Abstract
Hypothesized circuitry enabling information storage can be tested by attempting to implant memory directly in the brain in the absence of normal experience. Previously, we found that tone paired with activation of the cholinergic nucleus basalis (NB) does induce behavioral memory that shares cardinal features with natural memory; it is associative, highly specific, rapidly formed, consolidates and shows intermediate retention. Here we determine if implanted memory also exhibits long-term consolidation and retention. Adult male rats were first tested for behavioral responses (disruption of ongoing respiration) to tones (1–15 kHz), yielding pre-training behavioral frequency generalization gradients. They next received three days of training with a conditioned stimulus (CS) tone (8.0 kHz, 70 dB, 2 s) either paired (n = 7) or unpaired (n = 6) with moderate electrical stimulation of the nucleus basalis (~65 μA, 100 Hz, 0.2 s, co-terminating with CS offset). Testing for long-term retention was performed by obtaining post-training behavioral frequency generalization gradients 24 h and 2 weeks after training. At 24 h post-training, the Paired group exhibited specific associative behavioral memory, manifested by larger responses to the CS frequency band than the Unpaired group. This memory was retained 2 weeks post-training. Moreover, two weeks later, the specificity and magnitude of memory had become greater, indicating that the implanted memory had undergone consolidation. Overall, the results demonstrate the validity of NB-implanted memory for understanding natural memory and that activation of the cholinergic nucleus basalis is sufficient to form natural associative memory.
Keywords: Acetylcholine, Association, Behavioral state, Nucleus basalis
1. Introduction
Several standard approaches are used to investigate neural circuitry that is hypothesized to enable memory storage. These include recording changes in the activity of a putative involved structure consequent to learning, pre or post-training inactivation (permanent or reversible), direct stimulation (electrical or chemical) to facilitate or impair memory and targeting the neural structure by molecular genetics methods. However, if the hypothesized mechanism is sufficient to normally form behavioral memory, then it should be possible to implant memory directly into the brain by appropriate activation of that circuitry.
We have used this approach in a series of experiments to test the hypothesis that experience-based activation of the nucleus basalis (NB) (which is the major source of cortical acetylcholine [ACh]; Mesulam, Mufson, Levey, & Wainer, 1983), is engaged in the formation of at least some types of behavioral memory. The model under test postulates that activation of the cholinergic nucleus basalis serves as a “final common path” that is sufficient to promote or induce long-term memory based on the formation of information storage in the cerebral cortex, and perhaps elsewhere. In this schema, the nucleus basalis is “downstream” of motivational and emotional systems (Weinberger, 1998; Weinberger, Ashe, Metherate, McKenna, Diamond, Bakin, Lennartz, & Cassady, 1990). It is derived, in part, from long-standing evidence that the cholinergic system is involved in the formation of memory (Deutsch, 1971; Flood, Landry, & Jarvik, 1981) and memory-related cortical plasticity (Edeline, 2003).
We explicitly distinguish behavioral memory from associative neural plasticity, which was previously known to be induced by tone paired with electrical stimulation of the nucleus basalis (NBstm) (Bakin & Weinberger, 1996, Bjordahl, Dimyan, & Weinberger, 1998; Dimyan & Weinberger, 1999; Kilgard, Pandya, Vazquez, Gehi, Schreiner, & Merzenich, 2001). Unfortunately, such neural plasticity is often erroneously assumed to constitute “memory” rather than a memory substrate. Insofar as memory is validated at the behavioral level, assuming that a neural process is memory constitutes a “category error”, i.e., equating a part with the whole (Ryle, 1963).
We emphasize that putative implanted memory must past the test of having the same attributes as natural memory. Indeed, it should be difficult to determine whether memory-dependent changes in behavior reflect natural or implanted memory based on standard behavioral measures. Another important criterion is that memory-enabling stimulation of the brain not be mnemonically effective simply because it replaces either rewarding or punishing effects of standard reinforcers. Such motivational substitution effects have long been known (e.g., reward, Olds, 1962; nociception, Guilbaud, 1985).
The initial study in this program of research revealed that pairing a tone with NBstm produced associative memory. Furthermore, this implanted memory was specific to the frequency of the conditioned stimulus (CS) (McLin, Miasnikov, & Weinberger, 2002). Subsequent studies revealed that this putative implanted memory shares other cardinal features of natural associative memory, including rapid development, consolidation and retention and that NBstm was motivationally neutral (Weinberger, 2007).
To provide for a comprehensive characterization of implanted memory, the goal of this experiment was to determine if it exhibits a critical feature of natural associative memory, viz., long-term retention, herein defined as maintenance of specific memory for two weeks. In so doing, it was possible to also determine the consolidation dynamics of NBstm-implanted memory, i.e., the extent to which it changes in specificity and strength without any further training, attributes previously found for auditory cortical specific associative plasticity (Galván & Weinberger, 2002).
2. Materials and Methods
The materials and methods were mainly identical to those previously reported (Weinberger, Miasnikov, & Chen, 2009), and will be described only briefly. All procedures were performed in accordance with the University of California Irvine Animal Research Committee and the NIH Animal Welfare guidelines. During training and testing, subjects were continuously monitored by video cameras.
2.1. Subjects and surgery
The subjects were 13 adult male Sprague–Dawley rats (396 ± 33 g, mean ± sd), housed individually with ad libitum food and water, on a 12/12 h light–dark cycle (lights on at 6:15 am). Following several days of adaptation to the vivarium, they were handled and learned to sit calmly during attachment of a thermistor assembly and a cable to their skull pedestal. Under general anesthesia (sodium pentobarbital, 40 mg/kg, i.p.), a stainless steel epidural screw electrode was inserted over the right primary auditory cortex at the locus showing the largest amplitude evoked potential to a contralateral noise burst. Two screws over the frontal sinus served as reference electrodes. The EEG from the auditory cortex and respiration recordings were used to assess arousal state during training and testing. The EEG was also used to insure that NBstm-elicited cortical activation, which is an index of the cortical release of acetylcholine during natural behavior and by NBstm (e.g., Celesia & Jasper, 1966; Détári, Rasmusson, & Semba, 1997, 1999; Duque, Balatoni, Détári, & Zaborszky, 2000). A concentric bipolar stainless steel stimulating electrode was implanted either through the contralateral (left) hemisphere or vertically into the right nucleus basalis, aimed at the caudal nucleus basalis (ventrolateral internal capsule, ventromedial lateral globus pallidus and nucleus basalis of Meynert), which are sites of cholinergic projections to the auditory cortex (Bigl, Woolf, & Butcher, 1982; Moriizumi & Hattori, 1992). The effective locus was confirmed by obtaining at least a few seconds of auditory cortical EEG activation to NBstm (pairs of 0.2 ms opposite polarity pulses, 100 Hz, 200 ms trains; S88 stimulator, PSIU6 isolation units, Grass Instrument Co., Quincy, MA). A dental acrylic pedestal was built with two aluminum hex threaded standoffs embedded therein, and all leads connected to a miniature socket that could be led to a commutator via a multi-conductor cable. Subjects were allowed 1–2 weeks to recover from surgery.
2.2. Stimuli, recording and data analyses
Training and testing took place while each subject was in an acoustic-damping box (23 × 23 × 31 cm) supplied with fresh bedding, contained in a double-walled acoustic chamber. Acoustic stimuli were pure tones (1.0–15.0 kHz, 2 s duration, cosine 10 ms rise/fall time [10% to 90%], 70 dB SPL) produced by Tucker–Davis Technologies (TDT, Alachua, FL) System 3 components and delivered to a calibrated loudspeakers positioned about 35 cm above the floor of the box. NBstm current used during training was ~65 μA, a moderate level that elicited no muscular or behavioral responses but was known to be sufficient to induce specific associative memory (e.g., Weinberger, Miasnikov, & Chen, 2006).
