Abstract
Because angiogenesis underlies the pathogenesis of numerous conditions (cancer, psoriasis, macular degeneration), there is a pressing need for continued investigations into angiogenic signaling and potential drug targets. Antiangiogenic agents can be classified as either direct or indirect. Direct antiangiogenics act on untransformed endothelial cells to prevent differentiation and proliferation; indirect antiangiogenics act to inhibit factors involved in proangiogenic signaling. Agents currently available with dermatologic indications are few, while several established and novel biologics targeting various proangiogenic factors are currently being investigated for potential dermatologic uses, but the jury is still out on their efficacy and safety. In this review, we highlight our experience with a group of existing and novel, small molecules that combine several modes of action against angiogenesis in addition to other properties – triarylmethane dyes and fulvene derivatives.
Keywords: Angiogenesis, Angiopoietin-2, NADPH Oxidase, Triarylmethane, Gentian Violet, Fulvene-5
1. Introduction
Antiangiogenic agents can be classified as either direct or indirect. Direct antiangiogenics act on untransformed endothelial cells to prevent differentiation and proliferation; indirect antiangiogenics act to inhibit factors involved in proangiogenic signaling [1]. Agents currently available with dermatologic indications are few: interferon-α 2B (Kaposi's sarcoma, condyloma acuminata), thalidomide (erythema nodosum leprosum), imiquimod (actinic keratosis, basal cell carcinoma, condyloma acuminata), corticosteroids (various autoimmune syndromes and papulosquamous disorders), and becaplermin (diabetic ulcers) [1]. Several established and novel biologics targeting various proangiogenic factors are currently being investigated for potential dermatologic uses, but the jury is still out on their efficacy and safety [reviewed in 30]. Moreover, given that multiple factors are involved in stimulating vessel growth, it is unclear how targeted specific signal mediators will impact complex pathologic systems. What is more likely is that antiangiogenic therapy will exist in the context of a multi-targeted approach along with chemotherapeutic agents or other immunomodulators [2].
Because angiogenesis underlies the pathogenesis of numerous conditions (cancer, psoriasis, macular degeneration), there is a pressing need for continued investigations into angiogenic signaling and potential drug targets. The purpose of the following discussion, then, is to highlight our experience with a group of existing and novel, small molecules that combine several modes of action against angiogenesis in addition to other properties.
2. Triarylmethane Dyes
2.1 Historical Perspectives
The synthetic dye industry owes its existence to the Industrial Revolution. Prior to this period, the source of dyes was limited to natural products – plants, trees, lichens, and insects. Moreover, methods for isolating and combining pigments were closely guarded trade secrets and varied tremendously from maker to maker. Increasing demands on the textile industry brought on by the Industrial Revolution meant the pressing need for dye compounds that were readily available, inexpensive, reproducible, and easy to apply. It is somewhat ironic but no less meaningful that the discovery of the first synthetic dye by the British chemist Sir William H. Perkin in 1856 would come as an accident. While attempting to synthesis the naturally occurring, antimalarial compound quinine, he observed that a purple compound could be extracted with alcohol from a crude mixture of transformed aniline. He termed the new compound mauveine and found that it dyed silk in ways that were resistant to washing and light exposure. He quickly realized the significane of his discovery and filed for a patent in August of 1856 at the age of eighteen. With combinations of foresight, uncanny business acumen, and timely luck, Perkin made the dye a great commercial success. With the textile industry clamoring for more, mauveine was quickly followed up by the synthesis of a number of other dyes – fuchsine (Francois-Emmanuel Verguin, 1858), malachite green, magenta, and gentian violet (Charles Lauth, 1861) [3].
Based on organic compounds isolated from coal tar, initial attempts at dye synthesis were very much trial and error. However, efforts by the German chemist August Wilhelm von Hofmann greatly advanced the understanding of dye structure and led to the development of standardizations and high-quality reproducibility. This, in turn, allowed for the creation of an entirely new industry that by 1900, a mere 45 years after the first synthesis, produced 90 percent of all industrial dyes [3].
