Abstract
Alendronate (AL) is commonly used for prevention and treatment of osteoporotic fractures. Little is known about the effects of AL administration on osteoclast differentiation from human marrow progenitor cells. We used marrow discarded during orthopedic surgery to test the hypothesis that cultures of bone marrow-derived stem cells (BMCs) from subjects receiving AL (+AL) may differ from control subjects with respect to in vitro osteoclast differentiation and regulatory factors. The number of osteoclasts generated in BMC cultures from control subjects was 4.7-fold greater than +AL subjects (p = 0.015). The expression of RANKL in +AL BMCs was 57% of that in controls (p = 0.001), and the expression of OPG in +AL BMCs was greater than in controls (153%, p = 0.01). The mean RANKL/OPG ratio in BMCs was 0.65 ± 0.35 for +AL specimens and 1.28 ± 0.53 for Controls (p = 0.031). In addition, we assessed the direct effect of AL on expression of RANKL and OPG in marrow stromal cells isolated from 9 control women. Treatment with AL downregulated RANKL expression and upregulated expression of OPG, with an average 50% decrease in RANKL/OPG ratio at 10−7 M (p = 0.004). These studies show that osteoclast differentiation is dysregulated in marrow isolated from +AL subjects. Furthermore, these results demonstrate that AL may inhibit human osteoclastogenesis by affecting the key regulatory genes in marrow cells.
Keywords: Bisphosphonates, Alendronate, Osteoclasts, RANKL, OPG
Introduction
Alendronate (AL), a nitrogen-containing bisphosphonate (N-BP), is widely used for the treatment of osteoporosis [1, 2]. A number of clinical trials showed that AL decreases biomarkers of osteoclastic bone resorption, increases bone density, and reduces the incidence of fractures in osteoporotic patients [3–5].
Osteoclasts are large, multinucleated cells that resorb bone. Bone marrow contains non-adherent cells of the hematopoietic lineage with the capacity to differentiate into osteoclasts. MacDonald et al. were the first to develop methods for in vitro differentiation of human osteoclasts from bone marrow [6]. They also reported that marrow cultures from a patient with hyperparathyroidism and renal failure generated large numbers of osteoclasts and that the number was decreased with marrow taken after parathyroidectomy [6]. Subsequently, we reported an age-related increase in osteoclastogenesis in human marrow cultures [7]. Human marrow may reflect other aspects of the clinical status of the subject.
It has been established that differentiation of osteoclasts is regulated by factors produced by osteoblasts and marrow stromal cells, including the pro-osteoclastogenic receptor activator of nuclear factor-κB ligand (RANKL) and the inhibitory decoy receptor osteoprotegerin (OPG) [8].
Amino-bisphosphonates (N-BPs) inhibit bone resorption by inactivating osteoclasts. The major mechanism by which they inhibit bone resorption is by inducing apoptosis of mature osteoclasts [9]. In human bone biopsies from women treated with alendronate, Weinstein et al. described large, multinucleated, apoptotic cells as poorly-resorbing and detached from the bone [10]. In vitro, N-BPs induce apoptosis in murine osteoclasts [11] and their progenitors [12]. It has been shown that N-BP inhibition of protein prenylation is the mechanism of the apoptotic effect. After being internalized by osteoclasts, N-BPs inhibit farnesyl pyrophosphate synthase in the mevalonate pathway and block biosynthesis of small GTPases, such as Ras, Rho, and Rab [12, 13].
There is some evidence that BPs inhibit differentiation of osteoclasts in cultured segments of murine metatarsals [14]. The effects of BPs on human osteoclastogenesis are not clear. One study with human bone marrow cells showed that in vitro treatment with bisphosphonates inhibited the formation of osteoclast-like cells [15]. In this study, we tested the hypothesis that bone marrow cells from subjects who were receiving alendronate may differ from Control subjects with respect to in vitro osteoclast differentiation and expression of RANKL, OPG, and M-CSF, three key factors that regulate this process. In addition, we tested the direct effect of alendronate on RANKL and OPG expression in adherent human marrow stromal cells (MSCs).
Materials and Methods
Human bone marrow
Bone marrow samples were obtained with IRB approval as femoral tissue discarded from postmenopausal female subjects during primary hip arthroplasty for osteoarthritis [16, 17]. Criteria for exclusion were rheumatoid arthritis, cancer, and other comorbid conditions that may influence skeletal metabolism, such as renal insufficiency, alcoholism, active liver disease, malabsorption, hyperthyroidism, ankylosing spondylitis, aseptic necrosis, hyperparathyroidism, morbid obesity, and diabetes. Also excluded were patients who were taking medications other than bisphosphonates that may influence skeletal metabolism (e.g. thyroid hormone, glucocorticoids, and NSAIDs).
Bone Marrow Cultures for Osteoclast Differentiation
Marrow from 11 postmenopausal women who were receiving alendronate at the time of surgery (+AL) and 12 women without history of receiving bisphosphonates (Control) were used in the first part of the study. All reagents used in these studies were certified by suppliers as endotoxin-free. Low-density bone marrow cells (BMCs) were isolated as previously described [16, 17]. In brief, bone marrow was mixed with Ficoll-Histopaque 1077 (Sigma, St Louis, MO, USA) and centrifuged to isolate the low-density mononuclear population that is enriched for undifferentiated cells of the adherent mesenchymal and non-adherent hematopoietic lineages. It is common to use density centrifugation to remove mature marrow cells that may influence the progenitors [6, 15–18]. The seeding density used here (1.4 × 106 cells/cm2) was higher than in studies from other laboratories [6, 15] in order to optimize spontaneous generation of osteoclasts. In a preliminary time-course experiment, cells were cultured in 35-mm tissue culture dishes (1.4 × 106 cells/cm2) with α-MEM medium (Gibco, Grand Island, NY, USA) containing 10% Fetal Bovine Serum-heat inactivated (FBS-HI) (Invitrogen, Carlsbad, CA, USA), 100 U/ml penicillin, and 100 μg/ml streptomycin (Gibco, Grand Island, NY, USA) in a humidified atmosphere of 5% CO2, 95% air, at 37 °C. The cells were incubated ± 25 ng/ml recombinant human M-CSF (R&D Systems Inc., Minneapolis, MN, USA) [19–21], or 100 ng/ml recombinant human sRANKL (Pepro Tech, Inc., Rocky Hill, NJ, USA) [22] and osteoclasts were enumerated at intervals. In subsequent experiments, cells were seeded at 30 × 106 cells in 60-mm tissue culture dishes (1.4 × 106 cells/cm2) and incubated ± 25 ng/ml M-CSF for 14 days. Some samples were treated with 10−7 M alendronate (Sigma, St Louis, MO, USA), a concentration shown to inhibit bone resorption by in vitro generated osteoclasts [23]. Half-media was changed twice weekly with care to not remove non-adherent cells. Photomicrographs were captured with a Nikon inverted photomicroscope with Hoffman modulation contrast optics in order to emphasize margins of the cells.
