UV radiation is difficult to avoid. It should be emphasized that the approach of using natural agents could be an add-onto the existing strategies of preventing damage from excessive exposure to sun because of their safety, low cost, and oral bioavailability. In this review article the findings from preclinical and clinical studies of natural agents (tea polyphenols, pomegranate, delphinidin, cyanidin, resveratrol, genistein, silymarin, quercetin, luteolin, kampferol, lycopene, sulforaphane, honkiol, caffeine, grape seed proanthocyanidins, and capsiate, etc.) in the photoprotection of skin at the cellular and molecular levels (Figure 2, Table 1) are summarized and discussed.
Figure 2.
Natural agents have the ability to inhibit/reduce the adverse biological effects of UVB radiation.
Table 1.
Effect of natural agents on UVB-mediated modulation in cellular signaling targets
| Commercial and/or Chemical Names |
Source | Cellular Signaling Targets | References |
|---|---|---|---|
| Green tea poyphenols and/or EGCG | Camellia sinensis | Inhibition of UVB-induced phosphorylation of MAPK and NF-κB. Reduction in UVB-induced promoter-binding activities of AP-1 and NF-κB. |
[44,58,104] [105] |
| Pomegranate fruit extract | Pomegranates | Inhibition of UVB-mediated phosphorylation of ERK1/2, JNK1/2 and p38 proteins, degradation and phosphorylation of IκBα, activation of IKKα, nuclear translocation and phosphorylation of NF-κB/p65 and STAT3. | [21, 76, 96, 97] |
| Resveratrol [3,4',5-transtrihydroxystilbene] | Grape skins, Cranberries, Peanuts and Red wine | Reduction in UVB-mediated activation of NF-κB, phosphorylation and degradation of IκBα, and activation of IKKα. | [103] |
| Silibinin [2R,3R)-3,5,7-trihydroxy-2-[(2R,3R)-3-(4-hydroxy-3-methoxyphenyl)- 2-(hydroxymethyl)-2,3-dihydrobenzo[b][1,4] dioxin-6-yl]chroman-4-one] |
Artichoke | Reduction in phosphorylation of STAT 3 (Tyr705) and NF-κB/p65 (Ser536). Inhibition of mitogenic and survival signaling involving AP-1 and NF-κB transcription factors. |
[66] [100] |
| Quercetin [2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one] | Onions | Suppression of UVB-induced transactivation of AP-1, NF-κB and phosphorylation of MAPK. | [99] |
| Genistein [5,7,4-trihydroxyisoflavone] | Soybeans | Prevents UVB-induced enhancement of the DNA-binding activity of AP-1 by acting as a tyrosine kinase inhibitor, thus limiting LPO and increase in ROS formation. Reduce c-fos and c-jun expression. |
[101] [102] |
| Grape seed proanthocyanidins | Grape seeds | Inhibition of UVB-induced activation of MAPK and NF-κB. | [51] |
| Kaempferol [3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one] | Broccoli, Grapes, Sprouts and Apples | Reduction in UVB-induced transcriptional activity of AP-1. Decrease in UVB-induced COX-2 expression by blocking Src kinase activity. |
[62] [62] |
| Caffeic acid [3-(3,4-Dihydroxyphenyl 2-propenoic acid] | White grapes, White wine, Olives, Spinach, Asparagus and Coffee | Inhibition of UVB-induced COX-2 expression through suppression of AP-1 and NF-κB transcription activities. | [68] |
| Capsiate | Sweet peppers | Inhibition of UVB-mediated phosphorylation of ERK1/2 and phosphorylation and nuclear translocation of NF-κB/p65 | [63] |
| Curcumin [(1E,6E)-1,7-bis (4-hydroxy-3-methoxyphenyl) -1,6-heptadiene-3,5-dione] | Turmeric | Inhibition of COX- 2 expression by suppressing p38 MAPK and JNK activities. | [67] |
| Delphinidin [2-(3,4,5-trihydroxyphenyl)chromenylium-3,5,7-triol] | Pomegranates, Grapes and Cranberries | Suppression of UVB-induced COX-2 expression and PGE2 production by blocking MAPKK4 and PI3K pathways and AP-1 and NF-κB activities. | [70] |
| Cyanidin [2-(3,4-Dihydroxyphenyl) chromenylium-3,5,7-triol] | Grapes, Berries and Pomegranates | Inhibition of UVB-induced COX-2 expression and PGE2 secretion by suppressing the transactivation of NF-κB and AP-1. | [71] |
| Luteolin [2-(3,4-Dihydroxyphenyl)- 5,7-dihydroxy-4-chromenone] | Celery, Green pepper, Broccoli, Thyme, Carrots and Olive oil | Inhibition of UVB-induced phosphorylation of MAPK and the Akt signaling pathway. | [107] |
| Sulforaphane [1-Isothiocyanato-4-methylsulfinylbutane] | Broccoli sprouts, Cabbages, Kales and Collards | Inhibition of UVB-mediated activation of p38, ERK and SAPK/JNK. Reduction in UVB-induced AP-1 activation and AP-1 DNA binding activity. |
[73] [108] |
| Honokiol [2-(4-hydroxy-3-prop-2-enyl-phenyl)- 4-prop-2-enyl-phenol] | Cones, bark, and leaves of Magnolia grandifloris | Delay in skin cancer development by activating proapoptotic proteins through both intrinsic and extrinsic pathways. | [106] |
2. Effect of natural agents on UVB-induced DNA damage
UVB-induced DNA damage has been documented as an important molecular trigger for the initiation of UVB-induced carcinogenesis [16]. UVB is directly absorbed by cellular DNA bases leading to the formation of DNA lesions mainly cyclobutane primidine dimers (CPDs) and pyrimidyne-(6-4)-pyrimidone photoproducts [2,17]. Nucleotide excision repair (NER) machinery plays and important role in the repair of these bulky DNA photo products formed after UV irradiation [18]. In contrast, UVB has also been shown to cause oxidation of guanine residues resulting in the formation of 8-oxo-7,8-dihydro-2'-deoxyguanosine (8-oxodG) a process mediated by reactive oxygen species (ROS) and has been proposed as a key biomarker of oxidative DNA damage relevant to carcinogenesis [19]. In response to DNA damage elicited by UVB, cells respond by activation of surveillance mechanisms that leads to cell cycle arrest and DNA repair to prevent genomic instability. However, highly damaged cells are often eliminated by apoptosis as a protective mechanism. Furthermore, cellular responses to DNA-damage by UVB radiation are usually multifaceted and often regulated by multiple signaling pathways, which initiate DNA repair, cell cycle arrest and apoptosis [20].
Recently, we have demonstrated that oral feeding of pomegranate fruit extract (PFE) in drinking water to SKH-1 hairless mice resulted in marked reduction in the number of CPDs and 8-oxodG positive cells and these may be due to enhanced DNA repair. In addition, PFE also enhanced UVB-mediated increase in p53 and p21 proteins, thus shutting off cell replication and DNA synthesis, allowing extended time for DNA repair [21]. Pretreatment of human reconstituted skin with pomegranate-derived products resulted in reduction of UVB-induced formation of CPDs and 8-oxodG [22]. PFE was also effective at protecting human skin fibroblasts from cell death following UV exposure and this is likely related with reduced activation of the pro-inflammatory transcription factor nuclear factor-kappa B (NF-κB), a downregulation of caspase-3, and an increased G0/G1 phase associated with DNA repair [23]. Treatment of HaCaT cells and mouse skin with delphinidin, an anthocyanidin present in pigmented fruits and vegetables, reduced UVB-mediated DNA damage [24]. Pretreatment with anthocyanins reduced UVB-induced ROS levels and inhibited UVB-induced apoptotic cell death through the prevention of caspase-3 pathway activation and reduction of pro-apoptotic Bax protein levels both in vitro and in vivo [25].
