Abstract
Background & Aims
Natural killer (NK) cells are innate immune effector cells first characterized by their ability to lyse susceptible tumor cells. Recent studies demonstrated their role in initiating and modulating adaptive immunity. NK cells represent a larger percentage of the lymphoid population in liver than other organs suggesting that hepatic NK cells express some unique function. Here, we examined the response of NK cells to liver injury that occurs in a mouse model of biliary obstruction.
Methods
Bile duct ligations (BDL) were performed on mice previously depleted or not depleted of NK cells. NK cell activation, interleukin (IL)-6 mRNA expression and protein production by Kupffer cells, and the ability of exogenous IL-6 to ameliorate liver injury in NK cell-depleted mice were determined.
Results
The number of activated hepatic NK cells increased markedly following BDL. Activation was suppressed in mice rendered Kupffer cell-depleted prior to ligation. Increased liver injury occurred in NK cell-depleted mice correlating with a reduction in IL-6 production. Purified Kupffer cells obtained from NK cell-depleted or anti-interferon (IFN)-γ monoclonal antibody-pretreated mice following BDL produced less IL-6 in culture than did Kupffer cells derived from control animals. In culture, hepatic NK cells derived from BDL mice stimulated IFN-γ-dependent IL-6 production by Kupffer cells; splenic NK cells obtained from the same animals had a negligible effect. Treatment with recombinant murine IL-6 reduced liver injury in BDL, NK cell-depleted mice.
Conclusion
Hepatic NK cells suppress cholestatic liver injury by stimulating Kupffer cell-dependent IL-6 production.
Keywords: biliary obstruction, NK cell, Kupffer cell, interleukin-6
1. Introduction
Natural killer (NK) cells, a lymphocyte subset capable of expressing diverse functions, were originally characterized on the basis of their large granular morphology, lack of conventional T and B cell markers, and ability to lyse certain susceptible tumor cell lines in the absence of antigen-specific recognition [1]. Their role in innate host defenses to a variety of viral and intracellular bacterial pathogens was described more recently [2]. In addition to being an important component of the innate immune system, NK cells play a critical role in initiating and modulating adaptive immunity by interacting with a number of different cell types. In culture, for example, NK cells can either promote or inhibit dendritic cell (DC) maturation dependent upon the DC/NK cell ratio and, thus, modify the biological response of T cells [3]. In vivo, activated NK cells recruited to the secondary lymphoid organs secrete interferon (IFN)-γ and induce a type I helper T cell response [4]. In addition, NK cells can modulate the biological activity of mononuclear phagocytes. In a cecal ligation and puncture model of sepsis in mice, NK cells promoted phagocytosis, bacterial clearance and the production of nitric oxide, interleukin (IL)-6 and IL-12 by macrophages [5]. Human NK cells stimulated the contact-dependent production of tumor necrosis factor-α by monocytes in culture; monocytes, in turn, promoted IFN-γ production by NK cells [6]. NK cells, therefore, can serve an immuno-regulatory role, bridging innate and adaptive immunity.
On average, a healthy human liver contains approximately 1 × 1010 lymphocytes, 25–30% of these are NK cells; NK cells represent a much smaller percentage of total lymphoid cells circulating in human peripheral blood [7]. Similarly, NK cells constitute a large percentage (~20%) of the hepatic lymphocyte population in mice where they reside primarily within the sinusoids adherent to Kupffer and endothelial cells [8]. In contrast to their purported beneficial role in tumor surveillance and host defenses to infectious agents, NK cells have been implicated in the pathogenesis and liver injury that occur in a number of experimental models of disease. For example, activated hepatic NK cells contributed to progression of Pseudomonas exotoxin A-induced hepatitis and were a key factor in the liver injury induced in mice by polyinosinicpolycytidylic acid [9,10].
Previously, we reported that Kupffer cells exerted a beneficial effect in a mouse model of biliary obstruction and cholestatic liver injury. Liver injury was increased and the production of IL-6 was diminished in mice rendered Kupffer cell-depleted prior to common bile duct ligation (BDL); injury was reversed in depleted mice administered recombinant (r)IL-6 [11]. Given both the beneficial and detrimental roles played by NK cells in different experimental models referenced above, we undertook a series of experiments to determine the function of NK cells in a mouse model of biliary obstruction. Here we report BDL resulted in the Kupffer cell-dependent activation of hepatic NK cells. IL-6 production by Kupffer cells was diminished and the severity of liver injury was increased in mice rendered NK cell-depleted prior to BDL. NK cells promoted IL-6 production by Kupffer cells in vivo and in vitro by an IFN-γ-dependent mechanism. As in the case of Kupffer cell-depleted mice in the study cited, administration of rIL-6 reversed the injury assessed in NK cell-depleted animals. These findings delineate the obligate interaction of NK cells and Kupffer cells, and the role of NK cells in promoting IL-6 production and, thus, suppressing the liver damage attending biliary obstruction and cholestasis.
2. Materials and Methods
2.1 Mice
Wild-type female, C57BL/6J mice and female C57BL/6 mice expressing a targeted mutation in the gene encoding IL-6 [B6;129S-Il6tm1Kopf [12]] were purchased from Jackson Laboratories (Bar Harbor, ME). Vα14Jα18−/− (invariant NKT cell-deficient) mice on a C57BL/6 background, obtained from Dr. M. Taniguchi (Riken Research Center for Allergy) and bred in our animal facility, were used as a source of NK cells in the in vitro experiments described [13]. All animals were treated humanely in accordance with the guidelines set forth by the Rhode Island Hospital animal care and use committee.
2.2 Common bile duct ligation
BDL was performed by a slight modification of methods previously (detailed in the Supplementary Materials section) [11,14]. Sham-operated mice underwent laparotomy and bile duct exposure without ligation.
2.3 Cell preparation, purification and culture
Livers were perfused in situ via the portal vein with calcium- and magnesium-free HBSS supplemented with 2% heat-inactivated FBS, dissected and teased through 70 μm nylon mesh cell strainers (BD Biosciences, San Diego, CA) [14]. The hepatic leukocyte population was purified on a two-step (40/70%) discontinuous Percoll gradient (GE Healthcare Bio-sciences Corp., Piscataway, NJ), washed and analyzed by flow cytometry.
