Abstract
The gut hormone peptide YY(3–36)-amide [PYY(3–36)-NH2] is significantly more potent than PYY(1–36)-NH2 in reducing food intake in rats and humans. Other Gly-extended and Ser13-phosphorylated PYY forms have been detected or predicted based upon known cellular processes of PYY synthesis and modification. Here we compared the effects of 3-h IV infusion of PYY(1–36)-NH2, PYY(3–36)-NH2, PYY(1–36)-Gly-OH, PYY(3–36)-Gly-OH, Ser13(PO3)-PYY(1–36)-NH2, Ser13(PO3)-PYY(3–36)-NH2, Ser13(PO3)-PYY(1–36)-Gly-OH, and Ser13(PO3)-PYY(3–36)-Gly-OH during the early dark period on food intake in freely-feeding rats. PYY(3–36)-NH2 and Ser13(PO3)-PYY(3–36)-NH2 reduced food intake similarly at 50 pmol/kg/min, while only PYY(3–36)-NH2 reduced food intake at 15 pmol/kg/min. PYY(1–36)-NH2 and Ser13(PO3)-PYY(1–36)-NH2 reduced food intake similarly at 50 and 150 pmol/kg/min. In contrast, PYY(1–36)-Gly-OH, PYY(3–36)-Gly-OH, Ser13(PO3)-PYY(3–36)-Gly-OH, and Ser13(PO3)-PYY(1–36)-Gly-OH had no effect on food intake at doses of 50 or 150 pmol/kg/min. Taken together, these results indicate that i) PYY(3–36)-NH2 is significantly more potent than PYY(1–36)-NH2 in reducing food intake, ii) Gly-extended forms of PYY are significantly less potent than non-extended forms, and iii) Ser13-phosphorylation of PYY(3–36)-NH2 decreases the anorexigenic potency PYY(3–36)-NH2, but not PYY(1–36)-NH2. Thus, PYY(3–36)-NH2 appears to be the most potent PYY form for reducing food intake in rats.
Keywords: peptide YY(3-36), molecular forms, satiety
1.1 INTRODUCTION
The 36 amino acid gut hormone peptide YY(1–36)-amide [PYY(1–36)-NH2] was first discovered using an assay to detect peptides with carboxyl-terminal amides in porcine intestinal extracts [16, 17]. In 1989, Eberlein et al. [4] discovered a new molecular form of PYY, PYY(3–36)-NH2. In 2002, Batterham et al. [1] reported that PYY(3–36)-NH2 inhibits food intake in humans and rodents. Numerous groups have since confirmed that PYY(3–36)-NH2 reduces food intake in several species including rodents, monkeys, and humans [2, 11, 13]. We and others have demonstrated that PYY(3–36)-NH2 is significantly more potent than PYY(1–36)-NH2 in reducing food intake in rats [2] and humans [13].
The proposed processing of proPYY to PYY(3–36)-NH2 is as follows: i) the amino terminus of PYY is formed as it enters the endoplasmic reticulum by the action of signal peptidase; ii) the carboxyl terminus of PYY(1–36)-Gly-OH and PYY(1–36)-NH2 is formed in sequential steps in the golgi and secretory vesicle by the actions of prohormone convertase [14], carboxypeptidase E [12], and peptidylglycine-α-amidating monooxygenase (PAM) [5], and iii) dipeptidyl peptidase-4 (DPP-4) converts PYY(1–36)-Gly-OH to PYY(3–36)-Gly-OH and PYY(1–36)-NH2 to PYY(3–36)-NH2 [10]. While the relative proportions of the various intermediate forms of proPYY processing have been determined in intestinal tissue in dog [7] and rat [8], the relative bioactivities of the forms to reduce food intake have not been determined.
