Abstract
Acute in vivo measurements are often the initial, most practicable approach used to investigate the effects of novel compounds or genetic manipulations on the regulation of gastric motility. Such acute methods typically involve either surgical implantation of devices or require intragastric perfusion of solutions, which can substantially alter gastric activity and may require extended periods of time to allow stabilization or recovery of the preparation. We validated a simple, non-invasive novel method to measure acutely gastric contractility, using a solid-state catheter pressure transducer inserted orally into the gastric corpus, in fasted, anesthetized rats or mice. The area under the curve of the phasic component (pAUC) of intragastric pressure (IGP) was obtained from continuous manometric recordings of basal activity and in responses to central or peripheral activation of cholinergic pathways, or to abdominal surgery. In rats, intravenous ghrelin or intracisternal injection of the thyrotropin-releasing hormone agonist, RX-77368, significantly increased pAUC while coeliotomy and caecal palpation induced a rapid onset inhibition of phasic activity lasting for the 1-h recording period. In mice, RX-77368 injected into the lateral brain ventricle induced high-amplitude contractions, and carbachol injected intraperitoneally increased pAUC significantly, while coeliotomy and caecal palpation inhibited baseline contractile activity. In wild-type mice, cold exposure (15-min) increased gastric phasic activity and tone, while there was no gastric response in corticotorpin releasing factor (CRF)-over-expressing mice, a model of chronic stress. Thus, the novel solid-state manometric approach provides a simple, reliable means for acute pharmacological studies of gastric motility effects in rodents. Using this method we established in mice that the gastric motility response to central vagal activation is impaired under chronic expression of CRF.
Keywords: cold, CRF; ghrelin; intragastric pressure; surgery; TRH agonist
1. Introduction
Gastric motility has been widely investigated as an end point parameter to characterize impaired patterns of gut activity associated with a variety of functional gastrointestinal disorders in humans [47]. In experimental studies, the regulation of gastric motility and the influence of drugs on gastric propagative events have been assessed using several animal models and methodologies to record motility [45,46,70]. Among them, rodents are presently preferentially selected over dogs or pigs for a variety of ethical and technical reasons, as well as the availability of well-defined rodent brain atlases to investigate central neural regulation of gastric motility and the availability of genetically-modified mice [32].
In rats, however, most acute preparations to monitor gastric motility are invasive and entail surgical interventions to implant or introduce intragastric monitoring devices. Intra-gastric pressure (IGP) recording using either an intraluminal pressure transducer, or balloon positioned intragastrically requires laparotomy and opening of the stomach [12,53]. A stable baseline intraluminal pressure is typically then established by intragastric injection of fluids under conditions of pyloric or duodenal ligation [12,53]. Likewise, side-hole catheter manometry requires a constant liquid perfusion into the stomach and implantation of the catheter through a gastric fistula [31]. Non-manometric methods for measuring gastric motility in rats include the use of strain-gauge force transducers [7,9,33], electromyography electrodes [6,41] or ultrasonomicrometry piezoelectric crystals [1]. While all of these non-manometric methods are useful, they involve the opening of the abdominal cavity for surgical implantation of transducers, electrodes or piezoelectric crystals onto the gastric wall. It is well-established that abdominal surgery and manipulation of the gut alter the patterns of gastric motility and induce immunological changes [4,19]. More importantly, although there is an increasing use of genetically modified mice to assess gut function [32,67], gastric motility recording methods used in rats have been so far applied to mice only in a few reports [43,62,73].
In mice the assessment of gastric propulsive activity is typically derived from the monitoring of gastric emptying of liquid or solid meal [10,38,67], and from in vitro studies on gastric relaxation by recording from isolated mouse stomach preparations [2,32,44] or motility from isolated muscular strips [17,32]. Recent studies have recorded changes in gastric motility in mice, however all required abdominal surgery either to implant electrodes or strain gauges on serosal surface of the gastric antrum [57,73] or to insert a deflated balloon with tubing into the stomach through a small incision into the fundus [43,62].
In the present study, we first validated the use of a novel non-invasive method to monitor phasic gastric contractility using micro-tip pressure transducer catheter inserted into the gastric cavity via oral intubation to avoid any surgical procedures in anesthetized rats. We first tested treatments that are reproducibly known to stimulate gastric contractions in rats through vagal and/or peripheral cholinergic pathways namely intracisternal (ic) injection of the stable thyrotropin-releasing hormone (TRH) agonist, RX-77368 [9,21,55], and intravenous (iv) ghrelin [40,48,66] and extended these treatment to mice. We then assessed the inhibitory influence of abdominal surgery on basal gastric contractility in rats and mice which so far has not been investigated in naïve rodent without prior surgical interventions to record gastric motility. Lastly, we investigated in mice the gastric contractile response to central vagal stimulation induced by acute cold exposure [39] in corticotropin releasing factor overexpressing (CRF-OE) mice, an experimental model which displays behavioral, endocrine, immunological, autonomic and visceral phenotype of chronic stress [11,15,42,59,60].
2. Material and methods
2.1. Animals
Adult male mice (C57BL/6) and Sprague Dawley rats (250–300 g) were obtained from Harlan (San Diego, CA). The female and male CRF-OE mice (UCLA animal Core, Dr. M. Fanzelow) were derived from the transgenic line generated on C57BL/6 X SJL background by Stenzel-Poor et al. [59,60] and backcrossed over 10 generations onto C57BL/6. Their wild type littermates were used as control. Animals were housed 3-4/cages and maintained under controlled conditions of temperature (20–24 °C) and light (6AM on: 6 PM off) and fed ad libitum with standard rodent chow (Prolab RMH 2500-5P14; Ralson-Purina LabDiet, St. Louis, MO) and tap water. All protocols were approved by the Veterans Administration Animal Component of Research Protocols 9906-820 in accordance with principles and procedures outlined in the National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals.
2.2. Substances and treatments
Aliquots of human ghrelin (Dept. of Chemistry, Québec University, Montréal, Canada) and of the stable TRH analog, RX-77368 (Ferring Pharmaceuticals, Feltham, Middlesex, UK) were stored at −80 °C and diluted in saline before use. Carbachol (Sigma, St Louis, MO) in powder form was stored at 4°C and dissolved in saline immediately before use. Intravenous (iv) injections in rats were made in 0.1 ml/rat followed by 0.1 ml flush. Intracisternal (ic) injection in rats was performed through an ic catheter (Intramedics PE-10 polyethylene tubing, 0.28 mm ID, 0.61 mm OD, and 4 cm length) introduced into the cisterna magna and secured to the occipital brain membrane with instant Krazy® glue in rats positioned in a stereotaxic instrument (Model 1404, David Kopf Instruments, Tujanga, CA, USA) as previously described [9]. Occurrence of clear cerebrospinal fluid into the lumen of the catheter ascertained successful intracisternal cannulation. The end of the catheter was connected to a Hamilton microliter syringe and ic injection was made in a volume of 10 μl/rat followed by 10 μl flush with saline (0.9% NaCl). In mice, intracerebroventricular (icv) injection of 5 μl was performed manually with a 10 μl Hamilton syringe as detailed in our previous studies [37] and intraperitoneal (ip) injection was delivered in 100 μl/mouse.
2.3. Intra-gastric pressure recording in rats and mice
2.3.1. Animal preparation
In urethane (25%, 1.5 g/kg, ip) anesthetized rats or mice, a miniaturized solid-state pressure transducer catheter (Mikro-Tip catheter model SPR-524, Millar Instruments, Houston, TX) was inserted into the gastric lumen through the mouth. The catheter had a total length of 100 cm, a diameter of 0.75 mm, and was tipped with a transducer capsule approximately 4.5 mm long, 1.1 mm in diameter, and having an active transducer zone approximately 1 mm in length situated in the center of the capsule. The capsule was placed ~ 0.5 cm and ~ 1 cm below the lower esophageal sphincter (LES), in mice and rats respectively. The position of the LES was identified by observing the elevated pressure registered by the transducer as it passed through it. Occasionally (1/10 cases), phenomenon of high amplitude respiratory signal which we attributed to contact/shearing between the gastric wall and the active zone of the transducer occurred which we addressed by gently twisting the catheter until the respiratory excursions return to their typical signal. In rat studies, the trachea was cannulated prior pressure catheter insertion (PE-240 catheter, Intramedics), and an iv catheter (Intramedics PE-90 polyethylene tubing, 0.86 mm ID, 1.27 mm OD, and 8 cm length) was inserted into the right external jugular vein to allow continuous sterile saline infusion (0.4 ml/h) to maintain hydration and perform iv injections.
2.3.2. Data acquisition and analysis
Each miniature pressure transducer catheter (MPTC) was connected to a transducer control unit (model TCB-600; Millar Instruments, Houston, TX), whose output signal was subsequently amplified further using a transducer amplifier in differential mode (TBM4, World Precision Instruments, Boca Raton, FL). This output was acquired via a Micro1401 mk II A/D interface (Cambridge Electronic Design) and recorded using Spike 2 version 5 data acquisition software. After smoothing raw traces (2 s time constant) to reduce the contribution of the respiratory component of IGP, phasic and tonic components of IGP were determined using a modification of methods previously described [1,23].
