Abstract
The failure to eliminate self-reactive T cells during negative selection is a prerequisite for autoimmunity. To escape deletion, autoreactive T-cell receptors (TCRs) may form unstable complexes with self-peptide–MHC by adopting suboptimal binding topologies compared with anti-microbial TCRs. Alternatively, escape can occur by weak binding between self-peptides and MHC. We determined the structure of a human autoimmune TCR (MS2-3C8) bound to a self-peptide from myelin basic protein (MBP) and the multiple sclerosis-associated MHC molecule HLA-DR4. MBP is loosely accommodated in the HLA-DR4-binding groove, accounting for its low affinity. Conversely, MS2-3C8 binds MBP–DR4 as tightly as the most avid anti-microbial TCRs. MS2-3C8 engages self-antigen via a docking mode that resembles the optimal topology of anti-foreign TCRs, but is distinct from that of other autoreactive TCRs. Combined with a unique CDR3β conformation, this docking mode compensates for the weak binding of MBP to HLA-DR4 by maximizing interactions between MS2-3C8 and MBP. Thus, the MS2-3C8–MBP–DR4 complex reveals the basis for an alternative strategy whereby autoreactive T cells escape negative selection, yet retain the ability to initiate autoimmunity.
Keywords: autoimmunity, MHC class II, multiple sclerosis, structure, T-cell receptor
Introduction
The ability of the immune system to distinguish between non-self pathogens and self-antigens involves the elimination or inactivation of autoreactive T cells during T-cell ontogeny in the thymus, thereby avoiding immune responses to self. However, in autoimmune diseases such as multiple sclerosis (MS) and type 1 diabetes, the presence of autoreactive T cells in the periphery demonstrates that this filtering process is imperfect. Thymic selection is based on recognition of self-peptide–MHC complexes, whereby weak interactions with self-peptide–MHC promote T-cell survival (positive selection), whereas strong interactions induce apoptosis (negative selection) (Kappler et al, 1987; Goodnow et al, 2005; Daniels et al, 2006; Zehn and Bevan, 2006). Failure of negative selection may result from reduced T-cell receptor (TCR) affinity for self-peptide–MHC ligands, such that the complex with TCR is too short-lived to permit negative selection, yet sufficiently stable for activation in the periphery at high antigen concentrations (Ohashi, 2003; Goodnow et al, 2005) or activation by cross-reactive foreign peptide ligands (Hemmer et al, 1997). Alternatively, unusually weak binding of the self-peptide to MHC could effectively destabilize the complex with TCR (Anderton et al, 2001; Stadinski et al, 2010). Both these affinity-based mechanisms for escaping negative selection are supported by animal models of autoimmune disease (Ohashi, 2003; Goodnow et al, 2005), and by binding measurements which have demonstrated correlations between the longevity (half-life) of TCR–peptide–MHC complexes and selection outcome (Alam et al, 1996; Williams et al, 1999; Daniels et al, 2006).
Structural studies of autoimmune TCRs bound to self-peptide–MHC ligands have begun to reveal how such TCRs escape negative selection in the thymus, but still retain the ability to productively engage self-antigens in the periphery (Deng and Mariuzza, 2007; Wucherpfennig et al, 2009). Five structures have been reported to date, involving two human TCRs from MS patients (Hahn et al, 2005; Li et al, 2005) and three mouse TCRs from the experimental autoimmune encephalomyelitis (EAE) model of MS (Maynard et al, 2005; Feng et al, 2007). The human TCRs are Ob.1A12, which recognizes myelin basic protein (MBP) peptide 85–99 in the context of HLA-DR2b (Hahn et al, 2005), and 3A6, which recognizes MBP 89–101 presented by HLA-DR2a (Li et al, 2005). Both Ob.1A12 and 3A6 engage their MBP–HLA-DR ligands with docking topologies that differ substantially from the way most TCRs specific for microbial or other foreign epitopes bind peptide–MHC. Whereas anti-microbial TCRs typically dock on peptide–MHC in a diagonal orientation over the centre of the antigenic peptide, Ob.1A12 and 3A6 are displaced towards the N-terminus of MBP (Wucherpfennig et al, 2009). This suboptimal binding mode results in fewer specific interactions with the self-peptide, which is manifested by the much lower affinities of Ob.1A12 and 3A6 compared with anti-microbial TCRs. Although anti-microbial TCRs usually adopt a central diagonal orientation, deviations from this topology have been described (Ely et al, 2008). For example, an MHC class I-restricted TCR (CF34) specific for an Epstein-Barr virus peptide presented by HLA-B8 was reported, in which the TCR is shifted towards the N-terminus of the bound peptide in a manner reminiscent of 3A6 (Gras et al, 2009). Moreover, TCR CF34 binds its ligand with reasonably high affinity. Nevertheless, an emerging pattern for autoimmune TCRs appears to be to form suboptimal interactions with self-ligands that ultimately permit escape from thymic deletion (Ely et al, 2008; Wucherpfennig et al, 2009).
The three mouse TCRs (172.10, 1934.4 and cl19) recognize acetylated MBP 1–11 (MBP Ac1–11) presented by I-Au. The MBP–I-Au ligand is structurally defective in that the peptide only partially fills the MHC-binding groove (He et al, 2002). As a result, these mouse TCRs, like Ob.1A12 and 3A6, also exhibit suboptimal interactions with the MBP self-peptide, marked by a paucity of specific contacts (Maynard et al, 2005; Feng et al, 2007). Together, these studies suggest that structural alterations which destabilize the TCR–peptide–MHC recognition unit may enable certain self-reactive T cells to escape negative selection, without necessarily compromising their ability to cause autoimmune disease. Indeed, mice transgenic for TCR Ob.1A12 and HLA-DR2b developed central nervous system (CNS) inflammation (Madsen et al, 1999), and transfer into mice of T-cell clones recognizing MBP–I-Au induced EAE (Goverman, 2009).
Despite these advances, the database of autoimmune TCR–peptide–MHC complexes is still too limited to describe the recognition properties of autoreactive TCRs at the repertoire level, or to fully assess the structural mechanisms underlying escape from negative selection (Wucherpfennig et al, 2009). Accordingly, we determined the structure of a human autoimmune TCR (MS2-3C8) in complex with a self-peptide from MBP (MBP 111–129) and the MS-associated MHC class II molecule HLA-DR4 (HLA-DRB1*0401). Notably, this peptide represents an immunodominant epitope of MBP in HLA-DR4-positive MS patients (Muraro et al, 1997; Sospedra and Martin, 2005). Unlike the MBP peptides recognized by TCRs Ob.1A12 and 3A6, which bind with high affinity to HLA-DR2 and elicit a diverse TCR repertoire, MBP 111–129 binds weakly to HLA-DR4 and is recognized by a restricted TCR repertoire. The MBP 111–129-specific T-cell clone MS2-3C8 was repeatedly isolated from the peripheral blood of a patient with relapsing-remitting MS over a 2-year period (Muraro et al, 1997). In addition, the clonal overrepresentation and persistent expansion of MS2-3C8 T cells in this patient suggested an involvement in the disease process. The pathogenic potential of TCR MS2-3C8 was demonstrated using transgenic mice expressing this autoimmune TCR and HLA-DR4 (Quandt et al, 2004). These humanized mice readily developed EAE in adoptive transfer experiments without antigen administration. Interestingly, the clinical phenotype of TCR MS2-3C8/HLA-DR4 transgenic mice with brain stem involvement reiterated clinical findings in the MS patient from whom the TCR was derived (Quandt et al, 2004).
The structure of the MS2-3C8–MBP–DR4 complex revealed the basis for the weak binding of MBP to HLA-DR4, which likely enabled MS2-3C8 T cells to survive thymic deletion. Furthermore, the structure showed how, at the atomic level, an autoreactive TCR can compensate for an unstable self-antigen to achieve sufficiently high affinity for T-cell activation in the periphery and the induction of autoimmunity.
Results
Interaction of TCR MS2-3C8 with MBP–HLA-DR4
To characterize the interaction between MS2-3C8 (Vα4.1Jα32, Vβ2.1Jβ1.6) and MBP–DR4, we expressed recombinant TCR by in vitro folding from bacterial inclusion bodies. To produce soluble MBP–DR4, we first attempted folding the MHC class II α and β chains in vitro in the presence of MBP 111–129 or MBP 114–126, which is indistinguishable from MBP 111–129 in terms of MHC binding and T-cell stimulation (Muraro et al, 1997). However, yields were extremely low and the HLA-DR4 α and β chains dissociated during purification, presumably due to the weak interaction between peptide and MHC. Indeed, the low affinity of MBP 111–126 for HLA-DR4 is well documented in the literature. In one study, MBP 111–126 was found to bind HLA-DR4 ∼75-fold less tightly than influenza virus hemagglutinin peptide 307–319 (HA), a good binder (Muraro et al, 1997). In another study, the affinity of this MBP peptide for HLA-DR4 was estimated at 2000 nM, which is in the low range for peptide–MHC interactions (Valli et al, 1993). To permit in vitro folding, we covalently attached MBP 114–126 (FSWGAEGQRPGFG) to the N-terminus of the HLA-DR4 β chain via a 16-mer peptide linker (Fremont et al, 1996).
