Abstract
Resveratrol, a phytoalexin antioxidant found in red grapes, has been shown to have both chemopreventive and therapeutic effects against many diseases and disorders, including those of the skin. Studies have shown protective effects of resveratrol against ultraviolet radiation mediated oxidative stress and cutaneous damages including skin cancer. Because many of the skin conditions stem from ultraviolet radiation and oxidative stress, this antioxidant appears to have promise and prospects against a wide range of cutaneous disorders including skin aging and skin cancers. However, there are a few roadblocks in the way of this promising agent regarding its translation from the bench to the bedside. This review discusses the promise and prospects of resveratrol in the management of skin disorders and the associated challenges.
Keywords: Resveratrol, Skin, Ultraviolet, Antioxidant
Introduction
The skin is the body’s largest organ, and is its foremost line of defense against disease. Because of this importance, anything that compromises the skin’s integrity can be detrimental to the overall health of the individual. Also, since the skin is so easily seen by others, even non-life-threatening disorders can cause significant social problems, which can be almost as devastating to those affected with the disease. Most skin disorders and diseases have a complex set of processes responsible for their inception, which can be genetic and/or environmental, and the importance of each factor is different for every disease. However, exposure to solar ultraviolet (UV) radiation is a very important factor in the pathogenesis of many skin disorders, including aging and cancer [1]. UV light can directly cause DNA damage, as well as start a cascade of oxidative stress and related signaling leading to mutation and irreparable damages to the cells. For this reason, it is important to develop novel approaches aimed at fighting the stresses and insults that skin comes into contact with every day, as well as managing the cutaneous disorders that are developed.
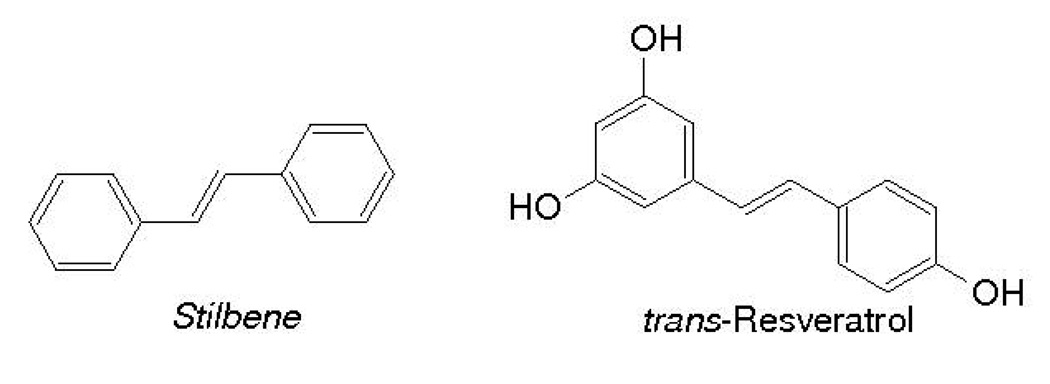
Resveratrol, a trihydroxy derivative of stilbene (3,5,4’-trihydroxystilbene) (Fig. 1), is a naturally occurring phytoalexin antioxidant present in grapes, berries, peanuts and red wine [2]. Trans-resveratrol is found in many plants, and is produced as a form of defense against harmful environmental stimuli, such as a fungus or infection. The earliest work on resveratrol was done examining its effects on cardiovascular health and heart disease. Researchers noticed that the people in France had a much lower incidence of coronary heart diseases than populations in other countries, despite their high saturated fat intake. This was attributed to an increased red wine intake by the French population and was termed the “French Paradox” by Renaud and Lorgeril in 1992 [3]. Although earlier work was done examining the effects of resveratrol on cardiovascular disease, much of the recent research done with this antioxidant has been in the areas of cancer chemoprevention and treatment, inflammation, and oxidative stress [4–16].
Figure 1.
Chemical Structures of Stilbene and Resveratrol (Trans-3,5,4’-trihydroxystilbene)
Skin and Skin Disorders
The skin is made up of three main layers: the hypodermis, the dermis and the epidermis [17]. The hypodermis is the deepest section of skin, and is primarily a place of connection and fat storage. The dermis contains most of the connective tissues of the skin, as well as nerve endings, sweat glands, and hair follicles. The primary function of the epidermis is to provide a weather- and water-proof layer to protect the body. The epidermis is made up mostly of keratinocytes, but also contains melanocytes, Merkel cells, and Langerhans cells [17, 18]. Because of the large amount of cells and structures contained within the skin, as well as it being the first line of defense, it is not surprising that many different things can go wrong and cause a variety of skin diseases. However, skin cancer is the most important cutaneous condition that needs aggressive research and new approaches for its management.
Skin cancer is estimated to affect one out of every seven Americans each year, making it the most prevalent form of cancer [19, 20]. Light skin color and excessive exposure to solar UV radiation have been identified as major risk factors in developing this disease. The two most frequent types of skin cancer, collectively known as non-melanoma skin cancer (NMSC), are Basal Cell Carcinoma (BCC) and Squamous Cell Carcinoma (SCC). More than a million new cases of non-melanoma skin cancers are diagnosed annually in the United States, with the number increasing every year [21, 22]. BCC is the most common form of NMSC, and is responsible for about 80% of new cases every year [23], while SCC presents as about 16% of new NMSC cases annually. In addition to NMSC, it is estimated that about 68,130 new cases of malignant melanoma, the most serious form of skin cancer, will be diagnosed in the United States this year, with an estimated 8,700 deaths [22]. Another disturbing fact about skin cancer comes from some epidemiological observations showing an increased risk of other lethal cancer types in individuals who have a history of skin cancer [24–27]. This fact combined with the high incidence of skin cancer provides a great impetus for finding new approaches for treating and preventing skin cancer.
Oxidative Stress and Antioxidants in the Skin
In normal, unstressed cells, there is a constant production of reactive oxygen from the mitochondria, which is balanced by the production of antioxidant enzymes in the cell, such as superoxide dismutase (SOD), catalase, and glutathione peroxidase [28]. When a cell comes under stress, this balance is interrupted, and the reactive oxygen species can overwhelm the cells and lead to a change in normal cellular behaviors [29, 30]. Oxidative stress can be generated by a variety of factors, including cigarette smoke, extreme temperature change, and exposure to UV radiation [31–33]. Exposure to UV radiation is one of the most important factors in many skin disorders and diseases, including aging and cancer [1, 34–39]. There are three main types of UV radiation; UVA, UVB, and UVC [1]. UVA is the longest wavelength and is thought to be responsible for many skin conditions including skin aging and skin carcinogenesis [40, 41]. UVB, a mid-range wavelength, is thought to contribute mostly to the development of skin cancer, although it has been shown to play a role in aging and other skin damage [1, 42]. UVC is the shortest wavelength that does not usually penetrate the atmosphere. However, UVC can also play a role in mutagenesis by imparting DNA damage [1]. In many cases, the visible signs of skin aging, such as wrinkling, dryness, and discoloration, are caused by or exacerbated by UV exposure [34, 35, 43]. Additionally, other skin conditions that can result from chronic sun and UV exposure, such as actinic keratosis, have been linked with increasing an individual’s risk of skin cancer, [44] which can also be induced by UV exposure as well. UV radiation can also cause oxidative stress in skin cells, which is thought to be another contributor to skin disease and skin carcinogenesis [1, 45].
Because of the critical role of oxidative stress in cancer and other cutaneous conditions [46], studies have attempted to assess if exogenous antioxidants can have preventive and/or therapeutic effects against skin cancers, especially since the skin consistently encounters factors that can cause oxidative stress. Obviously, there are many antioxidants that have been studied with varying success rates. Several antioxidants, including vitamins C, E, and the green tea polyphenol (−)-epigallocatechin-3-gallate (EGCG) [47–51], have been shown to possess protective effects against cutaneous disorders. As described below, a number of studies have shown that resveratrol also possesses promise against certain cutaneous pathologies, including skin cancer, in preventive as well as in therapeutic settings.