Treatment of SKH-1 hairless mice with green tea enhanced UVB-induced expression of p53, p21, and apoptotic sunburn in the epidermis [26]. Oral feeding of green tea to mice as the sole source of drinking fluid starting immediately after discontinuation of UVB treatment enhanced the rate and extent of disappearance of the mutant p53-positive patches [27]. In another study, Kramata et al. [28] have shown that mice treated with green tea during chronic UVB irradiation changed the mutation profile of the p53 gene in early mutant p53 positive epidermal patches. Studies have demonstrated that green tea polyphenols (GTP), and its major constituent (−)- epigallocatechin-3-gallate (EGCG), reduced the risk for skin cancer in a murine model of photocarcinogenesis, and this was accompanied by a reduction in UVB-induced DNA damage. These studies suggest that interleukin-12 (IL-12)-associated reduction in UVB-specific CPDs is due to induction of DNA repair, and particularly enhancement of NER [29,30]. Treatment of human skin with GTP before a single dose of UVB exposure decreased dose dependently the formation of UVB-induced CPDs, and this may be, at least in part, responsible for the inhibition of photocarcinogenesis [31]. Schwarz et al. [32] demonstrated that GTP containing EGCG reduced UVB-induced DNA damage in human cells and this effect appear to be mediated via IL-12, most likely through induction of DNA repair.
Lu et al. [33] have shown that administration of caffeine enhances the removal of DNA-damaged cells by inhibiting the ataxia-telangiectasia mutated- and Rad3-related (ATR)-mediated phosphorylation of Chk1 and prematurely increasing the number of cyclin B1-containing cells. Topical application of resveratrol to SKH-1 hairless mouse skin resulted in inhibition of UVB-induced cellular proliferation, phosphorylation of survivin and upregulation of pro-apoptotic Smac/DIABLO protein [34]. Oral or topical application of silibinin to SKH-1 hairless mice prior to, or immediately after, UV irradiation remarkably reduced UVB-induced CPDs positive cells in epidermis via an activation of p53-p21/Cip1 cascade [35,36]. Pretreatment of human reconstituted skin with genistein prior to UVB irradiation preserved cutaneous proliferation and repair mechanics [37]. Supplementation of grape seed proanthocyanidins (GSPs) with AIN76A control diet significantly reduced the levels of CPD+ cells in UVB-exposed mouse skin of wild type. However GSPs did not significantly reduce UVB-induced CPD+ cells in the skin of IL-12 knockout mice, suggesting that IL-12 is required for the repair of CPDs by GSPs. In addition, GSPs repaired UV-induced CPD+ cells in xeroderma pigmentosum complementation group A (XPA)-proficient fibroblasts from a healthy individual but did not repair in XPA-deficient fibroblasts from XPA patients [38].
Topical application of Baicalin on Balb/C mice skin significantly decreased the amount of epidermal CPDs after UVB irradiation as compared with untreated mice. UVB-induced apoptosis was less pronounced in Baicalin-treated mouse epidermis, which was accompanied by less p53 accumulation and higher Bcl-2/Bax ratio compared to control group [39]. Topical application of olive oil on mouse skin effectively reduced UVB-mediated formation of 8-oxodG, but had no effect on CPDs and (6-4) photoproducts. The reduced formation of UV-induced 8-oxodG in epidermal cells can be attributed to the scavenging activity of olive oil against ROS [40]. Treatment of HaCaT cells with Prunella vulgaris extract and its main phenolic acid component, rosmarinic acid before and after irradiation significantly reduced UVB mediated formation of single strand breaks [41]. Pre- and post-treatment of keratinocytes with Litoria caerulea and Vaccinium myrtillus phenolic fractions efficiently reduced the extent of DNA breakage [42].
3. Effect of natural agents on UVB-mediated formation of reactive oxygen species and oxidative stress
UVB irradiation damages skin cells also through indirect mechanisms by the formation of ROS. Overproduction of ROS results in oxidative stress a process that can serve as an important mediator of damage to cell structures, including lipids and membranes, proteins, and DNA [8,10]. A growing body of evidence suggests that ROS within cells can also act as secondary messengers in intracellular signaling cascades that can induce and maintain the oncogenic phenotype of cancer cells [43]. Paradoxically, the skin cells are rich in ROS detoxifying enzymes and in low-molecular-mass antioxidant molecules, to protect cells against ROS-induced oxidative stress and re-establish or maintain “redox balance” termed also “redox homeostasis”.
Pretreatment of normal human epidermal keratinocytes (NHEK) with EGCG inhibited UVB-induced hydrogen peroxide (H2O2) production and H2O2-mediated phosphorylation of mitogen activated protein kinases (MAPK) signaling pathways. These findings suggest that EGCG could be useful in attenuation of oxidative stress-mediated and MAPK-caused skin disorders in humans [44]. Topical application of EGCG to C3H/HeN mice before a single dose of UVB exposure inhibited UVB-induced H2O2 and nitric oxide (NO) in both epidermis and dermis [45]. Topical treatment of GTP or its most chemopreventive constituent EGCG in hydrophilic ointment before single or multiple UVB exposures resulted in significant prevention of UVB-induced depletion of antioxidant enzymes such as glutathione peroxidase (GPx), catalase and glutathione (GSH) level. Furthermore, treatment of GTP or EGCG also inhibited UVB-induced oxidative stress when measured in terms of lipid peroxidation (LPO) and protein oxidation [46]. Mittal et al. [47] have demonstrated that CD11b+ cell population from UV-irradiated skin resulted in higher production of total H2O2 in both epidermis and dermis in comparison with CD11b− cell population, suggesting a possible role of infiltrating CD11b+ cells in oxidative stress. EGCG treatment to human skin reduced UV-induced production of H2O2 and NO in both epidermis and dermis in a time-dependent manner. In addition, EGCG was found to restore the UV-induced decrease in GSH level and GPx enzyme activity [48].
We have show that pretreatment of human reconstituted skin with pomegranate-derived products reduced UVB-mediated formations of carbonyl groups on proteins. [22]. Treatment of HaCaT cells with delphinidin, inhibited UVB-mediated increase in LPO [24]. Treatment of human dermal fibroblasts with genistein protected against UVB-induced senescence-like characteristics via maintenance of antioxidant enzyme activities and modulation of mitochondrial oxidative stress through down-regulation of a p66Shc-dependent signaling pathway [49]. Caffeic acid, a dietary phenolic compound, treatment significantly reduced the levels of LPO, lipid hydroperoxide, conjugated diene and maintained antioxidant status in UVB-irradiated lymphocytes [50]. Feeding of dietary GSPs to mice exposed to either to acute or chronic UVB irradiation resulted in inhibition of depletion of endogenous antioxidant defense enzymes, such as GPx, GSH, and catalase; suppression of oxidative stress in terms of H2O2 and NO production, and lipid and protein oxidation [51]. Treatment of NHEK with GSPs inhibited UVB-induced H2O2 production, LPO, protein oxidation, and depletion of antioxidant defense components, such as GPx, catalase, superoxide dismutase, and GSH [52].