Enumeration of multinucleated osteoclasts
Cultures were evaluated for development of multinucleated cells positive for tartrate-resistant acid phosphatase (TRAP) stain. In a time-course experiment, the BMC cells from Control and Alendronate subjects were cultured ± M-CSF (25 ng/ml) for 7, 10, and 14 days, or sRANKL (100ng/ml) for 14 days. For subsequent 14-day studies, BMC cultures from 5 Control subjects were cultured ± M-CSF (25 ng/ml) or AL (10−7 M). The BMCs from 5 Alendronate subjects (+AL) were cultured ± M-CSF. Cytochemical stain for TRAP was performed. Cultures were washed with phosphate-buffered saline (PBS; Gibco) and fixed for 15 minutes with 2% paraformaldehyde. They were rinsed twice with PBS, following which 3 ml of freshly filtered incubation medium (pH 5.0) was added for 45 minutes at 37°C. Incubation medium consisted of 50 ml 0.1 M acetate buffer, 10 ml 0.3 M sodium tartrate, 1 ml 10 mg/ml Naphthol AS-MX phosphate, 100 μl Triton X-100, 38.9 ml distilled water, and 0.3 mg/ml Fast Red Violet LB (Sigma, St Louis, MO, USA). The plates were rinsed with PBS (Gibco) and coverslipped with GVA Medium (Invitrogen, Carlsbad, CA, USA). The number of TRAP-positive multinucleated cells (cells with ≥ 3 nuclei) was counted in 90 contiguous fields, each representing an area of 1.23 mm2, with a Nikon Labophot microscope. This was done without knowledge of sample identity. The total number was expressed per cm2.
Similar cultures of BMCs from 10 Control and 7 +AL subjects were used for RT-PCR evaluation of expression of TRAP, Cathepsin-K, RANKL, and osteoprotegerin (OPG) genes.
Human marrow stromal cells
For the second part of this study, adherent marrow stromal cells from 9 control female subjects were used. The BMC preparations were plated as above, but non-adherent stem cells of the hematopoietic/osteoclast lineage were rinsed away after the first 24 hours of culture. The adherent marrow stromal cells (hMSCs) were expanded to passage 2 or 3 in 100-mm dishes with α-MEM medium containing 10% FBS-HI (Invitrogen), 100 U/ml penicillin, and 100 μg/ml streptomycin (Gibco). Upon 80% confluence, hMSCs were treated with AL (10−7 or 10−9 M) or 25 ng/ml M-CSF for 3 days. Total RNA was isolated and expression of RANKL, OPG, and M-CSF genes was evaluated.
RNA isolation and PCR
Total RNA was isolated from dishes of BMCs and hMSCs with Trizol reagent (Invitrogen). For RT-PCR, 2 μg of RNA was reverse-transcribed with Superscript II (Invitrogen) following the manufacturer’s instructions. One-twentieth of the cDNA was used in each 50 μl PCR reaction (30–40 cycles) with specific primers including GAPDH [24], Cathepsin-K [25], TRAP [25], OPG [24], RANKL (F:AACACGCGTATTTACAGCC,R:CCCCTTCAGATGATCCTTCA), and M-CSF (F:ATGACAGACAGGTGGAACTGCCAGTGTAGAGG,R:TCACACAACTTCAGTAGGTT CAGGTGATGGGC). Concentration of cDNA and amplification conditions were optimized for each gene product to reflect the exponential phase of amplification. PCR products were quantified by densitometry of captured agarose gel images with Kodak Gel Logic 200 imaging system and measured by Kodak molecular Imaging System (New Haven, CT, USA). Gene expression was calculated as relative to GAPDH.
Statistical Analyses
The data are expressed as means ± standard deviation (SD). Statistical analyses were performed with GraphPad InStat 3 (GraphPad Software Inc. La Jolla, CA, USA). Values were analyzed with paired Student’s t test and Bonferroni correction for multiple comparisons, Wilcoxon matched-pairs signed rank tests, ANOVA, Mann-Whitney test for non-parametric data, and Spearman correlation tests.
Results
Effect of bisphosphonate therapy in vivo on marrow differentiation of osteoclasts in vitro
A time-course study with cells from a Control (80-year-old) and +Al (84-year-old) subject showed that osteoclasts generated spontaneously (Figure 1), due to the establishment of a supportive stromal monolayer. Addition of exogenous M-CSF or sRANKL appeared to increase the number of osteoclasts. In subsequent experiments with more specimens, osteoclasts were enumerated at 14 days (Table 1). The mean age of the Control subjects was 70.4 ± 6.0 years and of the +AL subjects was 73.8 ± 10.5 years. More osteoclasts were generated in BMC cultures from Control subjects (546 ± 295 per cm2) than from +AL subjects (116 ± 99, p=0.015). As expected, addition of M-CSF increased the number of osteoclasts in the Control group (238%), but with the numbers available this was not statistically significant (p=0.187, paired t-test), because of one unresponsive specimen. The other samples showed stimulation by M-CSF (Figure 2A, B). Addition of M-CSF tended to increase osteoclast number in the +AL specimens 5.8-fold, but not with statistical significance (p=0.062, paired t-test). Some BMC cultures from Control subjects were treated with 10−7 M alendronate (Figure 2C). The number of osteoclasts generated in the presence of alendronate was 53% of basal osteoclastogenesis.
Figure 1.