Antioxidant Resveratrol in Disease Management
Resveratrol was initially found to have antioxidant abilities and to be protective against cardiovascular disease [52–54]. The cancer chemopreventive properties of resveratrol were first demonstrated when it was found to possesses chemopreventive activity against all the three major stages of carcinogenesis i.e. initiation, promotion and progression [12]. Further, resveratrol has been shown to have low toxicity and limited side effects, two much sought-after properties in therapeutic settings, especially since a wide range of available drugs are toxic to normal cells. It has also been suggested that this specificity may be due to pro-oxidant activities of resveratrol when exposed to the elevated copper levels in cancer cells [55]. Because of these properties, several clinical trials were initiated with this agent, including one recently completed trial on melanoma [56]. Much of the recently published data on resveratrol in humans has been summarized in Table 1. As shown, most of the studies are focused on determining the safety, pharmacokinetics, and dosing, and not much has been done studying effects against specific cancers. It is expected that as these initial trials are finished up and some of the challenges discussed later in this review are addressed, many more clinical trials will initiated and conducted to study the potential of resveratrol against many disease conditions including cancers.
Table 1.
Human studies with resveratrol
| Aim of Study | Dose | N= | Result | Ref |
|---|---|---|---|---|
| Safety and Dose-Finding | 0–5 g orally | 10 | Safe up to 5 g, highest levels in blood were 1.5 hours after intake. Urinary excretion of resveratrol was rapid, so a high dose of resveratrol maybe insufficient for chemopreventative proterties. | [93] |
| Safety, pharmacokinetics, dosing with quercetin or alcohol | 2000 mg twice daily with food | 8 | Resveratrol was well tolerated, but 6/8 subjects reported diarrhea | [89] |
| Effects of food on resveratrol absorption | 400 mg | 24 | The extent of absorption wasn’t affected by food, but the rate of reveratrol absorption was delayed in the presence of food. | [94] |
| Pharmacokinetics of multiple doses | 0–150 mg, 6x/day | 40 | Repeated dosing was well tolerated, but still no high plasma concentrations of resveratrol. Bioavailability was higher after morning administration | [95] |
| Pharmacokinetics of repeated dosing versus age | 200 mg, 3x/day | 24 | No difference in pharmacokinetics was seen in young versus old patients, and resveratrol was well tolerated by all. | [79] |
| Bioavailability from red wine consumption | 246 µg-1.92 mg, in 3 different wines | 25 | The meal content did not affect resveratrol bioavailability. Only trace amounts of resveratrol were found (in only some subjects) 30 minutes after ingestion. | [96] |
| Absorption and metabolism | 0.36 mg/kg (resveratrol only) | 12 | Urine excretion of glucuronide and sulfate conjugates. Peak concentrations of polyphenols does not appear high enough for chemopreventive activity | [97] |
| Pharmacokinetics and specific protein interactions | 0.5, 1.0, 2.5, or 5.0 g | 40 | Resveratrol decreased levels of IGF-1 and IGFBP-3, which may contribute to chemoprevention. Resveratrol was safe but at higher doses caused some gastrointestinal symptoms. Plasma levels of metabolites exceeded those of resveratrol | [88] |
| Effects on pharmacologic doses of drug- and carcinogen-metabolizing enzymes | 1 g | 42 | Resveratrol affects enzymes involved in cancer activation and detoxification, and therefore my provide some chemopreventative properties. However, resveratrol also altered drug efficacy. | [56] |
As described above, the first and landmark study regarding the chemopreventive effects of resveratrol was done by Jang and colleagues [12]. In this study, resveratrol was found to i) act as an antioxidant and antimutagen, ii) induce phase II drug-metabolizing enzymes (anti-initiation activity), iii) mediate anti-inflammatory effects, iv) inhibit cyclooxygenase and hydroperoxidase functions (antipromotion activity), and iv) induce human promyelocytic leukemia cell differentiation (antiprogression activity). In addition, resveratrol also inhibited i) the development of preneoplastic lesions in carcinogen-treated mouse mammary glands in culture, and ii) tumorigenesis in a chemically-induced skin carcinogenesis mouse model [12]. Since the appearance of this study in the year 1997, evidence accumulated from a plethora of studies, both in vitro and in vivo, have suggested that resveratrol could be developed as a strong chemopreventive and/or therapeutic agent for the management of cancer [2, 5, 12–15, 57–62].
Resveratrol and Skin Diseases: In Vitro Studies
Because resveratrol has strong antioxidant properties, and oxidative stress is believed to be a critical factor in a variety of cutaneous conditions including skin cancers [1, 48, 63], a number of in vitro studies have been done to determine the anti-proliferative effects as well as photo-protective effects of resveratrol. Table 2 summarizes some of these in vitro studies. In two studies from this laboratory, we demonstrated that resveratrol, via modulating cyclin kinase inhibitor (cki)-cyclin-cyclin dependent kinase (cdk) network [15] and retinoblastoma (pRb)-E2F/DP machinery [14], resulted in a G1-phase arrest of the cell cycle and apoptosis of human epidermoid carcinoma (A431) cells. Another in vitro study demonstrated that resveratrol was able to induce apoptosis in two human melanoma cell lines [64]. Yang and colleagues showed that resveratrol inhibits APE/Ref-1 and significantly decreases AP-1/JunD, MMP-1, Bcl-2, and iNOS protein levels in melanoma cells [16]. Since, in this study, nitric oxide was found to initiate progression of human melanoma via a feedback loop mediated by apurinic/apyrimidinic endonuclease-1/redox factor-1, the authors suggested that resveratrol could be an appropriate choice for combining with other compounds that develop resistance by up-regulation of these molecules [16]. This group also demonstrated that that resveratrol inhibited anchorage-independent growth of melanoma cell lines [65]. Further, the authors also noted that following treatment with resveratrol, the cells appeared to act more “normal”, as if resveratrol had caused the cells to differentiate. Resveratrol did not have any cytotoxic effects on the lines tested, but did change the morphology of the cells, as well as elevate the MHC class I antigen and Fas expression levels, and decreased AP-1 binding and transcriptional activities [65]. It was also found that resveratrol decreased intracellular reactive oxygen species in melanoma cells. In another study, Osmond and colleagues showed that resveratrol significantly decreased the viability of melanoma cell lines (DM738 and DM443) without similar effects in fibroblast cells [66]. Further, resveratrol significantly enhanced the cytotoxicity of temozolomide in melanoma cells [66].
Table 2.
In Vitro Studies on Resveratrol and Skin
| Cells used | Brief description | Conclusion | Ref |
|---|---|---|---|
| NHEK | UVB induced skin damage | Chemo preventative properties of resveratrol may be due to blocked activation of NF-κB | [13] |
| NHEK, normal dermal fibroblasts | Effect of Sirt1 agonists on matrix metalloproteases (MMPs) | Resveratrol inhibited MMP-9 expression and protected collagen from UV radiation degradation | [98] |
| A431 cells | Effect of resveratrol on the pRb-E2F/DP pathway | Resveratrol down regulated pRb-E2F/DP pathway members and caused a G0/G1 phase cell cycle arrest and then apoptosis. | [14] |
| A431 | Investigate sensitization ability to UVB-induced cell death | Resveratrol inhibited UVB-induced proliferation via disruption of NF-κB pathway | [99] |
| A431 | Effects on apoptosis and growth | Regulates apoptosis through JAK/STAT pathway | [100] |
| A431 | Investigate antiproliferative mechanism | Modulates MEK1 and AP-1 pathways | [101] |
| A431 | Modulations in cyclindependent kinase (cdk) inhibitor-cyclin-cdk machinery | Causes cell apoptosis as a result of G(1)-phase cell cycle arrest | [15] |
| SK-mel28, A375 | Effects on melanoma cell growth | Resveratrol inhibited growth of both cell lines, inhibiting A375 cell growth more | [64] |
| 3PC, M 1/2, Ca3/7 | Effect of phytochemicals on oxidative DNA damage | Resveratrol protected cells from oxidative DNA damage | [102] |
| HaCaT | UVA induced oxidative stress | Resveratrol protected cells from UVA induced oxidative stress and increased cell viability after UVA exposure | [67] |
| HaCaT | Effect of resveratrol pretreatment | Confirmed photoprotective properties of resveratrol, found caspase-3 and -8 were decreased in pretreated cells | [103] |
| HaCaT | Effect of resveratrol on levels of p66Shc | Resveratrol induces ERK1/2 and p66Shc-Ser36 phosphorylation, as well as AKT dephosphorylation | [104] |
| c83-2c | Determine if levels of APE/Ref-1 are increased in melanoma, and can be exploited by resveratrol | Resveratrol inhibited APE/Ref-1 actions in the cell and increased sensitivity to dacarbazine treatment | [74] |
In an in vitro study, we have also shown that resveratrol treatment blocked UVB-mediated activation of NF-κB pathway in the normal human epidermal keratinocytes (NHEK), in a dose- as well as time- dependent fashion [13]. In a study done by Liu and colleagues, it was shown that resveratrol protects human HaCaT keratinocytes from UVA-induced oxidative stress damage by downregulating Keap1 expression [67]. Recently, in another study it was found that there are specific polyphenol receptor sites in the skin that bind resveratrol, which then exert protective effects against the nitric oxide free radical donor sodium nitroprusside (SNP) [68]. To determine the receptor binding, the authors treated human skin tissue sections with [3H]-resveratrol followed by studying localization of signals. A significant binding of resveratrol was seen in the epidermis, with some binding in the dermis. The study also determined that the resveratrol binding site was also present in vitro, in HaCaT cells, an immortalized human keratinocyte cell line [68]. This study gives promise to the notion that skin diseases and cancers can be treated with resveratrol because of the resveratrol-specific binding sites in the skin.