We recently developed the RAPID (Reduced temperature, Acidified, Peptidase inhibited, Isotopically-enriched mass spectrometry standards and Diluted) method for extracting and purifying peptides from tissue [7, 15]. This method minimizes enzymatic breakdown of peptides and uses internal standards to monitor their recovery during extraction and purification. Using this method and high-resolution mass spectrometry to quantify PYY forms, we determined that PYY(1–36)-NH2, PYY(3–36)-NH2, and PYY(1–36)-Gly-OH comprise about 79, 5, and 16%, respectively, of total PYY in canine ileum [7], and 64, 6, and 1%, respectively, of total PYY in rat ileum [8].
Chen et al. [3] used HPLC and MALDI-TOF and electrospray tandem mass spectrometry to discover a post-translationally modified form of PYY(1–36)-NH2 from porcine intestine that was phosphorylated at Ser13. These investigators also demonstrated that Ser13(PO3)-PYY(1–36)-NH2 exhibits high affinity for binding to neuropeptide Y receptors Y1, Y2 and Y5, and inhibits forskolin-stimulated cAMP accumulation in SK-N-MC cells.
Here we compared the effects of 3-h intravenous (IV) infusions of the various detected and predicted forms of PYY [PYY(1–36)-NH2, PYY(3–36)-NH2, PYY(1–36)-Gly-OH, PYY(3–36)-Gly-OH, Ser13(PO3)-PYY(1–36)-NH2, Ser13(PO3)-PYY(3–36)-NH2, Ser13(PO3)-PYY(1–36)-Gly-OH, Ser13(PO3)-PYY(3–36)-Gly-OH] at dark onset on food intake in rats with free access to food.
2.1 METHODS AND PROCEDURES
2.1.1 Peptides synthesis and purification
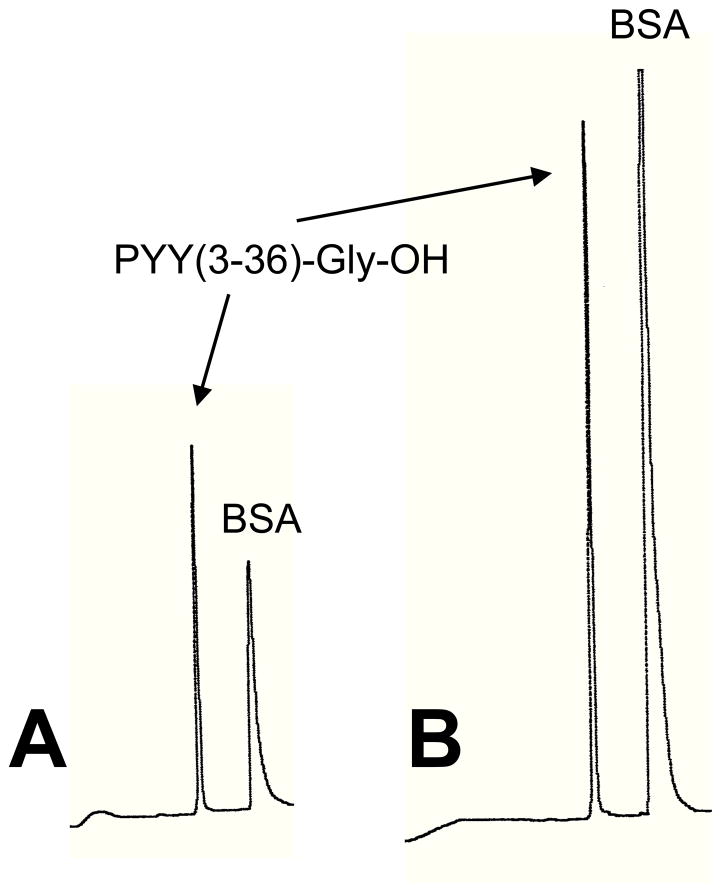
PYY(1–36)-NH2 was purchased from Phoenix Pharmaceuticals (Burlingame, CA). PYY(1–36)-Gly-OH, PYY(3–36)-Gly-OH, Ser13(PO3)-PYY(1–36)-NH2, Ser13(PO3)-PYY(3–36)-NH2, Ser13(PO3)-PYY(1–36)-Gly-OH, and Ser13(PO3)-PYY(3–36)-Gly-OH were synthesized and delivered to us as desalted crude material by GL Biochem (Shanghai) Ltd. (Shanghai, China). PYY(3–36)-NH2 was synthesized in our laboratory by Fmoc solid-phase methodology. Purification of the crude material from GL Biochem (Shanghai) L.t.d. (Shanghai, China) and the PYY(3–36) synthesized in our laboratory was accomplished by reverse phase high performance liquid chromatography. Purity of each peptide was greater than 98%. Proof of structure for each peptide was verified by electro-spray mass spectrometry. Stock solutions were prepared by dissolving each peptide in 0.15 M NaCl, 0.1% BSA. Single-use aliquots were stored at −70°C. Effects of thawing of peptide aliquots and incubation of peptides in infusate (0.15 M NaCl, 0.1% BSA) for 6 h at room temperature on