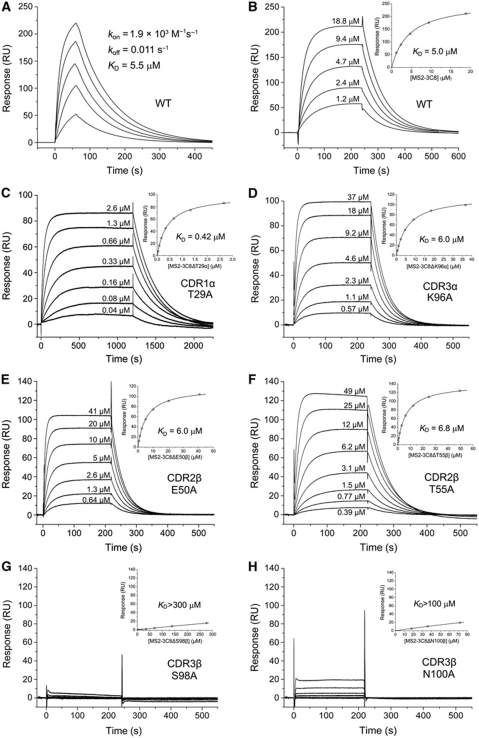
We used surface plasmon resonance (SPR) to measure the affinity and kinetics of TCR MS2-3C8 binding to MBP–DR4. To permit directional coupling to a streptavidin-coated biosensor surface, a biotinylation sequence was added to the C-terminus of the DR4 α chain. Kinetic parameters (on- and off-rates) for the binding of soluble TCR MS2-3C8 to immobilized HLA-DR4 were kon=1.9 × 103 M−1 s−1 and koff=0.011 s−1, corresponding to a dissociation constant (KD) of 5.5 μM (Figure 1A). Under equilibrium binding conditions, a KD of 5.0 μM was obtained (Figure 1B), in close agreement with the KD from kinetic analysis. The affinity of MS2-3C8 for its self-antigen is therefore at the high end of the range for TCR interactions with peptide–MHC class I or peptide–MHC class II (1 to >100 μM) (van der Merwe and Davis, 2003; Cole et al, 2007). Indeed, MS2-3C8 binds MBP–DR4 about as tightly as the most avid anti-microbial class I-restricted TCRs bind their respective ligands, and considerably more tightly than do anti-microbial class II-restricted TCRs, such as HA1.7, which recognizes HA–DR4 with KD=40 μM (Cole et al, 2007). It also binds far more tightly than do human autoimmune TCRs 3A6 and Ob.1A12 (KD>100 μM) (Appel et al, 2000; Li et al, 2005), but similarly to mouse autoimmune TCR 172.10 (6 μM) (Garcia et al, 2001). Moreover, the off-rate of the interaction (0.011 s−1, corresponding to a half-life of 69 s) is exceptionally slow, compared with the off-rates of all other TCR–peptide–MHC interactions characterized to date (0.02 to >1 s−1) (Cole et al, 2007). For TCR 172.10, the off-rate of its interaction with MBP–I-Au is ∼20-fold faster (0.22 s−1) (Garcia et al, 2001). The slow off-rate of MS2-3C8 is counterbalanced by a very slow on-rate (1.9 × 103 M−1 s−1) relative to the on-rates of other TCRs (2 × 103 to >1 × 106 M−1 s−1), including 172.10 (3.7 × 104 M−1 s−1), which may imply conformational changes in MS2-3C8 and/or MBP–DR4 during complex formation.
Figure 1.
SPR analysis of the binding of TCR MS2-3C8 to MBP–DR4. (A) For kinetic measurements, TCR MS2-3C8 at concentrations of 2.9, 5.8, 11.5, 23 and 46 μM was injected over immobilized MBP–DR4 (320 RU). Sensograms were fitted to a 1:1 binding model to obtain on- and off-rates: kon=1.9 × 103±0.01 M−1 s−1 and koff=0.011±0.0003 s−1. KD was calculated as koff/kon. (B) For equilibrium measurements, TCR MS2-3C8 at concentrations of 1.2, 2.4, 4.7, 9.4 and 18.8 μM was injected over immobilized MBP–DR4 (400 RU). Inset shows the fitting curve for equilibrium binding that resulted in a KD of 5.0±0.1 μM. Equilibrium measurements for TCR MS2-3C8 mutants are also shown: (C) CDR1α T29A, (D) CDR3α K96A, (E) CDR2β E50A, (F) CDR2β T55A, (G) CDR3β S98A and (H) CDR3β N100A. For weakly binding mutants CDR3β S98A and CDR3β N100A, KDs were estimated at >300 and >100 μM, respectively.
Although these results indicate that the intrinsic affinity of MS2-3C8 for MBP–DR4 is surprisingly high, it is important to note that SPR measurements were conducted using an engineered version of the ligand designed to overcome the low affinity of MBP for HLA-DR4. However, the instability of the natural MBP–DR4 ligand would effectively decrease the half-life of the overall complex with TCR, which most likely allowed MS2-3C8 T cells to survive thymic deletion. At the same time, the tight binding of MS2-3C8 to MBP–DR4 at least partially compensates for the weak binding of MBP to HLA-DR4, thereby explaining the ability of this autoreactive TCR to induce CNS inflammation in MS2-3C8–DR4 transgenic mice (Quandt et al, 2004).
Overview of the MS2-3C8–MBP–DR4 complex
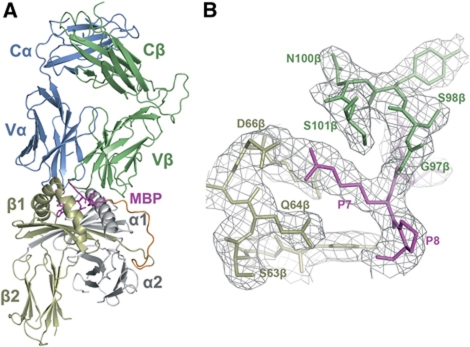
To understand why the MBP self-peptide binds weakly to HLA-DR4, and to test the hypothesis that the canonical docking mode of anti-foreign TCRs has evolved to provide optimal binding to peptide–MHC (Rudolph et al, 2006; Marrack et al, 2008), we determined the structure of MS2-3C8 in complex with MBP–DR4 to 2.8 Å resolution (Table I; Figure 2A). The interface between TCR and peptide–MHC was in unambiguous electron density for each of the two molecules in the asymmetric unit of the crystal (Figure 2B). Several loops in the MHC α2β2 and TCR CαCβ modules lacked electron density; however, these disordered regions are distant from the interface. The 16-mer peptide linking MBP to the N-terminus of the HLA-DR4 β chain exhibited clear electron density in one of the two complexes in the asymmetric unit, probably due to crystal contacts with the Cβ domain of a neighbouring complex molecule; no density could be detected for the linker peptide in the other complex, indicating flexibility. The r.m.s. deviation in α-carbon positions for the TCR VαVβ and MHC α1β1 modules, including the MBP peptide, is 0.22 Å for the two complexes in the asymmetric unit. Based on this close similarity, the following description of TCR–peptide–MHC interactions applies to both complex molecules.
Table 1. Data collection and refinement statistics.
| Data collection | |
| Space group | P21212 |
| Resolution (Å) | 49.2–2.80 |
| Unit cell (Å) | a=102.5, b=218.4, c=98.4 |
| Unique reflections | 55 156 |
| Redundancya | 14.2 (5.0) |
| Completeness (%)a | 100 (100) |
| Mean I/σ (I)a | 29.7 (5.2) |
| Rmerge (%)a,b | 8.8 (35.5) |
| Refinement | |
| Resolution range (Å) | 49.2–2.80 |
| Rwork (%)c | 23.9 |
| Rfree (%)c | 27.9 |
| Protein atoms | 12 876 |
| Water molecules | 27 |
| R.m.s.d. from ideality | |
| Bond lengths (Å) | 0.010 |
| Bond angles (deg) | 1.339 |
| Ramachandran plot statistics | |
| Favoured (%) | 92.0 |
| Allowed (%) | 7.8 |
| Outliers (%) | 0.2 |
| aValues in parentheses correspond to the highest resolution shell (2.80–2.90). | |
| bRmerge(I)=(Σ∣I(i)−<I(h)>∣/ΣI(i)), where I(i) is the ith observation of the intensity of the hkl reflection and <I> is the mean intensity from multiple measurements of the hkl reflection. | |
| cRwork (Rfree)=Σ∣∣Fo∣−∣Fc∣∣/Σ∣Fo∣; 5% of data were used for Rfree. | |
Figure 2.
Structure of the MS2-3C8–MBP–DR4 complex. (A) Side view of the MS2-3C8–MBP–DR4 complex (ribbon diagram), including the 16-mer peptide linking MBP to the HLA-DR4 β chain. TCR α chain, blue; TCR β chain, green; MHC α chain, grey; MHC β chain, yellow; MBP peptide (stick representation), magenta; linker peptide, orange. (B) Electron density in the interface of the MS2-3C8–MBP–DR4 complex. Density from the final 2Fo−Fc map at 2.8 Å resolution is contoured at 1 σ.