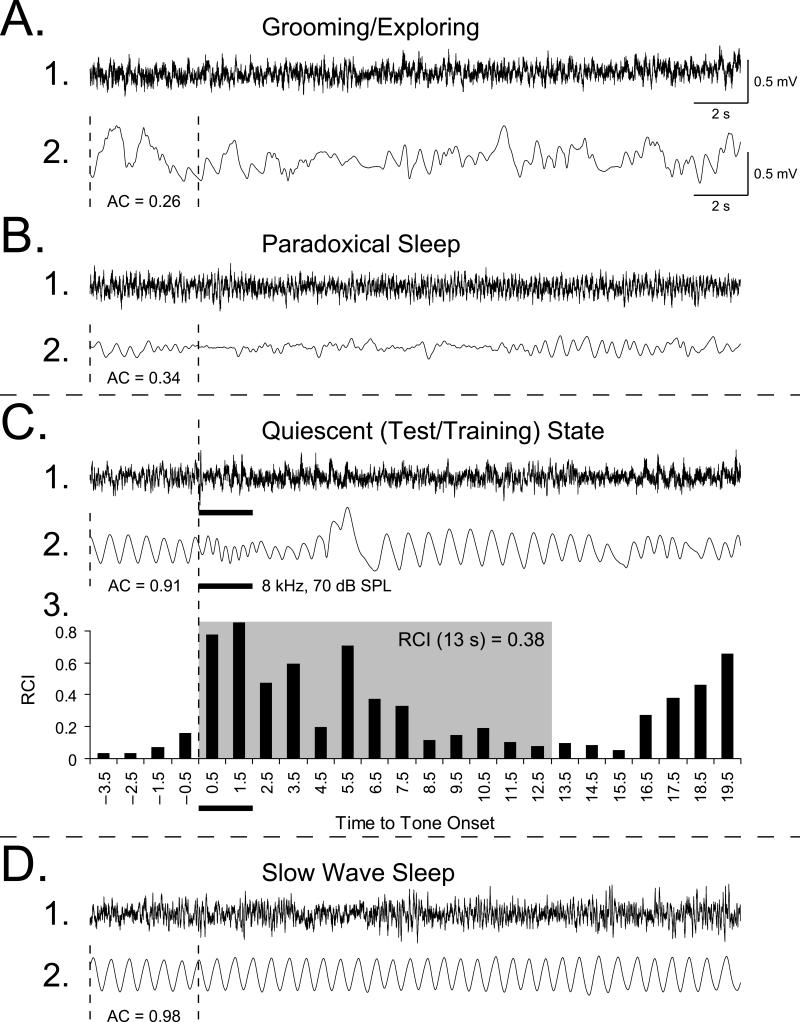
To assess the implantation of memory, we measured disruption of the ongoing pattern of regular respiration to various tones, before and after training. Respiration is a sensitive measure of state and associative learning (see 4.2. Changes of respiratory behavior in the detection of implanted memory). Respiration was detected as breathing-related thermal fluctuations with a glass-encapsulated thermistor attached to a lightweight pedestal-mounted assembly positioned in front of a naris, as described previously (McLin et al., 2002). Usage of such noninvasive method to measure ventilatory variables without restraining the animals is important because restriction is a potent stressor that strongly affects breathing (Dauger, Nsegbe, Vardon, Gaultier, & Gallego, 1998). The amplified output signal was fed to an ADC module, and the autocorrelation function (AC) was calculated on-line. The AC was used to present tones only when the subject was in a quiescent behavioral state (Miasnikov, Chen, & Weinberger, 2008b), thus excluding states such as exploration/grooming and paradoxical (REM) sleep (Figs. 1A, B and D). The pattern of respiration can serve as a reliable marker for each state (Weinberger et al., 2006). Trials meeting the criterion of regular baseline (0.700 < AC < 0.975) for over 4 s (Fig. 1C) were presented if the scheduled inter-trial interval period had passed. This state control was employed to avoid giving stimuli when very high levels of ACh were being released in the cortex, as during exploration or REM sleep, or very low, as during slow-wave sleep (Giovannini, Rakovska, Benton, Pazzagli, Bianchi, & Pepeu, 2001; Jasper & Tessier, 1971) to prevent a ceiling or floor effects, thus promoting a physiologically-effective release of ACh by NBstm.
Fig. 1. Behavioral state control and quantification of changes in ongoing respiration.
Examples of measures of respiration corresponding to major behavioral states: exploring/grooming, paradoxical (REM) sleep, quiet waking and slow wave sleep. Shown are the EEGs from the primary auditory cortex (line 1) and the respiration records (line 2). (A) During periods of preoccupation with ongoing activity, such as exploration or grooming, while the EEG is low-voltage fast (A1), the respiration pattern is chaotic (A2: AC = 0.26). (B) During paradoxical (REM) sleep, the EEG is low-voltage fast (B1) and respiration is irregular although less chaotic (B2: AC = 0.34) with many high-frequency shallow breathing movements. (C) During quiet waking (Quiescent State), which is the state used for presenting tone during testing and training, the EEG is less desynchronized (C1), animals are not moving, respiration is very regular (C2: AC = 0.91) and respiration can be easily disrupted by tones (thick horizontal bars). (D) During deep slow wave sleep, the EEG is synchronized with many high-voltage waveforms (D1), animals are not moving and respiration is extremely regular (D2: AC = 0.98). The respiration autocorrelation function (AC) was continuously calculated on-line over 4-s long epochs. When a randomly-selected inter-trial interval had passed, the software compared the current value of an AC with pre-selected thresholds (0.70 • AC • 0.925) and triggered a stimulus. C3: Quantification of a regular sinusoidal baseline respiration record (first 4 s) disrupted by tone presentation. The “Respiration Change Index” (RCI, Methods) is sensitive to both increases and decreases in signal amplitude and frequency. This example shows a response of a Paired animal to the CS tone, recorded while obtaining the behavioral frequency generalization gradient 24 h following completion of the pairing session. The shaded area indicates the first 13-s portion of the peri-stimulus respiratory record containing the majority of the tone-evoked response. The RCI values found within this epoch were used in the behavior data analysis.
Major evoked changes in respiration occurred within the first 13 s after tone onset (although certain changes in respiration can be detected at up to 20 s). The collected data were used to calculate a “Respiration Change Index” (RCI), on a second-by-second basis. The index was sensitive to increases and decreases of both frequency and amplitude of respiration response. RCIs were calculated as: RCIi = (|Posti – Pre|) / (Posti + Pre) where Post and Pre were the values of power of respiration signal (McLin et al., 2002). An RCI value of zero would indicate no change and a value of 1.0 would indicate complete cessation of respiration. An example of a record of the tone-elicited disruption of respiration is provided in Fig. 1C2 and its RCI quantification in Fig. 1C3. Statistical analyses used SPSS v. 17 software (SPSS, Chicago, IL).