The phasic component of IGP was obtained by removing the DC component of the trace using the DC remove channel process available in Spike2, with a time constant of 10 s, after first reducing the respiratory component of the original trace by smoothing with a 1 s time constant. A 1 s time constant for smoothing is equivalent to taking the mean value over a 2 s window centered around each point. The “area under the curve” (AUC) of this phasic component (pAUC) was then determined every minute using the Spike2 “modulus” function, within a custom script written by us (DWA) for the purpose. Thus, pAUC is a reflection of the magnitude of excursions events whose rate of change is in the interval 2–20 s, which includes the period of the gastric pacemaker in each species. In rats, the high-amplitude contractions (HAC) were defined as ≥ 10 mmHg as previously reported during the fasted state [18]. In mice, we arbitrarily used the same HAC criteria as in rats, i.e., ≥10 mmHg. The tonic component in mice was assessed by calculating for each minute the mean of the 20-s smoothed trace. Results are expressed either by the mean pAUC or the mean tonic IGP before and after each treatment. In addition, the time course of the phasic as well as the tonic components of IGP are represented for each minute by calculating the mean pAUC or the mean tonic pressure for every minute from the rolling average of pAUC for the period between 2 min before and 2 min after each minute’s pAUC. In experiments lasting over 60 min, pAUC is expressed as the mean from a 10 min bin.
In the present study, the built-in “channel processes” of smoothing and DC removal present in the Spike 2 software were used to allow rapid processing of the traces and extraction of the appropriate components. The limitations of this computational approach should, however, be kept in mind. For example, if the component of the signal changing more rapidly than the width of the smoothing window (e.g. the respiratory component) is symmetrical, i.e. excursions above the more slowly changing component of the signal are the same magnitude as excursions below it, then the more rapidly changing components will be effectively removed by the smoothing operation. If, however, the more rapidly changing component is asymmetrical, then after smoothing, a “DC offset” will exist in the resulting smoothed curve, whose magnitude will be related to the magnitude of this asymmetry. Such an offset will not typically be important, as long as it is constant throughout the recording. But if the size of this offset changes over time due to a change in the magnitude of respiratory excursions, for example, resulting from some treatment, and if the magnitude of this asymmetry is substantial compared to the changes being measured in the more slowly-changing components of the trace, then it may be necessary to use the more involved procedures available in Spike 2 for digital filtering. Similar concerns apply to the invasion of asymmetric components of the phasic component of IGP into the tonic component.
2.4. Experimental protocols
All experimental studies were conducted in adult overnight fasted rats and mice which were anesthetized with urethane (25%,1.5 g/kg, intraperitoneal, ip) and thereafter kept on a thermostatically controlled electric heating pad for rodents to maintain rectal temperature within 35.5–37.5° C except as otherwise stated. Animals were left undisturbed for at least 30 min after intragastric placement of the MPTC. At the end of the experiment and after euthanasia by an overdose of iv urethane, laparotomy and opening of rat and mouse stomachs were performed to verify the exact position of the tip of the probe, which was always located in the gastric corpus.
2.4.1. Intragastric pressure changes in response to central vagal activation, ghrelin, and surgery in rats
The basal IGP profile was recorded by MPTC for 1 h (n=56) or 3 h (n=7). To assess the effects of ic RX-77368, a stable TRH agonist [25] or iv ghrelin, rats were first acutely implanted with an ic cannula and/or with iv catheter respectively, followed by the oral insertion of MPTC into the gastric corpus cavity. RX-77368 (30 ng, ic, n=4), ghrelin (30 μg/kg, iv, n=6) or vehicle (saline 0.9%, iv, n=10) was injected after 30 min basal recording and changes in IGP were monitored for the subsequent 30 min post-injection. Rats with low basal gastric motility were selected for these studies Doses of peptides used were based on previous dose-response studies showing maximal gastric motor response in urethane-anesthetized rats [40,52,68]. To examine the influence of abdominal surgery and bowel manipulation, in rats with ongoing basal activity (n=6), basal recording was performed for 30 min, then laparotomy (~ 4 cm) was performed after which IGP was monitored for an additional 30 min. Thereafter, caecal palpation for 1 min was performed as previously described [22,58] and IGP was recorded for another 30 min.
2.4.2. Intragastric pressure changes induced by carbachol and central vagal activation in mice
Mice (22.1 ± 0.8 g; 3 months old) were injected ip with carbachol (6 μg/kg) or saline (n=4 in each group). In a separate experiment, mice (26.7 ± 1.8 g, 6 months old), were injected icv with RX-77368 (30 ng; n=5). IGP was recorded for 15 min before and 30 min after the ip or icv injection.
2.4.3. Intragastric pressure changes induced by cold exposure in WT and CRF-OE mice
Littermate wild type (WT, n=8 [4 males, 4 females]; 27.2 ± 1.1 g; 6 months old) and CRF-OE mice (n=8 [4 males, 4 females]; 31.1 ± 2.0 g; 6 months old) were first placed on a heating pad to maintain the core temperature between 35.5–37.5° C, while basal IGP was recorded for at least 30 min. Then, mice were placed in supine position on an impermeable pad under which ice was placed (refrigerant pack). A drop in rectal temperature to 26°C was achieved within 2 to 3 min in each mouse. Mice were maintained at 26 ± 0.5°C using a heating lamp to prevent further decline in rectal temperature. The pAUC was measured for 5 min before cold exposure and for 15 min after reaching 26 ± 0.5°C. In a few animals, after 15 min cold exposure, mice were returned to a heating pad and, with additional warming provided by the heating lamp, so the recovering of body temperature above 33°C was reached within 5 min and gastric motility was monitored for 30 min thereafter. Rectal temperature was monitored continuously in WT and CRF-OE mice using a lubricated thermistor probe (YSI, Yellow Springs, OH) inserted 2 cm into the rectum.
2.3.4. Intragastric pressure change to coeliotomy and caecal palpation in mice
In mice (n=6; 22.3 ± 1.2 g; 3 months old), IGP was monitored for a 30 min basal recording period, after which a midline coeliotomy (~ 2 cm) was performed, abdominal tissues were covered with saline-soaked gauze, and IGP was recorded for an additional 30 min. Within 30 – 35 min after the coeliotomy, caecal palpation was performed for 1 min as previously described [24,34] and the IGP response was monitored for the subsequent 30 min.
2.5. Statistical analysis
Intra-individual pairwise comparisons (baseline vs post-treatment) were analyzed using a non-parametric Wilcoxon paired-test while inter-individual pairwise comparisons were performed using a non-parametric Mann-Whitney test. Interaction between genotypes and treatments was analyzed by two-way repeated ANOVA followed by Bonferroni post-test. Values are expressed as mean ± SEM and a P value <0.05 was considered significant.
3. Results
3.1. Gastric contractile activity monitored with a non-invasive method in fasted urethane anesthetized rats
3.1.1. Basal phasic gastric contractile activity patterns
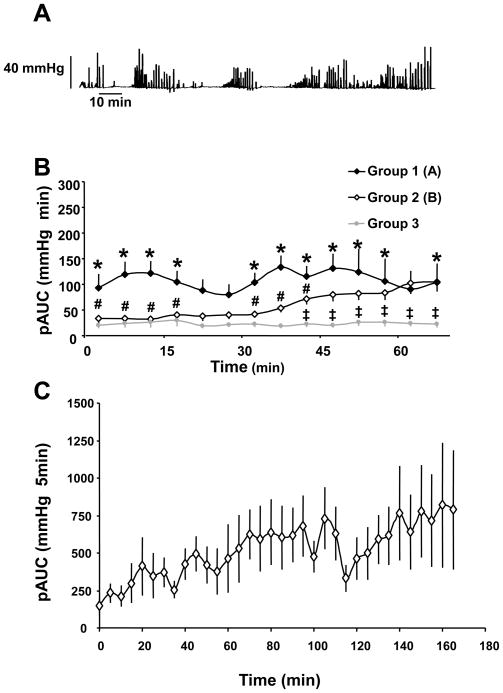
Spontaneous IGP was monitored for 1 h after the non-invasive insertion of miniaturized pressure transducer catheter into the gastric corpus in a total of 56 fasted urethane anesthetized rats. Three types of spontaneous activity patterns were observed under these conditions (Fig. 1B). In the first group 1, (n=11, 20%), spontaneous activity consisted of phasic high-amplitude (> 10 mm Hg) contractions occurring in clusters at a rate of 0.5–1 per min (0.66 ± 0.08 min−1, n=287) which prevailed from the start of the recording period (Fig. 1A, B). Such a pattern lasted throughout the 60-min experimental period (Fig. 1). In group 2 (n=36, 64%), spontaneous activity comprises low amplitude contractions (<10 mmHg) that occurred at a rate of 4–6 min−1 from the onset of the recording period for the following 30 min (Fig. 1B); thereafter the phasic contractile pattern described in group 1 developed to reach a plateau at 40 to 60 min period post the start of recording (Fig. 1B). Group 3 (n=9, 16%) did not display any spontaneous phasic activity during the first 1-h period of recording (Fig. 1B). Recordings of IGP for 170 min showed the occurrence of high amplitude contractions organized in cyclic activity (Fig. 1A) that occurred immediately (group 1: n=1), or within 30 min (group 2; n=5) (Fig. 1C).
Figure 1.
Basal patterns of gastric phasic contractile activity monitored with a novel non-invasive miniaturized pressure transducer method in fasted urethane anesthetized rats. A. Representative trace of spontaneous intragastric pressure showing phase III-like activity of long duration occurring from the start of the recording (group 1). B. Phasic component of the intragastric pressure represented as the mean ± SEM area under the curve (pAUC) over the time for 1h of group 1 (n=11), 2 (n=36) and 3 (n=9). C. Phasic component of the intragastric pressure represented as the mean ± SEM area under the curve (pAUC) over 3-h time monitored in 6 rats (group 1: n=1; group 2: n=5). *P<0.05 vs group 3; # p<0.05 vs group 1; ‡ p<0.05 vs group 2 (2-way ANOVA for repeated measures).
