Abstract
Previous studies suggested that endoplasmic reticulum (ER) stress–associated apoptosis plays an important role in the pathogenesis of ischemic heart disease. Gene transfer of sarco/endoplasmic reticulum Ca2+ ATPase 2a (SERCA2a) attenuates myocardial apoptosis in a variety of heart failure models. This study is to investigate the effects of SERCA2a gene delivery on the myocardial apoptosis and ER stress pathway in a porcine ischemic heart disease model. Eighteen pigs were either subjected to ameroid implantation in the coronary artery or sham operation. Eight wks after gene delivery, the protein level and activity of SERCA2a were measured. Myocardial apoptosis was determined using terminal deoxynucleotidyl transferase–mediated DNA nick-end labeling assay. Regional myocardial perfusion and function were evaluated by 99m Tc-sestamibi (99m Tc-MIBI) single photon emission computed tomography and echocardiography. The ER stress signaling was assessed by Western blot. SERCA2a protein level and activity were significantly decreased in the ischemic myocardium and restored to normal after SERCA2a gene transfer. Restoration of SERCA2a expression significantly improved the cardiac function, although no improvement of regional myocardial perfusion was detected. Restoration of SERCA2a significantly attenuated myocardial apoptosis and reversed the activation of unfolded protein response (UPR) pathway and the ER stress–associated apoptosis pathways. These findings demonstrate a robust role of SERCA2a in attenuation of ischemic myocardial apoptosis, correlating with reverse activation of the ER stress–associated apoptosis pathways, suggesting that the beneficial effects of SERCA2a gene transfer may involve the attenuation of ER stress–associated myocardial apoptosis.
INTRODUCTION
Chronic myocardial ischemia has become the leading cause of heart failure. In China, more than half of the patients with heart failure also have coronary artery heart disease (1). These patients suffer from ischemic heart disease (IHD), which leads to the further deterioration of cardiac function. The pathogenesis of IHD is a chronic and complex process, which may involve abnormalities in energy metabolism, altered expression or function of contractile proteins, ventricular remodeling and myocardial apoptosis (2). Growing evidence suggests that a defect of myocardial Ca2+ transport system with cytosolic Ca2+ overload is a major contributor to ischemic myocardial injury (3). Sarco/endoplasmic reticulum Ca2+ ATPase 2a (SERCA2a), which is primarily responsible for sarcoplasmic reticulum Ca2+ uptake, was reported to be decreased in protein level or activity in the ischemic myocardium in various animals and humans (4–7). Additionally, vector-mediated gene transfer to increase SERCA2a expression or the use of transgenic animals with SERCA2a overexpression have clearly established the potential beneficial effects in ischemic heart, and thus promoted the field of potential SERCA2a gene therapy in IHD (8–11). Recently, evidence from our group and others suggests that the beneficial effects of SERCA2a gene transfer to animals with heart failure may involve a decrease of myocardial apoptosis (12,13). However, the exact mechanisms are still not clear.
Endoplasmic reticulum (ER) is recognized as an organelle that participates in the folding of secretory and membrane proteins (14). Recent evidence suggests that another important function of the ER is apoptotic regulation (15–17). Various stimuli, such as disturbance of Ca2+ homeostasis, ischemia, hypoxia, exposure to free radicals, oxidative stress, elevated protein synthesis and gene mutation—all of which can potentially cause ER dysfunction—are designated as ER stress (14,17,18). To prevent deleterious effects of ER stress, cells have various protective strategies such as the unfolded protein response (UPR) through the mediation of ER transmembrane receptors: protein kinase R–like ER kinase (PERK), activating transcription factor 6 (ATF6) and inositol-requiring enzyme (IRE) (19). These transmembrane receptors are maintained in an inactive state by glucose-regulated protein 78 (GRP78). However, if the stress cannot be resolved, signaling switches from prosurvival to proapoptotic through the mediation of downstream molecules such as CCAAT/enhancer binding protein homology protein (CHOP), c-Jun N-terminal kinases (JNK) and caspase-12 (20–22). Accumulating evidences have demonstrated that apoptosis initiated by excessive ER stress is involved in the ischemic injury of cardiomyocytes in vitro and pathogenesis of IHD in vivo (23–25).
Early observations showed that alteration of sarcoplasmic reticulum/ER Ca2+ levels below functional acceptable limits enhances the ER stress, and SERCA2 expression and activity could be induced during ER stress in cardiomyocytes and other cells, suggesting a potential role of SERCA2a protein in ER stress (26–28). Because the major function of SERCA2a is to replenish the sarcoplasmic reticulum Ca2+ load during the contraction-relaxation cycle of the heart (29), which is fundamental to the ER function of protein folding, we hypothesized that restoration of SERCA2a expression by recombinant adeno-associated virus 1 (rAAV1)- mediated gene delivery could maintain the ER function and attenuate ER stress–associated myocardial apoptosis in an IHD pig model. The effects of SERCA2a gene transfer on regional myocardial function and perfusion were also investigated.
MATERIALS AND METHODS
Construction and Production of rAAV1 Vectors
The rAAV1 vectors carrying human SERCA2a or the enhanced green fluorescent protein (EGFP) reporter gene were constructed and produced by AGTC Gene Technology Company (Beijing, China). In each vector, gene expression was under control of the cytomegalovirus promoter and polyadenylation signal provided by simian virus 40. The vector preparations used in this study were diluted to titers of 1 × 1012 vector genomes (v.g.)/mL in phosphate buffered saline (PBS, pH 7.4) and were stored at 4°C.
Animal Experiment and Gene Delivery
Eighteen purebred male pigs (Animal Experimental Center of Chinese PLA General Hospital, Beijing, China), weighing 24.5–28.5 kg, 3 months of age, were housed at the institution for a minimum of 1 wk before use. Standard swine food was used for feeding. The animals were randomized into three groups. Four normal pigs underwent sham operation and served as the control group. The remaining 14 pigs served as 2 viral-administered groups and underwent ameroid constrictor implantation around the proximal of left anterior descending (LAD) coronary artery and received intramyocardial viral injection 4 wks later.
Ameroid-induced progressive coronary occlusion was performed as described previously (30). Briefly, initial sedation was achieved with intramuscularly ketamine hydrochloride (20 mg/kg), diazepam (0.05 mg/kg) and atropine (0.05 mg/kg). An ear vein was then cannulated for administration of an infusion of pentobarbital as needed to maintain anesthesia. The mini-pig was intubated and ventilated. A left lateral thoracotomy was performed through the fifth intercostal space. After opening the pericardium, an ameroid constrictor (2.5 mm, Research Instruments, Lebanon, OR, USA) was implanted around the proximal LAD for the viral-administered groups, but was not for the control group. Chronic myocardial ischemia was induced by progressive coronary artery occlusion with ameroid coronary constriction. The chest was closed after a stable position of the constrictor was confirmed.
