Abstract
Background and Aims
HCV related liver disease is one of the most important complications in persons with HIV, with accelerated fibrosis progression in coinfected persons compared to those with HCV alone. We hypothesized that HIV coinfection increases HCV related hepatocyte apoptosis and that HCV and HIV influence TRAIL signaling in hepatocytes.
Methods
We analyzed the effect of HIV on JFH1-infected Huh 7.5.1 cells. Apoptosis was measured by Caspase-Glo 3/7 assay and Western blot for cleaved PARP. TRAIL, TRAIL receptor 1 (DR4) and 2 (DR5) mRNA and protein levels were assessed by real-time PCR and Western blot. We also investigated activation of caspase pathways using caspase inhibitors and assessed expression of Bid and cytochrome C.
Results
We found increased caspase 3/7 activity and cleaved PARP in JFH1 HCV-infected Huh7.5.1 cells in the presence of heat-inactivated HIV compared to Huh7.5.1 cells infected with JFH1 or exposed to heat-inactivated HIV alone. Both DR4 and DR5 mRNA and protein expression were increased in JFH1-infected cells in the presence of inactivated HIV compared to Huh7.5.1 cells infected with JFH1 or exposed to heat-inactivated HIV alone. Pancaspase, Caspase-8, and caspase-9 inhibition blocked apoptosis induced by HCV, inactivated HIV and HCV plus inactivated HIV. A caspase-9 inhibitor blocked apoptosis induced by HCV, HIV and HCV-HIV comparably to pancaspase and caspase-8 inhibitors. HCV induced the activation of Bid cleavage and cytochrome C release. The addition of HIV substantially augmented this induction.
Conclusions
Our findings indicate that hepatocyte apoptosis is increased in the presence of HCV and HIV compared to HCV or HIV alone, and that this increase is mediated by DR4 and DR5 up-regulation. They provide an additional mechanism for the observed accelerated liver disease progression observed in HCV-HIV coinfection.
Keywords: HCV-HIV coinfection, Apoptosis, TRAIL receptor
Introduction
Hepatitis C virus (HCV) infects about 170 million people and is a leading cause of chronic liver disease worldwide [1,2]. It is a major cause of cirrhosis, a significant cause of hepatocellular carcinoma (HCC), and is the leading reason for liver transplantation worldwide.
HCV and human immunodeficiency virus (HIV) frequently coexist because of their common routes of transmission, and liver disease caused by HCV has rapidly become one of the most important complications in persons with HIV [3]. Indeed, end-stage liver disease is the most frequent cause of death among HIV-infected hospitalized patients [4]. Although the cause of liver injury in HIV-infected individuals is likely multifactorial (i.e., due to coinfection and medications), a number of reports have documented histological liver abnormalities that occurred solely as a result of HIV infection [5,6]. Moreover, HIV-1 RNA [5] and p24 antigen have been isolated from the livers of infected individuals [7], although direct evidence that HIV infects hepatocytes is lacking. These observations suggest that HIV may indirectly result in hepatocellular injury. The hallmark of HIV infection is depletion and exhaustion of the immune system.
Hepatocyte cell death by apoptosis is emerging as a fundamental component of virtually all acute and chronic liver diseases. The ensuing responses of cell repair, inflammation, regeneration, and fibrosis may all be triggered by apoptosis [8–11]. An increasing body of evidence from both experimental and clinical studies suggests that hepatocyte apoptosis may contribute to liver fibrogenesis. Engulfment of apoptotic bodies by hepatic stellate cells stimulates the fibrogenic activity of these cells and may be one mechanism by which hepatocyte apoptosis promotes fibrosis [12]. Furthermore, recent studies in chronic hepatitis C virus (HCV) infection demonstrate that hepatocyte apoptosis correlates with disease severity [13] and stage of fibrosis in patients with steatosis [14,15].
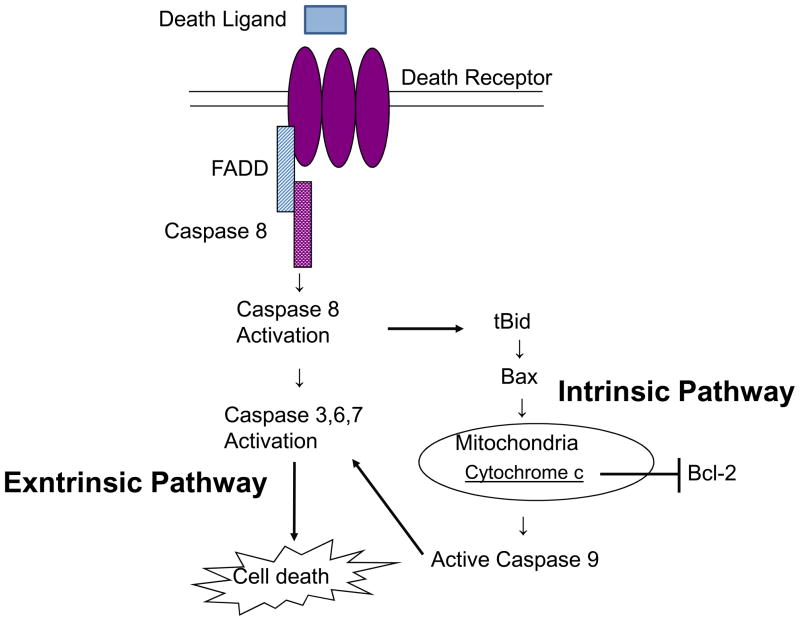
Death receptors 4 and 5 (DR4 and DR5), also called tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-R1 and R2, are members of the tumor necrosis factor-receptor (TNFR) family [16,17] and receptors for TNF-related apoptosis-inducing ligand (TRAIL) [18,19]. TRAIL selectively induces apoptosis in cancer cells in vitro and in vivo with little or no toxicity toward normal cells [20–22]. DR4 and DR5 mediate TRAIL-induced apoptosis through the formation of a death inducing signaling complex (DISC) containing the death receptor, adapter proteins such as Fas-associated death domain (FADD), and initiator caspases such as pro-caspase-8 [23] or pro-caspase-10 [24,25]. Consequently, pro-caspase-8 or pro-caspase-10 is activated by autoproteolytic processing, which then cleaves and activates downstream effector caspases, such as caspase-3 (extrinsic pathway). Additionally, the Bcl-2-interacting protein Bid is also cleaved by caspase-8 [26]. Truncated-Bid causes the loss of mitochondrial membrane potential and caspase-9 cleavage, resulting in apoptosis. Thus, truncated-Bid is a mediator that transduces the proapoptotic signal from the extrinsic pathway to the intrinsic (mitochondrial) pathway (Figure 1).
Figure 1. Pathways of Cellular Apoptosis.