3.1.2 .Stimulation by intravenous injection of ghrelin and intracisternal injection of TRH agonist
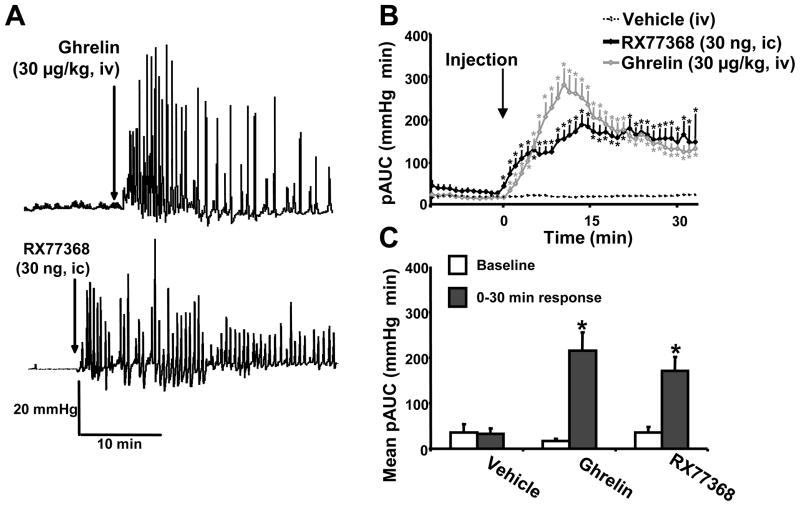
The effects of iv ghrelin and ic RX-77368 injection was investigated in group 3 which did not display any spontaneous phasic contractility during the first 1-h period of recording in fasted urethane anesthetized rats. Baseline activity was not significantly different between the treatment groups (Fig. 2B). Injection of iv saline did not modify the phasic component of IGP (pAUC, mmHg×min = 36 ± 18 at baseline vs 32 ± 12, p>0.05, Fig. 2C). Ghrelin (30 μg/kg, iv) produced a sustained increase in the phasic component of IGP for the 30-min period post injection (pAUC, mmHg×min = 17 ± 4 at baseline vs 215 ± 40; p<0.05, Fig. 2A,C). The peak response occurred within 8 min (316 ± 45 mmHg×min) and thereafter, values declined but still remained significantly elevated at 30 min (148 ± 26 mmHg×min) (Fig. 2B). Likewise, RX-77368 injected ic at 30 ng/rat induced a sustained increase in the phasic activity for the 30-min period post injection (pAUC, mmHg×min = 35 ± 13 at baseline vs 170 ± 31; p=0.05, Fig. 2C) with a plateau response reached at 11 min (211 ± 27 mmHg×min) that was maintained throughout the following 15-min recording period (Fig. 2B).
Figure 2.
Stimulation of gastric phasic contractility induced by intravenous ghrelin and intracisternal TRH agonist, RX-77368 monitored with a non non-invasive miniaturized pressure transducer method in fasted urethane anesthetized rats. Representative traces of intragastric pressure in rats after intravenous (iv) injection of ghrelin (A) and intracisternal (ic) injection of RX-77368 (B) (injection time shown by the arrow). C. Area under the curve of the phasic component of intragastric pressure (pAUC) over 30 min after the injection of either iv ghrelin (n=6), or vehicle (n=10) and ic injection of RX77369 (n=4). The pAUC is expressed as the mean ± SEM of the 2 min preceding and following each time point (injection made at time 0). D. Mean pAUC for the 15 min of baseline and 30 min response after iv injection of either ghrelin or vehicle and ic injection of RX77369. * P<0.05 vs baseline (Wilcoxon test).
3.1.3. Inhibition by coeliotomy and caecal palpation
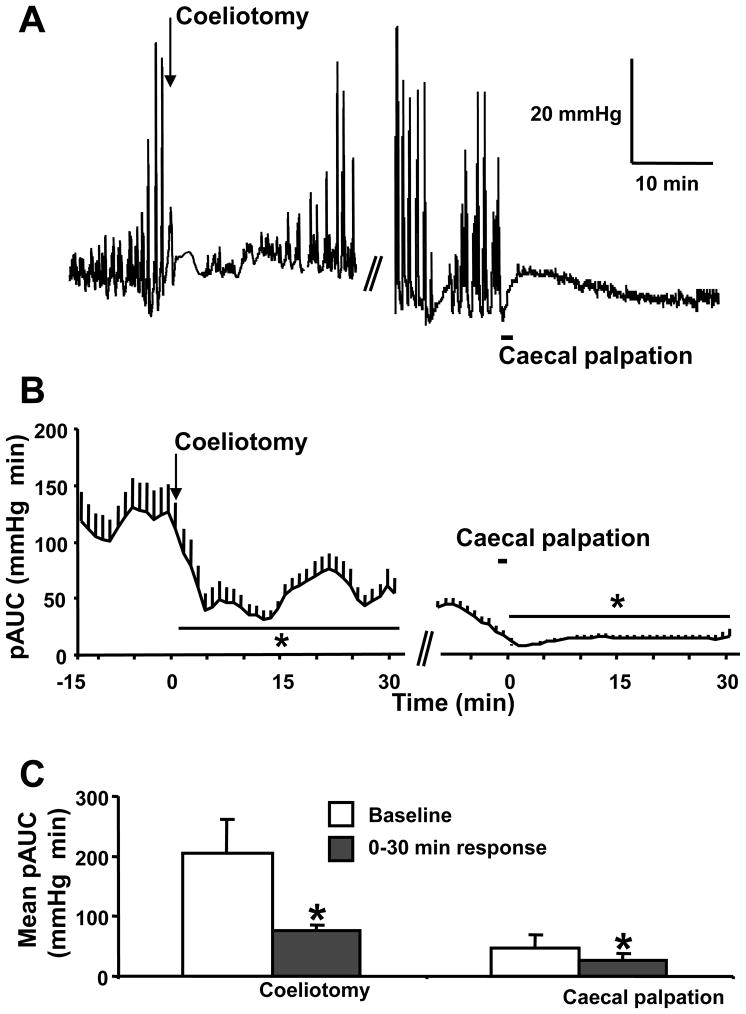
Under conditions of on going basal gastric contractile activity, midline coeliotomy produced a significant decrease in the phasic component of the IGP compared to baseline (pAUC, mmHg×min = 206 ± 56 at baseline vs 75 ± 21; p<0.01) that lasted for 30 min (Fig. 3C) with a rapid onset and peak inhibition occurring during the 5–15 min period post coeliotomy (Fig. 3A,B). Caecal palpation performed 30 min after the laparotomy induced a further rapid in onset drop in the phasic component of the IGP compared to baseline (Fig. 3AB), which lasted throughout the 30-min recording period (pAUC, mmHg×min = 47 ± 9 at baseline vs 27 ± 11; p<0.05, Fig. 3C).
Figure 3.
Inhibition of gastric phasic contractility induced by abdominal surgery monitored with a non non-invasive miniaturized pressure transducer method in fasted urethane anesthetized rats. A representative trace of intragastric pressure (A) and its phasic component (pAUC) (B) over the time in rats after coeliotomy (as shown by the arrow) and caecal palpation (as shown by the black line) realized 30 min later. The pAUC is expressed as the mean ± SEM of the 2 min preceding and following each time point. C. Mean pAUC ± SEM (n=6) for the 15 min of baseline and 30 min response after coeliotomy and caecal palpation * P<0.05 vs baseline (Wilcoxon test).
3.2. Gastric contractile activity monitored with a non-invasive method in fasted urethane anesthetized mice
3.2.1. Basal and stimulated pattern by intraperitoneal injection of carbachol
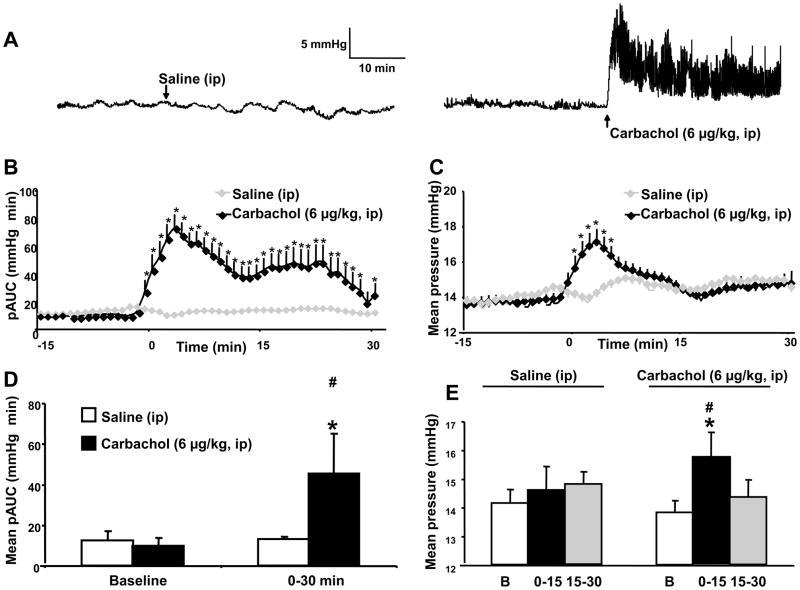
Spontaneous IGP was mainly composed of low amplitude phasic contractions (<10 mmHg) occurring at a rate of 5–6 min−1, and no high amplitude contractions was observed under basal conditions in fasted urethane anesthetized mice (Fig. 4A). Injection of carbachol (6 μg/kg, ip) induced a rise in the phasic component of IGP with a peak response occurring within the first 3 min (pAUC, mmHg×min = 10.2 ± 1.2 at baseline vs 72.9 ± 10.1; p = 0.05). There was a progressive return to basal values after 30 min while ip injection of saline had no effects on basal pAUC throughout the 30 min experimental period (Fig. 4A–B). The values of the pAUC (mmHg×min) induced by ip carbachol for the 30 min-post injection period reached 45.5 ± 19.7 from 10 ±1.2 at baseline (p=0.05) (Fig. 4C). Furthermore, carbachol administered ip increased significantly the tonic component of IGP from 2 min post injection with a peak response reached at 5 min and a return to ip vehicle value at 8 min (Fig. 4C). During the 30 min after carbachol injection, the mean gastric pressure increased significantly during the first 15 min period post injection and not thereafter compared with basal or saline ip which had no effect (Fig. 4E).
