Abstract
Many behavioural states are modulated by food availability and nutritional status. Here, we report that in Caenorhabditis elegans, the presence of an external food source enhances avoidance responses to soluble repellents sensed by the polymodal ASH neurons. This enhancement requires dopamine signalling and is mimicked by exogenous dopamine. Food modulation is dependent on the mechanosensory cilia of the dopaminergic neurons, indicating that dopamine is released in response to sensation of bacteria. Activation of the dopamine neurons leads within seconds to a transient state of increased sensory acuity. In vivo imaging experiments indicate that this dopamine-dependent sensitization results in part from modality-specific increases in the magnitude and duration of gustatory responses in the ASH neurons. The D1-like dopamine receptor DOP-4 acts cell autonomously in ASH to mediate effects on response magnitude. Thus, dopamine functions as a direct signal of the presence of food to control context-dependent behavioural states.
Keywords: C. elegans , dopamine, modulation, neuropeptide, nociception
Introduction
Sensory systems in both simple and complex animals are not static, but can switch between alternative behavioural states. This plasticity allows animals to adjust their behaviours in response to changes in the internal or external environment, such as feeding state, nutritional status, sleep deprivation and stress. To switch between behavioural states, the nervous system utilizes neuromodulators such as biogenic amines and neuropeptides. Neuromodulators act by altering the properties of specific neurons, resulting in changes in information processing and behavioural responses.
The nematode Caenorhabditis elegans offers a useful system to study neuromodulation at the behavioural, cellular and molecular levels. C. elegans has a compact nervous system consisting of 302 neurons, of which 32 are chemosensory and 28 are mechanosensory (Bargmann and Mori, 1997). Despite this small number, nematodes can sense a diverse variety of chemical and mechanical stimuli and generate relatively complex behaviours. C. elegans uses a large number of neuromodulators, including the monoamines serotonin, dopamine, octopamine and tyramine, as well as many neuropeptides (Rand and Nonet, 1997). In addition, a range of sophisticated genetic tools exists for studying behavioural states at the molecular level.
One particularly important factor that guides behaviour is feeding state. In the laboratory, C. elegans is grown using the E. coli strain OP50 as a food source, and various types of behaviours have been shown to be affected by the presence or absence of bacterial food. Specifically, the presence of food stimulates feeding (Avery and Horvitz, 1990) and egg laying (Trent et al, 1983), slows the rate of locomotion (Sawin et al, 2000), and modifies responses to sensory stimuli (Saeki et al, 2001; Chao et al, 2004; Hilliard et al, 2005; Mohri et al, 2005; Harris et al, 2009). These behavioural changes depend on a variety of neuromodulators, in particular, monoamines. For example, serotonin has been implicated in the food-dependent modulation of feeding (Horvitz et al, 1982) and locomotion (Sawin et al, 2000); tyramine affects foraging and the control of egg laying (Rex et al, 2004; Alkema et al, 2005); and octopamine is important in starvation-induced changes in gene expression (Suo et al, 2006). Neuropeptides also appear to be involved in food modulation of behaviour; for example, the flp-1 neuropeptide gene is required for the stimulation of egg laying by bacteria (Waggoner et al, 2000), and insulin-like peptides are important for some forms of food-dependent sensory plasticity (Kodama et al, 2006; Tomioka et al, 2006). For all these examples, however, it remains unclear whether these modulators are directly mobilized by the presence or absence of food, and if so what the neural and molecular mechanisms might be.
Another molecule strongly implicated as a direct signal of food in C. elegans is dopamine. Dopamine is required for the two effects of food on locomotion: a decrease in locomotion speed when worms encounter a food source (Sawin et al, 2000) and an increase in turn frequency when worms leave food (Hills et al, 2004). Dopamine is also required for the suppression of CREB-dependent gene expression in well-fed animals (Suo et al, 2009), as well as for the slowing of touch habituation on food (Sanyal et al, 2004; Kindt et al, 2007). There are only eight dopaminergic neurons in C. elegans: four CEPs in the nose, two ADEs in the head and two PDEs in the body (Sulston et al, 1975). All are ciliated neurons with putative mechanosensory dendrites, and the CEPs in particular have been shown to respond directly to mechanical stimuli (Kindt et al, 2007; Kang et al, 2010). This and other evidence suggest that the dopaminergic neurons directly sense the presence of bacteria using a mechanosensory modality.
In this study, we identify a new dopamine-mediated effect of feeding state on C. elegans behaviour. We find that responses to soluble repellents are enhanced in the presence of food, an effect dependent on direct food sensing by the dopamine neurons. Optical activation of the dopaminergic neurons mimics this effect within seconds, indicating that dopamine acutely modulates food-regulated behaviours. The enhancement of repellent responses is mediated in part by DOP-4, a D1-like dopamine receptor that functions in the ASH nociceptors to increase the magnitude of sensory responses. These results reveal that dopamine acts to signal the presence of food and to modify sensory perception in response to feeding state.
Results
Food enhances avoidance responses in ASH neurons
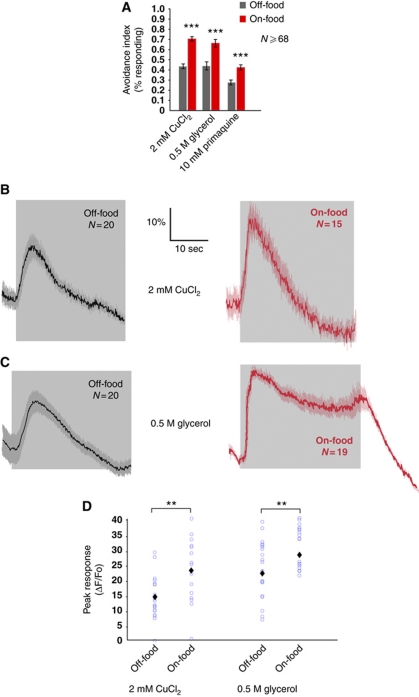
To investigate the effect of feeding state on avoidance responses, we tested how food affects behavioural responses to the soluble repellents copper, primaquine and glycerol. We measured the avoidance behaviour by applying a drop of repellent-containing solution to freely moving animals and scoring repellent-evoked reversals, an assay known as the drop test (Hilliard et al, 2002). We observed that wild-type animals showed significantly stronger avoidance of 2 mM CuCl2 in the presence of food than in the absence of food (Figure 1A). Likewise, we observed a significant increase in escape behaviour in response to glycerol (0.5 M) and primaquine (10 mM) in the presence of food compared with that in food-free conditions (Figure 1A). These results indicate that food enhances avoidance of soluble repellents in wild-type animals.
Figure 1.
Food enhances taste responses in ASH neurons. (A) Avoidance of soluble repellents is increased by food in wild-type animals. Fraction of animals on- and off-food reversing after delivery of a drop with repellent. Avoidance to 2 mM CuCl2, 10 mM primaquine and 0.5 M glycerol is increased on-food. (***P⩽0.0001, t-test), N⩾68. (B, C) Average neuronal responses in ASH. Grey shading indicates the duration of the stimulus. (B) Neuronal responses to 2 mM CuCl2 are increased by food. (C) Neuronal responses to 0.5 M glycerol are increased by food. (D) Quantification of ASH responses to 2 mM CuCl2 and 0.5 M glycerol on- and off-food. Blue circles represent individual animals assayed. Black diamonds indicate average value. Food increases the magnitude of ratio changes in wild-type animals. Statistical significance according to the Mann-Whitney test is indicated (**P<0.01), N⩾15.
Avoidance of copper, glycerol and primaquine is mainly mediated by the nociceptive neuron ASH (Bargmann et al, 1990; Sambongi et al, 1999; Hilliard et al, 2004). Transient activation of ASH generates more prolonged activation of the backward command interneurons, which evoke escape responses (reversals) by triggering a transition between backward and forward locomotion (Faumont and Lockery, 2006; Guo et al, 2009). In principle, the presence of food could enhance avoidance of these compounds by increasing the sensitivity or excitability of the ASH neurons themselves; alternatively, the effect could be the result of changes elsewhere in the escape circuit. To investigate these possibilities, we used calcium imaging to measure repellent-evoked neuronal activity in ASH on- and off-food. We found that in the presence of food, the magnitude of the neuronal responses to copper and glycerol was increased (Figure 1B–D). The presence of food also increased the duration of the response to 0.5 M glycerol (Figure 1C and D), as indicated by the integral of the ratio trace over the course of the response (Supplementary Figure 1). Thus, the presence of food appears to enhance avoidance of soluble repellents by increasing and in some cases by prolonging ASH sensory responses.