Death receptor mediate death ligand-induced apoptosis through the formation of a death inducing signaling complex (DISC) containing the death receptor, adapter proteins such as Fas-associated death domain (FADD), and initiator caspases such as pro-caspase-8 or pro-caspase-10. Consequently, pro-caspase-8 or pro-caspase-10 is activated by autoproteolytic processing, which then cleaves and activates downstream effector caspases, such as caspase-3 (extrinsic pathway). Additionally, the Bcl-2-interacting protein Bid is also cleaved by caspase-8.Truncated-Bid causes the loss of mitochondrial membrane potential and caspase-9 cleavage, resulting in apoptosis. Thus, truncated-Bid is a mediator that transduces the proapoptotic signal from the extrinsic pathway to the intrinsic (mitochondrial) pathway.
We hypothesized that HIV coinfection augments hepatocyte apoptosis attributable to HCV and that this represents an additional mechanism of accelerated liver disease in coinfection. We tested this hypothesis using an infectious HCV cell culture model.
Materials and Methods
Cell Cultures and Transfection
Huh 7.5.1 cells (human hepatocellular carcinoma cells) were grown in Dulbeco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (FBS) medium. Japanese Fulminant Hepatitis 1 (JFH1) is a known tissue culture infectious strain of HCV. We transfected JFH1 RNA into Huh7.5.1 cells and collected culture medium at 72 hours post transfection as described previously [27]. We then inoculated naïve Huh7.5.1 cells with these culture supernatants. We then confirmed JFH1 virus infection of newly inoculated cells by real-time PCR and western blot for HCV RNA and core protein. NL4-3 is a CXCR4-tropic (T lymphocyte-tropic) HIV strain. BaL is a CCR5-tropic (macrophage-tropic) HIV strain. Heat-inactivated HIV (CXCR4 and CCR5-tropic virus) (56°C for 30 minutes) was incubated with Huh7.5.1 cells or JFH1-infected Huh7.5.1 cells for 72 hours at 1:4 dilution (11.25 ng/mL p24 for NL4-3; 8.75 ng/mL p24 for BaL. Naive Huh7.5.1 cells were seeded at a density of 2 × 105 cells/well in 6-well plates 24 hours before transfection. Infection of JFH1 and incubation with heat inactivated HIV were performed simultaneously. Subsequently, cells were washed with PBS and collected 72 hours later after transfection.
HIV Stocks
Heat-inactivated HIV was prepared as previously described [28]. Laboratory-adapted HIV-1 strains were obtained from the NIH AIDS Research and Reference Reagent Program. A plasmid encoding the CXCR4-tropic virus NL4-3 was used to transfect HEK293T cells, and supernatant virus was propagated using a CEM-derived T-cell line that expresses CD4, CXCR4, and CCR5 (CEM-GXR) [28]. The CCR5-tropic virus BaL [29] was also propagated using CEM-GXR cells. Viral stocks were assayed for HIV-1 p24 using the Alliance p24 Antigen ELISA Kit (PerkinElmer, Waltham, MA). Previously, we found that the increase in HCV levels mediated by heat-inactivated CCR5-tropic HIV and CXCR4-tropic HIV was abrogated by preincubation with neutralizing antibody to CXCR4 and CCR5 [30]. These data provided evidence for the indirect effect of HIV virus on HCV infection signaling through engagement of its coreceptors on the surface of Huh 7.5.1 cells rather than through direct infection of hepatocytes.
Real-Time Polymerase Chain Reaction Quantification
Total cellular RNA was harvested using the RNeasy kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. Total cDNA was synthesized by reverse transcription using the GeneAmp RNA PCR Kit (Applied Biosystems, Branchburg, New Jersey). DR4, DR5, TRAIL and beta-actin mRNA levels were quantified by real-time PCR. Human DR4 (GeneBank#: NM_003844), Human DR5 (GeneBank# NM_003842) and Human TRAIL (GeneBank# NM_003810) were measured using the following primers: DR4 sense primer-CTGAGCAACGCAGACTCGCTGTCCAC; DR4 antisense primer -TCAAAGGACACGGCAGAGCCTGTGCCA; DR5 sense primer -GGGAGCCGCTCATGAGGAAGTTGG; DR5 antisense primer -GGCAAGTCTCTCTCCCAGCGTCTC; TRAIL sense primer -AATCATCAAGGAGTGGGCATTC; TRAIL antisense primer -ATGACCAGTTCACCATTCCTCAA. Human beta-actin sense primer -GCACTCTTCCAGCCTTCCT; human beta-actin antisense primer AGGTCTTTGCGGATGTCCAC. Beta-actin was used as a control for basal RNA levels. DR4, DR5, TRAIL and beta-actin levels were quantified by real-time PCR using the Bio-Rad IQ5 (Bio-Rad Laboratories, Hercules, CA) and Finnzymes SYBR green I dye (New England Biolabs, Ipswich, MA) was used for detection as previously described [31].
Protein sample preparation
At the time of harvest, cells were washed twice with phosphate-buffered saline, and whole-cell protein samples were extracted with lysis buffer (50mM Tris-HCl, 1mM EDTA, 50mM NaF, 1mM Na3VO4, 1% Triton X-100, 150mM NaCl, 10ug/ml aprotinin, phosphatase inhibitor cocktail and protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN)).
Western blotting
Proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE) with NuPAGE Novex Bis-Tris precast 4 to 12% gradient gels (Invitrogen Life Science, Carlsbad, CA) and transferred to nitrocellulose membranes. The primary antibodies used for Western blots were as follows: rabbit anti-cleaved PARP (1:1,000)(Cell signaling Technology, Inc., Beverly, MA), mouse anti-DR4 (1:1,000) (Abcam Inc., Cambridge, MA), rabbit anti-DR5, rabbit anti-TRAIL, mouse anti-Bid, rabbit anti-cytochrome C (all at 1:1,000, Cell signaling Technology, Inc., Beverly, MA), mouse anti-HCV core (1:1000)(Affinity BioReagents Inc., Golden, CO), and mouse anti-actin (1:10,000)(Sigma Life Science and Biochemicals, St. Louis, MO). The secondary antibody was horseradish peroxidase-conjugated ECL donkey anti-rabbit IgG (1:2,000) or sheep anti-mouse IgG (1:10,000) (Amersham Biosciences, Piscataway, NJ). Chemiluminescent signals were detected using the ECL Western blotting detection kit (Amersham Biosciences, Piscataway, NJ). Densitometry was performed using the Universal Hood II (Bio-Rad, Hercules, CA).
Poly (ADP-Ribose) polymerase assay
PARP, a 116 kDa nuclear poly (ADP-ribose) polymerase, appears to be involved in DNA repair in response to environmental stress. This protein can be cleaved by many ICE-like caspases in vitro and is one of the main cleavage targets of caspase-3 in vivo. In human PARP, the cleavage occurs between Asp214 and Gly215, which separates the PARP amino-terminal DNA binding domain (24 kDa) from the carboxy-terminal catalytic domain (89 kDa). PARP helps cells to maintain their viability; cleavage of PARP facilitates cellular disassembly and serves as a marker of cells undergoing apoptosis (32).