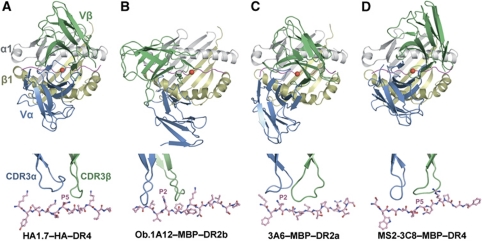
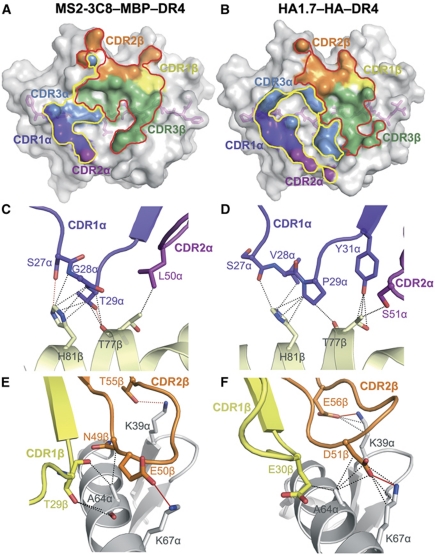
Compared with anti-microbial TCRs such as HA-specific TCR HA1.7 (Hennecke and Wiley, 2002), which typically dock over the central portion of the foreign peptide and interact symmetrically with the MHC α1 and β1 α helices (Figure 3A), the autoimmune TCRs Ob.1A12 and 3A6 (Hahn et al, 2005; Li et al, 2005) display substantially altered binding geometries, in which the TCR is shifted towards the N-terminus of the self-peptide and towards the MHC β1 α helix (Figure 3B and C). In sharp contrast to Ob.1A12 and 3A6, MS2-3C8 engages its self-ligand via a binding mode which closely resembles that of anti-foreign TCRs (Figure 3D), as exemplified by the HA1.7–HA–DR4 complex (Figure 3A). Thus, MS2-3C8 docks symmetrically over MBP–DR4 in a canonical diagonal orientation, with a crossing angle of TCR to peptide–MHC (Reinherz et al, 1999) of 65°, compared with 70° for the HA1.7–HA–DR4 complex. Importantly, mouse autoimmune TCR 172.10 also recognizes its self-ligand in a canonical manner (Maynard et al, 2005), implying that MS2-3C8 and 172.10 use similar mechanisms to escape negative selection (see Discussion). In addition, MS2-3C8 is positioned directly over the central P5 residue of MBP, rather than being displaced towards the N-terminus of the self-peptide, as in the Ob.1A12–MBP–DR2b and 3A6–MBP–DR2a complexes. As discussed later, this docking mode, along with a unique CDR3β conformation, optimizes interactions between MS2-3C8 and the self-peptide.
Figure 3.
Comparison of human anti-microbial and autoimmune TCR–peptide–MHC class II complexes. (A) Upper panel: top view of the anti-microbial HA1.7–HA–DR4 complex (PDB accession code 1J8H) (Hennecke and Wiley, 2002). Colours of TCR, MHC and peptide are the same as in Figure 2. The central P5 residue of the peptide is shown as a red sphere. Bottom panel: position of the TCR CDR3 loops over the HA peptide. The peptide is drawn in ball-and-stick representation with carbon atoms in pink, oxygen atoms in red and nitrogen atoms in blue. The CDR loops are positioned above the central P5 residue of the peptide. (B) The autoimmune Ob.1A12–MBP–DR2b complex (1YMM) (Hahn et al, 2005). (C) The autoimmune 3A6–MBP–DR2a complex (1ZGL) (Li et al, 2005). (D) The autoimmune MS2-3C8–MBP–DR4 complex. The comparison shows that autoimmune TCR MS2-3C8, like anti-microbial TCR HA1.7, docks symmetrically over peptide–MHC in a central diagonal orientation, whereas autoimmune TCRs Ob.1A12 and 3A6 are displaced towards the peptide N-terminus and the HLA-DR β1 helix. The CDR3 loops of HA1.7 and MS2-3C8 are centred over peptide residue P5, those of Ob.1A12 and 3A6 are positioned over P2.
Interaction of MBP with HLA-DR4
Based on the structure, the primary anchor residues for MBP 114–126 bound to HLA-DR4 are Trp116 (P1) and Gln121 (P6); the secondary anchor residues are Glu119 (P4), Arg122 (P7) and Gly124 (P9). Although Trp116 fulfills the need for an aromatic residue at P1 for efficient binding to HLA-DR4 and Glu119 at P4 is a suitable anchor as well, Gln121 does not meet the requirement for a small residue at P6 (Hammer et al, 1993, 1995; Southwood et al, 1998). Secondary anchor residue P7 Arg122 also does not conform to the optimal binding motif for HLA-DR4, which calls for an aliphatic residue at this position.
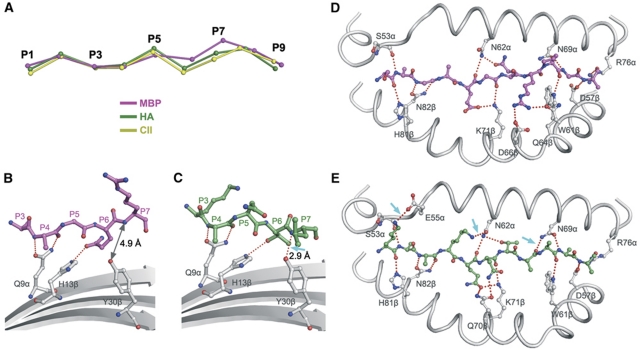
To understand the basis for the weak binding of MBP to HLA-DR4, the conformation of the MBP peptide was directly compared with those of two peptides that bind HLA-DR4 with high affinity: HA and collagen II peptide 1168–1180 (CII) (Hammer et al, 1993, 1995). HA and CII display very similar main-chain conformations in their corresponding complexes with HLA-DR4, from residues P1 to P9 (Dessen et al, 1997; Hennecke and Wiley, 2002) (Figure 4A). However, MBP diverges from HA and CII at residues P6 and P7, with the result that MBP sits less deeply in the peptide-binding groove than HA or CII.
Figure 4.
Basis for low-affinity binding of MBP to HLA-DR4. (A) Conformation of high- and low-affinity peptides bound to HLA-DR4. The α-carbon backbone of MBP (magenta) was compared with the α-carbon backbones of hemagglutinin peptide (HA) (green) and collagen II peptide (CII) (yellow) by superposing the α1β1 domains of HLA-DR4 in the MBP–DR4, HA–DR4 (1J8H) (Hennecke and Wiley, 2002) and CII–DR4 (2SEB) (Dessen et al, 1997) complexes. The peptides are viewed from the side of the β1 helix. HA and CII bind HLA-DR4 with much higher affinity than MBP. MBP diverges from HA and CII at residues P6 and P7. (B, C) Interactions between MBP (B) or HA (C) and the β-sheet floor of the HLA-DR4-binding groove (grey). The peptides are oriented the same as in (A). Hydrogen bonds are shown as dotted red lines. The arrow in (C) indicates the hydrogen bond between P7 and Tyr30β in the HA–DR4 complex that is absent in the MBP–DR4 complex, where the corresponding atoms are too distant (4.9 Å) for hydrogen bond formation (B). (D, E) Interactions between MBP (D) or HA (E) and the α1 and β1 helices of HLA-DR4. The arrows in (E) indicate three additional hydrogen bonds in the HA–DR4 complex not present in the MBP–DR4 complex (D).
In HA and CII, anchor residues P6 and P7 have short side chains (HA: Thr-Leu; CII: Ala-Ala), whereas in MBP these residues have long side chains (Gln-Arg). The shallower binding of MBP is mainly attributable to these longer side chains, but for different reasons. Thus, the side chain of P6 Gln pushes up the MBP main chain by pointing directly into the P6 pocket of HLA-DR4 (Figure 4B), in the same direction as the shorter side chain of HA P6 Thr (Figure 4C). By contrast, the long side chain of P7 Arg projects out towards the top of the DR4 β1 helix, rather than into the P7 pocket, in order to avoid colliding with the α1β1 platform if it pointed in the same direction as the side chain of HA P7 Leu (Figure 4B and D). Therefore, P7 Arg is not positioned as a typical anchor in MBP–DR4. Instead, this residue interacts extensively with TCR (see below).
In the HA–HLA-DR4 structure, the HA peptide forms four hydrogen bonds with β-sheet residues on the floor of the peptide-binding groove (Figure 4C). However, the elevation of MBP results in loss of the hydrogen bond between the main-chain nitrogen of P7 and the Oη atom of Tyr30β (Figure 4B). In addition, a twist in MBP eliminates a hydrogen bond between P7 and DR4 Asn69α found in the HA–DR4 complex (Figure 4D and E). The MBP–DR4 complex also lacks two side-chain–side-chain hydrogen bonds to Glu55α and Asn62α, due to the absence of suitable hydrogen bond donors at P-1 and P3. This net loss of four hydrogen bonds, along with 35 fewer van der Waals between peptide and MHC, likely explains the 75-fold lower affinity of MBP than HA for HLA-DR4 (Muraro et al, 1997).