Figure 4.
Basal pattern of basal or carbachol-stimulated gastric phasic and tonic contractile activity monitored with a non non-invasive miniaturized pressure transducer method in fasted urethane anesthetized mice. Representative traces of intragastric pressure (A) after either intraperitoneal (ip) injection of carbachol (6 μg/kg, ip) or saline (ip). B Phasic component of the intragastric pressure (pAUC) in mice after injection of carbachol (6 μg/kg, ip) or saline (ip) made at time 0. The pAUC is expressed as the mean ± SEM of the 2 min preceding and following each time point. C. Tonic component of the intragastric pressure in mice expressed as the mean pressure ± SEM over the time after injection of carbachol (6 μg/kg, ip) or saline (ip) made at time 0. D. Quantification of the phasic (mean pAUC ± SEM) components of intragastric pressure during baseline and 30 min after ip injections of either carbachol or saline. E. Quantification of the tonic (mean pressure ± SEM) components of intragastric pressure during baseline (B) and the consecutive 15 min periods after ip injections of either carbachol or saline. * P<0.05 vs baseline (Wilcoxon test). #: P<0.05 vs saline (Mann & Whitney test)
3.3.2. Stimulation by intracerebroventricular injection of TRH agonist or cold exposure: differential responses in wild type and CRF-OE mice
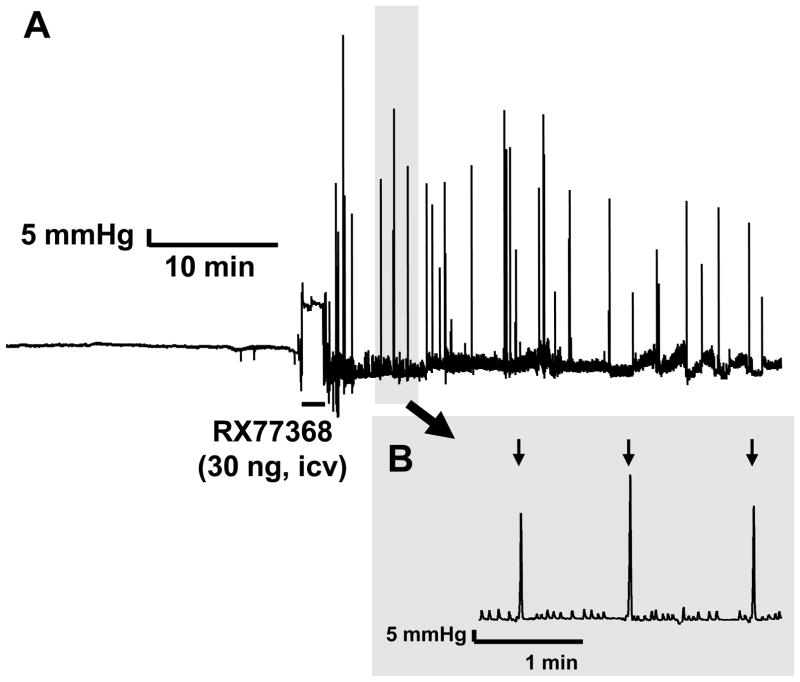
The icv injection of RX-77368 (30 ng) induced a rapid onset (50.1 ± 9.3 sec) occurrence of HAC at a rate of 0.71 ± 0.20 min−1 with a mean amplitude of 81.6 ± 3.2 mmHg and duration of 1.50 ± 0.05 s over the 30 min post injection period while the phasic AUC component was not significantly modified due to the short duration of the event (Fig. 5).
Figure 5.
Stimulation of phasic gastric contractions induced by intracerebroventricular (icv) injection of TRH agonist monitored with a non non-invasive miniaturized pressure transducer method in fasted urethane anesthetized mice. A. Representative trace of intragastric pressure change in fasted mice induced by icv injection of RX-77368. Time of injection is represented by a black line. B. Large scale representation of intragastric pressure recording from shaded area in A. High amplitude contractions (shown by black arrows) occurred at a frequency of about 1/min as monitored 30 min after icv injection.
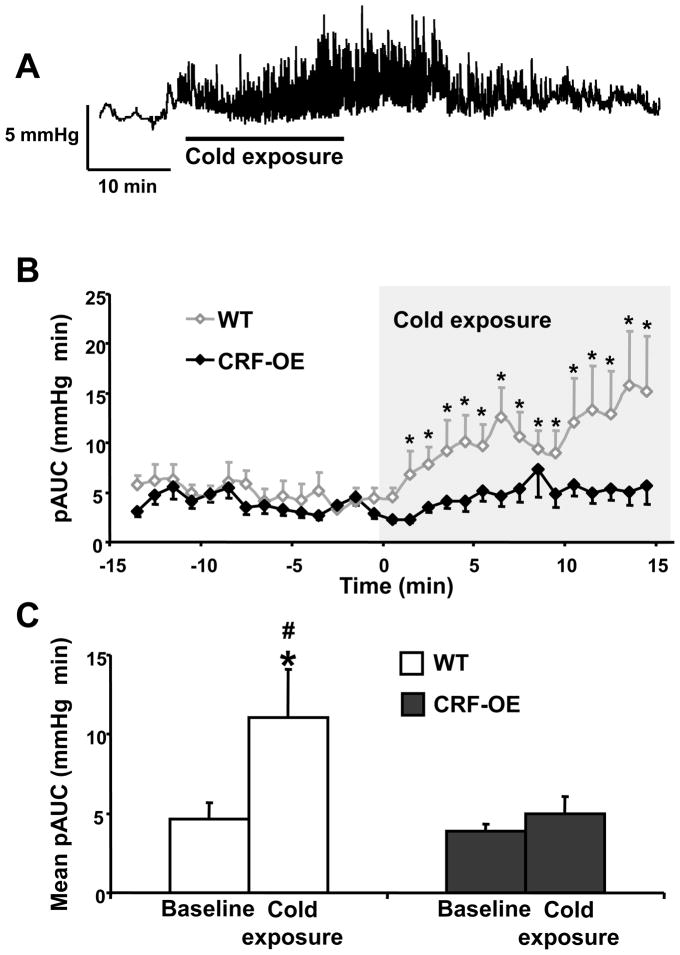
Mice either WT or CRF-OE exposed to ice cold pad for 15 min dropped their rectal temperature within 5 min which was thereafter maintained at 26.0 ± 0.5°C with manual heating control. Compared to baseline at room temperature, cold exposure induced a rapid onset (2–3 min, Fig. 6A–B) increase in the phasic component of the IGP in WT mice that lasted for the 15 min period of cold exposure (pAUC, mmHg×min = 4.6 ± 1.1 at baseline vs 11.0 ± 3.1; p<0.05, Fig. 6B–C) with a return to basal activity when cold exposure ended (Fig. 6A). In contrast, no significant change in pAUC was observed in CRF-OE mice during the 15 min of cold stress exposure compared to baseline (pAUC, mmHg×min = 3.9 ± 0.5 at baseline vs 5.0 ± 1.1; p>0.05, Fig. 6B–C).
Figure 6.
Acute exposure to cold induces increase in phasic gastric contractions in fasted urethane anesthetized wild type mice but not in CRF-OE mice as monitored with a non non-invasive miniaturized pressure transducer method. A. Representative trace of intragastric pressure before, during and after cold exposure in wild type (WT) mice; the cold exposure (period during which the animal body temperature was at 26 ± 0.5°C) is represented as a black line. B. Area under the curve of the phasic component of intragastric pressure (pAUC) over the time in WT (open square line) or CRF overexpressing (CRF-OE; filled square line) mice before and during cold stress exposure for 15 min. The pAUC is expressed as the mean ± SEM of the 2 min preceding and following each time point. C. Mean pAUC ± SEM pAUC 15 min before and during cold exposure in WT and CRF-OE mice (n=8/per group), * P<0.05 vs baseline. #: p<0.05 vs saline (Two-Way Repeated Measure ANOVA.)