Caspase 3/7 activity assay
The Caspase-Glo® 3/7 Assay provides a homogeneous luminescent assay that measures caspase-3/7 activity. Naïve Huh7.5.1 cells were seeded at a density of 104 cells/well (100 μl of DMEM with 10% FBS) in 96-well plates 24 hours before transfection. Infection of JFH1 and incubation with heat-inactivated HIV were performed simultaneously. Caspase-Glo 3/7 reagent (Promega Corporation, Madison. WI) was then added to each well 72 hours after transfection. The contents of the plate were gently mixed using a plate shaker at 300–500 rpm for 30s. The cells were then lysed at room temperature for 1h. Luminescence activities (relative caspase 3 and 7 activities) were measured with a BioTek Synergy 2 Microplate Reader (Winooski, VT). We measured caspase-3/7 activities as a function of JFH1 concentration using the Caspase-Glo® 3/7 Assay. We found that this assay sensitively detected apoptosis at very low concentrations of JFH1 (data not shown). Because of its sensitivity, we used caspase-3/7 activity as an apoptotic marker for this study.
Caspase inhibitors
Cells were preincubated with caspase inhibitors (pan-caspase inhibitor: Z-VAD-FMK (20uM), caspase-8 inhibitor: Z-IETD-FMK (20uM), caspase-9 inhibitor: Z-LEHDFMK (20uM); all from R&D Systems) for 6 hrs prior to the caspase Glo–3/7 assay. Caspase inhibitors were subsequently diluted in DMSO before use.
siRNA and transfection
To further explore the relationship between apoptosis and TRAIL receptor expression, we performed RNAi to knock down TRAIL receptor (DR4 and DR5) expression. The siRNAs were transfected into cells using Lipofectamine™ RNAiMAX Transfection Reagent (Invitrogen, Carlsbad, CA). The siRNAs used for gene knockdown were as follows: On-Target SMARTpool DR4 siRNA, On-Target SMARTpool DR5 siRNA (Dharmacon, Inc., Lafayette, CO). siGENOME Non-Targeting siRNA #1(Dharmacon, Inc., Lafayette, CO) was used as a negative control for siRNA-mediated knockdown. The knockdown of each gene was confirmed by real-time PCR and the extent of apoptosis was confirmed by Caspase-Glo 3/7 assay.
Statistics
Data analysis was carried out using paired t-test. Data were expressed as mean ± SD of at least three sample replicates, unless stated otherwise.
Results
Inactivated HIV increases apoptosis in JFH1-infected Huh7.5.1 cells
To assess whether HCV and HIV induce apoptosis in hepatocytes, we performed a quantitative caspase activity assay and Western blot in Huh 7.5.1 cells, JFH1-infected Huh7.5.1 cells, Huh7.5.1 cells grown in the presence of inactivated HIV (CCR5- and CXCR4-tropic strains), and JFH1-infected Huh7.5.1 cells grown in the presence of inactivated HIV (referred to as JFH1-infected/inactivated-HIV treated Huh7.5.1 cells). Using the Caspase–Glo 3/7 assay, we examined the apoptotic effects of JFH1 HCV and heat-inactivated HIV on Huh7.5.1 cells.
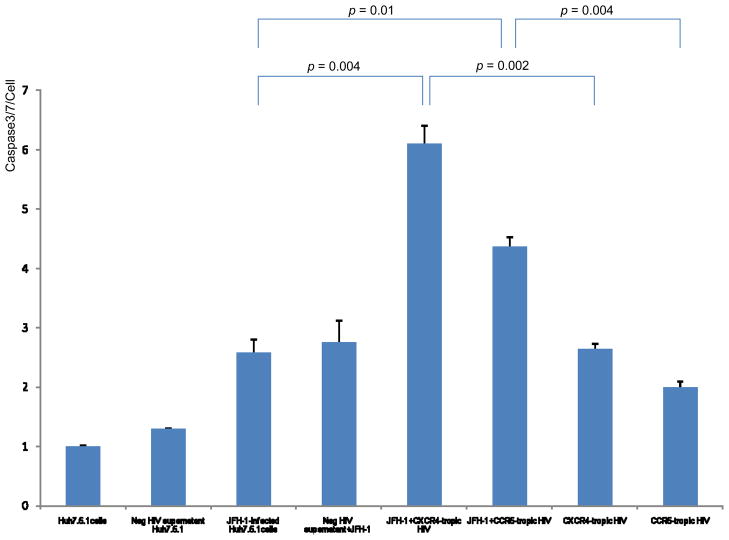
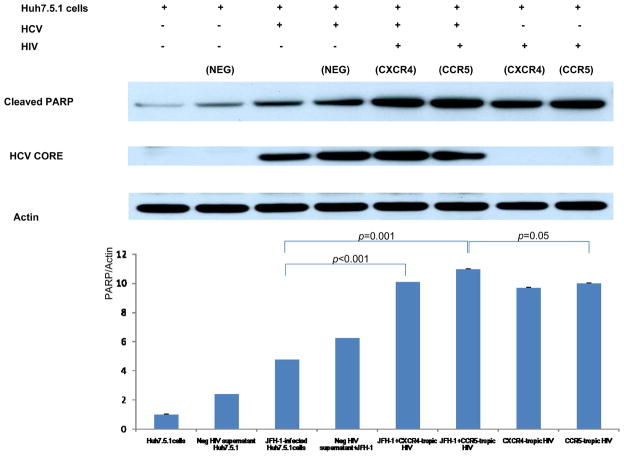
Both JFH1 infection and inactivated HIV increased caspase 3/7 activity in Huh7.5.1 cells compared to uninfected controls (Figure 2) (p<0.01 for each). Furthermore, JFH1-infected/inactivated-HIV, both CCR5- and CXCR4-tropic strains, produced a greater than 2-fold increase of caspase 3/7 activity compared to either JFH1-infected or inactivated-HIV Huh7.5.1 cells (Figure 2) (p<0.05 for each). Western blot for cleaved PARP demonstrated that both JFH1-infected and inactivated-HIV Huh7.5.1 cells increased cleaved PARP expression compared to negative controls (Figure 3) (p<0.01 for each); the expression of cleaved PARP was further increased in JFH1-infected/inactivated-HIV Huh7.5.1 cells (p<0.05 for each). From these experiments, we demonstrate that HIV increases HCV-induced hepatocyte apoptosis, and that HIV indirectly promotes hepatocyte apoptosis.
Figure 2. Increased caspase 3/7 activity in JFH1-infected, heat-inactivated HIV-treated Huh 7.5.1 cells compared to JFH1-infected or HIV-treated Huh 7.5.1 cells alone.
To assess the effects of HCV and HIV infection on apoptosis, 10,000 cells/well of Huh7.5.1 cells were added to 96-well plates in 100 μl 10 % FBS DMEM 24 hours before transfection. Transfection of JFH1 and incubation with heat-inactivated HIV were performed simultaneously. Caspase-Glo 3/7 reagent was added to each well 72 hours later. Each treatment was performed in triplicate. Caspase 3/7 activity was increased by over 2-fold in JFH1-infected, heat-inactivated HIV-treated Huh 7.5.1 cells compared to JFH1-infected or HIV-treated Huh 7.5.1 cells (p < 0.05 for each). Y axis refers to caspase 3/7 activity per cell. Lane#1 Huh7.5.1, #2 Huh 7.5.1 + negative supernatant HIV, #3 JFH1, #4 JFH1+ negative supernatant HIV, #5 JFH1+ CXCR4 tropic HIV, #6 JFH1+ CCR5 tropic HIV, #7 CXCR4 tropic HIV, #8 CCR5 tropic HIV
Figure 3. Expression of cleaved PARP was increased in JFH1-infected, heat-inactivated HIV-treated Huh 7.5.1 cells compared to JFH1-infected or HIV-treated Huh 7.5.1 cells alone.