Four weeks after ameroid placement, second thoracotomies were performed on the animal of the viral-administered groups, and rAAV1-SERCA2a and rAAV1-EGFP (1 × 1012 v.g. per pig, in 1 mL PBS solution, pH 7.4) were injected directly into the ischemic area along the two sides of the constricted LAD coronary artery (10 sites for each animal and 5 for each side with a 1-cm interval). The injection sites were marked with India ink for subsequent identification and protein analysis. Experimental protocols complied with the Guide for the Care and Use of Laboratory Animals of Chinese PLA General Hospital and were approved by the Chinese Academy of Sciences.
Assessment of Regional Myocardial Perfusion
Regional myocardial perfusion was evaluated at 4 wks (before viral administration) and 12 wks (8 wks after viral administration) after ameroid implantation by means of 99m Tc-sestamibi (99m Tc-MIBI) single photon emission computed tomography (SPECT). The pigs were placed in the dorsal (supine) position, and PET scans were performed with 15-cm axial field of view (Discovery LS, GE, Milwaukee, WI, USA) over the cardiac region. PET images were acquired in two- dimensional mode, starting 20 min after intravenous injections of a 740-MBq (20-mCi) bolus of 99m Tc-MIBI. During the PET scans, animals were maintained under general anesthesia as described above. Transaxial cardiac images were then reoriented into horizontal long-axis, vertical long-axis and short-axis images with a thickness of 4.25 mm by an experienced expert in nuclear medicine who was blinded to the treatment group. Three axis views were analyzed further.
Echocardiographic Assessment of Regional Myocardial Function
The same zones for each animal were analyzed by echocardiography at baseline, 4 wks and 12 wks after ameroid implantation. The pigs were sedated and placed in the left lateral decubitus position, and standard two-dimensional and M-mode transthoracic images were obtained with a Vivid 7 Dimension echocardiographic machine (GE Healthcare, Waukesha, WI, USA) and a 1.5- to 4.0-MHz frequency transthoracic transducer. From the right parasternal approach, short-axis, midpapillary views were obtained at rest for 3 min. Regional wall thickening, myocardial contractile function and wall motion were determined by a single experienced investigator in a blinded fashion. These images and parameters were recorded for subsequent review and analysis.
Measurement of Serum Brain Natriuretic Peptide Concentration
A blood sample was collected from each animal and put into plastic tubes containing anticoagulant (1:9, 0.129 mol/L trisodium citrate) at baseline, 4 wks and 12 wks after ameroid implantation. The citrated blood was immediately centrifuged at 1,760g for 10 min at room temperature to obtain plasma. The serum brain natriuretic peptide (BNP) concentrations were assayed using radioimmunoassay kits (Radioimmunity Institute, Chinese PLA General Hospital, Beijing, China) according to the procedure described by the manufacturer.
SERCA2a Activity Measurements
To investigate the effects of rAAV1-SERCA2a treatment on myocardial SERCA2a activity, proteins were extracted and quantified as previously described from heart tissue samples taken from the region of the left ventricular around the previously marked sites 8 wks after viral administration. Tissues in the same areas were taken in the control group for subsequent analysis (31). SERCA2a activity was measured using a Ca2+- ATPase assay kit (Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions. SERCA2a activity was normalized to protein concentration.
TUNEL and Immunohistochemistry Staining
Heart tissue samples were taken from the region of the left ventricle around the previously marked sites 8 wks after viral administration and cut into 4-μm-thick sections. Tissues in the same areas are taken in the control group. Some sections were stained with hematoxylin and eosin. Some sections were used for apoptotic assessment with a terminal deoxynucleotidyl transferase (TDT)-mediated DNA nick-end labeling (TUNEL) assay. Some sections were used for immunohistochemistry staining with GRP78 antibody.
Assessment for apoptosis was conducted using a commercially available TUNEL assay kit (Promega, Madison, WI, USA). Briefly, sections were deparaffinized, digested with proteinase K (20 mg/mL) at room temperature for 15 min and soaked in PBS for 5 min. Each section was covered with a TDT enzyme solution and incubated for 1 h at 37°C in a humidified chamber. The sections were immersed in stop buffer to terminate the enzymatic reaction and then gently rinsed with PBS. Streptavidin–horseradish peroxidase solution was applied to each section and then incubated at room temperature for 30 min in the darkness. Slides were washed in PBS and exposed to 3,3-diaminobenzidine (Golden Bridge Biotechnology, Beijing, China) for 5–7 min. The slides were then rinsed in water and counterstained with hematoxylin. The number of TUNEL-positive cells was counted in 10 randomly selected fields (400× magnifications) for each animal under ocular micrometers (Olympus Optical, Tokyo, Japan) by a blinded investigator without knowledge of groups.
The tissue expression of GRP78 was assessed immunohistochemically using an antibody (Santa Cruz, CA, USA). After deparaffinization, endogenous peroxidase activity was quenched with 30% methanol and 0.3% hydrogen PBS. The slides were then boiled in citrate buffer with microwaves. After blocking nonspecific binding with 5% BSA, the slides were incubated with primary antibodies overnight at 4°C (dilutions for anti-GRP78 1:400). The following day, the sections were thoroughly washed in PBS and incubated with a peroxidase-conjugated polymer that carries antibodies to goat (1:200) immunoglobulin (Golden Bridge Biotechnology) for 30 min. After rinsing with PBS, the sections were exposed to 3,3-diaminobenzidine for 7 min. The slides were rinsed in water and counter-stained with hematoxylin. The sections were examined using light microscopy and analyzed with a computer-assisted color image analysis system (Image- ProPlus 7.0, Media Cybernetics, MD, USA). The positive areas were assessed in at least 16 randomly selected tissue sections from each group studied.
Western Blotting
All five samples were taken from the ischemic heart region around the previously marked sites in one pig 8 wks after vector administration, and tissues in the same areas were taken in the control group for protein analysis. To investigate the effects of rAAV1-SERCA2a treatment on myocardial tissue protein expression, proteins were extracted, quantified and separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) as described previously (31). Blots were incubated with primary antibodies: anti-SERCA2a and anti-phospho-IRE1α (SER 724) (from Abcam, Cambridge, MA, USA) and anti-Bip/GFP78, anti-XBP1, anti-phospho PERK (Thr 981), anti-ATF6α, anti-phospho eIF2α (Ser 52), anti-PERK, anti-IRE1α, anti-CREB-2, anti-GADD 153, anti-caspase-12, anti-JNK, anti-phospho JNK (Thr183/Tyr185), anti-caspase-9, anti-Bcl-2, anti-Bax and anti-β-actin (all from Santa Cruz, CA, USA). The intensities of the various protein bands were quantified by densitometry.
Statistical Analysis
Data were expressed as the mean ± SEM. Comparisons of parameters were performed by one-way analysis of variance, followed by the Newman-Keuls test for unpaired data. Comparisons of parameters between two groups were made by unpaired Student t test. P values of <0.05 were regarded as statistically significant.