peptide stability were assessed using reverse phase HPLC. Analysis was performed on a C18 Vydac (0.46 × 15 cm) analytical column using a Waters 600 HPLC system. Elution gradient was from 94% Buffer A/6% Buffer B to 40% Buffer A/60% Buffer B over 30 min. Buffer A was 0.1% trifluoroacetic acid (TFA)/H2O and Buffer B was 0.095% TFA/acetonitrile. Flow rate was 1 ml/min and detection was with UV at 214 nm. Single peaks were observed for each peptide after both thawing and incubation in infusate. HPLC results for PYY(3–36)-Gly-OH are shown in Fig. 1. Thus, freeze-thawing and incubation of peptides in infusate at room temperature for 6 h appear not to affect the stability of the peptides.
Figure 1.
HPLC traces of PYY(3–36)-Gly-OH thawed from −70°C (A) and incubated at room temperature for 6 h after dilution (1:1) with infusate (0.15 M NaCl, 0.1% BSA) (B). Analysis was performed on a C18 Vydac (0.46 × 15 cm) analytical column using a Waters 600 HPLC system. Elution gradient was from 94% Buffer A/6% Buffer B to 40% Buffer A/60% Buffer B over 30 min. Buffer A was 0.1% trifluoroacetic acid (TFA)/H2O and Buffer B was 0.095% TFA/acetonitrile. Flow rate was 1 ml/min and detection was with UV at 214 nm.
2.1.2 Animals
Male Sprague-Dawley rats (Charles River, Kingston, NY) weighing 350–500 g (12 to 20 weeks of age) were housed individually in hanging wire-mesh cages in a room with controlled temperature (19-21C) and a 12:12-h light-dark cycle (time of onset of light cycle varied across experiments). Rats were provided pelleted or powdered rat chow (Labdiet®, 5001 Rodent diet, PMI® Nutrition International, Brentwood, MO) and water ad libitum. The Animal Studies Subcommittee of the Omaha Veterans Affairs Medical Center approved the experimental protocol.
2.1.3 Surgical procedures
The procedure for implantation of a jugular vein catheter for peptide infusions has been described previously [18]. Catheters were kept patent by flushing with 0.2 ml of heparinised saline (20 U/ml) on alternate days, and plugged with stainless steel wire. The animals were allowed at least 1 week to recover from surgery. Rats were then adapted to being chronically tethered to infusion swivels for at least 1 week before start of experiments. The jugular vein catheter was connected to a 40-cm length of tubing passed through a protective spring coil connected between a light-weight saddle (IITC, Woodland Hills, CA) worn by the rat and an infusion swivel. Rats had ad libitum access to ground chow that was provided fresh each day 3 h before dark onset.