Application of topical formulations containing quercetin on the dorsal skin of hairless mice inhibited UVB-mediated GSH depletion and proteinases secretion/activity [53]. Single topical application of resveratrol to SKH-1 hairless mice prior to UVB irradiation reduced UVB-mediated skin edema, generation of H2O2, infiltration of leukocytes and LPO [54]. Topical treatment of silymarin to C3H/HeN mice reduced UVB-induced H2O2 producing cells and inducible nitric oxide synthase (iNOS) expressing cells concomitant with decrease in H2O2 and NO production [55]. Prunella vulgaris extract and its main phenolic acid component, rosmarinic acid, treatment reduced ROS production and decreased IL-6 release in HaCaT cells exposed to UVB irradiation. [41]. Studies have shown that lutein accumulated in the skin of mice following dietary supplementation was found to decrease ROS generation following UVR exposure [56]. Dinkova-Kostova et al. [57] have shown that topical application of broccoli sprout extracts containing sulforaphane to the skin of mice and healthy human subjects elevates NAD(P)H:quinone oxidoreductase 1, a representative cytoprotective enzyme.
4. Effect of natural agents on UVB-induced inflammation
UVB-induced inflammatory changes are characterized by the development of edema, production of inflammatory mediators, and infiltration of inflammatory cells and ROS production [2,15]. UVB-mediated inflammation plays an important role in the development of skin cancer by enhancing epidermal hyperplasia through proinflammatory cytokines, growth factors, and induction of the cyclooxygenase-2 (COX-2) enzyme resulting in increased prostaglandin (PG) levels [2,15]. Studies have suggested that ROS produced by UVB irradiation act as a second messangers in the signaling pathways that plays an important role in inflammation [2,10,15]. NF-κB and activator protein-1 (AP-1), such as c-Jun, c-fos act independently or coordinately to regulate expression of target genes involved in inflammation. These transcription factors are regulated by upstream MAPK (such as ERK1/2, p38 and JNK1/2). UVB-induced ROS production is involved in the activation of MAPK in NHEK and is closely related to inflammation and carcinogenesis [1,2,15].
Treatment of SKH-1 hairless mice to GTP resulted in significant decrease in UVB-induced bifold-skin thickness, skin edema and infiltration of leukocytes [58]. Administration of GTP in drinking water to mice significantly reduced UVB-induced markers of inflammation and pro-inflammatory cytokines in chronically UVB-exposed skin and skin tumors [59]. Topical application of EGCG before UVB exposure to human skin significantly blocked UVB-induced infiltration of leukocytes and reduced myeloperoxidase activity. In addition, EGCG significantly reduced PG metabolites, particularly PGE2 [60]. Administration of standardized black tea extract prior to UVB exposure reduced the incidence and severity of erythema in murine and human skin [61].
Kaempferol, a flavonoid, reduced UVB-induced COX-2 protein expression and transcriptional activities of COX-2 and AP-1 in mouse skin epidermal JB6 P+ cells. Furthermore, in vivo data from mouse skin demonstrated that kaempferol suppressed UVB-induced COX-2 expression by blocking Src kinase activity [62]. Capsiate, one of the major capsaicinoids, treatment inhibited UVB-mediated increase in intracellular ROS and phosphorylation of ERK1/2 and phosphorylation and nuclear translocation of NF-κB/p65 in keratinocytes. Capsiate was also found to inhibit UVB-induced expression of COX-2, pro-inflammatory cytokines and potent angiogenic factors in vitro and in vivo [63]. Orally administered lutein and zeaxanthin decreased significantly the edematous cutaneous response as determined by the reduction of the UVB-induced increase of ear bifold thickening. In addition, dietary carotenoids were efficient in reducing the UVB-induced increases in the percentage of PCNA, bromodeoxyuridine and terminal dUTP nick end-labeling-positive cells [64]. Luteolin diminished UVB-induced release of PGE2 and IL-1α in keratinocytes, indicating a protection against the UVB-induced sunburn response [65]. Topical or dietary silibinin treatment to SKH-1 hairless reduced UVB-mediated iNOS and COX-2. In addition, there was a concomitant decrease in phospho-signal transducers and activators of transcription 3 (STAT3) (Tyr705) and phosphoNF-κB/p65 (Ser536), which are potential up-stream regulators of iNOS and COX-2 in mice that received silibinin treatment [66]. Treatment of HaCaT cells with curcumin inhibited COX-2 mRNA and protein expressions, p38 MAPK and JNK activities, as well as DNA-binding activity of AP-1 transcription factor. These results collectively suggest that curcumin may inhibit COX-2 expression by suppressing p38 MAPK and JNK activities [67]. Kang et al. [68] have recently shown that that Fyn, one of the members of the non-receptor protein tyrosine kinase family, is required for UVB-induced COX-2 expression. However, caffeic acid treatment inhibited UVB-induced COX-2 expression and PGE2 production by directly targeting Fyn, both in JB6 P+ cells and mouse skin. In addition, caffeic acid treatment induced the downregulation of COX-2 expression at the transcriptional level through the inhibition of AP-1 and NF-κB transcription activities.
Treatment of HaCaT cells with anthocyanins inhibited UVB-induced COX-2 and PGE2 production through a nuclear NF-κB-dependent pathway and regulation of the PI3K/Akt pathway. Furthermore, topical application of anthocyanins prior to UVB irradiation of hairless mice also reduced induction of COX-2 and PGE2 [69]. Delphinidin treatment suppressed UVB-induced COX-2 expression and PGE2 production in JB6 P+ mouse epidermal cells. These effects were mediated by blocking the MAPKK4 and PI3K pathways and subsequently suppressing AP-1 and NF-κB activities [70]. Cyanidin inhibited UVB-induced COX-2 expression and PGE2 secretion in the JB6 P+ cells by suppressing the transactivation of NF-κB and AP-1 which are regulated by MAPK. Furthermore, this study demonstrates that cyanidin inhibits UVB-induced COX-2 expression in JB6 P+ cells by blocking the MKK4, MEK1, and Raf-1 pathways [71]. Topical treatment of mice with honokiol in a hydrophilic cream-based topical formulation before or after UVB irradiation resulted in significant inhibition of UVB-induced expression of COX-2, PGE2, proliferating cell nuclear antigen and pro-inflammatory cytokines, such as tumor necrosis factor α (TNF-α, interleukin (IL)-1β, and IL-6 in the skin as well as in skin tumors. These results suggest that honokiol hold promise for the prevention of UVB-induced skin cancer by targeting inflammatory mediators [72]. Treatment of HaCaT cells with sulforaphane reduced UVB-induced IL-6, IL-1β, COX-2 and PGE2 levels. Furthermore, sulforaphane also inhibited UVB-mediated activation of p38, ERK and SAPK/JNK, indicating that the inhibition of MAPK by sulforaphane would attenuate the expression of COX-2, thereby reducing inflammatory responses [73].
Topical application of lycopene inhibited UVB-induced ornithine decarboxylase and myeloperoxidase, and significantly reduced bifold skin thickness [74]. Dietary GSPs inhibited UVB-induced infiltration of pro-inflammatory leukocytes and the levels of myeloperoxidase, COX-2, and PGE2 in mouse skin and skin tumors [75]. Oral feeding of PFE to SKH-1 hairless was found to inhibit single and multiple UVB exposure-mediated epidermal hyperplasia, infiltration of leukocytes, LPO, and protein oxidation [21,76]. Studies have shown that a water extract of Zingiber officinale, gingerol, and shogaol inhibited production of cytokines in UVB-irradiated HaCaT cells. In addition, treatment with Z. officinale attenuated UVB-induced hyperplasia, infiltration of leukocytes, and dilation of blood vessels in the dermis of mice [77]. Topical application of standardized black raspberry extract to mice significantly reduced UVB-induced edema and neutrophil activation [78].