RESULTS
Overall Assessment
Of the 18 pigs in the study, 16 (control group, n = 4; IHD+EGFP group, n = 6; IHD+SERCA2a, n = 6) survived throughout the study, without any clinical signs of toxicity; 2 pigs died during the experimental surgery (1 died of ventricular fibrillation and the other died of uncontrollable hemorrhage caused by vessel injury during the procedure). The two viral administered groups (ameroid constriction of LAD coronary artery) exhibited evidence of chronic myocardial ischemia, as demonstrated by (a) a fixed defect in the LAD coronary artery zone of the 99m Tc-MIBI SPECT images; (b) a thinned, akinetic region of the left ventricular and decreased cardiac systolic and diastolic function during echocardiography; and (c) myocardial fibrosis and extensive focal coalescent areas of ischemic cardiomyocyte degeneration, in the hematoxylin and eosin staining of ischemic myocardial tissue.
SERCA2a Gene Delivery Restores the Expression and Activity of SERCA2a Proteins
The in vivo expression and activity of SERCA2a proteins were significantly decreased in the IHD+EGFP group compared with the control group. However, there were no significant differences in SERCA2a protein expression or activities between the control and IHD+SERCA2a group (Figure 1A, B). These results indicate that rAAV1-mediated SERCA2a gene delivery restores both the expression and activities of SERCA2a proteins in myocardial tissue.
Figure 1.
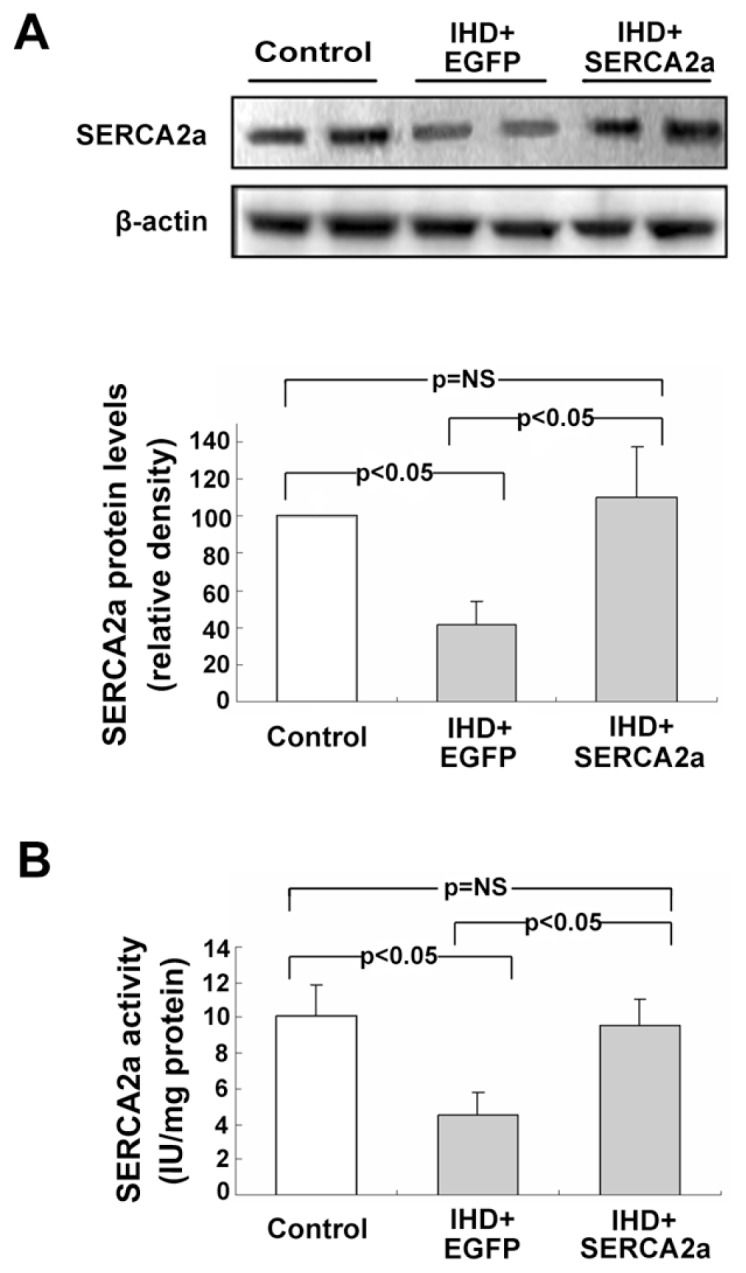
Myocardial expression and activity of SERCA2a protein after rAAV1-SERCA2a gene delivery. (A) Western blot and quantitative analysis of SERCA2a protein expression. β-Actin was used as an internal control. (B) Analysis of myocardial SERCA2a activity. Results show substantial decreases of SERCA2a protein and activity in the IHD+EGFP group compared with the control group, but activity was restored in the IHD+SERCA2a group. Data are presented as mean ± SEM (n = 4~6 per group).
rAAV1-SERCA2a Attenuates Apoptosis in Ischemic Myocardium
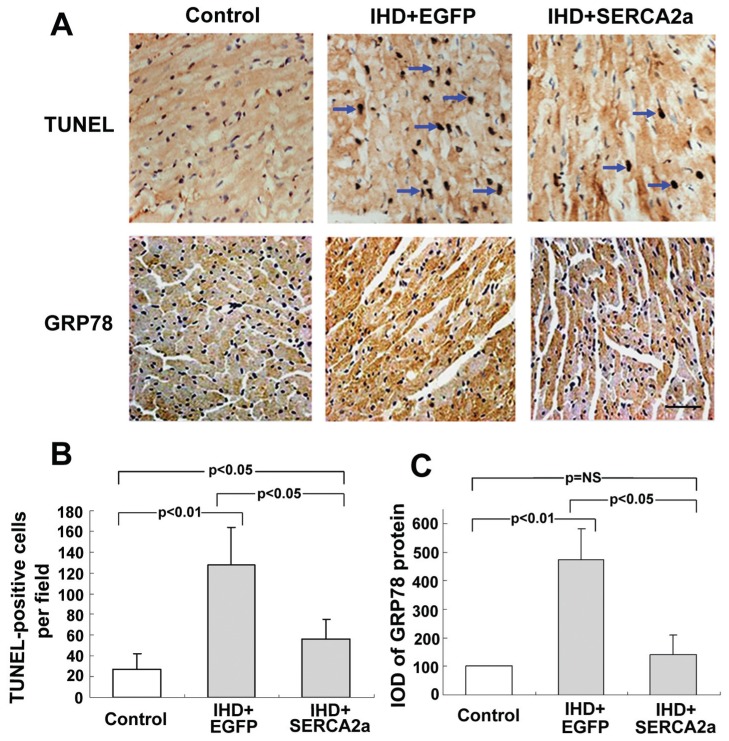
Apoptosis of myocardial cells was evaluated by TUNEL staining assay 8 wks after the viral transduction (Figure 2A, C). The SERCA2a gene, transferred to ischemic myocardium, attenuates the apoptosis of ischemic myocardium compared with the IHD+EGFP group, although the number of apoptotic myocardial cells is still greater in the IHD+SERCA2a group than in the control group.