Applications for these new synthetic dyes quickly extended over into the medical communities who had also been looking for reliable dyes for histological staining. Gentian violet (GV), perhaps the most recognizable triarylmethane dye, had been synthesized by the French chemist Charles Lauth in 1861 under the name of “Violet de Paris” [4]; what was then a mixture of various pararosaniline dyes, the dye proved popular as a histological stain. Because of its reliability and ease of use, Hans Christian Gram, a Danish bacteriologist, thought it might prove useful in staining bacteria. His method was successful and proved useful in differentiated bacterial species when paired with a fuchsine counterstain. The protocol, known as the Gram stain, was quickly adopted by the larger scientific community and remains a central microbiologic tool.
The first, clinical application of GV is credited to the German ophthalmologist Jakob Stilling. In a brief monograph published in 1890, he describes the bactericidal effects of a solution of aniline dyes (which contained GV) he termed “pyoktanin” [5]. He later set up a collaboration with E. Merck & Co. who developed a product labeled “pyoktanin caeruleum” and sold as an antiseptic. Increasing availability paired with clinical efficacy quickly set the stage for GV to become an often prescribed agent and a home remedy for all kinds of uncomplicated infections including impetigo, tinea, and candidal thrush. As a medical treatment, GV has even been safely administered intravenously in cases of overwhelming sepsis and leprosy, both with measurable efficacy [6,7]. However, investigations of GV quickly dropped off in the early 1950s with the discovery of penicillin and the advent of the antibiotic era, and its clinical role has largely faded from mainstream medicine.
2.2 Current and Investigational Uses
2.2.1 Antisepsis
The continued emergence and development of antibiotic resistance has led to a resurgence in the use of GV. It is estimated that in the United States, 2.6 million people are nasal carriers of methicillin-resistant Staphylococcus aureus (MRSA) and that the incidence of acquiring MRSA in the community approaches 15–75% [8,9]. While not all acquisition of MRSA results in disease, when MRSA is implicated in clinically significant presentations, it is associated with higher morbidity and increased treatment costs. Oral agents currently available to treat MRSA-associated infections include clindamycin, trimethoprim-sulfamethoxazole, tetracyclines, and, less commonly, linezolid; importantly, linezolid resistance has already emerged in a hospital intensive care setting in Spain [10]. Not only do any of these agents carry the risk of eventual resistance, they come with significant side effect profiles that are not easily overlooked – Clostridium difficile colitis with clindamycin, necrotic skin reactions with trimethoprim-sulfamethoxazole, and myelosuppresion with linezolid.
As a pharmaceutical agent, GV is readily available, cheap ($0.08 USD/mL of a 2% solution) [11] and efficacious against a wide variety of uncomplicated pyodermas [12]. Its utility against MRSA-associated infections was first demonstrated by Saji et al. in the setting of decubitus ulcers. Investigators used a dual approach where they bathed patients in solutions of GV (0.1%) then applied a ointment containing 0.1% GV under occlusion. No patients experienced any appreciable side effects, and average time to eradiction of MRSA was 10.8 days (+/− 2.7) [13]. These results have been expanded more recently by the work of Okano et al. Their study demonstrated that GV is effective against MRSA in a broad array of pyodermas including impetigo, infected erosions and ulcers, angular cheilitis, paronychia, and clearance of nasal carriage. For example, in eight cases of impetigo, eradication was achieved in an average of 6.8 days (+/− 3.7, range 4–15); time to eradication was virtually the same in the setting of infected erosions (6.9 +/− 3.9 days) while infected ulcers took an average of 16.3 days (+/− 11.1) to fully clear. The agent was well tolerated in all 37 cases, and no significant side effects were reported. Investigators also determined the in vitro minimum inhibitory concentration (MIC) of GV to be 0.0225 μg/mL [14].