To assess apoptosis in HCV and HIV coinfected Huh 7.5.1 cells we assessed cleaved PARP, HCV core, and beta-actin levels by Western blot and corresponding densitometry. We confirmed that expression of cleaved PARP was increased in JFH1-infected, heat-inactivated HIV-treated Huh 7.5.1 cells compared to JFH1-infected or HIV-treated Huh 7.5.1 cells alone (p < 0.05 for each). Lane#1 Huh7.5.1, #2 Huh 7.5.1 + negative supernatant HIV, #3 JFH1, #4 JFH1+ negative supernatant HIV, #5 JFH1+ CXCR4-tropic HIV, #6 JFH1+ CCR5-tropic HIV, #7 CXCR4-tropic HIV, #8 CCR5-tropic HIV
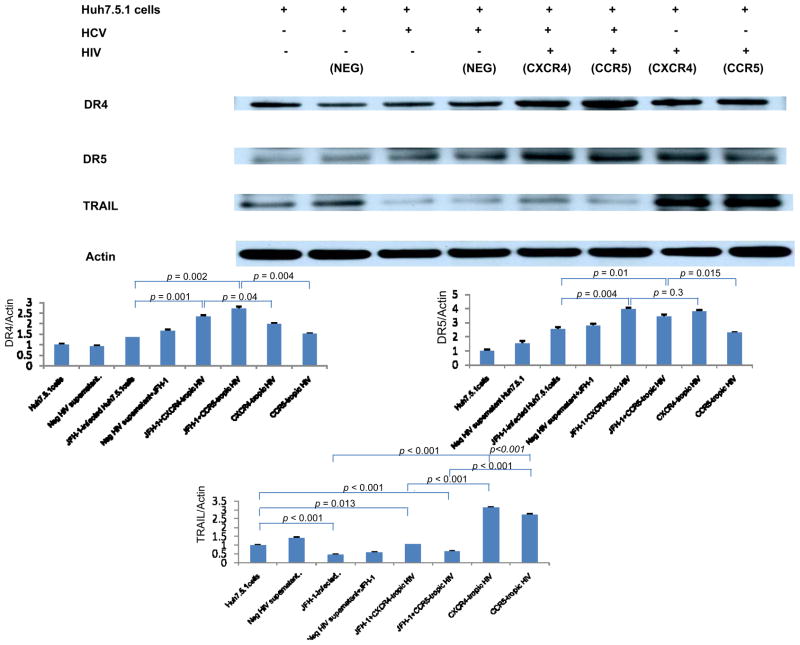
Increased expression of TRAIL receptor 1 and 2 is observed in HCV-infected Huh7.5.1 cells in the presence of HIV compared to HCV-infected or HIV-treated Huh7.5.1 cells
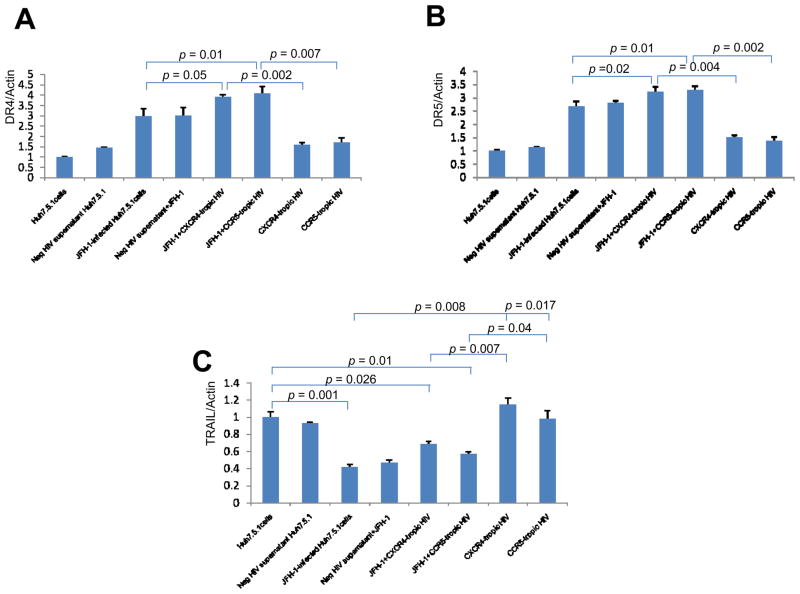
To further examine the molecular mechanisms of apoptosis induced by these viruses, we examined known mediators of apoptotic signaling, specifically TRAIL and TRAIL receptor 1, 2 (DR4, DR5). We first measured levels of TRAIL receptor 1 (DR4), 2 (DR5) and TRAIL using real-time PCR. We found that DR4 and DR5 mRNA levels were increased in HCV-infected Huh7.5.1 cells in the presence of heat-inactivated HIV compared to Huh7.5.1 cells infected with JFH1 or exposed to heat-inactivated HIV alone (Figure 4A, 4B). DR5 was significantly increased in JFH1-infected, heat-inactivated HIV-treated Huh 7.5.1 cells compared to JFH1 or heat-inactivated HIV-treated Huh 7.5.1 cells alone (p < 0.05) (1.23 fold (vs HCV), 2.41 fold (vs HIV)) and DR4 was moderately increased (p=0.05) (1.37 fold (vs HCV), 2.48 fold (vs HIV). In the case of TRAIL, mRNA levels were decreased in the presence of HCV compared to Huh 7.5.1 cells and HIV-incubated Huh 7.5.1 cells (Figure 4C). For further evaluation of TRAIL signaling, we performed Western blot for DR4, DR5 and TRAIL. As shown in Figure 5, DR 4 and DR 5 induction was observed in HCV-infected Huh7.5.1 cells in the presence of heat-inactivated HIV compared to either JFH1-infected or heat inactivated HIV-treated Huh7.5.1 cells (DR4 (p < 0.01) (2.02 fold (vs HCV), 1.80 fold (vs HIV)) (DR5 (p < 0.01) (1.55 fold (vs HCV), 1.50 fold (vs HIV)). Protein expression of TRAIL was decreased in the presence of HCV. These results suggest that HIV increases HCV-induced hepatocyte apoptosis, and that this increase in apoptosis is induced by up-regulation of the TRAIL receptors DR4 and DR5.
Figure 4. Increased DR4 and DR5 mRNA level in HCV-infected Huh7.5.1 cells in the presence of heat-inactivated HIV compared to JFH1-infected or HIV-treated Huh7.5.1 cells.