Figure 2.
Restoration of SERCA2a expression attenuates myocardial apoptosis and reverses the increase of GRP78. (A) TUNEL staining of apoptotic myocardial cells (pointed by the arrows). (B) Immunohistochemical staining of GRP78 protein. The GRP78 proteins are stained in brown. (C) Quantitative analysis of TUNEL-positive cells. (D) Quantitative analysis of GRP78 density. Data are presented as mean ± SEM (n = 4~6 per group). IOD, integrated optical density.
Effects of rAAV1-SERCA2a Delivery on Myocardial Perfusion
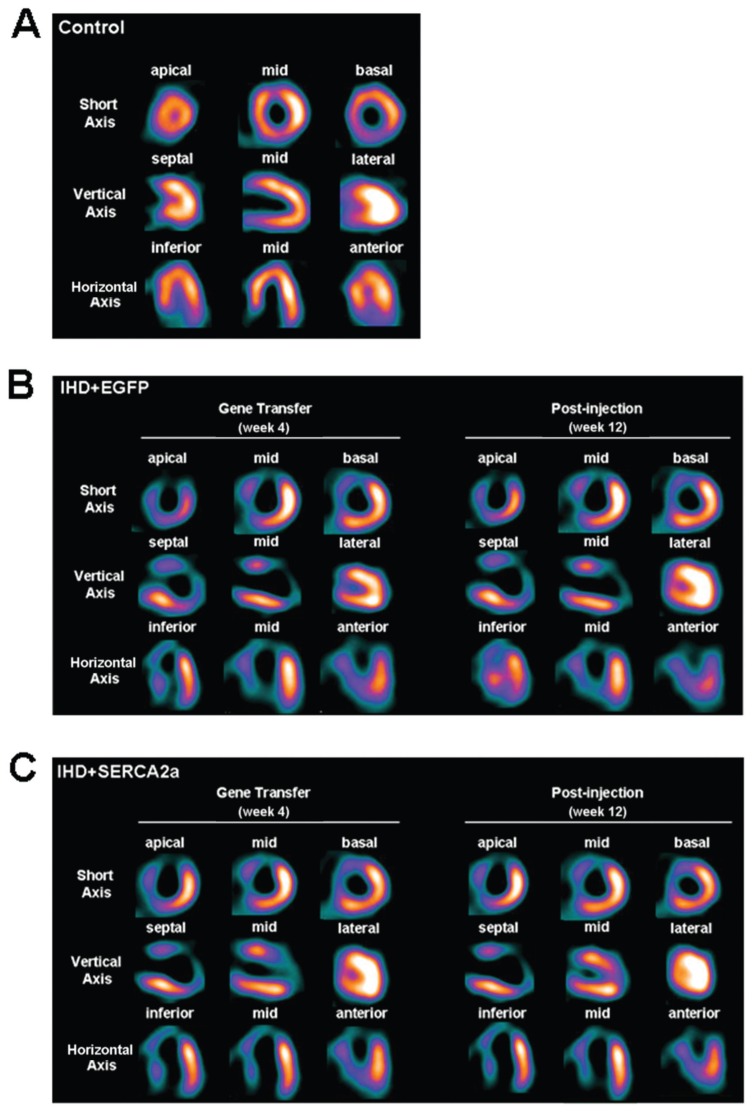
The 99m Tc-MIBI SPECT image data were used to display the myocardial perfusion and the severity of myocardial ischemia. The control group for myocardial perfusion in sham-operated pigs exhibited no detectable defect (Figure 3A). In the two viral transduced groups, SPECT images obtained at 4 wks after ameroid implantation displayed similar myocardial perfusion with a characteristic perfusion defect, mainly at apical, anterior and some septal regions. At 8 wks after viral administration, no significant improvement of myocardial perfusion could be detected in IHD+EGFP or IHD+SERCA2a groups (Figure 3B, C). Together, these data indicate that rAAV1-SERCA2a treatment has no obvious effect on myocardial blood perfusion after chronic myocardial ischemia.
Figure 3.
Effects of rAAV1-SERCA2a transfer in regional myocardial perfusion. (A) 99m Tc-MIBI SPECT images of control group. (B and C) 99m Tc-MIBI SPECT images of the IHD+EGFP and the IHD+SERCA2a groups. The control group exhibited no detectable defect. At 4 wks after ameroid implantation, the two viral-administered groups exhibited a significant myocardial perfusion defect in the LAD coronary artery–dependent zone. No significant improvement of myocardial perfusion was detected at 8 wks after rAAV1-SERCA2a transfer in the IHD+SERCA2a group.
Effects of rAAV1-SERCA2a Delivery on Myocardial Function and Serum BNP Levels
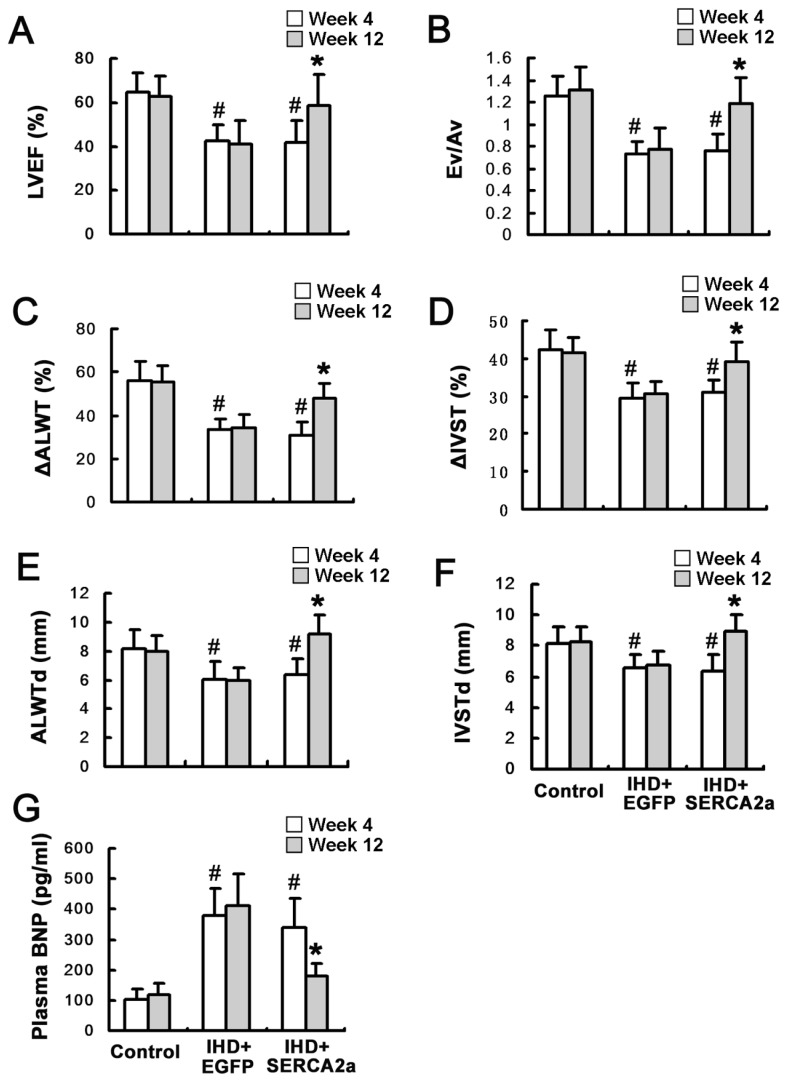
The effects of rAAV1-SERCA2a delivery on the myocardial function were examined by echocardiography. Cardiac systolic and diastolic function, regional wall motion and ventricular wall thickness were reduced 4 wks after ameroid implantation in the two viral administration groups compared with the control group (Figure 4A–F).