Interaction of TCR MS2-3C8 with HLA-DR4
The MS2-3C8–MBP–DR4 complex buries a total solvent-accessible surface of 2010 Å2, comparable to that in other MHC class II complexes (Rudolph et al, 2006). The buried surface area on Vβ (670 Å2, 66%) is nearly twice that on Vα (350 Å2, 34%) (Figure 5A). Such dominance by Vβ is unusual among TCR–peptide–MHC complexes, in which Vα and Vβ typically contribute roughly equal buried surfaces, as in the HA1.7–HA–DR4 complex (Vα: 47%; Vβ: 53%) (Figure 5B), or in which Vα dominates. Indeed, only three other complexes displaying a similar degree of Vβ dominance as MS2-3C8–MBP–DR4 have been reported, involving the HLA-A2-restricted TCR JM22 (67%) (Stewart-Jones et al, 2003), the H-2Kb-restricted TCR BM3.3 (63%) (Reiser et al, 2000) and the MBP-specific TCR 3A6 (61%) (Li et al, 2005). Overall, Vβ makes 57 van der Waals contacts with HLA-DR4, compared with only 19 by Vα. These contacts are mediated by 11 Vβ and 6 Vα residues and involve 15 MHC residues (Table II), of which 12 are contacted by HA1.7 and 10 by 3A6 (Supplementary Table S1).
Figure 5.
Comparison of TCR footprints and interactions by CDR1 and CDR2 loops of MS2-3C8 and HA1.7. (A) Footprint of autoimmune TCR MS2-3C8 on MBP–DR4. The top of the MHC molecule is represented as a grey surface; the bound peptide (pink) is shown in stick format under the transparent surface. The areas contacted by individual CDR loops are colour-coded: CDR1α, violet; CDR2α, purple; CDR3α, blue; CDR1β, yellow; CDR2β, orange; CDR3β, green. The contact areas of the V domains are contoured: Vα, yellow; Vβ, red. (B) Footprint of anti-microbial TCR HA1.7 on HA–DR4. (C, D) Conserved interactions between the DR4 β1 helix and the CDR1α/2α loops of MS2-3C8 (C) or HA1.7 (D). CDR1α, violet; CDR2β, purple; DR4 β chain, yellow. (E, F) Conserved interactions between the DR4 α1 helix and the CDR1β/2β loops of MS2-3C8 (E) or HA1.7 (F). CDR1β, yellow; CDR2β, orange; DR4 α chain, grey. Van der Waals contacts are shown as dotted black lines, hydrogen bonds as dotted red lines and salt bridges as solid red lines.
Table 2. Interactions between TCR MS2-3C8 and MBP–HLA-DR4.
| TCR | Hydrogen bonds | Number of van der Waals contacts | |||
|---|---|---|---|---|---|
| TCR-DR4 contacts | |||||
| CDR1α | Ser 27α | Oγ | His 81β | Nε2 | 1 |
| Gly 28α | His 81β | 3 | |||
| Thr 29α | O | Thr 77β | Oγ | 3 | |
| His 81β | 6 | ||||
| CDR2α | Leu 50α | Thr 77β | 1 | ||
| CDR3α | Asn 95α | Gly 58α | 1 | ||
| Ala 61α | 1 | ||||
| Lys 96α | Nη | Gln 57α | Oε | 3 | |
| CDR1β | Thr 29β | Ala 61α | 1 | ||
| Ala 64α | 3 | ||||
| Val 65α | 6 | ||||
| Thr 30β | Ala 61α | 1 | |||
| CDR2β | Asn 49β | Leu 60α | 2 | ||
| Ala 61α | 6 | ||||
| Ala 64α | 1 | ||||
| Glu 50β | Oε | Lys 67α | Nη | 1 | |
| Thr 55β | Oγ | Lys 39α | Nη | ||
| Gln 57α | 1 | ||||
| CDR3β | Arg 95β | Ala 61α | 1 | ||
| Gly 97β | Val 65α | 2 | |||
| Ser 98β | Gly 58α | 1 | |||
| Ala 61α | 5 | ||||
| Oγ | Asn 62α | Oδ | 4 | ||
| Val 65α | 1 | ||||
| Tyr 99β | Phe 54α | 2 | |||
| Gly 58α | 9 | ||||
| Ala 59α | 1 | ||||
| Asn 62α | 2 | ||||
| Asn 100β | O | Gln 70β | Nε | 6 | |
| Pro 102β | Asp 66β | 1 | |||
| TCR-MBP contacts | |||||
| CDR1α | Ser 27α | Ser P-1 | 3 | ||
| CDR3α | Gly 92α | Trp P1 | 1 | ||
| Gly P2 | 3 | ||||
| O | Ala P3 | N | 6 | ||
| Ala 93α | Ser P-1 | 1 | |||
| CDR3β | Gly 96β | Arg P7 | 4 | ||
| Pro P8 | 2 | ||||
| Gly 97β | Gln P6 | 2 | |||
| Ser 98β | N | Gln P6 | O | ||
| Tyr 99β | Ala P3 | 7 | |||
| Gly P5 | 1 | ||||
| Asn 100β | Arg P7 | 1 | |||
| Ser 101β | Oγ | Arg P7 | Nε | 6 | |
| Pro 102β | Arg P7 | 1 | |||
| Hydrogen bonds were calculated using a cutoff distance of 3.4 Å. The cutoff distance for van der Waals contacts was 4.0 Å. | |||||
All six CDR loops of MS2-3C8 participate in binding HLA-DR4. Although the germline-encoded CDR1 and CDR2 loops of MS2-3C8 (Vα4.1, Vβ2.1) and HA1.7 (Vα1.2, Vβ3.1) differ in both sequence and conformation, they contact similar sites on HLA-DR4, consistent with the conserved docking orientation. Thus, in both the MS2-3C8–MBP–DR4 and HA1.7–HA–DR4 complexes, CDR1α and CDR2α interact extensively with DR4 α-helical residues Thr77β and His81β (Figure 5C and D). Regarding CDR1β and CDR2β, these loops contact Lys39α, Ala64α and Lys67α in both the MS2-3C8–MBP–DR4 and HA1.7–HA–DR4 structures (Figure 5E and F). By contrast, the overall shift by autoimmune TCR 3A6 towards the N-terminus of the MBP self-peptide relative to the central position of MS2-3C8 results in engagement of different MHC residues by structurally equivalent CDR1 and CDR2 residues (Supplementary Table S1). This is also the case for TCR Ob.1A12, which uses the same Vβ segment (Vβ2.1) as MS2-3C8.
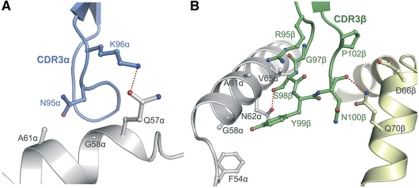
Whereas the CDR3α loop of MS2-3C8 forms only a few contacts with the α1 helix of HLA-DR4 (Figure 6A), the CDR3β loop straddles the peptide-binding groove and interacts extensively with both the α1 and β1 helices (Figure 6B). These interactions, which are mediated by CDR3β residues 95–102, include 35 van der Waals contacts (46% of the total to HLA-DR4) to α1 residues Phe54α, Gly58α, Ala59α, Ala61α, Asn62α and Val65α, and to β1 residues Asp66β and Gln70β, in addition to two hydrogen bonds (Table II). Of particular note are CDR3β Gly97 and Ser98, which contact the α1 helix (Figure 6B). These somatically generated junctional residues were found to be highly conserved among 23 TCRs recognizing MBP–DR4 (Muraro et al, 1997), even for TCRs using different Vβ segments; this suggests similar interactions between CDR3β and the DR4 helices to those observed for MS2-3C8.
Figure 6.
Interaction of CDR3 loops of MS2-3C8 with HLA-DR4. (A) Interactions between CDR3α and the α1 helix of HLA-DR4. (B) Interactions between CDR3β and the α1 and β1 helices of HLA-DR4. CDR3α, blue; CDR3β, orange; DR4 α chain, grey; DR4 β chain, yellow. Hydrogen bonds are shown as dotted red lines.
Of the total buried surface on HLA-DR4, excluding MBP, CDR1α, CDR2α and CDR3α contribute 19, 4 and 12%, respectively, compared with 10, 23 and 32%, respectively, for CDR1β, CDR2β and CDR3β. Hence, CDR3β of MS2-3C8 accounts for more of the binding interface with MHC than any other CDR. The dominance of CDR3β in the MS2-3C8–MBP–DR4 complex is further emphasized by comparing the number of contacts made by this loop in other TCR–peptide–MHC class II structures (Supplementary Table S2).
Peptide recognition by TCR MS2-3C8
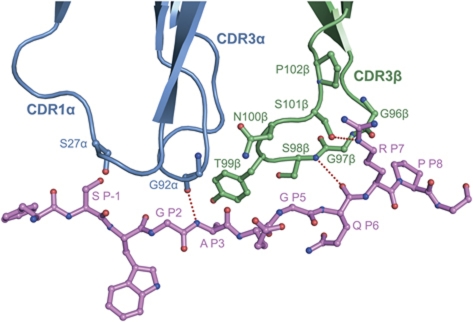
TCRs specific for foreign peptides presented by MHC class I or class II molecules typically utilize four of six CDR loops for peptide recognition: CDR1α, CDR3α, CDR1β and CDR3β (Rudolph et al, 2006). By contrast, most autoimmune TCRs contact peptide using only CDR3α and CDR3β, due to altered binding topology or to partial occupancy of the peptide-binding groove (Wucherpfennig et al, 2009). In this respect, MS2-3C8 is clearly more akin to autoimmune than to anti-foreign TCRs, despite its canonical docking mode and full occupancy of the peptide-binding groove of HLA-DR4 by MBP. Except for some minor contacts between CDR1α Ser27 and P-1 Ser, all interactions between MS2-3C8 and MBP are mediated by the CDR3 loops, primarily CDR3β (Table II). As shown in Figure 7, CDR3α contacts MBP residues P1–P3, while CDR3β contacts residues P3–P8. In agreement with the crystal structure, these residues comprise the minimal epitope recognized by MS2-3C8, as defined by T-cell proliferation assays (Muraro et al, 1997).