2.3. Experimental design
The subjects were assigned to two groups, Paired (n = 7) and Unpaired (n = 6). There was no difference in age (t(11) = 1.308, p > 0.20, two-tailed t-test; 92 ± 7 days of age, mean ± sd for the population) or weight (t(11) = 0.027, p > 0.95; 396 ± 34 g) between the Paired and Unpaired groups. After recovery from surgery, NBstm thresholds were determined while subjects were in the state of slow-wave sleep. NBstm was delivered every few minutes at increasing levels starting at ~30 μA (100 Hz bipolar, 200 ms train) until stimulation reliably elicited 3–5 s epoch of cortical activation (decrease in low frequency activity often accompanied by increase in gamma activity). The current levels used in subsequent training with NB stimulation did not change any ongoing behavior.
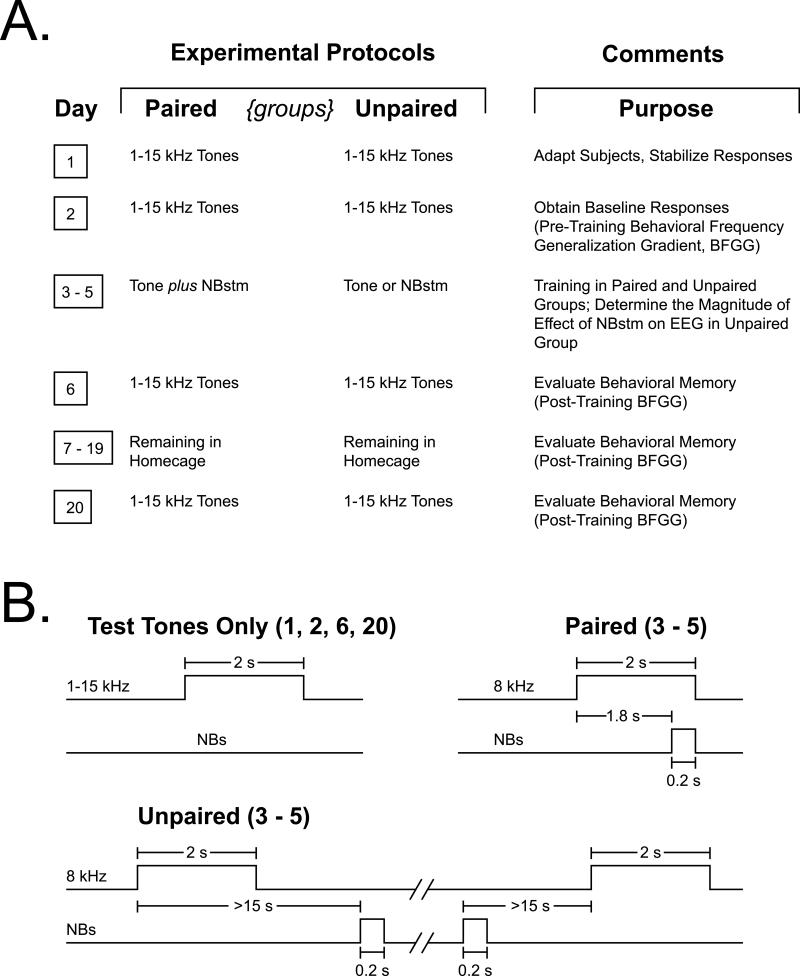
To induce and subsequently evaluate stimulus-specific implanted memory, we used the approach of acquiring behavioral baseline responses to many frequencies, then training with one frequency and later testing the training effects with many frequencies, 24 h and 2 weeks post-training (Fig. 2). This yielded pre- and post-training behavioral frequency generalization gradients. Specificity of memory would be indicated by a peaked (rather than flat) frequency generalization gradient (Mostofsky, 1965). The protocol required twenty consecutive days (Fig. 2A). Days 1–2: pre-training baseline response to test tones (Day 1 was acclimatization and data were not analyzed). Data from Day 2 constituted the pre-training baseline. Days 3–5 each had one training session, in which a CS tone was paired with NBstm in the Paired group (200 trials/day = 200 tone + 200 NBstm presentations), or the same number of tones and NBstm presented in the Unpaired group. In the Paired group, the CS tone (8.0 kHz, 2 s, 70 dB SPL) was followed by 0.2 s of NBstm co-terminating with CS offset (i.e., the CS–US interval was 1.8 s). In the Unpaired group, tone and NBstm never overlapped. Inter-trial intervals were 54.3 ± 3.6 s (mean ± sd) during pairing sessions, and 28.7 ± 1.8 s during unpaired training sessions (Fig. 1B). Potential transfer between training and frequency testing sessions was reduced by using different contexts for the two types of session. Thus, animals were delivered to the lab via different circuitous routes and they were trained in the dark (red light) but tested (pre- and post-training) in the light. On Day 6, post-training responses to tones were obtained. The animals were then left for 13 days (Days 7–19) in their home cages undisturbed and re-tested on Day 20.
Fig. 2. Experimental design.
(A) The stages of the experiment used to obtain pre-training and post-training behavioral frequency generalization gradients for the Paired and Unpaired groups. (B) Detailed temporal relationships of stimuli for the various phases of the experiment: delivery of test tones (Days 1, 2, 6 and 20) and tone vs. NBstm paired and unpaired (Days 3–5).
On frequency test days, subjects received random presentation of tones of nine different frequencies (1.00, 2.75, 4.50, 6.25, 8.00, 9.75, 11.50, 13.25, and 15.00 kHz, 70 dB SPL, constrained only by presenting not more than two stimuli of the same frequency in a row) for 200 trials total (Fig. 2B). Intervals between tone presentations averaged 54.5 ± 2.5 s (mean ± sd) and were not different statistically (t(8) = 1.17, p > 0.25, two-tailed t-test) between the Paired and Unpaired groups. The initial statistical analyses of respiration responses were based on averaging the data for triplets of frequencies: 1.00–4.50 kHz (low band), 6.25–9.75 kHz (middle band) and 11.50–15.00 kHz (upper band). The middle frequency band (6.25, 8.0 [CS], 9.75 kHz) is referred to as the “CS band”. Some subsequent detailed analysis involved responses to individual test frequencies.
2.4. Histology
Following the completion of the experiment, an electrolytic lesion (4 ms pulses at 100 Hz, 500 μA for 10–20 s) was made with bipolar current through the stimulating electrode while the animal was under sodium pentobarbital anesthesia. Perfusion, processing of tissue and location of the recording and stimulating electrodes were as previously reported (Miasnikov, Chen, & Weinberger, 2009). The recording and stimulation sites were plotted on coordinates derived from the atlas of Paxinos and Watson (1997).