Total cellular RNA was harvested and DR4 (Figure 4A), DR5 (Figure 4B), TRAIL (Figure 4C) and beta-actin mRNA levels were quantified by real-time PCR. Each treatment was performed in triplicate. DR4, DR5 and TRAIL levels were normalized to actin levels to calculate DR4, DR5 and TRAIL/actin arbitrary units. Real-time PCR demonstrates increased relative DR4 and DR5 mRNA levels in HCV-infected, HIV-treated Huh7.5.1 cell compared to JFH1-infected or HIV treated Huh7.5.1 cells. DR5 mRNA levels were significantly increased in HCV-infected Huh7.5.1 cells in the presence of heat-inactivated HIV compared to JFH1-infected or HIV-treated Huh7.5.1 cells (p < 0.05) and DR4 mRNA was moderately increased (p=0.05). In the case of TRAIL, mRNA levels were decreased in the presence of HCV. Lane#1 Huh7.5.1, #2 Huh 7.5.1 + negative supernatant HIV, #3 JFH1, #4 JFH1+ negative supernatant HIV, #5 JFH1+ CXCR4-tropic HIV, #6 JFH1+ CCR5 tropic HIV, #7 CXCR4-tropic HIV, #8 CCR5-tropic HIV
Figure 5. Increased DR4 and DR5 protein expression in HCV-infected Huh7.5.1 cells in the presence of heat-inactivated HIV compared to JFH1-infected or HIV-treated Huh7.5.1 cells.
Protein lysates were harvested for Western blot for DR4, DR5 and TRAIL. Corresponding densitometry was performed. We found that protein expression of DR4 and DR5 was increased in JFH1-infected, heat-inactivated HIV-treated Huh 7.5.1 cells compared to JFH1 or HIV-treated Huh 7.5.1 cells alone (p < 0.01 for each). Protein expression of TRAIL was decreased in the presence of HCV. Lane#1 Huh7.5.1, #2 Huh 7.5.1 + negative supernatant HIV, #3 JFH1, #4 JFH1+ negative supernatant HIV, #5 JFH1+ CXCR4-tropic HIV, #6 JFH1+ CCR5-tropic HIV, #7 CXCR4-tropic HIV, #8 CCR5-tropic HIV
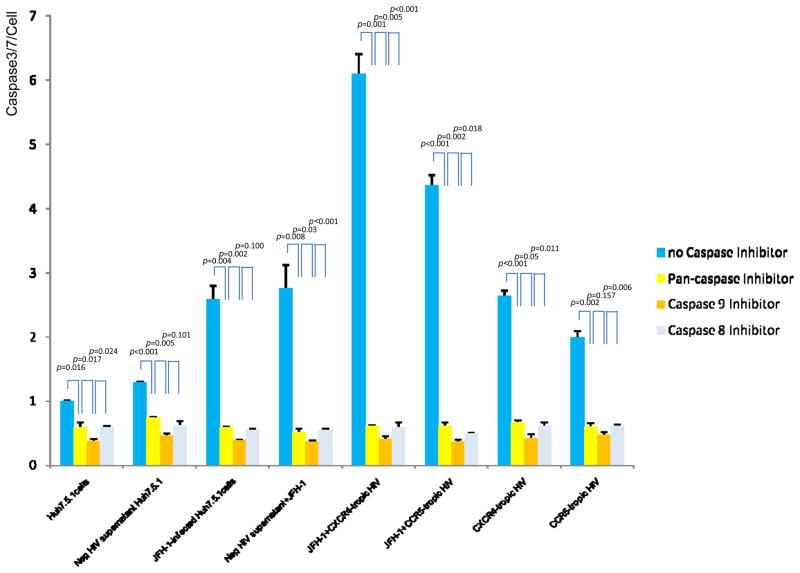
HCV-HIV induced apoptosis is dependent on caspases within the mitochondrial pathway
To further delineate the intracellular apoptotic pathway modulated by HCV and HIV, we investigated activation of caspase pathways during apoptosis using several caspase inhibitors. We performed Caspase-Glo 3/7 assay after preincubation with several caspase inhibitors. The pan-caspase inhibitor zVAD-fmk decreased HCV, HIV or HCV-HIV induced caspase 3/7 activity by over 4-fold (p<0.01). A caspase-8 inhibitor blocked HCV, HIV or HCV-HIV induced apoptosis to the same degree as that inhibited by zVAD-fmk. In addition, a caspase-9 inhibitor blocked apoptosis induced by HCV, HIV or HCV-HIV comparably to pancaspase or caspase-8 inhibition (Figure 6). Taken together, these data indicate that HCV, HIV or HCV-HIV induced apoptosis are dependent on both extrinsic caspases and caspases related to the mitochondrial pathway.
Figure 6. HCV-HIV induced apoptosis is dependent on caspases related to the mitochondrial pathway.
Naïve Huh 7.5.1 cell and HCV and/or HIV treated Huh7.5.1 cells were incubated with 20 μM of a variety of caspase inhibitors in 96-well plates (10,000 cell/well) for 6 hrs prior to Caspase–Glo 3/7 assay. Inhibitors used included the pan-caspase inhibitor Z-VAD-FMK, the caspase 8 inhibitor Z-IETD-FMK, and the caspase 9 inhibitor Z-LEHDFMK. The pan-caspase inhibitor zVAD-fmk decreased HCV, HIV or HCV-HIV induced caspase 3/7 activity by over 4-fold (p < 0.01). The caspase 8 inhibitor blocked HCV, HIV or HCV-HIV induced apoptosis to the same degree as that inhibited by zVAD-fmk. In addition, a caspase 9 inhibitor significantly blocked apoptosis induced by HCV, HIV or HCV-HIV comparably to the pancaspase and caspase 8 inhibitors. The y axis refers to caspase 3/7 activity per cell. Lane#1 Huh7.5.1, #2 Huh 7.5.1 + negative supernatant HIV, #3 JFH1, #4 JFH1+ negative supernatant HIV, #5 JFH1+ CXCR4 tropic HIV, #6 JFH1+ CCR5 tropic HIV, #7 CXCR4-tropic HIV, #8 CCR5-tropic HIV
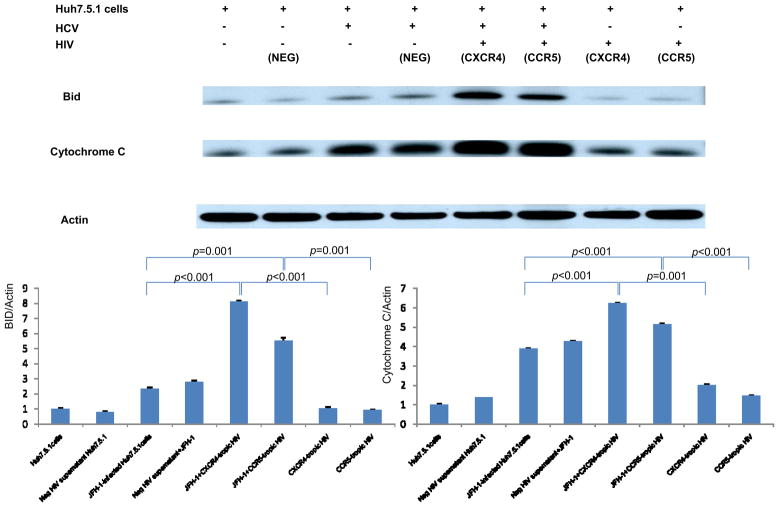
Analysis of Bid cleavage and Cytochrome C levels
To confirm the activation of the mitochondrial pathway upon HCV-HIV infection of Huh7.5.1 cells, we performed Western blot of the mitochondrial apoptosis activating protein Bid and cytochrome C. While HCV alone induced the activation of Bid cleavage and cytochrome C release, addition of HIV substantially augmented this induction (3.5-fold for BID,1.6-fold for cytochrome C, Figure 7). These data confirm that HCV-HIV related apoptosis utilizes the mitochondrial pathway.