Figure 7.
Interaction of MS2-3C8 with the MBP peptide. Contact residues are drawn and labeled. TCR α chain, blue; TCR β chain, green; MBP peptide (ball-and-stick representation), magenta. Hydrogen bonds are indicated as dotted red lines.
The CDR3β loop resembles a horseshoe stepping on the C-terminal three-quarters of the MBP peptide (Figure 7), which is positioned higher in the binding groove compared with other DR4-bound peptides (Figure 4A). As a result, the contact interface between CDR3β and MBP is large (250 Å2) and flat, with good shape complementarity. Thus, the shape correlation statistic (Sc) (Lawrence and Colman, 1993) for the interface between CDR3β and MBP–DR4 is 0.78 (Sc=1.0 for interfaces with geometrically perfect fits), which is significantly higher than the Sc for the whole interface (0.65). In comparison, the corresponding Sc values for the HA1.7–HA–DR4 complex, which has six-fold lower affinity than the MS2-3C8–MBP–DR4 complex (Cole et al, 2007), are both 0.56. As the tip of CDR3β is directed towards CDR3α rather than the peptide, MS2-3C8 lacks the open pocket between CDR3 loops found in other TCRs that typically accommodates the side chain of residue P5. In MBP, P5 is glycine, whose lack of a side chain nonetheless permits this residue to pack against the CDR3β flap sealing off the CDR3 pocket of MS2-3C8 (Figure 7). Indeed, the interaction of CDR3β with MBP is distinguished by the multiple close contacts made by this loop to the main chain of the self-peptide. These factors, combined with the engagement of both DR4 helices by CDR3β, are probably key to the unexpectedly high affinity of MS2-C38 for its self-antigen.
As described above, the long side chain of secondary anchor residue P7 Arg is not situated in the P7 pocket of HLA-DR4. Rather, it is sandwiched between the DR4 β1 helix and the CDR3β loop. P7 Arg alone accounts for half (12 of 24) the van der Waals contacts made by CDR3β to MBP, as well as a side-chain–side-chain hydrogen bond linking the guanidinium group of P7 Arg to the Oγ atom of CDR3β Ser101 (Figure 7). Collectively, these interactions underscore the importance P7 Arg to complex formation. We therefore conclude that P7 Arg compensates for destabilizing the MBP–DR4 self-ligand, which likely enabled MS2-3C8 T cells to escape thymic deletion, by reinforcing the interaction between MBP and TCR, which permitted activation of these autoreactive cells in the periphery.
Mutational analysis of TCR MS2-3C8 binding to MBP–HLA-DR4
To assess the functional contribution of germline-encoded CDR1/CDR2 versus somatically generated CDR3 loops to complex formation, six CDR residues were selected for alanine mutagenesis: CDR1α Thr29, CDR3α Lys96, CDR2β Glu50, CDR2β Thr55, CDR3β Ser98 and CDR3β Asn100. In the MS2-3C8–MBP–DR4 structure, CDR1α Thr29 makes multiple contacts with HLA-DR4 Thr77β and His81β (Figure 5C), CDR2β Glu50 forms a salt bridge with HLA-DR4 Lys67α (Figure 5D), and CDR2β Thr55 makes a side-chain–side-chain hydrogen bond with HLA-DR4 Lys39α (Figure 5D). In addition, all three germline-encoded residues occur at positions believed to control the conserved diagonal orientation of TCR on MHC (Marrack et al, 2008). Surprisingly, mutation of CDR2β Glu50 or CDR2β Thr55 to alanine had minimal effect on binding (KD=6.0 and 6.8 μM, respectively, compared with 5.0 μM for wild-type MS2-3C8) (Figure 1E and F; Table III), while mutation of CDR1α Thr29 to alanine actually increased affinity 12-fold (0.42 μM) (Figure 1C). Mutation of CDR3α Lys96 also had little effect on binding (KD=6.0 μM) (Figure 1D), despite the involvement of this residue in a side-chain–side-chain hydrogen bond with HLA-DR4 Gln57α (Figure 6A). In sharp contrast, alanine substitution of CDR3β Ser98 or CDR3β Asn100 abolished binding nearly completely (Figure 1G and H). In the MS2-3C8–MBP–DR4 structure, CDR3β Ser98 makes 14 van der Waals contacts and one side-chain–side-chain hydrogen bond with HLA-DR4 (Figure 6B; Table II), as well as one main-chain–main-chain hydrogen bond with the MBP peptide (Figure 7). As truncation of CDR3β Ser98 to alanine should still permit formation of the main-chain–main-chain hydrogen bond, this mutation should mostly affect interactions with HLA-DR4. Similarly, CDR3β Asn100 makes six contacts and one hydrogen bond with HLA-DR4 (Figure 6B; Table II), compared with only one contact with MBP.
Table 3. Binding parameters of wild-type and mutant TCR MS2-3C8 for MBP–HLA-DR4.
| CDR | Mutants | MHC contacts | Peptide contacts | KDa (μM) | kon ( × 103 M−1 s−1) | koff ( × 10−3 s−1) |
|---|---|---|---|---|---|---|
| Wild type | 5.0±0.1 | 1.93±0.01 | 10.6±0.03 | |||
| CDR1α | T29A | T77β, H81β | None | 0.42±0.02 | 9.88±0.02 | 4.14±0.00 |
| CDR3α | K96A | Q57β | None | 6.0±0.1 | ||
| CDR2β | E50A | K67α | None | 6.0±0.1 | ||
| T55A | K39α | None | 6.8±0.1 | |||
| CDR3β | S98A | G58α, A61α, N62α, V65α | Q P6 | >300 | ||
| N100A | Q70β | R P7 | >100 | |||
| aAll KDs were measured at equilibrium. | ||||||
These mutagenesis experiments, though not exhaustive, support our conclusion from the crystal structure that CDR3β, which accounts for more of the binding interface with HLA-DR4 than any other CDR (see above), has a major role in MHC recognition. In this respect, MS2-3C8 differs from the mouse MHC class I-restricted TCR 2C, whose most important energetic interactions are contributed by CDR1 and CDR2 (Manning et al, 1998; Lee et al, 2000). It is, however, remarkably reminiscent of the human MHC class I-restricted TCR LC13, whose CDR3 loops dominate peptide–MHC recognition, with CDR1 and CDR2 having only minor energetic roles (Borg et al, 2005).
Discussion
Negative selection in the thymus is based on the functional avidity of T cells for self-antigens, which is comprehensively determined by (1) the affinity of TCR for self-peptide–MHC, (2) the affinity of peptide for MHC and (3) the availability of and need for co-receptors and signaling molecules (Anderton and Wraith, 2002). To undergo positive selection and be in principle pathogenic, autoreactive T cells must meet two competing criteria: they must escape thymic deletion, but nevertheless signal with sufficient strength in the periphery to cause autoimmune disease. While only tiny amounts of antigen are generally available for negative selection (Anderson et al, 2002; Anderson and Kuchroo, 2003), many of the antigens recognized by autoimmune TCRs, including MBP, type II collagen and insulin, are abundant in the target organ of the disease (Brand et al, 2003; Nakayama et al, 2005; Sospedra and Martin, 2005). Pathogenic T cells can thus take advantage of the low abundance of such antigens in the thymus to avoid negative selection, but become activated when they encounter much higher densities of self-peptide–MHC complexes in the target organ (Wucherpfennig et al, 2009). This compensatory mechanism may overcome the low affinity of TCRs that engage self-peptide–MHC via suboptimal docking modes, such as Ob.1A12 and 3A6. It may also allow TCRs to recognize relatively unstable ligands, such as MBP Ac1–11–I-Au (Garcia et al, 2001) and MBP 111–129–DR4, studied here.
Based on the available structures of autoimmune TCR–peptide–MHC complexes, two broad categories of self-reactive TCRs may now be distinguished: (1) TCRs with altered binding topologies to self-peptide–MHC (Ob.1A12, 3A6) and (2) TCRs that bind self-peptide–MHC in the canonical diagonal orientation, but where there are structural defects or suboptimal anchors in the self-ligand (172.10, MS2-3C8). For both categories, however, the overall stability of the TCR–peptide–MHC recognition unit is markedly reduced, allowing the autoreactive T cells to escape negative selection (Wucherpfennig et al, 2009).