3. Results
3.1. Location of electrodes, stimulation current
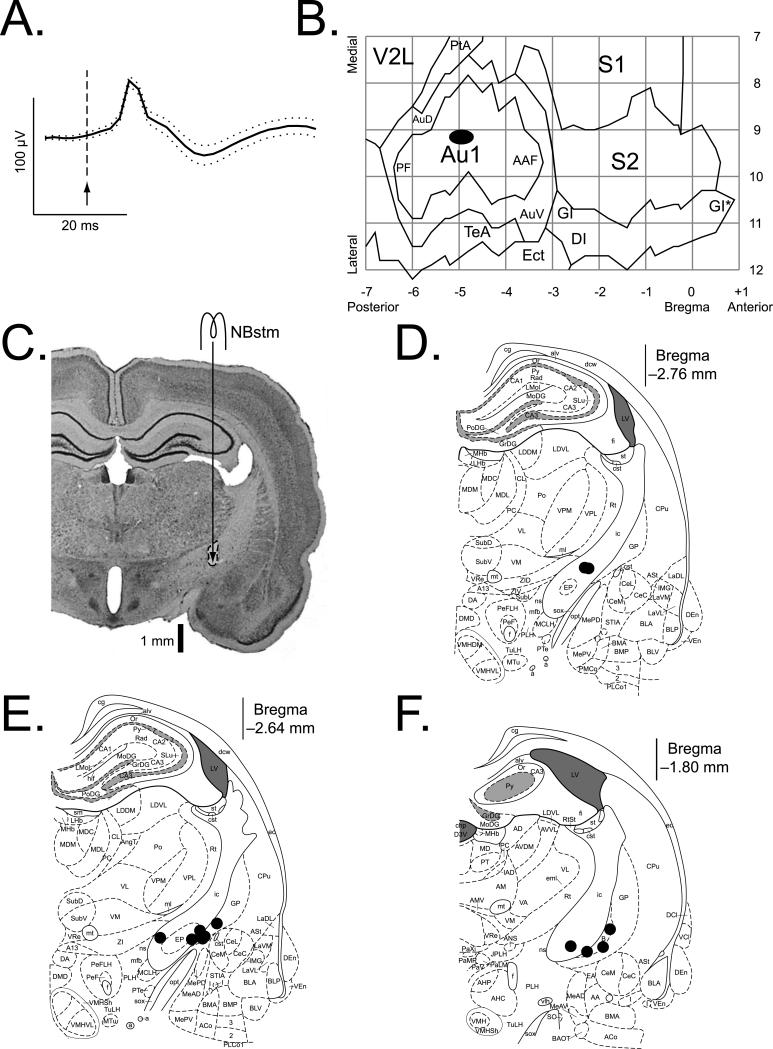
The grand mean auditory cortical evoked potential is given in Fig. 3A. It exhibits the standard initial positivity observed in surface and upper layer recording and is consistent with activity evoked in the primary field (A1). Consistent with this, cortical recording electrodes were all located above A1 (Fig. 3B). The recording sites of the Paired and Unpaired groups were intermingled and differed neither in the AP (t(11) = 0.25, p > 0.80, two-tailed t-test) nor ML (t(11) = 1.20, p > 0.25) dimensions. The NB stimulation sites of the Paired and Unpaired groups were intermingled and did not differ in the AP (t(11) = 2.12, p > 0.05, two-tailed t-test), ML (t(11) = 0.78, p > 0.45) or DV (t(11) = 0.72, p > 0.45) dimensions (Figs. 3C–F). There was no difference in NBstm current between the groups (t(11) = 0.11, p > 0.90; 67 ± 6 μA, mean ± sd).
Fig. 3. Location of recording and stimulation sites.
(A) Grand average (± sd) of the local field potential (LFP) for all animals, recorded over the auditory cortex during surgery (3 ms bursts of white noise, 15/subject, 13 subjects, n = 195). (B) The auditory cortex EEG recording locations. The black oval indicates the location of epidural recordings based on their stereotaxic coordinates using a cortical map derived from Paxinos and Watson (1997). The center of the oval corresponds to the X–Y coordinates of the mean for the group on the flattened standardized cortical surface, and the horizontal and vertical spreads of the oval correspond to the ranges from mean – se to mean + se for the AP (–4.98 ± 0.25 mm, mean ± se) and the ML (9.14 ± 0.13 mm) coordinates, respectively. The sites of recording in Paired and Unpaired groups overlapped, did not differ statistically (see Results) and thus are shown as a single group. Abbreviations: Au1, primary auditory cortex; PF, posterior auditory field; AAF, anterior auditory field; AuD, secondary auditory cortex, dorsal; AuV, secondary auditory cortex, ventral; PtA, parietal association cortex; V2L, secondary visual cortex, lateral area; S1, primary somatosensory cortex; S2, secondary somatosensory cortex; TeA, temporal association cortex; Ect, ectorhinal cortex; DI, dysgranular insular cortex; GI, granular insular cortex; (*), an extension of GI that was arbitrarily cut to fit the diagram. (C) Nissl section showing the trajectory of the stimulating electrode and location of lesion site (area of stimulation during training) in the nucleus basalis. (D–F) Diagrams of the three coronal sections (AP = –2.76, AP = –2.64 and AP = –1.80 mm) from the atlas showing the NB stimulation sites (Paxinos & Watson, 2007). The stimulation sites in the Paired and the Unpaired groups were intermingled and did not differ statistically (see Results) and thus are shown as a single group. In all animals, stimulation was within the caudal nucleus basalis (ventrolateral internal capsule, ventromedial lateral globus pallidus and nucleus basalis of Meynert) which projects to the auditory cortex. All stimulation sites were in the basal forebrain within structures containing corticopetal cholinergic cells (Bigl, Woolf, & Butcher, 1982; Johnston, McKinney, & Coyle, 1979; Mesulam, Mufson, Levey, & Wainer, 1983). In general, the coordinates of the area of stimulation, as referenced to the coronal plane, were as follows: AP, –2.38 ± 0.43 mm (mean ± sd); ML, 3.37 ± 0.41 mm; DV, 7.52 ± 0.15 mm. Abbreviations: B, basal nucleus of Meynert; CeM, amygdala central nucleus, medial; CeL, amygdala central nucleus, lateral; CPu, caudate–putamen; IC, internal capsule; IPAC, interstitial nucleus of posterior limb of anterior commissure; GP, lateral globus pallidus; LH, lateral hypothalamus; EP, medial globus pallidus; SI, substantia innominata; Rt, reticular thalamic nucleus.
3.2. Effects of training on NB-induced memory
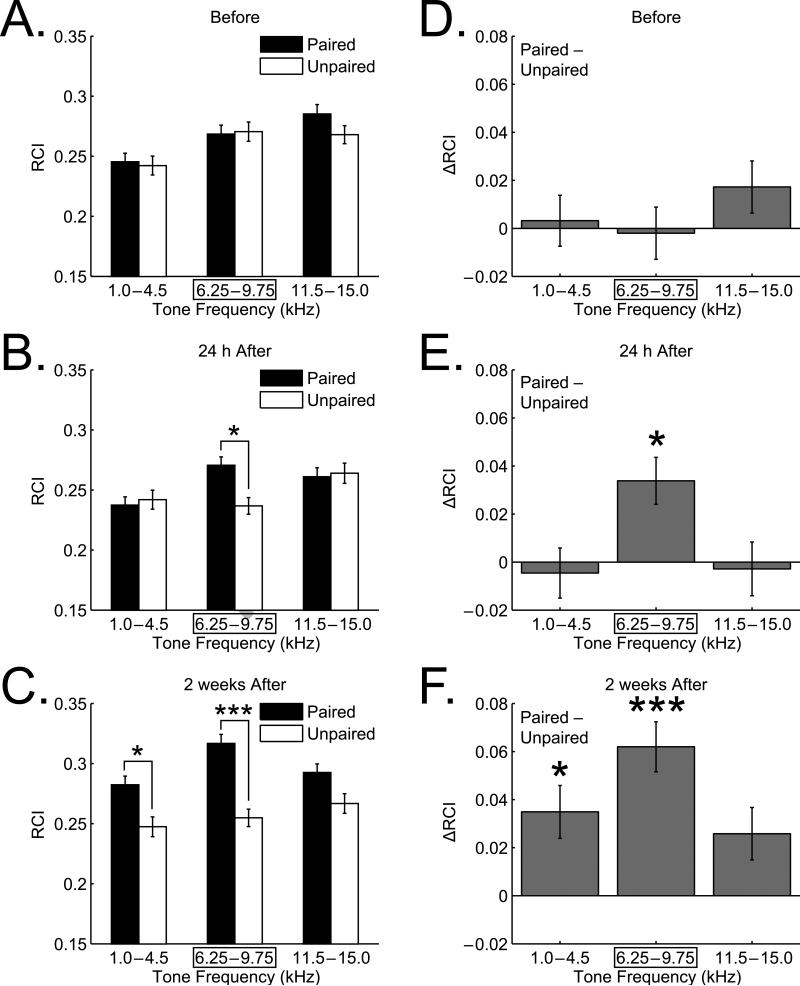
Fig. 4 summarizes the behavioral group data. Before training, the animals were differentially sensitive to acoustic frequency (two-way ANOVA, Frequency factor: F(2, 2601) = 10.08, p < 0.00005), as expected by the rat audiogram (Heffner, Heffner, Contos, & Ott, 1994) and previously found as measured by disruption of the ongoing pattern of respiration (e.g., Miasnikov et al., 2009). However, there were no significant differences between the Paired and Unpaired groups across frequency (F(1, 2601) = 0.96, p > 0.30). Moreover, the Group × Frequency interaction was not significant (F(2, 2601) = 0.86, p > 0.40) (Figs. 4A and D).
