Abstract
Learning is ubiquitous in the animal kingdom but has been studied extensively in only a handful of species. Moreover, learning studied under laboratory conditions is typically unrelated to the animal’s natural environment or life history. Here, we designed a task relevant to the natural behavior of male African cichlid fish (Astatotilapia burtoni), to determine if they could be trained on a spatial task to gain access to females and shelter. We measured both how successfully animals completed this task over time and whether and how immediate early gene and hormone expression profiles were related to success. While training fish in a maze, we measured time to task completion, circulating levels of three key hormones (cortisol, 11-ketotestosterone, and testosterone) and mRNA abundance of seven target genes including three immediate early genes (that served proxies for brain activity) in nine brain regions. Data from our subjects fell naturally into three phenotypes: fish that could be trained (learners), fish that could not be trained (non-learners) and fish that never attempted the task (non-attempters). Learners and non-learners had lower levels of circulating cortisol compared to fish that never attempted the task. Learners had the highest immediate early gene mRNA levels in the homologue of the hippocampus (dorsolateral telencephalon; Dl), lower cortisol (stress) levels and were more motivated to accomplish the task as measured by behavioral observations. Fish that never attempted the task showed the lowest activity within the Dl, high stress levels and little to no apparent motivation. Data from non-learners fell between these two extremes in behavior, stress, and motivation.
Keywords: Fish, spatial learning, stress, cortisol, motivation
1. Introduction
Learning in non-humans has been the subject of philosophical speculation for nearly 100 years. In the late nineteenth century, Thorndike assessed the behaviors animals display, their performance, and how they “feel” upon enacting such behavior to elucidate the psychology behind animal learning (Thorndike, 1911). However, the capacity to learn is not restricted to mammals as was assumed in the early 1900s. Studies of non-mammalian vertebrates have revealed the ability to learn in a variety of domains, including a large variety of social and spatial tasks (Kieffer and Colgan, 1992). Birds are a prime example of a non-mammalian vertebrate where learning and memory are vital to communication and reproduction. For many oscine bird species, learned songs are used to find and attract mates, directly influencing reproductive success. In white-crowned sparrows (Zonotrichia leucophrys), for example, songs vary by “dialect” depending upon region so that males settling in new environments must learn a new song in order to attract mates. Male sparrows can memorize songs from a variety of different regions, incorporate these songs into their repertoire, and practice them. However, birds retain only the dialect from the area that matched the song of the male birds within that region (Nelson and Marler, 1994).
Fish, too, can learn throughout life and are capable of complex learning strategies, including social and spatial learning (Keiffer and Colgan, 1992). Vision plays an important role in orienting a fish in its environment, a skill vital to spatial learning. For example, many migrating fish species frequently use the sun for orientation (Dodson, 1988). Butterflyfish (Chaetodon trifascialis) use coral landmarks, or “coral crown heads,” to identify foraging locations and territory, as demonstrated by removing a crown head and showing that the animals search for the landmark while traversing its foraging path (Reese, 1989). Spatial learning is particularly important for animals that remain in the same habitat, like butterflyfish, who need to remember where they can feed or possibly take over a territory.
In all vertebrates, the hippocampus is known to be centrally important for spatial learning and memory, but other areas of the brain, including the cerebellum, striatum, and basal forebrain, are also involved. The functional roles of these have been identified primarily by the effects of lesions on learned behaviors both in mammals (D’Hooge and De Deyn, 2001; Eichenbaum, 2000) and in fish (e.g. Vargas et al, 2009). While the mammalian hippocampus is implicated in spatial learning, the cerebellum and the amygdala are involved in implicit learning and memory across many animal species including rats and fish, particularly with regards to fear conditioning (Krebs et al., 1989; Portavella et al., 2004; Saito and Watanabe, 2006; 2004; Vargas et al., 2009). Brain regions known to play a central role in a variety of social interactions in mammals, birds and fish are called the “social behavior network” (SBN). The SBN is comprised of six brain areas: the pre-optic area, lateral septum, anterior hypothalamus, ventromedial hypothalamus, periaqueductal gray, and dorsomedial telencephalon (Newman, 1999). The SBN was identified in fish, and homologous nuclei were identified (Goodson, 2005). These areas overlap with some nuclei involved in learning but it is not known how they interact functionally.
The African cichlid Astatotilapia burtoni is a good model for studying learning because of its complex social structure and adaptability within a changing environment. A. burtoni live in Lake Tanganyika in eastern Africa, where reproductively active territorial (T) males establish territories over a food resource in which they court females and feed. Non-territorial (NT) males are reproductively inactive and are socially subordinate to the T males. Based on changes in social hierarchy or in the natural habitat, NT males can quickly assume social dominance and become reproductively active. Correspondingly, T males become NT after losing a social challenge. T males can also use transitive inference to determine their likelihood of winning a fight, showing they are capable of very complex learning and logical strategies (Grosenick, Clement, and Fernald, 2007). In addition to having to quick responses to social changes, A. burtoni must also adapt to local environmental changes, finding new shelter and food due to disruption that naturally occurs in its habitat (Fernald and Hirata, 1977). Since learning strategies are most important to species with relatively unstable environments like A. burtoni (Keiffer and Colgan, 1992), it seemed likely that these fish could learn to use social and environmental cues to adapt to their changing situations (Hofmann, Benson and Fernald, 1999). Characteristics of their social environment also allowed us to establish paradigm with a biologically relevant reward, namely access to a shelter and close proximity to females. Male A. burtoni are very social and when reproductively mature are attracted to females by both visual and olfactory cues (Fernald and Hirata, 1977; de Caprona, 1974; Hofmann and Fernald, 2001).
Here we tested whether territorial A. burtoni could be trained on a spatial task and, if so, what physiological and neural substrates were involved. To do this, we trained fish to swim through an uncued hole in a transparent barrier, motivated by proximity to a shelter and to females. We quantified levels of circulating steroid hormones (e.g., androgens: testosterone and 11-ketotestosterone, and cortisol, the main stress hormone), 3 immediate early genes (bdnf, c-fos and egr-1; as a proxy for brain activity) and the corticotropin releasing factor (CRF) family of genes (crf, crfbp, crf-r1, crf-r2) in nine micro-dissected regions of the brain. Brain areas chosen were the six nodes of the social behavior network (SBN) (Newman, 1999; Goodson, 2005), namely: pre-optic area (POA), lateral septum (LS), anterior hypothalamus (AH), ventromedial hypothalamus (Vm), periaqueductal gray (PAG), dorsomedial telencephalon (Dm). We also measured gene expression in the cerebellum (Cce), the raphe nucleus (R) and the dorsolateral telencephalon (Dl). Based on prior work, we hypothesized that systematic variance in the ability to be trained on a task would be reflected in distinct physiological and neural expression patterns.