Resveratrol and Skin Diseases: In Vivo Studies
A few in vivo studies have also evaluated the potential of resveratrol for skin conditions. Some of these studies have been summarized in Table 3.
Table 3.
In Vivo Studies on Resveratrol and Skin
| Animal | Brief description | Result | Ref |
|---|---|---|---|
| SKH-1 mice | UVB mediated skin damage | Resveratrol decreased leukocyte infiltration, bi-fold skin thickness, and hyperplasia | [8] |
| SKH-1 mice | UVB exposure damage | Resveratrol decreased bi-fold skin thickness, skin edema, and inhibited markers of tumor progression | [57] |
| p53(+/−)/SKH-1 mice | UV induced skin tumorigenesis | Resveratrol delayed UV induced tumorigenesis and transformation of benign papillomas to Squamous cell carcinomas | [105] |
| SKH-1 mice | UVB radiation-mediated skin tumorigenesis | Resveratrol reduced the incidence of tumors and delayed tumorigenesis | [69] |
| CD-1 mice | Chemopreventative activity of Resveratrol | Resveratrol reduced tumor incidence by at least 50% | [12] |
| CD-1 mice | Anti-cancer activity of 4 wine polyphenols | Trans-resveratrol reduced the amount of DMBA-induced skin tumors and was absorbed more effectively than the other polyphenols | [106] |
| syngeneic C57BL/6N mice | Effects of polyphenols on B16-BL6 melanoma cells | Resveratrol (injected) inhibited the growth of B16-BL6 cells | [107] |
| C57BL/6J mice, Wistar rats, ESD NZW rabbits | Bioavailability of resveratrol and its effect on tumor growth | Oral resveratrol did not inhibit growth of B16M cells | [108] |
| SENCAR mice | Resveratrol combination treatment of DMBA-induced skin cancer | Resveratrol showed a greater effect on chemopreventive markers when used in combination with other phytochemicals than when used alone | [109] |
| B16 cells in mice | Chemopreventative effects of Resveratrol on resistant melanoma cells | Resveratrol induced apoptosis in B16 cells after an artificial arrest of the G(1) phase of the cell cycle was imposed | [110] |
| C918 and Mum2b cells in athymic nu/nu mice | Effect of resveratrol against uveal melanoma tumor growth | Peritumoral injection of resveratrol had better effects than systemic, oral administration. Resveratrol induces | [72] |
| SKH-1 mice | Assess the involvement of Survivin in UVB-mediated skin cancer induction | Resveratrol inhibited UVB-mediated skin cancer development by inhibition of Survivin and associated apoptotic proteins | [6] |
| Swiss albino mice | Assess chemopreventive potential against DMBA-induced skin carcinogenesis | Resveratrol delayed tumor onset, reduced tumor number, regulated apoptosis and cell survival, likely through the PI3k/AKT pathway | [111] |
| Swiss albino mice | Assess potential against DMBA-induced skin carcinogenesis | Resveratrol induces apoptosis through p53 activity | [112] |
| C3H/HeN and C3H/HeJ mice | Effect of resveratrol on DMBA-induced skin carcinogenesis and TLR4 interaction | Resveratrol inhibited angiogenesis and tumor size better in TLR4-competent mice | [113] |
| SirT1-null mice | Chemopreventative effects in SirT1-null mice | Topically applied resveratrol reduced tumorigenesis more effectively in normal SirT1 genotype mice. | [114] |
In a study from this laboratory, we have shown that a topical application of resveratrol to SKH-1 hairless mice results in significant inhibitions of UVB-mediated increases in (i) skin edema, (ii) inflammation, (iii) cyclooxygenase (COX) and ornithine decarboxylase (ODC) induction, and (iv) generation of hydrogen peroxide (H2O2) and lipid peroxidation, in the skin [57]. In another study [8], we determined the photoprotective effects of resveratrol against multiple UVB exposure mediated damages to the skin of SKH-1 hairless mouse. Our data demonstrated that a topical pre-application of resveratrol (10 µ mole/0.2 ml/mouse) 30 minutes prior to UVB-exposures (180 mJ/cm2 × 7 exposures on alternate days) to mouse skin resulted in a significant decrease in UVB mediated increases i) in skin edema and hyperplasia, ii) infiltration of leukocytes into the epidermis and dermis, and iii) protein levels of PCNA in epidermis [8]. We also found that UVB-exposures to mouse skin resulted in significant i) increases in cdk-2, cdk-4, cdk-6 and cyclin D2 protein levels in epidermis; whereas, resveratrol pre-treatment significantly reversed these effects [8]. Interestingly, resveratrol pre-treatment was found to result in a further enhancement in UVB-exposure mediated increase in WAF1/p21 and p53 proteins [8]. Finally, we also found that repeated UVB exposures resulted in an upregulation of mitogen activated protein-kinase (MAPK) 1/2 and MAPK kinase (MEK)-1; whereas topical application of resveratrol prior to UVB exposures significantly reversed the UVB-mediated responses in these proteins [8]. Taken together, this study suggested that the anti-proliferative effects of resveratrol might be mediated via modulation in i) cki-cyclin-cdk network, and ii) MAPK-pathway [8].
In a follow up study [6], we evaluated the involvement of IAP protein Survivin during the protective effects of resveratrol against multiple UVB exposure mediated damages in SKH-1 hairless mouse skin. The data from this study demonstrated that topical pre-treatment of resveratrol (10 µmol in 200 µl acetone per mouse) resulted in significant inhibition of UVB exposure-mediated increases in i) cellular proliferations (Ki-67 immunostaining), ii) protein levels of epidermal cyclooxygenase (COX)-2 and ornithine decarboxylase (ODC), established markers of tumor promotion, iii) protein and mRNA levels of Survivin, and iv) phosphorylation of Survivin; in the skin of SKH-1 hairless mouse [6]. Resveratrol pre-treatment also resulted in i) reversal of UVB-mediated decrease of Smac/DIABLO, and ii) enhancement of UVB-mediated induction of apoptosis, in mouse skin [6]. Taken together, this study suggested that resveratrol imparts chemopreventive effects against UVB exposure-mediated damages in SKH-1 hairless mouse skin via inhibiting Survivin-pathway.
In another recent study [69], we evaluated the skin cancer chemopreventive effects of resveratrol in a photocarcinogenesis model of skin cancer. In this study, we employed a UVB initiation-promotion protocol in which the control mice were subjected to chronic UVB exposure (180 mJ/cm2, twice weekly, for 28 weeks). The experimental animals received either a pretreatment (30 min before each UVB) or post-treatment (5 min after UVB) of resveratrol (25 or 50 µmole/0.2 ml acetone/mouse). The mice were followed for skin tumorigenesis and were killed at 24 h after the last UVB exposure, for further studies. Our data demonstrated that the topical application of skin with resveratrol (both pre- and post- treatment) resulted in a highly significant 1) inhibition in tumor incidence, and 2) delay in the onset of tumorigenesis [69]. Interestingly, the posttreatment of resveratrol was found to impart equal protection than the pretreatment; suggesting that resveratrol-mediated responses may not be sunscreen effects [69]. Further, our data also demonstrated a significant i) up-regulation of Survivin (both at protein- and mRNA- levels), ii) up-regulation of phospho-Survivin protein, and iii) down-regulation of proapoptotic Smac/DIABLO protein in skin tumors; whereas treatment with resveratrol resulted in the attenuation of these responses [69]. Our study also suggested that resveratrol enhanced apoptosis in UVB-exposure-mediated skin tumors [69]. This study, for the first time, suggested that i) resveratrol imparts strong chemopreventive effects against UVB exposure-mediated skin carcinogenesis, and ii) the chemopreventive effects of resveratrol may, at least in part, be mediated via modulations in Survivin and other associated events [69].