Food modulation of ASH sensory responses is mediated by dopamine
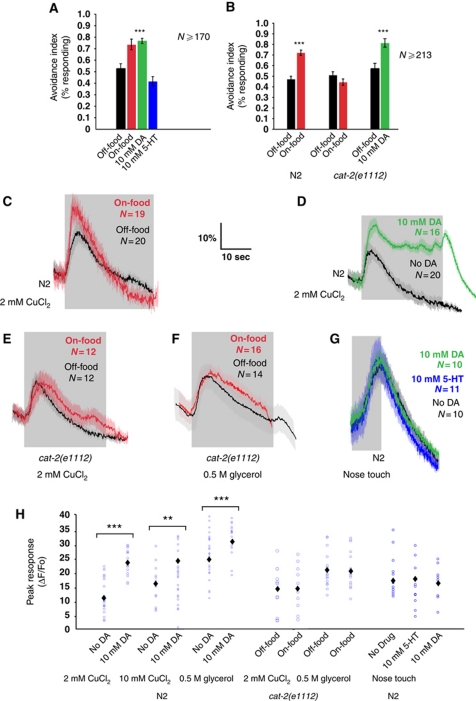
Monoamines, in particular dopamine and serotonin, have been implicated in food-related behavioural plasticity in C. elegans (Chao et al, 2004; Harris et al, 2009; Ezak and Ferkey, 2010). We therefore investigated whether dopamine and/or serotonin are involved in mediating the enhancing effect of food on avoidance responses. Previous studies have found that exogenous serotonin and dopamine added to the experimental plates or the buffer can mimic the effects of aminergic signalling on many behaviours (Horvitz et al, 1982; Schafer and Kenyon, 1995; Chao et al, 2004; Ezak and Ferkey, 2010). Therefore, we performed the drop test on wild-type animals on food-free plates containing 10 mM dopamine or 10 mM serotonin. We observed that exogenous dopamine increased avoidance responses to copper; in contrast, exogenous serotonin did not affect avoidance responses to copper (Figure 2A). We also used calcium imaging to test whether addition of 10 mM dopamine or 10 mM serotonin to the recording buffer affects neuronal responses in ASH. We found that whereas serotonin does not affect ASH calcium transients (data not shown), 10 mM dopamine increases both the magnitude and the duration of responses to 2 mM CuCl2 (Figure 2D and H and Supplementary Figure 1), 10 mM CuCl2 (Figure 2H and Supplementary Figure 1) and 0.5 M glycerol (Figure 2H and Supplementary Figure 1). We reported previously that responses to a non-chemical stimulus, nose touch, are enhanced by exogenous serotonin (Hilliard et al, 2005). However, in this study, we could not find any differences between nose touch responses off-food and in the presence of exogenous dopamine or serotonin (Figure 2G and H). Thus, exogenous dopamine mimics many of the effects of food on ASH responses, though some of these (e.g., the prolonging of CuCl2 responses) may be non-physiological.
Figure 2.
Food modulation of ASH involves dopamine. (A) Avoidance is increased by exogenous dopamine (DA). Avoidance is not increased by exogenous serotonin. Fraction of wild-type animals off-food, on-food, on 10 mM DA and on 10 mM serotonin, reversing after delivery of a drop with CuCl2. (***P<0.0001 ratio t-test), N⩾170. (B) Avoidance is not increased by food but by exogenous DA in cat-2(e1112). Fraction of wild-type and cat-2(e1112) animals, on- and off–food, and on and off 10 mM DA, reversing after delivery of a drop with 2 mM CuCl2. N⩾213. (C–F) Average neuronal responses in ASH. Grey shading indicates the duration of the stimulus. (C) Neuronal responses to 2 mM CuCl2 are increased by food in wild-type animals. (D) Responses to 2 mM CuCl2 in wild-type animals are increased by 10 mM exogenous DA. (E) Neuronal responses to 2 mM CuCl2 are not affected in cat-2(e1112). (F) Neuronal responses to 0.5 M glycerol are not affected in cat-2(e1112). (G) Responses to nose touch are not affected by 10 mM serotonin and 10 mM DA in wild-type animals. (H) Quantification of ASH responses. Blue circles represent individual animals assayed. Black diamonds indicate average value. Exogenous DA increases the magnitude of the ratio change in wild-type animals. In cat-2(e1112), ASH responses to 2 mM CuCl2 and 0.5 M glycerol are not increased by food. Exogenous DA or serotonin does not affect the ratio change in wild-type animals in response to nose touch. (***P<0.001, **P<0.01, Mann–Whitney rank sum test), N⩾12.
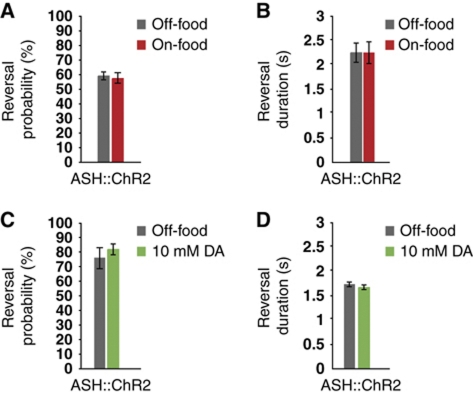
The modality-specific effects of dopamine on stimulus-evoked calcium transients suggest that dopamine modulation specifically affects sensitivity to chemical repellents rather than the general excitability or synaptic activity of the ASH neurons. To investigate this possibility further, we used a channelrhodopsin 2 (ChR2) transgene expressed specifically in ASH to test the effects of food and dopamine on reversals evoked by non-specific depolarization of ASH. We observed that exposure to blue light evoked reversals in this strain that were dependent on the ChR2 cofactor retinal, indicating that ChR2 could robustly activate ASH. Neither the frequency nor the magnitude of these reversals was detectably affected by the presence or absence of food. Likewise, addition of exogenous dopamine to animals off-food did not enhance ChR2-evoked reversal responses (Figure 3A–D). These results further support the hypothesis that dopamine modulation of ASH is repellent-specific and most likely acts at the level of the sensory response.
Figure 3.
Food and dopamine do not affect reversals caused by optogenetic activation of the ASH neurons. (A–D) Reversals following optogenetic activation of the ASH neurons. (A) Reversal probability is not increased by food. (B) Reversal duration is not increased by food. (C) Reversal probability is not increased by DA. (D) Reversal duration is not increased by DA.
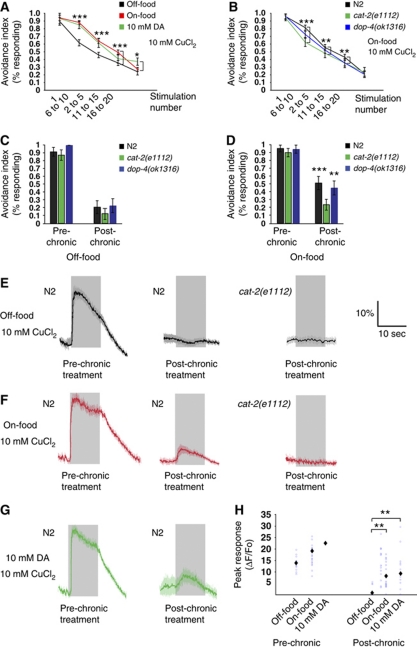
Together, these results suggest that endogenous dopamine signalling might specifically enhance ASH responses to chemical repellents in response to food. To test this possibility, we investigated the phenotype of cat-2(e1112) animals, which are defective in dopamine biosynthesis (Lints and Emmons, 1999). In behavioural experiments, we observed that cat-2(e1112) animals showed indistinguishable avoidance responses to copper in the presence and absence of food (Figure 2B). In contrast, on plates containing 10 mM dopamine, the avoidance response of cat-2(e1112) animals was increased (Figure 2B). Thus, endogenous dopamine appears to be necessary for the enhancement of avoidance responses by food. We also analysed ASH neuronal responses in cat-2 mutants by calcium imaging. In these experiments, neither the magnitude nor the duration of calcium transients evoked by 2 mM CuCl2 or 0.5 M glycerol were altered by the presence of food (Figure 2E, F and H and Supplementary Figure 1). Together, these results indicate that endogenous dopamine is necessary for food modulation of ASH-mediated avoidance of soluble repellents, and suggest that dopamine signalling directly or indirectly modulates ASH sensory responses generated by food. We also tested whether food affected the ASH sensory adaptation to repeated or continuous stimulation. We showed previously that ASH-mediated avoidance responses attenuate after receiving repeated stimuli or after prolonged incubation in 10 mM CuCl2 (Hilliard et al, 2005). We found that in the presence of food, wild-type animals adapt more slowly to repeated stimulation with 10 mM CuCl2 as assayed by the drop test (Figure 4A). Addition of exogenous dopamine to food-free assay plates slowed habituation to copper, mimicking the effect of food (Figure 4A). Conversely, dopamine-deficient cat-2 mutants adapted rapidly in the presence of food (Figure 4B), suggesting that endogenous dopamine is required for the inhibitory effect of food on adaptation. We further tested whether food also affects adaptation to continuous stimulation using a different assay (Hilliard et al, 2002), in which the animals are tested after 15 s incubation in 10 mM CuCl2. Also, in these conditions, we found that adaptation in wild-type animals is inhibited in the presence of food (Figure 4C and D), and that cat-2 mutants adapt rapidly both in the absence and in the presence of food (Figure 4C and D).