3.3.3. Inhibition of gastric contractile activity by coeliotomy and caecal palpation in mice
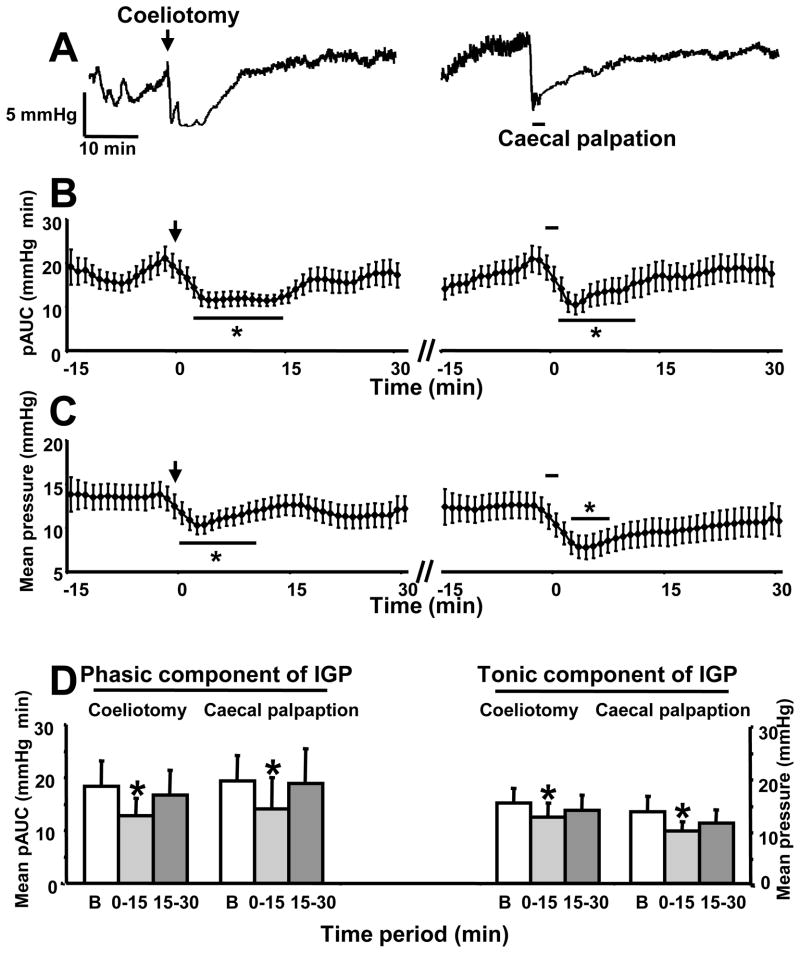
Coeliotomy resulted in a short lasting (15 min) significant reduction of the phasic and tonic components of the IGP compared to baseline (Fig. 7A). The plateau decrease occurred at 5 min after the intervention (pAUC: mmHg×min, 18.3 ± 4.8 at baseline vs 12.8 ± 3.3; mean pressure: mmHg, 15.3 ± 2.7 vs 12.5 ± 2.7; p<0.05, Fig. 7A–C) and was maintained for 15 min with a return to basal values during the 15–30 min time period (Fig. 7A–D). Cecal palpation for 1 min performed 30–35 min after the coeliotomy resulted in a similar pattern of inhibition of phasic and tonic components of IGP (pAUC: mmHg×min, 19.3 ± 4.8 at baseline vs 14.0 ± 6.0; mean pressure: mmHg, 13.6 ± 2.8 vs 9.8 ± 1.9; 0–15 min post caecal palpation; p<0.05, Fig. 7A–D).
Figure 7.
Both coeliotomy and caecal palpation inhibit phasic and tonic gastric contractile activity monitored with a non non-invasive miniaturized pressure transducer method in fasted urethane anesthetized mice A. Representative traces of intragastric pressure in mice after coeliotomy (as shown by the arrow) and caecal palpation (as shown by the black line). B. Area under the curve of the phasic component of intragastric pressure (pAUC) over the time in mice after coeliotomy (left) and caecal palpation (right) realized 30 min later. pAUC expressed as the mean ± SEM of the 2 min preceding and following each time point. C. Tonic component of the intragastric pressure expressed as the mean pressure ±SEM over the time in mice after coeliotomy (left) and caecal palpation (right) realized 30 min later. D. Quantification of the phasic (mean pAUC ± SEM per 15 min period) and tonic (mean pressure ± SEM per 15 min period, n=6) components of intragastric pressure during baseline (B) and the first consecutive 15 min periods after coeliotomy and caecal palpation. * p<0.05 vs baseline (Wilcoxon test).
4. Discussion
In the present study, we validated a novel non-invasive still-manometry method to measure acutely the phasic components of IGP in fasted urethane anesthetized mice and rats. We positioned the pressure transducer of the Mikro-Tip catheter into the gastric corpus through insertion into the oral cavity and monitored the well-established stimulatory effect of iv injection of ghrelin and central vagal cholinergic activation in rats. Similarly we demonstrated for the first time in mice that icv TRH agonist or acute cold exposure induced a rapid onset stimulation of gastric phasic contractile activity and that CRF-OE mice are not responsive to central vagal stimulation. In addition we delineated the different components of gastric motility inhibition induced by coeliotomy alone followed by bowel manipulation in rats and mice naïve of prior surgery.
In fasted urethane anesthetized rats, spontaneous IGP recorded under these conditions exhibited phasic contractile activity, composed of high amplitude contractions over 10 mmHg at a frequency of 0.5–1/min in 84% of the case. Such activity is compatible with the pattern of gastric motility previously reported using mechanical, myoelectrical or manometric methods for recording motility in conscious rats chronically implanted with strain-gauges or electrodes in the corpus, or side hole perfused manometry catheters in the gastric lumen respectively [18,20,49]. For instance in fasted conscious rats, the frequency of high-amplitude contractions was reported in the range of 0.4–1/min contractions/min in the proximal corpus [20,49]. In the present study, we found that high amplitude contractions were organized in cyclic activity, although during the first 30 min there was no clear return to basal activity between cycles. In the fasting state, migrating motor complex (MMC) has been characterized in human and dog stomachs as well as the small intestine of most mammals [28,35]. It remains, however, controversial whether rats have a similar MMC in the stomach under fasting conditions [3,9,16,49,65]. For instance, Tatewaki M et al. [65] found no cyclic grouping of strong antral contractions in 57% of fasted rats while contractions were organized in cyclic phase III-like activity in the remaining 43% while in others report, no obvious gastric MMC in fasted rats was observed [30,49]. In the other 16% of cases, no spontaneous activated phasic motility was observed during the first 1-h period of recording basal activity.. In previous studies, a low basal activity with an amplitude <10 mmHg was the main pattern observed in urethane anesthetized rats when gastric motility was monitored using a water filled balloon-catheter assembly that was inserted acutely into the stomach through laparotomy and incision into the forestomach [40]. As we demonstrated using pressure microsensor catheter inserted orally that each step of abdominal surgery (i.e. laparotomy and gut manipulation) inhibits the phasic component of IGP, these data suggest that the commonly used gastric pressure measurements methods under acute conditions involving surgery induce confounding factors for IGP measurement. Under our conditions without surgery, the low basal gastric motility was observed only in 16% of animals and may be related to differential sensitivity of rats to increased sympathetic activity induced by urethane [8,36].
Under low basal gastric contractility, intravenous injection of ghrelin induces a rapid in onset and sustained pattern of phasic IGP activity characterized by regular high amplitude contractions similar to phase III-like contractions [3] throughout the 30 min experimental period. These data are in line with the initial report showing that rat ghrelin injected iv at a similar dose range as used in the present study, evoked a rapid increase in the amplitude and frequency of gastric contractions lasting 50 min as monitored by a manometric method with a water filled balloon-catheter acutely inserted into the stomach through an incision in the duodenum of urethane anesthetized fasted rats [40]. Similarly in conscious rats, iv injection of ghrelin was reported to induce a rapid onset increase in antral phase-III like contractions when recorded using chronically implanted manometric catheter into the antrum to measure pressure changes [18], strain gauge sutured on the serosal surface of the antrum to measure antral wall motion [64] or with chronically implanted wire electrode on the surface of the stomach (electrogastrography) [66]. In addition, we showed that central vagal activation induced by the ic injection of stable TRH agonist, RX-77368 induced a rapid increase in phasic gastric contractions that lasted during the 30-min recording period. The pattern of response to RX-77368 is similar to that described in urethane anesthetized rats by recording from acutely implanted strain gauges on the gastric corpus or manometrically by fluid filled catheter placed into the stomach through the pylorus or in conscious rats using strain gauges [9,13,52]. Previous time course studies established that RX- 77368 injected intracisternally at a similar dose as used in the present study (30 ng) stimulates gastric motility for 70 min post injection [9,52]. Both gastric motility stimulatory response to iv ghrelin and ic RX-77368 have been characterized to be vagal dependent and atropine sensitive in conscious or anesthetized rats [21,40,66]. Collectively the data obtained in rats using prokinetic peptides such as iv ghrelin and central vagal cholinergic activation in fasted urethane anesthetized rats validate the sensitivity and reliability of the novel non surgical acute method with pressure microsensor catheter inserted orally. Of note is that urethane anesthesia was used to conduct the validation of the non-invasive method for comparison purpose to a number of reports showing that iv injection of ghrelin or central vagal activation stimulates high amplitude phasic contractions monitored by different recording approaches in urethane anesthetized rats [1,9,27,40,55,55]. In addition, the stimulation induced by central vagal activation and ghrelin or other transmitters initially observed in urethane anesthetized rats have been largely reproduced in conscious rats [18,21,63,64]. However urethane results in the activation of sympathetic outflow [8], hyperglycemia [27] and gastric somatostatin release [71] that can impact on gastric motor function [51]. Therefore it will be relevant to expand this non-invasive pressure microsensor method under other anesthetic conditions (inactin, pentobarbital) also used to record gastric motility.