In a recent study, Kim et al demonstrated that oral administration of resveratrol to p53(+/−)/SKH-1 mice delayed UV-induced skin tumorigenesis and reduced the malignant conversion of benign papillomas to squamous cell carcinomas (SCCs)[70]. This study also showed the involvement of transforming growth factor-beta2 (TGF-β2) signaling as a mechanism of resveratrol’s action [70]. Kundu et al demonstrated that resveratrol pretreatment resulted in a decrease in the phosphorylation of extracellular signal-regulated protein kinase (ERK) as well as the catalytic activity of ERK and p38 MAPK (mitogen activated protein kinase) [71]. Further, resveratrol prevented TPA-induced DNA binding of activator protein-1 (AP-1) [71]. This study suggested that suppression of COX-2 expression by blocking the activation of MAPKs and AP-1 may represent possible molecular mechanisms responsible for previously reported anti-tumor promoting effects of resveratrol on mouse skin carcinogenesis.
Another in vivo study by Van Ginkel and colleagues demonstrated that peritumoral injection of resveratrol inhibited tumor growth in animal models of uveal melanoma via early mitochondrial dysfunction [72]. In this study, in vitro experiments with uveal melanoma cell lines showed that resveratrol causes apoptosis through a mitochondrial pathway i.e. decrease in mitochondrial membrane potential associated with an activation of caspase-3 [72]. The authors suggested that the nontoxic nature of the drug makes resveratrol an attractive candidate for the treatment of uveal melanoma [72].
Resveratrol and Skin Diseases: Promises and Prospects
As discussed above, some useful research with promising outcomes have been done regarding the effectiveness of resveratrol in photoprotection and against skin cancer. Based on these studies, it appears that the prospects are very bright for the possible use of resveratrol in skin diseases such as UV light mediated skin aging, skin cancer and other inflammatory and hyper-proliferative disorders. Based on studies in skin as well as other model systems, it appears that resveratrol may have prospects against pre-cancerous conditions such as actinic keratosis, as well as in enhancing the therapeutic ability of other drugs that are currently being used to treat skin disorders. For example, melanoma is one of the most drug-resistant cancers and the standard treatment options used for metastatic melanoma are interferon and dacarbazine, which have not shown a good therapeutic outcome. Some recent pre-clinical studies have suggested that polyphenols in combination with standard treatment options may be a viable approach for melanoma [73]. Further, Yang and colleagues demonstrated that resveratrol is an APE/Ref-1 inhibitor and enhances the therapeutic efficacy of dacarbazine for melanoma [74]. Thus, it appears that resveratrol may have bright prospects as an adjuvant therapy for melanoma management.
Another direction for researchers is to create resveratrol analogs with high efficacy against skin conditions. For example, Wong et al synthesized 4'-ester analogs of resveratrol via decarbonylative Heck coupling to assemble the protected stilbene core structure [75]. These analogs were then tested against melanoma cells [75]. It was found that four of the synthesized compounds are more effective in killing the melanoma cells than resveratrol, and that two out of four compounds had no cytotoxic effects on normal human dermal fibroblasts. Similarly, Moran and colleagues showed that fluorinated analogues of resveratrol had better growth inhibitory potential against melanoma cells [76]. Choi and colleagues synthesized an analogue of resveratrol, 5-(6-hydroxy-2-naphthyl)-1,2,3-benzenetriol (5HNB), which has a potent tyrosinase inhibitory activity [77]. This analogue did not show any cytotoxic effects on B16 melanoma; however, it was found to suppress melanin production by at least 50% [77]. The authors suggested that 5HNB might have skin-whitening effects as well as therapeutic potential for treating skin pigmentation disorders [77].
Resveratrol and Skin Diseases: Challenges
The biggest challenge that resveratrol researchers are currently facing is its poor in vivo bioavailability. In mammals, resveratrol is metabolized by the intestine and liver, mainly into glucuronides and sulfonates [78–83]. Resveratrol is very quickly metabolized, usually within 30–60 minutes after administration, although by changing some variables in how the drug is given, the peak plasma concentration can be delayed slightly. Because it is so quickly metabolized, it is difficult to use systemically as a drug treatment since it disappears from the plasma in such a short time [78]. There are several ways that researchers are trying to combat this problem to make resveratrol treatment viable against skin cancers and diseases.
The problem of quick metabolism of resveratrol and the impact it has on treatment of melanoma is easily illustrated by two studies conducted by Niles et al published in 2003 and 2006 [64, 84]. The same group demonstrated in vitro that resveratrol induced apoptosis in melanoma cells [64]. The authors then studied the effects of resveratrol in A375 cells implanted tumors in athymic (nu/nu) mice [84]. Several modes of resveratrol administration used in this study were via drinking water, oral feeding, and slow-release pellet implants in tumor proximity [84]. Surprisingly, the authors did not find any effect of resveratrol against melanoma tumors in vivo[84]. The authors then administered a 75 mg/kg bolus dose via gavage to non-tumor bearing athymic mice to determine the plasma concentration of resveratrol 5 minutes following treatment. It was found that resveratrol levels were significantly reduced in plasma and it was converted into metabolites such as resveratrol glucuronide and piceatannol. Similar problems were also reported in other cancers, including leukemia [85], lung [86], and intestine [87]. Also, gastrointestinal problems have been reported in some patients who have taken resveratrol as part of clinical trials [88, 89].
One possible way to circumvent the problem of bioavailability for skin disorders is to apply resveratrol topically to the skin. This would allow resveratrol to come into direct contact with the area of interest, without the side effects associated with systemic metabolism. Currently, several topical formulations of resveratrol are being developed. Hung and colleagues have studied several different solutions and hydrogel patches as delivery routes for resveratrol [90]. Hydrogel patches were successfully used in an effort to ensure that the resveratrol stayed at the site of interest, instead of diffusing into the body. Already, a number of resveratrol supplemented skin care products and cosmetics are available in the market. However, these products have not been rigorously tested for their claims. One problem with the resveratrol formulation used in cosmetics is that in order to allow resveratrol to incorporate into the creams or oils, usually microparticles are used that supposedly prolong its release into the skin. The drawback of this approach is that it also reduces the amount of resveratrol available for penetration into the skin. Recently, Kobierski and colleagues have tried using several different stabilizers and surfactants to produce a stable nanosuspension of resveratrol, and found that two of the non-ionic stabilizers they tested worked very well [91]. The nano-formulations can possibly improve resveratrol transport across the membrane as well as increase solubility. These nanosuspensions were also found to be stable at room temperature for at least 30 days [91].
As mentioned above, the use of resveratrol via systemic means is marred by its poor bioavailability due to its rapid metabolism in mammals. Therefore, efforts are needed to enhance its bioavailability in humans. We have earlier stressed on certain specific directions to combat this issue [92]. The possible scenarios for aggressive future research in this direction include, i) the strategy of combining resveratrol with agents that can inhibit the in vivo metabolism of resveratrol; ii) use of nanoparticlemediated delivery; iii) synthesis and/or evaluation analogues of resveratrol with improved bioavailability; and iv) careful evaluation of conjugated metabolites of resveratrol which may be deconjugated at the target organ to elicit a biological response.
Conclusion
Resveratrol is a promising antioxidant that is currently being investigated for a variety of disease conditions. It is not a surprise that resveratrol is also being evaluated for the management of skin disorders such as skin aging and skin cancers. Skin is particularly well suited for the use of this promising agent because the antioxidant properties of resveratrol work well against the high oxidative stress that skin cells come under frequently. Resveratrol has shown promise against skin diseases and even more prospects are yet to be explored. Indeed, concerted and multidisciplinary efforts are needed to take-up the challenges which hinder the prospects of this promising agent, in order to take it to the next level i.e. from ‘bench-to-bedside’.