Topical application of sulforaphane-rich extracts of broccoli sprouts protected against UVR-induced inflammation and edema in mice, and reduced susceptibility to erythema in humans [79]. Studies have shown that a carotenoid supplement and a combination of the carotenoid supplement and vitamin E provided protection against erythema in humans [80]. A natural dietary tomato paste rich in lycopene protected against UV-induced erythema in humans [81]. Supplementation with tomato-based products increases lycopene levels in human serum and protects against UV-light-induced erythema [82].
5. Effect of natural agents on UVB-mediated immunosuppression
As UVB penetrates into the epidermis, the energy from the photons is absorbed by chromophores (such as DNA, RNA, urocanic acid, protein aromatic amino acids, lipids, melanin, quinones, flavins and porphyrins) in the skin that modify gene expression profiles and alters the immune system [83]. UVB induces immunosupression by formation of pyrimidine dimer and isomerization of trans-urocanic acid to cis-urocanic acid. CPDs formation is the initial molecular step that leads to immune suppression. It is well documented that cis-urocanic acid affects keratinocytes, fibroblasts, Langerhans cells, fibroblasts, T lymphocytes, macrophages and natural killer cells [12,84]. UVB radiation alters cellular redox equilibrium leading to ROS formation and membrane LPO and may also contribute to immunosuppression [12,15,84]. UV exposure has also been shown to suppress wide variety of immune responses, including contact hypersensitivity (CHS) to chemical haptens [85], and delayed-type hypersensitivity to bacterial [86], fungal [87] and viral [88] antigens. CHS is a special experimental form of delayed-type hypersensitivity (DTH) where haptens (e.g. dinitrofluorbenzene or oxazolone) are applied epicutanously to the skin. UVB-induced immunosuppression is generally defined as local and systemic. In local immunosuppression the hapten is applied directly to the UVB-irradiated skin. In systemic immune suppression, the UVB radiation is applied to one site, and the hapten or antigen is applied to unexposed site. It is generally believed that local and systemic immunosuppression may share common mechanisms in the initial stage, however may differ in some later aspects. Experimental evidence suggests that the production of IL-10 from keratinocytes and other target cells in the skin plays a critical role in UVB radiation-induced immune suppression. It has also been shown that IL-10 plays a key role in UVB-mediated systemic suppression of DTH by preventing the secretion of interferon (IFN)-γ by T helper 1 cells or by the preferential expansion of T helper 2 cells [89,90].
Pre- and Post-application of GTP resulted in significant protection against local and systemic suppression of CHS in mouse skin [91]. Topical application of EGCG before a single low dose UVB exposure to C3H/HeN mice prevented UVB-induced inhibition of the contact hypersensitivity response and tolerance induction to the contact sensitizer 2,4-dinitrofluorobenzene (DNFB). In this study, topical application of EGCG blocked UVB-induced infiltration of CD11b+ cells into the skin, and also reduced IL-10 production in skin as well as in draining lymph nodes (DLN). In addition, EGCG markedly increased IL-12 production in DLN [60]. In another study, topical application application of EGCG before UVB exposure inhibited the migration, depletion, or death of antigen presenting cells (APCs) (Ia1cells). This observation suggests that EGCG protects APCs from the adverse effect of UVB irradiation [45].
Lutein-supplemented diet protected C3H/HeJ mice from UVB-induced reduction of the CHS response to DNFB in the local model of UV-induced immune suppression. However, dietary lutein does not prevent the deleterious effects of UVR on CHS in the systemic model of UV-induced immunosuppression [56]. Topical application of silymarin to C3H/HeN mice inhibited UVB-induced suppression of CHS response to contact sensitizer DNFB and was found to be associated with the inhibition of infiltrating leukocytes, particularly CD11b+ cell type, and myeloperoxidase activity as well as suppression of cytokine IL-10 producing cells and its production [55]. Meeran et al. [92], have demonstrated that treatment of C3H/HeN mice with topically applied silymarin or silibinin, a major component of silymarin, markedly inhibited UVB-induced suppression of CHS response in a local model of immunosuppression and had a moderate inhibitory effect in a systemic model of contact hypersensitivity. Silymarin was found to reduce UVB-induced expression of immunosuppressive cytokine, IL-10 in the skin and draining lymph nodes and enhanced the levels of the immunostimulatory cytokine, IL-12. In addition, silymarin treatment did not prevent UVB-induced suppression of the contact hypersensitivity response in IL-12 knockout mice but prevented it in their wild-type counterpart. Dietary GSPs was also found to be effective in reducing UVB-induced increase in the production of IL-10 in skin and draining lymph nodes and enhanced the level of IL-12 in the draining lymph nodes compared with mice that did not receive GSPs [93].
Feeding of lyophilized aged garlic extract incorporated into semipurified powdered diet to hairless mice protected from UVB-induced suppression of CHS [94]. Topical application of black raspberry extract reduced the number of tumor-infiltrating CD4+ T-cells within tumors when compared to vehicle treated mice. However, there was no significant difference in the number of infiltrating CD8+ T-cells in any tumor [78]. Oral administration of quercetin prevented the UV-induced suppression of the CHS and the reduction of the percentage of CD8+ cells in spleen and lymph nodes [95].
6. Effect of natural agents on UVB-mediated modulation in cellular signaling targets
Several studies have shown that UV radiation induces signal transduction pathways, some of which lead to apoptotic cell death while others protect against this process [1,2]. UV-induced responses depend on the type, dose and mode of UV-irradiation, cell type, duration of activation of the pathways and signal crosstalk between pathways. UV radiation causes the activation of members of the MAPK or MAPK family, including ERK1/2, JNK, and p38. UV-induced cell apoptosis and skin-damage are mediated by MAPKs while PI3K/AKT negatively regulates this process. UV can modulate signal transduction through the release of latent growth factors and cytokines from epidermal cells or infiltrating leukocytes that act in an autocrine or paracrine fashion to stimulate intracellular signaling or by modifying the activity of growth factor and cytokine receptors [14,16]. The UV response cause activation of the immediate early genes c-fos and c-jun and transcription factors including AP-1 and NF-κB. UV regulates the immediate early genes by activation of c-Jun and related transcription factors by members of the MAPK family, although many additional signaling pathways and transcription factors are now known to be activated [60,70,71].
Pretreatment of NHEK with PFE inhibited UVB-mediated phosphorylation of ERK1/2, JNK1/2 and p38 proteins, degradation and phosphorylation of IκBα, activation of IKKα, nuclear translocation and phosphorylation of NF-κB/p65 [96]. Oral administration of PFE protected the mouse skin from the adverse effects of single and multiple UVB radiation by modulating UVB-induced signaling pathways including NF-κB, MAPK, c-Jun as well as decreased expression of gelatinase (MMPs-2 and -9), and stromelysin (MMP-3) [21,76]. For relevance of this work to human skin we utilized three dimensional full thickness reconstituted human skin equivalent. Pretreatment of human skin equivalent with PFE resulted in inhibition of UVB-induced phosphorylation of c-Jun, protein expression of c-Fos and various MMPs protein expression [22]. More recently, we reported that PFE protected against UVB-induced skin tumorigenesis in in mouse model of photocarcinogenesis, at least in part, by modulating transcription factors STAT3, NF-κB and HIF-1α leading to decrease in inflammatory and angiogenic responses, and provide a molecular basis for its photochemopreventive effect [97].