Next we applied this approach to mice and report for the first time a new method to monitor IGP in anesthetized mice without any surgical involvement. Interestingly, we did not observed any high amplitude phasic contractions (i.e. >10 mmHg) compared with rats when anesthetized with the same dose/kg of urethane. Basal activity was composed of low amplitude contractions (<10 mmHg) occurring at a rate of 5–6 min−1. However, urethane mice preparation was responsive to stimulation by cholinergic mechanisms, which resulted in a marked rapid (<3 min) increase in phasic and tonic components of IGP and to inhibition by abdominal surgery. Only one report so far has monitored gastric contractility in urethane anesthetized mice [43]. Under central and peripheral cholinergic stimulated conditions, gastric motility increased as measured using a balloon surgically placed within the gastric lumen and similar changes were produced in un-anesthetized decerebrated mice [43]. However, the use of a miniaturized barostat only allowed the recording of changes in IGP at a rate of 1Hz, which is not sensitive enough to monitor precisely phasic changes in IGP as observed in the present study. More recent studies developed methods to record motility in conscious mice using miniaturized perfused manometry system inserted into the antrum through an intragastric incision or implanting miniature strain gauge transducer on the antrum or greater curvature [57,62,73]. Under these conditions, fasted mice showed a basal phase-III like activity in the antrum characterized by high amplitude contractions occurring at a rate of 8/h [62] or every 12–15 min [73]. The absence of spontaneous high amplitude contractions in the present method may be related to the greater sensitivity of the mice vs rats to urethane anesthesia as urethane can exert suppressant effect on contractile activity [36]. Recent studies provided evidence that endogenous ghrelin regulates the occurrence of phase III-like contractions in freely moving fasted mice [73]. We previously established that urethane is a potent releaser of gastric somatostatin which inhibits gastric endocrine hormone secretion such as gastrin [71] and ghrelin [14,56].
However the low basal activity can be stimulated by cold which induced a rapid onset (2–3 min) increase in the phasic component of the IGP in wild type mice that lasted for the 15 min period of cold exposure while CRF-OE mice had no change in gastric motility in response to cold. Consistent with increased gastric motility, functional studies showed that acute cold exposure stimulates gastric emptying in conscious mice [26]. Acute cold exposure is known to activate preganglionic vagal motor neurons through brainstem TRH receptor dependent mechanisms resulting in a vagal cholinergic stimulation of gastric secretory and motor function in rats [61]. In the present study, we demontrate that icv injection of TRH agonist also induces a rapid onset increase in high amplitude gastric contractions expanding to mice previous reports in rats [61]. We previously demonstrated a central action of CRF to inhibit cold-induced activation of neurons in the dorsal motor nucleus of the vagus and interaction between exogenous injection of CRF and TRH at this site whereby CRF suppressed TRH-induced increase in gastric motility in rats [29,69]. In mice, icv injection of CRF results in a delayed gastric emptying of liquid or solid meal [37,54]. Therefore the lack of gastric motility response to cold exposure in CRF-OE mice may reflect such interaction between endogenous brainstem TRH released by cold exposure [72] and CRF under conditions of CRF overexpression.
All previous methods to measure gastric motility in rodents required abdominal surgery and manipulation of the gut to implant the recording device, thus rendering them disadvantageous to study the influence of surgery itself in animals naïve of previous surgery. In the present study, the positioning of miniaturized pressure transducer catheter into the gastric corpus through oral insertion allowed to assess changes in gastric contractile activity without a confounding surgery effect. Under this non invasive condition, both anesthetized rats and mice undergoing midline coeliotomy had a significant acute decrease in the phasic component of IGP compared to baseline. Caecal palpation performed 30 min after the laparotomy induced a further drop in the phasic component of the IGP compared to baseline. These data provide insight to changes in gastric contractile events underlying functional studies showing delayed gastric emptying in rats and mice immediately following similar surgical intervention [34,58]. In particular the rapid onset of changes in gastric motility induced by midline coeliotomy and caecal palpation provide additional support for neurally mediated mechanisms initiating the post operative gastric ileus as shown by the role of capsaicin sensitive afferent and activation of brain CRF signaling pathways in the delayed gastric emptying observed within 2 h of coeliotomy and caecal palpation in rats or mice [5,34,50,74].
Interestingly, we also demonstrated in mice that our method could capture changes in the tonic component of IGP which is usually not possible using catheter manometry in higher species, including rats. This is most likely due to the smaller size of the gastric cavity. The responsiveness of our assay to monitor tonic component of IGP in mice was assessed using coeliotomy and caecal palpation that induced a decrease in gastric tonic activity, while injection of ip carbachol increased the tonic component of IGP.
In summary, we validate a novel and accurate method to monitor acutely the phasic component of gastric contractility in both in rats and mice, using a solid-state micro-tip pressure transducer catheter positioned through oral intubation into the gastric corpus. In addition, this technique does not require surgical procedure or gastric filling with any liquids contrasting with present methods employed to measure acutely gastric pressure in rats. Such method appears therefore particularly useful to investigate acutely gastric motility in rodents, especially in mice in the context of the increasing availability of transgenic models. Such an approach has allowed us to demonstrate that chronic overexpression of CRF in mice suppressed central vagal activation-induced rapid increased in high amplitude gastric contractions and the rapid onset decrease in gastric phasic contractions in response to laparotomy and the additive effect of intestinal manipulations in mice and rats.
Acknowledgments
This work was supported by NIH grants R01 DK-33061 (YT), Center grant DK-41301 (Animal Core; YT), VA Career Scientist and Merit Awards (YT), the French Society of Gastroenterology (S.N.F.G.E.; GG), and the French Foreign Office (EGIDE Program, GG). We thank Dr. Serge St Pierre (University of Quebec, Montreal, Canada for the generous supply of ghrelin, Dr Michael Fanzelow (Department of Psychology, UCLA, Los Angeles) for the supply of CRF-OE and wild type mice and Ms. Honghui Liang for technical assistance and Eugenia Hu for helping in the preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adelson DW, Million M, Kanamoto K, Palanca T, Taché Y. Coordintated gastric and sphincter motility evoked by intravenous CCK-8 as monitored by ultrasononomicrometry in rats. Am J Physiol Gastrointest Liver Physiol. 2004;283:G321–G332. doi: 10.1152/ajpgi.00057.2003. [DOI] [PubMed] [Google Scholar]
- 2.Amato A, Serio R, Mule F. Relaxation induced by N-terminal fragments of chromogranin A in mouse gastric preparations. Regul Pept. 2007;139:90–95. doi: 10.1016/j.regpep.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Ariga H, Imai K, Chen C, Mantyh C, Pappas TN, Takahashi T. Fixed feeding potentiates interdigestive gastric motor activity in rats: importance of eating habits for maintaining interdigestive MMC. Am J Physiol Gastrointest Liver Physiol. 2008;294:G655–G659. doi: 10.1152/ajpgi.00484.2007. [DOI] [PubMed] [Google Scholar]
- 4.Bauer AJ. Mentation on the immunological modulation of gastrointestinal motility. Neurogastroenterol Motil. 2008;20 (Suppl 1):81–90. doi: 10.1111/j.1365-2982.2008.01105.x. [DOI] [PubMed] [Google Scholar]
- 5.Bonaz B, Taché Y. Corticotropin-releasing factor and systemic capsaicin-sensitive afferents are involved in abdominal surgery-induced Fos expression in the paraventricular nucleus of the hypothalamus. Brain Res. 1997;748:12–20. doi: 10.1016/s0006-8993(96)01281-4. [DOI] [PubMed] [Google Scholar]
- 6.Buéno L, Ferre JP, Fioramonti J, Ruckesbusch M. Control of the antral motor response to feeding by gastric acid secretion in rats. J Physiol. 1982;325:43–50. doi: 10.1113/jphysiol.1982.sp014134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buéno L, Ferre JP, Ruckebusch M, Genton M, Pascaud X. Continuous electrical and mechanical activity recording in the gut of the conscious rat. J Pharmacol Methods. 1981;6:129–136. doi: 10.1016/0160-5402(81)90035-8. [DOI] [PubMed] [Google Scholar]