Figure 4.
Food and dopamine inhibit adaptation to repellents. (A, B) Fraction of animals responding to repeated drops of 10 mM CuCl2. (A) Wild-type animals adapt slower on-food and 10 mM DA (P⩽0.036, t-test), N⩾30. (B) cat-2(e1112) animals do not adapt slower on-food. cat-2(e1112) adapts faster than wild type on-food, dop-4(ok1321) is not significantly different from wild-type animals. (***P<0.001, **P<0.01, *P<0.05, t-test), N⩾37. (C,D) Fraction of animals responding before and after a chronic stimulation. (C) cat-2(e1112) and dop-4(ok1321) adapt like wild type to chronic stimulation off-food. (D) cat-2(e1112) adapts faster than wild type on-food. dop-4(ok1321) is not significantly different from wild type. (E–G) Average neuronal responses in ASH before and after chronic treatment with 10 mM CuCl2. Grey shading indicates the duration of the stimulus. (E) Wild-type and cat-2(e1112) animals adapt completely off-food, and show no post-chronic responses. (F) On-food, wild type does not fully adapt, and still displays post-chronic responses. cat-2(e1112) completely adapts on-food. (G) Wild type does not fully adapt and displays post-chronic responses in exogenous DA. (P<0.001, t-test), N⩾24. (H) Quantification of ASH responses before and after chronic treatment. Blue circles represent individual animals assayed. Black diamonds indicate average value. Wild type adapts completely to a chronic stimulation with 10 mM CuCl2 off-food, and adapts less on-food or 10 mM DA. (P<0.01, Mann–Whitney rank sum test), N⩾17.
To determine whether these behavioural effects of food result from changes in ASH sensory responses, we measured copper-evoked calcium transients in ASH before and after 15 s treatment with 10 mM CuCl2. In the presence of food or exogenous dopamine, ASH responses were significantly larger after the chronic treatment than in animals tested in the absence of food or dopamine (Figure 4E–H). Moreover, in cat-2 mutants subjected to chronic 10 mM CuCl2 exposure, calcium transients were not affected by the presence of food, and calcium transients were absent in both on-food and off-food conditions (Figure 4E, F and H). Together, these experiments indicate that food decreases adaptation to CuCl2 through dopamine signalling. Our calcium imaging data show that the initial responses also to 10 mM CuCl2 were increased by food and dopamine; however, we could not detect this in our behavioural assays as almost 100% of the tested animals respond to this concentration of copper.
Modulation of avoidance behaviours requires dopaminergic sensory cilia
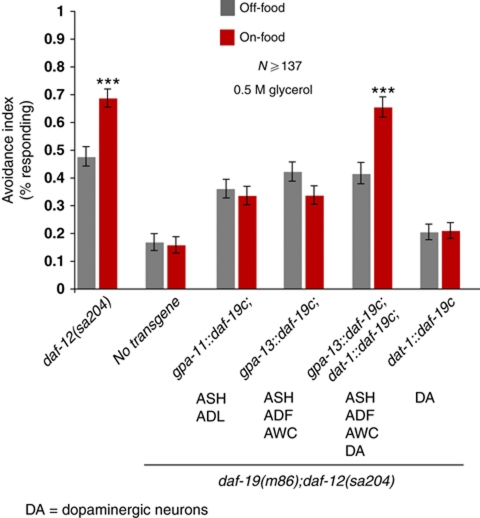
The dopaminergic neurons of C. elegans are mechanosensors that appear to directly sense the presence of a bacterial lawn (Sawin et al, 2000; Kindt et al, 2007). However, these neurons also receive input from other neurons via synapses and gap junctions, and could therefore in principle respond indirectly to internal signals of food availability. To determine whether the dopaminergic neurons sense and respond to food directly, we investigated whether the dopamine neurons’ sensory cilia are required for food modulation of repellent responses. We used a method called FRISSC (functional rescue in single sensory cilia), in which a null mutation in the ciliogenic RFX transcription factor DAF-19C, which is required for the production of cilia (Swoboda et al, 2000; Senti et al, 2009), is rescued cell specifically, allowing for restoration of ciliogenesis and sensory function in individual neurons in a daf-19 background (Senti et al, 2009). To suppress the dauer-formation phenotype in daf-19 animals, we also introduced a daf-12 mutation. As Figure 5 shows, daf-19;daf-12 animals have defective responses to glycerol. When we restored the cilia of the ASH neurons but not the dopamine neurons using either the gpa-11 or the gpa-13 promoter, we observed that responses to glycerol were of equal magnitude in the presence and absence of food (Figure 5). However, when we also restored cilia to the dopamine neurons using a dat-1∷daf-19c(+) transgene, we observed significantly stronger escape responses in the presence of food than in the absence of food (Figure 5). Thus, the cilia of the dopamine neurons are specifically required for food modulation, indicating that dopamine signalling is activated by external sensory cues.
Figure 5.
Dopaminergic neurons with intact cilia are required for increased avoidance on food. Fraction of worms reversing on- and off-food after a drop of 0.5 M glycerol. daf-12(sa204) animals show increased responses on food, whereas cilia-defective daf-19(m86);daf-12(sa204) animals exhibit defective avoidance on- and off-food. Rescuing DAF-19c under the gpa-11 (ASH, ADL) or gpa-13 (ASH, ADF, AWC, PHA and PHB) promoters results in normal responses off-food, but does not increase avoidance on food. Rescuing DAF-19c using both the gpa-13 and dat-1 (dopaminergic neurons) promoters leads to increased avoidance on food. (***P<0.001, t-test), N⩾103.
Dopamine is an acute signal of the presence of food
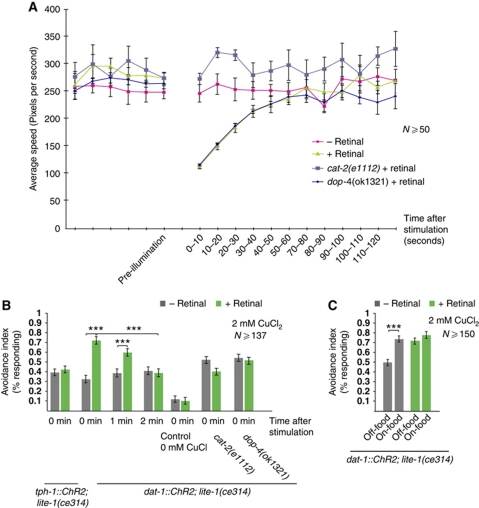
These genetic and pharmacological data suggest that dopamine is released in the presence of food to modulate avoidance behaviours and ASH activity. However, experiments using dopamine signalling-deficient mutants cannot address the dynamics of the dopamine-mediated food signal. In principle, dopamine signalling could act acutely to indicate the presence of food at a given moment; alternatively, dopamine signalling could act on a longer time scale to represent the animal's experience of food over the course of minutes or hours. To investigate the time scale of dopamine action, we generated transgenic lines expressing ChR2 (Nagel et al, 2005a, 2005b) under the dat-1 (dopamine neuron-specific) and tph-1 (serotonin neuron-specific) promoters, in a lite-1(ce314) background. LITE-1 mediates responses to ultraviolet light, and loss of lite-1 prevents native light responses (Edwards et al, 2008; Guo et al, 2009). We grew these animals in the presence or absence of the ChR2 cofactor retinal, and performed the behavioural tests on food-free media after a flash of blue light to activate the dopaminergic or serotonergic neurons, respectively. We observed that upon a 10 s blue-light stimulation of the dat-1∷ChR2 line, the animals’ rate of locomotion decreased within seconds, a response similar to the dopamine-dependent slowing response to a bacterial lawn (Sawin et al, 2000). In a cat-2 mutant background, this slowing response was not observed, indicating that the dopaminergic neurons inhibit locomotion speed through dopamine release. Blue-light stimulation of the tph-1∷ChR2 line likewise led to retinal-dependent slowing (data not shown), as expected from previous studies, indicating that serotonin inhibits locomotion. Blue-light exposure did not induce slowing in either line if the animals were grown in the absence of retinal. Next, we investigated the time course of the light-induced slowing of locomotion in the dat-1∷ChR2 line. We found that 60–70 s after the light flash, the speed had returned to its baseline level (Figure 6A). This is in line with other studies showing that within 5 min of being on food, animals display a decreased rate of locomotion (Sawin et al, 2000).
Figure 6.