2. Materials and Methods
2.1. Subjects
All subjects were descendents of wild caught Astatotilapia burtoni in Lake Tanganyika, Africa (Fernald, 1977). Prior to the experiment, fish were kept in aquaria under housing conditions that mimic their natural environment (28°C, pH 8, 12:12 h light:dark cycle) and were fed each morning ad libitum with cichlid pellets and flakes (AquaDine; Healdsburg, CA). Procedures described for catching and sacrificing the fish were in accordance with Stanford’s Administrative Panel for Laboratory Animal Care. Males used in training were transferred to a 151-liter isolation tank three days prior to the start of training. Subjects were dominant males, ranging from 4.80 cm to 7.49 cm in length, and from 2.72 g to 10.74 g in weight. Dominant males from community tanks were identified by observing coloration patterns and territorial or courtship behaviors. Fish were isolated prior to the experiment to prevent the community tank from having any effect upon the subject. In addition, this 3-day isolation period was also used to increase the likelihood that the fish were motivated to be trained in the task. As this fish are extremely social (Fernald, 1977) so that access to a group of conspecific individuals after a period of isolation is intrinsically rewarding. Isolation tanks were divided into six approximately equal sections with opaque barriers, each with a half terra cotta flowerpot for shelter to allow for the male to establish territory. Fish were fed daily in the morning while in isolation and prior to beginning training sessions for each day.
2.2. Behavioral Procedures
2.2.1. Apparatus
For the training tank, a 30-liter (51cm × 25 cm × 30 cm) fish tank was set up, consisting of three compartments (Figure 1). Two clear barriers separated each section from one another and the first barrier had a square opening (approximately 2.5 cm × 2.5 cm) that allowed the subject to swim from the left-most start compartment into the middle target compartment. Fish used in the experiment never touched both the top and the bottom of the 2.5 cm target hole whose location was held constant. The second clear barrier was perforated to allow water flow between sections. The square opening was not marked (e.g., uncued), as preliminary pilot tests with a color cued hole appeared to present an obstacle to fish successfully completing the maze. In addition, a removable opaque barrier was placed between the first and second chambers to allow the beginning of the experiment to be controlled. A flowerpot and an air bubbler were in the middle compartment.
Figure 1.
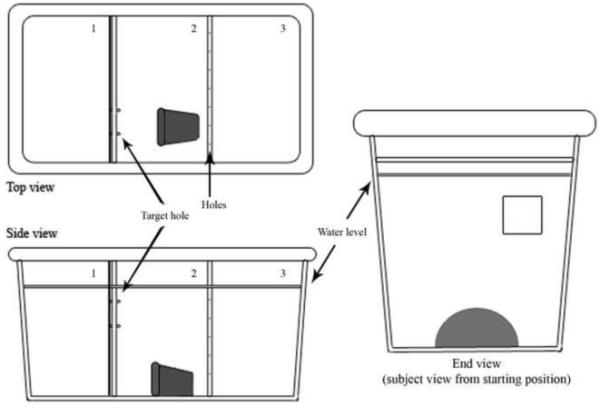
Behavioral tank from three perspectives (not to scale). Small notches and square between sections labeled 1 and 2 indicate the approximate location of the hole in the clear barrier: a 2.5 cm × 2.5 cm square hole placed approximately 2.5 cm from the surface of the water and 2.5 cm from the observer side of the tank. The left-most compartment in the top and side observer views (section 1) represents the start compartment, in which the subject started. The flowerpot (grey shaded object) in the middle section in the top and side views (section 2) was the goal for the subject: the target compartment. The flowerpot serves as a motivating factor to males as flowerpots are normally associated with a territory to claim. In addition, it is closer to the female fish. Since male fish had been kept in social isolation prior to placement in the behavioral tank, close proximity to females, is also motivating (Fernald and Hirata, 1977; Hofmann, Benson, and Fernald, 1999). Between compartments 1 and 2, a removable opaque barrier was present for an acclimation period of one minute. The right-most section in the top and side views (labeled “3”) represents the compartment in which 10 females were placed. The barrier separating the target compartment from the female compartment was clear and perforated, with holes drilled into the barrier as indicated by small Xs. All barriers permitted the flow of water between compartments.
2.2.2. Training
The training was based upon the subjects’ latency to locate a 2.5-cm × 2.5-cm square hole and swim from the leftmost compartment to the middle compartment, through the hole. Ten trials were run for each subject over the course of five consecutive days (“trial period”), with two trials per day. Behavioral data (latency to swim through the hole) was collected for a total of 73 fish. Prior to running each trial, 10 female fish varying in reproductive state (most were gravid) were moved from community tanks into the right-most compartment of the training tank. A flowerpot was placed in the middle compartment, where it allowed the male a potential place to establish a territory and seek shelter (Fernald and Hirata, 1977). Females were placed in the third compartment as motivation and a reward to the subject fish, since the subjects were held in isolation prior to the start of training and between training sessions (Hofmann, Benson and Fernald, 1999). Males were not used in the third compartment, as they might serve as a negative stimulus (e.g. competition for territories and resources) for the male test subject (Fernald and Hirata, 1977).
Each single trial consisted of an acclimation period (1 m), the trial period (15 m), and a reward period (1.5 m), based on pilot trials. Each subject was placed in the start compartment of the training tank and allowed to acclimate for one minute behind the opaque barrier. Fish were video recorded (Sony mini-DV HDR-HC5 Handycam; New York, NY) beginning when a fish was placed into the start compartment. After 1 minute, the opaque barrier was lifted to reveal the clear barrier with a 2.5-cm by 2.5-cm square hole. Timing for the trial period began once the opaque barrier was lifted. A maximum of 15 minutes were allowed for the fish to locate the hole and swim through to the second chamber. Time for each trial was stopped once the fish swam through the hole and reached the midpoint of the target compartment or the maximum 15 minutes was reached. One minute and 30 seconds was allotted for the subject to remain in the target compartment as the reward period. This reward period was not allowed for subjects who failed to find the target within 15 minutes. Fish that did not complete a given trial were assigned completion values of 15 minutes (900 seconds). Subjects were separated into three groups based on their performance in the task: Fish that were successfully trained in the task we have called learners (L), fish that attempted but were not successfully trained in the task we have called non-learners (NL), and those that did not attempt the task (non-attempting or NA) (Figure 2). These designations were based on their latency to find the hole in the first clear barrier (please see statistical analysis for further explanation).
Figure 2.
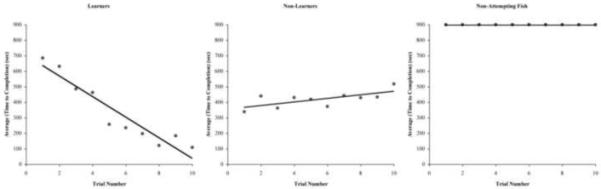
Average time for subjects to the complete task over ten trials for each fish type. The lines graphed are best-fit showing the statistical difference among the groups: Learners (n=22), Non-learners (n=45), and those that did not attempt the task (n=6).
2.3. Sample Collection
Fish were sacrificed immediately after completion of the last trial by cervical transection. Blood was collected and centrifuged for 8 minutes at 8000 RPM to separate plasma from red blood cells and plasma was kept and frozen at −20°C for later assay. Brains tissue was collected and mounted in optimal cutting temperature mounting medium (Neg50; Thermo Scientific, Waltham, MA) and stored at −80°C until sectioning.
2.4. Hormone Quantification Procedures
Hormone levels of cortisol, 11-ketotestosterone (11KT), and testosterone were measured in plasma samples (Cayman Chemical Enzyme Immunoassay (EIA), Ann Arbor, MI) applying well-established protocols (Parikh et al., 2006). Spectrophotometric readings were taken using the standard wavelength recommended by the EIA kits of 405 nm. The hormone present in the original sample was calculated using the standard curve and the volume of sample used for purification, in ng/ml.