2.1.4 Effects of 3-h IV infusion of different forms of PYY(3–36) on food intake in non-food-deprived rats
In a series of 4 experiments, rats (n=16) received a single, 3-h jugular vein infusion per day beginning 15 min before dark onset, of PYY(3–36)-NH2 (0, 15 pmol/kg/min), Ser13(PO3)-PYY(3–36)-NH2 (0, 15 pmol/kg/min), PYY(3–36)-NH2 (0, 50 pmol/kg/min), and Ser13(PO3)-PYY(3–36)-NH2 (0, 50 pmol/kg/min). Peptides were administered in 0.15 M NaCl containing 0.1% BSA at 50 μl/min via a syringe infusion pump (PHD2000, Harvard Apparatus, South Natick, MA); pumps were turned on and off by computer. Within each experiment, each rat received vehicle and peptide in counterbalanced order at approximately 48-h intervals. Food intake cumulated hourly for 20 h after dark onset was determined from continuous computer recording of changes in food bowl weight. At the end of an experiment, data from a rat were excluded if its jugular vein catheter was not patent. A catheter was deemed patent if the rat lost consciousness within 10 s of a bolus injection of the short-acting anesthetic Brevital into the catheter.
In 4 additional experiments of identical design, rats (n=16) received at dark onset, 3-h jugular vein infusions of PYY(3–36)-Gly-OH (0, 50 pmol/kg/min), Ser13(PO3)-PYY(3–36)-Gly-OH (0, 50 pmol/kg/min), PYY(3–36)-Gly-OH (0, 150 pmol/kg/min), and Ser13(PO3)-PYY(3–36)-Gly-OH (0, 150 pmol/kg/min).
2.1.5 Effects of 3-h IV infusion of different forms of PYY(1–36) on food intake in non-food-deprived rats
We previously determined that 3-h IV infusion of PYY(3–36)-NH2 at dark onset is an order of magnitude more potent than PYY(1–36)-NH2 in reducing food intake in rats. Here we compared the feeding effects of PYY(3–36)-NH2, PYY(1–36)-NH2, and Ser13(PO3)-PYY(1–36)-NH2 at 0 and 15 pmol/kg/min, the approximate mean effective dose for inhibition of food intake by PYY(3–36)-NH2. The experimental design was the same as that described above for PYY(3–36) forms. In 2 additional experiments of identical design, we compared the feeding effects of PYY(1–36)-NH2 and Ser13(PO3)-PYY(1–36)-NH2 at 2 higher doses, 50 and 150 pmol/kg/min.
The last 2 experiments determined the feeding effects of 3-h jugular vein infusion of PYY(1–36)-Gly-OH (0, 150 pmol/kg/min) and Ser13(PO3)-PYY(3–36)-Gly-OH (0, 150 pmol/kg/min).