As suggested by Okano et al., GV is also useful in the setting of secondary skin infections. Demonstrating the efficacy of GV against other bacterial pathogens, our group recently reported a case of eczema superinfected with group A Streptococcus successfully treated with a combination of oral doxycyline and daily applications of a 1% solution of GV. The patient experienced immediate relief of pain, tolerated the treatment well, and showed dramatic improvement in all lesions at 2 weeks; this suggests that GV should be considered as an alternative to topical corticosteroid in the setting of secondary infections [15].
This finding builds on the work of Brockow et al. who previously demonstrated the efficacy of GV alone in clearing S. aureus from colonized lesions of atopic eczema. In this study, a 0.3% solution of GV was applied twice daily to lesional and non-lesional skin for 4 days; when compared with topical steroids and tar preparations, GV was the only agent to immediately reduce bacterial density at both sites (p < 0.001). Not only did GV help clear bacterial presence but it also had the added effect of significantly reducing eczema severity [16].
2.2.2 Anti-inflammation
What underlies the process of inflammation is the ability of serous and cellular elements to cross the vessel wall. A structure that under normal circumstances is a tight barrier, when insulted, the vessel wall becomes pervious out of necessity to allow the immune response to progress. This phenomenon, known as vascular leak, is mediated by several factors, one of which is angiopoietin-2 (ang-2). Ang-2 is a 66-kD protein stored in the Weibel-Palade bodies (WPB) of endothelial cells; upon binding with the Tie2 receptor (a tyrosine kinase), ang-2 mediates a variety of downstream effects including vascular permeability, endothelial cell activation, and angiogenesis [17]. Several factors can trigger the release of ang-2 including thrombin, histamine, epinephrine, VEGF, and hypoxia, all of which abound in injured or infected sites [18].
However beneficial, vascular leak has an unintended consequence. As serous fluid accumulates at the injured site, the relative concentrations of soluble elements drops. This has direct implications for antibiotics, for example, where their degree of efficacy is dependent on their concentrations. If drug levels fall below their minimum inhibitory concentration (MIC), pathogens will continue to persist despite antibiotic therapy. A example of extreme vascular leak is septic shock. In this case, overwhelming pathogen loads result in decreased peripheral resistance, vascular leak, and what is known as “third-spacing” of serous fluid. Under these circumstances, high doses of antibiotics are necessary not only to treat the imminent infection but also because of the need to keep drugs levels at their MICs despite the degree of extravascular fluid. Ang-2 plays an important role in this case as well; serum levels of ang-2 have been shown to correlate directly with the degree of sepsis and inversely with nitric oxide-dependent microvascular reactivity, a measure of vascular tone [19].
In addition to their antimicrobial properties, triarylmethane dyes such as GV may play another role in fighting infection. As mentioned previously, a report from 1933 details the results of a series of sepsis patients successfully treated with intravenous GV. While this study has yet to be reproduced in the modern day, recent data on the effect of tryarylmethane dyes on ang-2 expression suggest another mechanism for the effects of GV in sepsis. Our group has shown that, in the setting of an in vitro model of hemangioma, exposure to GV and brilliant green (BG, another triarylmethane dye) reduces expression of ang-2 [20]. It is reasonable to postulate, then, that applications of GV to infected sites may be inhibiting expression of ang-2, thereby reducing local vascular leak and allowing for improved antibiotic delivery.
Triarylmethane dyes may also be acting more directly to inhibit the inflammatory response and some of its deleterious side effects. Peng and colleagues at the University of Rochester Medical Center recently published a study demonstrating the utility of brilliant blue G (BBG, FD&C Blue No. 1) in reducing secondary spinal cord injury [21]. Following primary spinal cord injury (SCI), initial and irreversible tissue loss is made worse by secondary expansion of tissue damage over time. Termed secondary SCI, this phenomenon is mediated by local release of adenosine triphosphate (ATP); in high concentrations, ATP exerts cytotoxic stress on viable motor neurons (“purinergic excitotoxicity”) leading to worsening of the initial trauma. Resultant inflammation also results in activation of astrocytes and microglial cells and migration of neutrophils to the injury site; eventual scar tissue forms a barrier to neuronal regrowth and reconnection. Inhibition of this secondary injury response could result in the salvaging of viable neurons and restoration of neural connections.