- 8.Carruba MO, Bondiolotti GP, Picotti GB, Catteruccia N, Da Prada M. Effects of diethyl ether, halothaneketamine and urethane on sympathetic activity in the rat. Eur J Pharmacol. 1987;134:15–24. doi: 10.1016/0014-2999(87)90126-9. [DOI] [PubMed] [Google Scholar]
- 9.Chen CY, Million M, Adelson DW, Martinez V, Rivier J, Taché Y. Intracisternal urocortin inhibits vagally stimulated gastric motility in rats: role of CRF(2) Br J Pharmacol. 2002;136:237–247. doi: 10.1038/sj.bjp.0704713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi KM, Zhu J, Stoltz GJ, Vernino S, Camilleri M, Szurszewski JH, Gibbons SJ, Farrugia G. Determination of gastric emptying in nonobese diabetic mice. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1039–G1045. doi: 10.1152/ajpgi.00317.2007. [DOI] [PubMed] [Google Scholar]
- 11.Coste SC, Murray SE, Stenzel-Poore MP. Animal models of CRH excess and CRH receptor deficiency display altered adaptations to stress. Peptides. 2001;22:733–741. doi: 10.1016/s0196-9781(01)00386-2. [DOI] [PubMed] [Google Scholar]
- 12.Cruz MT, Murphy EC, Sahibzada N, Verbalis JG, Gillis RA. A reevaluation of the effects of stimulation of the dorsal motor nucleus of the vagus on gastric motility in the rat. Am J Physiol Regul Integr Comp Physiol. 2007;292:R291–R307. doi: 10.1152/ajpregu.00863.2005. [DOI] [PubMed] [Google Scholar]
- 13.Davison JS, Wootton P. The effect of intracisternal injection of thyrotropin-releasing hormone on gastric and duodenal motility in the urethane- anaesthetized rat. Exp Physiol. 1991;76:983–986. doi: 10.1113/expphysiol.1991.sp003562. [DOI] [PubMed] [Google Scholar]
- 14.de la Cour CD, Norlen P, Hakanson R. Secretion of ghrelin from rat stomach ghrelin cells in response to local microinfusion of candidate messenger compounds: a microdialysis study. Regul Pept. 2007;143:118–126. doi: 10.1016/j.regpep.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Dirks A, Groenink L, Bouwknecht JA, Hijzen TH, Van Der GJ, Ronken E, Verbeek JS, Veening JG, Dederen PJ, Korosi A, Schoolderman LF, Roubos EW, Olivier B. Overexpression of corticotropin-releasing hormone in transgenic mice and chronic stress-like autonomic and physiological alterations. Eur J Neurosci. 2002;16:1751–1760. doi: 10.1046/j.1460-9568.2002.02245.x. [DOI] [PubMed] [Google Scholar]
- 16.Fioramonti J, Buéno L. Gastrointestinal myolectric activity disturbances in gastric ulcer disease in rats and dogs. Dig Dis Sci. 1980;25:575–580. doi: 10.1007/BF01318869. [DOI] [PubMed] [Google Scholar]
- 17.Forrest AS, Ordog T, Sanders KM. Neural regulation of slow-wave frequency in the murine gastric antrum. Am J Physiol Gastrointest Liver Physiol. 2006;290:G486–G495. doi: 10.1152/ajpgi.00349.2005. [DOI] [PubMed] [Google Scholar]
- 18.Fujino K, Inui A, Asakawa A, Kihara N, Fujimura M, Fujimiya M. Ghrelin induces fasted motor activity of the gastrointestinal tract in conscious fed rats. J Physiol. 2003;550:227–240. doi: 10.1113/jphysiol.2003.040600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukuda H, Tsuchida D, Koda K, Miyazaki M, Pappas TN, Takahashi T. Impaired gastric motor activity after abdominal surgery in rats. Neurogastroenterol Motil. 2005;17:245–250. doi: 10.1111/j.1365-2982.2004.00602.x. [DOI] [PubMed] [Google Scholar]
- 20.Garrick T, Buack S, Bass P. Gastric motility is a major factor in cold restraint-induced lesion formation in rats. Am J Physiol. 1986;250:G191–G199. doi: 10.1152/ajpgi.1986.250.2.G191. [DOI] [PubMed] [Google Scholar]
- 21.Garrick T, Buack S, Veiseh A, Taché Y. Thyrotropin-releasing hormone (TRH) acts centrally to stimulate gastric contractility in rats. Life Sci. 1987;40:649–657. doi: 10.1016/0024-3205(87)90266-9. [DOI] [PubMed] [Google Scholar]
- 22.Gourcerol G, Gallas S, Mounien L, Leblanc I, Bizet P, Boutelet I, Leroi AM, Ducrotte P, Vaudry H, Jegou S. Gastric electrical stimulation modulates hypothalamic corticotropin-releasing factor-producing neurons during post-operative ileus in rat. Neuroscience. 2007;148:775–781. doi: 10.1016/j.neuroscience.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Gourcerol G, Million M, Adelson DW, Wang Y, Wang L, Rivier J, St Pierre DH, Taché Y. Lack of interaction between peripheral injection of CCK and obestatin in the regulation of gastric satiety signaling in rodents. Peptides. 2006;27:2811–2819. doi: 10.1016/j.peptides.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Gourcerol G, Wang L, Adelson DW, Larauche M, Taché Y, Million M. Cholinergic giant migrating contractions in conscious mouse colon assessed by using a novel noninvasive solid-state manometry method: modulation by stressors. Am J Physiol Gastrointest Liver Physiol. 2009;296:G992–G1002. doi: 10.1152/ajpgi.90436.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffiths EC, McDermott JR, Smith AI. Mechanisms of brain inactivation of centrally-acting thyrotrophin-releasing hormone (TRH) analogues: a high performance liquid chromatography study. Regul Pept. 1982;5:1–11. doi: 10.1016/0167-0115(82)90070-2. [DOI] [PubMed] [Google Scholar]
- 26.Gué M, Fioramonti J, Buéno L. Comparative influences of acoustic and cold stress on gastrointestinal transit in mice. Am J Physiol. 1987;253:G124–G128. doi: 10.1152/ajpgi.1987.253.2.G124. [DOI] [PubMed] [Google Scholar]
- 27.Guerrini S, Raybould HE, Anselmi L, Agazzi A, Cervio E, Reeve JR, Jr, Tonini M, Sternini C. Role of galanin receptor 1 in gastric motility in rat. Neurogastroenterol Motil. 2004;16:429–438. doi: 10.1111/j.1365-2982.2004.00534.x. [DOI] [PubMed] [Google Scholar]
- 28.Hall KE, Greenberg GR, El-Sharkawy TY, Diamant NE. Relationship between porcine motilin-induced migrating motor complex-like activity, vagal integrity, and endogenous motilin release in dogs. Gastroenterology. 1984;87:76–85. [PubMed] [Google Scholar]
- 29.Heymann-Monnikes I, Taché Y, Trauner M, Weiner H, Garrick T. CRF microinjected into the dorsal vagal complex inhibits TRH analog- and kainic acid-stimulated gastric contractility in rats. Brain Res. 1991;554:139–144. doi: 10.1016/0006-8993(91)90181-t. [DOI] [PubMed] [Google Scholar]
- 30.Kaneko H, Tomomasa T, Watanabe T, Takahashi A, Tabata M, Hussein S, Morikawa A. Effect of vincristine on gastric motility in conscious rats. Dig Dis Sci. 2001;46:952–959. doi: 10.1023/a:1010785206315. [DOI] [PubMed] [Google Scholar]
- 31.Kihara N, Fujimura M, Yamamoto I, Itoh E, Inui A, Fujimiya M. Effects of central and peripheral urocortin on fed and fasted gastroduodenal motor activity in conscious rats. Am J Physiol Gastrointest Liver Physiol. 2001;280:G406–G419. doi: 10.1152/ajpgi.2001.280.3.G406. [DOI] [PubMed] [Google Scholar]
- 32.Kitazawa T, Hashiba K, Cao J, Unno T, Komori S, Yamada M, Wess J, Taneike T. Functional roles of muscarinic M2 and M3 receptors in mouse stomach motility: studies with muscarinic receptor knockout mice. Eur J Pharmacol. 2007;554:212–222. doi: 10.1016/j.ejphar.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 33.Krantis A, Glasgow I, McKay AE, Mattar K, Johnson F. A method for simultaneous recording and assessment of gut contractions and relaxations in vivo. Can J Physiol Pharmacol. 1996;74:894–903. [PubMed] [Google Scholar]
- 34.Luckey A, Wang L, Jamieson PM, Basa NR, Million M, Czimmer J, Vale W, Taché Y. Corticotropin-releasing factor receptor 1-deficient mice do not develop postoperative gastric ileus. Gastroenterology. 2003;125:654–659. doi: 10.1016/s0016-5085(03)01069-2. [DOI] [PubMed] [Google Scholar]
- 35.Luiking YC, van der Reijden AC, Berge Henegouwen GP, Akkermans LM. Migrating motor complex cycle duration is determined by gastric or duodenal origin of phase III. Am J Physiol. 1998;275:G1246–G1251. doi: 10.1152/ajpgi.1998.275.6.G1246. [DOI] [PubMed] [Google Scholar]
- 36.Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations. Part 3: other systems and conclusions. Experientia. 1986;42:531–537. doi: 10.1007/BF01946692. [DOI] [PubMed] [Google Scholar]
- 37.Martinez V, Wang L, Rivier J, Grigoriadis D, Taché Y. Central CRF, urocortins and stress increase colonic transit via CRF1 receptors while activation of CRF2 receptors delays gastric transit in mice. J Physiol. 2004;556.1:221–234. doi: 10.1113/jphysiol.2003.059659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez V, Wang L, Rivier JE, Vale W, Taché Y. Differential actions of peripheral corticotropin-releasing factor (CRF), urocortin II, and urocortin III on gastric emptying and colonic transit in mice: role of CRF receptor subtypes 1 and 2. J Pharmacol Exp Ther. 2002;301:611–617. doi: 10.1124/jpet.301.2.611. [DOI] [PubMed] [Google Scholar]
- 39.Martinez V, Wu SV, Taché Y. Intracisternal antisense oligodeoxynucleotides to the thyrotropin-releasing hormone receptor blocked vagal-dependent stimulation of gastric emptying induced by acute cold in rats. Endocrinology. 1998;139:3730–3735. doi: 10.1210/endo.139.9.6195. [DOI] [PubMed] [Google Scholar]