Optogenetic activation of the dopaminergic neurons induces slowing and increases avoidance. (A) Normalized speed in animals expressing ChR2 under the dat-1 promoter (dopaminergic neurons). Shown is the normalized speed measured every 10 s. Animals not grown on retinal are not affected by blue-light stimulation. In animals grown on retinal, a 10-s-long blue-light stimulation induces slowing, which lasts for 60 s. (P⩽0.0006, t-test), N⩾50. Blue-light stimulation does not induce slowing in cat-2(e1112). dop-4(ok1321) slows like wild type. (B) Fraction of animals reversing after blue-light stimulation, followed by a drop of 2 mM CuCl2. Time between light activation and drop delivery is indicated. Animals not grown on retinal are not affected by the blue-light stimulation. Animals expressing ChR2 under the tph-1 promoter (serotonergic neurons) and grown on retinal do not have increased avoidance after the light flash. Animals expressing ChR2 under the dat-1 promoter and grown on retinal show increased avoidance after blue-light activation, and this effect lasts 1–2 min after the light flash. (P⩽0.0002, t-test), N⩾137. Avoidance is not affected in dat-1∷ChR2 transgenes in cat-2(e1112) and dop-4(ok1321) backgrounds. (C) Food does not further enhance avoidance in animals expressing ChR2 under the dat-1 promoter. Fraction of animals reversing after blue-light stimulation, followed by a drop of 2 mM CuCl2. dat-1∷ChR2; lite-1(ce314) animals not grown on retinal have enhanced responses on food. dat-1∷ChR2; lite-1(ce314) animals grown on retinal have enhanced responses both off- and on-food, and the avoidance is not further increased in the presence of food. (***P<0.001, t-test), N⩾150.
We next tested how activation of dopaminergic and serotonergic neurons by ChR2 affected ASH-mediated avoidance responses. We observed that the CuCl2 responses of retinal-grown dat-1∷ChR2 animals were significantly enhanced by blue-light stimulation, whereas animals of the same genotype grown without retinal or tph-1∷ChR2 animals grown with or without retinal showed no enhancement (Figure 6B). Thus, the acute activation of the dopaminergic neurons quickly and robustly enhances avoidance to CuCl2, whereas activation of the serotonergic neurons does not. When we performed the dat-1∷ChR2 photoactivation experiments in a dopamine-deficient cat-2 mutant background, we did not observe enhanced avoidance in response to blue light. Thus, activation of the dopaminergic neurons appears to modulate ASH responses through dopamine release.
We then investigated the persistence of dopamine-evoked sensitization to repellents. We found that when dat-1∷ChR2 animals were stimulated with CuCl2, a minute after receiving a blue-light flash, their avoidance response was slightly less sensitized than that immediately after stimulation (Figure 6B). By 2 minutes after dat-1∷ChR2 activation, the CuCl2 avoidance response had returned to its normal off-food level (Figure 6B). These results show a similar time course to the dat-1∷ChR2 effect on locomotion speed. Activation of dat-1∷ChR2 in the presence of food did not further enhance CuCl2 avoidance (Figure 6C), indicating that food and the dopamine neurons affect the same modulatory process. Thus, activation of the dopamine neurons appears to cause an immediate and transient enhancement of ASH-mediated repellent avoidance, suggesting that dopamine signals the acute presence of food in the environment.
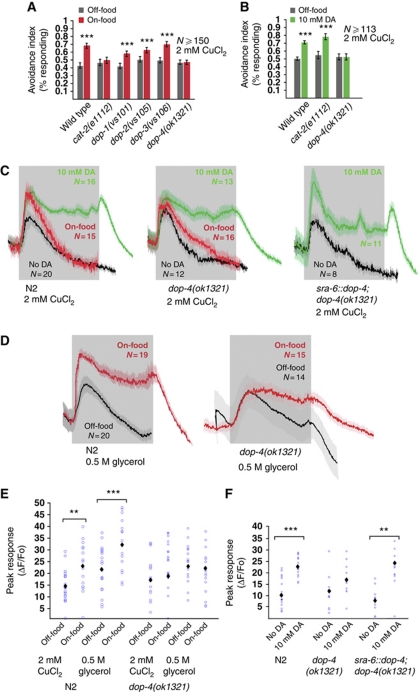
The DOP-4 dopamine receptor acts in ASH to enhance repellent responses
To identify the receptor mediating the effects of dopamine on ASH, we screened mutants defective in each of the four dopamine-activated G protein-coupled receptors (GPCRs) in the C. elegans genome. We first used the drop test to determine whether deletion mutations in each of these genes (dop-1, dop-2, dop-3 or dop-4) affected the enhancement of repellent avoidance by food. We observed that responses to copper in dop-1, dop-2 and dop-3 deletion mutants were still modulated by food, whereas responses to copper in dop-4 mutants were not (Figure 7A). Copper avoidance in dop-4 mutant animals was also unaffected by treatment with 10 mM dopamine (Figure 7B) or by photoactivation of the dopaminergic neurons (Figure 6B). Interestingly, dop-4 did not prevent photoactivation-induced slowing of locomotion (Figure 6A), indicating that it affects only a subset of dopamine-related phenotypes. Thus, DOP-4 is a candidate for mediating the enhancement of ASH responses by dopamine. To test DOP-4 directly, we imaged ASH calcium transients evoked by copper and glycerol in dop-4 mutants. We found that in dop-4 mutants, the magnitudes of ASH calcium transients evoked by copper or glycerol were not significantly increased by dopamine or by food (Figure 7C–F). Thus, DOP-4 appears to be important for modulation of ASH by dopamine. Interestingly, dopamine still increased the duration of repellent-evoked calcium transients (Figure 7C and D and Supplementary Figure 1); thus, this effect may involve a dopamine receptor other than DOP-4 or, alternatively, may reflect a non-physiological effect of exogenous dopamine.
Figure 7.
The dopamine receptor DOP-4 mediates the effect of food on avoidance. (A) Avoidance is not increased by food in dop-4(ok1321). Fraction of animals responding to 2 mM CuCl2. (***P<0.001, t-test), N⩾150. (B) Avoidance in dop-4(ok1321) is not increased by exogenous DA. 10 mM exogenous DA increases the avoidance to 2 mM CuCl2 in wild type and cat-2(e1112), but not in dop-4(ok1321). (***P<0.001, t-test), N⩾113. (C,D) Average neuronal responses in ASH. Grey shading indicates the duration of the stimulus. (C) Magnitude of ASH responses to 2 mM CuCl2 is not increased by food or exogenous DA in dop-4(ok1321). In wild-type animals, the magnitude of responses to 2 mM CuCl2 is increased by food and 10 mM exogenous DA, but not in dop-4(ok1321). Expression of genomic dop-4 DNA under the sra-6 promoter (ASH, ASI, PVQ) rescues ASH responses in dop-4(ok1321). Shown are the average traces. (D) Magnitude of ASH responses to 0.5 M glycerol is not increased by food in dop-4(ok1321). In wild-type animals, the magnitude of responses is increased by food, but not in dop-4(ok1321). (E, F) Quantification of ASH responses. Blue circles represent individual animals assayed. Black diamonds indicate average value. (***P<0.001, **P<0.01, Mann-Whitney rank sum test) (E) Wild-type animals have increased responses to 2 mM CuCl2 and 0.5 M glycerol on exogenous DA. In dop-4(ok1321), the responses are not increased. (F) Rescue of dop-4(ok1321) under the sra-6 promoter restores the magnitude of the response to 10 mM DA. (***P<0.001, **P<0.01, Mann–Whitney rank sum test), N⩾9.
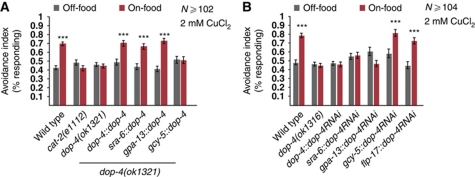
A dop-4∷gfp promoter fusion has been shown to be expressed in a number of neurons as well as non-neuronal tissues, but these do not include the ASH neurons (Sugiura et al, 2005). Additional dop-4 reporter transgenes constructed in our lab likewise did not show visible expression in ASH (data not shown). However, as gfp expression can be below the limit of detection, we used cell-specific rescue and knockdown experiments to determine whether DOP-4 might function directly in ASH. We first generated transgenic animals expressing wild-type dop-4(+) under the control of its own promoter and two additional promoters, sra-6 and gpa-13, whose expression patterns overlap only in ASH. Both ASH-expressed transgenes rescued the food-modulation defect (assayed by behaviour) of the dop-4 deletion mutant, as did expression under the dop-4 promoter (Figure 8A). In contrast, expressing dop-4(+) under a non-ASH promoter (gcy-5) did not rescue the mutant phenotype (Figure 8A). The sra-6∷dop-4(+) transgene also restored the ability of dopamine to enhance repellent-evoked calcium transients in ASH in the dop-4 deletion mutant (Figure 7C and F). These results suggest that the DOP-4 receptor can act cell autonomously in ASH to mediate dopamine enhancement of repellent responses.
Figure 8.