Basing their hypothesis on known inhibition of the ATP receptor P2X7 by BBG, researchers administered the drug to rats that had suffered primary spinal cord trauma via a weight drop model [22,23]. Surprisingly, rats that had received BBG were able to regain mobility while none of those that didn't receive the agent ever did; the only notable side effect was that the rats turned blue for several weeks. Additionally, not only is BBG cheap and readily available, it is already approved for and used in human consumption [24].
2.2.3 Antiangiogenesis
Vascular tumors represent the signaling abnormalities of pathogenic angiogenesis [25]. By understanding what goes wrong in vascular tumor pathology, we garner knowledge that may be applicable to other disease dependent on angiogenesis, such as cancer and macular degeneration.
Our group has looked extensively into the application of three triarylmethane dyes (GV, brilliant green, and eosin) to hemangiomas in the in vivo, in vitro, and clinical settings. In a study published in 2006, we demonstrated the efficacy of GV and brilliant green (BG) in reducing tumor volume in a bEnd.3 murine model of hemangiomas [20]. Following subcutaneous injections of bEnd.3 cells (neonatal endothelial cells transformed with the polyoma middle T antigen), mice were treated with intralesional administrations of vehicle control, GV (5 mg/kg), and BG (5 mg/kg) on days 9 and 14 following tumor loading. After euthanization on day 20, mice treated with GV and BG were found to have reductions in hemangioma volumes of 92.6% and 95.7%, respectively.
In efforts to explain how this occurs mechanistically, we looked into the effect of these agents on levels of angiopoetin-2 (ang-2) expression in vitro; not only is ang-2 a mediator of vascular leak but it is also a proangiogenic factor necessary for hemangiomagenesis via recruitment of host endothelial cells and precursor cells. We found that GV at 10 μM concentrations reduced ang-2 levels by 90% and that BG at 0.75 μM concentrations reduced ang-2 levels to undetectable. We also examined the effects of both agents on reactive oxygen species (ROS) production in Cos-phox and HEK293 Nox4-11 cells. Previously, we had shown that production of ROS by NADPH Oxidase (Nox) 1 is a potent trigger of angiogenesis via upregulation of VEGF production and activation of MMP-9. Here, we demonstrated that GV and BG both inhibit Nox 2 and 4 as evidenced by decreased hydrogen peroxide production. Together, these data suggest that triarylmethane dyes have may have clinical applications in angiogenic disorders such as hemangiomas as inhibitors of angiogenic signaling in two ways: inhibitions of ang-2 expression and ROS production by Nox 2 and 4.
While we are yet to extend these findings into the clinical setting, to that end, we conducted a small proof-of-concept case series of ulcerated infantile hemangiomas (IHs) using another triarylmethane dye, eosin. Ulceration is the most common complication of IHs and can be further complicated by secondary infection, worsening pain, and bleeding [26]. More definitively, recent data suggest that ulceration may in fact have a biochemical basis in an imbalance between ang-2 and VEGF production [27]. Given our previous demonstration of GV and BG as inhibitors of ang-2 and Nox 2/4, we hypothesized that topical application of eosin would enhance ulcer healing via correction of the ang-2/VEGF imbalance and induce involution of the lesion by reductions in proangiogenic, ang-2 stimulation.