Figure 7. Analysis of Bid cleavage and Cytochrome C levels.
Protein lysates were harvested for Western analysis of the mitochondrial apoptosis activating protein Bid and cytochrome C. Corresponding densitometry was performed. While HCV alone induced the activation of Bid cleavage and cytochrome C release, addition of heat-inactivated HIV substantially augmented this induction (3.47 fold for BID, 1.60 fold for cytochrome C by densitometry). Lane#1 Huh7.5.1, #2 Huh 7.5.1 + negative supernatant HIV, #3 JFH1, #4 JFH1+ negative supernatant HIV, #5 JFH1+ CXCR4-tropic HIV, #6 JFH1+ CCR5-tropic HIV, #7 CXCR4-tropic HIV, #8 CCR5-tropic HIV
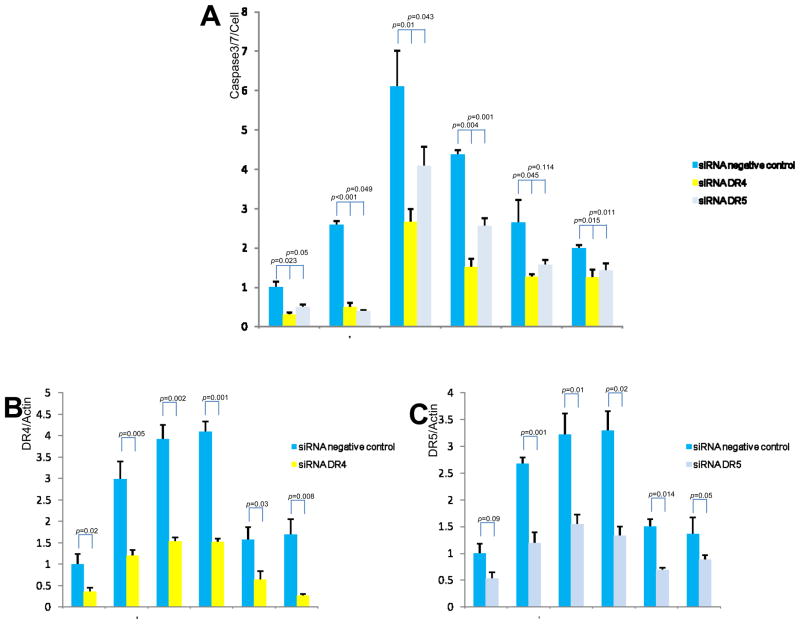
HCV-HIV induced apoptosis is decreased by DR4 and DR5 siRNA
To demonstrate that HCV and HIV-induced increases in apoptosis are mediated by DR4 and DR5, we performed siRNA-mediated knockdown of DR4 and DR5 (Figure 8). We found that HCV-HIV induced apoptosis was blocked by DR4- and DR5-specific siRNAs, but not by negative control siRNAs (Figure 8A). The data confirm that HCV-HIV induced apoptosis is dependent on DR4 and DR5 up-regulation. The finding of residual apoptosis that is not knocked down by DR4 or DR5 siRNAs in the presence of HIV or HIV-HCV infected cells raises the possibility of contribution to apoptosis from additional, non-DR4, DR5 pathways.
Figure 8. Effects of DR4 and DR5 knockdown on HCV-HIV induced apoptosis.
The indicated siRNAs were transfected into HCV-HIV infected Huh7.5.1 cells in 96-well plate. The siRNAs used for gene knockdown included DR4-, DR5-targeted or negative control siRNAs. Real-time PCR assay was performed to measure DR4 and DR5 expression levels in these siRNA-transfected cells. DR4 and DR5 mRNA levels were decreased by over 2-fold in DR4- and DR5-targeted siRNA-transfected cells compared to negative siRNA-transfected cells (Figure 8B and 8C). Caspase-Glo 3/7 staining revealed that HCV-HIV induced apoptosis was suppressed by nearly 2-fold in DR4- and DR5-targeted siRNA-transfected cells compared to negative control siRNA-transfected cells (Figure 8A). Lane#1 Huh7.5.1, #2 JFH1, #3 JFH1+ CXCR4-tropic HIV, #4 JFH1+ CCR5-tropic HIV, #5 CXCR4-tropic HIV, #6 CCR5-tropic HIV
Discussion
Fibrogenesis is a relatively late event in chronic liver injury, and occurs as a consequence of activation of hepatic stellate cells (HSC) and excessive deposition of extracellular matrix, under conditions of persistent inflammation. The first phase of liver injury, however, independent of the etiology, is almost always characterized by increased hepatocyte apoptosis. Since for many years apoptosis has been considered a mechanism of cell death not associated with an inflammatory response, these two phases have not been linked until recently [14].
Because of their shared routes of transmission, coinfection with HCV is increasingly a major cause of morbidity and mortality among HIV+ persons [4]. For instance, it is well established that HIV+/HCV+ persons experience more progressive liver fibrosis and higher rates of cirrhosis, liver failure and death than HIV−/HCV+ persons [33–38]despite successful control of HIV with antiretroviral therapy.
The mechanisms underlying the accelerated evolution of CHC in HCV/HIV coinfection are not known. We have previously shown [30] that addition of heat-inactivated HIV indirectly regulates HCV replication and, ultimately, the fibrogenic actions of HCV by augmenting TGF-β1 expression in HCV-infected hepatocytes. Furthermore, this increase in HCV replication was abrogated by pre-incubation with neutralizing antibody to CXCR4 and CCR5. These data thereby provided evidence for an indirect effect of HIV virus signaling through engagement of its coreceptor on the surface of Huh 7.5.1 cells rather than through infection itself. Another possible explanation for accelerated fibrogenesis is enhanced hepatocyte apoptosis. In the current study, we now extend our previous findings to demonstrate that heat-inactivated HIV can also augment HCV-mediated apoptosis. The observed increased apoptosis in JFH1-infected, heat-inactivated HIV-treated Huh 7.5.1 cells compared to JFH1 or heat-inactivated HIV-treated Huh 7.5.1 cells alone may contribute to more progressive liver fibrosis in HCV-HIV coinfected patients. Because we also found that HIV alone can moderately induce hepatocyte apoptosis, these data suggest that HIV predisposes the liver to a multitude of second hits, including HCV and HBV coinfection, both of whose natural history are accelerated in HIV-infected persons.