2.5. Microdissection
Frozen brain tissue was sliced coronally into 300 μm thick sections using a cryostat (−13°C; Microm HM550, Thermo Scientific, Waltham, MA) and mounted on glass microscope slides. Specimens were kept frozen in the cryostat throughout sectioning, and completed slides were stored at −80 °C for later microdissection (Korzan et al., 2000; Øverli et al., 2004; Korzan and Summers, 2004; Desjardins and Fernald, 2010). A frozen stage (BFS-30MP; Physitemp, Clifton, NJ) viewed through a dissection microscope was used to identify and microdissect specific brain regions. These microdissections were performed with a modified 27G needle with an internal diameter of 190 μm as a punch. Brain atlases from A. burtoni (Fernald and Shelton, 1985; Burmeister et al., 2009) and from other fish species (Maler et al., 1991; Reiner and Northcutt, 1992; Munoz-Cueto et al., 2001) were used to target the cerebellum (Cce), raphe (R), periaquiductal gray (PAG), anterior hypothalamus (AH), ventromedial hypothalamus (Vm), dorsolateral telencephalon (Dl), dorsomedial telencephalon (Dm), preoptic area (POA), and lateral septum (LS) for microdissection.
2.6. Real Time Quantitative Reverse Transcriptase Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted from the microdissected brain areas (Qiagen RNeasy Plus Micro Kit; Alameda, CA), cDNA synthesis was conducted (BioRad iScript cDNA; Alameda, CA), and quantitative reverse transcription polymerase chain reaction (qRT-PCR) was used to quantify mRNA expression in each region of the brain. The qRT-PCR protocol was based on procedures previously performed in our lab (Burmeister et al., 2007; Desjardins and Fernald, 2010). Primers for all target mRNA were designed according to published sequences (Greenwood et al., 2003; Burmeister and Fernald, 2005; Burmeister et al., 2007; c-fos: Genbank accession number: HQ232413; bdnf: Genbank Submission ID: 1398614). The qRT-PCR was performed using 30 μL duplicate reactions (SSoFast EvaGreen Supermix; Bio-Rad, Hercules, CA), 0.5 μL of each primer, and 0.5 μL of template cDNA (RNA equivalent) using a RT-PCR machine (Bio-Rad CFX96; Bio-Rad, Hercules, CA). Data were analyzed using a specialized qRT-PCR algorithms (Zhao and Fernald, 2005).
2.7. Statistical Analysis
Statistical analyses were conducted using JMP software (SAS institute; Cary, North Carolina). Subjects were separated into three groups based on their performance in the task: learners (L), non-learners (NL), and those that did not attempt the task (non-attempting or NA) (Figure 2). These designations were based on their latency to find the hole in the first clear barrier. A line was plotted which is the line of best fit. Those fish with an overall negative slope for a best-fit line (p<0.1) were labeled as those who were successfully trained and classified as “learners.” Fish with both overall negative and positive slopes for best-fit lines and p-values greater than 0.1 were considered to be not successfully trained and were labeled as “non-learners.” Those fish with P-values greater than 0.99 with data points at 900 seconds were labeled as non-attempting fish (NA).
Results from the qRT-PCR plate readings were analyzed using a special purpose curve-fitting algorithm to generate the number of cycles required to reach the threshold value of gene amplification (Zhao and Fernald, 2005). The geometric mean of the three housekeeping gene levels served as the normalized standard for the expression of the genes of interest. The amount of mRNA of the target gene present in the sample was determined by a formula incorporating the average reaction amplification efficiency for each gene and the number of cycles to threshold (R0). The relative levels of target mRNA expressed in the samples were calculated as a percentage of R0 of the geometric mean of the housekeeping genes.
Prior to any data analysis, hormone level and mRNA expression data were checked for violations of the assumptions of the parametric tests. All residuals from the mean were normally distributed and groups had equal variances. To determine whether hormone data and mRNA expression data could explain the probability that a male was a learner, non-learner or non-attempter, two logistic regressions were conducted with type (learner, non-learner, non-attempter) as the independent variable and 1) all three hormones (testosterone, 11-ketotestosterone and cortisol) and 2) mRNA expression normalized to housekeeping genes (bdnf, c-fos, crf, crfbp, crf-r1, crf-r2, egr-1) as dependent variables. Based on the results of these logistic regressions, some variables were removed from subsequent analyses because they were did not contribute significantly to the differences between the behavioral subgroups (see below). With the remaining variables, we conducted one-way ANOVAS and bonferroni corrected post-hoc tests to determine specific effects within brain regions for each hormone and mRNA level. Alpha levels for individual tests were adjusted so that the overall alpha level did not exceed 0.05. In order to explore relationships and possible connectivity between measured genes, we conducted a principal components analysis on mRNA expression levels. Subsequent to this principal components analysis and to explore (in more detail) the relationship between genes within brain areas, individual least squares regressions were conducted between mRNA expression levels within each brain area. All of the alpha levels for each individual regression were bonferroni corrected to maintain the overall alpha level at 0.05.
3. Results
To differentiate among the behavioral, physiological and molecular profiles of learners (L), non-learners (NL), and non-attempting (NA) fish, multiple comparisons and analyses were conducted where mRNA levels are shown in italics, and protein levels are shown in capital letters.
3.1. Behavioral Results
Behavioral data (e.g., latency to swim through the hole) revealed three distinct groups of fish (n=73): Learners (L: p < 0.1, n=22), Non-learners (NL: p > 0.1, n=45), and those that did not attempt the task (NA: p > 0.99, n=6) fish (Figure 2).
3.2. Gene and Hormone Expression
To determine the relationship between hormone level and learning type (learners, non-learners and non-attempters), logistic regression analysis revealed that overall hormone level varies between learning types (R2=0.32, X2=24.45, p=0.0004) indicating that hormone status influences learning type. Subsequent likelihood ratio tests, to determine which hormones were most related to learning type, revealed that only cortisol varied predictably with learning type (cort: L-R X2=15.001, p=0.0006; T: L-R X2=0.078, p=0.961; 11KT: L-R X2=3.262, p=0.196). Therefore, additional tests were only conducted with cortisol (see results below). Similarly for mRNA levels, logistic regression revealed that variation in learning types can be explained using gene expression (R2=0.10, X2=40.211, p=0.0003); however, likelihood ratio tests reveal that only bdnf (L-R X2=7.838, p=0.019), crf (L-R X2=9.027, p=0.011), crfbp (L-R X2=9.714, p=0.0078) and egr-1 (L-R X2=11.215, p=0.004) but not c-fos (L-R X2=7.237, p=0.539), crf-r1 (L-R X2=0.535, p=0.765) or crf-r2 (L-R X2=0.588, p=0.745) successfully predicted learning type. This indicated that only expression levels of bdnf, crf, crfbp and egr-1 were related to learning type and so were the only expression levels included in further analyses (see results below).
3.2.1. Hormone and Gene Expression in Learner Fish
Cortisol levels of learners were significantly lower than those found in NA fish (F(2,42) = 32.21; p < 0.0001; Figure 3).
Figure 3.