2.1.6 Statistical analyses
Values are presented as group means ± SE. Data were analyzed by repeated measures analysis of variance (ANOVA); planned comparisons of treatment means were evaluated by paired t-tests. Differences were considered significant if P<0.05.
3.1 RESULTS
3.1.1 Effects of 3-h IV infusion of different forms of PYY(3–36) on food intake in non-food-deprived rats
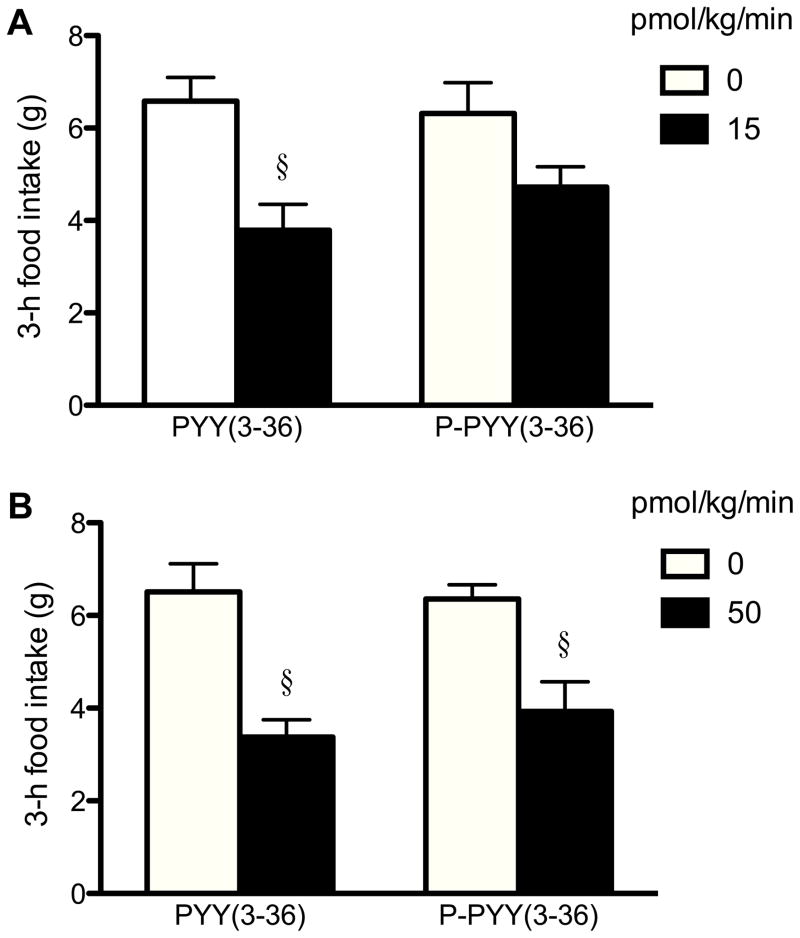
PYY(3–36)-NH2 was more potent than Ser13(PO3)-PYY(3–36)-NH2 in reducing food intake (Figs. 2A–D and 3). At 15 pmol/kg/min, PYY(3–36)-NH2 significantly reduced 3-h food intake by 29%, while Ser13(PO3)-PYY(3–36)-NH2 had no statistically significant effect (Fig. 3A). PYY(3–36)-NH2 also appeared to be more inhibitory than Ser13(PO3)-PYY(3–36)-NH2 at the 3-fold higher dose of 50 pmol/kg/min (42 vs. 31% inhibition, Fig. 3B). In contrast, PYY(3–36)-Gly-OH and Ser13(PO3)-PYY(3–36)-Gly-OH did not reduce food intake at 50 or 150 pmol/kg/min (Fig. 2E–H).
Figure 2.
Effects of 3-h IV infusion of different molecular forms of peptide YY(3–36) [PYY(3–36)] on cumulative food intake for 20 h in 11–16 rats. Rats that were normally fed received 3-h jugular vein infusions beginning 15 min before dark onset (time 0) of the following: PYY(3–36)-NH2 at 0 and 15 pmol/kg/min (A), Ser13(PO3)-PYY(3–36)-NH2 [PO3-PYY(3–36)-NH2] at 0 and 15 pmol/kg/min (B), PYY(3–36)-NH2 at 0 and 50 pmol/kg/min (C), PO3-PYY(3–36)-NH2 at 0 and 50 pmol/kg/min (D), PYY(3–36)-Gly-OH at 0 and 50 pmol/kg/min (E), PO3-PYY(3–36)-Gly-OH at 0 and 50 pmol/kg/min (F), PYY(3–36)-Gly-OH at 0 and 150 pmol/kg/min (G), and PO3-PYY(3–36)-Gly-OH at 0 and 150 pmol/kg/min (H). Food intake was determined from continuous computer recording of changes in food bowl weight. Values are means ± SE. *P < 0.05, †P < 0.01, §P < 0.001, compared with the vehicle-treated group.
Figure 3.
Effects of 3-h IV infusion of peptide YY(3–36)-NH2 [PYY(3–36)-NH2] and Ser13(PO3)-PYY(3–36)-NH2 [PO3-PYY(3–36)-NH2] on 3-h food intake in 11–16 rats. Data are a subset of those presented in Figs. 2A–D. Rats received PYY(3–36)-NH2 and PO3-PYY(3–36)-NH2 at 0 and 15 pmol/kg/min (A) and at 0 and 50 pmol/kg/min (B). Values are means ± SE. §P < 0.001 compared with the vehicle-treated group.