Hepatic and splenic NK cells were obtained from Vα14Jα18−/− mice inoculated i.p. two days previously with 0.5 mg rat IgG2b anti-mouse Thy-1.2 (CD90.2; clone 30-H12, ATCC, Manassas, VA) to eliminate T cells including NK1.1-expressing NKT cells. Total leukocyte populations were stained with biotin-conjugated mouse IgG2a anti-mouse NK1.1 (BD Biosciences), and the cells were isolated by positive selection using streptavidin-conjugated magnetic MicroBeads (Miltenyi Biotec, Auburn, CA) [11].
The total nonparenchymal liver cell (NPC) population was isolated and purified after perfusing the liver with collagenase using methods we described previously [11,15]. The F4/80+CD115+ Kupffer cells were subsequently isolated by positive selection using magnetic MicroBeads also by methods we previously reported and detail in the Supplementary Materials section) [11,15]. The purity of isolated population was routinely >90% (Supplementary Figure 1).
Purified NPCs, Kupffer cells and NK cells were cultured in HEPES-buffered RPMI 1640 medium supplemented with 10% heat-inactivated FBS (Sterile Systems, Inc. Logan, UT), 1 mmol/L L-glutamine, 1% essential and nonessential amino acids, 5 × 105 mol/L 2-mercaptoethanol, 100 U/ml penicillin and 100 μg/ml streptomycin.
2.4 Cell-depletion
Mice were rendered NK cell-deficient by inoculating i.v. 50 μl rabbit anti-mouse asialoganglio-N-tetraosylceramide (GM1) (Wako Chemicals Inc., Richmond, VA) on day 2 prior to surgery. Flow cytometric analysis confirmed >90% reduction in hepatic NK1.1+TcR− (NK) cells in treated mice (Supplementary Figure 2). In accordance with the results of others [16], NKT cells were not affected by anti-asialo-GM1-treatment. No change was observed in either the hepatic NK or NKT cell population of mice inoculated i.v. with normal rabbit serum. Mice were inoculated i.p. with 200 μg rat IgG2a anti-mouse CD8α (clone 53-6.7; BioLegend, San Diego, CA) on day 3 prior to surgery to deplete CD8+ T cells; depletion was verified by flow cytometry. Control mice received an equivalent concentration of normal rat IgG (Sigma-Alrich, St. Louis, MO). Kupffer cells were depleted in accordance with methods we described previously [11]. Mice were inoculated i.v. with 200 μl of 1 mg/ml multilamellar liposomes containing dichloromethylene diphosphonate (Cl2MDP-L) suspended in saline on day 3 prior to surgery. Mice administered 200 μl of liposome-encapsulated PBS served as controls.
2.5. Liver injury
Plasma samples were collected from anesthetized animals via the orbital plexus at the time of sacrifice. Plasma alanine aminotransferase (ALT) activity, a marker of hepatic injury, was quantified spectrophotometrically [11].
2.6. Histochemistry, immunohistochemistry and photoimage analysis
Methods are detailed in the Supplementary Materials section.
2.7 Enzyme-linked immunosorbent assay
Representative liver samples were homogenized in calcium- and magnesium-free HBSS containing 0.5% Triton X-100 and 2× complete protease inhibitor (Roche Diagnostics Corporation, Indianapolis, IN). Homogenates were centrifuged at 13,500 × g, and the supernates were stored at −20°C until analyses. IL-6 was quantified by ELISA as we described previously [11].
2.8 RNA extraction, purification, and quantitative real-time RT-PCR
Total cellular RNA in representative tissue samples was extracted and purified using TRIzol (Invitrogen Corporation, Carlsbad, CA). Real-time RT-PCR was conducted using the methods and RNA primers we described previously [11,14].
2.9 Flow cytometry
NK cells comprising the hepatic lymphocyte population were quantified and characterized using methods we described previously [14]. The numbers of cells constituting the livers of BDL and sham-operated mice were calculated by multiplying the number of leukocytes routinely recovered from Percoll gradients by the percent lymphocytes composing the purified leukocyte population (quantified by flow cytometry) X percent NK or NKT cells constituting that lymphocyte population (also quantified by flow). Dye-conjugated monoclonal antibodies (mAb) specific for the following determinants were purchased from eBioscience: TCRβ-chain mAb (clone H57-597), NK1.1 mAb (clone PK136), CD25 mAb (clone PC61.5) and CD69 mAb (clone H1.2F3). Stained cells were washed and analyzed (FACSCalibur; BD Biosciences, San Jose, CA). All analyses were conducted using the appropriate isotype controls to correct for non-specific staining.
2.10 Statistical analysis
Results were analyzed using the SigmaStat statistics program (Jandel Scientific, San Rafael, CA). Two groups were compared by the non-paired Student's t-test. Differences among multiple groups were determined by a one-way ANOVA using Dunnett's method and the Tukey test for post hoc analysis. P values < 0.05 were considered statistically different.
3. Results
3.1 Activated hepatic NK cells suppress cholestatic liver injury
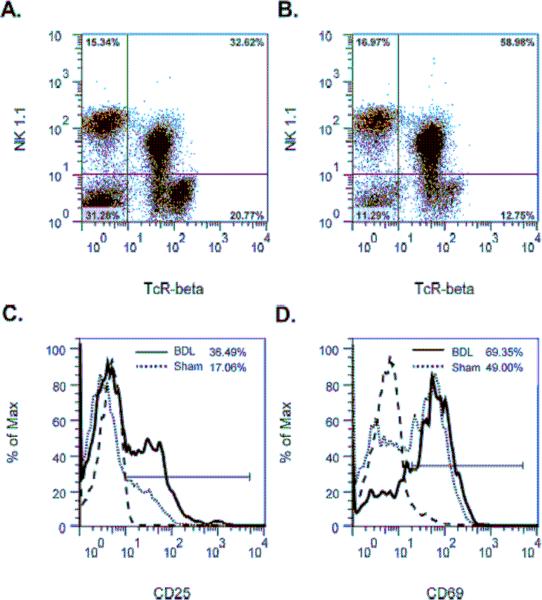
To delineate the response of NK cells to cholestasis, the hepatic leukocyte population was obtained from BDL or sham-operated mice and analyzed by flow cytometry. In contrast to the NKT cells shown previously [14], no difference was detected in the percentage of NK1.1+TcRβ− (NK) cells constituting the lymphoid population recovered from the livers of BDL (Figure 1A), relative to sham-operated (Figure 1B), mice at 18 hours post-surgery. Similarly, the numbers of NK cells estimated in the livers of BDL (2.68 × 105) and sham-operated (3.03 × 105) mice at this time were approximately the same. These findings correlate with a statistically insignificant difference in numbers of NKp46+ (NK) cells counted in stained liver sections derived from BDL (1.42 ± 0.8 NK cells/mm2) and sham-operated (0.98 ± 0.42 NK cells/mm2) animals. While the percentages and numbers were unchanged, however, the expression of CD25 (IL-2R alpha) (Figure 1C) and CD69 (an early activation marker) (Figure 1D) by hepatic NK cells was elevated markedly in the BDL animals.