- 40.Masuda Y, Tanaka T, Inomata N, Ohnuma N, Tanaka S, Itoh Z, Hosoda H, Kojima M, Kangawa K. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem Biophys Res Commun. 2000;276:905–908. doi: 10.1006/bbrc.2000.3568. [DOI] [PubMed] [Google Scholar]
- 41.Million M, Fioramonti J, Buéno L. Oral administration of Tyr-MIF-1 stimulates gastric emptying and gastrointestinal motility in rodents. Peptides. 1992;13:469–474. doi: 10.1016/0196-9781(92)90076-f. [DOI] [PubMed] [Google Scholar]
- 42.Million M, Wang L, Stenzel-Poore MP, Coste SC, Yuan PQ, Lamy C, Rivier J, Buffington T, Taché Y. Enhanced pelvic responses to stressors in female CRF-overexpressing mice. Am J Physiol Regul Integr Comp Physiol. 2006;292:R1429–R1438. doi: 10.1152/ajpregu.00626.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monroe MJ, Hornby PJ, Partosoedarso ER. Central vagal stimulation evokes gastric volume changes in mice: a novel technique using a miniaturized barostat. Neurogastroenterol Motil. 2004;16:5–11. doi: 10.1046/j.1365-2982.2003.00464.x. [DOI] [PubMed] [Google Scholar]
- 44.Mule F, Amato A, Baldassano S, Serio R. Involvement of CB1 and CB2 receptors in the modulation of cholinergic neurotransmission in mouse gastric preparations. Pharmacol Res. 2007;56:185–192. doi: 10.1016/j.phrs.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Nagell CF, Wettergren A, Orskov C, Holst JJ. Inhibitory effect of GLP-1 on gastric motility persists after vagal deafferentation in pigs. Scand J Gastroenterol. 2006;41:667–672. doi: 10.1080/00365520500408253. [DOI] [PubMed] [Google Scholar]
- 46.Orihata M, Sarna SK. Contractile mechanisms of action of gastroprokinetic agents: cisapride, metoclopramide, and domperidone. Am J Physiol. 1994;266:G665–G676. doi: 10.1152/ajpgi.1994.266.4.G665. [DOI] [PubMed] [Google Scholar]
- 47.Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592–1622. doi: 10.1053/j.gastro.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 48.Peeters TL. Central and peripheral mechanisms by which ghrelin regulates gut motility. J Physiol Pharmacol. 2003;54 (Suppl 4):95–103. [PubMed] [Google Scholar]
- 49.Pihan G, Kline TJ, Hollenberg NK, Szabo S. Duodenal ulcerogens cysteamine and propionitrile induce gastroduodenal motility alterations in the rat. Gastroenterology. 1985;88:989–997. doi: 10.1016/s0016-5085(85)80019-6. [DOI] [PubMed] [Google Scholar]
- 50.Plourde V, Wong HC, Walsh JH, Raybould HE, Taché Y. CGRP antagonists and capsaicin on celiac ganglia partly prevent postoperative gastric ileus. Peptides. 1993;14:1225–1229. doi: 10.1016/0196-9781(93)90180-o. [DOI] [PubMed] [Google Scholar]
- 51.Qualls-Creekmore E, Tong M, Holmes GM. Gastric emptying of enterally administered liquid meal in conscious rats and during sustained anaesthesia. Neurogastroenterol Motil. 2010;22:181–185. doi: 10.1111/j.1365-2982.2009.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raybould HE, Holzer P, Reddy NS, Yang H, Taché Y. Capsaicin-sensitive vagal afferents contribute to gastric acid and vascular responses to intracisternal TRH analog. Peptides. 1990;11:789–795. doi: 10.1016/0196-9781(90)90196-c. [DOI] [PubMed] [Google Scholar]
- 53.Raybould HE, Taché Y. Cholecystokinin inhibits gastric motility and emptying via a capsaicin-sensitive vagal pathways in rats. Am J Physiol. 1988;255:G242–G246. doi: 10.1152/ajpgi.1988.255.2.G242. [DOI] [PubMed] [Google Scholar]
- 54.Sheldon RJ, Qi JA, Porreca F, Fisher LA. Gastrointestinal motor effects of corticotropin-releasing factor in mice. Regul Pept. 1990;28:137–151. doi: 10.1016/0167-0115(90)90013-m. [DOI] [PubMed] [Google Scholar]
- 55.Shi M, Jones AR, Niedringhaus MS, Pearson RJ, Biehl AM, Ferreira M, Jr, Sahibzada N, Verbalis JG, Gillis RA. Glucose acts in the CNS to regulate gastric motility during hypoglycemia. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1192–R1202. doi: 10.1152/ajpregu.00179.2003. [DOI] [PubMed] [Google Scholar]
- 56.Shimada M, Date Y, Mondal MS, Toshinai K, Shimbara T, Fukunaga K, Murakami N, Miyazato M, Kangawa K, Yoshimatsu H, Matsuo H, Nakazato M. Somatostatin suppresses ghrelin secretion from the rat stomach. Biochem Biophys Res Commun. 2003;302:520–525. doi: 10.1016/s0006-291x(03)00178-5. [DOI] [PubMed] [Google Scholar]
- 57.Song GQ, Chen JD. Synchronized gastric electrical stimulation improves delayed gastric emptying in nonobese mice with diabetic gastroparesis. J Appl Physiol. 2007;103:1560– 1564. doi: 10.1152/japplphysiol.00319.2007. [DOI] [PubMed] [Google Scholar]
- 58.Stengel A, Goebel M, Luckey A, Yuan PQ, Wang L, Taché Y. Cold ambient temperature reverses abdominal surgery-induced delayed gastric emptying and decreased plasma ghrelin levels in rats. Peptides. 2010;31:2229–2235. doi: 10.1016/j.peptides.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stenzel-Poore MP, Cameron VA, Vaughan J, Sawchenko PE, Vale W. Development of Cushing's syndrome in corticotropin-releasing factor transgenic mice. Endocrinology. 1992;130:3378–3386. doi: 10.1210/endo.130.6.1597149. [DOI] [PubMed] [Google Scholar]
- 60.Stenzel-Poore MP, Heinrichs SC, Rivest S, Koob GF, Vale WW. Overproduction of corticotropin-releasing factor in transgenic mice: a genetic model of anxiogenic behavior. J Neurosci. 1994;14:2579–2584. doi: 10.1523/JNEUROSCI.14-05-02579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taché Y, Yang H, Miampamba M, Martinez V, Yuan PQ. Role of brainstem TRH/TRH- R1 receptors in the vagal gastric cholinergic response to various stimuli including sham-feeding. Auton Neurosci. 2006;125:42–52. doi: 10.1016/j.autneu.2006.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tanaka R, Inui A, Asakawa A, Atsuchi K, Ataka K, Fujimiya M. New method of manometric measurement of gastroduodenal motility in conscious mice: effects of ghrelin and Y2 depletion. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1028–G1034. doi: 10.1152/ajpgi.90692.2008. [DOI] [PubMed] [Google Scholar]
- 63.Taniguchi H, Ariga H, Zheng J, Ludwig K, Mantyh C, Pappas TN, Takahashi T. Endogenous ghrelin and 5-HT regulate interdigestive gastrointestinal contractions in conscious rats. Am J Physiol Gastrointest Liver Physiol. 2008;295:G403–G411. doi: 10.1152/ajpgi.90260.2008. [DOI] [PubMed] [Google Scholar]
- 64.Taniguchi H, Ariga H, Zheng J, Ludwig K, Takahashi T. Effects of ghrelin on interdigestive contractions of the rat gastrointestinal tract. World J Gastroenterol. 2008;14:6299–6302. doi: 10.3748/wjg.14.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tatewaki M, Harris M, Uemura K, Ueno T, Hoshino E, Shiotani A, Pappas TN, Takahashi T. Dual effects of acupuncture on gastric motility in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2003;285:R862–R872. doi: 10.1152/ajpregu.00715.2002. [DOI] [PubMed] [Google Scholar]
- 66.Tumer C, Oflazoglu HD, Obay BD, Kelle M, Tasdemir E. Effect of ghrelin on gastric myoelectric activity and gastric emptying in rats. Regul Pept. 2007 doi: 10.1016/j.regpep.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 67.Vanneste G, Dhaese I, Sips P, Buys E, Brouckaert P, Lefebvre RA. Gastric motility in soluble guanylate cyclase alpha 1 knock-out mice. J Physiol. 2007;584:907–920. doi: 10.1113/jphysiol.2007.140608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang L, Basa NR, Shaikh A, Luckey A, Heber D, St Pierre DH, Taché Y. LPS inhibits fasted plasma ghrelin levels in rats: role of IL-1 and PGs and functional implications. Am J Physiol Gastrointest Liver Physiol. 2006;291:G611–G620. doi: 10.1152/ajpgi.00533.2005. [DOI] [PubMed] [Google Scholar]
- 69.Wang L, Cardin S, Martinez V, Taché Y. Intracerebroventricular CRF inhibits cold restraint-induced c-fos expression in the dorsal motor nucleus of the vagus and gastric erosions in rats. Brain Res. 1996;736:44–53. doi: 10.1016/0006-8993(96)00726-3. [DOI] [PubMed] [Google Scholar]
- 70.Weisbrodt NW, Bass P. In vivo motility techniques. In: Gaginella TS, editor. Handbook of methods in gastrointestinal pharmacology. CRC press; Boca Raton: 1996. pp. 163–188. [Google Scholar]
- 71.Yang H, Wong H, Wu V, Walsh JH, Taché Y. Somatostatin monoclonal antibody immunoneutralization increases gastrin and gastric acid secretion in urethane-anesthetized rats. Gastroenterology. 1990;99:659–665. doi: 10.1016/0016-5085(90)90952-w. [DOI] [PubMed] [Google Scholar]
- 72.Yang H, Wu SV, Ishikawa T, Taché Y. Cold exposure elevates thyrotropin-releasing hormone gene expression in medullary raphe nuclei: relationship with vagally mediated gastric erosions. Neuroscience. 1994;61:655–663. doi: 10.1016/0306-4522(94)90442-1. [DOI] [PubMed] [Google Scholar]
- 73.Zheng J, Ariga H, Taniguchi H, Ludwig K, Takahashi T. Ghrelin regulates gastric phase III-like contractions in freely moving conscious mice. Neurogastroenterol Motil. 2009;21:78–84. doi: 10.1111/j.1365-2982.2008.01179.x. [DOI] [PubMed] [Google Scholar]
- 74.Zittel TT, Meile T, Huge A, Kreis ME, Becker HD, Jehle EC. Preoperative intraluminal application of capsaicin increases postoperative gastric and colonic motility in rats. J Gastrointest Surg. 2001;5:503–513. doi: 10.1016/s1091-255x(01)80088-3. [DOI] [PubMed] [Google Scholar]