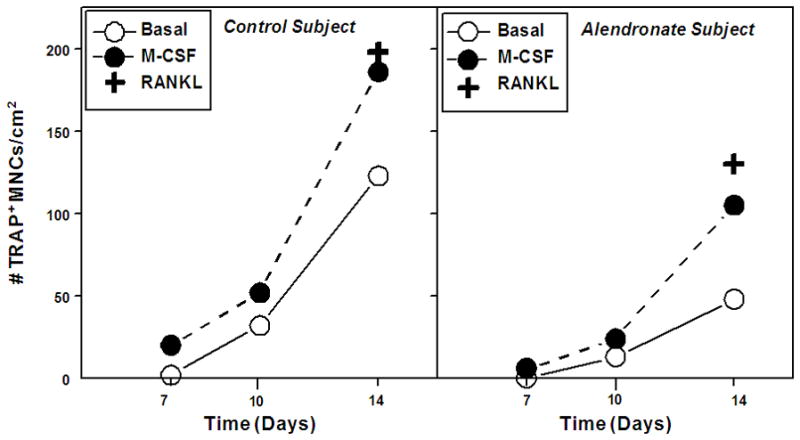
Time course of osteoclast generation in bone marrow cell cultures from Control (80-year-old woman) and Alendronate (84-year-old woman) subjects. Some cells were treated with 25 ng/ml M-CSF or 100 ng/ml sRANKL.
Table 1.
In vitro generation of TRAP-positive multinucleated cells in bone marrow cell cultures from age-matched patients who were receiving alendronate (+AL) (n=5) and Control group (n=5).
| Control | +AL | p value, Control vs. +AL | |
|---|---|---|---|
| Number of TRAP-Positive Multinucleated Cells/cm2 | |||
| Basal Medium | 547 ± 295 | 116 ± 99 | p=0.015 |
| M-CSF (25 ng/mL) | 964 ± 403 | 381 ± 206 | p=0.015 |
| % effect M-CSF/Basal (p value, paired) | 238 ± 174 (p=0.187) | 576 ± 450 (p=0.062) | |
| Alendronate (10−7 M) | 236 ± 257 | ||
| % effect Alendronate/Basal (p value, paired) | 53 ± 58 (p=0.187) | ||
Figure 2.
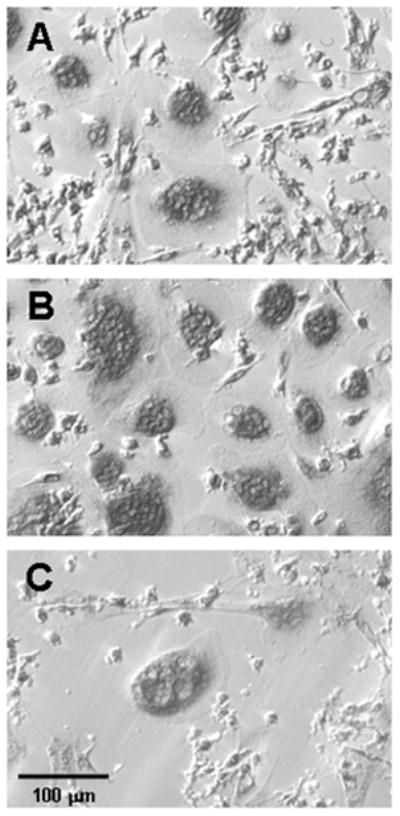
Photomicrograph of representative cultures of cells from a Control subject after staining for TRAP activity. Bone marrow cells were cultured for 2 weeks in (A) basal medium, (B) basal medium with 25 ng/ml M-CSF, and (C) basal medium with 10−7 M alendronate. Hoffman modulation contrast optics accentuate nuclear profiles and margins of the cells.
More sensitive than enumeration of cells, RT-PCR indices of osteoclast differentiation were determined for a series of BMC cultures from 10 Control and 7 +AL specimens (Figure 3). The mean age of these Control subjects was 70.8 ± 5.4 years and of the +AL subjects was 73.8 ± 6.8 years. Expression analysis of osteoclast marker genes, TRAP and Cathepsin-K (Cat-K), showed that +AL samples had lower expression of the osteoclast marker genes Cat-K (32%, p=0.01) and TRAP (48%, p=0.06). As expected, M-CSF significantly increased Cat-K and TRAP in both +AL (Cat-K: 2.7-fold, p=0.005) (TRAP: 3.3-fold, p=0.02) and Controls (Cat-K; 2.5-fold, p=0.01) (TRAP: 2.3-fold, p=0.01).
Figure 3.
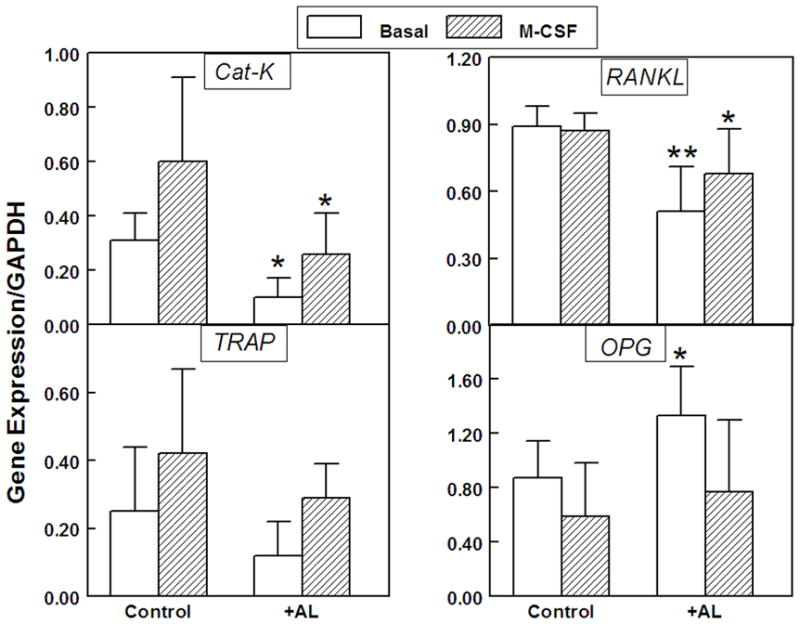
Expression of CAT-K and TRAP (left panel); and RANKL and OPG (right panel) after two weeks culture of bone marrow cells from women who were receiving alendronate (+AL, n=7) and Control group (n=10), with or without 25 ng/ml M-CSF. Data are shown as the mean ± standard deviation (SD) for each gene relative to expression of GAPDH. (**p=0.001, *p=0.01, for AL vs. Control)
For a set of 12 samples, data were available for all measures of osteoclastogenesis, including number of TRAP-positive multinucleated cells and expression of osteoclast marker genes, TRAP and Cat-K. Osteoclast cell counts were positively correlated with both osteoclast marker genes, TRAP (p=0.015) and Cat-K (p= 0.023) (Figure 4).