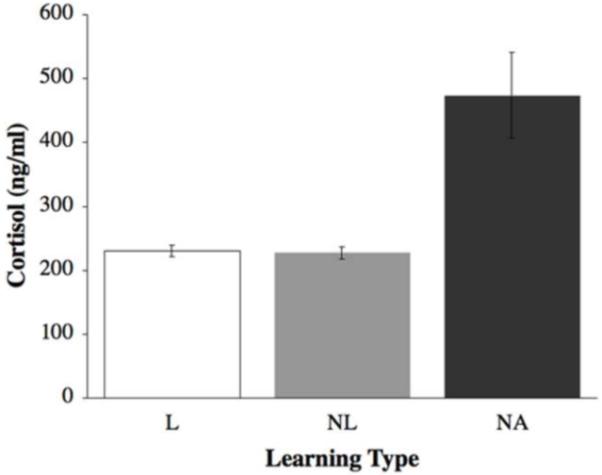
Mean (±SE) plasma cortisol levels by learning type. Non-attempting fish exhibited significantly higher levels of cortisol than either learners or non-learners, in ng/ml. Open bars represent learners (L); grey bars represent non-learners (NL); black bars represent non-attempting fish (NA). Sample sizes were L=11, NL=29, NA=5.
Across all brain areas of learners, mRNA levels of the immediate early genes (IEGs) bdnf and egr-1 were expressed at significantly higher levels within the dorsolateral telencephalon (Dl) as compared to the non-attempting (NA) and non-learner (NL) fish (bdnf: F(2,20) = 7.33, p = 0.004; Figure 4a; egr-1: F(2,17) = 8.72, p = 0.003; Figure 4b) than those found in NL. This suggests differential activation of the Dl in response to a learned spatial task.
Figure 4.
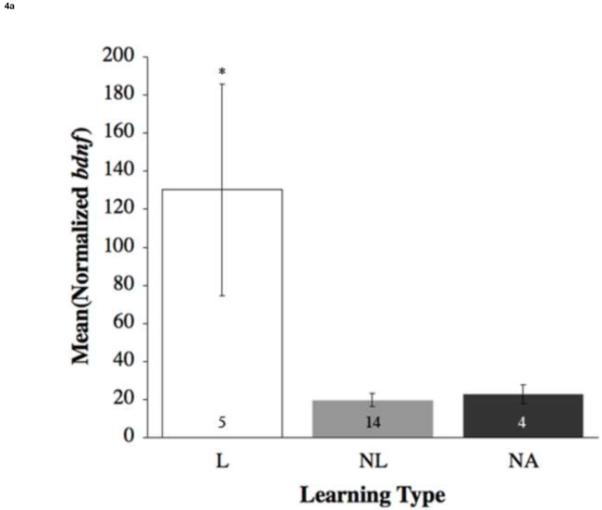
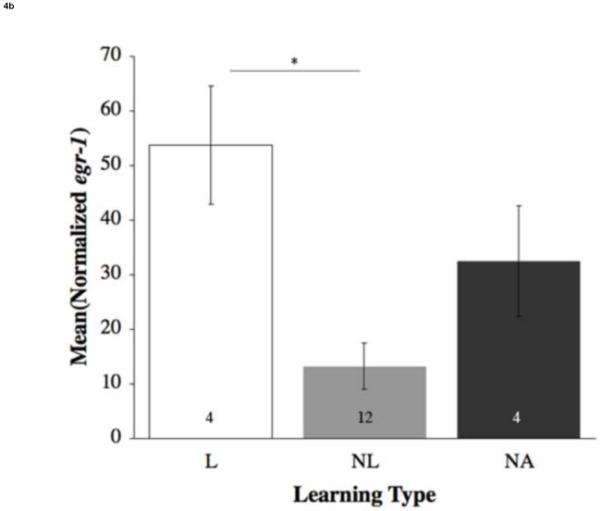
Mean (±SE) relative expression of genes within the dorsolateral telencephalon (Dl) by learning type (a) bdnf and (b) egr-1. Open bars represent learners (L); grey bars represent non-learners (NL); dark grey bars represent non-attempting fish (NA). Numbers within the bars are sample size. Asterisks and lines connecting two bars show significant differences (p < 0.05). L exhibited significantly higher levels of bdnf compared to NL and NA, egr-1 compared to NL.
Using pair-wise regression analyses, significant correlations were found across the entire brain and within specific brain regions. Throughout the entire brain of learner fish, positive correlations between all IEGs were found (Table A1): c-fos and bdnf (F(1,62) = 34.39, p < 0.0001); egr-1 and bdnf (F(1,62) = 6.52, p = 0.01); egr-1 and c-fos (F(1,61) = 28.76, p < 0.0001). Pair-wise comparisons were also made within specific brain areas and by learning types (Table A2). The lateral septum (LS) of learners exhibited a positive correlation between crf-r1 and crfbp (F(1,6) = 5.80, p = 0.05). Within the periaqueductal gray (PAG), a negative relationship between crf-r2 and bdnf appeared (F(1,6) 10.49, p = 0.02). crf-r2 showed a positive correlation with both egr-1 and c-fos in the preoptic area (POA) (F(1,4) = 12.64, p = 0.02; F(1,4) = 19.63, p = 0.01). The ventromedial hypothalamus (Vm) showed a positive correlation between crfbp and bdnf (F(1,6) = 5.98, p = 0.05).
3.2.2. Hormone and Gene Expression in Non-Learner Fish
Cortisol levels of non-learners were significantly lower than those found in NA fish (F(2,42) = 32.21; p < 0.0001; Figure 3).
Pair-wise gene regression analysis also revealed trends throughout the brain of non-learners, involving both CRF family genes and IEGs (Table A1). Non-learners demonstrated positive relationships between gene expression levels of crfbp and crf (F(1,102) = 5.80, p = 0.02). Within specific brain areas, additional significant correlations were found (Table A2). In the LS, there were positive correlations between crf and crfbp (F(1,11) = 7.77, p = 0.02; F(1,12) = 9.19, p = 0.01).
3.2.3. Hormone and Gene Expression in Fish Who Did Not Attempt the Task (NA)
The cortisol levels of NA fish were significantly higher than those found in L and NL (F(2,42) = 32.21; p < 0.0001; Figure 3).
In the brains of non-attempting (NA) fish, there was greater activation within brain areas associated with anxiety as well as increased levels of crf and crfbp. The immediate early gene bdnf was significantly differentially expressed in the PAG of NA fish as compared to both learners (L) and non-learners (NL) (F(2,21) = 6.40, p = 0.007; Figure 5). Furthermore, in the dorsomedial telencephalon (Dm) egr-1 (F(2,17) = 55.33; p < 0.0001; Figure 6) was expressed at significantly higher levels in no-try fish in comparison to the L and NL.
Figure 5.
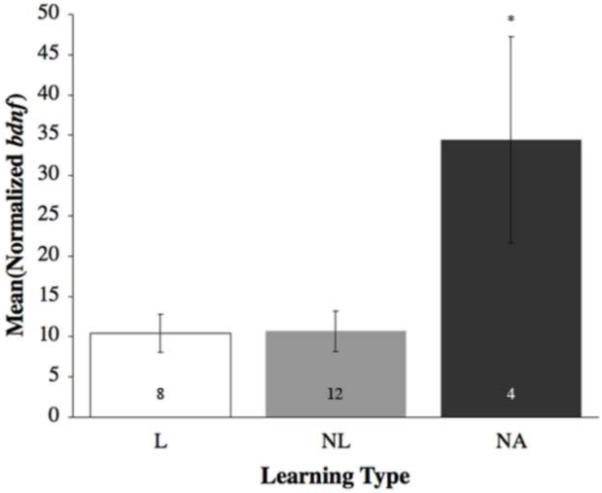
Mean (±SE) relative gene expression of bdnf within the periaqueductal gray (PAG) by learning type, with non-attempting fish (NA – black bars) higher than learner fish (L – open bars) and non-learner fish (NL – grey bars). Numbers within the bars are sample size. Asterisk denotes a significant difference (p < 0.05).