The dopamine receptor DOP-4 functions in ASH to modulate avoidance behaviours. (A) Transgenic rescue of dop-4(ok1321). Fraction of animals responding to 2 mM CuCl2. Expressing genomic dop-4 DNA under its own promoter (ASG, AVL, CAN and PQR), or the sra-6 (ASH, ASI and PVQ) or gpa-13 (ASH, ADL, AWC, PHA and PHB) promoters restores the avoidance on food. Expression under the gcy-5 promoter (ASER) does not rescue. (***P<0.001, t-test), N⩾126. (B) RNAi knockdown of DOP-4 in ASH results in defect avoidance on food. Fraction of animals responding to 2 mM CuCl2. Animals expressing dop-4 RNAi under its own promoter, or under the sra-6 and gpa-13 promoters, both of which express in ASH, do not have increased avoidance on food. Animals expressing dop-4 RNAi under the gcy-5 and flp-17 promoters, which do not express in ASH, display wild-type behaviour. (***P<0.001, t-test), N⩾94.
To further test whether DOP-4 functions in ASH for dopamine modulation, we knocked down dop-4 expression cell specifically using RNAi (Esposito et al, 2007). Although transgenic RNAi silencing can spread between C. elegans tissues (Jose et al, 2009), spreading of cell-specific RNAi has not been observed between different neurons (Esposito et al, 2007; Harris et al, 2009; Chatzigeorgiou et al, 2010). Therefore, we generated transgenes expressing sense and antisense dop-4 sequences (hence referred to as RNAi transgenes) under the control of the dop-4 promoter, and the sra-6 and gpa-13 promoters. As controls, we also generated RNAi transgenes expressed under the control of the flp-17 and gcy-5 promoters, which do not drive the expression in ASH. We observed that dop-4, sra-6- and gpa-13-driven RNAi transgenes led to a defect in dopamine modulation of repellent avoidance (Figure 8B). However, expressing a dop-4 RNAi transgene under the flp-17 and gcy-5 promoters did not affect dopamine modulation of repellent avoidance. These results are consistent with the cell-specific mutant rescue data indicating that DOP-4 acts cell autonomously in ASH to mediate the enhancement of repellent responses by food and dopamine.
Discussion
In this study, we show that the behavioural escape responses to soluble repellents are enhanced by food, and that this enhancement results at least in part from changes in the response properties of the primary chemosensory neurons. Food modulation potentially involves at least three different pathways that increase the sensory responsiveness of the ASH nociceptors. In the first pathway, dopamine acts directly on ASH through dopamine receptor DOP-4 to increase the magnitude of its acute responses to repellents. In the absence of food, dopamine signalling does not affect ASH responses; thus, activation of the dopaminergic pathway functions as a direct ‘on-food’ signal to facilitate larger sensory responses in ASH. In the second pathway, dopamine acts in a dop-4-independent manner to inhibit adaptation to repeated stimuli in the presence of food. Finally, dopamine also acts in a third, dop-4-independent pathway to prolong initial responses to repellents such as glycerol in the presence of food. The duration of responses to glycerol in dop-4 animals are unaffected by the presence of food, and the molecules required for this third pathway remain unidentified. As none of the dopamine-activated GPCRs affect response duration, we speculate that a dopamine-gated ion channel may be involved.
These studies add to the already remarkable number of food-dependent modulatory pathways acting on the ASH neurons. For example, several studies have shown that ASH-mediated avoidance behaviours are also modulated by food through serotonergic signalling. Responses to nose touch are enhanced in the presence of food; this process has been shown to require serotonin signalling and can be mimicked by provision of exogenous serotonin (Chao et al, 2004). However, when we tested nose touch responses using calcium imaging, we did not find exogenous serotonin to have an effect, suggesting that serotonin modulates ASH activity downstream of calcium signalling, possibly at the synapse. Food and serotonin also enhance responses to dilute octanol, a process requiring the activities of the G-proteins GPA-11 (Chao et al, 2004) and EGL-30 (Harris et al, 2010), and the serotonin receptor SER-5 (Harris et al, 2009). Conversely, octopamine appears to inhibit dilute octanol responses in the absence of food, a process that requires the OCT-1 receptor and the G-protein GOA-1 (Harris et al, 2010). Dopamine does not appear to be involved in enhancing octanol responses in ASH; indeed, exogenous dopamine dampens responses to dilute octanol in a process requiring the activity of dopamine receptor DOP-3 (Ezak and Ferkey, 2010). SER-5, OCT-1 and DOP-3 are required cell autonomously in ASH; thus, including DOP-4, there are at least four different GPCRs that directly modulate ASH activity in response to food. Calcium imaging experiments have not been reported for ser-5, oct-1 or dop-3 mutants for octanol; thus, it is not clear whether these pathways affect sensory transduction and neuronal excitability, or processes downstream of calcium influx such as synaptic transmission. However, it is clear that the targets of at least two of these pathways differ from those of DOP-4, as neither dop-3 nor ser-5 affects the calcium transients evoked by soluble repellents (data not shown). Thus, the molecular mechanisms by which a single environmental condition—food—modulates a single sensory neuron appear to be unexpectedly intricate and complex.
Both DOP-4 and DOP-3 act cell autonomously in ASH; thus, dopamine appears to act directly on the ASH neurons to modulate their activity. C. elegans has eight dopaminergic neurons, and activation of this set of neurons is sufficient to immediately induce increased avoidance. On the basis of the EM reconstruction of the C. elegans nervous system, the dopaminergic neurons make synapses with a total of 41 neurons; however, these neurons do not include ASH. This implies that dopamine appears to signal extrasynaptically to modulate ASH avoidance responses. Dopamine has been shown to act extrasynaptically in other C. elegans neural circuits. For example, the basal slowing response is regulated by DOP-1 and DOP-3 acting in the motor neurons, which receive no significant synaptic input from the dopaminergic neurons (Chase et al, 2004). Signalling through the DOP-1 receptor also acts in the ALM anterior body mechanoreceptors to modulate habituation to touch responses (Kindt et al, 2007); although the touch receptor neurons synapse onto the dopaminergic CEP neurons, there are no reported synapses from any of the dopaminergic neurons to the ALMs. Indeed, a survey of expression data indicated that of the 118 C. elegans neurons expressing dopamine-activated GPCRs, only 20 (17%) were post-synaptic to any of the dopaminergic neurons (Suo et al, 2003, 2009; Tsalik et al, 2003; Chase et al, 2004; Sanyal et al, 2004; Sugiura et al, 2005; Etchberger et al, 2007; Ezak and Ferkey, 2010). The prevalence of extrasynaptic signalling in C. elegans is not limited to the dopamine system; only 17 of 45 serotonin receptor-expressing cells are post-synaptic to serotonergic neurons (Tsalik et al, 2003; Carnell et al, 2005; Xiao, 2006 #2417; Hobson et al, 2006; Dernovici et al, 2007; Harris et al, 2009), and none of the 80 tyramine receptor-expressing neurons are post-synaptic to tyraminergic neurons (Tsalik et al, 2003; Rex et al, 2004, 2005). This suggests that most aminergic neurotransmission may involve extrasynaptic communication pathways that are not identified in the EM reconstruction of the C. elegans anatomy. Thus, even the nominally complete anatomical wiring diagram is likely to significantly underestimate the functional connectivity in the C. elegans nervous system.
Dopamine has been implicated in the control of food-dependent states with respect to many behaviours (Sawin et al, 2000; Hills et al, 2004; Kindt et al, 2007; Ezak and Ferkey, 2010). However, the mechanism and dynamics of how dopamine signalling is activated by food to modulate neural circuits has been difficult to discern. We show here that food modulation requires the sensory cilia of the dopamine neurons, indicating that dopamine signalling is directly activated by external sensation of bacteria. Using the light-activated channel ChR2, we found that optogenetic activation of the dopaminergic neurons causes an immediate increase in avoidance responses, an effect that lasts for 1–2 min. This demonstrates that the activation of dopaminergic neurons is sufficient to acutely modulate avoidance responses. Optogenetic activation of the dopaminergic neurons also induces immediate slowing of speed and, interestingly, this effect also lasts between 1 and 2 min, indicating that dopaminergic modulation of both behaviours has a similar time course. This correlates well with the time course of the effects exerted by food itself on behaviour; for example, differences in calcium transients could be observed within a few minutes after the animals are placed on- or off-food. Likewise, the basal slowing response, which requires dopamine, occurs within 5 min of being placed on food (Sawin et al, 2000), and increases in reversals and turning occur within 1 min of removal from food (Hills et al, 2004). Thus, it appears that dopamine is released immediately upon food encounter, and that this signal is only maintained for a prolonged period if the animal remains in a food-rich environment.
Although dopamine itself appears to be a direct, acute signal of food availability, dopamine signalling may interact with other monoamine and neuropeptide systems to integrate information about food and starvation across varying time scales. Previous studies have also indicated that dopamine acts indirectly by controlling the release of other neuromodulators; for example, dopamine appears to negatively regulate release of octopamine from neurons controlling CREB-dependent gene expression in interneurons (Suo et al, 2006). Interactions between neuromodulator systems have a critical role in the control of behavioural states in many organisms, including mammals. Future studies of these processes in C. elegans may provide insight into conserved mechanisms by which feeding state modifies neural circuits controlling complex behaviours.