By examining T cells with high functional avidity for various myelin antigens (MBP, proteolipid protein and myelin oligodendrocyte glycoprotein), it was shown that immunodominant epitopes in MS patients are clearly biased towards epitopes, such as MBP 111–129, that bind HLA-DR molecules with low affinity (Bielekova et al, 2004). This negative correlation between immunodominance and strength of HLA-DR binding, which concurs with animal studies (Vanderlugt et al, 2000; Anderton et al, 2001; Goverman, 2009), supports the hypothesis that disease-associated MHC molecules permit escape of potentially deleterious high-avidity autoreactive T cells due to their poor peptide-binding properties (DiPaolo and Unanue, 2002; Starr et al, 2003; Stadinski et al, 2010). The system we have chosen for structural analysis exemplifies this concept of immunodominance: MBP 111–129 is an immunodominant epitope in MS patients that binds very weakly to HLA-DR4 (Valli et al, 1993; Muraro et al, 1997), whereas TCR MS2-3C8 binds MBP–DR4 with as high an affinity (KD=5.5 μM) as TCRs recognizing foreign ligands, and with an even slower off-rate (koff=0.011 s−1) (van der Merwe and Davis, 2003; Cole et al, 2007). The exceptionally slow off-rate of MS2-3C8 is notable, as off-rate appears to be the binding parameter that correlates best with T-cell responsiveness (van der Merwe and Davis, 2003; Stone et al, 2009). We propose that the tight binding of MS2-3C8 to MBP–DR4, and especially the slow off-rate of this interaction, overcome the weak binding of MBP to HLA-DR4 to sufficiently stabilize the TCR–peptide–MHC ternary complex to permit T-cell activation at high myelin antigen concentrations, particularly once a naïve MS2-3C8-bearing T cell has differentiated into a memory cell with reduced co-receptor requirements for activation or at high myelin antigen concentrations, such as may be encountered in the CNS.
The structure of the MS2-3C8–MBP–DR4 complex revealed why the self-peptide binds weakly to HLA-DR4, and how the TCR overcomes this instability. Compared with peptides that bind HLA-DR4 with high affinity, MBP 114–129 lies less deeply in the binding groove, due to suboptimal anchor resides at positions P6 and P7. This elevation results in the net loss of several hydrogen bonds and numerous van der Waals contacts relative to high-affinity peptide–DR4 complexes, which translate into weak binding affinity (Valli et al, 1993; Muraro et al, 1997). We also found that MS2-3C8 engages its self-ligand via a docking mode that conforms to the central diagonal orientation of high-affinity anti-microbial TCRs, but is distinct from the unconventional docking topology of autoimmune TCRs Ob.1A12 and 3A6, which bind their self-ligands with very low affinity. These results support the view that the central diagonal orientation represents an optimal binding mode for maximizing interactions between TCR and peptide (Rudolph et al, 2006; Marrack et al, 2008), and that deviations from this orientation are likely to reduce affinity. Indeed, MS2-3C8 binds MBP–DR4 with six-fold higher affinity than HA1.7 binds HA–DR4 (Cole et al, 2007).
Of particular importance to complex formation is MBP residue P7 Arg, which appears to have the dual role of weakening the interaction of MBP with HLA-DR4 while strengthening that between MBP and MS2-3C8. In addition, the unusual conformation of CDR3β, which allows this loop to pack against the main chain of MBP, rather than simply contact sides chains, results in a large and very complementary interaction surface between CDR3β and the self-antigen, with more contacts than in other TCR–peptide–MHC class II complexes (Supplementary Table S2).
The MS2-3C8–MBP–DR4 structure also exhibits certain features that distinguish autoimmune from anti-foreign complexes (Deng and Mariuzza, 2007; Wucherpfennig et al, 2009). Most notably, MS2-3C8 contacts peptide almost exclusively through the CDR3 loops, with only minimal participation by CDR1α. In addition, as in the case of Ob.1A12 (Hahn et al, 2005), the somatically generated CDR3 loops of MS2-3C8 make about as many contacts to the MHC helices as the germline-encoded CDR1 and CDR2 loops, even though MS2-3C8, unlike Ob.1A12, engages peptide–MHC in the canonical diagonal orientation. Particularly prominent is the contribution of CDR3β, which straddles the peptide-binding groove and contacts both the DR4 α1 and β1 helices.
The human MS2-3C8–MBP–DR4 complex shares certain features with the complexes between three mouse TCRs (172.10, 1934.4 and cl19) and MBP–I-Au (Maynard et al, 2005; Feng et al, 2007), but also presents significant differences. The MBP–I-Au ligand is unusual in that the N-terminal one third of the binding groove is empty (He et al, 2002). The groove contains only the first seven residues of the MBP Ac1–11 peptide, leaving the P1 and P2 pockets of I-Au unoccupied, which likely explains the short half-life of the structurally defective MBP–I-Au complex. By contrast, the binding groove of HLA-DR4 is completely filled by MBP 114–129, although portions of the peptide are loosely accommodated. Like MS2-3C8, TCRs specific for MBP–I-Au recognize their self-ligand via a canonical docking orientation (Maynard et al, 2005; Feng et al, 2007). However, as a consequence of partial occupancy of the I-Au-binding groove by MBP Ac1–11, TCRs 172.10, 1934.4 and cl19 only recognize six peptide residues (P3–P8), compared with nine (P-1 to P8) in the case of MS2-3C8. In addition, whereas the interaction of TCRs 172.10, 1934.4 and cl19 with MBP Ac1–11 is characterized by a paucity of specific contacts between TCR and peptide (Maynard et al, 2005; Feng et al, 2007), such structural degeneracy is not evident in the interface of MS2-3C8 with MBP 114–129, which possesses high shape and chemical complementarity. In conclusion, the MS2-3C8–MBP–DR4 structure provides a framework for understanding how autoreactive T cells can successfully target pathogenic self-epitopes with unfavourable MHC-binding properties that may nevertheless be dominant in human autoimmune disease.
Materials and methods
Protein production and purification
Soluble MBP–DR4 was prepared by in vitro folding from inclusion bodies produced in Escherichia coli. The gene encoding extracellular residues Ile1–Ala182 of the HLA-DR4 α chain (DRA*0101) was inserted into the expression vector pET-26b(+) (Novagen). The construct for the HLA-DR4 β chain (DRB1*0401), which encoded a fusion protein comprising MBP 114–126 (FSWGAEGQRPGFG) connected by a 16-mer peptide linker (SGGGSLVPRGSGGGGS) to residues Gly1–Ser192 of the β chain, was cloned into the same vector. The HLA-DR4 α and β chains were expressed separately as inclusion bodies in E. coli BL21(DE3) cells (Novagen). Inclusion bodies were dissolved in 8 M urea, 50 mM Tris–HCl (pH 8.0) and 10 mM DTT, followed by purification on a Poros HQ50 anion exchange column (Perspective Biosystems) in 50 mM Tris–HCl, 8 M urea and 1 mM DTT at pH 8.0 (DRα) or pH 8.5 (DRβ), using a linear NaCl gradient. For in vitro folding, the purified subunits were diluted to a final concentration of 40 mg l−1 each in a folding solution containing 50 mM Tris–HCl, 30% (w/v) glycerol, 0.5 mM EDTA, 3 mM reduced glutathione and 0.9 mM oxidized glutathione (pH 8.0). After 2 weeks at 4°C, the folding solution was concentrated and dialysed against 50 mM MES (pH 6.0). Purification of MBP–DR4 was carried out with sequential Superdex S-200 and MonoQ FPLC columns (GE Healthcare).
For the production of biotinylated MBP–DR4, a 17-amino acid tag (GGGLNDIFEAQKIEWHE) was added to the C-terminus of the α chain. The tagged protein was produced in the same way as non-tagged protein. Biotinylation was performed using biotin protein ligase (Avidity); excess biotin and ligase were removed with a Superdex S-200 column.
The α and β chains of TCR MS2-3C8 (residues 1–206 and 1–245, respectively) were expressed separately as inclusion bodies in E. coli BL21(DE3) cells. The inclusion bodies were dissolved in 8 M urea, 50 mM Tris–HCl (pH 8.0) and 10 mM DTT. For in vitro folding, the MS2-3C8 α and β chains were mixed in a 1.2:1 molar ratio and diluted into a folding buffer containing 1.0 M L-arginine-HCl, 50 mM Tris–HCl (pH 8.5), 1 mM EDTA, 3 mM reduced glutathione and 0.9 mM oxidized glutathione to a final concentration of 50 mg l−1. After 72 h at 4°C, the folding mixture was dialysed against 50 mM MES (pH 6.0). Disulfide-linked MS2-3C8 heterodimer was purified using Superdex S-200 and MonoQ columns. Mutants of MS2-3C8 (CDR1α T29A, CDR3α K96A, CDR2β E50A, CDR2β T55A, CDR3β S98A and CDR3β N100A) were prepared similarly.
SPR analysis
The interaction of TCR MS2-3C8 (wild type and mutants) with MBP–DR4 was assessed by SPR using a BIAcore T100 biosensor at 25°C. Biotin-tagged MBP–DR4 was immobilized on a streptavidin-coated BIAcore SA chip, followed by blocking the remaining streptavidin sites with 20 μM biotin solution. An additional flow cell was injected only with free biotin to serve as a blank control. For analysis of TCR binding, solutions containing different concentrations of MS2-3C8 were flowed sequentially over the chips immobilized with MBP–DR4 and the blank. For equilibrium measurements, injections of MS2-3C8 were stopped at 30 s after SPR signals reached a plateau. Both equilibrium and kinetic data were fitted with a 1:1 binding model using BIAevaluation software (BIAcore).