Figure 4.
Restoration of SERCA2a protein expression improves myocardial function. (A and B) Left ventricular ejection fraction (LVEF) and the ratio between early and atrial peak filling velocities (Ev/Av) showed that cardiac systolic and diastolic function was improved in the IHD+SERCA2a group 8 wks after viral administration. (C and D) Anterior lateral wall systolic thickening fraction (ΔALWT) and anterior lateral wall systolic thickening fraction (ΔIVST) showed the regional motion of anterior lateral and interventricular septal wall was improved in the IHD+SERCA2a group 8 wks after viral administration. (E and F) Anterior lateral wall diastolic thickness (ALWTd) and interventricular septal diastolic thickness (IVSTd) showed the thickness of anterior lateral and interventricular septal wall was increased in the IHD+SERCA2a group 8 wks after viral administration. (G) Serum BNP level was decreased in the IHD+SERCA2a group 8 wks after viral administration. Data are presented as mean ± SEM (n = 4~6 per group). #P < 0.05 versus control group; *P < 0.05 versus IHD+EGFP group.
In contrast, by 8 wks after vector administration (12 wks after ameroid implantation), the increase of left ventricular ejection fraction and the ratio between early and atrial peak filling velocities (Ev/Av) were found in the IHD+SERCA2a group compared with the IHD+EGFP group, indicating improvement in both systolic and diastolic myocardial function (Figure 4A, B). The regional wall motion, verified by the anterior lateral wall systolic thickening fraction and the interventricular septal systolic thickening fraction, showed further improvement in the IHD+SERCA2a group than in the IHD+EGFP group (Figure 4C, D). The septal and anterior lateral wall diastolic thickness, according to anterior lateral wall diastolic thickness and interventricular septal diastolic thickness, were increased in the IHD+SERCA2a group (Figure 4E, F). These results demonstrate that rAAV1-SERCA2a transfer results in enormous improvement of cardiac function and wall motion and a significant increase in wall thickness.
Serum levels of BNP, a biomarker of cardiac dysfunction, were also found to increase significantly 4 wks after ameroid implantation in the two viral administration groups compared with the control group (Figure 4G). However, by 8 wk after vector administration, serum BNP levels were significantly reduced in the IHD+SERCA2a group, paralleling an improvement of cardiac function compared with the IHD+EGFP group.
Effects of rAAV1-SERCA2a Delivery on ER Stress Pathways
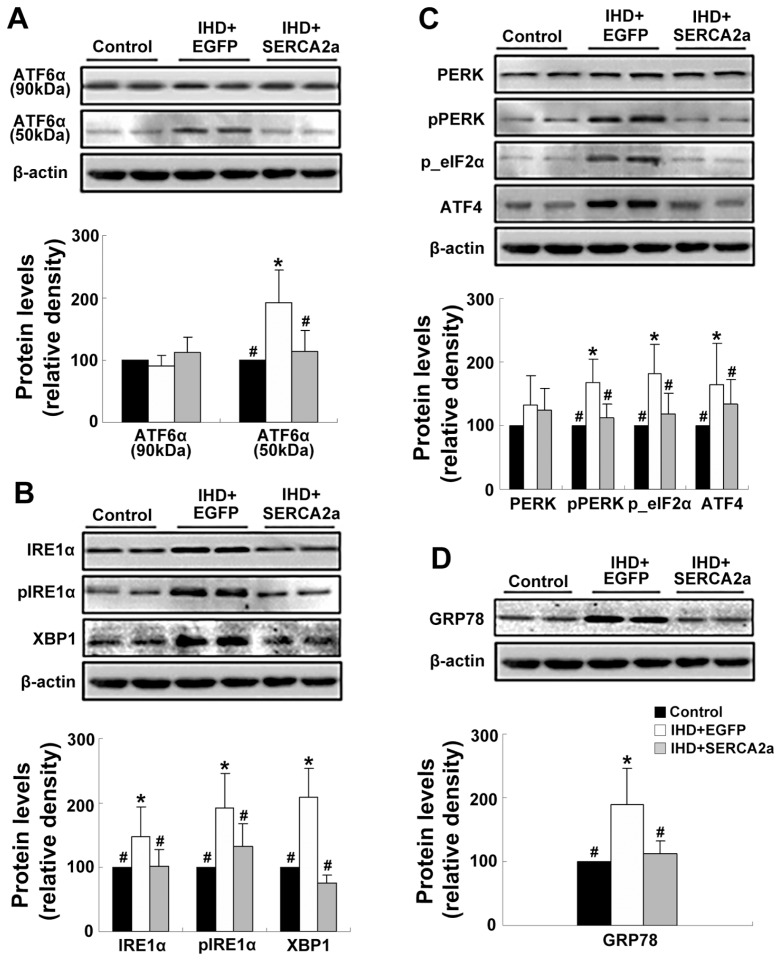
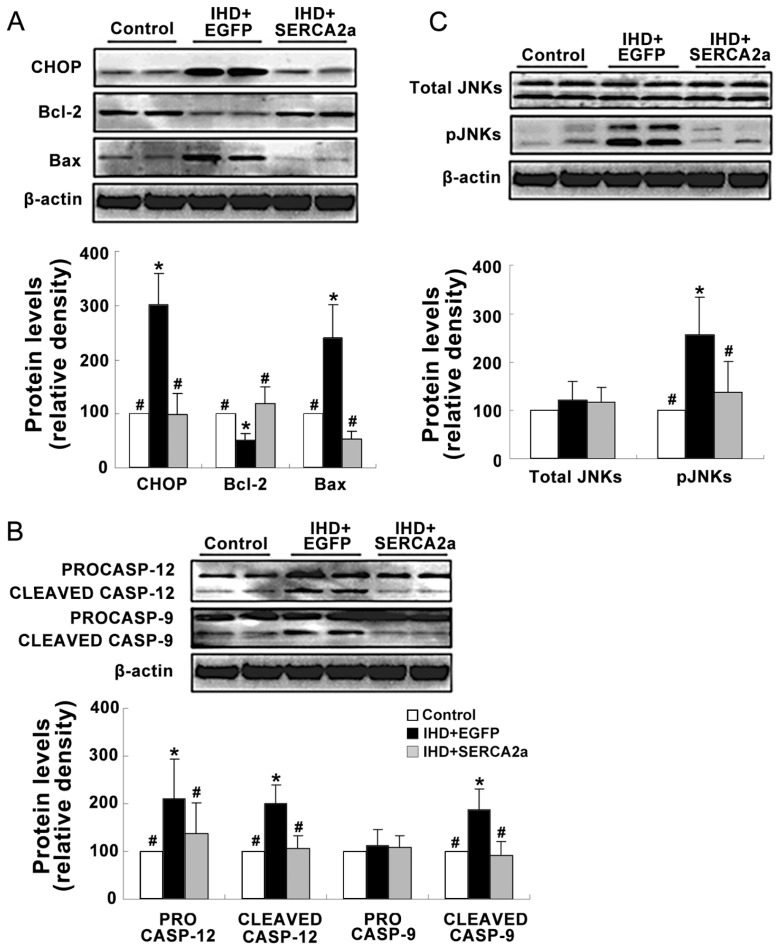
The effects of rAAV1-SERCA2a delivery on the UPR pathway and ER stress–related apoptosis pathways were examined. Western blot and immunohistochemistry staining results showed that the levels of the ER chaperones GRP78 and the components of the three arms of UPR pathway, such as ATF6α (50 kDa), phospho-IRE1α XBP1, phospho-PERK, phospho-eIFα and ATF4 were significantly increased in the IHD+EGFP group compared with the control group (Figure 2B, D; Figure 5). Moreover, the expressions of ER stress–related apoptotic proteins, including CHOP, cleaved caspase-12 and phospho-JNKs, were also enhanced in the IHD+EGFP group compared with the control group (Figure 6). However, changes of the above proteins were all reversed in the IHD+SERCA2a groups. Taken together, these results suggest that restoration of SERCA2a protein levels by rAAV1-SERCA2a treatment reverses the activation of UPR signals and ER stress–related apoptosis pathways in the ischemic myocardial tissue.
Figure 5.
Restoration of SERCA2a protein expression reverses the activation of UPR signaling pathways. (A) The protein levels of the ATF6α branch of UPR: ATF6α (90 kDa) and ATF6α (50 kDa). (B) The protein levels of the IRE1α branch of UPR: IRE1α, phospho-IRE1α and XBP-1. (C) The protein levels of the PERK branch of UPR: PERK, phospho-PERK, phospho-eIF2α and ATF4. (D) The protein levels of GRP78. Data are presented as mean ± SEM (n = 4~6 per group). *P < 0.05 versus control group; #P < 0.05 versus IHD+EGFP group.