Recently, Olsen et al. [98] reported that quercetin induces c-Fos mRNA and protein expression through activation of p38 and cAMP-responsive element binding protein, and also potentiates UVB-induced c-Fos expression in human keratinocyte cell line, HaCaT. However, addition of ascorbic acid in cell culture media stabilizes quercetin and completely prevented both quercetin- and UVB-induced c-fos expression, a cellular event important for the promotion phase of tumor development. Pretreatment of JB6 cells with quercetin reduced UVB-induced transactivation of AP-1, NF-κB and phosphorylation of MAPK. This study suggests that quercitrin contributes to the inhibition of neoplastic transformation by blocking activation of the cellular signaling pathway [99]. Luteolin treatment increased the survival of keratinocytes upon UVB irradiation through inhibition/suppression of the mitochondrial intrinsic apoptotic pathway. However, in malignant keratinocytes luteolin did not affect UVB-induced apoptosis [65]. Silibinin treatment before or immediately after UVB exposure inhibited both mitogenic and survival signaling involving AP-1 and NF-κB transcription factors in JB6 cells [100]. Genistein treatment of cultured human keratinocytes prevented UV-induced enhancement of the DNA-binding activity of AP-1 by acting as a tyrosine kinase inhibitor, thus limiting LPO and increase in ROS formation [101]. Topical application of genistein prior to UVB radiation was found to reduce c-fos and c-jun expression in the SENCAR mouse skin in a dose-dependent manner [102]. Adhami et al. [103] have shown that pretreatment of keratinocyes with resveratrol reduced UVB-mediated activation of NF-κB, phosphorylation and degradation of IκBα, and activation of IKKα in a dose- and time-dependent manner. Treatment of NHEK with EGCG prior to UVB irradiation inhibited UVB-induced oxidative stress mediated phosphorylation of MAPK [44]. In addition, EGCG also suppressed UVB-induced phosphorylation and degradation of IκBα and activation of IKKα and NF-κB in NHEK [104]. Topical application of GTP to SKH-1 hairless mice suppressed UVB-mediated phosphorylation of MAPK and activation of NF-κB [58]. EGCG treatment of human epidermal fibroblasts blocked the UV-induced increase of collagen secretion and collagenase mRNA level and the promoter-binding activities of AP-l and NF-κB [105]. Topical application of honokiol to SKH-1 hairless mice reduced skin cancer development possibly by activating pro-apoptotic proteins through both intrinsic and extrinsic pathways [106]. Recently, Byun et al. [107] have shown that luteolin exerts significant protective effects against UVB-induced skin tumorigenesis in SKH-1 hairless mice by directly suppressing PKCε and c-Src kinase activities and also subsequently inhibited UVB-induced phosphorylation of MAPK and the Akt signaling pathways. Treatment of HCL14 cells with sulforaphane reduced UVB-induced AP-1 activation, and this appears to be at least, in part, due to the direct inhibition of AP-1 DNA-binding activity [108].
7. Conclusions
UV radiation is one of the major and permanent environmental insults to the skin and is thought to contribute to a multiplicity of pathological consequences such as DNA damage, inflammation, ROS production, immunosuppression and photocarcinogenesis. Increased production of ROS results in oxidative stress, a process that can serve as an important mediator of damage to cell structures. Dysregulation of signaling pathways, disturbances in the apoptotic machinery, DNA damage and mutations in critical target genes and immunosuppression thereby results in photocarcinogenesis. The natural agents discussed in this review article abrogate dysfunction of the cellular signaling pathways, disturbances in the apoptotic machinery and various cellular and biochemical processes induced or mediated by the solar UVB radiation. Based on the laboratory and epidemiological studies, it is suggested that natural agents could be effective approach for reducing UV-induced photodamage and other skin disorders in humans by using as dietary sources, and/or supplementing skin care products or sunscreens.
Acknowledgement
This work was supported by Grant from USPHS R21 AT002429-02.
Abbreviations
- (UV)
Ultraviolet
- (CPDs)
Cyclobutane primidine dimers
- (8-oxodG)
8-oxo-7,8-dihydro-2'-deoxyguanosine
- (ROS)
Reactive oxygen species
- (GPx)
Glutathione Peroxidase
- (GSH)
Glutathione
- (IL)
Interleukin
- (NF-κB)
Nuclear Factor-kappa B
- (AP-1)
Activator Protein-1
- (MAPK)
Mitogen Activated Protein Kinases
- (iNOS)
Inducible nitric oxide synthase
- (COX-2)
Cyclooxygenase-2
- (PG)
Prostaglandin
References
- [1].Bowden GT. Nat. Rev. Cancer. 2004;4:23–35. doi: 10.1038/nrc1253. [DOI] [PubMed] [Google Scholar]
- [2].Afaq F, Adhami VM, H Mutat. Res. 2005;571:153–173. doi: 10.1016/j.mrfmmm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- [3].Matsumura Y, Ananthaswamy HN. Toxicol. Appl. Pharmacol. 2004;195:298–308. doi: 10.1016/j.taap.2003.08.019. [DOI] [PubMed] [Google Scholar]
- [4].Jemal A, Siegel R, Xu J, Ward E. CA Cancer J. Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- [5].Brash DE, Ziegler A, Jonason AS, Simon JA, Kunala S, Leffell DJ. J. Investig. Dermatol. Symp. Proc. 1996;1:136–142. [PubMed] [Google Scholar]
- [6].Leffell DJ, Brash DE. Sci. Am. 1996;275:52–33. doi: 10.1038/scientificamerican0796-52. [DOI] [PubMed] [Google Scholar]