Figure 1. Bile duct ligation induces hepatic NK cell activation.
The hepatic leukocyte population was obtained from groups of 4 wild-type C57BL/6J mice at 18 hours following sham operation (A) or BDL (B). Expression of the cell-surface activation markers CD25 (C) and CD69 (D) by NK1.1+TcRβ− (NK) cells in the upper left quadrant is shown (isotype control;----). Data represent one of three experiments.
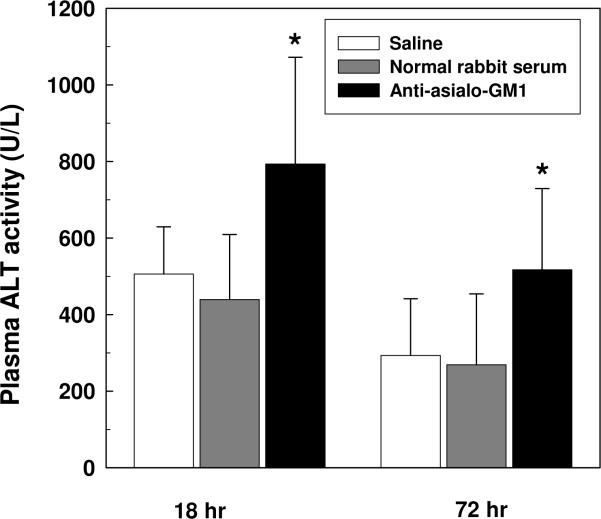
Ligation of the common bile duct in mice induces a marked and rapid increase in plasma ALT levels indicative of liver injury [11,14]. To ascertain the role of NK cells in this model, mice were rendered NK cell-deficient by treatment with anti-asialo-GM1 polyclonal antibody inoculated i.v. on day 2 prior to BDL. Relative to non-treated animals, NK cell-depleted mice exhibited a significant increase in liver injury evidenced by elevated plasma ALT levels assessed at 18 and 72 hours post-BDL (Figure 2). Plasma ALT levels in sham operated animals pretreated with α-asialo-GM1 were an approximate 40 IU/L at these times. These data indicate that hepatic NK cells, activated in response to biliary obstruction, suppress liver injury.
Figure 2. Activated NK cells ameliorate cholestatic liver injury.
Groups of 6 wild-type C57BL/6 mice were inoculated i.v. with 50 μl of saline, polyclonal rabbit anti-mouse asialo-GM1, or an equivalent concentration (37 mg/ml) of normal rabbit serum on day 2 prior to BDL. Plasma was collected from each group at 18 and 72 hours post-BDL, and the ALT levels were quantified. Data (means ± SD) are the combined results of 2 experiments (n=12). *Significantly greater than both other groups at comparable time points: P<0.05 (Kruskal-Wallis one way analysis of variance on ranks).
3.2 Kupffer cell-dependent activation of hepatic NK cells in cholestatic livers
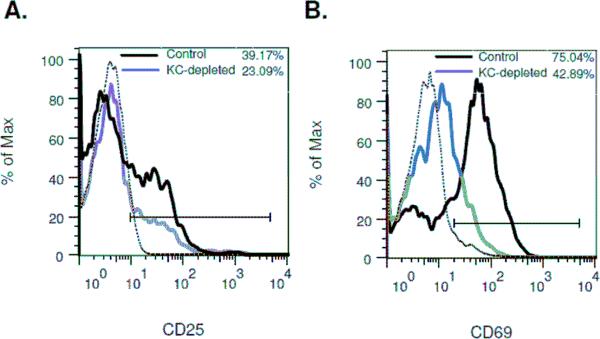
It has been suggested that Kupffer cells play an essential role in the activation and differentiation of hepatic NK cells [17]. Given the close physical relationship between NK cells and Kupffer cells within the hepatic sinusoids, and the ability of both populations to suppress cholestatic liver injury, we undertook a series of experiments to explore the interaction of NK cells and Kupffer cells in mice following BDL. To determine the role of Kupffer cells in NK cell activation, Kupffer cells were eliminated by intravenous inoculation of Cl2MDP-L. Mice pretreated with Cl2MDP-L were subjected to BDL, and an enriched hepatic leukocyte population was obtained at 18 hours post-surgery and analyzed by flow cytometry. The expression of both CD25 and CD69 by NK cells was suppressed in the Kupffer cell-depleted animals (Figure 3) demonstrating the role of Kupffer cells in hepatic NK cell activation following biliary obstruction.
Figure 3. Kupffer cell-depletion suppresses hepatic NK cell activation in bile duct obstructed mice.
The hepatic leukocyte populations were obtained from groups of 4 control and 4 Kupffer cell (KC)-depleted wild-type C57BL/6J mice at 18 hours post-BDL; expression of cell-surface activation markers CD25 (A) and CD69 (B) by NK cells was quantified by flow cytometric analysis (isotype control; -----). Data represent one of two experiments.