Research Highlights
The review provides a discussion on the following:
-
➢
Background on skin, skin cancer, and oxidative stress
-
➢
Resveratrol’s effects on skin disease in vitro and in vivo
-
➢
Promise and prospects of resveratrol in skin disorders
-
➢
Challenges of using resveratrol, and ways to combat them
Acknowledgements
This work was partially supported by the NIH grants 1R21CA149560, R01CA114060, and 1R01AR059130.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302:71–83. doi: 10.1007/s00403-009-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ren S, Lien EJ. Natural products and their derivatives as cancer chemopreventive agents. Prog Drug Res. 1997;48:147–171. doi: 10.1007/978-3-0348-8861-5_6. [DOI] [PubMed] [Google Scholar]
- 3.Renaud S, Lorgeril Mde. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 4.Reagan-Shaw S, Mukhtar H, Ahmad N. Resveratrol imparts photoprotection of normal cells and enhances the efficacy of radiation therapy in cancer cells. Photochem Photobiol. 2008;84:415–421. doi: 10.1111/j.1751-1097.2007.00279.x. [DOI] [PubMed] [Google Scholar]
- 5.Aziz MH, Kumar R, Ahmad N. Cancer chemoprevention by resveratrol: in vitro and in vivo studies and the underlying mechanisms (review) Int J Oncol. 2003;23:17–28. [PubMed] [Google Scholar]
- 6.Aziz MH, Afaq F, Ahmad N. Prevention of ultraviolet-B radiation damage by resveratrol in mouse skin is mediated via modulation in survivin. Photochem Photobiol. 2005;81:25–31. doi: 10.1562/2004-08-13-RA-274. [DOI] [PubMed] [Google Scholar]
- 7.Aziz MH, Nihal M, Fu VX, Jarrard DF, Ahmad N. Resveratrol-caused apoptosis of human prostate carcinoma LNCaP cells is mediated via modulation of phosphatidylinositol 3'-kinase/Akt pathway and Bcl-2 family proteins. Mol Cancer Ther. 2006;5:1335–1341. doi: 10.1158/1535-7163.MCT-05-0526. [DOI] [PubMed] [Google Scholar]
- 8.Reagan-Shaw S, Afaq F, Aziz MH, Ahmad N. Modulations of critical cell cycle regulatory events during chemoprevention of ultraviolet B-mediated responses by resveratrol in SKH-1 hairless mouse skin. Oncogene. 2004;23:5151–5160. doi: 10.1038/sj.onc.1207666. [DOI] [PubMed] [Google Scholar]
- 9.Shankar S, Singh G, Srivastava RK. Chemoprevention by resveratrol: molecular mechanisms and therapeutic potential. Front Biosci. 2007;12:4839–4854. doi: 10.2741/2432. [DOI] [PubMed] [Google Scholar]
- 10.Jang M, Pezzuto JM. Cancer chemopreventive activity of resveratrol. Drugs Exp Clin Res. 1999;25:65–77. [PubMed] [Google Scholar]
- 11.Park JW, Choi YJ, Jang MA, Lee YS, Jun DY, Suh SI, Baek WK, Suh MH, Jin IN, Kwon TK. Chemopreventive agent resveratrol, a natural product derived from grapes, reversibly inhibits progression through S and G2 phases of the cell cycle in U937 cells. Cancer Lett. 2001;163:43–49. doi: 10.1016/s0304-3835(00)00658-3. [DOI] [PubMed] [Google Scholar]
- 12.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 13.Adhami VM, Afaq F, Ahmad N. Suppression of ultraviolet B exposure-mediated activation of NF-kappaB in normal human keratinocytes by resveratrol. Neoplasia. 2003;5:74–82. doi: 10.1016/s1476-5586(03)80019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adhami VM, Afaq F, Ahmad N. Involvement of the retinoblastoma (pRb)-E2F/DP pathway during antiproliferative effects of resveratrol in human epidermoid carcinoma (A431) cells. Biochem Biophys Res Commun. 2001;288:579–585. doi: 10.1006/bbrc.2001.5819. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad N, Adhami VM, Afaq F, Feyes DK, Mukhtar H. Resveratrol causes WAF-1/p21-mediated G(1)-phase arrest of cell cycle and induction of apoptosis in human epidermoid carcinoma A431 cells. Clin Cancer Res. 2001;7:1466–1473. [PubMed] [Google Scholar]
- 16.Yang Z, Yang S, Misner BJ, Chiu R, Liu F, Meyskens FL., Jr Nitric oxide initiates progression of human melanoma via a feedback loop mediated by apurinic/apyrimidinic endonuclease-1/redox factor-1. which is inhibited by resveratrol, Mol Cancer Ther. 2008;7:3751–3760. doi: 10.1158/1535-7163.MCT-08-0562. [DOI] [PubMed] [Google Scholar]
- 17.Brohem CA, Da Silva Cardeal LB, Tiago M, Soengas MS, De Moraes Barros SB, Maria-Engler SS. Artificial skin in perspective: concepts and applications. Pigment Cell Melanoma Res. 2010 doi: 10.1111/j.1755-148X.2010.00786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen DE, Rice RH. Toxic Responses of the Skin. In: Klassen CD, editor. Casarett and Doull"s Toxicology: The Basic Science of Poisons. McGraw-Hill; 2001. pp. 653–671. [Google Scholar]
- 19.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 20.Rogers HW, Weinstock MA, Harris AR, Hinckley MR, Feldman SR, Fleischer AB, Coldiron BM. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 21.Rigel DS, Friedman RJ, Kopf AW, Polsky D. ABCDE--an evolving concept in the early detection of melanoma. Arch Dermatol. 2005;141:1032–1034. doi: 10.1001/archderm.141.8.1032. [DOI] [PubMed] [Google Scholar]
- 22.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 23.Ridky TW. Nonmelanoma skin cancer. J Am Acad Dermatol. 2007;57:484–501. doi: 10.1016/j.jaad.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 24.Kahn HS, Tatham LM, Patel AV, Thun MJ, Heath CW., Jr Increased cancer mortality following a history of nonmelanoma skin cancer. JAMA. 1998;280:910–912. doi: 10.1001/jama.280.10.910. [DOI] [PubMed] [Google Scholar]
- 25.Levi F, La Vecchia C, Te VC, Randimbison L, Erler G. Incidence of invasive cancers following basal cell skin cancer. Am J Epidemiol. 1998;147:722–726. doi: 10.1093/oxfordjournals.aje.a009516. [DOI] [PubMed] [Google Scholar]
- 26.Levi F, Randimbison L, La Vecchia C, Erler G, Te VC. Incidence of invasive cancers following squamous cell skin cancer. Am J Epidemiol. 1997;146:734–739. doi: 10.1093/oxfordjournals.aje.a009349. [DOI] [PubMed] [Google Scholar]
- 27.Levi F, Randimbison L, Te VC, Conconi MM, La Vecchia C. Risk of prostate, breast and colorectal cancer after skin cancer diagnosis. Int J Cancer. 2008;123:2899–2901. doi: 10.1002/ijc.23816. [DOI] [PubMed] [Google Scholar]
- 28.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 29.Lee YJ, Shacter E. Oxidative stress inhibits apoptosis in human lymphoma cells. J Biol Chem. 1999;274:19792–19798. doi: 10.1074/jbc.274.28.19792. [DOI] [PubMed] [Google Scholar]
- 30.Sies H, Cadenas E. Oxidative stress: damage to intact cells and organs. Philos Trans R Soc Lond B Biol Sci. 1985;311:617–631. doi: 10.1098/rstb.1985.0168. [DOI] [PubMed] [Google Scholar]
- 31.Cantin AM. Cellular response to cigarette smoke and oxidants: adapting to survive. Proc Am Thorac Soc. 2010;7:368–375. doi: 10.1513/pats.201001-014AW. [DOI] [PubMed] [Google Scholar]