- [7].Bachelor MA, Bowden GT. Semin. Cancer Biol. 2004;14:131–138. doi: 10.1016/j.semcancer.2003.09.017. [DOI] [PubMed] [Google Scholar]
- [8].Afaq F, Mukhtar H. Exp. Dermatol. 2006;15:678–684. doi: 10.1111/j.1600-0625.2006.00466.x. [DOI] [PubMed] [Google Scholar]
- [9].Halliday GM, Lyons JG. Photochem Photobiol. 2008;84:272–283. doi: 10.1111/j.1751-1097.2007.00247.x. [DOI] [PubMed] [Google Scholar]
- [10].Bickers DR, Athar M. J. Invest. Dermatol. 2006;126:2565–2575. doi: 10.1038/sj.jid.5700340. [DOI] [PubMed] [Google Scholar]
- [11].Timares L, Katiyar SK, Elmets CA. Photochem. Photobiol. 2008;84:422–436. doi: 10.1111/j.1751-1097.2007.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Katiyar SK, Bergamo BM, Vyalil PK, Elmets CA. J. Photochem. Photobiol. B. 2001;65:109–114. doi: 10.1016/s1011-1344(01)00248-2. [DOI] [PubMed] [Google Scholar]
- [13].Ananthaswamy HN, Pierceall WE. Photochem. Photobiol. 1990;52:1119–1136. doi: 10.1111/j.1751-1097.1990.tb08452.x. [DOI] [PubMed] [Google Scholar]
- [14].Melnikova VO, Ananthaswamy HN. Mutat. Res. 2005;571:91–106. doi: 10.1016/j.mrfmmm.2004.11.015. [DOI] [PubMed] [Google Scholar]
- [15].Nichols JA, Katiyar SK. Arch. Dermatol. Res. 2010;302:71–83. doi: 10.1007/s00403-009-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].de Gruijl FR, Rebel H. Photochem. Photobiol. 2008;84:382–387. doi: 10.1111/j.1751-1097.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- [17].de Gruijl FR, van Kranen HJ, Mullenders LH. J Photochem Photobiol B. 2001;63:19–27. doi: 10.1016/s1011-1344(01)00199-3. [DOI] [PubMed] [Google Scholar]
- [18].Sarasin A. Mutat. Res. 1999;428:5–10. doi: 10.1016/s1383-5742(99)00025-3. [DOI] [PubMed] [Google Scholar]
- [19].Cooke MS, Loft S, Olinski R, Evans MD, Bialkowski K, Wagner JR, Dedon PC, Møller P, Greenberg MM, Cadet J. Chem. Res. Toxicol. 2010;23:705–707. doi: 10.1021/tx1000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Abd Elmageed ZY, Gaur RL, Williams M, Abdraboh ME, Rao PN, Raj MH, Ismail FM, Ouhtit A. J. Invest. Dermatol. 2009;129:175–183. doi: 10.1038/jid.2008.208. [DOI] [PubMed] [Google Scholar]
- [21].Afaq F, Khan N, Syed DN, Mukhtar H. Photochem. Photobiol. 2010 Sep 23; doi: 10.1111/j.1751-1097.2010.00815.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Afaq F, Zaid MA, Khan N, Dreher M, Mukhtar H. Exp. Dermatol. 2009;18:553–561. doi: 10.1111/j.1600-0625.2008.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pacheco-Palencia LA, Noratto G, Hingorani L, Talcott ST, Mertens-Talcott SU. J. Agric. Food Chem. 2008;56:8434–8441. doi: 10.1021/jf8005307. [DOI] [PubMed] [Google Scholar]
- [24].Afaq F, Syed DN, Malik A, Hadi N, Sarfaraz S, Kweon MH, Khan N, Zaid MA, Mukhtar H. J. Invest. Dermatol. 2007;127:222–232. doi: 10.1038/sj.jid.5700510. [DOI] [PubMed] [Google Scholar]
- [25].Tsoyi K, Park HB, Kim YM, Chung JI, Shin SC, Shim HJ, Lee WS, Seo HG, Lee JH, Chang KC, Kim HJ. J. Agric. Food Chem. 2008;56:10600–10605. doi: 10.1021/jf802112c. [DOI] [PubMed] [Google Scholar]
- [26].Lu YP, Lou YR, Li XH, Xie JG, Brash D, Huang MT, Conney AH. Cancer Res. 2000;60:4785–4791. [PubMed] [Google Scholar]
- [27].Lu YP, Lou YR, Liao J, Xie JG, Peng QY, Yang CS, Conney AH. Carcinogenesis. 2005;26:1465–1472. doi: 10.1093/carcin/bgi086. [DOI] [PubMed] [Google Scholar]
- [28].Kramata P, Lu YP, Lou YR, Cohen JL, Olcha M, Liu S, Conney AH. Carcinogenesis. 2005;26:1965–1974. doi: 10.1093/carcin/bgi162. [DOI] [PubMed] [Google Scholar]
- [29].Meeran SM, Mantena SK, Katiyar SK. Clin. Cancer Res. 2006;12:2272–2280. doi: 10.1158/1078-0432.CCR-05-2672. [DOI] [PubMed] [Google Scholar]
- [30].Meeran SM, Mantena SK, Elmets CA, Katiyar SK. Cancer Res. 2006;66:5512–5520. doi: 10.1158/0008-5472.CAN-06-0218. [DOI] [PubMed] [Google Scholar]
- [31].Katiyar SK, Perez A, Mukhtar H. Clin. Cancer Res. 2000;6:3864–3869. [PubMed] [Google Scholar]
- [32].Schwarz A, Maeda A, Gan D, Mammone T, Matsui MS, Schwarz T. Photochem. Photobiol. 2008;84:350–355. doi: 10.1111/j.1751-1097.2007.00265.x. [DOI] [PubMed] [Google Scholar]
- [33].Lu YP, Lou YR, Peng QY, Xie JG, Nghiem P, Conney AH. Cancer Res. 2008;68:2523–2529. doi: 10.1158/0008-5472.CAN-07-5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Aziz MH, Afaq F, Ahmad N. Photochem. Photobiol. 2005;81:25–31. doi: 10.1562/2004-08-13-RA-274. [DOI] [PubMed] [Google Scholar]
- [35].Dhanalakshmi S, Mallikarjuna GU, Singh RP, Agarwal R. Carcinogenesis. 2004;25:1459–1465. doi: 10.1093/carcin/bgh152. [DOI] [PubMed] [Google Scholar]
- [36].Gu M, Dhanalakshmi S, Singh RP, Agarwal R. Cancer Epidemiol. Biomarkers Prev. 2005;14:1344–1349. doi: 10.1158/1055-9965.EPI-04-0664. [DOI] [PubMed] [Google Scholar]
- [37].Moore JO, Wang Y, Stebbins WG, Gao D, Zhou X, Phelps R, Lebwohl M, Wei H. Carcinogenesis. 2006;27:1627–1635. doi: 10.1093/carcin/bgi367. [DOI] [PubMed] [Google Scholar]
- [38].Vaid M, Sharma SD, Katiyar SK. Cancer Prev. Res. (Phila) 2010 Oct 8; doi: 10.1158/1940-6207.CAPR-10-0137. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [39].Bing-Rong Z, Song-Liang J, Xiao EC, Xiang-Fei L, Bao-Xiang C, Jie G, Dan L. Photodermatol. Photoimmunol. Photomed. 2008;24:175–182. doi: 10.1111/j.1600-0781.2008.00356.x. [DOI] [PubMed] [Google Scholar]
- [40].Budiyanto A, Ahmed NU, Wu A, Bito T, Nikaido O, Osawa T, Ueda M, Ichihashi M. Carcinogenesis. 2000;21:2085–2090. doi: 10.1093/carcin/21.11.2085. [DOI] [PubMed] [Google Scholar]
- [41].Vostálová J, Zdarilová A, Svobodová A. Arch. Dermatol. Res. 2010;302:171–181. doi: 10.1007/s00403-009-0999-6. [DOI] [PubMed] [Google Scholar]