Figure 6.
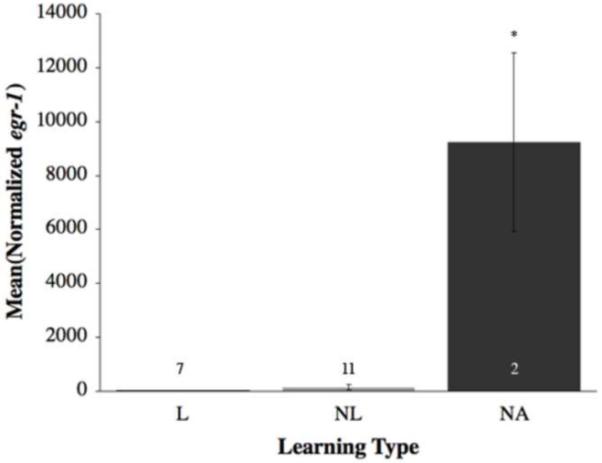
Mean (±SE) relative gene expression of egr-1 within the dorsomedial telencephalon (Dm) by learning type. Open bars represent learners (L); grey bars represent non-learners (NL); black bars represent non-attempting fish (NA). Numbers within or immediately above the bars are sample size. Asterisks signal a significant difference (p < 0.05). NA fish demonstrated significantly higher levels of egr-1 than both L and NL in the Dm.
Pair-wise comparisons conducted between gene expression level within brain areas, important correlations that emerged. In the Dl, crf and c-fos also showed a positive relationship (F(1,2) = 46.68, p = 0.02). A positive correlation was found in the POA crfbp and bdnf exhibited a negative relationship in the Vm (F(1,2) = 37.13, p = 0.03).
3.2.4 Relationships Between Genes
Principal components analysis on mRNA expression levels of bdnf, crf, crfbp and egr-1 revealed that the first 2 principal components explained 76.94% and 21.78% of the variation in these 4 genes. These 2 new variables combined successfully explain 98.72% of the variation in the four genes. The first principal component showed small positive loadings from bdnf (0.1) and crf (0.01), a large positive loading from egr-1 (99.44) and a small negative loading from crfbp (0.45). The second component showed small negative loadings from bdnf (−0.03) and crf (−0.12), a small positive loading from egr-1 (0.005) and a large positive loading from crfbp (99.85). While there are significant relationships between each one of these mRNA levels, the overall variation in the data seems to be mostly driven by egr-1 and crfbp.
4. Discussion
In this experiment we identified three distinct groups of fish, that differed in their ability to be trained in a task: Those that could be trained (learners; L), those that could not be trained (non-learners; NL) and those that never attempted the task (non-attempters; NA). We used both proximity to a shelter or potential territory and proximity to females as rewards in this task. Given the design of the apparatus, the fish could be using visual and/or olfactory cues of the females to guide their training.
4.1. Profile of Learners
Fish able to perform the spatial task successfully (learners) had specific gene and hormone expression profiles reflecting this group’s ability (Figure 7). Of particular importance is the immediate early gene (IEG) activity within the dorsolateral telencephalon (Dl) in the brains of learners. Learners showed higher levels of bdnf mRNA as compared to both non-learners (NL) and non-attempting (NA) fish, and higher levels of egr-1 mRNA as compared to NL fish. Correspondingly, increases in IEG levels have also been found in the hippocampi of rats trained in a spatial task. IEGs have also been shown to have necessary roles in learning and memory experimentally using animal gene knockouts and introduction of drugs to inhibit their expression (Kesslak et al., 1998; Mizuno et al., 2000; Jones et al., 2001; Vann et al., 2000). Ablation studies conducted in goldfish brains have shown that the Dl, homologous to the hippocampus, is vital to learning and memory (López et al., 1999; Rodríguez et al., 2002; Wullimann and Mueller, 2004; Portavella and Vargas, 2005). As suggested by previous work and as shown here in learners, bdnf, egr-1 upregulation within the Dl indicates it may play a key a role in training during a spatial task.
Figure 7.
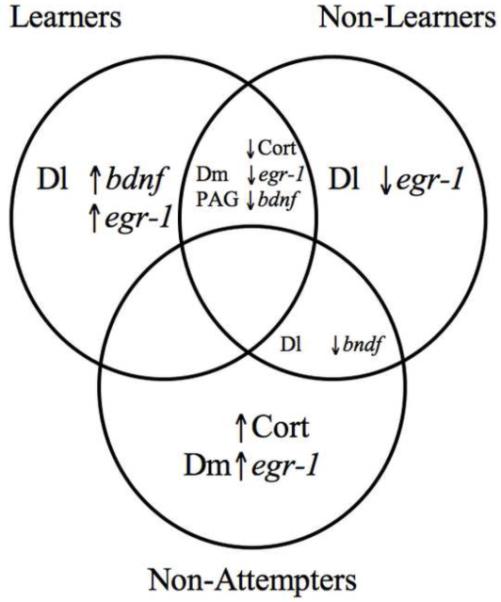
Venn diagram representing overlapping and distinct hormonal and gene expression profiles for learners, non-learners, and fish that did not attempt the spatial task. All relationships were determined by logistic regressional analyses. In learners, the dorsolateral telencephalon (Dl) showed higher levels of bdnf and egr-1. In non-learners, the Dl had decreased levels of egr-1. In non-attempters, individuals demonstrated higher levels of cortisol and the dorsomedial telencephalon (Dm) demonstrated higher levels of egr-1. Both learners and non-learners exhibited decreased levels of cortisol, egr-1 in the Dm, and bdnf in the periaqueductal gray (PAG). Both non-learners and non-attempters showed decreased levels of bdnf in the Dl. Note that there are no characteristics shared between all three types learning types nor are there any shared characteristics between learning and non-attempting fish.
Learners also had lower cortisol levels than the NA fish, suggesting lower stress levels. We interpret this to mean that elements of the environment or of the task did not appear to be stressors to the fish as confirmed by their behavior. Furthermore, the dorsomedial telencephalon (Dm) had lower levels of egr-1. Given the Dm’s hypothesized role as the homologous structure to the amygdala in regulating fear responses, lower levels of IEG markers for neural activity and stress hormone receptors in this area suggests a lower stress response in these animals (Wullimann and Mueller, 2004; Portavella et al., 2004; Portavella and Vargas, 2005; Vargas et al., 2009).
Learners had significantly different correlations between gene pairs when compared to NL and NA fish. These data support the notion of a lower stress profile as seen in these fish. Within the ventromedial hypothalamus (Vm), crfbp was positively correlated with two IEG mRNAs – bdnf, and egr-1 – suggesting that activity here could regulate the stress response by decreasing the amount of free CRF present and/or by preparing for a reaction to a stressful situation by allowing for quicker release of CRF, as opposed to synthesizing protein from genes (Jahn et al., 2002; Flik et al., 2006). While there were positive correlations between crf and IEGs within the Dm of learners, CRF is expected to be in an area associated with anxiety since it is considered homologous to the mammalian amygdala (Portavella et al., 2004; Vargas et al., 2009; Wullimann and Mueller, 2004). Low levels of IEGs in this area suggest that there is little activity despite the presence of CRF, therefore supporting the idea that these fish are less stressed than those from the other learning types.
