Abstract
EMBO J 30 6, 1123–1136 (2011); published online February 04 2011
NF-κB-dependent immune responses are tightly controlled so as to avoid runaway inflammation. One easy model to study the negative regulation of these pathways is provided by the Drosophila melanogaster immune deficiency (IMD) pathway, which regulates both systemic and epithelial immune responses against bacteria. Ragab et al (2011) report in this issue of the EMBO Journal that the Ras/MAPK pathway negatively regulates the IMD pathway when activated by the PDGF/VEGF receptor (PVR), a process mediated by the PIRK/Rudra/PIMS inhibitor. This study raises interesting questions regarding the biological significance of IMD negative regulation in diverse settings.
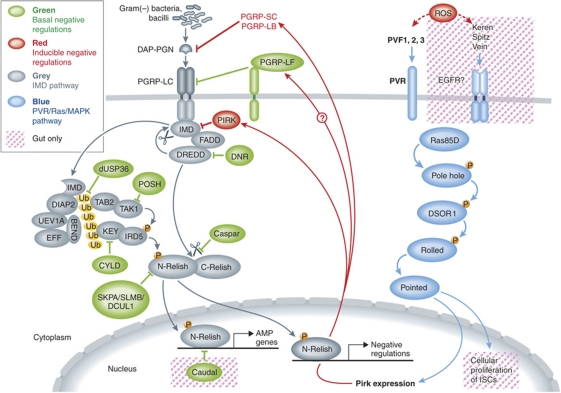
The immune deficiency (IMD) pathway is triggered upon the binding of peptidoglycan (PGN) to the PGRP-LC receptor, which further signals intracellularly through the death-domain containing adapters IMD, FADD, and the DREDD apical caspase (see Figure 1). DREDD cleaves IMD, thus allowing its K63-linked polyubiquitination by the Uev1A, Bendless, Effete, and DIAP2 complex. Polyubiquitinated IMD is then thought to recruit the TAK1/TAB2 and the IRD5/Kenny (IKKβ and NEMO fly homologues) kinase complexes, the latter of which ultimately activates the Rel transcriptional factor Relish. Relish is likely directly cleaved by DREDD, thus allowing the nuclear uptake of its N-terminal domain. This process thus leads to the induction of the expression of several antimicrobial peptide (AMP) genes as well as tens of other genes in response to a challenge by Gram(–) bacteria (Ferrandon et al, 2007; Ganesan et al, 2010).
Figure 1.
Negative regulation of the IMD pathway. Basal negative regulators (green) prevent the constitutive activation of the IMD pathway. Inducible negative regulators (red) (PGRP-LF excepted) modulate the amplitude of the IMD response. The PVR/RAS MAPK may inhibit the IMD pathway by upregulating the expression of the PIRK negative regulator. Arrows: positive interaction; dashed arrows: indirect activation; red boxes: interactions taking place in the gut epithelium; ROS: reactive oxygen species; DAP-PGN: diaminopimelic acid peptidoglycan.
Negative regulation occurs at several steps, including for instance hormonal control by the juvenile hormone. The activating signal is downregulated by PGRP-SC and PGRP-LB amidases that degrade PGN into nonstimulatory fragments. The PGRP-LF receptor competes with PGRP-LC for dimerization, thus producing inactive heterodimers (Basbous et al, 2011). The PIRK intracellular regulator disrupts the association of IMD with PGRP-LC and removes the receptor from the membrane. Several negative regulators are required to prevent the activation of the IMD pathway in the absence of an immune challenge. When these basal regulators are missing, there is a constitutive activation of the pathway. These are often ubiquitous factors that regulate the protein stability of defined IMD pathway members, often by regulating their ubiquitination status (SKPA/SLMB/DCUL1, dUSP36, CYLD, POSH, DNR-1, Caspar). An additional level of regulation is provided in the posterior midgut by the Caudal transcription factor that blocks the transcription of AMP genes, but not of other IMD targets. While the inhibition provided by basal regulators appears to be constitutive, the negative regulation brought by PGRP-SC/LB/LF and PIRK is the result of a negative feedback loop of the IMD pathway. As a result, these factors additively regulate the amplitude of the IMD response. Inactivating only one such regulator at a time is thus not sufficient to prolong IMD activation kinetics.
Many components of the IMD pathway have been identified through genetic screens, both in whole flies and by genome-wide RNAi screens in cell lines. A striking finding of the cell line screens was the large number of IMD pathway negative regulators that were discovered, as compared with positive regulators. In previous work, the Boutros laboratory identified 29 putative negative regulators, eight of which belong to the Ras/MAPK pathway. Ragab et al (2011) elegantly confirm this finding both in cell culture and in vivo, and also show along the way that this negative regulation is cell autonomous in the larval fat body. The activation of the MAPK pathway by overexpressing either the PDGF/VEGF receptor (PVR) ligands PVF1, 2, and 3, PVR itself, or the V12 activated version of Ras inhibits the IMD-dependent expression of AMP genes and renders the flies susceptible to Gram(–) bacteria. Conversely, inhibiting members of the Ras/MAPK pathway, including PVR, leads to an increased amplitude of AMP gene expression but does not affect the basal level of IMD activation nor its induction kinetics. This pattern of regulation is very similar to that of PIRK. Indeed, the authors show that the activation of the PVR/MAPK pathway can drive the expression of pirk in the absence of any immune challenge. pirk expression can however still be somewhat induced by an immune challenge in cultured cells when the MAPK pathway is genetically blocked, presumably by the IMD pathway. Finally, Ragab et al (2011) show that the higher induction of AMP expression observed when the Ras pathway is activated ectopically is blocked in pirk mutants, thus establishing that PVR pathway regulation on IMD is mediated by PIRK.