Fig. 4. Effect of training and retention interval on NB-implanted memory.
Graph bars show mean ± se of Respiration Change Index (RCI). (A) Pre-training (“Before”) frequency generalization gradients to tones in three frequency bands: “Low” (1.0–4.5 kHz), “CS” (6.25–9.75 kHz) and “High” (11.5–15.0 kHz). There were no significant differences between Paired and Unpaired groups before training. (B) Post-training at 24 h. Note the increased response of the Paired group in the CS frequency band. The interactions between groups and tone frequency were significant, due to the CS frequency band, indicating CS-band specific associative learning. (C) Post-training at 2 weeks following last training session. Note the marked increase in response of the Paired group for the CS-band compared to the 24 h value, with little change in response in the Unpaired group, indicative of greater memory strength due to consolidation processes over the intervening period. The Low frequency band also developed an increased response of lesser magnitude. (D–F) Between-group differences (Paired minus Unpaired) for each frequency band. (D) Before training, there were no significant differences for any frequency band. (E) Post-training at 24 h. Increased behavioral responses developed only for the Paired group and only for the CS frequency band. This was attributable largely to a significant increase in response at 9.75 kHz, but not at the CS frequency of 8.0 kHz. (F) Two weeks post-training, the Paired group developed even larger responses to the CS frequency band, now attributable to a significant response increase to the CS frequency of 8.0 kHz. Additionally, the high and low frequency bands exhibited increased responses compared to the 24 h retention test but only the low band difference reached statistical significance. Overall, the findings indicate CS-band specific associative memory 24 h post-training, which was increased in strength after a two-week period, accompanied by progressive enhancement in its specificity to the training frequency (8.0 kHz) with increased generalization to the lower frequency band. *p < 0.05, ***p < 0.005.
In contrast, 24 h after the end of training there was evidence of NB-implanted memory (Figs. 4B and E). As expected, the Frequency factor remained significant (F(2, 2600) = 4.76, p < 0.01) while the Group factor was still not significant (F(1, 2600) = 2.12, p > 0.10). However, the Group × Frequency interaction was significant (F(2, 2600) = 4.35, p < 0.02). Post-hoc analyses indicated that this was due to a between-group difference limited to the CS frequency band (Tukey's test: CS band, mean difference [MD] = 0.034, p < 0.015; low band, MD = –0.005, p > 0.99; high band, MD = –0.003, p > 0.99). These findings indicate that the Paired group had acquired a CS-specific associative memory following pairing of tone with stimulation of the nucleus basalis.
Further changes developed during the next 14 days (Figs. 4C and F). Specifically, the Group factor now reached significance (F(1, 2600) = 43.23, p < 0.00001), while the Frequency factor (F(2, 2600) = 3.99, p < 0.02) and the Group × Frequency interaction (F(2, 2600) = 3.10, p < 0.05) remained significant. The between-group difference was significant at the CS frequency band (Tukey's test: MD = 0.062, p < 0.00001) and at the low frequency band (MD = 0.035, p < 0.02) while responses did not reach significance at the high band (MD = 0.026, p > 0.15).
Additional analyses of between-group differences (Paired minus Unpaired), based on individual frequencies, further revealed the nature of the changes. While the differences between the Paired and Unpaired groups increased at the CS band one day post-training (Fig. 4E), this difference was not due to a change precisely at the CS frequency of 8.0 kHz, but to its adjacent high frequency of 9.75 kHz (Tukey's test, MD = 0.071, p < 0.01) (Fig. 5). Changes in response to other frequencies in the CS band were not significant (6.25 kHz, MD = 0.027, p > 0.98; 8.0 kHz, MD = 0.007, p > 0.99). Given prior findings of increased responses over four days (consolidation; Weinberger et al., 2009), we predicted increased responses at two weeks. Indeed, there were significant dynamics of responses at 2 weeks vs. 24 hours post-training. First, responses to all nine test tones taken together became stronger (t(16) = 2.944, p < 0.01, two-tailed t-test). Second, responses at the CS band become stronger (t(37) = 1.777, p < 0.05, one-tailed t-test). Finally, within the CS-band, only responses to the CS itself become stronger at 2 weeks vs. 24 hours (t(11) = 2.152, p < 0.05, one-tailed t-test).
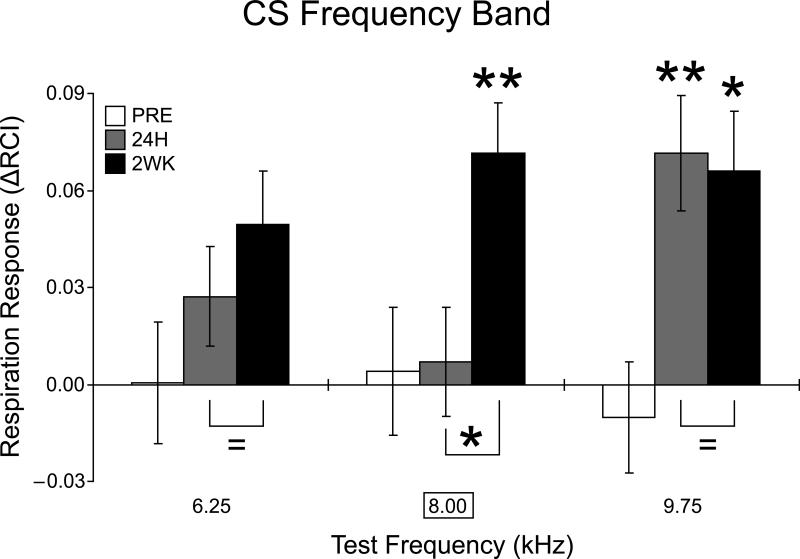
Fig. 5. Changes in response to individual frequencies within the CS band.
Note the absence of significant change to the CS frequency (8.0 kHz) 24 h after training vs. the large increase in response two weeks later. *p < 0.05, **p < 0.01.
Although the low frequency band developed increased responses after two weeks (see above), there were no significant differences among individual tones (1.0, 2.75, 4.50 kHz; all p values > 0.40) between the Paired and Unpaired groups 2 weeks post-training. In contrast, the dynamics were different for the CS band. Examination of the CS frequency band revealed increased response specificity. While responses to 6.25 kHz never exhibited any significant change after training whether 24 h or 2 weeks post-training (p > 0.40), and responses to 9.75 kHz remained significantly larger 2 weeks after training (p < 0.03) as they were 24 h after training, the responses to the CS frequency of 8.0 kHz, which were unchanged after 24 h, were markedly increased two weeks later (MD = 0.066, p < 0.01) (Fig. 5).