3.1.2 Effects of 3-h IV infusion of different forms of PYY(1–36) on food intake in non-food-deprived rats
At 15 pmol/kg/min, PYY(3–36)-NH2 significantly reduced food intake, while PYY(1–36)-NH2 had a significantly smaller effect and Ser13(PO3)-PYY(1–36)-NH2 had no effect (Figs. 4A and 5A). At higher doses of 50 and 150 pmol/kg/min, PYY(1–36)-NH2 and Ser13(PO3)-PYY(1–36)-NH2 similarly reduced food in a dose-dependent manner (Figs. 4B–C and 5B–C). In contrast, PYY(1–36)-Gly-OH and Ser13(PO3)-PYY(1–36)-Gly-OH did not reduce food intake at 150 pmol/kg/min (Fig. 4D–E).
Figure 4.
Effects of 3-h IV infusion of different molecular forms of peptide YY (PYY) on cumulative food intake for 20 h in 11–15 rats. Rats that were normally fed received 3-h jugular vein infusions beginning 15 min before dark onset (time 0) of the following: PYY(3–36)-NH2, PYY(1–36)-NH2, and Ser13(PO3)-PYY(1–36)-NH2 [PO3-PYY(1–36)-NH2] at 0 and 15 pmol/kg/min (A), PYY(1–36)-NH2 and PO3-PYY(1–36)-NH2 at 0 and 50 pmol/kg/min (B), PYY(1–36)-NH2 and PO3-PYY(1–36)-NH2 at 0 and 150 pmol/kg/min (C), PYY(1–36)-Gly-OH at 0 and 150 pmol/kg/min (D), and PO3-PYY(1–36)-Gly-OH at 0 and 150 pmol/kg/min (E). Food intake was determined from continuous computer recording of changes in food bowl weight. Values are means ± SE. *P < 0.05, †P < 0.01, §P < 0.001, compared with the vehicle-treated group.
Figure 5.
Effects of 3-h IV infusion of peptide YY(3–36)-NH2 [PYY(3–36)-NH2], PYY(1–36)-NH2, and Ser13(PO3)-PYY(1–36)-NH2 [PO3-PYY(3–36)-NH2] on 3-h food intake in 11–15 rats. Data are a subset of those presented in Figs. 4A–C. Rats received PYY(3–36)-NH2, PYY(1–36)-NH2 and PO3-PYY(1–36)-NH2 at 0 and 15 pmol/kg/min (A), PYY(1–36)-NH2 and PO3-PYY(1–36)-NH2 at 0 and 50 pmol/kg/min (B), and PYY(1–36)-NH2 and PO3-PYY(1–36)-NH2 at 0 and 150 pmol/kg/min (C). Values are means ± SE. Values with different letters are different (P<0.05).
4.1 DISCUSSION
The proposed processing of proPYY to PYY(3–36)-NH2 includes formation of the carboxyl terminus of PYY(1–36)-Gly-OH and PYY(1–36)-NH2 by prohormone convertase [14], carboxypeptidase E [12], and PAM [5], and conversion of PYY(1–36)-Gly-OH to PYY(3–36)-Gly-OH and PYY(1–36)-NH2 to PYY(3–36)-NH2 by DPP-4 [10]. We previously determined that 3-h IV infusion of PYY(3–36)-NH2 during the early dark period in freely-feeding rats is an order of magnitude more potent than PYY(1–36)-NH2 in reducing food intake [2]. Sloth et al. [13] reported similar results for PYY(3–36)-NH2 in humans. Our results here further support these findings for PYY(3–36)-NH2 and PYY(1–36)-NH2. No previous study has characterized the anorexigenic effects of the other possible molecular forms of PYY.
We recently developed the RAPID method for extracting and purifying peptides from tissue [7, 15]. Using this method and high-resolution mass spectrometry to quantify PYY forms, we determined that PYY(1–36)-NH2, PYY(3–36)-NH2, and PYY(1–36)-Gly-OH comprise about 79, 5, and 16%, respectively, of total PYY in canine ileum [7], and 64, 6, and 1%, respectively, of total PYY in rat ileum [8]. Here we demonstrate that PYY(1–36)-Gly-OH and PYY(3–36)-Gly-OH are significantly less anorexigenic than PYY(1–36)-NH2 and PYY(3–36)-NH2, respectively.