Materials and methods
Strains and culture
Strains were maintained as described (Brenner, 1974). All strains were grown at 20°C on NGM plates with OP50. The following strains were used: N2 (wild-type reference), CB1112 cat-2(e1112), LX636 dop-1(vs101), LX702 dop-2(vs105), LX703 dop-3 (vs106), RB1252 dop-4(ok1321), JT204 daf-12(sa204), JT6924 daf-19(m86); daf-12(sa204), OE3789 daf-19(m86);daf-12(sa204);ofEx588[gpa-13∷ daf-19c], OE3797 daf-19(m86);daf-12(sa204);ofEx596[gpa-11∷gfp; gpa-11∷daf-19c;che-13∷dsRed;elt-2∷mCherry], OE3793 daf-19(m86); daf-12(sa204);ofEx592[dat-1∷daf-19c]. The following strains were generated: AQ2353 dop-4(ok1321);ljEx241[sra-6∷dop-4, unc-122∷gfp], AQ2548 dop-4(ok1321); ljEx304[dop-4∷dop-4; unc-122∷gfp], AQ2465 dop-4(ok1321);ljEx292[gpa-13∷dop-4;unc-122∷gfp], AQ2477 dop-4 (ok1321);ljEx302[flp-17∷dop-4; unc-122∷gfp], AQ2475 daf-19(m86); daf-12(sa204);ofEx588[gpa-13∷daf-19c];ofEx592[dat-1∷daf-19c],AQ2483ljEx290[sra-6∷dop-4RNAi;elt-2∷mCherry], AQ2519 ljEx281[gpa-13∷dop-4RNAi; elt-2∷mCherry], AQ2484 ljEx291[flp-17∷dop-4RNAi; elt-2∷mCherry], AQ2546 ljEx303[gcy-5∷dop-4RNAi;elt-2∷mCherry], AQ2028 lite-1(ce314);ljIs100 [Pdat-1∷ChR2∷yfp,unc-122∷gfp], AQ2050 lite-1(ce314);ljIs102 [tph-1;;ChR2∷YFP;unc-122∷gfp], AQ2235 lite-1(ce314); ljIs114[Pgpa-13∷FLPase, Psra-6∷FTF∷ChR2∷YFP], AQ2673 lite-1(ce314); cat-2(e1112); ljIs100[Pdat-1∷ChR2∷YFP, unc-122∷gfp], AQ2235 lite-1(ce314); ljIs114[Pgpa-13∷FLPase;Psra-6∷FTF∷ChR2∷YFP], AQ2242 dop-4(ok1321) ljEx95[sra-6∷yc2.12, lin-15(+)], AQ2180 cat-2(e1112); ljEx95[sra-6∷yc2.12, lin-15(+)], AQ2241 dop-4(ok1321) ljEx95[sra-6∷yc2.12, lin-15(+)], AQ2303 dop-4(ok1321) ljEx241[sra-6∷dop-4, Punc-122∷gfp]; ljEx95[sra-6∷yc2.12; lin-15(+)].
Generation of transgenic animals
Cameleon strains were generated by crossing lin-15(n765);ljEx95[sra-6∷YC2.12] (Hilliard et al, 2005) with cat-2(e1112) and dop-4(ok1321), and by crossing dop-4(ok1321);ljEx95[sra-6∷YC2.12] with dop-4(ok1321);ljEx241[sra-6∷dop-4]. cat-2(e1112) was confirmed by PCR, followed by digestion using StyI. dop-4(ok1321) was confirmed by PCR. daf-19(m86);daf-12(sa204);gpa-11∷daf-19;dat-1∷daf-19c was generated by crossing daf-19(m86);daf-12(sa204); gpa-11∷daf-19c (Senti et al, 2009) with daf-19(m86);daf-12(sa204); dat-1∷daf-19c (Senti et al, 2009).
Rescue constructs
Plasmids were constructed using MultiSite Gateway Three-Fragment Vector Construction Kit (Invitrogen). Promoters were inserted into pDONR P4-P1R, except gpa-13, which was inserted into pDEST4926. Genomic DNA of dop-4 was inserted into pDONR 221. A pENTRY P2R-P3 containing the unc-54 3′-UTR (kindly provided by Ithai Rabinowitch) was used. Rescue constructs were generated by recombining the pENTRY and pDESTR4-R3 vectors using LR Clonase II Plus. sra-6 pEntry was kindly provided by Emanuel Busch and contains a 3 kb region of the promoter. flp-17 pEntry and gcy-5 pEntry were kindly provided by Andrew Bretscher and contain a 3 kb and 2.2 kb region of the promoter, respectively. The primers used are listed in Supplementary data. ljEx305[sra-6∷npr-1], ljEx304[dop-4∷dop-4] and ljEx241[sra-6∷dop-4] were injected (Mello et al, 1991) at 50 ng/μl with co-injection marker unc-122∷gfp at 50 ng/μl. All other rescue constructs were injected at 50 ng/μl with co-injection marker elt-2∷mCherry at 50 ng/μl.
ChR lines
Promoters for dat-1 and tph-1 were PCR amplified and cloned into pDONRP4-P1R (Invitrogen). ChR2 (Nagel et al, 2003)-coding sequence without a stop codon was PCR amplified from a plasmid MGW16-3 (a kind gift from Emanuel Busch) and cloned into pDONR221 (Invitrogen). These plasmids were recombined in conjunction with a plasmid including YFP and unc-54 3′-UTR (a generous gift from Mario de Bono), and pDESTR4-R3 (Invitrogen) using LR clonase II Plus (Invitrogen) to generate pTNZ13 Pdat-1∷ChR2∷YFP and pTNZ31 Ptph-1∷ChR2∷YFP. pTNZ13 and pTNZ31 were injected (Mello et al, 1991) into wild-type to generate transgenic animals, which were subsequently irradiated with 365 nm UV light at an intensity of 2 mJ/cm2 in the presence of 0.033 mg/ml trimethylpsoralen (Sigma-Aldrich, MO) to generate integrated transgenic arrays ljIs100 and ljIs102, respectively. Obtained integrants were outcrossed five times with wild type, and then the transgenes were transferred to KG1180 lite-1(ce314) to establish AQ2028 (with ljIs100) and AQ2050 (with ljIs102). FLP recombinase (Davis et al, 2008) was used to express ChR2 specifically in ASH by generating pTNZ141(Pgpa-13∷FLPase) and pTNZ109(Psra-6∷FTF∷ChR2∷YFP), and injecting them at 60 ng/μl and 140 ng/μl, respectively, into lite-1(ce314). The obtained line was irradiated as described above and the integrated strain was outcrossed with lite-1(ce314) seven times.
Cell-specific RNAi
The sense and antisense RNAi strands spanning a 1.6 kb region of the dop-4 gene (Keating et al, 2003) were inserted into pDONR221 (Invitrogen). The cell-specific RNAi constructs were generated by recombining the RNAi plasmids with the pENTRY containing promoters for sra-6, gpa-11, gcy-5 and flp-17, pENTRY containing unc-54 3′-UTR, and pDESTR4-R3. The primers used to amplify the RNAi are listed in Supplementary data. For all RNAi constructs, the sense and antisense strand constructs were injected at 100 ng/μl each with co-injection marker elt-2∷mCherry at 50 ng/μl.
Drop test assays
For all behavioural assays, L4 stage animals were picked 20 h before the assay. The drop test and the adaptation drop test were performed on NGM plates and prepared for off- and on-food assays as follows: for on-food plates, 25 μl of overnight culture of OP50 in LB (A=0.5) was spread on each plate. For off-food plates, 25 μl of LB was spread on each plate. All plates were allowed to dry for 1 h without lids, and used after an additional hour for assays. Animals were picked from the culture plate using an eyelash pick, and placed on a plate without food for a few seconds to prevent food from being transferred to the assay plates. The animals were then placed on the assay plate. Animals were allowed to settle for 10 min and then assayed using a capillary to deliver the repellent drop as described previously (Hilliard et al, 2002). For the drop test, 10 animals were assayed on each plate. All animals were stimulated every 60 s, and the fraction of worms reversing was recorded. In the adaptation drop test, a single animal was picked to each plate and assayed 20 times every 10 s. The response to every drop was recorded. Responses to chronic stimulation were tested using a previously described protocol (Hilliard et al, 2002). A drop of the repellent was first delivered to a single animal to test the pre-chronic stimulation. The animal was then placed in a drop of the repellent for 15 s, followed by a recovery time of 2 min. After the recovery, the animal was tested again for post-chronic responses. The tested repellents were CuCl2 (copper(II)chloride dihydrate, Sigma), glycerol (Fisher) and primaquine diphosphate (Sigma-Aldrich). All repellents were dissolved and diluted in M13 buffer. For behavioural assays with exogenous dopamine and serotonin, fresh assay plates containing 10 mM dopamine or 10 mM serotonin were prepared by spreading dopamine (dopamine hydrocomplex, Sigma-Aldrich) or serotonin (serotonin creatinin sulphate complex, Sigma-Aldrich) dissolved in M9 on each assay plate. The same volume of M9, without the drug, was spread on control plates for assays without drugs. All plates were dried without lids for 1 h and used after an additional hour. Dopamine plates were kept in dark until the assay to prevent oxidation. In all serotonin experiments, addition of serotonin stimulated rapid pharyngeal pumping, indicating that it was biologically active.