Several studies have postulated an autocrine TRAIL-dependent apoptosis of hepatocytes [39][40]. Other studies suggest that a TRAIL autocrine loop does not play a major role for TRAIL-induced sensitization [41]. HCV-dependent up-regulation of TRAIL and apoptosis induction in a novel hepatoma cell line has been described [42]. Others [43] have described that HIV selectively upregulates TRAIL-R2 expression on hepatocytes and confers an acquired sensitivity to TRAIL-mediated apoptosis.
In the present study, we report that HCV-infected, HIV-treated Huh7.5.1 cells express higher levels of TRAIL receptor (DR4 and DR5) and apoptosis than do HCV or HIV-treated Huh7.5.1 cells alone. However, TRAIL was decreased in the presence of HCV, suggesting that an autocrine loop is not operative. Rather, our data suggest either that increased DR4 or DR5 expression or sensitivity may compensate for decreases in TRAIL release or that additional death receptor pathways contribute to this finding. The finding that circulating TRAIL levels are increased in HIV monoinfection [44] compared to no infection or HCV monoinfection suggests another basis for the predisposition of the HIV-infected liver to enhanced injury from other sources.
We investigated the participation of caspase pathways using several caspase inhibitors. Lan et al [41] previously demonstrated that the mitochondrially-activated caspase pathway is triggered in HCV infection by use of selective caspase inhibitors. We also sought to assess the operative pathways in HCV-HIV coinfection using these inhibitors. Our results indicate that the HCV-HIV mediated apoptosis signal is transduced from DR4 and DR5 through activation of caspase 8, which in turn activates the mitochondrial signaling pathway, whose involvement was confirmed by our demonstration of Bid cleavage and cytochrome c activation after HCV and HIV coinfection. Furthermore, because a caspase-8 inhibitor was equally as effective as pancaspase and caspase-9 inhibitors, our data imply that TRAIL-mediated apoptosis engages both the caspase-8 and the mitochondrial pathways.
Our study provides evidence, using an infectious tissue culture model, that HCV and HIV infection increase hepatocyte apoptosis compared to HCV or HIV alone. We propose a model in which HCV and HIV each induces TRAIL receptor (DR4 and DR5), which in turn activate caspase 8 and the mitochondrial pathway. They further provide an additional plausible mechanism for the observed accelerated disease progression observed in HCV-HIV coinfection. Efforts to specifically inhibit this apoptotic program may be warranted to retard liver disease progression in coinfected persons.
Acknowledgments
This work was supported in part by NIH grants AI069939 and AI082630 (RTC).
We gratefully acknowledge: Dr. Ralf Bartenschlager, University, Heidelberg, Germany; and Dr. Takaji Wakita, Second Department of Virology, National Institute of Infectious Diseases, Tokyo, Japan (infectious HCV virus JFH1 DNA construct); and Dr. Francis Chisari, Scripps Institute, CA (Huh7.5.1 cells). This work was support by NIH grants R01 AI069939 and U19 AI082630 (to RTC).
Abbreviations
- JFH-1
Japanese Fulminant Hepatitis 1
- TRAIL
TNF-related apoptosis-inducing ligand
- PARP
Poly (ADP-Ribose)polymerase
- TGF-β1
Transforming growth factor beta1
- DR4
Death receptor 4
- DR5
Death receptor 5
Footnotes
There are no conflicts to disclose for the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alter MJ. Epidemiology of viral hepatitis and HIV coinfection. J Hepatol. 2006;44:S6–9. doi: 10.1016/j.jhep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 3.Munshi N, Balasubramanian A, Koziel M, Ganju RK, Groopman JE. Hepatitis C and Human Immunodeficiency Virus Envelope Proteins Cooperatively Induce Hepatocytic Apoptosis via an Innocent Bystander Mechanism. J Infect dis. 2003;188:1192–1204. doi: 10.1086/378643. [DOI] [PubMed] [Google Scholar]
- 4.Bica I, McGovern B, Dhar R, Stone D, McGowan K, Scheib R, et al. Increasing mortality due to endstage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32:492–497. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 5.Cao YZ, Dieterich D, Thomas PA, Huang YX, Mirabile M, Ho DD. Identification and quantitation of HIV-1 in the liver of patients with AIDS. AIDS. 1992;6:65–70. doi: 10.1097/00002030-199201000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Lefkowitch JH. Pathology of AIDS-related liver disease. Dig Dis. 1994;12:321–330. doi: 10.1159/000171468. [DOI] [PubMed] [Google Scholar]
- 7.Housset C, Boucher O, Girard PM, Leibowitch J, Saimot AG, Bréchot C, et al. Immunohistochemical evidence for human immunodeficiency virus–1 infection of liver Kupffer cells. Hum Pathol. 1990;21:404–408. doi: 10.1016/0046-8177(90)90202-g. [DOI] [PubMed] [Google Scholar]
- 8.Rust C, Gores GJ. Apoptosis and liver disease. Am J Med. 2000;108:567–574. doi: 10.1016/s0002-9343(00)00370-3. [DOI] [PubMed] [Google Scholar]
- 9.Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65:166–176. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- 10.Lawson JA, Fisher MA, Simmons CA, Farhood A, Jaeschke H. Parenchymalcell apoptosis as a signal for sinusoidal sequestration and transendothelial migration of neutrophils in murine models of endotoxin and Fas-antibody-induced liver injury. Hepatology. 1998;28:761–767. doi: 10.1002/hep.510280324. [DOI] [PubMed] [Google Scholar]
- 11.Faouzi S, Burckhardt BE, Hanson JC, Campe CB, Schrum LW, Rippe RA, et al. Anti-Fas induces hepatic chemokines and promotes inflammationby an NF-kappa B-independent, caspase-3-dependent pathway. J Biol Chem. 2001;276:49077–49082. doi: 10.1074/jbc.M109791200. [DOI] [PubMed] [Google Scholar]
- 12.Canbay A, Friedman S, Gores GJ. Apoptosis: the nexus of liver injury and fibrosis. Hepatology. 2004;39:273–278. doi: 10.1002/hep.20051. [DOI] [PubMed] [Google Scholar]
- 13.Bantel H, Lugering A, Poremba C, Lugering N, Held J, Domschke W, et al. Caspase activation correlates with the degree of inflammatory liver injury in chronic hepatitis C virus infection. Hepatology. 2001;34:758–767. doi: 10.1053/jhep.2001.28229. [DOI] [PubMed] [Google Scholar]
- 14.Walsh MJ, Vanags DM, Clouston AD, Richardson MM, Purdie DM, Jonsson JR, et al. Steatosis and liver cell apoptosis in chronic hepatitis C: a mechanism for increased liver injury. Hepatology. 2004;39:1230–1238. doi: 10.1002/hep.20179. [DOI] [PubMed] [Google Scholar]