3.3 Hepatic NK cells promote IL-6 production by Kupffer cells in vivo
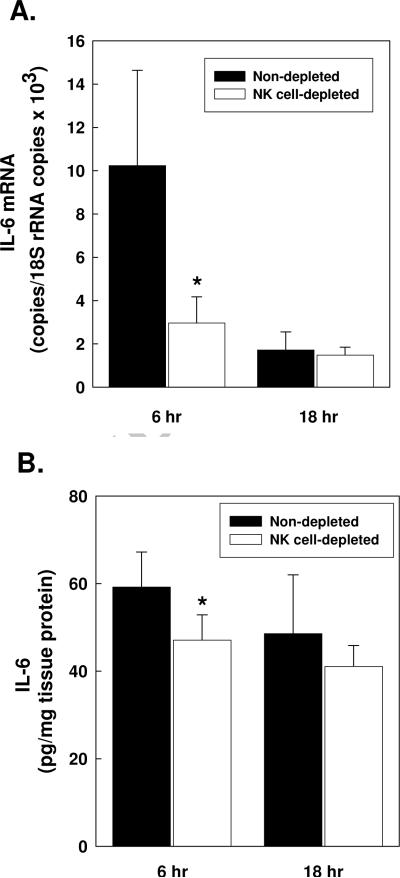
Previously, we reported that Kupffer cells ameliorate cholestatic liver injury by an IL-6-dependent mechanism [11]. Thus, it was interesting to find here that IL-6 production was significantly diminished in the livers of BDL, NK cell-depleted mice. Total liver RNA extracted at 6 hours following surgery showed a marked decrease in IL-6 mRNA expression in their livers, relative to the controls (Figure 4A). The disparate expression of IL-6 mRNA at 6 hours post-BDL was consistent with the presence of significantly less IL-6 protein in the livers of NK cell-depleted animals determined at the same time (Figure 4B). CD8+ T cells failed to exert a similar effect; IL-6 message expression and protein production were comparable in control and CD8+ T cell-depleted mice at 6 hours post-BDL, evidencing the unique function of hepatic NK cells (data not shown).
Figure 4. Hepatic NK cells up-regulate IL-6 message expression and protein production in the livers of bile duct ligated mice.
Groups of 6 wild-type C57BL/6J mice were non-depleted or NK cell-depleted as indicated in the key. Represent liver samples were obtained at 6 and 18 hours following BDL. The RNA was extracted and purified; IL-6 mRNA and 18S rRNA were quantified by real-time RT-PCR (A). IL-6 in clarified homogenates was quantified by ELISA (B). In each case, data are the means ± SD derived from one of two comparable experiments. Zero time control values for groups of non-depleted mice inoculated i.v. with 1.85 mg normal rabbit serum and NK cell-depleted mice pretreated with equivalent concentration of anti-asialo-GM1 (n=6) were: 0.32 ± 0.16 and 0.23 ± 0.06 IL-6 mRNA copies/18S rRNA copies × 103, respectively; 10.07 ± 1.85 and 12.44 ± 3.44 pg IL-6/mg tissue protein, respectively. *Significantly less than non-depleted or rat IgG pre-treated controls: panel A, p=0.032; panel B, p=0.049 (Student's t test).
Inasmuch as Kupffer cells constitute the principal source of IL-6 produced in the liver shortly following BDL [11], experiments were undertaken to assess specifically the ability of NK cells to regulate IL-6 production by Kupffer cells. Both the total NPC and purified Kupffer cell populations derived from NK cell-depleted mice at 6 hours post-BDL produced significantly less IL-6 than did comparable populations derived from BDL controls (Table I). Similarly, cultured NPCs and Kupffer cells derived from mice administered anti-IFN-γ mAb prior to BDL produced substantially less IL-6 indicating the intermediary role of IFN-γ in promoting IL-6 production by Kupffer cells subsequent to biliary obstruction. The role of soluble factors (and not cell-to-cell contact) in mediating the interaction of NK cells and Kupffer cells is supported, albeit indirectly, by confocal image analyses demonstrating only ≈20% of NK cells co-localized with Kupffer cells at 18 hours post-surgery regardless of whether the animals underwent BDL or sham operations (Supplementary Figure 3). Microscopic examination revealed no apparent differences in the distribution of NK cells in the livers of ligated and non-ligated mice.
Table 1.
NK cell depletion suppresses IL-6 production by Kupffer cellsa
| Treatment | Cell population | IL-6 (pg/ml) | |
|---|---|---|---|
| Experiment 1 | control | total NPC | 142.6 ± 21.1 |
| Kupffer cell | 1,172.2 ± 167 | ||
| NK cell-depleted | total NPC | 94.8 ± 19.7b | |
| Kupffer cell | 515.2 ± 61.5b | ||
| Experiment 2 | control | total NPC | 126.7 ± 23.7 |
| Kupffer cell | 1,683.3 ± 31.5 | ||
| anti-IFN-γ | total NPC | 61.4 ± 6.8b | |
| Kupffer cell | 743.6 ± 37.9b |
Total NPC and purified Kupffer cells were isolated from groups of 5 control, NK cell-depleted (Experiment 1), or anti-IFN-γ-treated [500 μg/mouse i.v 1 hour prior to surgery; control animals received 500 μg normal rat IgG (Experiment 2)] mice at 6 hours post-BDL and cultured at 1×105 cells/well in 96-well tissue culture plates. The culture supernates were collected after an approximate 40-hour incubation period; IL-6 was quantified by ELISA. Data are the mean ± SD pg/ml derived from triplicate wells. Both experiments were performed twice yielding similar results.
3.4 Hepatic NK cells stimulate IFN-γ dependent, IL-6 production by Kupffer cells in vitro
Other investigators reported the ability of NK cells to stimulate cytokine production by mononuclear phagocytes [6]. To demonstrate directly the effects of hepatic NK cells on IL-6 production by Kupffer cells, purified Kupffer cells were incubated with or without NK cells. Hepatic NK cells obtained from BDL animals stimulated a significant increase in IL-6 production (Table 2). The addition of neutralizing, anti-IFN-γ mAb reduced the production of IL-6 production significantly supporting the intermediary role of IFN-γ indicated above. Purified splenic NK cells derived from the same bile duct ligated animals, on the other hand, exerted a negligible effect on IL-6 synthesis by Kupffer cells.
Table 2.