Figure 4.
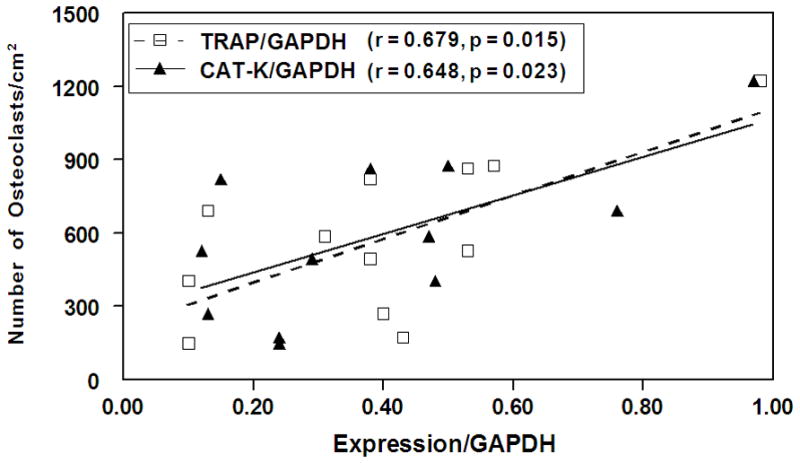
Spearman correlation of number of TRAP-positive osteoclasts with gene expression (Cat-K or TRAP) in bone marrow cell cultures (n=12). The number of osteoclasts was positively correlated with both Cat-K and TRAP expression.
Effect of bisphosphonate therapy in vivo on marrow expression of osteoclast regulatory genes in vitro
Expression of the pro-osteoclastogenic factor, RANKL, and the anti-osteoclastogenic factor, OPG, was assessed in BMC cultures for which TRAP and Cat-K expression were measured (Figure 3). Expression of pro-osteoclastogenic RANKL in +AL samples (0.51 ± 0.20) was 57% of that in Controls (0.89 ± 0.09, p=0.001). Expression of OPG in +AL samples (1.33 ± 0.36) was 153% of that in Controls (0.87 ± 0.27, p=0.01). The means of the RANKL/OPG ratios in BMCs were 0.65 ± 0.35 for +AL and 1.28 ± 0.53 for Controls (p = 0.031). Treatment with M-CSF resulted in suppression of OPG in the +AL group (50%, p=0.003) and in 8 of 10 Controls (62%, p=0.02).
Effects of alendronate in vitro on expression of osteoclast regulatory genes in human marrow stromal cells
To assess the direct in vitro effect of AL on the factors that regulate osteoclastogenesis, we treated human marrow stromal cell (hMSCs) from 9 Control subjects (mean age = 67.1 ± 8.1) with AL. First, we demonstrated that all samples of hMSCs expressed the pro-osteoclastic factors, RANKL and M-CSF, and the anti-osteoclastic factor, OPG, during 5 days of culture (data not shown). There was a wide range of basal expression of osteoclast regulatory genes. Basal expression of RANKL ranged 3.8-fold and expression of OPG ranged 7-fold, for the 9 samples. The mean of the RANKL/OPG ratios was 1.84 ± 0.40 (Figure 5). Analysis of effects of AL on RANKL/OPG ratio in MSCs showed significant decreases (p = 0.012 at 10−9 M and p = 0.004 at 10−7 M, ANOVA and Wilcoxon matched pairs signed rank tests). In this experiment, treatment of the cells with AL did not have significant effects on M-CSF expression at 1, 3, or 5 days (data not shown).
Figure 5.
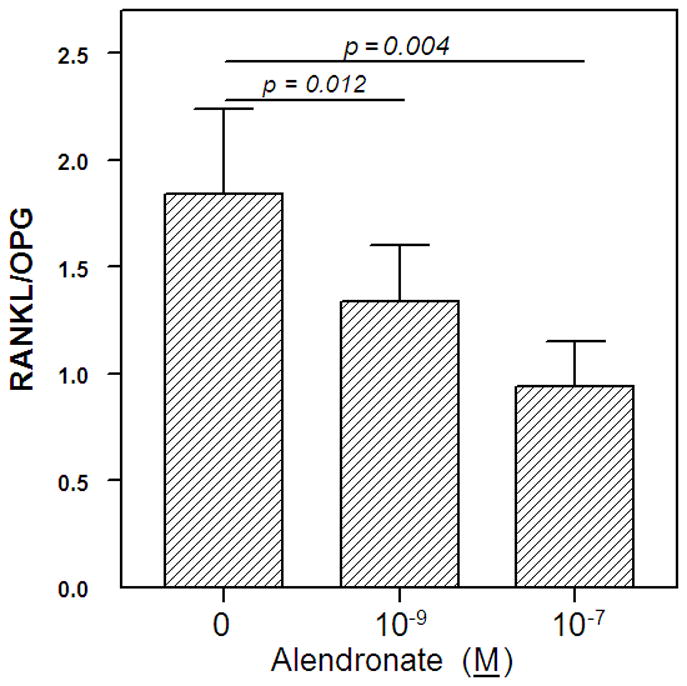
Effect of Alendronate on the RANKL/OPG ratio in marrow stromal cells. MSCs from 9 control subjects were treated with alendronate at 10−9 or 10−7 M for 3 days. Expression levels of RANKL and OPG were normalized to GAPDH; data are presented as the mean ± SEM for the ratios of RANKL/GAPDH to OPG/GAPDH.