Crystallization and data collection
Purified TCR MS2-3C8 and MBP–DR4 were mixed at a 1:1 molar ratio and concentrated to 10 mg ml−1. Initially, small needle-shaped crystals were obtained in hanging drops at room temperature in 10% (w/v) polyethylene glycol 8000, 0.2 M calcium acetate and 0.1 M imidazole (pH 8.0). Larger plate-like crystals were grown against a reservoir of 21% (w/v) polyethylene glycol 4000, 0.14 M MgCl2 and 70 mM Tris–HCl (pH 8.5) by seeding crushed crystals into a mixture of 1 μl protein solution and 1 μl 10% (w/v) polyethylene glycol 8000, 0.2 M calcium acetate and 0.1 M imidazole (pH 8.5). For data collection, a crystal with dimensions 0.5 × 0.2 × 0.05 mm3 was briefly soaked in a cryoprotectant solution (mother liquor plus 25% (v/v) glycerol) and flash-cooled in liquid nitrogen. Diffraction data were recorded at beamline X29 of the National Synchrotron Light Source with an ADSC Quantum-315 CCD detector. The data were processed and scaled using the HKL2000 programs (Otwinowski and Minor, 1997). Data collection statistics are shown in Table I.
Structure determination and refinement
The structure of the MS2-3C8–MBP–DR4 complex was determined by molecular replacement with the program Phaser (Storoni et al, 2004). Two MBP–DR4 molecules were found immediately using HA–DR4 (Protein Data Bank accession code 1J8H) (Hennecke and Wiley, 2002) as the search model, but only one MS2-3C8 molecule was found in a different asymmetric unit cell from the one containing the MBP–DR4 molecules using various TCRs (1YMM, 3DX9, 1KGC) (Kjer-Nielsen et al, 2002; Hahn et al, 2005; Archbold et al, 2009) as search models. A new search model consisting of a complex of MS2-3C8 and MBP–DR4 was then created by positioning a symmetry mate of the TCR opposite one of the found MBP–DR4 molecules. Two complete complexes were then readily located by Phaser (Storoni et al, 2004). Rigid-body and simulated-annealing refinements were conducted using CNS1.2 (Brunger et al, 1998). Manual model fitting was performed with the program Coot (Emsley et al, 2010). The 16-mer peptide connecting MBP to the HLA-DR4 β chain had visible electron density in one of the two complexes and was built accordingly. Subsequent refinements of positional and atomic displacement parameters were carried out using Phenix-1.5.2 (Adams et al, 2010). Water molecules were added with a distance cutoff of 3.4 Å. The final Rwork and Rfree values are 23.9 and 27.9%, respectively. Refinement statistics are summarized in Table I. Solvent-accessible surface areas were calculated using the program AREAIMOL (Collaborative Computational Project No. 4, 1994), with a probe radius of 1.4 Å. Contacts were identified by CONTACT using a cutoff distance of 4.0 Å. All structure figures were prepared using PyMOL (http://pymol.org/). Atomic coordinates and structure factors have been deposited in the Protein Data Bank under accession code 3O6F.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (AI036900 and AI073654) and the National Multiple Sclerosis Society (RG2747). We thank H Robinson (Brookhaven National Synchrotron Light Source) for X-ray data collection. Support for beamline X29 comes from the Offices of Biological and Environmental Research and of Basic Energy Sciences of the US Department of Energy, and from the National Center for Research Resources of the National Institutes of Health.
Author contributions: YY designed and performed the experiments and analysed the results. YL and RAM supervised the research. MCK carried out experimental work. RM provided reagents and contributed to manuscript writing. YY and RAM wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66: 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam SM, Travers PJ, Wung JL, Nasholds W, Redpath S, Jameson SC, Gascoigne NRJ (1996) T-cell receptor affinity and thymocyte positive selection. Nature 381: 616–620 [DOI] [PubMed] [Google Scholar]
- Anderson AC, Kuchroo VK (2003) Expression of self-antigen in the thymus: a little goes a long way. J Exp Med 198: 1627–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dietrich A, Benoist C, Mathis D (2002) Projection of an immunological self shadow within the thymus by the AIRE protein. Science 298: 1395–1401 [DOI] [PubMed] [Google Scholar]
- Anderton SM, Radu CG, Lowrey PS, Ward ES, Wraith DC (2001) Negative selection during peripheral immune response to antigen. J Exp Med 193: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderton SM, Wraith DC (2002) Selection and fine-tuning of the autoimmune T-cell repertoire. Nat Rev Immunol 2: 487–498 [DOI] [PubMed] [Google Scholar]
- Appel H, Gauthier L, Pyrdol J, Wucherpfennig KW (2000) Kinetics of T-cell receptor binding by bivalent HLA-DR. Peptide complexes that activate antigen-specific human T-cells. J Biol Chem 275: 312–321 [DOI] [PubMed] [Google Scholar]
- Archbold JK, Macdonald WA, Gras S, Ely LK, Miles JJ, Bell MJ, Brennan RM, Beddoe T, Wilce MC, Clements CS, Purcell AW, McCluskey J, Burrows SR, Rossjohn J (2009) Natural micropolymorphism in human leukocyte antigens provides a basis for genetic control of antigen recognition. J Exp Med 206: 209–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielekova B, Sung MH, Kadom N, Simon R, McFarland H, Martin R (2004) Expansion and functional relevance of high-avidity myelin-specific CD4+ T cells in multiple sclerosis. J Immunol 172: 3893–3904 [DOI] [PubMed] [Google Scholar]
- Borg NA, Ely LK, Beddoe T, Macdonald WA, Reid HH, Clements CS, Purcell AW, Kjer-Nielsen L, Miles JJ, Burrows SR, McCluskey J, Rossjohn J (2005) The CDR3 regions of an immunodominant T cell receptor dictate the ‘energetic landscape’ of peptide-MHC recognition. Nat Immunol 6: 171–180 [DOI] [PubMed] [Google Scholar]
- Brand DD, Kang AH, Rosloniec EF (2003) Immunopathogenesis of collagen arthritis. Springer Semin Immunopathol 25: 3–18 [DOI] [PubMed] [Google Scholar]
- Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL (1998) Crystallography and NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr 54: 905–921 [DOI] [PubMed] [Google Scholar]
- Cole DK, Pumphrey NJ, Boulter JM, Sami M, Bell JI, Gostick E, Price DA, Gao GF, Sewell AK, Jakobsen BK (2007) Human TCR-binding affinity is governed by MHC class restriction. J Immunol 178: 5727–5734 [DOI] [PubMed] [Google Scholar]
- Collaborative Computational Project, Number 4 (1994) The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr 50: 240–255 [DOI] [PubMed] [Google Scholar]
- Daniels MA, Teixeiro E, Gill J, Hausmann B, Roubaty D, Holmberg K, Werlen G, Hollander GA, Gascoigne NRJ, Palmer E (2006) Thymic selection threshold defined by compartmentalization of Ras/MAPK signaling. Nature 444: 724–729 [DOI] [PubMed] [Google Scholar]
- Deng L, Mariuzza RA (2007) Recognition of self-peptide–MHC complexes by autoimmune T cell receptors. Trends Biochem Sci 32: 500–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessen A, Lawrence CM, Cupo S, Zaller DM, Wiley DC (1997) X-ray crystal structure of HLA-DR4 (DRA*0101, DRB1*0401) complexed with a peptide from human collagen II. Immunity 7: 473–481 [DOI] [PubMed] [Google Scholar]
- DiPaolo RJ, Unanue ER (2002) Cutting edge: chemical dominance does not relate to immunodominance: studies of the CD4+ response to a model antigen. J Immunol 169: 1–4 [DOI] [PubMed] [Google Scholar]
- Ely LK, Burrows SR, Purcell AW, Rossjohn J, McCluskey J (2008) T cells behaving badly: structural insights into alloreactivity and autoimmunity. Curr Opin Immunol 20: 575–580 [DOI] [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, Cowtan K (2010) Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66: 486–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D, Bond CJ, Ely LK, Maynard J, Garcia KC (2007) Structural evidence for a germline-encoded T cell receptor–major histocompatibility complex interaction ‘codon’. Nat Immunol 8: 975–983 [DOI] [PubMed] [Google Scholar]
- Fremont DH, Hendrickson WA, Marrack P, Kappler J (1996) Structures of an MHC class II molecule with covalently bound single peptides. Science 272: 1001–1004 [DOI] [PubMed] [Google Scholar]
- Garcia KC, Radu CG, Ho J, Ober RJ, Ward ES (2001) Kinetics and thermodynamics of T cell receptor-autoantigen interactions in murine experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA 98: 6818–6823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnow CC, Sprent J, Fazekas de St Groth B, Vinuesa CG (2005) Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature 435: 590–597 [DOI] [PubMed] [Google Scholar]