Furthermore, lower IEG activity in the periaqueductal gray (PAG) suggests less stress in learners than in the other fish types in the presence of a potentially fearful situation. Although levels of c-fos had been shown to be upregulated in this area after a fear response in rats, the role of the PAG in an anxiogenic environment may carry over even to a spatial learning task in A. burtoni (Campeau et al., 1997). Specifically, learners had lower levels of bdnf as compared to NA fish in the PAG. As illustrated by the behavioral and hormonal data, learners had lower cortisol levels (e.g., less stress) suggesting that learners had lower activity in the PAG than animals with higher cortisol levels.
The activity within the preoptic area (POA) suggests increased motivation or a mechanism for conserving the motivation in learners over the training period, as this area has been shown to play a role in the reward and motivation pathway, particularly sexual motivation and drive (Ikemoto, 2010; Hull and Dominguez, 2006). Learners’ higher levels of IEG activity, decreased stress response, and increased motivation may indicate successful a profile to learning a spatial task, particularly as compared to those of NL and NA fish.
4.2. Profile of Non-Learners
Fish that were not successfully trained in the task (non-learners) also exhibited a distinctive profile, offering clues about why they were behaviorally unsuccessful at this task. As in the brains of the learners (L), IEG expression levels within specific brain regions makes these individuals distinct from the others. Non-learners exhibited significantly lower levels of bdnf and egr-1 within the Dl as compared to L. The lower levels of expression may indicate an inability to maintain neural pathways associated with learning the task, showing a marked difference from the profile of L fish in both the brain and behavior (Kesslak et al., 1998; Jones et al., 2001).
The stress profile of non-learners was somewhat similar to that of L fish, but relationships between gene pairs suggest a possible enhanced sensitivity to the stress response. Non-learners exhibited significantly lower levels of cortisol in plasma in comparison to non-attempting (NA) fish, suggesting that cortisol may not have contributed to the differences in behavior seen in these fish compared to L fish; these differences may have occurred within the brain instead. Like L fish, non-learners had lower levels of egr-1 within the Dm when compared to NA fish, perhaps providing for less stressed behavior and some variable performance on the task.
The PAG of the non-learners showed an “intermediate” gene expression profile. As seen in L fish, the non-learners showed lower levels of bdnf in comparison to the NA fish, indicating that non-learners may not be as stressed by their environment and therefore do not need to involve an area of the brain associated with fear conditioning (Campeau et al., 1997).
The LS of non-learners also resembles that of L fish but demonstrates relationships that may indicate a heightened stress response. Non-learners had positive correlations between crf and crfbp, suggesting that there may more extensive regulation for CRF needed. Given the variability in performance in the non-learners, levels of motivation appeared to vary from subject to subject, from trial to trial.
Despite some similarities in the profile of non-learners when compared to that of the L, distinct differences in areas of the brain associated with learning, stress response, and motivation may also reveal why these fish were unable to be trained as well as the learners.
4.3. Profile of Non-attempting (NA) Fish
The gene and hormone expression profiles of NA (fish that never attempted the task) fish may reveal the source of their inability to complete the task during training. As with both learners (L) and non-learners (NL), the IEGs expressed in the Dl may play a role in explaining why these fish did not appear to learn in our spatial task. bdnf levels were significantly lower in the Dl of non-attempting fish in comparison to L fish, perhaps indicating lower levels of activity within this region and an inability to maintain the neural pathway involved in learning the task as seen in NL fish (Kesslak et al., 1998).
The stress and behavioral profiled of NA fish differed from both L and NL fish as they did not even attempt to complete the task. In comparison, all NL fish completed the task at some point over ten trials. NA fish exhibited significantly higher levels of cortisol in comparison to the levels found in the plasma of L and NL fish, indicating a high level of activity within the stress axis of the NA fish, which may have an effect upon the behavior of the fish. At the level of the brain, egr-1 was significantly higher in the Dm in comparison to both NL and L, perhaps indicating increased activity in and sensitivity to the stress response. The Vm of NA fish exhibited a negative correlation between crfbp and bdnf, the opposite relationship to the one seen in the Vm of L. This suggests that free CRF may accumulate and stimulate the stress axis.
Activity within the PAG of NA fish further supports the idea of enhanced fear and stress within these fish. The PAG in NA fish expressed significantly higher levels of bdnf than in both the L and NL fish, supporting its role in contextual fear conditioning (Liu et al., 2004; Kim et al., 1993); perhaps the task represented a fearful context for the fish, thus recruiting PAG activity.
With regards to motivation, the POA exhibited trends that suggest lower motivation in NA fish than was seen in the other two fish types. The behavior of the animals suggested a complete lack of motivation, despite the social reward made available to the fish upon completion of the task. NA fish demonstrate a profile, which may have led to consistent failure of the task. That is, low IEG activity in brain areas associated with learning, heightened stress response, and little to no motivation.
Interestingly, studies with different vertebrates, using both pharmacological and genetic tools have shown that stress, in relation to the amygdala, facilitates and might even be indispensable for good learning and memory performance (Oitzl and de Kloet, 1992; Sandi and Rose, 1994; Roozendall and McGaugh, 1996; Sandi, 1997; Oitzl et al. 2001; Joëls et al., 2006). This has also been demonstrated in humans (Lupien et al., 2002; Cahill et al., 2003), however, stress has also been associated with impaired cognitive performance such that people who have experienced a very stressful event, often show unreliable memory for details (Christianson, 1992). Furthermore, learning impairments have been observed in chronic conditions, or in predisposed individuals linked to hyperactivity of the stress systems, such as major depression or aging (Shors, 2006; McGaugh, 2004). Here we have shown that fish with the highest circulating cortisol levels and high IEG activity in the amygdala do not attempt the learning task or appear to be unable to be trained in the task. It is likely that these fish may resemble more individuals who have a chronic stress disorder, rather than normal individuals who undergo mild stress during or prior to learning.
5. Conclusion
We found a continuum in learning types in our spatial task, in the learning, stress and motivation profiles amongst the fish we tested. L fish demonstrated high levels of activity within the Dl, compared to both NL and NA fish, indicating that fish within these two groups relied less on this area typically associated with learning and memory. L fish exhibited little to no stress in their behavior, gene or hormone expression profiles, whereas NL and NA fish exhibited higher levels of stress in most (NL) and all (NA) of these areas. Finally, L fish demonstrated high motivation by the tenth trial as shown behaviorally and by activity in the POA, while NL and NA fish showed much less motivation in both behavior and gene expression. It is possible that the results derived from these fish reflect the gene expression profiles as of the ninth trial, given the time course of maximal IEG expression after neuronal activity ranging from 15 to 60 minutes (Yamada et al., 2002; Burmeister et al., 2005; Morgan et al., 1987). However, by the ninth trial, test fish have already demonstrated their ability to successfully perform the task, or not, by this point. Since our research focuses mainly on the ability to complete a spatial task, as opposed to the stage of learning we suggest that future work can address “adeptness” of the subjects. Our results suggest that stress may have a larger effect on performance in a spatial task, and perhaps learning and memory than is suggested by the literature. CRF is believed to have a positive effect upon learning when introduced to CRF-R1 in the hippocampus of rats (Radulovic et al., 1999). However, for extremely stressed animals – as in our NA fish – learning appears to depend on the effects of stress on motivation and, ultimately, behavior. A third type of CRF receptor has been discovered in fish, but we have focused on the first two receptor types since most research has been on these receptor types (Arai, Assil, and Abou-Samra, 2001).