ChR2 assays
For ChR2 experiments, transgenic animals were grown in the dark at 20°C on NGM plates seeded with OP50 with or without all-trans retinal (ATR; Sigma-Aldrich) dissolved in ethanol. For locomotion and drop test assays, adult animals were transferred to an unseeded NGM plate without ATR and left undisturbed for 10 min before illumination. After the recovery, blue light (440–460 nm) from Luxeon III LXHL-PR09 (Lumileds, CA) was used to illuminate the entire plate for 10 s during the recording in order to activate ChR2. The animals were then tested for either speed or avoidance.
To grow animals for speed assays, 5 μM ATR was used and 20 adult animals were tested in each experiment. Movies were recorded with a USB-connected camera AM413TL (AnMo, Taiwan) at the frequency of 2 frames per second, and analysed using Parallel Worm Tracker (Ramot et al, 2008) running on MATLAB (Mathworks, MA). The speed of all the animals in the movie frame was averaged to generate one speed value at each time point. Assays were done for 11 different sets of animals for each condition (with/without ATR). For avoidance assays, 62.5 μM ATR was used to grow the animals, and 10 adult animals were stimulated with repellent drops as described for the drop test. The drop was delivered 0, 1 or 2 min after the illumination. As a control for reversals caused by the ChR activation, the same assay was performed delivering drops with only M9 buffer. To test for reversals following ChR activation of ASH, adult animals were transferred to plates that were unseeded or seeded, or that containing 10 mM dopamine. The animals were allowed to recover for 5 min and illuminated for 1 s. Reversals were analysed using the Parallel Worm Tracker (Ramot et al, 2008).
Calcium imaging experiments
Sample preparation and delivery of repellent
Animals were glued on 2% agarose pads made with neuronal buffer using cyanoacrylate glue (Nexaband S/C, Abbott Laboratories). The pad was briefly placed on ice during the gluing to constrict the animal's movements. The animals were placed under the microscope in a perfusion chamber (RC-26GLP, Warner Instruments) under constant flow rate (0.4 ml/min) of neuronal buffer using a perfusion pencil (AutoMate). Outflow was regulated using a peristaltic pump (Econo Pump, Biorad). Repellents were delivered using the perfusion pencil and automated valves (EW-98302-20, Cole Parmer Ltd), controlled by Motorway software 2.5. The neuronal buffer contained 40 mM NaCl, 10 mM HEPES-NaOH pH 7.1 and 1 mM MgSO4, and the osmolarity was adjusted to 350 mOsmo using glycerol. Copper and glycerol were dissolved in M13 buffer. For recordings with food, OP50 was grown overnight in LB (A=0.5), and 50 ml cultures were pelleted by centrifugation. The pellets were dissolved in neuronal buffer or repellent, to a final volume of 25 ml, and used within 1 h.
Calcium imaging and image analysis
Optical recordings were performed on a Zeiss Axioskop 2 upright compound microscope using a × 63 Zeiss Achroplan water immersion objective. Filter/dichroic pairs were: excitation, 420/40; excitation dichroic 455; CFP emission, 480/30; emission dichroic 505; YFP emission, 535/30 (Chroma). The microscope was fitted with a Hamamatsu Orca ER CCD camera, a Hamamatsu W-View emission image splitter and a Uniblitz Shutter (Vincent Associates). Images were acquired at 10 Hz using MetaVue 4.6 (Universal Imaging). Image analysis was performed using a custom programme written in Java parameterized and averaged using scripts written in Matlab 6.5.1 (Mathworks).
Supplementary Material
Acknowledgments
We thank the Caenorhabditis Genetics Center for strains, Mario de Bono, Emanuel Busch, Andrew Bretscher and Ithai Rabinowitch for plasmids, and Ithai Rabinowitch for Matlab scripts. We thank Robyn Branicky, Ithai Rabinowitch and Denise Walker for useful comments on the manuscript. Work in the authors labs was supported by the Medical Research Council (UK), grants from NIDA (USA), and a Marie Curie postdoctoral fellowship (to YT). PS received grant support from the Swedish Research Council and from the NordForsk Nordic C. elegans Network.
Footnotes
The authors declare that they have no conflict of interest.
References
- Alkema MJ, Hunter-Ensor M, Ringstad N, Horvitz HR (2005) Tyramine functions independently of octopamine in the Caenorhabditis elegans nervous system. Neuron 46: 247–260 [DOI] [PubMed] [Google Scholar]
- Avery L, Horvitz HR (1990) Effects of starvation and neuroactive drugs on feeding in Caenorhabditis elegans. J Exp Zool 253: 263–270 [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Mori I (1997) Chemotaxis and thermotaxis. In C. elegans II, Riddle DL, Blumenthal T, Meyer BJ, Priess JR (eds), pp 717–737. Cold Spring Harbor: Cold Spring Harbor Laboratory Press [PubMed] [Google Scholar]
- Bargmann CI, Thomas JH, Horvitz HR (1990) Chemosensory cell function in the behavior and development of Caenorhabditis elegans. Cold Spring Harb Symp Quant Biol 55: 529–538 [DOI] [PubMed] [Google Scholar]
- Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77: 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnell L, Illi J, Hong SW, McIntire SL (2005) The G-protein-coupled serotonin receptor SER-1 regulates egg laying and male mating behaviors in Caenorhabditis elegans. J Neurosci 25: 10671–10681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MY, Komatsu H, Fukuto HS, Dionne HM, Hart AC (2004) Feeding status and serotonin rapidly and reversibly modulate a Caenorhabditis elegans chemosensory circuit. Proc Natl Acad Sci USA 101: 15512–15517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzigeorgiou M, Yoo S, Watson JD, Lee WH, Spencer WC, Kindt KS, Hwang SW, Miller DM 3rd, Treinin M, Driscoll M, Schafer WR (2010) Specific roles for DEG/ENaC and TRP channels in touch and thermosensation in C. elegans nociceptors. Nat Neurosci 13: 861–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase DL, Pepper JS, Koelle MR (2004) Mechanism of extrasynaptic dopamine signaling in Caenorhabditis elegans. Nat Neurosci 7: 1096–1103 [DOI] [PubMed] [Google Scholar]
- Davis MW, Morton JJ, Carroll D, Jorgensen EM (2008) Gene activation using FLP recombinase in C. elegans. PLoS Genet 4: e1000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dernovici S, Starc T, Dent JA, Ribeiro P (2007) The serotonin receptor SER-1 (5HT2ce) contributes to the regulation of locomotion in Caenorhabditis elegans. Dev Neurobiol 67: 189–204 [DOI] [PubMed] [Google Scholar]
- Edwards SL, Charlie NK, Milfort MC, Brown BS, Gravlin CN, Knecht JE, Miller KG (2008) A novel molecular solution for ultraviolet light detection in Caenorhabditis elegans. PLoS Biol 6: e198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, Di Schiavi E, Bergamasco C, Bazzicalupo P (2007) Efficient and cell specific knock-down of gene function in targeted C. elegans neurons. Gene 395: 170–176 [DOI] [PubMed] [Google Scholar]
- Etchberger JF, Lorch A, Sleumer MC, Zapf R, Jones SJ, Marra MA, Holt RA, Moerman DG, Hobert O (2007) The molecular signature and cis-regulatory architecture of a C. elegans gustatory neuron. Genes Dev 21: 1653–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezak MJ, Ferkey DM (2010) The C. elegans D2-like dopamine receptor DOP-3 decreases behavioral sensitivity to the olfactory stimulus 1-octanol. PLoS One 5: e9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faumont S, Lockery SR (2006) The awake behaving worm: simultaneous imaging of neuronal activity and behavior in intact animals at millimeter scale. J Neurophysiol 95: 1976–1981 [DOI] [PubMed] [Google Scholar]
- Guo ZV, Hart AC, Ramanathan S (2009) Optical interrogation of neural circuits in Caenorhabditis elegans. Nat Methods 6: 891–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris G, Mills H, Wragg R, Hapiak V, Castelletto M, Korchnak A, Komuniecki RW (2010) The monoaminergic modulation of sensory-mediated aversive responses in Caenorhabditis elegans requires glutamatergic/peptidergic cotransmission. J Neurosci 30: 7889–7899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GP, Hapiak VM, Wragg RT, Miller SB, Hughes LJ, Hobson RJ, Steven R, Bamber B, Komuniecki RW (2009) Three distinct amine receptors operating at different levels within the locomotory circuit are each essential for the serotonergic modulation of chemosensation in Caenorhabditis elegans. J Neurosci 29: 1446–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard MA, Apicella AJ, Kerr R, Suzuki H, Bazzicalupo P, Schafer WR (2005) In vivo imaging of C. elegans ASH neurons: cellular response and adaptation to chemical repellents. EMBO J 24: 63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard MA, Bargmann CI, Bazzicalupo P (2002) C. elegans responds to chemical repellents by integrating sensory inputs from the head and the tail. Curr Biol 12: 730–734 [DOI] [PubMed] [Google Scholar]