Hepatic NK cells stimulate IFN-γ-dependent IL-6 production by Kupffer cells in vitroa
| NK cells | IL-6 (pg/ml) | ||
|---|---|---|---|
| Source | Surgery | Treatment | |
| none | - | rat IgG | 414.0 ± 52.2 |
| Liver | control | rat IgG | 451.2 ± 34 |
| BDL | rat IgG | 609.7 ± 29.2b | |
| BDL | anti-IFN-γ | 523.6 ± 35.4 | |
| Spleen | control | rat IgG | 430.4 ± 15.2 |
| BDL | rat IgG | 409.1 ± 23.8 | |
Kupffer cells (2 × 104/100 μl medium/half-area 96-well tissue culture plate) derived from wild-type C57BL/6J mice were cultured with or without 6 × 104 hepatic or splenic NK cells obtained from anti-Thy1.2-pretreated, T cell-depleted control Vα14Jα18−/− mice or T cell-depleted Vα14Jα18−/− mice at 18 hours post-BDL. Additionally, cultures were treated with 10 μg/ml normal rat IgG or monoclonal rat anti-mouse IFN-γ (clone R4-6A2; American Type Culture Collection, Manassas, VA) as indicated. Supernates were collected after 40 hours incubation and IL-6 was quantified by ELISA. Data are the means ± SD pg/ml IL-6 obtained from quadruplicate wells in a single experiment representative of 2 experiments.
Significantly greater than all the other groups; p<0.05; one-way ANOVA.
3.5 Exogenous IL-6 abrogates liver injury in BDL, NK cell-depleted mice
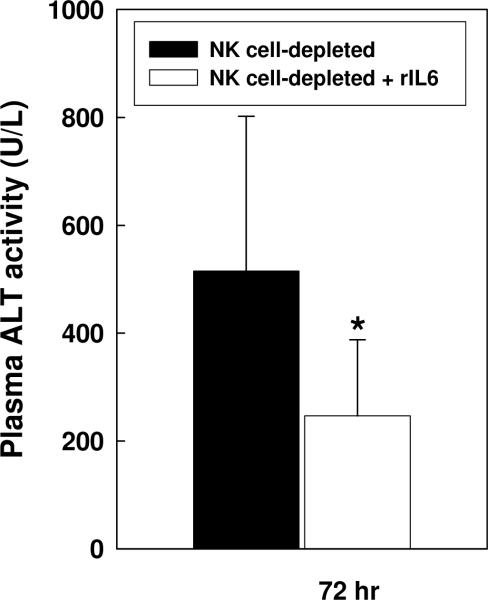
To ascertain the contribution of diminished IL-6 production to the increase in liver injury found in NK cell-deficient mice, deficient mice were administered recombinant IL-6 subcutaneously one hour prior to BDL. As shown in Figure 5, the administration of exogenous IL-6 resulted in a significant reduction in the elevated plasma ALT levels normally assessed in BDL, NK cell-depleted animals. Taken together, these results demonstrate the ability of activated hepatic NK cells to suppress cholestatic liver injury by stimulating Kupffer cell-dependent IL-6 production.
Figure 5. Recombinant murine IL-6 suppresses liver injury in BDL, NK cell-depleted mice.
Groups of five NK cell-depleted wild-type C57BL/6J mice were not treated or pre-treated with 2 μg recombinant murine IL-6 (Peprotech Inc., Rocky Hill, NJ) inoculated s.c. 1 hour prior to BDL. Plasma was collected on day 3 post-surgery and ALT levels were quantified. Data (means ± SD) are the combined results of 2 experiments. *Significantly less than mice not administered IL-6 prior to surgery: p=0.029 (Student's t test).
4. Discussion
The liver represents a unique anatomical and immunological site through which ~30% of the total blood volume passes each minute [18]. Twenty percent of this blood derives from the hepatic artery; the remainder comes from the splanchnic organs via the hepatic portal vein. Blood originating from the intestines contains a variety of substances including bacterial products, environmental toxins and food antigens that are capable of eliciting immune responses. The failure of these substances to elicit such responses has lead other investigators to describe the liver as an immunologically privileged site, tolerant to antigenic stimulation; the specific mechanisms that underlie liver tolerance remain to be clarified [19]. Studies to date have focused in part on the contribution of Kupffer cells, NK cells and NKT cells, which are selectively enriched and represent much larger percentages of the leukocyte population in liver than in other organs [20]. Collectively, our data suggest that the interaction of these same three cell types suppresses liver injury attending biliary obstruction and cholestasis [11,14].
Kupffer cells, located preferentially in the periportal region of the liver, constitute the first macrophage population to contact materials derived from the gut via the portal vein [21]. Undoubtedly, they play a critical role in modulating the inflammatory response to these materials, as well as the responses observed in a number of experimental models of liver disease [22]. In this regard, we reported previously that Kupffer cells abrogate cholestatic liver injury by a cytokine-dependent mechanism [11]. Mice rendered Kupffer cell-depleted prior to BDL exhibited significant increases in serum ALT levels relative to BDL, non-depleted animals assessed on day 3 post-surgery. Histologic examination revealed portal inflammation, neutrophil infiltration and bile duct proliferation. Photoimage analyses verified more hepatocellular necrosis in the livers of Kupffer cell-depleted animals correlating directly with the elevated serum ALT levels assessed. Notably, plasma ALT levels were elevated, but not significantly different in Kupffer cell-depleted and non-depleted mice assessed at an earlier (18 hour post-BDL) time point in the experiments reported herein (Supplementary Figure 4).
Kupffer cells serve as the principal source of IL-6 produced immediately following BDL; liver injury is exacerbated in the absence of either Kupffer cells or IL-6 [11]. rIL-6 administered at the time of BDL completely reversed the negative impact of Kupffer cell depletion, demonstrating the intermediary role of IL-6 in the Kupffer cell-dependent abrogation of cholestatic liver injury in this model. The experiments presented herein demonstrate the capacity of NK cells to stimulate IL-6 production by Kupffer cells.