Eighteen patients with ulcerated hemangiomas were enrolled to receive topical application of a 2% solution of eosin three times a day unoccluded for superficial ulcers or once a day under hydrocolloid dressing for deeper ulcers. Following 4 weeks of treatment, complete healing of the ulcer was achieved in 16 patients. Four patients required adjuvant therapy with either pulsed dye laser and/or topical antibiotics while one patient required surgical intervention; notably, none of the ulcers recurred. Finally, we confirmed that eosin acts similar to GV and BG in reducing ang-2 production in vivo in bEnd.3 cells [28].
3. Fulvene Derivatives
Apart from topical application and intralesional administration, many triarylmethane dyes are poorly tolerated when administered systemically. Our experience with diphenyliodonium (DPI), GV and BG suggests that conjugated aromaticity (and the ability to delocalize electrons that imparts) is central to their shared antiangiogenic properties. The desire to retain aromaticity without the triarylmethane backbone led to the development of fulvene derivatives. Fulvenes are aromatic, 5-cardon ring molecules that have the advantage of being highly water soluble and very chemically reactive. Conjugation of sodium cyclopentadiene with the ketone indigo led to the formation of what we termed fulvene-5 [29]. Given its aromaticity and enhanced water solubility, we hypothesized that fulvene-5 would also act as an inhibitor of ang-2 and Nox 2 and 4 but be better tolerated. Following establishment of bEnd.3 tumors in vitro, fulvene-5 (120 mg/kg/day) was administered via intraperitoneal injection daily for two weeks. No toxicities were noted after administration of the agent, and, following euthanization on day 14, tumor volumes were found to be reduced by approximately 75% when compared to vehicle controls. In confirming our hypothesis, we found that fulvene-5 did indeed inhibit Nox 2 and 4 activity as measured by in vivo hydrogen peroxide production. We also confirmed that this novel agent downregulated in vivo expression of ang-2 and several downstream targets of ang-2 including Notch-related ankyrin repeat protein (Nrarp), delta-like ligand 4 (DII4), and placental growth factor (PLGF). Fulvene-5, then, represents a novel class of small molecule inhibitors of angiogenesis that act in similar ways to traditional tryarylmethane dyes but are more systemically tolerable.
4. Conclusion
Altogether, our experience with triarylmethane dyes and fulvene-5 as potent inhibitors of angiogenic signaling suggest that they have a role in the management of hemangiomas and possibly other disorders dependent on angiogensis. In the case of triarylmethane dyes, not only are most of them relatively inexpensive and readily available, they combine several modes of action including antisepsis and anti-inflammation along with antiangiogensis. The goal, then, should be to expand these results into larger, clinical trials with aims to establish efficacy and optimal dosing protocols for these various disorders. Table 1 summarizes the structures and medicinal uses of fulvene-5 and some triarylmethane dyes.
Table 1.
Novel antiangiogenic agents, including fulvene-5 and examples of triarylmethane dyes with accepted/investigational medical uses, dermatologic uses, and pertinent side effects. Adapted with permission from the authors from Dermatol Clin 2011;29(1):69–73.
| Compound | Structure | Medical Uses | Dermatologic Uses | Potential Side Effects |
|---|---|---|---|---|
| Fulvene-5 C26H22N2 |
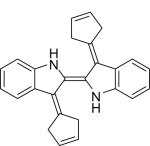
|
|
|
|
| Gentian Violet C25H30ClN3 |

|
|
|
|
| Brilliant Green C27H33N2·HO4S |

|
|
|
|
| Eosin Y C20H6Br4Na2O4 |
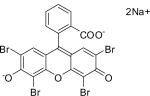
|
|
|
|
| Malachite Green C23H25ClN2 |

|
|
|
|
| Fuchsine C20H20N3·HCl |
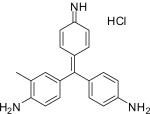
|
|
|
|
| Brilliant Blue G “Coomassie” C47H49N3O7S2 |
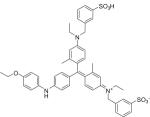
|
|
|
|
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- [1].Reddy K, Arbiser J. Anti-angiogenic agents. In Fitzpatrick's Dermatology in General Medicine. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, et al., editors. 7th Ed. McGraw-Hill; New York: 2008. [Google Scholar]
- [2].Arbiser JL. Why targeted therapy hasn't worked in advanced cancer. J Clin Invest. 2007 Oct;117(10):2762–5. doi: 10.1172/JCI33190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dye [Accessed December 1, 2010];In Encyclopedia Britannica. http://www.britannica.eom/EBchecked/topic/174980/dye.