- 15.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 16.MacFarlane M, Ahmad M, Srinivasula SM, Fernandes-Alnemri T, Cohen GM, Alnemri ES. Identification and molecular cloning of two novel receptors for the cytotoxic ligand TRAIL. J Biol Chem. 1997;272:25417–25420. doi: 10.1074/jbc.272.41.25417. [DOI] [PubMed] [Google Scholar]
- 17.Walczak H, Degli-Esposti MA, Johnson RS, Smolak PJ, Waugh JY, Boiani N, et al. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J. 1997;16:5386–5397. doi: 10.1093/emboj/16.17.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 19.Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 20.Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence D, Shahrokh Z, Marsters S, Achilles K, Shih D, Mounho B, et al. Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nat Med. 2001;7:383–385. doi: 10.1038/86397. [DOI] [PubMed] [Google Scholar]
- 23.Hersey P, Zhang XD. How melanoma cells evade trail-induced apoptosis. Nat Rev Cancer. 2001;1:142–150. doi: 10.1038/35101078. [DOI] [PubMed] [Google Scholar]
- 24.Kischkel FC, Lawrence DA, Tinel A, LeBlanc H, Virmani A, Schow P, et al. Death receptor recruitment of endogenous caspase-10 and apoptosis initiation in the absence of caspase-8. J Biol Chem. 2001;276:46639–46646. doi: 10.1074/jbc.M105102200. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Chun HJ, Wong W, Spencer DM, Lenardo MJ. Caspase-10 is an initiator caspase in death receptor signaling. Proc Natl Acad Sci USA. 2001;98:13884–13888. doi: 10.1073/pnas.241358198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada H, Tada- Oikawa S, Uchida A, Kawanishi S. TRAIL causes cleavage of bid by caspase-8 and loss of mitochondrial membrane potential resulting in apoptosis in BJAB cells. Biochem Biophys Res Commun. 1999;265:130–133. doi: 10.1006/bbrc.1999.1641. [DOI] [PubMed] [Google Scholar]
- 27.Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z, et al. production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11:791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brockman MA, Tanzi GO, Walker BD, Allen TM. Use of a novel GFP reporter cell line to examine replication capacity of CXCR4- and CCR5-tropic HIV-1 by flow cytometry. J Virol Methods. 2006;131:134–142. doi: 10.1016/j.jviromet.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Gartner S, Markovits P, Markovitz DM, Kaplan MH, Gallo RC, Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986;233:215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 30.Lin W, Weinberg EM, Tai AW, Peng LF, Brockman MA, Kim KA, et al. HIV Increases HCV Replication in a TGF-β1–Dependent Manner. Gastroenterology. 2008;134:803–811. doi: 10.1053/j.gastro.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Lin W, Choe WH, Hiasa Y, Kamegaya Y, Blackard JT, Schmidt EV, et al. Hepatitis C virus expression suppresses interferon signaling by degrading STAT1. Gastroenterology. 2005;128:1034–1041. doi: 10.1053/j.gastro.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Oliver FJ, de la Rubia G, Rolli V, Ruiz-Ruiz MC, de Murcia G, Murcia JM. Importance of poly(ADP-ribose) polymerase and its cleavage in apoptosis. Lesson from an uncleavable mutant. J Biol Chem. 1998;273(50):33533–33539. doi: 10.1074/jbc.273.50.33533. [DOI] [PubMed] [Google Scholar]
- 33.Martinez-Sierra C, Arizcorreta A, Díaz F, Roldán R, Martín-Herrera L, Pérez-Guzmán E, et al. Progression of chronic hepatitis C to liver fibrosis and cirrhosis in patients coinfected with hepatitis C virus and human immunodeficiency virus. Clin Infect Dis. 2003 Feb 15;36(4):491–498. doi: 10.1086/367643. [DOI] [PubMed] [Google Scholar]
- 34.Lesens O, Deschênes M, Steben M, Bélanger G, Tsoukas CM. Hepatitis C virus is related to progressive liver disease in human immunodeficiency virus-positive hemophiliacs and should be treated as an opportunistic infection. J Infect Dis. 1999 May;179(5):1254–1258. doi: 10.1086/314720. [DOI] [PubMed] [Google Scholar]
- 35.Ragni MV, Belle SH. Impact of human immunodeficiency virus infection on progression to end-stage liver disease in individuals with hemophilia and hepatitis C virus. J Infect Dis. 2001 Apr 1;183(7):1112–1115. doi: 10.1086/319273. [DOI] [PubMed] [Google Scholar]
- 36.Tedaldi EM, Baker RK, Moorman AC, Alzola CF, Furhrer J, McCabe RE, et al. HIV Outpatient Study (HOPS) Investigators. Influence of coinfection with hepatitis C virus on morbidity and mortality due to human immunodeficiency virus infection in the era of highly active antiretroviral therapy. Clin Infect Dis. 2003 Feb 1;36(3):363–367. doi: 10.1086/345953. [DOI] [PubMed] [Google Scholar]
- 37.Puoti M, Bonacini M, Spinetti A, Putzolu V, Govindarajan S, Zaltron S, et al. HIV-HCV Coinfection Study Group. Liver fibrosis progression is related to CD4 cell depletion in patients coinfected with hepatitis C virus and human immunodeficiency virus. J Infect Dis. 2001 Jan 1;183(1):134–137. doi: 10.1086/317644. [DOI] [PubMed] [Google Scholar]
- 38.Benhamou Y, Bochet M, Di Martino V, Charlotte F, Azria F, Coutellier A, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999 Oct;30(4):1054–1058. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 39.Mundt B, Kuhnel F, Zender L, Paul Y, Tillmann H, Trautwein C, et al. Involvement of TRAIL and its receptors in viral hepatitis. FASEB J. 2003;17:94–96. doi: 10.1096/fj.02-0537fje. [DOI] [PubMed] [Google Scholar]
- 40.Volkmann X, Fischer U, Bahr MJ, Ott M, Lehner F, Macfarlane M, et al. Increased hepatotoxicity of tumor necrosis factor-related apoptosis- inducing ligand in diseased human liver. Hepatology. 2007;46:1498–1508. doi: 10.1002/hep.21846. [DOI] [PubMed] [Google Scholar]
- 41.Lan L, Gorke S, Rau SJ, Zeisel MB, Hildt E, Himmelsbach K, et al. Hepatitis C virus infection sensitizes human hepatocytes to TRAIL-induced apoptosis in a caspase 9-dependent manner. J Immunol. 2008 Oct 1;181(7):4926–4935. doi: 10.4049/jimmunol.181.7.4926. [DOI] [PubMed] [Google Scholar]
- 42.Zhu H, Dong H, Eksioglu E, Hemming A, Cao M, Crawford JM, et al. Hepatitis C virus triggers apoptosis of a newly developed hepatoma cell line through antiviral defense system. Gastroenterology. 2007;133:1649–1659. doi: 10.1053/j.gastro.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 43.Babu CK, Suwansrinon K, Bren GD, Badley AD, Rizza SA. HIV Induces TRAIL Sensitivity in Hepatocytes. PLoS ONE. 2009;4(2):e4623. doi: 10.1371/journal.pone.0004623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herbeuval JP, Boasso A, Grivel JC, Hardy AW, Anderson SA, Dolan MJ, et al. TNF-related apoptosis-inducing ligand (TRAIL) in HIV-1-infected patients and its in vitro production by antigen-presenting cells. Blood. 2005;105(6):2458–2464. doi: 10.1182/blood-2004-08-3058. [DOI] [PubMed] [Google Scholar]