- [42].Svobodová A, Zdarilová A, Vostálová J. J. Dermatol. Sci. 2009;56:196–204. doi: 10.1016/j.jdermsci.2009.08.004. [DOI] [PubMed] [Google Scholar]
- [43].Dröge W. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- [44].Katiyar SK, Afaq F, Azizuddin K, Mukhtar H. Toxicol Appl Pharmacol. 2001;176:110–117. doi: 10.1006/taap.2001.9276. [DOI] [PubMed] [Google Scholar]
- [45].Katiyar SK, Mukhtar H. J. Leukoc Biol. 2001;69:719–726. [PubMed] [Google Scholar]
- [46].Vayalil PK, Elmets CA, Katiyar SK. Carcinogenesis. 2003;24:927–936. doi: 10.1093/carcin/bgg025. [DOI] [PubMed] [Google Scholar]
- [47].Mittal A, Elmets CA, Katiyar SK. Photochem. Photobiol. 2003;77:259–264. doi: 10.1562/0031-8655(2003)077<0259:ccatms>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- [48].Katiyar SK, Afaq F, Perez A, Mukhtar H. Carcinogenesis. 2001;22:287–294. doi: 10.1093/carcin/22.2.287. [DOI] [PubMed] [Google Scholar]
- [49].Wang YN, Wu W, Chen HC, Fang H. J. Dermatol. Sci. 2010;58:19–27. doi: 10.1016/j.jdermsci.2010.02.002. [DOI] [PubMed] [Google Scholar]
- [50].Prasad NR, Jeyanthimala K, Ramachandran S. J. Photochem. Photobiol B. 2009;95:196–203. doi: 10.1016/j.jphotobiol.2009.03.007. [DOI] [PubMed] [Google Scholar]
- [51].Sharma SD, Meeran SM, Katiyar SK. Mol. Cancer Ther. 2007;6:995–1005. doi: 10.1158/1535-7163.MCT-06-0661. [DOI] [PubMed] [Google Scholar]
- [52].Mantena SK, Katiyar SK. Free Radic. Biol. Med. 2006;40:1603–1614. doi: 10.1016/j.freeradbiomed.2005.12.032. [DOI] [PubMed] [Google Scholar]
- [53].Casagrande R, Georgetti SR, Verri WA, Jr, Dorta DJ, dos Santos AC, Fonseca MJ. J. Photochem. Photobiol. B. 2006;84:21–27. doi: 10.1016/j.jphotobiol.2006.01.006. [DOI] [PubMed] [Google Scholar]
- [54].Afaq F, Adhami VM, Ahmad N. Toxicol. Appl. Pharmacol. 2003;186:28–37. doi: 10.1016/s0041-008x(02)00014-5. [DOI] [PubMed] [Google Scholar]
- [55].Katiyar SK. Int. J. Oncol. 2002;21:1213–1222. [PubMed] [Google Scholar]
- [56].Lee EH, Faulhaber D, Hanson KM, Ding W, Peters S, Kodali S, Granstein RD. J. Invest. Dermatol. 2004;122:510–517. doi: 10.1046/j.0022-202X.2004.22227.x. [DOI] [PubMed] [Google Scholar]
- [57].Dinkova-Kostova AT, Jenkins SN, Fahey JW, Ye L, Wehage SL, Liby KT, Stephenson KK, Wade KL, Talalay P. Cancer Lett. 2006;240:243–252. doi: 10.1016/j.canlet.2005.09.012. [DOI] [PubMed] [Google Scholar]
- [58].Afaq F, Ahmad N, Mukhtar H. Oncogene. 2003;22:9254–9264. doi: 10.1038/sj.onc.1207035. [DOI] [PubMed] [Google Scholar]
- [59].Meeran SM, Akhtar S, Katiyar SK. J. Invest. Dermatol. 2009;129:1258–1270. doi: 10.1038/jid.2008.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Katiyar SK, Challa A, McCormick TS, Cooper KD, Mukhtar H. Carcinogenesis. 1999;20:2117–2124. doi: 10.1093/carcin/20.11.2117. [DOI] [PubMed] [Google Scholar]
- [61].Zhao J, Jin X, Yaping E, Zheng ZS, Zhang YJ, Athar M, DeLeo VA, Mukhtar H, Bickers DR, Wang ZY. Photochem. Photobiol. 1999;70:637–644. doi: 10.1562/0031-8655(1999)070<0637:peobte>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- [62].Lee KM, Lee KW, Jung SK, Lee EJ, Heo YS, Bode AM, Lubet RA, Lee HJ, Dong Z. Biochem. Pharmacol. 2010;80:2042–2049. doi: 10.1016/j.bcp.2010.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lee EJ, Jeon MS, Kim BD, Kim JH, Kwon YG, Lee H, Lee YS, Yang JH, Kim TY. Free Radic. Biol. Med. 2010;48:1133–1143. doi: 10.1016/j.freeradbiomed.2010.01.034. [DOI] [PubMed] [Google Scholar]
- [64].González S, Astner S, An W, Goukassian D, Pathak MA MA. J. Invest. Dermatol. 2003;121:399–405. doi: 10.1046/j.1523-1747.2003.12355.x. [DOI] [PubMed] [Google Scholar]
- [65].Verschooten L, Smaers K, Van Kelst S, Proby C, Maes D, Declercq L, Agostinis P, Garmyn M. J. Invest. Dermatol. 2010;130:2277–2285. doi: 10.1038/jid.2010.124. [DOI] [PubMed] [Google Scholar]
- [66].Gu M, Singh RP, Dhanalakshmi S, Agarwal C, Agarwal R. Cancer Res. 2007;67:3483–3491. doi: 10.1158/0008-5472.CAN-06-3955. [DOI] [PubMed] [Google Scholar]
- [67].Cho JW, Park K, Kweon GR, Jang BC, Baek WK, Suh MH, Kim CW, Lee KS, Suh SI. Exp. Mol. Med. 2005;37:186–192. doi: 10.1038/emm.2005.25. [DOI] [PubMed] [Google Scholar]
- [68].Kang NJ, Lee KW, Shin BJ, Jung SK, Hwang MK, Bode AM, Heo YS, Lee HJ, Dong Z. Carcinogenesis. 2009;30:321–330. doi: 10.1093/carcin/bgn282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Tsoyi K, Park HB, Kim YM, Chung JI, Shin SC, Lee WS, Seo HG, Lee JH, Chang KC, Kim HJ. J. Agric. Food. Chem. 2008;56:8969–8974. doi: 10.1021/jf801345c. [DOI] [PubMed] [Google Scholar]
- [70].Kwon JY, Lee KW, Kim JE, Jung SK, Kang NJ, Hwang MK, Heo YS, Bode AM, Dong Z, Lee HJ. Carcinogenesis. 2009;30:1932–1940. doi: 10.1093/carcin/bgp216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kim JE, Kwon JY, Seo SK, Son JE, Jung SK, Min SY, Hwang MK, Heo YS, Lee KW, Lee HJ. Biochem. Pharmacol. 2010;79:1473–1482. doi: 10.1016/j.bcp.2010.01.008. [DOI] [PubMed] [Google Scholar]
- [72].Vaid M, Sharma SD, Katiyar SK. Carcinogenesis. 2010 Sep 7; doi: 10.1093/carcin/bgq186. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [73].Shibata A, Nakagawa K, Yamanoi H, Tsuduki T, Sookwong P, Higuchi O, Kimura F, Miyazawa T. J. Nutr. Biochem. 2010;21:702–709. doi: 10.1016/j.jnutbio.2009.04.007. [DOI] [PubMed] [Google Scholar]
- [74].Fazekas Z, Gao D, Saladi RN, Lu Y, Lebwohl M, Wei H. Protective effects of lycopene against ultraviolet B-induced photodamage. Nutr. Cancer. 2003;47:181–187. doi: 10.1207/s15327914nc4702_11. [DOI] [PubMed] [Google Scholar]