Chen et al. [3] used HPLC and mass spectrometry to detect Ser13(PO3)-PYY(1–36)-NH2 in extracts of porcine intestine. They noted that while the amount of phosphorylated PYY could not be determined from mass spectrometry, phospho-PYY appears to be a minor form compared to non-phosphorylated PYY. We did not detect significant amounts of either Ser13(PO3)-PYY(1–36)-NH2 or Ser13(PO3)-PYY(3–36)-NH2 in dog or rat distal small intestine [7, 8]. Chen et al. showed that Ser13(PO3)-PYY(1–36)-NH2 binds with high affinity to Y1, Y2 and Y5 receptors, and inhibits forskolin-stimulated cAMP accumulation in SK-N-MC cells. Our work here extends these findings by showing that Ser13(PO3)-PYY(1–36)-NH2 and PYY(1–36)-NH2 similarly reduce food intake, while Ser13(PO3)-PYY(3–36)-NH2 is less potent than PYY(3–36)-NH2.
Previous studies of PYY forms in blood have reported that PYY(3–36)-NH2 levels are equal to or greater than those of PYY(1–36)-NH2 [6, 9]. However, these studies did not use methods to monitor possible alteration of PYY forms during processing of blood samples, nor did they use mass spectrometry to identify and quantify the various molecular forms of PYY. Thus, it remains to be determined whether blood levels of PYY forms reflect those occurring in intestinal tissue [high PYY(1–36)-NH2 and low PYY(1–36)-Gly-OH and PYY(3–36)-NH2] or whether significant in vivo conversion of PYY(1–36)-NH2 to PYY(3–36)-NH2 by DPP-4 occurs following secretion of PYY(1–36)-NH2 from intestinal cells [10].
In summary, our results indicate that i) PYY(3–36)-NH2 is significantly more potent than PYY(1–36)-NH2 in reducing food intake in rats, ii) Gly-extended forms of PYY(3–36)-NH2 and PYY(1–36)-NH2 are significantly less potent than their non-extended forms, and iii) phosphorylation of Ser13 slightly reduces the anorexigenic potency of PYY(3–36)-NH2, but not PYY(1–36)-NH2. Thus, PYY(3–36)-NH2 is more potent than other processing intermediates of PYY for reducing food intake.
Acknowledgments
We thank Krista Anders, Bettye Apenteng, Lucille Waite, and Sharalyn Steenson for their technical assistance. This research was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Biomedical Laboratory Research and Development, and by National Institutes of Health grants DK73152 and P20RR16469.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6.1 REFERENCES
- 1.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature. 2002;418:650–4. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 2.Chelikani PK, Haver AC, Reidelberger RD. Intravenous infusion of peptide YY(3–36) potently inhibits food intake in rats. Endocrinology. 2005;146:879–88. doi: 10.1210/en.2004-1138. [DOI] [PubMed] [Google Scholar]
- 3.Chen Z, Eriste E, Jonsson AP, Norberg A, Nepomuceno D, Lovenberg TW, Bergman T, Efendic S, Jörnvall H, Sillard R. Ser(13)-phosphorylated PYY from porcine intestine with a potent biological activity. FEBS Lett. 2001;492:119–22. doi: 10.1016/s0014-5793(01)02234-7. [DOI] [PubMed] [Google Scholar]
- 4.Eberlein GA, Eysselein VE, Schaeffer M, Layer P, Grandt D, Goebell H, Niebel W, Davis M, Lee TD, Shively JE. A new molecular form of PYY: structural characterization of human PYY(3–36) and PYY(1–36) Peptides. 1989;10:797–803. doi: 10.1016/0196-9781(89)90116-2. [DOI] [PubMed] [Google Scholar]