- [4].Lauth C. On the new aniline dye, Violet de Paris. Laboratory. 1867;1:138–39. [Google Scholar]
- [5].Stilling J. Anilin Farbstoffe als Antiseptica und ihre Anwendung in der Praxis. Mittheilung; Strassbourg: 1890. [Google Scholar]
- [6].Hinton D. Results of the intravenous use of gentian violet in cases of extreme septicemia. Ann Surg. 1925 March;81(3):687–692. doi: 10.1097/00000658-192503010-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ryrie GA. A preliminary report on the action of certain dyes in leprosy. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1933 June;27(1):85–92. [Google Scholar]
- [8].Graham PL, 3rd, Lin SX, Larson EL. A U.S. population-based survey of Staphylococcus aureus colonization. Ann Intern Med. 2006 Mar 7;144(5):318–25. doi: 10.7326/0003-4819-144-5-200603070-00006. [DOI] [PubMed] [Google Scholar]
- [9].Odell CA. Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) skin infections. Curr Opin Pediatr. 2010 Jun;22(3):273–7. doi: 10.1097/MOP.0b013e328339421b. [DOI] [PubMed] [Google Scholar]
- [10].Sánchez García M, De la Torre MA, Morales G, Peláez B, et al. Clinical outbreak of linezolid-resistant Staphylococcus aureus in an intensive care unit. JAMA. 2010 Jun 9;303(22):2260–4. doi: 10.1001/jama.2010.757. [DOI] [PubMed] [Google Scholar]
- [11].Gentian Violet [accessed October 15, 2010];Amazon.com. http://www.amazon.com/GENTIAN-VIOLET-SOL-2-HUM/dp/B000GCQ05G;
- [12].Berrios RL, Arbiser JL. Effectiveness of gentian violet and similar products commonly used to treat pyodermas. Dermatol Clin. 2011 Jan;29(1):69–73. doi: 10.1016/j.det.2010.08.009. [DOI] [PubMed] [Google Scholar]
- [13].Saji M, Taguchi S, Uchiyama K, et al. Ef®cacy of gentian violet in the eradication of methicillin-resistant Staphylococcus aureus from skin lesions. J Hosp Infect. 1995;31:225–228. doi: 10.1016/0195-6701(95)90070-5. [DOI] [PubMed] [Google Scholar]
- [14].Okano M, Noguchi S, Tabata K, Matsumoto Y. Topical gentian violet for cutaneous infection and nasal carriage with MRSA. Int J Dermatol. 2000 Dec;39(12):942–4. doi: 10.1046/j.1365-4362.2000.00128.x. [DOI] [PubMed] [Google Scholar]
- [15].Arbiser JL, MacKelfresh J, Stoff B. A nonsteroidal alternative to impetiginized eczema in the emergency room. J Am Acad Dermatol. 2010 Sep;63(3):537–9. doi: 10.1016/j.jaad.2009.05.027. [DOI] [PubMed] [Google Scholar]
- [16].Brockow K, Grabenhorst P, Abeck D, Traupe B, Ring J, Hoppe U, Wolf F. Effect of gentian violet, corticosteroid and tar preparations in Staphylococcus-aureus-colonized atopic eczema. Dermatology. 1999;199(3):231–6. doi: 10.1159/000018253. [DOI] [PubMed] [Google Scholar]
- [17].Fiedler U, Augustin HG. Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol. 2006;27:552–558. doi: 10.1016/j.it.2006.10.004. [DOI] [PubMed] [Google Scholar]
- [18].Lowenstein CJ, Morrell CN, Yamakuchi M. Regulation of Weibel-Palade body exocytosis. Trends Cardiovasc Med. 2005;15:302–308. doi: 10.1016/j.tcm.2005.09.005. [DOI] [PubMed] [Google Scholar]