4. Discussion
4.1. Summary of results
The major finding is that specific associative memory induced by pairing a tone with stimulation of the nucleus basalis exhibits long-term retention, tested here at two weeks post-training. In a related study using different parameters of NBstm to implant specific associative memory (below), we found retention when tested at four days post-training (Weinberger et al., 2009), a period that may be considered to index “intermediate-term” memory.
A related finding is that the specificity of this memory consolidates over a two-week period and in so doing exhibits both increased specificity for the CS frequency and a general reduction in specificity across the frequency domain. Consolidation is herein used to indicate a strengthening or increased specificity, or both, of memory after initial learning in the absence of additional training. This formulation is consistent with the core meaning of consolidation, although such time-dependent processes in memory storage are more often studied by the use of post-training modulatory treatments that generally reveal increased resistance to change over time (McGaugh, 2000). Evidence for memory consolidation is provided by measures of frequency specificity of learning and the magnitude of behavioral change. Twenty-four hours after the end of training, partial specificity was evident as a statistically significant increase in responses to the CS band, which proved to be attributable to response facilitation at 9.75 kHz, the tone adjacent to the 8.0 kHz CS. However, at this time there was no significant change at the CS frequency. There is no a priori reason to expect that specificity would be exact, i.e., manifest at the CS frequency, insofar as there was no discrimination training, which would promote specificity (Mackintosh, 1974). In contrast, responses to 8.0 kHz increased during the two-week retention period, while responses to 9.75 kHz did not further change. Furthermore, the magnitude of difference between the Paired and Unpaired groups increased for responses to CS band frequencies.
However, the temporal dynamics are more complicated. In addition to increased specificity within the CS frequency band, the two-week retention test revealed increased responses to the low and high frequency bands. The high band did not attain statistical significance. Increased low frequency responding was significant, however, although responses to the three low frequencies (1.0, 2.75 and 4.50 kHz) increased as a group, no single frequency showed a significant increase.
Previously, we studied consolidation using weak NB stimulation (~45 μA) to induce associative memory lacking any specificity, i.e., responses to all tones were increased at 24 h after training in the paired group alone. This permitted detection of consolidation in the form of increased specificity over the following three days, by which time only the CS band exhibited an associative increase in response (Weinberger et al., 2009). In the present study, we used a moderate level of stimulation (67 ± 6 μA), which produced evidence of specificity at 24 h post-training as seen in a significant increase in response restricted to the CS-band. Nonetheless, further specificity could develop over two weeks, i.e., increased responses to the CS frequency of 8.0 kHz. Thus, consolidation processes for implanted memory can be enhanced. Moreover, the time course of consolidation can be extended by using a higher level of NB stimulation (e.g., ~65 μA), which presumably increases the amount of acetylcholine that is released.
4.2. Changes of respiratory behavior in the detection of implanted memory
The behavioral measure used in this and previous demonstrations of NBstm-implanted memory is the interruption of ongoing respiration. Although conditioned respiratory response is not currently a commonly employed indicator of animal associative learning, it has a long and distinguished history. First reported by Sherrington (1900), the study of respiration was exploited by many key workers (e.g., Bekhterev, 1932; Watson, 1916) and even used in classic studies that determined the limits of animal hearing (Wever, 1930). Findings of respiratory conditioned responses in the rat (Kappauf & Schlosberg, 1937) were extended to all mammals tested, as well as to fish and reptiles (Lennartz & Weinberger, 1992). Respiration responses continue to find useful application in studies of human learning, psychophysiology and psychopathology as the only vital function under both voluntary and involuntary control (Ley, 1999). As explained in Methods, the respiratory autocorrelogram provides a sensitive and accurate measure of the state of arousal, enabling the presentation of tone trials during periods in which the release of acetylcholine in the cerebral cortex is neither too low nor too high (Fig. 2). In summary, the use of changes in respiratory behavior is both appropriate for the detection of implanted memory and of general applicability.
Respiratory and autonomic conditioned responses (CRs) (e.g., blood pressure, heart rate, galvanic skin response, papillary dilation) are not, strictly speaking, “conditioned” in the same sense that, e.g., the eyeblink is conditioned. Rather, they constitute a constellation of highly sensitive modifications of ongoing processes that develop conditioned changes rapidly, and index the detection of stimulus–stimulus (S–S) associations; critically, and in contradistinction of specific somatic CRs (e.g., eyeblink, limb flexion), respiratory and autonomic CRs develop more or less simultaneously for all unconditioned stimuli (UCS) regardless of their locus of application (Lennartz & Weinberger, 1992; Winters, 2002). In contrast, specific somatic CRs develop more slowly, are specific to the locus and nature of the UCS, indicate stimulus–response (S–R) associations and can be considered attempts to reduce the impact of nociceptive reinforcers (Konorski, 1967). The circuitry underlying specific S–R associations is more amenable to comprehensive identification, the most notable example is the eyeblink CR (Thompson & Steinmetz, 2009).
4.3. The validity of “implanted memory”
It is appropriate to question if the behavioral changes in the Paired group, reported here and previously using NBstm, are indicative of actual associative memory. If so, then such behavior should be accepted as indicating the validity of implanted memory in the same way that the same changes in behavior are accepted as criteria for natural memory.
It might be thought that the behavioral effect (i.e., CS-specific generalization gradient) is non-associative. However, the two groups did not differ behaviorally before training but differed thereafter. Insofar as the only difference in treatment was whether the tone was paired or not with NBstm, non-associative effects such as sensitization or pseudo-conditioning can be ruled out. Moreover, the level of NBstm current (67 μA) and the loci of stimulation were the same for the two groups. Therefore, the effects can be attributed to associative processes.
A related critique might be that although the effects are associative, the NBstm simply caused pain or pleasure, e.g., by directly activating nociceptive or reward structures (see Introduction). In this case, memory would not have been “implanted” but simply formed in the natural manner, albeit with positive or negative reinforcement produced by brain stimulation. However, implanted memory reported here was induced using NBstm parameters shown not to possess any positive or negative motivational significance in a previous study (Miasnikov, Chen, Gross, Poytress, & Weinberger, 2008a).
Another potential concern is that, despite induction by NBstm, implanted memory might be due to current spread over neural tissue and not actually involve the cholinergic system. However, pharmacological studies have shown that NB-implanted memory does require the engagement of central muscarinic receptors (Miasnikov et al., 2008b).
A systematic change in behavior due to associative processes is a major criterion for classical (Pavlovian) conditioning, which is the appropriate domain for this study. As noted above, this criterion has been satisfied. In addition to associativity, NB-induced memory is specific, rapidly acquired, is retained over weeks and exhibits the same behavioral generalization found with natural memory (McLin, Miasnikov, & Weinberger, 2002, 2003; Miasnikov, Chen, & Weinberger, 2006, 2008b; Weinberger et al., 2006, 2009). It also exhibits reversal when contingencies are reversed (Miasnikov et al., 2009). Thus, memory implanted by NBstm satisfies the criteria used to accept behavioral changes as evidence for the formation of natural memory. Therefore, although a unique and therefore unfamiliar phenomenon, the present and past findings indicate that genuine behavioral memory can be implanted in the brain by pairing a tone with stimulation of the cholinergic nucleus basalis.