In vivo, both IL-6 mRNA expression and protein production were decreased significantly in mice rendered NK cell-depleted prior to BDL (Figure 4); rIL-6 inoculated s.c. reversed the increased liver injury assessed otherwise (Figure 5). Kupffer cells derived from BDL, NK cell-depleted mice produced less IL-6 in culture than did Kupffer cells obtained from control, BDL animals (Table I). Similarly, Kupffer cells derived from animals treated with anti-IFN-γ mAb prior to BDL produced less IL-6, supporting the intermediary role of IFN-γ. In vitro experiments demonstrating the enhanced, IFN-γ-dependent production of IL-6 by Kupffer cells co-cultured with hepatic, but not splenic, NK cells corroborate these findings (Table II). Recently, Tu and co-workers reported the capacity of human Kupffer cells to stimulate IFN-γ production by human NK cells in culture [23]. The data presented herein are the first to document the reciprocal interaction and ability of hepatic NK cells to stimulate cytokine, i.e., IL-6, production by Kupffer cells in vivo, as well as in vitro. IL-6 was not a factor in the Kupffer cell-dependent activation of NK cells subsequent to BDL. CD25 and CD69 expression analyzed by flow cytometry was comparably expressed by NK cells obtained from groups (n=6) of wild-type and IL-6-deficient mice at 18 hours post-BDL (data not shown).
IFN-γ plays a critical role in suppressing cholestatic liver injury attending biliary obstruction; hepatocellular necrosis was increased sharply in IFN-γ-receptor chain-deficient, relative to wild-type, mice following BDL [24]. A significant increase in the proliferative activity found in the livers of BDL wild-type mice, led the authors to conclude in part that IFN-γ promoted hepatocyte replication. Our results demonstrating the elevated, IFN-γ-dependent production of IL-6 by Kupffer cells co-cultured with hepatic NK cells suggest that IL-6 may play an intervening role. Experimental approaches that diminish IL-6 production following BDL dramatically impair S-phase progression and DNA synthesis by hepatocytes [25]. Moreover, IL-6 stimulates Bcl-xL expression by hepatocytes and exerts anti-apoptotic effects that are essential for liver regeneration [26]. In this regard, de novo expression of anti-apoptotic genes (i.e., A1, and cIAP2) by hepatocytes is observed in bile duct ligated rats suggesting the existence of mechanisms that provoke an adaptive response to pro-apoptotic factors such as toxic bile salts [27]. These latter findings are particularly relevant inasmuch as apoptosis by hepatocytes is not a significant factor effecting cholestatic liver injury in experimental mouse models [28].
Recently, Wang and co-workers reported that liver regeneration was reduced significantly in hepatocyte-specific (STAT3Hep−/−) STAT3-deficient mice following partial hepatectomy [29]. Although the role of IL-6 was not addressed directly, it was implied that one mechanism by which IL-6 stimulated liver regeneration was mediated by the STAT3-dependent inhibition of STAT1 signaling, signaling shown previously to suppress hepatocyte proliferation [29,30]. Thus, in addition to inducing the expression of anti-apoptotic genes, it is conceivable that IL-6 suppresses cholestatic liver injury by down-regulating STAT1 activity.
While their critical role in tumor immunity and innate host defenses to a variety of viral and intracellular bacterial pathogens in animal models is well-documented, the contribution of hepatic NK cells to liver injury and disease is a matter of controversy [31]. Kahraman and coworkers recently reported, for example, a marked reduction in liver injury assessed at ≥7 days post-bile duct ligation in mice administered anti-NK1.1 mAb and rendered deficient in NK1.1-expressing cells (i.e., NK cells and NKT cells, as well as subsets of CD8+ T cells and dendritic cells not addressed) [32–34]. Although these results suggest the adverse contribution of NK1.1-expressing cells to injury; the specific role of NK cells remains unclear. Indeed, other investigators reported that anti-NK1.1 monoclonal (but not anti-asialo-GM1 polyclonal) antibody treatment activated NK cells which, in turn, promoted the macrophage-dependent elimination of bacteria in a mouse model of sepsis [35]. Thus, it is conceivable that NK cells activated as a consequence of anti-NK1.1 treatment exert a beneficial effect on cholestatic liver injury in agreement with the results presented herein, rather than the detrimental effect previously concluded by other investigators [32]. Alternatively, the difference in the results presented herein and those reported by others might reflect the disparate functions of NK cells at different time points following biliary obstruction and during the pathogenesis of liver injury.
Accumulating evidence suggests that NK cells play a protective role during fibrogenesis. In mouse models of chronic alcohol consumption and/or hepatotoxin ingestion, for example, NK cells abrogated liver fibrosis by IFN-γ- and TRIAL-dependent mechanisms that suppress hepatic stellate cell proliferation and that kill activated stellate cells, respectively [36,37]. The experiments reported herein evidence the beneficial effects of NK cells in a short-term, experimental model of cholestatic liver injury. Their potential role in abrogating fibrosis attending chronic biliary obstruction is a matter of ongoing investigation in our laboratory. Notably, in preliminary experiments, treatment with a single dose of anti-asialo-GM1 polyclonal antibody to deplete NK cells on day 2 prior to surgery exerted a negligible effect on the level of fibrosis assessed on day 10 post-BDL (Supplementary Figure 5). In depth analysis of the role of NK cells in the development of fibrosis attending biliary obstruction will require repeated inoculation of anti-asialo-GM1 to maintain NK cell-deficiency over the entire course of fibrogenesis.
In conclusion, the hepatic leukocyte population includes large percentages of resident macrophages, NK and NKT cells that differ dramatically from the population found in peripheral blood and other organs. This suggests that it may serve a unique function in addition or unrelated to tumor immunity and innate host defenses to microbial pathogens proposed by others [38]. Rather, we speculate that a primary function of these hepatic leukocytes is to suppress inflammation and tissue damage that would otherwise occur consequent to the accumulation of toxins, metabolic products, microorganisms, etc., and particularly during periods of exacerbated liver injury. This speculation is supported in part by the present study in which NK cells, activated in response to biliary obstruction, stimulated the IFN-γ-dependent production of IL-6 by Kupffer cells; IL-6, in turn, suppressed cholestatic liver injury. Conversely, NK cells activation depended upon Kupffer cells. While IL-6 was not involved, Kupffer cells can synthesize a number of cytokines, e.g. IL-12 and IL-18, that possess NK cell activating activity [38]. The factors that mediate the interactions and activities of these two hepatic cell populations are the focus of continued investigation.