- 32.Ali SS, Marcondes MC, Bajova H, Dugan LL, Conti B. Metabolic depression and increased reactive oxygen species production by isolated mitochondria at moderately lower temperatures. J Biol Chem. 2010;285:32522–32528. doi: 10.1074/jbc.M110.155432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rasmussen C, Gratz K, Liebel F, Southall M, Garay M, Bhattacharyya S, Simon N, Vander Zanden M, Van Winkle K, Pirnstill J, Pirnstill S, Comer A, Allen-Hoffmann BL. The StrataTest(R) human skin model, a consistent in vitro alternative for toxicological testing. Toxicol In Vitro. 2010;24:2021–2029. doi: 10.1016/j.tiv.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto T, Ikuta N, Mori M, Nagayama K. Mechanics of wrinkle formation: micromechanical analysis of skin deformation during wrinkle formation in ultraviolet-irradiated mice. Skin Res Technol. 2010;16:179–189. doi: 10.1111/j.1600-0846.2009.00419.x. [DOI] [PubMed] [Google Scholar]
- 35.Ichihashi M, Ueda M, Budiyanto A, Bito T, Oka M, Fukunaga M, Tsuru K, Horikawa T. UV-induced skin damage. Toxicology. 2003;189:21–39. doi: 10.1016/s0300-483x(03)00150-1. [DOI] [PubMed] [Google Scholar]
- 36.Mukhtar H, Elmets CA. Photocarcinogenesis: mechanisms, models and human health implications. Photochem Photobiol. 1996;63:356–357. doi: 10.1111/j.1751-1097.1996.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 37.Mukhtar H, Forbes PD, Ananthaswamy HN. Photocarcinogenesis--models and mechanisms. Photodermatol Photoimmunol Photomed. 1999;15:91–95. doi: 10.1111/j.1600-0781.1999.tb00065.x. [DOI] [PubMed] [Google Scholar]
- 38.de Gruijl FR, Van der Leun JC. Estimate of the wavelength dependency of ultraviolet carcinogenesis in humans and its relevance to the risk assessment of a stratospheric ozone depletion. Health Phys. 1994;67:319–325. doi: 10.1097/00004032-199410000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Berneburg M, Krutmann J. Photoimmunology, DNA repair and photocarcinogenesis. J Photochem Photobiol B. 2000;54:87–93. doi: 10.1016/s1011-1344(00)00024-5. [DOI] [PubMed] [Google Scholar]
- 40.Rass K, Reichrath J. UV damage and DNA repair in malignant melanoma and nonmelanoma skin cancer. Adv Exp Med Biol. 2008;624:162–178. doi: 10.1007/978-0-387-77574-6_13. [DOI] [PubMed] [Google Scholar]
- 41.Matsui MS, DeLeo VA. Longwave ultraviolet radiation and promotion of skin cancer. Cancer Cells. 1991;3:8–12. [PubMed] [Google Scholar]
- 42.Burke KE. Photoaging: the role of oxidative stress. G Ital Dermatol Venereol. 2010;145:445–459. [PubMed] [Google Scholar]
- 43.Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, Voorhees JJ. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138:1462–1470. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- 44.Cohen JL. Actinic keratosis treatment as a key component of preventive strategies for nonmelanoma skin cancer. J Clin Aesthet Dermatol. 2010;3:39–44. [PMC free article] [PubMed] [Google Scholar]
- 45.Brenneisen P, Sies H, Scharffetter-Kochanek K. Ultraviolet-B irradiation and matrix metalloproteinases: from induction via signaling to initial events. Ann N Y Acad Sci. 2002;973:31–43. doi: 10.1111/j.1749-6632.2002.tb04602.x. [DOI] [PubMed] [Google Scholar]
- 46.Cross CE, van der Vliet A, Louie S, Thiele JJ, Halliwell B. Oxidative stress and antioxidants at biosurfaces: plants, skin, and respiratory tract surfaces. Environ Health Perspect. 1998;106 Suppl 5:1241–1251. doi: 10.1289/ehp.98106s51241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujiki H, Yoshizawa S, Horiuchi T, Suganuma M, Yatsunami J, Nishiwaki S, Okabe S, Nishiwaki-Matsushima R, Okuda T, Sugimura T. Anticarcinogenic effects of (−)-epigallocatechin gallate. Prev Med. 1992;21:503–509. doi: 10.1016/0091-7435(92)90057-o. [DOI] [PubMed] [Google Scholar]
- 48.Gruber JV, Holtz R. Examining the genomic influence of skin antioxidants in vitro. Mediators Inflamm. 2010;2010 doi: 10.1155/2010/230450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katiyar SK, Mukhtar H. Tea antioxidants in cancer chemoprevention. J Cell Biochem Suppl. 1997;27:59–67. [PubMed] [Google Scholar]
- 50.Katiyar SK, Agarwal R, Wang ZY, Bhatia AK, Mukhtar H. (−)-Epigallocatechin-3-gallate in Camellia sinensis leaves from Himalayan region of Sikkim: inhibitory effects against biochemical events and tumor initiation in Sencar mouse skin. Nutr Cancer. 1992;18:73–83. doi: 10.1080/01635589209514207. [DOI] [PubMed] [Google Scholar]
- 51.Murray JC, Burch JA, Streilein RD, Iannacchione MA, Hall RP, Pinnell SR. A topical antioxidant solution containing vitamins C and E stabilized by ferulic acid provides protection for human skin against damage caused by ultraviolet irradiation. J Am Acad Dermatol. 2008;59:418–425. doi: 10.1016/j.jaad.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 52.Frankel EN, Waterhouse AL, Kinsella JE. Inhibition of human LDL oxidation by resveratrol. Lancet. 1993;341:1103–1104. doi: 10.1016/0140-6736(93)92472-6. [DOI] [PubMed] [Google Scholar]
- 53.Kimura Y, Okuda H, Arichi S. Effects of stilbenes on arachidonate metabolism in leukocytes. Biochim Biophys Acta. 1985;834:275–278. [PubMed] [Google Scholar]
- 54.Bertelli AA, Giovannini L, Giannessi D, Migliori M, Bernini W, Fregoni M, Bertelli A. Antiplatelet activity of synthetic and natural resveratrol in red wine. Int J Tissue React. 1995;17:1–3. [PubMed] [Google Scholar]
- 55.Hadi SM, Ullah MF, Azmi AS, Ahmad A, Shamim U, Zubair H, Khan HY. Resveratrol mobilizes endogenous copper in human peripheral lymphocytes leading to oxidative DNA breakage: a putative mechanism for chemoprevention of cancer. Pharm Res. 2010;27:979–988. doi: 10.1007/s11095-010-0055-4. [DOI] [PubMed] [Google Scholar]
- 56.Chow HH, Garland LL, Hsu CH, Vining DR, Chew WM, Miller JA, Perloff M, Crowell JA, Alberts DS. Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev Res (Phila) 2010;3:1168–1175. doi: 10.1158/1940-6207.CAPR-09-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Afaq F, Adhami VM, Ahmad N. Prevention of short-term ultraviolet B radiation-mediated damages by resveratrol in SKH-1 hairless mice. Toxicol Appl Pharmacol. 2003;186:28–37. doi: 10.1016/s0041-008x(02)00014-5. [DOI] [PubMed] [Google Scholar]
- 58.Surh YJ, Lee E, Lee JM. Chemoprotective properties of some pungent ingredients present in red pepper and ginger. Mutat Res. 1998;402:259–267. doi: 10.1016/s0027-5107(97)00305-9. [DOI] [PubMed] [Google Scholar]
- 59.Craig WJ. Phytochemicals: guardians of our health. J Am Diet Assoc. 1997;97:S199–S204. doi: 10.1016/s0002-8223(97)00765-7. [DOI] [PubMed] [Google Scholar]
- 60.Chun YJ, Kim MY, Guengerich FP. Resveratrol is a selective human cytochrome P450 1A1 inhibitor. Biochem Biophys Res Commun. 1999;262:20–24. doi: 10.1006/bbrc.1999.1152. [DOI] [PubMed] [Google Scholar]
- 61.Ciolino HP, Daschner PJ, Yeh GC. Resveratrol inhibits transcription of CYP1A1 in vitro by preventing activation of the aryl hydrocarbon receptor. Cancer Res. 1998;58:5707–5712. [PubMed] [Google Scholar]
- 62.Pezzuto JM. Resveratrol as an Inhibitor of Carcinogenesis. Pharm Biol. 2008;46:443–573. [Google Scholar]
- 63.Packer L, Valacchi G. Antioxidants and the response of skin to oxidative stress: vitamin E as a key indicator. Skin Pharmacol Appl Skin Physiol. 2002;15:282–290. doi: 10.1159/000064531. [DOI] [PubMed] [Google Scholar]