- Goverman J (2009) Autoimmune T cell responses in the central nervous system. Nat Rev Immunol 9: 393–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras S, Burrows SR, Kjer-Nielsen L, Clements CS, Liu YC, Sullivam LC, Bell MJ, Brooks AG, Purcell AW, McCluskey J, Rossjohn J (2009) The shaping of T cell repertoire recognition by self-tolerance. Immunity 30: 193–203 [DOI] [PubMed] [Google Scholar]
- Hahn M, Nicholson MJ, Pyrdol J, Wucherpfennig KW (2005) Unconventional topology of self peptide-major histocompatibility complex binding by a human autoimmune T cell receptor. Nat Immunol 6: 490–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer J, Valsasnini P, Tolba K, Bolin D, Higelin J, Takacs B, Sinigaglia F (1993) Promiscuous and allele-specific anchors in HLA-DR-binding peptides. Cell 74: 197–203 [DOI] [PubMed] [Google Scholar]
- Hammer J, Gallazzi F, Bono E, Karr RW, Guenot J, Valsasnini P, Nagy ZA, Sinigaglia F (1995) Peptide binding specificity of HLA-DR4 molecules: correlation with rheumatoid arthritis association. J Exp Med 181: 1847–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XL, Radu C, Sidney J, Sette A, Ward ES, Garcia KC (2002) Structural snapshot of aberrant antigen presentation linked to autoimmunity: the immunodominant epitope of MBP complexed with I-Au. Immunity 17: 83–94 [DOI] [PubMed] [Google Scholar]
- Hemmer B, Fleckenstein BT, Vergelli M, Jung G, McFarland H, Martin R, Wiesmuller K-H (1997) Identification of high potency microbial and self ligands for a human autoreactive class II-restricted T cell clone. J Exp Med 185: 1651–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennecke J, Wiley DC (2002) Structure of a complex of the human α/β T cell receptor (TCR) HA1.7, influenza hemagglutinin peptide, and major histocompatibility complex class II molecule, HLA-DR4 (DRA*0101 and DRB1*0401): insight into TCR cross-restriction and alloreactivity. J Exp Med 195: 571–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler JW, Roehm N, Marrack P (1987) T cell tolerance by clonal elimination in the thymus. Cell 49: 273–280 [DOI] [PubMed] [Google Scholar]
- Kjer-Nielsen L, Clements CS, Brooks AG, Purcell AW, McCluskey J, Rossjohn J (2002) The 1.5 Å crystal structure of a highly selected antiviral T cell receptor provides evidence for a structural basis of immunodominance. Structure 10: 1521–1532 [DOI] [PubMed] [Google Scholar]
- Lawrence MC, Colman PM (1993) Shape complementarity at protein-protein interfaces. J Mol Biol 234: 946–950 [DOI] [PubMed] [Google Scholar]
- Lee PU, Churchill HR, Daniels M, Jameson SC, Kranz DM (2000) Role of 2C T cell receptor residues in the binding of self- and allo-major histocompatibility complexes. J Exp Med 191: 1355–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Huang Y, Lue J, Quandt JA, Martin R, Mariuzza RA (2005) Structure of a human autoimmune TCR bound to a myelin basic protein self-peptide and a multiple sclerosis-associated MHC class II molecule. EMBO J 24: 2968–2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen LS, Andersson EC, Jansson L, Krogsgaard M, Andersen CB, Engberg J, Strominger JL, Svejgaard A, Hjorth JP, Holmdahl R, Wucherpfennig KW, Fugger L (1999) A humanized model for multiple sclerosis using HLA-DR2 and a human T-cell receptor. Nat Genet 23: 343–347 [DOI] [PubMed] [Google Scholar]
- Manning TC, Schlueter CJ, Brodnicki TC, Parke EA, Speir JA, Garcia KC, Teyton L, Wilson IA, Kranz DM (1998) Alanine scanning mutagenesis of an αβ T cell receptor: mapping the energy of antigen recognition. Immunity 8: 413–425 [DOI] [PubMed] [Google Scholar]
- Marrack P, Scott-Browne JP, Dai S, Gapin L, Kappler JW (2008) Evolutionarily conserved amino acids that control TCR-MHC interaction. Annu Rev Immunol 26: 171–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard J, Petersson K, Wilson DH, Adams EJ, Blondelle SE, Boulanger MJ, Wilson DB, Garcia KC (2005) Structure of an autoimmune T cell receptor complexed with class II peptide-MHC: insights into MHC bias and antigen specificity. Immunity 22: 81–92 [DOI] [PubMed] [Google Scholar]
- Muraro PA, Vergelli M, Kalbus M, Banks DE, Nagle JW, Tranquill LR, Nepom GT, Biddison WE, McFarland HF, Martin R (1997) Immunodominance of a low-affinity major histocompatibility complex-binding myelin basic protein epitope (residues 111-129) in HLA-DR4 (B1*0401) subjects is associated with a restricted T cell receptor repertoire. J Clin Invest 100: 339–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, Yu L, Wegmann DR, Hutton JC, Elliott JF, Eisenbarth GS (2005) Prime role of an insulin epitope in the development of type 1 diabetes in NOD mice. Nature 435: 220–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi P (2003) Negative selection and autoimmunity. Curr Opin Immunol 15: 668–676 [DOI] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W (1997) Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol 276: 307–326 [DOI] [PubMed] [Google Scholar]
- Quandt JA, Baig M, Yao K, Kawamura K, Huh J, Ludwin SK, Bian H-J, Bryant M, Quigley L, Nagy ZA, McFarland HF, Muraro PA, Martin R, Ito K (2004) Unique clinical and pathological features in HLA-DRB1*0401-restricted MBP 111-129-specific humanized TCR transgenic mice. J Exp Med 200: 223–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz EL, Tan K, Tang L, Kern P, Liu J, Xiong Y, Hussey RE, Smolyar A, Hare B, Zhang R, Joachimiak A, Chang HC, Wagner G, Wang J (1999) The crystal structure of a T cell receptor in complex with peptide and MHC class II. Science 286: 1913–1921 [DOI] [PubMed] [Google Scholar]
- Reiser J-B, Darnault C, Guimezanes A, Gregoire C, Mosser T, Schmitt-Verhulst AM, Fontecilla-Camps JC, Mazza G, Malissen B, Housset D (2000) Crystal structure of a T cell receptor bound to an allogeneic MHC molecule. Nat Immunol 1: 291–297 [DOI] [PubMed] [Google Scholar]
- Rudolph MG, Stanfield RL, Wilson IA (2006) How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol 24: 419–466 [DOI] [PubMed] [Google Scholar]
- Sospedra M, Martin R (2005) Immunology of multiple sclerosis. Annu Rev Immunol 23: 683–747 [DOI] [PubMed] [Google Scholar]
- Southwood S, Sidney J, Kondo A, del Guercio MF, Appella E, Hoffman S, Kubo RT, Chesnut RW, Grey HM, Sette A (1998) Several common HLA-DR types share largely overlapping peptide binding repertoires. J Immunol 160: 3363–3373 [PubMed] [Google Scholar]
- Stadinski BD, Zhang L, Crawford F, Marrack P, Eisenbarth GS, Kappler JW (2010) Diabetogenic T cells recognize insulin bound to IAg7 in an unexpected, weakly binding register. Proc Natl Acad Sci USA 107: 10978–10983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr TK, Jameson SC, Hogquist KA (2003) Positive and negative selection of T cells. Annu Rev Immunol 21: 139–176 [DOI] [PubMed] [Google Scholar]
- Stewart-Jones GB, McMichael AJ, Bell JI, Stuart DI, Jones EY (2003) A structural basis for immunodominant human T cell receptor recognition. Nat Immunol 4: 657–663 [DOI] [PubMed] [Google Scholar]
- Stone JD, Chervin AS, Kranz DM (2009) T-cell receptor binding affinities and kinetics: impact on T-cell activity and specificity. Immunology 126: 165–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storoni LC, McCoy AJ, Read RJ (2004) Likelihood-enhanced fast rotation functions. Acta Crystallogr D Biol Crystallogr 60: 432–438 [DOI] [PubMed] [Google Scholar]
- Valli A, Sette A, Kappos L, Oseroff C, Sidney J, Miescher G, Hochberger M, Albert ED, Adorini L (1993) Binding of myelin basic protein peptides to human histocompatibility leukocyte antigen class II molecules and their recognition by T cells from multiple sclerosis patients. J Clin Invest 91: 616–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Merwe PA, Davis SJ (2003) Molecular interactions mediating T cell antigen recognition. Annu Rev Immunol 21: 659–684 [DOI] [PubMed] [Google Scholar]
- Vanderlugt CL, Neville KL, Nikcevich KM, Eagar TN, Bluestone JA, Miller SD (2000) Pathologic role and temporal appearance of newly emerging autoepitopes in relapsing experimental autoimmune encephalomyelitis. J Immunol 164: 670–678 [DOI] [PubMed] [Google Scholar]
- Williams CB, Engle DL, Kersh GJ, Michael White J, Allen PM (1999) A kinetic threshold between negative and positive selection based on the longevity of the T cell receptor–ligand complex. J Exp Med 189: 1531–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig KW, Call MJ, Deng L, Mariuzza R (2009) Structural alterations in peptide-MHC recognition by self-reactive T cell receptors. Curr Opin Immunol 21: 590–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehn D, Bevan MJ (2006) Cells with low avidity for tissue-restricted antigen routinely evade central and peripheral tolerance and cause autoimmunity. Immunity 25: 261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.