Discussion
These data show that osteoclast differentiation was diminished in bone marrow cell cultures that were isolated from post-menopausal women receiving alendronate, compared with Control subjects. It is not entirely surprising that marrow behavior in vitro was correlated with in vivo status of the subjects. Many in vitro studies with human marrow show that isolated marrow cells retain aspects of the clinical status of the subject, such as an age-related increase in osteoclast differentiation [7] and in osteoclastogenic cytokines [26]; reduction of those cytokines in cells from women receiving estrogen replacement therapy [26]; an age-related decline in OPG expression [24]; reduced in vitro osteoclast differentiation following parathyroidectomy of a subject with hyperparathyroidism [6]; age and gender differences in WNT gene expression [27]; and age-related differences in proliferation, senescence markers, and osteoblast potential [17]. To our knowledge this is the first study to evaluate bone marrow cells from subjects receiving alendronate.
Large numbers of TRAP-positive multinucleated osteoclasts were generated spontaneously in BMC cultures from control subjects in basal medium. This is expected because of the high seeding density that was used and because of the advanced age of the subjects whose marrow was studied. We [7, 28] and others [29] reported that human osteoclast formation with bone marrow cells increases with subject age.
This study is the first to show that in vivo treatment with alendronate decreases in vitro differentiation of bone marrow cells to develop into osteoclasts. In contrast to this finding with human cells, Van Beek et al. reported that alendronate given to mice for 4 weeks suppressed in vivo bone resorption but did not affect the in vitro capacity of isolated bone marrow to develop into bone-resorbing osteoclasts [30]. In a more recent study with a different culture model, Van Beek et al. reported that alendronate and other N-BPs inhibited in vitro development of bone-resorbing osteoclasts in fetal mouse metatarsals [14]. M-CSF is often used in vitro with marrow cells to stimulate differentiation and activation of osteoclast progenitors [19–21]. In this study, treatment with M-CSF increased osteoclast differentiation in the control group, as expected. Because addition of M-CSF or RANKL also increased osteoclast differentiation of cells from +AL subjects, we conclude that suppression of in vitro osteoclastogenesis by in vivo administration of AL was not due to complete obliteration of osteoclast progenitors.
We showed that in vitro treatment with alendronate reduced generation of osteoclasts in specimens from Control subjects. This confirms the only related previous study with human bone marrow cell cultures [15]. Studies with co-culture models provide different conclusions. Breuil et al. used a co-culture model of human peripheral blood mononuclear cells with murine ST2 stromal cells on bone slices, and reported that alendronate inhibited bone resorption at ≥ 10−7 M but with an inconsistent effect on osteoclast number [23]. Murakami et al., using co-cultures of murine marrow and osteoblasts, concluded that alendronate inhibited osteoclastic resorption but did not inhibit murine osteoclast recruitment [31]. Both of those studies supplemented the medium with 1α,25-dihydroxyvitamin D3, which may alter responses to alendronate. There may be different effects for different N-BPs and differences attributable to species or source of osteoclast progenitors.
These experiments do not reveal the contribution of alendronate’s inhibition of osteoclastogenesis to its net effects on bone in vivo. In a recent analysis of bone biopsies from subjects in an alendronate trial, Weinstein et al. reported an unexpected apparent increase in the number of osteoclasts, including distinctive giant, hypernucleated, detached, and apoptotic osteoclasts in samples from subjects treated with 10 mg/day alendronate, compared with placebo [10]. Because markers of bone resorption were reduced in the treated group, the authors concluded that the osteoclast number was not an index of bone resorption and that the observed osteoclasts were not functionally resorptive. They speculated that alendronate may prolong the duration of osteoclast apoptosis. The in vitro findings described herein of reduced osteoclast differentiation with marrow from subjects receiving alendronate may appear to contrast with the biopsy findings. We speculate that there may have been an apparent increase in osteoclast number in the biopsies because of impaired efferocytosis of abnormal osteoclasts; the phagocytes that should be recruited to dispose of the apoptotic osteoclasts may themselves be inhibited by alendronate present in the bone microenvironment [32, 33]. It is possible that impaired osteoclast differentiation may predominate in the early course of bisphosphonate therapy and the in vivo accumulation of apoptotic and dysfunctional osteoclasts may prevail with prolonged treatment.
This study shows that alendronate in vivo is associated with reduced pro-osteoclastogenic RANKL and elevated anti-osteoclastogenic factor, OPG, in bone marrow. There is some information about clinical effects of alendronate on these factors. Dobnig et al. demonstrated a significant increase in serum levels of OPG in osteoporotic women who were treated with alendronate or risedronate for 6 and 12 months [34]. They reported that sRANKL levels remained unchanged at those times. D’Amelio et al. showed that after three months of treatment with risedronate, the number and activity of osteoclasts generated from peripheral blood mononuclear cells were significantly lower compared with a Control group [35]. They suggested that risedronate is able to reduce osteoclast recruitment from peripheral blood probably by altered production of regulatory cytokines. For a group of patients with Paget’s disease, Martini et al. reported increases in OPG levels in blood after 3 and 6 months treatment with pamidronate [36]. They also showed a trend toward a decrease in RANKL levels. Other data suggest that circulating OPG may reflect OPG activity in the bone [37, 38]. Given that OPG is a secreted molecule produced by marrow stromal cells and osteoblasts, it is conceivable that the circulating level of OPG is a meaningful indicator of the effects of bisphosphonates on bone marrow. The RANKL/OPG ratio is considered by many to be useful to monitor clinical suppression of osteoclastic bone resorption by bisphosphonates [34–36].
Our data indicate that in addition to direct stimulation of osteoclasts, M-CSF may downregulate the expression of OPG in human bone marrow cells. M-CSF was shown to be an important factor for the survival of osteoclasts and, in fact, is one of the mediators produced by osteoblasts and stromal cells that regulate osteoclasts [19–21]. Yamada et al. reported that M-CSF suppresses the production of OPG in mouse bone marrow macrophages and may act to increase the sensitivity of osteoclast progenitors to RANKL [39]. Many of the agents that stimulate osteoclast development, like 1,25-dihydroxyvitamin D and interleukins, act through a dual capacity to stimulate RANKL and inhibit OPG [40]. The data presented herein indicate that bisphosphonates act in reverse. This culture system intentionally does not include supplements that promote osteoblast differentiation, i.e. dexamethasone, β-glycerophosphate, and ascorbate-phosphate. Evidence suggests that marrow stromal cells [41] and osteoblasts [42] support osteoclast differentiation by producing regulatory factors and that the RANKL/OPG ratio varies under different conditions [8]. The data presented herein demonstrate that constitutive levels of expression of RANKL and OPG in marrow depend on whether the subject had been treated with alendronate.