4.4. The nature of implanted memory
What are the contents of this implanted memory; what has been learned and remembered? We have discussed this previously (Weinberger et al., 2009) and so will be brief. We have emphasized the multidimensional character of memory, which includes the storage of information about the motivational situation, emotional state and nature of reinforcement as well as the major sensory–perceptual information (such as that of a CS and its context) that constitute its central subject matter. An understanding of the substrates of the actual contents of memory, in contrast to processes that enable memories in general to be formed, is hampered by its multidimensional character. For example, memory of a traumatic experience necessarily involves both the sensory/perceptual aspects of the experience and the affect. The study of implanted memory has the advantage of isolating the sensory–perceptual contents of memory from the other aspects of experience, principally because memory can be implanted by stimulation of the nucleus basalis that itself is motivationally neutral. We believe that this simplification of the learning situation commends the use of NB-implanted memory to understand the neural bases of the salient sensory content of associative memory.
We suggest that the contents of memory implanted in this study are that a particular tonal frequency (or range of frequencies) is behaviorally important but that the normal reason for this importance is absent. In other words, certain sounds are now stored in the brain as more relevant or salient to the animal than otherwise despite the absence of any actual natural experience that would render them of increased significance. This outcome, while perhaps unprecedented, is what would be expected if the nucleus basalis is naturally engaged by motivationally-related systems (whether rewarding or aversive) to release acetylcholine that would promote storage of the events that have just occurred. The same would hold true for post-training events consequent to the release of stress hormones and their modulatory effects on memory strength via the basolateral amygdala, which acts through a cholinergic link (McGaugh, 2006; McGaugh, McIntyre, & Power, 2002).
4.5. Implications
That stimulation of the nucleus basalis can implant specific, associative memory supports a model of the storage of information in the cerebral cortex for which cholinergic activation constitutes a sufficient final link (Weinberger, 2003). The detailed implications of NBstm-implanted specific, associative memory for the role of the cholinergic system in memory have been discussed elsewhere (Weinberger et al., 2009). However, it is important to note the high degree of control of the time course and level of specificity of implanted memory by parametric manipulation of NB stimulation. This factor provides unique opportunities for experimental control and determination of the neural mechanisms of memory and underscores the close linkage that can be found between the cholinergic system and the formation of associative memory. NB stimulation serves as a proxy for the release of acetylcholine in the auditory cortex, and such release has been verified by direct measurement during auditory associative learning (Butt, Chavez, Flesher, Kinney-Hurd, Araujo, Miasnikov, & Weinberger, 2009). Future studies that combine implantation of memory with the measurement of acetylcholine release are needed to more closely link the presumptive cholinergic cause with the formation of specific behavioral memory.
Insofar as implanted memory has now been established as genuine specific associative memory, which affords neurophysiological control of both the sensory/perceptual contents of memory and its consolidation dynamics, it affords a novel direct attack on the understanding of mnemonic mechanisms. Future exploitation of implanted memory awaits.
Acknowledgements
We are grateful to Gabriel K. Hui, Jacquie D. Weinberger and Steven Clifford (CED) for assistance. This study was funded by the following research grants to NMW: NIDCD/NIH, DC-02938 and DC-010013.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(20):11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhterev VM. General principles of human reflexology. International Publishers; New York: 1932. [Google Scholar]
- Bigl V, Woolf NJ, Butcher LL. Cholinergic projections from the basal forebrain to frontal, parietal, temporal, occipital, and cingulate cortices: A combined fluorescent tracer and acetylcholinesterase analysis. Brain Research Bulletin. 1982;8(6):727–749. doi: 10.1016/0361-9230(82)90101-0. [DOI] [PubMed] [Google Scholar]
- Bjordahl TS, Dimyan MA, Weinberger NM. Induction of long-term receptive field plasticity in the auditory cortex of the waking guinea pig by stimulation of the nucleus basalis. Behavioral Neuroscience. 1998;112(3):467–479. doi: 10.1037//0735-7044.112.3.467. [DOI] [PubMed] [Google Scholar]
- Butt AE, Chavez CM, Flesher MM, Kinney-Hurd BL, Araujo GC, Miasnikov AA, Weinberger NM. Association learning-dependent increases in acetylcholine release in the rat auditory cortex during auditory classical conditioning. Neurobiology of Learning and Memory. 2009;92:400–409. doi: 10.1016/j.nlm.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Celesia GG, Jasper HH. Acetylcholine released from cerebral cortex in relation to state of activation. Neurology. 1966;16(11):1053–1063. doi: 10.1212/wnl.16.11.1053. [DOI] [PubMed] [Google Scholar]
- Dauger S, Nsegbe E, Vardon G, Gaultier C, Gallego J. The effects of restraint on ventilatory responses to hypercapnia and hypoxia in adult mice. Respiration Physiology. 1998;112(2):215–225. doi: 10.1016/s0034-5687(98)00027-9. [DOI] [PubMed] [Google Scholar]
- Détári L, Rasmusson DD, Semba K. Phasic relationship between the activity of basal forebrain neurons and cortical EEG in urethane-anesthetized rat. Brain Research. 1997;759(1):112–121. doi: 10.1016/s0006-8993(97)00252-7. [DOI] [PubMed] [Google Scholar]
- Détári L, Rasmusson DD, Semba K. The role of basal forebrain neurons in tonic and phasic activation of the cerebral cortex. Progress in Neurobiology. 1999;58(3):249–277. doi: 10.1016/s0301-0082(98)00084-7. [DOI] [PubMed] [Google Scholar]
- Deutsch JA. The cholinergic synapse and the site of memory. Science. 1971;174(11):788–794. doi: 10.1126/science.174.4011.788. [DOI] [PubMed] [Google Scholar]
- Dimyan MA, Weinberger NM. Basal forebrain stimulation induces discriminative receptive field plasticity in the auditory cortex. Behavioral Neuroscience. 1999;113(4):691–702. doi: 10.1037//0735-7044.113.4.691. [DOI] [PubMed] [Google Scholar]
- Duque A, Balatoni B, Détári L, Zaborszky L. EEG correlation of the discharge properties of identified neurons in the basal forebrain. Journal of Neurophysiology. 2000;84(3):1627–1635. doi: 10.1152/jn.2000.84.3.1627. [DOI] [PubMed] [Google Scholar]
- Edeline J-M. The thalamo-cortical auditory receptive fields: Regulation by the states of vigilance, learning and the neuromodulatory systems. Experimental Brain Research. 2003;153(4):554–572. doi: 10.1007/s00221-003-1608-0. [DOI] [PubMed] [Google Scholar]
- Flood JF, Landry DW, Jarvik ME. Cholinergic receptor interactions and their effects on long-term memory processing. Brain Research. 1981;215(1–2):177–185. doi: 10.1016/0006-8993(81)90500-x. [DOI] [PubMed] [Google Scholar]
- Galván VV, Weinberger NM. Long-term consolidation and retention of learning-induced tuning plasticity in the auditory cortex of the guinea pig. Neurobiology of Learning and Memory. 2002;77(1):78–108. doi: 10.1006/nlme.2001.4044. [DOI] [PubMed] [Google Scholar]
- Giovannini MG, Rakovska A, Benton RS, Pazzagli M, Bianchi L, Pepeu G. Effects of novelty and habituation on acetylcholine, GABA, and glutamate release from the frontal cortex and hippocampus of freely moving rats. Neuroscience. 2001;106(1):43–53. doi: 10.1016/s0306-4522(01)00266-4. [DOI] [PubMed] [Google Scholar]