- 5.Eipper BA, Mains RE, Glembotski CC. Identification in pituitary tissue of a peptide alpha-amidation activity that acts on glycine-extended peptides and requires molecular oxygen, copper, and ascorbic acid. Proc Natl Acad Sci U S A. 1983;80:5144–8. doi: 10.1073/pnas.80.16.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grandt D, Schimiczek M, Beglinger C, Layer P, Goebell H, Eysselein VE, Reeve JR., Jr Two molecular forms of peptide YY (PYY) are abundant in human blood: characterization of a radioimmunoassay recognizing PYY 1–36 and PYY 3–36. Regul Pept. 1994;51:151–9. doi: 10.1016/0167-0115(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 7.Keire DA, Whitelegge JP, Bassilian S, Faull KF, Wiggins BW, Mehdizadeh OB, Reidelberger RD, Haver AC, Sayegh AI, Reeve JR. A new endogenous form of PYY isolated from canine ileum: Gly-extended PYY(1–36) Regul Pept. 2008;151:61–70. doi: 10.1016/j.regpep.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Keire DA, Whitelegge JP, Souda P, Faull KF, Bassilian S, Reidelberger R, Haver AC, Reeve JR. PYY(1–36) is the major form of PYY in rat distal small intestine: Quantification using high-resolution mass spectrometry. Regul Pept. doi: 10.1016/j.regpep.2010.06.006. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Roux CW, Batterham RL, Aylwin SJ, Patterson M, Borg CM, Wynne KJ, Kent A, Vincent RP, Gardiner J, Ghatei MA, Bloom SR. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology. 2006;147:3–8. doi: 10.1210/en.2005-0972. [DOI] [PubMed] [Google Scholar]
- 10.Medeiros MD, Turner AJ. Processing and metabolism of peptide-YY: pivotal roles of dipeptidylpeptidase-IV, aminopeptidase-P, and endopeptidase-24.11. Endocrinology. 1994;134:2088–94. doi: 10.1210/endo.134.5.7908871. [DOI] [PubMed] [Google Scholar]
- 11.Moran TH, Smedh U, Kinzig KP, Scott KA, Knipp S, Ladenheim EE. Peptide YY(3–36) inhibits gastric emptying and produces acute reductions in food intake in rhesus monkeys. Am J Physiol Regul Integr Comp Physiol. 2005;288:R384–8. doi: 10.1152/ajpregu.00535.2004. [DOI] [PubMed] [Google Scholar]
- 12.Rawdon BB, Larsson LI. Development of hormonal peptides and processing enzymes in the embryonic avian pancreas with special reference to co-localisation. Histochem Cell Biol. 2000;114:105–12. doi: 10.1007/s004180000169. [DOI] [PubMed] [Google Scholar]
- 13.Sloth B, Holst JJ, Flint A, Gregersen NT, Astrup A. Effects of PYY1-36 and PYY3-36 on appetite, energy intake, energy expenditure, glucose and fat metabolism in obese and lean subjects. Am J Physiol Endocrinol Metab. 2007;292:E1062–8. doi: 10.1152/ajpendo.00450.2006. [DOI] [PubMed] [Google Scholar]
- 14.Steiner DF, Rouillé Y, Gong Q, Martin S, Carroll R, Chan SJ. The role of prohormone convertases in insulin biosynthesis: evidence for inherited defects in their action in man and experimental animals. Diabetes Metab. 1996;22:94–104. [PubMed] [Google Scholar]
- 15.Stengel A, Keire D, Goebel M, Evilevitch L, Wiggins B, Taché Y, Reeve JR. The RAPID method for blood processing yields new insight in plasma concentrations and molecular forms of circulating gut peptides. Endocrinology. 2009;150:5113–8. doi: 10.1210/en.2009-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tatemoto K. Isolation and characterization of peptide YY (PYY), a candidate gut hormone that inhibits pancreatic exocrine secretion. Proc Natl Acad Sci U S A. 1982;79:2514–8. doi: 10.1073/pnas.79.8.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tatemoto K, Mutt V. Isolation of two novel candidate hormones using a chemical method for finding naturally occurring polypeptides. Nature. 1980;285:417–8. doi: 10.1038/285417a0. [DOI] [PubMed] [Google Scholar]
- 18.Weeks JR, Myers RD. Methods in Psychobiology. London: Academic Press; 1972. Long-term intravenous infusion. [Google Scholar]