In the second part of this study, we tested whether alendronate directly affected RANKL, OPG, and M-CSF expression in cultures of adherent human marrow stromal cells (hMSCs). There is no obvious explanation for the range of basal expression in this series of hMSCs from post-menopausal women who met the inclusion criteria, but it may reflect the range of inherent rates of bone turnover. The effect of alendronate in the 9 samples of MSCs tested here was related to the basal level of gene expression. Alendronate’s inhibition of RANKL was more consistent in samples that had higher basal levels of RANKL. More information may be gained by evaluation of other marrow cells, such as lymphocytes. Eghbali-Fatourechi et al. noted that expression of RANKL in MSCs, as well as in marrow-derived T cells and B-cells, was related to bone turnover, age, and estrogen status of the subject whose cells were studied [43]. The effects of alendronate on human marrow cells may be different from those reported for murine cells, for osteoblasts, or for other N-BPs. Nishida et al. demonstrated that minodronic acid inhibited osteoclast formation by downregulating RANKL in murine ST2 stromal cells [44]. Using murine calvarial osteoblasts, however, Kim et al. reported that the inhibitory action of alendronate and pamidronate on bone resorption did not involve the expression of RANKL and OPG after one day of treatment; after 2 days, RANKL was unexpectedly increased by both N-BPs and OPG expression was slightly increased by 10−7 and 10−6 M of pamidronate [45]. Because of the resulting increase, rather than decrease in the ratio of RANKL/OPG, the authors suggested that RANKL and OPG may not be the mechanism by which bisphosphonates inhibit bone resorption. On the other hand, Viereck et al. reported that zoledronic acid and pamidronate upregulated OPG expression in primary human osteoblasts after 3 days [46]. Pan et al. showed that treatment of primary human osteoblasts with zoledronate led to a significant decrease in the expression of transmembrane RANKL and a significant increase in the level of secreted OPG [47]. Mackie et al. reported that pamidronate downregulated RANKL in UMR-106 rat osteosarcoma cells and had no effect on OPG expression [48]. Kobayashi et al. showed that alendronate inhibited secretion of dexamethasone-induced sRANKL protein by MG-63 cells, a line of human osteosarcoma cells [49]. Other cytokines, such as TNF [50], are involved in modulating osteoclast differentiation, but were not evaluated in this study.
In conclusion, these studies demonstrate that marrow from subjects receiving alendronate had lower in vitro differentiation of osteoclasts and altered expression of osteoclast regulatory factors RANKL and OPG, compared with marrow from age- and gender-matched controls. These results show that osteoclast differentiation is dysregulated in marrow isolated from subjects receiving alendronate. These findings show that alendronate has inhibitory effects on osteoclast differentiation in addition to its known action to promote apoptosis of mature osteoclasts. Furthermore, this study affirms the usefulness of human bone marrow cultures to investigate at the cellular level the clinical effects of therapeutic interventions.
Acknowledgments
The authors are indebted to the orthopedic surgeons and operating room staff at the Brigham and Women’s Hospital for their assistance in specimen procurement.
Funding Source: This work was supported by grants from the NIH (R01 AG025015 and R01 AG028114).
Footnotes
Disclosures: None
References
- 1.Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, Rodriguez-Portales JA, Downs RW, Gupta J, Santora AC, Liberman UA. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189–1199. doi: 10.1056/NEJMoa030897. [DOI] [PubMed] [Google Scholar]
- 2.Bone HG, Downs RW, Jr, Tucci JR, Harris ST, Weinstein RS, Licata AA, McClung MR, Kimmel DB, Gertz BJ, Hale E, Polvino WJ. Dose-response relationships for alendronate treatment in osteoporotic elderly women. Alendronate Elderly Osteoporosis Study Centers. J Clin Endocrinol Metab. 1997;82:265–74. doi: 10.1210/jcem.82.1.3682. [DOI] [PubMed] [Google Scholar]
- 3.Liberman UA, Weiss SR, Bröll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs RW, Jr, Dequeker J, Favus M. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995;333:1437–1443. doi: 10.1056/NEJM199511303332201. [DOI] [PubMed] [Google Scholar]
- 4.Tonino RP, Meunier PJ, Emkey R, Rodriguez-Portales JA, Menkes CJ, Wasnich RD, Bone HG, Santora AC, Wu M, Desai R, Ross PD. Skeletal benefits of alendronate: 7-year treatment of postmenopausal osteoporotic women. Phase III Osteoporosis Treatment Study Group. J Clin Endocrinol Metab. 2000;85:3109–3115. doi: 10.1210/jcem.85.9.6777. [DOI] [PubMed] [Google Scholar]
- 5.Orwoll E, Ettinger M, Weiss S, Miller P, Kendler D, Graham J, Adami S, Weber K, Lorenc R, Pietschmann P, Vandormael K, Lombardi A. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343:604–610. doi: 10.1056/NEJM200008313430902. [DOI] [PubMed] [Google Scholar]
- 6.MacDonald BR, Takahashi N, McManus LM, Holahan J, Mundy GR, Roodman GD. Formation of multinucleated cells that respond to osteotropic hormones in long term human bone marrow cultures. Endocrinology. 1987;120:2326–2333. doi: 10.1210/endo-120-6-2326. [DOI] [PubMed] [Google Scholar]
- 7.Glowacki J. Influence of age on human marrow. Calcif Tissue Int. 1995;1:S50–51. [Google Scholar]