- Hilliard MA, Bergamasco C, Arbucci S, Plasterk RH, Bazzicalupo P (2004) Worms taste bitter: ASH neurons, QUI-1, GPA-3 and ODR-3 mediate quinine avoidance in Caenorhabditis elegans. EMBO J 23: 1101–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills T, Brockie PJ, Maricq AV (2004) Dopamine and glutamate control area-restricted search behavior in Caenorhabditis elegans. J Neurosci 24: 1217–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson RJ, Hapiak VM, Xiao H, Buehrer KL, Komuniecki PR, Komuniecki RW (2006) SER-7, a Caenorhabditis elegans 5-HT7-like receptor, is essential for the 5-HT stimulation of pharyngeal pumping and egg laying. Genetics 172: 159–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz HR, Chalfie M, Trent C, Sulston JE, Evans PD (1982) Serotonin and octopamine in the nematode Caenorhabditis elegans. Science 216: 1012–1014 [DOI] [PubMed] [Google Scholar]
- Jose AM, Smith JJ, Hunter CP (2009) Export of RNA silencing from C. elegans tissues does not require the RNA channel SID-1. Proc Natl Acad Sci USA 106: 2283–2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Gao J, Schafer WR, Xie Z, Xu XZ (2010) C. elegans TRP family protein TRP-4 is a pore-forming subunit of a native mechanotransduction channel. Neuron 67: 381–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating CD, Kriek N, Daniels M, Ashcroft NR, Hopper NA, Siney EJ, Holden-Dye L, Burke JF (2003) Whole-genome analysis of 60 G protein-coupled receptors in Caenorhabditis elegans by gene knockout with RNAi. Curr Biol 13: 1715–1720 [DOI] [PubMed] [Google Scholar]
- Kindt KS, Quast KB, Giles AC, De S, Hendrey D, Nicastro I, Rankin CH, Schafer WR (2007) Dopamine mediates context-dependent modulation of sensory plasticity in C. elegans. Neuron 55: 662–676 [DOI] [PubMed] [Google Scholar]
- Kodama E, Kuhara A, Mohri-Shiomi A, Kimura KD, Okumura M, Tomioka M, Iino Y, Mori I (2006) Insulin-like signaling and the neural circuit for integrative behavior in C. elegans. Genes Dev 20: 2955–2960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lints R, Emmons SW (1999) Patterning of dopaminergic neurotransmitter identity among Caenorhabditis elegans ray sensory neurons by a TGFB family signaling pathway and a Hox gene. Development 126: 5819–5831 [DOI] [PubMed] [Google Scholar]
- Mello CC, Kramer JM, Stinchcomb D, Ambros V (1991) Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J 10: 3959–3970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohri A, Kodama E, Kimura KD, Koike M, Mizuno T, Mori I (2005) Genetic control of temperature preference in the nematode Caenorhabditis elegans. Genetics 169: 1437–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G, Brauner M, Liewald JF, Adeishvili N, Bamberg E, Gottschalk A (2005a) Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol 15: 2279–2284 [DOI] [PubMed] [Google Scholar]
- Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E (2003) Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci USA 100: 13940–13945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G, Szellas T, Kateriya S, Adeishvili N, Hegemann P, Bamberg E (2005b) Channelrhodopsins: directly light-gated cation channels. Biochem Soc Trans 33: 863–866 [DOI] [PubMed] [Google Scholar]
- Ramot D, Johnson BE, Berry TL Jr, Carnell L, Goodman MB (2008) The Parallel Worm Tracker: a platform for measuring average speed and drug-induced paralysis in nematodes. PLoS One 3: e2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand JB, Nonet ML (1997) Neurotransmitter assignments for specific neurons. In C. elegans II, Riddle DL, Blumenthal T, Meyer BJ, Priess JR (eds) Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- Rex E, Hapiak V, Hobson R, Smith K, Xiao H, Komuniecki R (2005) TYRA-2 (F01E11.5): a Caenorhabditis elegans tyramine receptor expressed in the MC and NSM pharyngeal neurons. J Neurochem 94: 181–191 [DOI] [PubMed] [Google Scholar]
- Rex E, Molitor SC, Hapiak V, Xiao H, Henderson M, Komuniecki R (2004) Tyramine receptor (SER-2) isoforms are involved in the regulation of pharyngeal pumping and foraging behavior in Caenorhabditis elegans. J Neurochem 91: 1104–1115 [DOI] [PubMed] [Google Scholar]
- Saeki S, Yamamoto M, Iino Y (2001) Plasticity of chemotaxis revealed by paired presentation of a chemoattractant and starvation in the nematode Caenorhabditis elegans. J Exp Biol 204: 1757–1764 [DOI] [PubMed] [Google Scholar]
- Sambongi Y, Nagae T, Liu Y, Yoshimizu T, Takeda K, Wada Y, Futai M (1999) Sensing of cadmium and copper ions by externally exposed ADL, ASE, and ASH neurons elicits avoidance response in Caenorhabditis elegans. Neuroreport 10: 753–757 [DOI] [PubMed] [Google Scholar]
- Sanyal S, Wintle RF, Kindt KS, Nuttley WM, Arvan R, Fitzmaurice P, Bigras E, Merz DC, Hebert TE, van der Kooy D, Schafer WR, Culotti JG, Van Tol HH (2004) Dopamine modulates the plasticity of mechanosensory responses in Caenorhabditis elegans. EMBO J 23: 473–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin ER, Ranganathan R, Horvitz HR (2000) C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26: 619–631 [DOI] [PubMed] [Google Scholar]
- Schafer WR, Kenyon CJ (1995) A calcium channel homologue required for adaptation to dopamine and serotonin in Caenorhabditis elegans. Nature 375: 73–78 [DOI] [PubMed] [Google Scholar]
- Senti G, Ezcurra M, Lobner J, Schafer WR, Swoboda P (2009) Worms with a single functional sensory cilium generate proper neuron-specific behavioral output. Genetics 183: 595–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura M, Fuke S, Suo S, Sasagawa N, Van Tol HH, Ishiura S (2005) Characterization of a novel D2-like dopamine receptor with a truncated splice variant and a D1-like dopamine receptor unique to invertebrates from Caenorhabditis elegans. J Neurochem 94: 1146–1157 [DOI] [PubMed] [Google Scholar]
- Sulston J, Dew M, Brenner S (1975) Dopaminergic neurons in the nematode Caenorhabditis elegans. J Comp Neurol 163: 215–226 [DOI] [PubMed] [Google Scholar]
- Suo S, Culotti JG, Van Tol HH (2009) Dopamine counteracts octopamine signalling in a neural circuit mediating food response in C. elegans. EMBO J 28: 2437–2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo S, Kimura Y, Van Tol HH (2006) Starvation induces cAMP response element-binding protein-dependent gene expression through octopamine-Gq signaling in Caenorhabditis elegans. J Neurosci 26: 10082–10090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo S, Sasagawa N, Ishiura S (2003) Cloning and characterization of a Caenorhabditis elegans D2-like dopamine receptor. J Neurochem 86: 869–878 [DOI] [PubMed] [Google Scholar]
- Swoboda P, Adler HT, Thomas JH (2000) The RFX-type transcription factor DAF-19 regulates sensory neuron cilium formation in C. elegans. Mol Cell 5: 411–421 [DOI] [PubMed] [Google Scholar]
- Tomioka M, Adachi T, Suzuki H, Kunitomo H, Schafer WR, Iino Y (2006) The insulin/PI 3-kinase pathway regulates salt chemotaxis learning in Caenorhabditis elegans. Neuron 51: 613–625 [DOI] [PubMed] [Google Scholar]
- Trent C, Tsung N, Horvitz HR (1983) Egg-laying defective mutants of the nematode Caenorhabditis elegans. Genetics 104: 619–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsalik EL, Niacaris T, Wenick AS, Pau K, Avery L, Hobert O (2003) LIM homeobox gene-dependent expression of biogenic amine receptors in restricted regions of the C. elegans nervous system. Dev Biol 263: 81–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner LE, Hardaker LA, Golik S, Schafer WR (2000) Effect of a neuropeptide gene on behavioral states in Caenorhabditis elegans egg-laying. Genetics 154: 1181–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Hapiak VM, Smith KA, Lin L, Hobson RJ, Plenefisch J, Komuniecki R (2006) SER-1, a Caenorhabditis elegans 5-HT2-like receptor, and a multi-PDZ domain containing protein (MPZ-1) interact in vulval muscle to facilitate serotonin-stimulated egg-laying. Dev Biol 298: 379–391 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.