- 64.Niles RM, McFarland M, Weimer MB, Redkar A, Fu YM, Meadows GG. Resveratrol is a potent inducer of apoptosis in human melanoma cells. Cancer Lett. 2003;190:157–163. doi: 10.1016/s0304-3835(02)00676-6. [DOI] [PubMed] [Google Scholar]
- 65.Yang S, Meyskens FL., Jr Alterations in activating protein 1 composition correlate with phenotypic differentiation changes induced by resveratrol in human melanoma. Mol Pharmacol. 2005;67:298–308. doi: 10.1124/mol.104.006023. [DOI] [PubMed] [Google Scholar]
- 66.Osmond GW, Augustine CK, Zipfel PA, Padussis J, Tyler DS. Enhancing Melanoma Treatment with Resveratrol. J Surg Res. 2010 doi: 10.1016/j.jss.2010.07.033. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 67.Liu Y, Chan F, Sun H, Yan J, Fan D, Zhao D, An J, Zhou D. Resveratrol protects human keratinocytes HaCaT cells from UVA-induced oxidative stress damage by downregulating Keap1 expression. Eur J Pharmacol. 2010 doi: 10.1016/j.ejphar.2010.10.009. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 68.Bastianetto S, Dumont Y, Duranton A, Vercauteren F, Breton L, Quirion R. Protective action of resveratrol in human skin: possible involvement of specific receptor binding sites. PLoS One. 2010;5:e12935. doi: 10.1371/journal.pone.0012935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aziz MH, Reagan-Shaw S, Wu J, Longley BJ, Ahmad N. Chemoprevention of skin cancer by grape constituent resveratrol: relevance to human disease? FASEB J. 2005;19:1193–1195. doi: 10.1096/fj.04-3582fje. [DOI] [PubMed] [Google Scholar]
- 70.Kim KH, Back JH, Zhu Y, Arbesman J, Athar M, Kopelovich L, Kim AL, Bickers DR. Resveratrol Targets Transforming Growth Factor-beta2 Signaling to Block UV-Induced Tumor Progression. J Invest Dermatol. 2010 doi: 10.1038/jid.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kundu JK, Chun KS, Kim SO, Surh YJ. Resveratrol inhibits phorbol ester-induced cyclooxygenase-2 expression in mouse skin: MAPKs and AP-1 as potential molecular targets. Biofactors. 2004;21:33–39. doi: 10.1002/biof.552210108. [DOI] [PubMed] [Google Scholar]
- 72.van Ginkel PR, Darjatmoko SR, Sareen D, Subramanian L, Bhattacharya S, Lindstrom MJ, Albert DM, Polans AS. Resveratrol inhibits uveal melanoma tumor growth via early mitochondrial dysfunction. Invest Ophthalmol Vis Sci. 2008;49:1299–1306. doi: 10.1167/iovs.07-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nihal M, Ahsan H, Siddiqui IA, Mukhtar H, Ahmad N, Wood GS. (−)-Epigallocatechin-3-gallate (EGCG) sensitizes melanoma cells to interferon induced growth inhibition in a mouse model of human melanoma. Cell Cycle. 2009;8:2057–2063. doi: 10.4161/cc.8.13.8862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang S, Irani K, Heffron SE, Jurnak F, Meyskens FL., Jr Alterations in the expression of the apurinic/apyrimidinic endonuclease-1/redox factor-1 (APE/Ref-1) in human melanoma and identification of the therapeutic potential of resveratrol as an APE/Ref-1 inhibitor. Mol Cancer Ther. 2005;4:1923–1935. doi: 10.1158/1535-7163.MCT-05-0229. [DOI] [PubMed] [Google Scholar]
- 75.Wong Y, Osmond G, Brewer KI, Tyler DS, Andrus MB. Synthesis of 4'-ester analogs of resveratrol and their evaluation in malignant melanoma and pancreatic cell lines. Bioorg Med Chem Lett. 2010;20:1198–1201. doi: 10.1016/j.bmcl.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 76.Moran BW, Anderson FP, Devery A, Cloonan S, Butler WE, Varughese S, Draper SM, Kenny PT. Synthesis, structural characterisation and biological evaluation of fluorinated analogues of resveratrol. Bioorg Med Chem. 2009;17:4510–4522. doi: 10.1016/j.bmc.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 77.Choi J, Bae SJ, Ha YM, No JK, Lee EK, Lee JS, Song S, Lee H, Suh H, Yu BP, Chung HY. A newly synthesized, potent tyrosinase inhibitor: 5-(6-hydroxy-2-naphthyl)-1,2,3-benzenetriol. Bioorg Med Chem Lett. 2010;20:4882–4884. doi: 10.1016/j.bmcl.2010.06.087. [DOI] [PubMed] [Google Scholar]
- 78.Cottart CH, Nivet-Antoine V, Laguillier-Morizot C, Beaudeux JL. Resveratrol bioavailability and toxicity in humans. Mol Nutr Food Res. 2010;54:7–16. doi: 10.1002/mnfr.200900437. [DOI] [PubMed] [Google Scholar]
- 79.Nunes T, Almeida L, Rocha JF, Falcao A, Fernandes-Lopes C, Loureiro AI, Wright L, Vaz-da-Silva M, Soares-da-Silva P. Pharmacokinetics of trans-resveratrol following repeated administration in healthy elderly and young subjects. J Clin Pharmacol. 2009;49:1477–1482. doi: 10.1177/0091270009339191. [DOI] [PubMed] [Google Scholar]
- 80.Burkon A, Somoza V. Quantification of free and protein-bound trans-resveratrol metabolites and identification of trans-resveratrol-C/O-conjugated diglucuronides - two novel resveratrol metabolites in human plasma. Mol Nutr Food Res. 2008;52:549–557. doi: 10.1002/mnfr.200700290. [DOI] [PubMed] [Google Scholar]
- 81.de Santi C, Pietrabissa A, Mosca F, Pacifici GM. Glucuronidation of resveratrol, a natural product present in grape and wine, in the human liver. Xenobiotica. 2000;30:1047–1054. doi: 10.1080/00498250010002487. [DOI] [PubMed] [Google Scholar]
- 82.De Santi C, Pietrabissa A, Spisni R, Mosca F, Pacifici GM. Sulphation of resveratrol, a natural compound present in wine, and its inhibition by natural flavonoids. Xenobiotica. 2000;30:857–866. doi: 10.1080/004982500433282. [DOI] [PubMed] [Google Scholar]
- 83.De Santi C, Pietrabissa A, Spisni R, Mosca F, Pacifici GM. Sulphation of resveratrol, a natural product present in grapes and wine, in the human liver and duodenum. Xenobiotica. 2000;30:609–617. doi: 10.1080/004982500406435. [DOI] [PubMed] [Google Scholar]
- 84.Niles RM, Cook CP, Meadows GG, Fu YM, McLaughlin JL, Rankin GO. Resveratrol is rapidly metabolized in athymic (nu/nu) mice and does not inhibit human melanoma xenograft tumor growth. J Nutr. 2006;136:2542–2546. doi: 10.1093/jn/136.10.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gao X, Xu YX, Divine G, Janakiraman N, Chapman RA, Gautam SC. Disparate in vitro and in vivo antileukemic effects of resveratrol, a natural polyphenolic compound found in grapes. J Nutr. 2002;132:2076–2081. doi: 10.1093/jn/132.7.2076. [DOI] [PubMed] [Google Scholar]
- 86.Hecht SS, Kenney PM, Wang M, Trushin N, Agarwal S, Rao AV, Upadhyaya P. Evaluation of butylated hydroxyanisole, myo-inositol, curcumin, esculetin, resveratrol and lycopene as inhibitors of benzo[a]pyrene plus 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in A/J mice. Cancer Lett. 1999;137:123–130. doi: 10.1016/s0304-3835(98)00326-7. [DOI] [PubMed] [Google Scholar]
- 87.Ziegler CC, Rainwater L, Whelan J, McEntee MF. Dietary resveratrol does not affect intestinal tumorigenesis in Apc(Min/+) mice. J Nutr. 2004;134:5–10. doi: 10.1093/jn/134.1.5. [DOI] [PubMed] [Google Scholar]