- 8.Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142:5050–5055. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- 9.Russell RG. Bisphosphonates: mode of action and pharmacology. Pediatrics. 2007;119:150–62. doi: 10.1542/peds.2006-2023H. [DOI] [PubMed] [Google Scholar]
- 10.Weinstein RS, Roberson PK, Manolagas SC. Giant osteoclast formation and long-term oral bisphosphonate therapy. N Engl J Med. 2009;360:53–62. doi: 10.1056/NEJMoa0802633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher JE, Rogers MJ, Halasy JM, Luckman SP, Hughes DE, Masarachia PJ, Wesolowski G, Russell RG, Rodan GA, Reszka AA. Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro. Proc Natl Acad Sci USA. 1999;96:133–138. doi: 10.1073/pnas.96.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13:581–589. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- 13.Dunford JE, Thompson K, Coxon FP, Luckman SP, Hahn FM, Poulter CD, Ebetino FH, Rogers MJ. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther. 2001;296:235–242. [PubMed] [Google Scholar]
- 14.Van Beek ER, Löwik CW, Papapoulos SE. Bisphosphonates suppress bone resorption by a direct effect on early osteoclast precursors without affecting the osteoclastogenic capacity of osteogenic cells: the role of protein geranylgeranylation in the action of nitrogen-containing bisphosphonates on osteoclast precursors. Bone. 2002;30:64–70. doi: 10.1016/s8756-3282(01)00655-x. [DOI] [PubMed] [Google Scholar]
- 15.Hughes DE, MacDonald BR, Russell RG, Gowen M. Inhibition of osteoclast-like cell formation by bisphosphonates in long-term cultures of human bone marrow. J Clin Invest. 1989;83:1930–1935. doi: 10.1172/JCI114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon CM, LeBoff MS, Glowacki J. Adrenal and gonadal steroids inhibit IL-6 secretion by human marrow cells. Cytokine. 2001;16:178–186. doi: 10.1006/cyto.2001.0962. [DOI] [PubMed] [Google Scholar]
- 17.Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, Leboff MS, Glowacki J. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7:335–343. doi: 10.1111/j.1474-9726.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thavarajah M, Evans DB, Binderup L, Kanis JA. 1,25(OH)2D3 and calcipotriol (MC903) have similar effects on the induction of osteoclast-like cell formation in human bone marrow cultures. Biochem Biophys Res Commun. 1990;171:1056–63. doi: 10.1016/0006-291x(90)90791-k. [DOI] [PubMed] [Google Scholar]
- 19.MacDonald BR, Mundy GR, Clark S, Wang EA, Kuehl TJ, Stanley ER, Roodman GD. Effects of human recombinant CSF-GM and highly purified CSF-1 on the formation of multinucleated cells with osteoclast characteristics in long-term bone marrow cultures. J Bone Miner Res. 1989;1:227–233. doi: 10.1002/jbmr.5650010210. [DOI] [PubMed] [Google Scholar]
- 20.Liggett W, Jr, Shevde N, Anklesaria P, Sohoni S, Greenberger J, Glowacki J. Effects of macrophage colony stimulating factor and granulocyte-macrophage colony stimulating factor on osteoclastic differentiation of hematopoietic progenitor cells. Stem Cells. 1993;11:398–411. doi: 10.1002/stem.5530110507. [DOI] [PubMed] [Google Scholar]
- 21.Sarma U, Flanagan AM. Macrophage colony-stimulating factor induces substantial osteoclast generation and bone resorption in human bone marrow cultures. Blood. 1996;88:2531–2540. [PubMed] [Google Scholar]
- 22.Mabilleau G, Sabokbar A. Interleukin-32 promotes osteoclast differentiation but not osteoclast activation. PLoS One. 2009;4:e4173. doi: 10.1371/journal.pone.0004173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breuil V, Cosman F, Stein L, Horbert W, Nieves J, Shen V, Lindsay R, Dempster DW. Human osteoclast formation and activity in vitro: effects of alendronate. J Bone Miner Res. 1998;13:1721–1729. doi: 10.1359/jbmr.1998.13.11.1721. [DOI] [PubMed] [Google Scholar]
- 24.Makhluf HA, Mueller SM, Mizuno S, Glowacki J. Age-related decline in osteoprotegerin expression by human bone marrow cells cultured in three-dimensional collagen sponges. Biochem Biophys Res Commun. 2000;268:669–672. doi: 10.1006/bbrc.2000.2182. [DOI] [PubMed] [Google Scholar]
- 25.Wittrant Y, Couillaud S, Theoleyre S, Dunstan C, Heymann D, Rédini F. Osteoprotegerin differentially regulates protease expression in osteoclast cultures. Biochem Biophys Res Commun. 2002;293:38–44. doi: 10.1016/S0006-291X(02)00179-1. [DOI] [PubMed] [Google Scholar]
- 26.Cheleuitte D, Mizuno S, Glowacki J. In vitro secretion of cytokines by human bone marrow: effects of age and estrogen status. J Clin Endocrinol Metab. 1998;83:2043–2051. doi: 10.1210/jcem.83.6.4848. [DOI] [PubMed] [Google Scholar]
- 27.Shen L, Zhou S, Glowacki J. Effects of age and gender on WNT gene expression in human bone marrow stromal cells. J Cellular Biochem. 2009;106:337–43. doi: 10.1002/jcb.22010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung P-lB, Shen L, Glowacki J. Effect of age on regulation of human osteoclast differentiation. J Bone Mineral Res. 2009;24:S192. doi: 10.1002/jcb.24792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koshihara Y, Suematsu A, Feng D, Okawara R, Ishibashi H, Yamamoto S. Osteoclastogenic potential of bone marrow cells increases with age in elderly women with fracture. Mech Ageing Dev. 2002;123:1321–31. doi: 10.1016/s0047-6374(02)00071-4. [DOI] [PubMed] [Google Scholar]
- 30.Van Beek ER, Löwik CW, Papapoulos SE. Effect of alendronate treatment on the osteoclastogenic potential of bone marrow cells in mice. Bone. 1997;20:335–340. doi: 10.1016/s8756-3282(97)00006-9. [DOI] [PubMed] [Google Scholar]