Underlying the differing performance levels on the task may result from a spectrum of personalities within A. burtoni. A “shyness-boldness continuum” has been observed and proposed in a variety of fish – from sunfish to South American cichlids – suggesting variability amongst individuals, and some research has applied human personality dimensions to animal behavior (Sih, Bell and Johnson, 2004; Brick and Jakobsson, 2002; Gosling and John, 1999). A fish’s boldness may allow the individual to be more willing to explore its environment and be more likely to successfully complete a spatial task such as that used here. Whether learning ability results from its stress and motivation profile or its personality, these results show the profile – or personality – that is optimal for a fish to be trained in a spatial task.
Research Highlights.
Learners are unstressed and highly motivated.
Stressed animals do not attempt to solve a maze.
Immediate early genes are a proxy for brain activity.
The dorsolateral telencephalon, the teleost’s homologous structure to the mammalian hippocampus, and the gene BDNF are involved in spatial learning in fish
Supplementary Material
Acknowledgements
This research was supported by grants from the Vice Provost for Undergraduate Education at Stanford University (LSW), Rose Hills Foundation (LSW), NSERC (JKD), NIH NS 034950 (RDF) and NSF IOS-0923588 (RDF). We thank Russ Carpenter for contributing useful comments to this manuscript; Greg Wood for editing; Holly Cooper, Jina Nam, and Max Simon for assisting with various aspects of the project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arai M, Assil IQ, Abou-Samra AB. Characterization of Three Corticotropin-Releasing Factor Receptors in Catfish: A Novel Third Receptor Is Predominantly Expressed in Pituitary and Urophysis. Endocrinology. 2001;142(1):446–454. doi: 10.1210/endo.142.1.7879. [DOI] [PubMed] [Google Scholar]
- Brick O, Jakobsson S. Individual variation in risk taking: the effect of a predatory threat on fighting behavior in Nannacara anomala. Behavioral Ecology. 2002;13(4):439–442. [Google Scholar]
- Burmeister SS, Fernald RD. Evolutionary conservation of the egr-1 immediate-early gene response in a teleost. The Journal of Comparative Neurology. 2005;481(2):220–32. doi: 10.1002/cne.20380. [DOI] [PubMed] [Google Scholar]
- Burmeister SS, Kailasanath V, Fernald RD. Social dominance regulates androgen and estrogen receptor gene expression. Hormones and Behavior. 2007;51(1):164–70. doi: 10.1016/j.yhbeh.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister SS, Munshi RG, Fernald RD. Cytoarchitecture of a cichlid fish telencephalon. Brain, Behavior and Evolution. 2009;74:110–20. doi: 10.1159/000235613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Gorski L, Le K. Enhanced human memory consolidation with post-learning stress: Interaction with the degree of arousal at encoding. Learning and Memory. 2003;10:270–274. doi: 10.1101/lm.62403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Falls WA, Cullinan WE, Helmreich DL, Davis M, Watson SJ, et al. Elicitation and reduction of fear: behavioural and neuroendocrine indices and brain induction of the immediate-early gene c-fos. Neuroscience. 1997;78(4):1087–104. doi: 10.1016/s0306-4522(96)00632-x. [DOI] [PubMed] [Google Scholar]
- Christianson SA. Emotional stress and eyewitness memory: a critical review. Psychological Bulletin. 1992;112:284–309. doi: 10.1037/0033-2909.112.2.284. [DOI] [PubMed] [Google Scholar]
- Crapon de Caprona MD. The effect of chemical stimuli from conspecifics on the behavior of Haplochromis burtoni (Cichlidae, Pisces) Cellular and Molecular Life Sciences. 1974;30(12):1394–1395. doi: 10.1007/BF01919654. [DOI] [PubMed] [Google Scholar]
- Desjardins JK, Fernald RD. What do fish make of mirror images? Biology Letters. 2010 doi: 10.1098/rsbl.2010.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Hooge R, De Deyn P. Applications of the Morris water maze in the study of learning and memory. Brain Research Reviews. 2001;36(1):60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- Dodson JJ. The nature and role of learning in the orientation and migratory behavior of fishes. Environmental Biology of Fishes. 1988;23(3):161–82. [Google Scholar]
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nature Reviews Neuroscience. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Fernald RD, Hirata NR. Field study of Haplochromis burtoni: Quantitative behavioural observations. Animal Behavior. 1977;25:964–975. [Google Scholar]
- Flik G, Klaren PH, Van den Burg EH, Metz JR, Huising MO. CRF and stress in fish. General and comparative Endocrinology. 2006;146(1):36–44. doi: 10.1016/j.ygcen.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Goodson JL. The vertebrate social behavior network: evolutionary themes and variations. Hormones and Behavior. 2005;48(1):11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosling SD, John OP. Personality Dimensions in Nonhuman Animals: A Cross-Species Review. Current Directions in Psychological Science. 1999;8(3):69–75. [Google Scholar]
- Greenwood AK, Butler PC, White RB, DeMarco U, Pearce D, Fernald RD, et al. Multiple corticosteroid receptors in a teleost fish: distinct sequences, expression patterns, and transcriptional activities. Endocrinology. 2003;144(10):4226–36. doi: 10.1210/en.2003-0566. [DOI] [PubMed] [Google Scholar]
- Grosenick L, Clement TS, Fernald RD. Fish can infer social rank by observation alone. Nature. 2007;445:429–32. doi: 10.1038/nature05511. [DOI] [PubMed] [Google Scholar]
- Hofmann HA, Benson ME, Fernald RD. Social status regulates growth rate: Consequences for life-history strategies. Proceedings of the National Academy of Sciences. 1999;96(24):14171–14176. doi: 10.1073/pnas.96.24.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann HA, Fernald RD. What cichlids tell us about the social regulation of brain and behavior. Journal of Aquariculture and Aquatic Sciences. 2001;9:1–15. [Google Scholar]
- Hull EM, Dominguez JM. Getting his act together: roles of glutamate, nitric oxide, and dopamine in the medial preoptic area. Brain Research. 2006;1126(1):66–75. doi: 10.1016/j.brainres.2006.08.031. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Brain reward circuitry beyond the mesolimbic dopamine system: A neurobiological theory. Neuroscience and Biobehavioral Reviews. 2010 doi: 10.1016/j.neubiorev.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn O, Eckart K, Brauns O, Tezval H, Spiess J. The binding protein of corticotropin-releasing factor: ligand-binding site and subunit structure. Proceedings of the National Academy of Sciences. 2002;99(19):12055–60. doi: 10.1073/pnas.192449299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: How does it work? Trends in Cognitive Sciences. 2006;10:152–158. doi: 10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Jones MW, Errington ML, French PJ, Fine A, Bliss TV, Garel S, et al. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nature Neuroscience. 2001;4(3):289–296. doi: 10.1038/85138. [DOI] [PubMed] [Google Scholar]
- Kesslak JP, So V, Choi J, Cotman CW, Gomez-Pinilla F. Learning upregulates brain-derived neurotrophic factor messenger ribonucleic acid: a mechanism to facilitate encoding and circuit maintenance? Behavioral Neuroscience. 1998;112(4):1012–9. doi: 10.1037//0735-7044.112.4.1012. [DOI] [PubMed] [Google Scholar]