Supplementary Material
Acknowledgements
We gratefully acknowledge Drs. Jorge Albina, Laurent Brossay and Surendra Sharma for their critiques and helpful discussion. Tissue sectioning, staining and image analyses were performed by Paul Monfils, Carol Ayala and Virginia Hovasian (Core Research Laboratories, Rhode Island Hospital). This study was supported by National Institutes of Health Research Grants DK068097 and funds provided by Rhode Island Hospital. Dr. Wintermeyer was supported, in part, by Deutsche Forschungsgemeinschaft Grant WI2683/1-1.
Abbreviations used
- NK
natural killer
- DC
dendritic cell
- IFN
interferon
- IL
interleukin
- BDL
bile duct ligation
- NPC
nonparenchymal liver cells
- Cl2MDP-L
liposome encapsulated dichloromethylene diphosphonate
- ALT
alanine aminotransferase
- asialo-GM1
asialo-ganglio-N-tetraosylceramide
- mAb
monoclonal antibody
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts: The authors have no financial conflicts of interest to disclose.
References
- [1].Herberman RB, Nunn ME, Holden HT, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer. 1975;16:230–9. doi: 10.1002/ijc.2910160205. [DOI] [PubMed] [Google Scholar]
- [2].Vales-Gomez M, Reyburn H, Strominger J. Interaction between the human NK receptors and their ligands. Crit Rev Immunol. 2000;20:223–44. [PubMed] [Google Scholar]
- [3].Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002;195:335–41. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5:1260–5. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- [5].Godshall CJ, Scott MJ, Burch PT, Peyton JC, Cheadle WG. Natural killer cells participate in bacterial clearance during septic peritonitis through interactions with macrophages. Shock. 2003;19:144–9. doi: 10.1097/00024382-200302000-00010. [DOI] [PubMed] [Google Scholar]
- [6].Dalbeth N, Gundle R, Davies RJ, Lee YC, McMichael AJ, Callan MF. CD56bright NK cells are enriched at inflammatory sites and can engage with monocytes in a reciprocal program of activation. J Immunol. 2004;173:6418–26. doi: 10.4049/jimmunol.173.10.6418. [DOI] [PubMed] [Google Scholar]
- [7].Doherty DG, Norris S, Madrigal-Estebas L, McEntee G, Traynor O, Hegarty JE, O'Farrelly C. The human liver contains multiple populations of NK cells, T cells, and CD3+CD56+ natural T cells with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. J Immunol. 1999;163:2314–21. [PubMed] [Google Scholar]
- [8].Klugewitz K, Adams DH, Emoto M, Eulenburg K, Hamann A. The composition of intrahepatic lymphocytes: shaped by selective recruitment? Trends Immunol. 2004;25:590–4. doi: 10.1016/j.it.2004.09.006. [DOI] [PubMed] [Google Scholar]
- [9].Muhlen KA, Schumann J, Wittke F, Stenger S, van Rooijen N, van Kaer L, Tiegs G. NK cells, but not NKT cells, are involved in Pseudomonas aeruginosa exotoxin A-induced hepatotoxicity in mice. J Immunol. 2004;172:3034–41. doi: 10.4049/jimmunol.172.5.3034. [DOI] [PubMed] [Google Scholar]
- [10].Dong Z, Wei H, Sun R, Hu Z, Gao B, Tian Z. Involvement of natural killer cells in PolyI:C-induced liver injury. J Hepatol. 2004;41:966–73. doi: 10.1016/j.jhep.2004.08.021. [DOI] [PubMed] [Google Scholar]
- [11].Gehring S, Dickson EM, San Martin ME, van Rooijen N, Papa EF, Harty MW, Tracy TF, Jr., Gregory SH. Kupffer cells abrogate cholestatic liver injury in mice. Gastroenterology. 2006;130:810–22. doi: 10.1053/j.gastro.2005.11.015. [DOI] [PubMed] [Google Scholar]
- [12].Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G. Impaired immune and acute-phase response in interleukin-6-deficient mice. Nature. 1994;368:339–41. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- [13].Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–6. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- [14].Wintermeyer P, Cheng CW, Gehring S, Hoffman BL, Holub M, Brossay L, Gregory SH. Invariant natural killer T cells suppress the neutrophil inflammatory response in a mouse model of cholestatic liver damage. Gastroenterology. 2009;136:1048–59. doi: 10.1053/j.gastro.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Holub M, Cheng CW, Mott S, Wintermeyer P, van Rooijen N, Gregory SH. Neutrophils sequestered in the liver suppress the proinflammatory response of Kupffer cells to systemic bacterial infection. J Immunol. 2009;183:3309–16. doi: 10.4049/jimmunol.0803041. [DOI] [PubMed] [Google Scholar]
- [16].Miyamoto M, Emoto M, Brinkmann V, van Rooijen N, Schmits R, Kita E, Kaufmann SH. Cutting edge: contribution of NK cells to the homing of thymic CD4+NKT cells to the liver. J Immunol. 2000;165:1729–32. doi: 10.4049/jimmunol.165.4.1729. [DOI] [PubMed] [Google Scholar]
- [17].Vanderkerken K, Bouwens L, van Rooijen N, Van den Berg K, Baekeland M, Wisse E. The role of Kupffer cells in the differentiation process of hepatic NK cells. Hepatology. 1995;22:283–90. [PubMed] [Google Scholar]
- [18].Sheth K, Bankey P. The liver as an immune organ. Curr Opin Crit Care. 2001;7:99–104. doi: 10.1097/00075198-200104000-00008. [DOI] [PubMed] [Google Scholar]