- 31.Murakami H, Takahashi N, Sasaki T, Udagawa N, Tanaka S, Nakamura I, Zhang D, Barbier A, Suda T. A possible mechanism of the specific action of bisphosphonates on osteoclasts: tiludronate preferentially affects polarized osteoclasts having ruffled borders. Bone. 1995;17:137–144. doi: 10.1016/s8756-3282(95)00150-6. [DOI] [PubMed] [Google Scholar]
- 32.Glowacki J. The deceiving appearances of osteoclasts. New Engl J Med. 2009;360:80–82. doi: 10.1056/NEJMe0806271. [DOI] [PubMed] [Google Scholar]
- 33.Glowacki J. Whither goest the lifeless osteoclast? Intl J Clin Rheumatol. 2009;4:510–22. [Google Scholar]
- 34.Dobnig H, Hofbauer LC, Viereck V, Obermayer-Pietsch B, Fahrleitner-Pammer A. Changes in the RANK ligand/osteoprotegerin system are correlated to changes in bone mineral density in bisphosphonate-treated osteoporotic patients. Osteoporos Int. 2006;17:693–703. doi: 10.1007/s00198-005-0035-4. [DOI] [PubMed] [Google Scholar]
- 35.D’Amelio P, Grimaldi A, Di Bella S, Tamone C, Brianza SZ, Ravazzoli MG, Bernabei P, Cristofaro MA, Pescarmona GP, Isaia G. Risedronate reduces osteoclast precursors and cytokine production in postmenopausal osteoporotic women. J Bone Miner Res. 2008;23:373–379. doi: 10.1359/jbmr.071031. [DOI] [PubMed] [Google Scholar]
- 36.Martini G, Gennari L, Merlotti D, Salvadori S, Franci MB, Campagna S, Avanzati A, De Paola V, Valleggi F, Nuti R. Serum OPG and RANKL levels before and after intravenous bisphosphonate treatment in Paget’s disease of bone. Bone. 2007;40:457–463. doi: 10.1016/j.bone.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Yano K, Tsuda E, Washida N, Kobayashi F, Goto M, Harada A, Ikeda K, Higashio K, Yamada Y. Immunological characterization of circulating osteoprotegerin/osteoclastogenesis inhibitory factor: increased serum concentrations in postmenopausal women with osteoporosis. J Bone Miner Res. 1999;14:518–527. doi: 10.1359/jbmr.1999.14.4.518. [DOI] [PubMed] [Google Scholar]
- 38.Ueland T, Brixen K, Mosekilde L, Mosekilde L, Flyvbjerg A, Bollerslev J. Age-related changes in cortical bone content of insulin-like growth factor binding protein (IGFBP)-3, IGFBP-5, osteoprotegerin, and calcium in postmenopausal osteoporosis: a cross-sectional study. J Clin Endocrinol Metab. 2003;88:1014–1018. doi: 10.1210/jc.2002-020977. [DOI] [PubMed] [Google Scholar]
- 39.Yamada N, Tsujimura T, Ueda H, Hayashi S, Ohyama H, Okamura H, Terada N. Down-regulation of osteoprotegerin production in bone marrow macrophages by macrophage colony-stimulating factor. Cytokine. 2005;31:288–297. doi: 10.1016/j.cyto.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 40.Aubin JE, Bonnelye E. Osteoprotegerin and its ligand: a new paradigm for regulation of osteoclastogenesis and bone resorption. Osteoporos Int. 2000;11:905–913. doi: 10.1007/s001980070028. [DOI] [PubMed] [Google Scholar]
- 41.Gori F, Hofbauer LC, Dunstan CR, Spelsberg TC, Khosla S, Riggs BL. The expression of osteoprotegerin and RANK ligand and the support of osteoclast formation by stromal-osteoblast lineage cells is developmentally regulated. Endocrinology. 2000;141:4768–4776. doi: 10.1210/endo.141.12.7840. [DOI] [PubMed] [Google Scholar]
- 42.Atkins GJ, Kostakis P, Welldon KJ, Vincent C, Findlay DM, Zannettino AC. Human trabecular bone-derived osteoblasts support human osteoclast formation in vitro in a defined, serum-free medium. J Cell Physiol. 2005;203:573–82. doi: 10.1002/jcp.20255. [DOI] [PubMed] [Google Scholar]
- 43.Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest. 2003;111:1221–1230. doi: 10.1172/JCI17215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishida S, Tsubaki M, Hoshino M, Namimatsu A, Uji H, Yoshioka S, Tanimori Y, Yanae M, Iwaki M, Irimajiri K. Nitrogen-containing bisphosphonate, YM529/ONO-5920 (a novel minodronic acid), inhibits RANKL expression in a cultured bone marrow stromal cell line ST2. Biochem Biophys Res Commun. 2005;328:91–97. doi: 10.1016/j.bbrc.2004.12.145. [DOI] [PubMed] [Google Scholar]
- 45.Kim YH, Kim GS, Jeong-Hwa B. Inhibitory action of bisphosphonates on bone resorption does not involve the regulation of RANKL and OPG expression. Exp Mol Med. 2002;34:145–151. doi: 10.1038/emm.2002.21. [DOI] [PubMed] [Google Scholar]
- 46.Viereck V, Emons G, Lauck V, Frosch KH, Blaschke S, Gründker C, Hofbauer LC. Bisphosphonates pamidronate and zoledronic acid stimulate osteoprotegerin production by primary human osteoblasts. Biochem Biophys Res Commun. 2002;291:680–686. doi: 10.1006/bbrc.2002.6510. [DOI] [PubMed] [Google Scholar]
- 47.Pan B, Farrugia AN, To LB, Findlay DM, Green J, Lynch K, Zannettino AC. The nitrogen-containing bisphosphonate, zoledronic acid, influences RANKL expression in human osteoblast-like cells by activating TNF-alpha converting enzyme (TACE) J Bone Miner Res. 2004;19:147–154. doi: 10.1359/jbmr.2004.19.1.147. [DOI] [PubMed] [Google Scholar]
- 48.Mackie PS, Fisher JL, Zhou H, Choong PF. Bisphosphonates regulate cell growth and gene expression in the UMR 106-01 clonal rat osteosarcoma cell line. Br J Cancer. 2001;84:951–958. doi: 10.1054/bjoc.2000.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kobayashi A, Hirano F, Makino I. The inhibitory effect of bisphosphonates on glucocorticoid-induced RANKL expression in human cells. Scand J Rheumatol. 2005;34:480–484. doi: 10.1080/03009740510026788. [DOI] [PubMed] [Google Scholar]
- 50.Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000;106:1481–8. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]