Figure 6.
Restoration of SERCA2a protein expression reverses the activation of ER stress–related apoptosis pathways. (A) The protein levels of CHOP, Bcl-2 and Bax. (B) The protein levels of procaspase 9, 12 (CASP9 and CASP12) and cleaved caspase 9, 12. (C) The protein levels of total and phospho-JNKs. Data are presented as mean ± SEM (n = 4~6 per group). *P < 0.05 versus control group; #P < 0.05 versus IHD+EGFP group.
DISCUSSION
In the present study, with a porcine IHD model induced by chronic occlusion of LAD coronary artery, we found that the protein level and activity of SERCA2a in the ischemic myocardium was decreased. Furthermore, restoration of SERCA2a expression by intramyocardial rAAV1-SERCA2a gene delivery improved regional myocardial function and cardiac systolic and diastolic function. Further investigation demonstrated that all three arms of UPR and the ER stress–associated apoptosis pathways were activated in the myocardium of IHD pigs, and enhanced myocardial apoptosis was observed in the ischemic myocardium. However, restoration of SERCA2a expression reversed the activation of UPR and the ER stress–associated apoptosis pathways and attenuated myocardial apoptosis in IHD pigs, suggesting that the beneficial effects of SERCA2a gene transfer to IHD myocardium are associated with the reduction of ER stress–related myocardial apoptosis.
Abnormal calcium handling associated with a decrease in the expression and activity of SERCA2a is suggested to be one of the key abnormalities in a variety of cardiovascular disorders, including heart failure, acute myocardial ischemia and ischemia/perfusion, and chronic myocardial ischemia (3,29,32). A large number of studies in isolated cardiomyocytes and animal models of heart failure showed that restoring SERCA2a expression by gene transfer corrects the contractile dysfunction and energetic and electrical remodeling (29,32). After a long line of investigation, two clinical trials are underway using rAAV-SERCA2a to restore SERCA2a expression in patients with heart failure (33). However, the effects of SERCA2a gene delivery on IHD are still unknown. In this study, with ameroid constrictor–induced progressive occlusion of the LAD coronary artery, we made a chronic ischemic heart disease model in large animals to examine the effects of SERCA2a gene transfer. The results showed that intramyocardial injection of rAAV1 can lead to the efficient local gene delivery in a patchy pattern, with the highest level in the area of the injection sites, as indicated by EGFP fluorescence (data not shown), (consistent with our previous study in dogs with the same vectors [31]). Restoration of SERCA2a expression significantly improved the regional myocardial contractile function and wall thickness in the ischemic area and the overall cardiac systolic and diastolic function, indicating pro-hypertrophy may involve in the beneficial effects of SERCA2a. However, the heart weight/body weight (HW/BW) was not significantly different among the three groups 8 wks after gene delivery (data not shown), and perhaps more animals and longer observational periods are needed to show the difference. Recent evidence suggests that SERCA2a gene transfer increases the expression and activity of the endothelial isoform of nitric oxide synthase (eNOS) and improves vascular activity and coronary flow in the setting of mitral regurgitation–induced heart failure (34). We went on to examine the effects of SERCA2a gene transfer on regional myocardial perfusion. However, no significant improvement of myocardial perfusion could be detected by 99m Tc-MIBI SPECT at 8 wks after gene delivery. Because the LAD coronary artery in our model was occluded eventually, it was not a surprise to find that even if SERCA2a gene transfer could potentially improve the vascular activity, little beneficial effects could be detected on the regional myocardial perfusion.