- [19].Crispe IN, Giannandrea M, Klein I, John B, Sampson B, Wuensch S. Cellular and molecular mechanisms of liver tolerance. Immunol Rev. 2006;213:101–18. doi: 10.1111/j.1600-065X.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- [20].Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:S54–S62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- [21].Billiar TR, Maddaus MA, West MA, Dunn DL, Simmons RL. The role of intestinal flora on the interactions between nonparenchymal cells and hepatocytes in coculture. J Surg Res. 1988;44:397–403. doi: 10.1016/0022-4804(88)90182-5. [DOI] [PubMed] [Google Scholar]
- [22].Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int. 2006;26:1175–86. doi: 10.1111/j.1478-3231.2006.01342.x. [DOI] [PubMed] [Google Scholar]
- [23].Tu Z, Bozorgzadeh A, Pierce RH, Kurtis J, Crispe IN, Orloff MS. TLR-dependent cross talk between human Kupffer cells and NK cells. J Exp. 2008;205:233–44. doi: 10.1084/jem.20072195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sewnath ME, van der PT, van Noorden CJ, ten Kate FJ, Gouma DJ. Endogenous interferon γ protects against cholestatic liver injury in mice. Hepatology. 2002;36:1466–77. doi: 10.1053/jhep.2002.37196. [DOI] [PubMed] [Google Scholar]
- [25].Ezure T, Sakamoto T, Tsuji H, Lunz JG, III, Murase N, Fung JJ, Demetris AJ. The development and compensation of biliary cirrhosis in interleukin-6- deficient mice. Am J Pathol. 2000;156:1627–39. doi: 10.1016/s0002-9440(10)65034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wuestefeld T, Klein C, Streetz KL, Betz U, Lauber J, Buer J, Manns MP, Muller W, Trautwein C. Interleukin-6/glycoprotein 130-dependent pathways are protective during liver regeneration. J Biol Chem. 2003;278:11281–8. doi: 10.1074/jbc.M208470200. [DOI] [PubMed] [Google Scholar]
- [27].Schoemaker MH, Gommans WM, de la Rosa LC, Homan M, Klok P, Trautwein C, van Goor H, Poelstra K, Haisma HJ, Jansen PL, Moshage H. Resistance of rat hepatocytes against bile acid-induced apoptosis in cholestatic liver injury is due to nuclear factor-kappa B activation. J Hepatol. 2003;39:153–61. doi: 10.1016/s0168-8278(03)00214-9. [DOI] [PubMed] [Google Scholar]
- [28].Gujral JS, Liu J, Farhood A, Jaeschke H. Reduced oncotic necrosis in Fas receptor-deficient C57BL/6J-lpr mice after bile duct ligation. Hepatology. 2004;40:998–1007. doi: 10.1002/hep.20380. [DOI] [PubMed] [Google Scholar]
- [29].Wang H, Park O, Lafdil F, Shen K, Horiguchi N, Yin S, Fu XY, Kunos G, Gao B. Interplay of hepatic and myeloid signal transducer and activator of transcription 3 in facilitating liver regeneration via tempering innate immunity. Hepatology. 2010;51:1354–62. doi: 10.1002/hep.23430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sun R, Park O, Horiguchi N, Kulkarni S, Jeong WI, Sun HY, Radaeva S, Gao B. STAT1 contributes to dsRNA inhibition of liver regeneration after partial hepatectomy in mice. Hepatology. 2006;44:955–66. doi: 10.1002/hep.21344. [DOI] [PubMed] [Google Scholar]
- [31].Notas G, Kisseleva T, Brenner D. NK and NKT cells in liver injury and fibrosis. Clin Immunol. 2009;130:16–26. doi: 10.1016/j.clim.2008.08.008. [DOI] [PubMed] [Google Scholar]
- [32].Kahraman A, Barreyro FJ, Bronk SF, Werneburg NW, Mott JL, Akazawa Y, Masuoka HC, Howe CL, Gores GJ. TRAIL mediates liver injury by the innate immune system in the bile duct-ligated mouse. Hepatology. 2008;47:1317–30. doi: 10.1002/hep.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].McMahon CW, Zajac AJ, Jamieson AM, Corral L, Hammer GE, Ahmed R, Raulet DH. Viral and bacterial infections induce expression of multiple NK cell receptors in responding CD8(+) T cells. J Immunol. 2002;169:1444–52. doi: 10.4049/jimmunol.169.3.1444. [DOI] [PubMed] [Google Scholar]
- [34].Chen L, Calomeni E, Wen J, Ozato K, Shen R, Gao JX. Natural killer dendritic cells are an intermediate of developing dendritic cells. J Leukoc Biol. 2007;81:1422–33. doi: 10.1189/jlb.1106674. [DOI] [PubMed] [Google Scholar]
- [35].Scott MJ, Hoth JJ, Gardner SA, Peyton JC, Cheadle WG. Natural killer cell activation primes macrophages to clear bacterial infection. Am Surg. 2003;69:679–86. [PubMed] [Google Scholar]
- [36].Melhem A, Muhanna N, Bishara A, Alvarez CE, Ilan Y, Bishara T, Horani A, Nassar M, Friedman SL, Safadi R. Anti-fibrotic activity of NK cells in experimental liver injury through killing of activated HSC. J Hepatol. 2006;45:60–71. doi: 10.1016/j.jhep.2005.12.025. [DOI] [PubMed] [Google Scholar]
- [37].Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology. 2006;130:435–52. doi: 10.1053/j.gastro.2005.10.055. [DOI] [PubMed] [Google Scholar]
- [38].Seki S, Habu Y, Kawamura T, Takeda K, Dobashi H, Ohkawa T, Hiraide H. The liver as a crucial organ in the first line of host defense: the roles of Kupffer cells, natural killer (NK) cells and NK1.1 Ag+ T cells in T helper 1 immune responses. Immunol Rev. 2000;174:35–46. doi: 10.1034/j.1600-0528.2002.017404.x. [DOI] [PubMed] [Google Scholar]
- [39].Gregory SH, Sagnimeni AJ, Wing EJ. Bacteria in the bloodstream are trapped in the liver and killed by immigrating neutrophils. J Immunol. 1996;157:2514–20. [PubMed] [Google Scholar]
- [40].Gregory SH, Cousens LP, van Rooijen N, Döpp EA, Carlos TM, Wing EJ. Complemetary adhesion molecules promote neutrophil-Kupffer cell interaction and the elimination of bacteria taken up by the liver. J Immunol. 2002;168:308–15. doi: 10.4049/jimmunol.168.1.308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.