While the detrimental effects of runaway inflammation in mammals are well established, the situation is less clear with regard to Drosophila. In some, but not all cases, the absence of basal regulators leads to a reduced lifespan, which however cannot be ascribed specifically to the constitutive activation of the IMD pathway, as these regulators act upon multiple targets. It is well established that the uncontrolled activation of the IMD pathway during ontogenesis leads to developmental defects and the induction of apoptosis (Georgel et al, 1995; Bischoff et al, 2006; Maillet et al, 2008). This provides an additional level of complexity, as increased susceptibility of mutants might be due to impaired development. One clear example of the harmful effects of IMD overexpression is provided by caudal mutants, which display a much decreased lifespan provoked by the preferential colonization of the gut by a noxious commensal bacterium that is resistant to AMPs (Ryu et al, 2008). This susceptibility phenotype is reversed in caudal-Dredd double mutants, and importantly, in germ-free flies. To establish rigorously that the systemic, constitutive activation of the IMD pathway is detrimental in the absence of microbiota, the acid test would be to compare the lifespan of flies mutant for a negative regulator to that of flies doubly mutant for that regulator and the IMD pathway, in sterile and normal conditions. Inactivating PGRP-LF only in adults may be the best option as this is the one negative regulator that may specifically interfere with the IMD pathway.
PVR downregulation of the IMD pathway appears to be constitutive during the systemic immune response. The induction of the Ras/MAPK pathway by infections is well documented in the midgut. Indeed, the EGFR pathway that signals through the Ras/MAPK pathway is required first in enterocytes for their delamination when damaged by the host oxidative response against pathogens and, later, for the compensatory proliferation of intestinal stem cells (ISC; Buchon et al, 2010). Interestingly, PVF2 expression is also induced in ISCs and enteroblast in response to oxidative damage (Choi et al, 2008). Ragab et al (2011) establish that the inactivation of the Ras/MAPK pathway by RNAi in ISCs and enteroblasts leads to an enhanced expression of AMPs in enterocytes, enteroblasts, and possibly also in ISCs, although this latter point requires confirmation with specific stem cell markers. It is not clear whether the enterocyte phenotype is due to a nonautonomous effect caused by signalling through PVF2 originating from ISCs and enteroblasts or to the perdurance of the RNAi effect during differentiation of enteroblasts into enterocytes. Indeed, the exposure of the intestinal epithelium to Erwinia carotovora causes the death of 50% of the enterocytes following oxidative damage and leads to the sustained differentiation of enteroblasts and rapid compensatory proliferation of ISCs through EGFR signalling. Ragab et al (2011) propose that the inhibition of IMD signalling in ISCs may help them preserve their function in epithelial homeostasis, a hypothesis that should be tested using lineage tracing experiments. The work of Ragab et al (2011) paves the way for a thorough dissection of the relationships between the immune response in intestinal cells and tissue homeostasis of the midgut.
Acknowledgments
We apologize to our colleagues that we could not cite here due to space restrictions. Work in the Ferrandon laboratory is funded by CNRS, ANR, NIH (grant PO1 AI44220), and Fondation pour la Recherche médicale (Equipe FRM).
Footnotes
The authors declare that they have no conflict of interest.
References
- Basbous N, Coste F, Leone P, Vincentelli R, Royet J, Kellenberger C, Roussel A (2011) The Drosophila peptidoglycan recognition protein LF interacts with peptidoglycan recognition protein LC to down regulate the Imd pathway. EMBO Rep (advance online publication; doi:; DOI: 10.1038/embor.2011.19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff V, Vignal C, Duvic B, Boneca IG, Hoffmann JA, Royet J (2006) Downregulation of the Drosophila immune response by peptidoglycan-recognition proteins SC1 and SC2. PLoS Pathog 2: e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Kuraishi T, Lemaitre B (2010) Drosophila EGFR pathway coordinates stem cell proliferation and gut remodeling following infection. BMC Biol 8: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi NH, Kim JG, Yang DJ, Kim YS, Yoo MA (2008) Age-related changes in Drosophila midgut are associated with PVF2, a PDGF/VEGF-like growth factor. Aging Cell 7: 318–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandon D, Imler JL, Hetru C, Hoffmann JA (2007) The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat Rev Immunol 7: 862–874 [DOI] [PubMed] [Google Scholar]
- Ganesan S, Aggarwal K, Paquette N, Silverman N (2010) NF-kappaB/Rel proteins and the humoral immune responses of Drosophila melanogaster. Curr Top Microbiol Immunol (; DOI: 10.1007/82_2010_107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgel P, Kappler C, Langley E, Gross I, Nicolas E, Reichhart J-M, Hoffmann JA (1995) Drosophila immunity. A sequence homologous to mammalian interferon consensus response elements enhances the activity of the diptericin promoter. Nuc Acid Res 23: 1140–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet F, Bischoff V, Vignal C, Hoffmann J, Royet J (2008) The Drosophila peptidoglycan recognition protein PGRP-LF blocks PGRP-LC and IMD/JNK pathway activation. Cell Host Microbe 3: 293–303 [DOI] [PubMed] [Google Scholar]
- Ragab A, Buechling T, Gesellchen V, Spirohn K, Boettcher A-L, Boutros M (2011) Drosophila Ras/MAPK signalling regulates innate immune responses in immune and intestinal stem cells. EMBO J 30: 1123–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu JH, Kim SH, Lee HY, Bai JY, Nam YD, Bae JW, Lee DG, Shin SC, Ha EM, Lee WJ (2008) Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science 319: 777–782 [DOI] [PubMed] [Google Scholar]