- [75].Sharma SD, Katiyar SK. Pharm. Res. 2010;27:1092–1102. doi: 10.1007/s11095-010-0050-9. [DOI] [PubMed] [Google Scholar]
- [76].Afaq F, Zaid MA, Khan N, Syed D, Hafeez BB, Yun J, Sarfaraz S, Mukhtar H. Am. Assoc. Cancer Res. Vol. 2573. Los Angeles, CA: 2007. p. 613. [Google Scholar]
- [77].Guahk GH, Ha SK, Jung HS, Kang C, Kim CH, Kim YB, Kim SY. J. Med. Food. 2010;13:673–680. doi: 10.1089/jmf.2009.1239. [DOI] [PubMed] [Google Scholar]
- [78].Duncan FJ, Martin JR, Wulff BC, Stoner GD, Tober KL, Oberyszyn TM, Kusewitt DF, Van Buskirk AM. Cancer Prev. Res. (Phila) 2009;2:665–672. doi: 10.1158/1940-6207.CAPR-08-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Talalay P, Fahey JW, Healy ZR, Wehage SL, Benedict AL, Min C, Dinkova-Kostova AT. Proc. Natl. Acad. Sci. USA. 2007;104:17500–17505. doi: 10.1073/pnas.0708710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Stahl W, Heinrich U, Jungmann H, Sies H, Tronnier H. Am. J. Clin. Nutr. 2000;71:795–798. doi: 10.1093/ajcn/71.3.795. [DOI] [PubMed] [Google Scholar]
- [81].Stahl W, Heinrich U, Wiseman S, Eichler O, Sies H, Tronnier H. J. Nutr. 2001;131:1449–1451. doi: 10.1093/jn/131.5.1449. [DOI] [PubMed] [Google Scholar]
- [82].Aust O, Stahl W, Sies H, Tronnier H, Heinrich U. Int. J. Vitam. Nutr. Res. 2005;75:54–60. doi: 10.1024/0300-9831.75.1.54. [DOI] [PubMed] [Google Scholar]
- [83].Trautinger F. Clin. Exp. Dermatol. 2001;26:573–577. doi: 10.1046/j.1365-2230.2001.00893.x. [DOI] [PubMed] [Google Scholar]
- [84].Schade N, Esse C, Krutmann J. Photochem. Photobiol. Sci. 2005;4:699–708. doi: 10.1039/b418378a. [DOI] [PubMed] [Google Scholar]
- [85].Jessup JM, Hanna N, Palaszynski E, Kripke ML. Cell Immunol. 1978;38:105–115. doi: 10.1016/0008-8749(78)90036-9. [DOI] [PubMed] [Google Scholar]
- [86].Jeevan A, Kripke ML. Effect of a single exposure to ultraviolet radiation on Mycobacterium bovis bacillus Calmette–Guerin infection in mice. J. Immunol. 1989;143:2837–2843. [PubMed] [Google Scholar]
- [87].Denkins Y, Fidler IJ, Kripke ML. Photochem. Photobiol. 1989;49:615–619. doi: 10.1111/j.1751-1097.1989.tb08432.x. [DOI] [PubMed] [Google Scholar]
- [88].Howie SEM, Norval M, Maingay J. J. Invest. Dermatol. 1986;86:125–128. doi: 10.1111/1523-1747.ep12284128. [DOI] [PubMed] [Google Scholar]
- [89].Mosmann TR, Schumacher JH, Street NF, Buss R, O'Garra A, Fong TAT, Bond MW, Moore KW, Sher A, Fiorentino DF. Immunol. Rev. 1991;123:209–229. doi: 10.1111/j.1600-065x.1991.tb00612.x. [DOI] [PubMed] [Google Scholar]
- [90].Rivas JM, Ullrich SE. J. Immunol. 1992;149:3865–3871. [PubMed] [Google Scholar]
- [91].Katiyar SK, Elmets CA, Agarwal R, Mukhtar H. Photochem. Photobiol. 1995;62:855–861. doi: 10.1111/j.1751-1097.1995.tb09147.x. [DOI] [PubMed] [Google Scholar]
- [92].Meeran SM, Katiyar S, Elmets CA, Katiyar SK. Mol. Cancer Ther. 2006;5:1660–1668. doi: 10.1158/1535-7163.MCT-06-0095. [DOI] [PubMed] [Google Scholar]
- [93].Sharma SD, Katiyar SK. Carcinogenesis. 2006;27:95–102. doi: 10.1093/carcin/bgi169. [DOI] [PubMed] [Google Scholar]
- [94].Reeve VE, Bosnic M, Rozinova E, Boehm-Wilcox C. Photochem. Photobiol. 1993;58:813–817. doi: 10.1111/j.1751-1097.1993.tb04975.x. [DOI] [PubMed] [Google Scholar]
- [95].Steerenberg PA, Garssen J, Dortant PM, van der Vliet H, Geerse E, Verlaan AP, Goettsch WG, Sontag Y, Bueno-de-Mesquita HB, Van Loveren H. Cancer Lett. 1997;114:187–189. doi: 10.1016/s0304-3835(97)04659-4. [DOI] [PubMed] [Google Scholar]
- [96].Afaq F, Malik A, Syed D, Maes D, Matsui MS, Mukhtar H. Photochem Photobiol. 2005;81:38–45. doi: 10.1562/2004-08-06-RA-264. [DOI] [PubMed] [Google Scholar]
- [97].Afaq F, Zaid MA, Khan N, Syed DN, Yun J, Sarfaraz S, Suh Y, Mukhtar H. Proc Amer Assoc Cancer Res. 2008;49:1246. [Google Scholar]
- [98].Olson ER, Melton T, Dickinson SE, Dong Z, Alberts DS, Bowden GT. Cancer Prev. Res. (Phila) 2010;3:876–884. doi: 10.1158/1940-6207.CAPR-09-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Ding M, Zhao J, Bowman L, Lu Y, Shi X. Int. J. Oncol. 2010;36:59–67. [PubMed] [Google Scholar]
- [100].Singh RP, Dhanalakshmi S, Mohan S, Agarwal C, Agarwal R. Mol. Cancer Ther. 2006;5:1145–1153. doi: 10.1158/1535-7163.MCT-05-0478. [DOI] [PubMed] [Google Scholar]
- [101].Maziere C, Dantin F, Dubois F, Santus R, Maziere J. Free Radic. Biol. Med. 2000;28:1430–1437. doi: 10.1016/s0891-5849(00)00264-1. [DOI] [PubMed] [Google Scholar]
- [102].Wang Y, Zhang X, Lebwohl M, DeLeo V, Wei H. Carcinogenesis. 1998;19:649–654. doi: 10.1093/carcin/19.4.649. [DOI] [PubMed] [Google Scholar]
- [103].Adhami VM, Afaq F, Ahmad N. Neoplasia. 2003;5:74–82. doi: 10.1016/s1476-5586(03)80019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Afaq F, Adhami VM, Ahmad N, Mukhtar H. Oncogene. 2003;22:1035–1044. doi: 10.1038/sj.onc.1206206. [DOI] [PubMed] [Google Scholar]
- [105].Kim J, Hwang JS, Cho YK, Han Y, Jeon YJ, Yang KH. Skin Pharmacol. Appl. Skin Physiol. 2001;14:11–19. doi: 10.1159/000056329. [DOI] [PubMed] [Google Scholar]
- [106].Chilampalli S, Zhang X, Fahmy H, Kaushik RS, Zeman D, Hildreth MB, Dwivedi C. Anticancer Res. 2010;30:777–783. [PubMed] [Google Scholar]
- [107].Byun S, Lee KW, Jung SK, Lee EJ, Hwang MK, Lim SH, Bode AM, Lee HJ, Dong Z. Cancer Res. 2010;70:2415–2423. doi: 10.1158/0008-5472.CAN-09-4093. [DOI] [PubMed] [Google Scholar]
- [108].Zhu M, Zhang Y, Cooper S, Sikorski E, Rohwer J, Bowden GT. Mol. Carcinog. 2004;41:179–186. doi: 10.1002/mc.20052. [DOI] [PubMed] [Google Scholar]