It has been suggested that apoptosis plays an important role in the pathogenesis of IHD (35,36). Ischemia induces myocardial apoptosis, which causes the loss of cardiomyocytes, leading to the impairment of cardiac systolic and diastolic functions. A few studies demonstrated that ischemia, along with deprivation of oxygen, nutrition and energy supply, led to the activation of UPR-and ER stress–associated pathways in cultured cardiomyocytes or different animal models (23–25). In this study, we found that apoptosis was significantly enhanced in the ischemic myocardium, and the three arms of the UPR pathway mediated by the three transmembrane receptors, namely PERK, ATF6 and IRE1, were also activated as shown by the detection of their target molecules (37). We detected marked induction of XBP1 protein, a marker for the coordinated action of active ATF6 and IRE1 in the ischemic myocardium, suggesting the activation of these two branches. The PERK branch was also activated because the expression of phosphorylated eIF2α and subsequent ATF4 proteins were also enhanced. Additionally, GRP78, the central regulator of ER function and the master modulator for the UPR network by binding to the above three ER stress sensors, were also induced in the ischemic myocardium.
Although the UPR is primarily an adaptive response, if the stress persists, the ER stress receptors can also trigger proapoptotic pathways to initiate cell death (18). CHOP, caspase-12 and JNK are three well-defined apoptotic pathways related to ER stress. In our study, these apoptotic pathways were activated in the myocardium of IHD models, as shown by the induction of CHOP and cleaved caspase-12 proteins and enhanced phosphorylation of JNKs. The CHOP protein belongs to the C/EBP family of transcription factors, and it is transcriptionally induced during the development of ER stress by ATF6, PERK and IRE1 signaling (38,39). Our results are consistent with the earlier findings that showed that CHOP sensitized the cells to ER stress–induced apoptosis via downregulation of Bcl-2 expression (40,41). Caspase-12–mediated apoptosis was a specific apoptotic pathway of ER, and apoptosis that occurred as a result of membrane- or mitochondrial-targeted signals did not activate it (42). Cleaved caspase-12 reportedly activates caspase-9, followed by activation of caspase-3 (43). JNKs were activated during ER stress through phosphorylation mediated by the formation of the IRE1–tumor necrosis factor receptor–associated factor 2–apoptosis signal–regulating kinase 1 complex (44). Activation of JNKs is a common response to many forms of stress and is known to influence the cell-death machinery through the regulation of BCL2 family proteins (45). In our study, the above apoptotic pathways were all activated with the enhanced myocardial apoptosis, suggesting ER stress–associated apoptosis may be involved in the pathogenesis of IHD.
Interestingly, we found that SERCA2a gene delivery reversed the activation of the above UPR- and ER stress–related apoptotic pathways, with significant attenuation of apoptosis in the ischemic myocardium. These results suggested that the beneficial effects of SERCA2a gene transfer on IHD maybe involved the attenuation of ER stress–associated myocardial apoptosis. It has been reported that the expression and activity of SERCA2b, the other spliced protein of the SERCA2 gene, could be induced by the UPR pathway in the PC12 cell line during ER stress (26,27). In the cardiomyocytes, SERCA2a expression could be upregulated as a potential physiologically important compensatory mechanism by ATF6 in response to sarcoplasmic reticulum calcium depletion–induced ER stress (28). In our study, although the ATF6 branch of UPR was activated, we did not find an increased SERCA2a protein level in the IHD+EGFP group. This is not surprising, mainly because ischemia is a more complex pathophysiological process compared with in vitro sarcoplasmic reticulum calcium depletion, and many other mechanisms may affect SERCA2 expression besides the activation of ATF6. However, previous evidence suggests that SERCA2a protein may be an ER stress–induced protein, which may contribute to the restoration of ER calcium homeostasis to attenuate ER stress. Considering its important role in calcium uptake to the ER, it is likely that intramyocardial delivery of SERCA2a in our IHD model restored the ER calcium homeostasis and potentially attenuated ER stress–associated myocardial apoptosis. Further studies are needed to detect the direct connection of cellular calcium disturbance and ER stress–associated apoptosis, and the mechanisms and signal pathways involved in the beneficial effects of SERCA2a gene transfer in this setting are to be investigated.
Although the effects of SERCA2a gene transfer on the other apoptotic pathways still need to be determined, it can be concluded on the basis of our study that SERCA2a gene delivery in the myocardium of the porcine IHD model improves the regional myocardium function and overall cardiac systolic and diastolic function. The beneficial effects of SERCA2a to ischemic myocardium may involve the attenuation of ER stress–associated myocardial apoptosis. Our findings suggest the potential value of SERCA2a gene delivery as a treatment of chronic ischemic heart disease. Further studies are needed to determine the dose and time effects and the safety of rAAV1-SERCA2a gene transfer on ischemic heart disease.
ACKNOWLEDGMENTS
This study was supported by the National Basic Research Development Program of China, namely “973” Program (number 2007CB512004), and the National Science Foundation of China (numbers 30600236 and 30770900).
Footnotes
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
Online address: http://www.molmed.org
REFERENCES
- 1.Jiang H, Ge J. Epidemiology and clinical management of cardiomyopathies and heart failure in China. Heart. 2009;95:1727–31. doi: 10.1136/hrt.2008.150177. [DOI] [PubMed] [Google Scholar]
- 2.Rahimtoola SH, Dilsizian V, Kramer CM, Marwick TH, Vanoverschelde JL. Chronic ischemic left ventricular dysfunction: from pathophysiology to imaging and its integration into clinical practice. JACC Cardiovasc. Imaging. 2008;1:536–55. doi: 10.1016/j.jcmg.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Talukder MA, Zweier JL, Periasamy M. Targeting calcium transport in ischaemic heart disease. Cardiovasc Res. 2009;84:345–52. doi: 10.1093/cvr/cvp264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krause S, Hess ML. Characterization of cardiac sarcoplasmic reticulum dysfunction during short-term, normothermic, global ischemia. Circ Res. 1984;55:176–84. doi: 10.1161/01.res.55.2.176. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan P, Hendrikx M, Mattheussen M, Mubagwa K, Flameng W. Effect of ischemia and reperfusion on sarcoplasmic reticulum calcium uptake. Circ Res. 1992;71:1123–30. doi: 10.1161/01.res.71.5.1123. [DOI] [PubMed] [Google Scholar]
- 6.Mubagwa K. Sarcoplasmic reticulum function during myocardial ischaemia and reperfusion. Cardiovasc Res. 1995;30:166–75. [PubMed] [Google Scholar]
- 7.Zucchi R, et al. Sarcoplasmic reticulum calcium uptake in human myocardium subjected to ischemia and reperfusion during cardiac surgery. J Mol Cell Cardiol. 1996;28:1693–701. doi: 10.1006/jmcc.1996.0159. [DOI] [PubMed] [Google Scholar]