- Guilbaud G. Thalamic nociceptive systems. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 1985;308(1136):339–345. doi: 10.1098/rstb.1985.0034. [DOI] [PubMed] [Google Scholar]
- Heffner HE, Heffner RS, Contos C, Ott T. Audiogram of the hooded Norway rat. Hearing Research. 1994;73(2):244–247. doi: 10.1016/0378-5955(94)90240-2. [DOI] [PubMed] [Google Scholar]
- Jasper HH, Tessier J. Acetylcholine liberation from cerebral cortex during paradoxical (REM) sleep. Science. 1971;172(983):601–602. doi: 10.1126/science.172.3983.601. [DOI] [PubMed] [Google Scholar]
- Johnston MV, McKinney M, Coyle JT. Evidence for a cholinergic projection to neocortex from neurons in basal forebrain. Proceedings of the National Academy of Sciences of the United States of America. 1979;76(10):5392–5396. doi: 10.1073/pnas.76.10.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappauf WE, Schlosberg H. Conditioned responses in the white rat: III. Conditioning as a function of the length of the period of delay. Pedagogical Seminary and Journal of Genetic Psychology. 1937;50:27–45. [Google Scholar]
- Kilgard MP, Pandya PK, Vazquez J, Gehi A, Schreiner CE, Merzenich MM. Sensory input directs spatial and temporal plasticity in primary auditory cortex. Journal of Neurophysiology. 2001;86(1):326–338. doi: 10.1152/jn.2001.86.1.326. [DOI] [PubMed] [Google Scholar]
- Konorski J. Integrative activity of the brain: An interdisciplinary approach. University of Chicago Press; Chicago: 1967. [Google Scholar]
- Lennartz RC, Weinberger NM. Analysis of response systems in Pavlovian conditioning reveals rapidly versus slowly acquired conditioned responses: Support for two factors, implications for behavior and neurobiology. Psychobiology. 1992;20(2):93–119. [Google Scholar]
- Ley R. The modification of breathing behavior. Pavlovian and operant control in emotion and cognition. Behavior Modification. 1999;23(3):441–479. doi: 10.1177/0145445599233006. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. The psychology of animal learning. Academic Press; New York: 1974. [Google Scholar]
- McGaugh JL. Memory — A century of consolidation. Science. 2000;287(5451):248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Make mild moments memorable: Add a little arousal. Trends in Cognitive Sciences. 2006;10(8):345–347. doi: 10.1016/j.tics.2006.06.001. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, McIntyre CK, Power AE. Amygdala modulation of memory consolidation: Interaction with other brain systems. Neurobiology of Learning and Memory. 2002;78(3):539–552. doi: 10.1006/nlme.2002.4082. [DOI] [PubMed] [Google Scholar]
- McLin DE, 3rd, Miasnikov AA, Weinberger NM. Induction of behavioral associative memory by stimulation of the nucleus basalis. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(6):4002–4007. doi: 10.1073/pnas.062057099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLin DE, 3rd, Miasnikov AA, Weinberger NM. CS-specific gamma, theta, and alpha EEG activity detected in stimulus generalization following induction of behavioral memory by stimulation of the nucleus basalis. Neurobiology of Learning and Memory. 2003;79(2):152–176. doi: 10.1016/s1074-7427(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: Cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (Substantia innominata), and hypothalamus in the rhesus monkey. Journal of Comparative Neurology. 1983;214(2):170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Gross N, Poytress BS, Weinberger NM. Motivationally neutral stimulation of the nucleus basalis induces specific behavioral memory. Neurobiology of Learning and Memory. 2008a;90(1):125–137. doi: 10.1016/j.nlm.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Weinberger NM. Rapid induction of specific associative behavioral memory by stimulation of the nucleus basalis in the rat. Neurobiology of Learning and Memory. 2006;86(1):47–65. doi: 10.1016/j.nlm.2005.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Weinberger NM. Specific auditory memory induced by nucleus basalis stimulation depends on intrinsic acetylcholine. Neurobiology of Learning and Memory. 2008b;90(2):443–454. doi: 10.1016/j.nlm.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Weinberger NM. Behavioral memory induced by nucleus basalis stimulation: Effects of contingency reversal. Neurobiology of Learning and Memory. 2009;91(3):298–309. doi: 10.1016/j.nlm.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriizumi T, Hattori T. Separate neuronal populations of the rat globus-pallidus projecting to the subthalamic nucleus, auditory-cortex and pedunculopontine tegmental area. Neuroscience. 1992;46(3):701–710. doi: 10.1016/0306-4522(92)90156-v. [DOI] [PubMed] [Google Scholar]
- Mostofsky DI, editor. Stimulus generalization. Stanford University Press; Stanford, CA: 1965. [Google Scholar]
- Olds J. Hypothalamic substrates of reward. Physiological Reviews. 1962;42:554–604. doi: 10.1152/physrev.1962.42.4.554. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3rd ed. Academic Press; San Diego: 1997. [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed. Academic Press; San Diego: 2007. [Google Scholar]
- Ryle G. The concept of mind. Hutchinson, Ltd.; London: 1963. [Google Scholar]
- Sherrington CS. Experiments on the value of vascular and visceral factors for the genesis of emotion. Proceedings of the Royal Society of London. 1900;66:390–403. [Google Scholar]
- Thompson RF, Steinmetz JE. The role of the cerebellum in classical conditioning of discrete behavioral responses. Neuroscience. 2009;162(3):732–755. doi: 10.1016/j.neuroscience.2009.01.041. [DOI] [PubMed] [Google Scholar]
- Watson JB. The place of the conditioned-reflex in psychology. Psychological Review. 1916;23(2):89–116. [Google Scholar]
- Weinberger NM. Physiological memory in primary auditory cortex: Characteristics and mechanisms. Neurobiology of Learning and Memory. 1998;70(1–2):226–251. doi: 10.1006/nlme.1998.3850. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. The nucleus basalis and memory codes: Auditory cortical plasticity and the induction of specific, associative behavioral memory. Neurobiology of Learning and Memory. 2003;80(3):268–284. doi: 10.1016/s1074-7427(03)00072-8. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Associative representational plasticity in the auditory cortex: A synthesis of two disciplines. Learning and Memory. 2007;14(1–2):1–16. doi: 10.1101/lm.421807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM, Ashe JH, Metherate R, McKenna TM, Diamond DM, Bakin JS, Lennartz RC, Cassady JM. Neural adaptive information processing: A preliminary model of receptive-field plasticity in auditory cortex during Pavlovian conditioning. In: Gabriel M, Moore J, editors. Learning and computational neuroscience: Foundations of adaptive networks. MIT Press; Cambridge, MA: 1990. pp. 91–138. chap. 3. [Google Scholar]
- Weinberger NM, Miasnikov AA, Chen JC. The level of cholinergic nucleus basalis activation controls the specificity of auditory associative memory. Neurobiology of Learning and Memory. 2006;86(3):270–285. doi: 10.1016/j.nlm.2006.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM, Miasnikov AA, Chen JC. Sensory memory consolidation observed: Increased specificity of detail over days. Neurobiology of Learning and Memory. 2009;91(3):273–286. doi: 10.1016/j.nlm.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wever EG. The upper limit of hearing in the cat. Journal of Comparative Psychology. 1930;10(2):221–233. [Google Scholar]
- Winters RW, McCabe PM, Schneiderman N. Functional utility and neurobiology of conditioned autonomic responses. In: Moore JW, editor. A neuroscientist's guide to classical conditioning. Springer; New York: 2002. pp. 46–85. [Google Scholar]