- [19].Davis JS, Yeo TW, Piera KA, Woodberry T, Celermajer DS, Stephens DP, Anstey NM. Angiopoietin-2 is increased in sepsis and inversely associated with nitric oxide-dependent microvascular reactivity. Crit Care. 2010 May 18;14(3):R89. doi: 10.1186/cc9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Perry BN, Govindarajan B, Bhandarkar SS, Knaus UG, et al. Pharmacologic blockade of angiopoietin-2 is efficacious against model hemangiomas in mice. J Invest Dermatol. 2006 Oct;126(10):2316–22. doi: 10.1038/sj.jid.5700413. [DOI] [PubMed] [Google Scholar]
- [21].Peng W, Cotrina ML, Han X, Yu H, et al. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc Natl Acad Sci USA. 2009 Jul 28;106(30):12489–93. doi: 10.1073/pnas.0902531106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Remy M, Thaler S, Schumann RG, May CA, et al. An in vivo evaluation of Brilliant Blue G in animals and humans. Br J Ophtalmol. 2008;92:1142–47. doi: 10.1136/bjo.2008.138164. [DOI] [PubMed] [Google Scholar]
- [23].Jiang LH, Mackenzie AB, North RA, Surprenant A. Brilliant Blue G selectively blocks ATP-gated rat P2X7 receptors. Mol Pharmacol. 2000;58:82–88. [PubMed] [Google Scholar]
- [24].Borzelleca JF, Depukat K, Hallagan JB. Lifetime toxicity/carcinogenicity studies of FD&C Blue No. 1 (brilliant blue FCF) in rats and mice. Food Chem Toxicol. 1990;28:221–34. doi: 10.1016/0278-6915(90)90034-k. [DOI] [PubMed] [Google Scholar]
- [25].Arbiser JL, Bonner MY, Berrios RL. Hemangiomas, angiosarcomas, and vascular malformations represent the signaling abnormalities of pathogenic angiogenesis. Curr Mol Med. 2009 Nov;9(8):929–34. doi: 10.2174/156652409789712828. [DOI] [PubMed] [Google Scholar]
- [26].Chamlin SL, Haggstrom AN, Drolet BA, Baselga E, et al. Multicenter prospective study of ulcerated hemangiomas. J Pediatr. 2007 Dec;151(6):684–9. doi: 10.1016/j.jpeds.2007.04.055. [DOI] [PubMed] [Google Scholar]
- [27].Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thurston G, Gale NW, et al. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med. 2006;12:235–39. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- [28].Lapidoth M, Ben-Amitai D, Bhandarkar S, Fried L, Arbiser JL. Efficacy of topical application of eosin for ulcerated hemangiomas. J Am Acad Dermatol. 2009 Feb;60(2):350–1. doi: 10.1016/j.jaad.2008.10.034. [DOI] [PubMed] [Google Scholar]
- [29].Bhandarkar SS, Jaconi M, Fried LE, Bonner MY. Fulvene-5 potently inhibits NADPH oxidase 4 and blocks the growth of endothelial tumors in mice. J Clin Invest. 2009 Aug;119(8):2359–65. doi: 10.1172/JCI33877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nguyen A, Hoang V, Laquer V, Kelly KM. Angiogenesis in cutaneous disease: part I. J Am Acad Dermatol. 2009 Dec;61(6):921–42. doi: 10.1016/j.jaad.2009.05.052. [DOI] [PubMed] [Google Scholar]