- 8.Miyamoto MI, et al. Adenoviral gene transfer of SERCA2a improves left-ventricular function in aortic-banded rats in transition to heart failure. Proc Natl Acad Sci U S A. 2000;97:793–8. doi: 10.1073/pnas.97.2.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.del MF, et al. Abrogation of ventricular arrhythmias in a model of ischemia and reperfusion by targeting myocardial calcium cycling. Proc Natl Acad Sci U S A. 2004;101:5622–7. doi: 10.1073/pnas.0305778101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrne MJ, et al. Recirculating cardiac delivery of AAV2/1SERCA2a improves myocardial function in an experimental model of heart failure in large animals. Gene Ther. 2008;15:1550–7. doi: 10.1038/gt.2008.120. [DOI] [PubMed] [Google Scholar]
- 11.Niwano K, et al. Lentiviral vector-mediated SERCA2 gene transfer protects against heart failure and left ventricular remodeling after myocardial infarction in rats. Mol Ther. 2008;16:1026–32. doi: 10.1038/mt.2008.61. [DOI] [PubMed] [Google Scholar]
- 12.Li XY, Hui HP, Lu XC, Guo YT. Treatment of chronic heart failure by overexpressing sarcoplasmic reticulum calcium ATPase through gene therapy: an experiment with rats. Zhonghua Yi Xue Za Zhi. 2006;86:1174–8. [PubMed] [Google Scholar]
- 13.Gupta D, et al. Improved exercise capacity and reduced systemic inflammation after adenoviral-mediated SERCA-2a gene transfer. J Surg Res. 2008;145:257–65. doi: 10.1016/j.jss.2007.03.081. [DOI] [PubMed] [Google Scholar]
- 14.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–33. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 15.Ferri KF, Kroemer G. Organelle-specific initiation of cell death pathways. Nat Cell Biol. 2001;3:E255–63. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- 16.Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002;110:1389–98. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ron D. Translational control in the endoplasmic reticulum stress response. J Clin Invest. 2002;110:1383–88. doi: 10.1172/JCI16784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115:2656–64. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernales S, Papa FR, Walter P. Intracellular signaling by the unfolded protein response. Annu Rev Cell Dev Biol. 2006;22:487–508. doi: 10.1146/annurev.cellbio.21.122303.120200. [DOI] [PubMed] [Google Scholar]
- 20.Wang XZ, et al. Signals from the stressed endoplasmic reticulum induce C/EBP-homologous protein (CHOP/GADD153) Mol Cell Biol. 1996;16:4273–80. doi: 10.1128/mcb.16.8.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hetz C, Russelakis-Carneiro M, Maundrell K, Castilla J, Soto C. Caspase-12 and endoplasmic reticulum stress mediate neurotoxicity of pathological prion protein. EMBO J. 2003;22:5435–45. doi: 10.1093/emboj/cdg537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hotamisligil GS. Role of endoplasmic reticulum stress and c-Jun NH2-terminal kinase pathways in inflammation and origin of obesity and diabetes. Diabetes 54 Suppl. 2005;2:S73–8. doi: 10.2337/diabetes.54.suppl_2.s73. [DOI] [PubMed] [Google Scholar]
- 23.Azfer A, Niu J, Rogers LM, Adamski FM, Kolattukudy PE. Activation of endoplasmic reticulum stress response during the development of ischemic heart disease. Am J Physiol Heart Circ Physiol. 2006;291:H1411–20. doi: 10.1152/ajpheart.01378.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szegezdi E, et al. ER stress contributes to ischemia-induced cardiomyocyte apoptosis. Biochem Biophys Res Commun. 2006;349:1406–11. doi: 10.1016/j.bbrc.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Thuerauf DJ, et al. Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes. Circ Res. 2006;99:275–82. doi: 10.1161/01.RES.0000233317.70421.03. [DOI] [PubMed] [Google Scholar]
- 26.Caspersen C, Pedersen PS, Treiman M. The sarco/endoplasmic reticulum calcium-ATPase 2b is an endoplasmic reticulum stress-inducible protein. J Biol Chem. 2000;275:22363–72. doi: 10.1074/jbc.M001569200. [DOI] [PubMed] [Google Scholar]
- 27.Hojmann LA, Frandsen A, Treiman M. Upregulation of the SERCA-type Ca2+ pump activity in response to endoplasmic reticulum stress in PC12 cells. BMC Biochem. 2001;2:4. doi: 10.1186/1471-2091-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thuerauf DJ, et al. Sarco/endoplasmic reticulum calcium ATPase-2 expression is regulated by ATF6 during the endoplasmic reticulum stress response: intracellular signaling of calcium stress in a cardiac myocyte model system. J Biol Chem. 2001;276:48309–17. doi: 10.1074/jbc.M107146200. [DOI] [PubMed] [Google Scholar]
- 29.Lipskaia L, Chemaly ER, Hadri L, Lompre AM, Hajjar RJ. Sarcoplasmic reticulum Ca(2+) ATPase as a therapeutic target for heart failure. Expert Opin Biol Ther. 2010;10:29–41. doi: 10.1517/14712590903321462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tuzun E, et al. Correlation of ischemic area and coronary flow with ameroid size in a porcine model. J Surg Res. 2009 doi: 10.1016/j.jss.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 31.Mi YF, et al. Improvement in cardiac function after sarcoplasmic reticulum Ca2+-ATPase gene transfer in a beagle heart failure model. Chin. Med. J. (Engl.) 2009;122:1423–8. [PubMed] [Google Scholar]
- 32.Kawase Y, Hajjar RJ. The cardiac sarcoplasmic/endoplasmic reticulum calcium ATPase: a potent target for cardiovascular diseases. Nat Clin Pract Cardiovasc Med. 2008;5:554–65. doi: 10.1038/ncpcardio1301. [DOI] [PubMed] [Google Scholar]
- 33.Hajjar R, Fuster V. Cardiac cell and gene therapies: two trajectories, one goal. Nat. Clin. Pract. Cardiovasc. Med. 2008;5:749. doi: 10.1038/ncpcardio1411. [DOI] [PubMed] [Google Scholar]
- 34.Hadri L, et al. SERCA2a gene transfer enhances eNOS expression and activity in endothelial cells. Mol Ther. 2010;18:1284–92. doi: 10.1038/mt.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dorn GW., 2nd Apoptotic and non-apoptotic programmed cardiomyocyte death in ventricular remodelling. Cardiovasc Res. 2009;81:465–73. doi: 10.1093/cvr/cvn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee Y, Gustafsson AB. Role of apoptosis in cardiovascular disease. Apoptosis. 2009;14:536–48. doi: 10.1007/s10495-008-0302-x. [DOI] [PubMed] [Google Scholar]
- 37.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–89. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 38.Wang XZ, et al. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J. 1998;17:5708–17. doi: 10.1093/emboj/17.19.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshida H, et al. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol Cell Biol. 2000;20:6755–67. doi: 10.1128/mcb.20.18.6755-6767.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zinszner H, et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–95. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oyadomari S, et al. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest. 2002;109:525–32. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakagawa T, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 43.Rao RV, et al. Coupling endoplasmic reticulum stress to the cell death program: an Apaf-1-independent intrinsic pathway. J Biol Chem. 2002;277:21836–42. doi: 10.1074/jbc.M202726200. [DOI] [PubMed] [Google Scholar]
- 44.Urano F, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–6. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 45.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–52. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]