- 88.Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, Vasilinin G, Sen A, Schinas AM, Piccirilli G, Brown K, Steward WP, Gescher AJ, DE B. Repeat Dose Study of the Cancer Chemopreventive Agent Resveratrol in Healthy Volunteers: Safety, Pharmacokinetics, and Effect on the Insulin-like Growth Factor Axis. Cancer Res. 2010 doi: 10.1158/0008-5472.CAN-10-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.la Porte C, Voduc N, Zhang G, Seguin I, Tardiff D, Singhal N, Cameron DW. Steady-State pharmacokinetics and tolerability of trans-resveratrol 2000 mg twice daily with food, quercetin and alcohol (ethanol) in healthy human subjects. Clin Pharmacokinet. 2010;49:449–454. doi: 10.2165/11531820-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 90.Hung CF, Lin YK, Huang ZR, Fang JY. Delivery of resveratrol, a red wine polyphenol, from solutions and hydrogels via the skin. Biol Pharm Bull. 2008;31:955–962. doi: 10.1248/bpb.31.955. [DOI] [PubMed] [Google Scholar]
- 91.Kobierski S, Ofori-Kwakye K, Muller RH, Keck CM. Resveratrol nanosuspensions for dermal application--production, characterization, and physical stability. Pharmazie. 2009;64:741–747. [PubMed] [Google Scholar]
- 92.Ndiaye M, Kumar R, Ahmad N. Resveratrol in Cancer Management: Where are we and where we go from here? Ann NY Acad Sci. 2010 doi: 10.1111/j.1749-6632.2010.05851.x. In Press. [DOI] [PubMed] [Google Scholar]
- 93.Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M, Gescher AJ, Steward WP, Brenner DE. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007;16:1246–1252. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- 94.Vaz-da-Silva M, Loureiro AI, Falcao A, Nunes T, Rocha JF, Fernandes-Lopes C, Soares E, Wright L, Almeida L, Soares-da-Silva P. Effect of food on the pharmacokinetic profile of trans-resveratrol. Int J Clin Pharmacol Ther. 2008;46:564–570. doi: 10.5414/cpp46564. [DOI] [PubMed] [Google Scholar]
- 95.Almeida L, Vaz-da-Silva M, Falcao A, Soares E, Costa R, Loureiro AI, Fernandes-Lopes C, Rocha JF, Nunes T, Wright L, Soares-da-Silva P. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol Nutr Food Res. 2009;53 Suppl 1:S7–S15. doi: 10.1002/mnfr.200800177. [DOI] [PubMed] [Google Scholar]
- 96.Vitaglione P, Sforza S, Galaverna G, Ghidini C, Caporaso N, Vescovi PP, Fogliano V, Marchelli R. Bioavailability of trans-resveratrol from red wine in humans. Mol Nutr Food Res. 2005;49:495–504. doi: 10.1002/mnfr.200500002. [DOI] [PubMed] [Google Scholar]
- 97.Goldberg DM, Yan J, Soleas GJ. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin Biochem. 2003;36:79–87. doi: 10.1016/s0009-9120(02)00397-1. [DOI] [PubMed] [Google Scholar]
- 98.Lee JS, Park KY, Min HG, Lee SJ, Kim JJ, Choi JS, Kim WS, Cha HJ. Negative regulation of stress-induced matrix metalloproteinase-9 by Sirt1 in skin tissue. Exp Dermatol. 2010;19:1060–1066. doi: 10.1111/j.1600-0625.2010.01129.x. [DOI] [PubMed] [Google Scholar]
- 99.Roy P, Madan E, Kalra N, Nigam N, George J, Ray RS, Hans RK, Prasad S, Shukla Y. Resveratrol enhances ultraviolet B-induced cell death through nuclear factor-kappaB pathway in human epidermoid carcinoma A431 cells. Biochem Biophys Res Commun. 2009;384:215–220. doi: 10.1016/j.bbrc.2009.04.100. [DOI] [PubMed] [Google Scholar]
- 100.Madan E, Prasad S, Roy P, George J, Shukla Y. Regulation of apoptosis by resveratrol through JAK/STAT and mitochondria mediated pathway in human epidermoid carcinoma A431 cells. Biochem Biophys Res Commun. 2008;377:1232–1237. doi: 10.1016/j.bbrc.2008.10.158. [DOI] [PubMed] [Google Scholar]
- 101.Kim AL, Zhu Y, Zhu H, Han L, Kopelovich L, Bickers DR, Athar M. Resveratrol inhibits proliferation of human epidermoid carcinoma A431 cells by modulating MEK1 and AP-1 signalling pathways. Exp Dermatol. 2006;15:538–546. doi: 10.1111/j.1600-0625.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 102.Kowalczyk MC, Walaszek Z, Kowalczyk P, Kinjo T, Hanausek M, Slaga TJ. Differential effects of several phytochemicals and their derivatives on murine keratinocytes in vitro and in vivo: implications for skin cancer prevention. Carcinogenesis. 2009;30:1008–1015. doi: 10.1093/carcin/bgp069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Park K, Lee JH. Protective effects of resveratrol on UVB-irradiated HaCaT cells through attenuation of the caspase pathway. Oncol Rep. 2008;19:413–417. [PubMed] [Google Scholar]
- 104.Fabbrocini G, Kisslinger A, Iannelli P, Vitale N, Procaccini C, Sparaneo G, Chieffi P, Ayala F, Mancini FP, Tramontano D. Resveratrol regulates p66Shc activation in HaCaT cells. Exp Dermatol. 2010;19:895–903. doi: 10.1111/j.1600-0625.2010.01117.x. [DOI] [PubMed] [Google Scholar]
- 105.Kim KH, Back JH, Zhu Y, Arbesman J, Athar M, Kopelovich L, Kim AL, Bickers DR. Resveratrol Targets Transforming Growth Factor-beta2 Signaling to Block UV-Induced Tumor Progression. J Invest Dermatol. 2010 doi: 10.1038/jid.2010.250. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Soleas GJ, Grass L, Josephy PD, Goldberg DM, Diamandis EP. A comparison of the anticarcinogenic properties of four red wine polyphenols. Clin Biochem. 2002;35:119–124. doi: 10.1016/s0009-9120(02)00275-8. [DOI] [PubMed] [Google Scholar]
- 107.Caltagirone S, Rossi C, Poggi A, Ranelletti FO, Natali PG, Brunetti M, Aiello FB, Piantelli M. Flavonoids apigenin and quercetin inhibit melanoma growth and metastatic potential. Int J Cancer. 2000;87:595–600. doi: 10.1002/1097-0215(20000815)87:4<595::aid-ijc21>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 108.Asensi M, Medina I, Ortega A, Carretero J, Bano MC, Obrador E, Estrela JM. Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Radic Biol Med. 2002;33:387–398. doi: 10.1016/s0891-5849(02)00911-5. [DOI] [PubMed] [Google Scholar]
- 109.Kowalczyk MC, Kowalczyk P, Tolstykh O, Hanausek M, Walaszek Z, Slaga TJ. Synergistic effects of combined phytochemicals and skin cancer prevention in SENCAR mice. Cancer Prev Res (Phila) 2010;3:170–178. doi: 10.1158/1940-6207.CAPR-09-0196. [DOI] [PubMed] [Google Scholar]
- 110.Gatouillat G, Balasse E, Joseph-Pietras D, Morjani H, Madoulet C. Resveratrol induces cell-cycle disruption and apoptosis in chemoresistant B16 melanoma. J Cell Biochem. 2010;110:893–902. doi: 10.1002/jcb.22601. [DOI] [PubMed] [Google Scholar]
- 111.Roy P, Kalra N, Prasad S, George J, Shukla Y. Chemopreventive potential of resveratrol in mouse skin tumors through regulation of mitochondrial and PI3K/AKT signaling pathways. Pharm Res. 2009;26:211–217. doi: 10.1007/s11095-008-9723-z. [DOI] [PubMed] [Google Scholar]
- 112.Kalra N, Roy P, Prasad S, Shukla Y. Resveratrol induces apoptosis involving mitochondrial pathways in mouse skin tumorigenesis. Life Sci. 2008;82:348–358. doi: 10.1016/j.lfs.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 113.Yusuf N, Nasti TH, Meleth S, Elmets CA. Resveratrol enhances cell-mediated immune response to DMBA through TLR4 and prevents DMBA induced cutaneous carcinogenesis. Mol Carcinog. 2009;48:713–723. doi: 10.1002/mc.20517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Boily G, He XH, Pearce B, Jardine K, McBurney MW. SirT1-null mice develop tumors at normal rates but are poorly protected by resveratrol. Oncogene. 2009;28:2882–2893. doi: 10.1038/onc.2009.147. [DOI] [PubMed] [Google Scholar]