- Kieffer JD, Colgan PW. The role of learning in fish behaviour. Reviews in Fish Biology and Fisheries. 1992;2(2):125–143. [Google Scholar]
- Kim JJ, Rison R. a., Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear. Behavioral Neuroscience. 1993;107(6):1093–8. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Radulovic J, Radulovic M, Lin CR, Schrick C, Hooshmand F, et al. Deletion of crhr2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nature Genetics. 2000;24(4):415–9. doi: 10.1038/74271. [DOI] [PubMed] [Google Scholar]
- Krebs JR. Hippocampal Specialization of Food-Storing Birds. Proceedings of the National Academy of Sciences. 1989;86(4):1388–1392. doi: 10.1073/pnas.86.4.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu IY, Lyons WE, Mamounas L. a., Thompson RF. Brain-derived neurotrophic factor plays a critical role in contextual fear conditioning. The Journal of Neuroscience. 2004;24(36):7958–63. doi: 10.1523/JNEUROSCI.1948-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López J, Broglio C, Rodr F, Thinus-Blanc C, Salas C. Multiple spatial learning strategies in goldfish (Carassius auratus) Animal Cognition. 1999;2:109–120. [Google Scholar]
- Lupien SJ, Wilkinson CW, Brière S, Ménard C, Ng Ying Kin NMK, Nair NPV. The modulatory effects of corticosteroids on cognition: Studies in young human populations. Psychoneuroendocrinology. 2002;27(3):401–416. doi: 10.1016/s0306-4530(01)00061-0. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories and emotionally arousing experiences. Annual Review of Neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. The Journal of Neuroscience. 2000;20(18):7116–21. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J, Cohen D, Hempstead J, Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987;237(4811):192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- Nelson D, Marler P. Selection-based learning in bird song development. Proceedings of the National Academy of Sciences. 1994;91:10498–10501. doi: 10.1073/pnas.91.22.10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SW. The Medial Extended Amygdala in Male Reproductive Behavior: A Node in the Mammalian Social Behavior Network. Annals of the New York Academy of Sciences. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Oitzl MS, de Kloet ER. Selective corticosteroid antagonists modulate specific aspects of spatial orientation learning. Behavioral Neuroscience. 1992;106:62–71. doi: 10.1037//0735-7044.106.1.62. [DOI] [PubMed] [Google Scholar]
- Oitzl MS, Reichard HM, Joëls M, de Kloet ER. Point mutation in the mouse glucocorticoid receptor preventing DNA binding impairs spatial memory. Proceedings of the National Academy of Sciences. 2001;98:12790–12795. doi: 10.1073/pnas.231313998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh VN, Clement TS, Fernald RD. Androgen level and male social status in the African cichlid, Astatotilapia burtoni. Behavioural Brain Research. 2006;166(2):291–5. doi: 10.1016/j.bbr.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Portavella M, Torres B, Salas C. Avoidance response in goldfish: emotional and temporal involvement of medial and lateral telencephalic pallium. The Journal of Neuroscience. 2004;24(9):2335–42. doi: 10.1523/JNEUROSCI.4930-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portavella M, Vargas JP. Emotional and spatial learning in goldfish is dependent on different telencephalic pallial systems. European Journal of Neuroscience. 2005;21(10):2800–2806. doi: 10.1111/j.1460-9568.2005.04114.x. [DOI] [PubMed] [Google Scholar]
- Radulovic J, Rühmann A, Liepold T, Spiess J. Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: differential roles of CRF receptors 1 and 2. The Journal of Neuroscience. 1999;19(12):5016–25. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese ES. Orientation behavior of butterflyfishes (family Chaetodontidae) on coral reefs: spatial learning of route specific landmarks and cognitive maps. Environmental Biology of Fishes. 1989;25(1-3):79–86. [Google Scholar]
- Rodríguez F, López JC, Vargas JP, Gómez Y, Broglio C, Salas C, et al. Conservation of spatial memory function in the pallial forebrain of reptiles and ray-finned fishes. The Journal of Neuroscience. 2002;22(7):2894–903. doi: 10.1523/JNEUROSCI.22-07-02894.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendall B, McGaugh JL. Amygdaloid nuclei lesions differentially affect glucocorticoid-induced memory enhancement in an inhibitory avoidance task. Neurobiology of Learning and Memory. 1996;65:1–8. doi: 10.1006/nlme.1996.0001. [DOI] [PubMed] [Google Scholar]
- Saito K, Watanabe S. Spatial learning deficits after the development of dorsomedial telencephalon lesions in goldfish. NeuroReport. 2004;15(8):2695–2699. [PubMed] [Google Scholar]
- Saito K, Watanabe S. Deficits in acquisition of spatial learning after dorsomedial telencephalon lesions in goldfish. Behavioural Brain Research. 2006;172(2):187–194. doi: 10.1016/j.bbr.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Sandi C. Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. European Journal of Neuroscience. 1997;9:637–642. doi: 10.1111/j.1460-9568.1997.tb01412.x. [DOI] [PubMed] [Google Scholar]
- Sandi C, Rose SP. Corticosterone enhances long-term retention in one-day-old chicks trained in a weak passive avoidance learning paradigm. Brain Research. 1994;647:106–112. doi: 10.1016/0006-8993(94)91404-4. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Stressful experience and learning across the lifespan. Annual Review of Psychology. 2006;57:55–85. doi: 10.1146/annurev.psych.57.102904.190205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sih A, Bell A, Johnson JC. Behavioral syndromes: an ecological and evolutionary overview. Trends in Ecology and Evolution. 2004;19(7):372–378. doi: 10.1016/j.tree.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Thorndike E. Animal Intelligence: Experimental Studies. The Macmillan Company; New York: 1911. [Google Scholar]
- Timpl P, Spanagel R, Sillaber I, Kresse a., Reul JM, Stalla GK, et al. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nature Genetics. 1998;19(2):162–6. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- Vann SD, Brown MW, Erichsen JT, Aggleton JP. Fos imaging reveals differential patterns of hippocampal and parahippocampal subfield activation in rats in response to different spatial memory tests. The Journal of Neuroscience. 2000;20(7):2711–8. doi: 10.1523/JNEUROSCI.20-07-02711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas JP, López JC, Portavella M. What are the functions of fish brain pallium? Brain Research Bulletin. 2009;79:436–40. doi: 10.1016/j.brainresbull.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Wullimann MF, Mueller T. Teleostean and mammalian forebrains contrasted: evidence from genes to behavior. J. Comp. Neurol. 2004;475:143–162. doi: 10.1002/cne.20183. [DOI] [PubMed] [Google Scholar]
- Yamada K, Mizuno M, Nabeshima T. Role for brain-derived neurotrophic factor in learning and memory. Life Sciences. 2002;70(7):735–44. doi: 10.1016/s0024-3205(01)01461-8. [DOI] [PubMed] [Google Scholar]
- Zhao S, Fernald RD. Comprehensive algorithm for quantitative real-time polymerase chain reaction. Journal of Computational Biology. 2005;12(8):1047–64. doi: 10.1089/cmb.2005.12.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


